A comparative study of Bi, Sb, and BiSb for electrochemical nitrogen reduction leading to a new catalyst design strategy†
Youn Jeong
Jang‡
ab,
Taylor A.
Evans‡
a,
Bipasa
Samanta‡
c,
Keyu
Zeng
a,
Maytal Caspary
Toroker
 *cd and
Kyoung-Shin
Choi
*cd and
Kyoung-Shin
Choi
 *a
*a
aDepartment of Chemistry, University of Wisconsin–Madison, Madison, WI 53706, USA. E-mail: kschoi@chem.wisc.edu
bDepartment of Chemical Engineering, Hanyang University, Seongdong-gu, Seoul, 04763, Republic of Korea
cDepartment of Materials Science and Engineering, Technion−Israel Institute of Technology, Haifa 3200003, Israel. E-mail: maytalc@technion.ac.il
dThe Nancy and Stephen Grand Technion Energy Program, Technion−Israel Institute of Technology, Haifa 3200003, Israel
First published on 25th August 2021
Abstract
Recent studies identified Bi as one of the most promising non-noble metal elements that can promote the electrochemical N2 reduction reaction (ENRR) to produce NH3. The electronic features that make Bi a promising ENRR catalyst may also be owned by Sb that belongs to the same group as Bi. Thus, the ENRR properties of Bi, Sb, and a BiSb alloy were investigated comparatively to identify common characteristics that facilitate the ENRR. These catalysts were prepared as uniform coating layers on high surface area carbon felt electrodes, which could serve as both regular electrodes and pseudo-gas diffusion electrodes. The experimental results demonstrated that while Bi and Sb show comparable ENRR performances, the formation of a BiSb alloy distinctively increases the faradic efficiency for NH3 production. The X-ray photoelectron spectroscopy results revealed that Bi in BiSb possesses a partial positive charge while Sb in BiSb possesses a partial negative charge, which can impact the way the catalyst surface interacts with the reactants and reaction intermediates of the ENRR and hydrogen evolution reaction (HER), the major competing reaction with the ENRR. Computational investigations including the Bader charge analysis and Gibbs free energy calculations for the elemental steps of the ENRR and HER provided an explanation of how the formation of a BiSb alloy can change the selectivity for the ENRR. The combined experimental and theoretical results and discussion contained in this study lead to a new strategy for designing efficient metal catalysts for the ENRR. Additionally, this study investigated how the use of gas phase and dissolved N2 affected the ENRR performances of the Bi, Sb, and BiSb catalysts.
1. Introduction
Recently, the electrochemical nitrogen reduction reaction (ENRR) has greatly attracted research interest as a way to produce ammonia (NH3) owing to the reduced cost of electricity generated using renewable energy. NH3 is a key fertilizer, an important feedstock in industry, and a carbon-free energy source.1–3 Unlike the traditional Haber-Bosch process, the ENRR produces NH3 under ambient temperatures and pressures, using water as the hydrogen source.4 Thus, it can offer an environmentally benign and sustainable route to produce NH3. Furthermore, the ENRR can allow for the decentralized production of NH3 in various capacities to suit the needs of those in remote areas, leading to reduced transportation and investment costs.5The ENRR is currently impeded by the extremely low faradaic efficiency (FE) for the conversion of N2 to NH3. This low FE stems from two reasons. First, the standard reduction potential of N2 to NH3 (N2(g) + 8H+ + 6e− ⇌ 2NH4+(aq), E° = 0.275 V vs. SHE) is very close to the standard reduction potential of the hydrogen evolution reaction (HER) (2H+ + 2e− ⇌ H2(g), E° = 0 V vs. SHE).8 Second, N2 reduction to NH3 is a much more kinetically complex process than the HER. As a result, the HER dominates under the applied potentials required for the ENRR. Thus, the discovery of new catalysts and strategies that can promote the ENRR is critically needed. The development of catalysts that do not contain noble metal elements are of particular interest as it can enable the production of ENRR catalysts on a practical scale.
Recent studies showed that Bi is one of the most promising non-noble metal elements that can promote the ENRR.6–9 These observations may be related to a few features of Bi that can serve favorably for the ENRR. First, Bi has weak proton binding affinity and is poor at performing the HER.10 Second, Bi is a semimetal and its conductivity is lower than typical metals.11 It has been proposed that the rate of the HER is approximately first order with respect to the electron concentration, while the ENRR is zeroth order.12 This means that as the availability of electrons decreases, the ENRR rate will not be strongly affected while the HER rate will decrease exponentially. Thus, the low conductivity of Bi may favorably act to increase the FE for NH3 production. Third, it has also been demonstrated through density functional theory (DFT) calculations that the energy level of Bi 6p orbitals is advantageously positioned to interact with the N 2p orbitals, allowing for a stronger interaction between the Bi surface and a N2 molecule and weakening the N2 triple bond.6
The advantageous features of Bi for the ENRR motivated us to investigate the ENRR performance of Sb, another semi-metal directly above Bi in the periodic table, in comparison with that of Bi. Compared to Bi, Sb has received little attention as a possible ENRR catalyst, and there have been only a handful of studies of ENRR catalysts containing Sb.13–15 The Sb-based catalysts used in these studies were composed of rather complex compositions and interfaces (e.g. antimony sulfide/tin oxide, antimony/antimony oxide, antimony phosphate/carbon). The ENRR property of pure Sb metal has not been investigated to date. Thus, comparing the ENRR performance of pure Sb metal in comparison with that of Bi can provide interesting new insights to understand the features of metals that can promote the ENRR.
The goal of the current study is two-fold. First, we aimed to prepare Bi, Sb, and a solid solution alloy BiSb with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 atomic ratio of Bi
1 atomic ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb to comparatively study their ENRR performances. To obtain an accurate comparison, it is critical to prepare these catalysts with comparable morphologies and surface areas under similar synthesis conditions so that any difference observed in their ENRR performances is solely due to the difference in their compositions. Thus, we prepared Bi, Sb, and BiSb as uniform coating layers on a high surface area carbon felt substrate by electrodeposition and investigated their ENRR performances. Our experimental results show that the solid solution of BiSb shows a considerably enhanced ENRR performance compared with pure Bi and Sb. We conducted thorough computational investigations to elucidate the reason, which in turn provided a novel insight and general strategy that can be used to design efficient metal catalysts for the ENRR. The second goal is to examine if the performance of any of these catalysts can be improved if they can use N2 gas directly instead of N2 gas dissolved in the electrolyte for the ENRR. This allows us to study whether the low solubility of N2 in the aqueous electrolyte (ca. 1 mM at 20 °C and 1 bar)16 is one of the major limiting factors for the low FE for NH3 production. For this purpose, we developed a simple method to use the same Bi, Sb, and BiSb electrodes on carbon felt both as a regular electrode and as a pseudo-gas diffusion electrode (GDE) so that the effect of using N2 gas for the ENRR can be unambiguously identified.
Sb to comparatively study their ENRR performances. To obtain an accurate comparison, it is critical to prepare these catalysts with comparable morphologies and surface areas under similar synthesis conditions so that any difference observed in their ENRR performances is solely due to the difference in their compositions. Thus, we prepared Bi, Sb, and BiSb as uniform coating layers on a high surface area carbon felt substrate by electrodeposition and investigated their ENRR performances. Our experimental results show that the solid solution of BiSb shows a considerably enhanced ENRR performance compared with pure Bi and Sb. We conducted thorough computational investigations to elucidate the reason, which in turn provided a novel insight and general strategy that can be used to design efficient metal catalysts for the ENRR. The second goal is to examine if the performance of any of these catalysts can be improved if they can use N2 gas directly instead of N2 gas dissolved in the electrolyte for the ENRR. This allows us to study whether the low solubility of N2 in the aqueous electrolyte (ca. 1 mM at 20 °C and 1 bar)16 is one of the major limiting factors for the low FE for NH3 production. For this purpose, we developed a simple method to use the same Bi, Sb, and BiSb electrodes on carbon felt both as a regular electrode and as a pseudo-gas diffusion electrode (GDE) so that the effect of using N2 gas for the ENRR can be unambiguously identified.
We note that in this study, isotopically labeled 15N2 was used as the N2 source and only 15NH3 was quantified to calculate the FE for NH3 production. Thus, the effect of any environmental 14NH3 contamination, which could result in the overestimation of the FE for NH3 production, was completely eliminated.
2. Methods
2.1 Materials
Potassium antimony tartrate (K2Sb2(C4H2O6)2·H2O, 99%), bismuth(III) nitrate (Bi(NO3)3·5H2O, ≥98.0%), sodium hydroxide (NaOH, ≥97.0%), ethylenediaminetetraacetic acid (EDTA, 99.4–100.6%), boric acid (H3BO3, ≥99.5%), potassium pyrophosphate (K4P2O7, 97%), tartaric acid (C4H6O6, ≥99.5%), hydrochloric acid (HCl, 37%), 4-(dimethylamino)benzaldehyde (C9H11NO, 99%), hydrazine hydrate (N2H4·xH2O, 50–60%), phosphoric acid (H3PO4, 85%), deuterated water (D2O, 99.9%) and ammonium chloride (15NH4Cl, 99%) were all purchased from Millipore-Sigma. All chemicals were used as received and without further purification. Carbon felt (AvCarb C100) was purchased from Fuel Cell Store for use as the working electrode. Deionized (DI) water with a resistivity of >18.0 MΩ cm was used to prepare all solutions.2.2 Synthesis of Bi, Sb, and BiSb
Electrodeposition was carried out in an undivided three electrode cell composed of a carbon felt working electrode, a Ag/AgCl (4 M KCl) reference electrode and a carbon rod counter electrode using a VMP2 multichannel potentiostat (Princeton Applied Research). The working electrode was prepared by first cutting carbon felt into 5 cm × 1 cm × 0.3 cm strips. Then, the top portion of the carbon felt electrodes was tightly wrapped with Teflon tape (J.V. Converting Company) to prevent solution from traveling up the carbon felt and making direct contact with the conducting alligator clip, which connects the carbon felt to the lead from the potentiostat. The size of the carbon felt electrode exposed to the solution was 2 cm × 1 cm × 0.3 cm. All current densities reported in this study are the currents divided by the largest lateral area (2 cm × 1 cm) of the carbon felt substrate.For Bi deposition, the aqueous plating solution contained 20 mM Bi(NO3)3·5H2O and 1 M tartaric acid with the pH adjusted to 6 with NaOH. The films were deposited using a pulsed deposition where a short pulse of potential is applied followed by a resting time at the open circuit potential (OCP). Each cycle was composed of applying a potential of −0.9 V vs. AgCl for five seconds followed by a resting time of ten seconds, and the cycle was repeated for 24 times to pass approximately 1.6 C of charge. The pulsed deposition was critical for the deposition of Bi as a uniform layer. For Sb deposition, the aqueous plating solution contained 10 mM K2Sb2(C4H2O6)2·H2O and 1 M tartaric acid with the pH adjusted to 7.5 with NaOH. The films were deposited onto carbon felt by applying −1.4 V vs. Ag/AgCl for 20 seconds, passing approximately 1.6 C of charge. For the deposition of BiSb, an aqueous plating solution containing 20 mM Bi(NO3)3·5H2O, 30 mM K2Sb2(C4H2O6)2·H2O, and 1 M tartaric acid with the pH adjusted to 6 with NaOH was used. The films were deposited by applying −1.5 V vs. Ag/AgCl for 20 seconds to pass approximately 1.6 C of charge. Before deposition of any species onto the carbon felt substrate, the carbon felt working electrode was immersed in a tartate solution (pH ∼7) that did not contain Bi or Sb, and the potential was swept from the OCP to −1.7 V vs. Ag/AgCl. This process ensured sufficient wetting of the surface of the carbon felt substrate before electrodeposition, allowing for more uniform coverage of the deposits on the substrate.
2.3 Characterization
Powder X-ray diffraction (PXRD, D8 Discover, Bruker, Ni-filtered Cu Kα radiation, λ = 1.5418 Å) was used to examine the crystal structure of the electrodes. Scanning electron microscopy with an accelerating voltage of 2 keV (SEM; LEO Supra55 VP) and energy dispersive spectroscopy with an accelerating voltage of 22 keV (EDS; Noran System Seven, Thermo-Fisher, ultra-dry silicon drift detector) were used to investigate the morphology and bulk elemental ratios respectively. X-ray photoelectron spectroscopy (XPS) using a Thermo Scientific K-Alpha X-ray photoelectron spectrometer equipped with an Al Kα excitation source was used to determine surface elemental compositions.2.4 Electrochemical characterization
All electrochemical measurements were performed in an undivided three electrode cell. Either Sb, Bi or BiSb on carbon felt was used as the working electrode, with a Ag/AgCl (3 M KCl) reference electrode and a carbon rod counter electrode. The stainless-steel cell used in this study has a cover with a gas inlet and an outlet to allow for gas purging. Before any electrochemical measurement, the solution was saturated with Ar (99.999%) or 15N2 and during the measurement the gas was purged constantly. When the catalyst electrode was used as a regular electrode, 15N2 was purged through a needle inserted into the electrolyte. The purging needle was approximately 1 cm apart from the catalyst electrode, and the rate of gas flow was 100 mL min−1. When the catalyst electrode was used as a pseudo-GDE, the purging needle was inserted into the middle of the carbon felt substrate as illustrated in Scheme 1. The needle had multiple holes on the side to purge N2 to all directions within the substrate. The rate of gas flow was varied from 50 mL min−1 to 200 mL min−1 to find an optimal rate.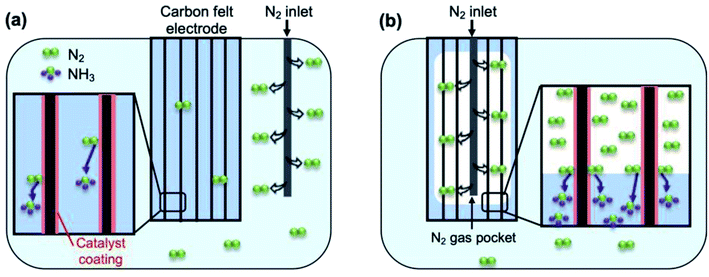 | ||
| Scheme 1 An illustration of how our Bi, Sb, and BiSb electrodes can be used as (a) a regular electrode and (b) a pseudo-GDE. | ||
In this study, all ENRR experiments were performed with isotopically labeled 15N2 as the N2 source and only 15NH3 (not 14NH3) was quantified to calculate the FE for NH3 production.17 This was to eliminate any concern from a possible environmental 14NH3 contaminant falsely affecting the FE for NH3 production. The purity of the isotopically labelled 15N2 gas is 98% 15N and ≥99.0% N2 (15N2 + 14N2) with the remaining <1% impurities identified by the manufacturer (Sigma-Aldrich) as Ar, O2, CO2, N2O, and C2H6. To remove any nitrogen-containing impurities in the N2 gas flow, the 15N2 gas was first passed through a Cu/SAPO trap as recommended in the literature.17
The linear sweep voltammograms (LSVs) were obtained in 0.5 M borate (pH 9.3) by sweeping the potential from the OCP to the negative direction with the scan rate of 25 mV s−1. Constant potential ENRR was performed in the same solution by applying a desired potential and passing 10 C. All constant potential ENRRs were repeated with Ar instead of 15N2 to confirm the produced 15NH3 came from the reduction of 15N2 gas. Before any measurements, a potential of −0.6 V vs. RHE for 1 minute was applied to reduce the naturally formed oxide passivation layer on the metal surfaces.
Proton nuclear magnetic resonance (1H-NMR) was used to quantify the produced 15NH3 on a Bruker Advance III HD 600 MHz spectrometer (Bruker Biospin Corp., Billerica, MA) equipped with a TCI-F cryoprobe with z-gradient. After the J–t measurement, the electrolyte was collected and acidified to pH ∼3 using H3PO4 and then condensed to 6 mL using reduced pressure distillation. After this, 5 v% D2O with trimethylsilylpropanoic acid (TSP) was mixed with the solution to act as the internal reference for locking. The 1H signal from the water was removed using water suppression with excitation sculpting. The quantification of 15NH3 was not affected by water suppression because the 15NH4+ signal appears at 7 ppm. Spectra were acquired using 1024 scans, a relaxation delay of 1.5 s and an acquisition time of 3 s. A calibration curve was prepared using solutions of a known concentration of 15NH4Cl in the same electrolyte used for the J–t measurements followed by the above acidification and mixing with D2O containing TSP for locking and integrating the observed 15NH4+ signal for each.
The ENRR can also produce N2H4, therefore the Watt and Crisp method was used to determine if any N2H4 formed during the ENRR.18 After the ENRR, 2 mL of the electrolyte was collected in a centrifuge tube and 2 mL of ethanol solution containing 0.7 M HCl and 0.12 M 4-(dimethylamino)benzaldehyde was added. The solution was left to react for 1 hour at room temperature and then the absorbance was measured at 455 nm using a UV-VIS spectrophotometer. The concentration of N2H4 was determined by comparing the measured absorbance to a calibration curve generated using standard solutions of N2H4 prepared by adding N2H4·xH2O into the electrolyte used in J–t measurements. No N2H4 was detected for any of the J–t measurements. The FE for NH3 was determined using the following equation where F is Faraday's constant (96485.33 C mol−1):
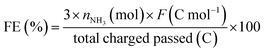 | (1) |
While a Ag/AgCl reference was used in each experiment performed, all results within this report are presented against the reversible hydrogen electrode (RHE) for direct comparison to other reports in aqueous solution but that contain different pH values. The conversion between Ag/AgCl and RHE reference electrode values can be done via the following equation:
| E(vs. RHE) = E(vs. Ag/AgCl) + E(Ag/AgCl)(reference) + 0.0591 V × pH (at 25 °C) | (2) |
| EAg/AgCl(reference, 4 M KCl) = 0.1976 V vs. NHE at 25 °C |
| EAg/AgCl(reference, 3 M KCl) = 0.209 V vs. NHE at 25 °C |
2.5 Computational methods
In order to understand the key factors that make BiSb a better ENRR catalyst than Bi and Sb, Density Functional Theory (DFT) calculations were performed using the Vienna ab Initio Simulation Package (VASP).19–21 Spin-polarized DFT22,23 calculations were performed using the generalized gradient approximation of Perdew, Burke, and Ernzerhof (PBE).24 The utility of the PBE functional for modeling the nitrogen reduction reaction (NRR) on the current system has been already well established.25–28 The frozen core electrons of Bi 1s2s2p3s3p3d4s4p4d5s5p4f, Sb 1s2s2p3s3p3d4s4p4d and N 1s are presented by the projected augmented wave (PAW) method.20For the initial bulk system, we have taken a rhombohedral structure with a R3m space group for all three systems (Bi, Sb and BiSb).29,30 After the initial convergence test for energy cut off and K-point, we have obtained an energy cut off value of 450 eV for the Bi and BiSb systems and 300 eV for the Sb system for a converged energy of 1 meV per atom (Fig. S1†). The converged K-point for the bulk systems is 16 × 16 × 6 gamma points. From the optimized bulk (Table S1†) we have obtained the (012) surface of Bi, Sb, and BiSb to study for the ENRR (Fig. S2†). The (012) plane was chosen because it is the most densely packed plane and is mentioned to be more catalytic for the ENRR than other low indexing planes.26,28,31 Due to these reasons, the (012) plane has been most intensively investigated for the ENRR on Bi.28,31,32 For the slab optimization we have taken 5 × 5 × 1 gamma K-points with the vacuum of 15 Å in the z direction. The energy and force convergence criteria of 1 × 10−5 eV and −0.02 eV Å−1 are employed respectively in all the calculations.
The Gibbs free energy (ΔG) of each elemental step is calculated using the following equation (Tables S2 and S3†).33
| ΔG = ΔE + ΔEZPE − TΔS | (3) |
3. Results and discussion
3.1 Synthesis of Bi, Sb, and BiSb electrodes
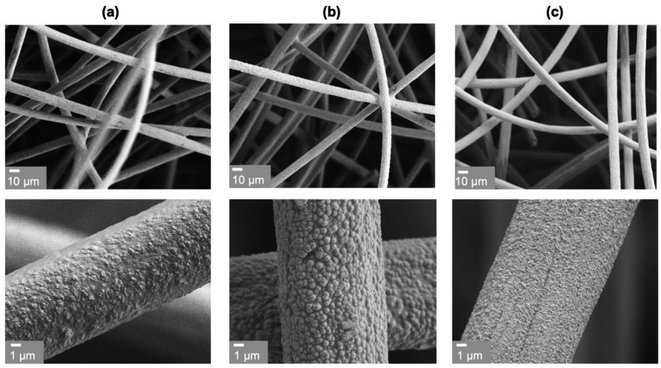
Bi, Sb and BiSb were deposited on the carbon felt substrate to investigate their ENRR performances. The carbon felt substrate not only provided a high surface area to deposit Bi, Sb and BiSb but also allowed the resulting Bi, Sb and BiSb electrodes to be tested both as regular electrodes and as pseudo-GDEs, as explained in detail below.In order to accurately compare the ENRR performances of Bi, Sb and BiSb, they must be prepared with comparable morphologies and surface areas so that the difference in their ENRR performances solely originates from the difference in their compositions, and is not affected by any difference in their morphologies. Thus, we aimed to deposit Bi, Sb and BiSb as featureless conformal coating layers on the carbon felt substrate using plating solutions whose compositions are comparable with each other. We discovered that tartrate solutions (pH 6–7.5) can solubilize both Bi3+ and Sb3+ ions and allow Bi, Sb, and BiSb to be deposited as uniform coating layers on the carbon felt substrate (Fig. 1). The same amount of charge was passed to deposit each of these layers to ensure that the thicknesses of all catalyst layers are comparable (150–200 nm) (Fig. S7†).
The XRD patterns of Bi, Sb, and BiSb deposited on carbon felt are shown in Fig. 2. In the XRD patterns, broad peaks originating from the carbon felt were commonly observed. The Bi and Sb electrodes show Bi peaks and Sb peaks, respectively, indicating that the as-deposited Bi and Sb layers are crystalline, although the crystallinity of the Sb layer appears to be significantly lower than that of the Bi layer. We note that Bi and Sb have the same crystal structure, and the only difference is that Sb has smaller cell parameters,36 which shift the Sb peaks to higher two theta values. BiSb also has the same crystal structure, and its (hkl) peaks appear exactly at the positions expected for a solid solution alloy of Bi and Sb with the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb ratio of 1
Sb ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whose unit cell parameters are the averages of the unit cell parameters of Bi and Sb.36
1, whose unit cell parameters are the averages of the unit cell parameters of Bi and Sb.36
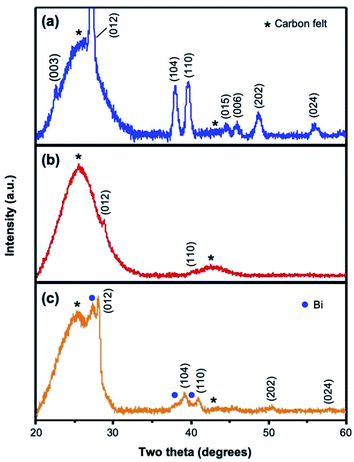 | ||
Fig. 2 XRD patterns of as-deposited (a) Bi, (b) Sb, and (c) BiSb on carbon felt. The (hkl) indices are assigned from the PDF cards for Bi (44-1246) and Sb (35-0732). The broad peaks corresponding to the carbon felt are denoted by an asterisk. Bi (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 0.454650 nm and c = 1.186294 nm), Sb (R m, a = 0.454650 nm and c = 1.186294 nm), Sb (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 0.430777 nm and c = 1.127662 nm) and BiSb (R m, a = 0.430777 nm and c = 1.127662 nm) and BiSb (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = 0.442680 nm and c = 1.160351 nm).36 m, a = 0.442680 nm and c = 1.160351 nm).36 | ||
The XRD of the BiSb electrode also shows peaks from Bi, meaning Bi is present as an impurity phase in the BiSb electrode. The EDS analysis, which analyzes the bulk composition of the sample, showed that the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb ratio in the BiSb electrode is 2
Sb ratio in the BiSb electrode is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, the XPS analysis, which analyzes the surface composition of the sample, showed that the Bi
1. However, the XPS analysis, which analyzes the surface composition of the sample, showed that the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb ratio in the BiSb electrode is 1
Sb ratio in the BiSb electrode is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which agrees with the Bi
1, which agrees with the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb ratio in the BiSb alloy. Combining the results from XRD, EDS, and XPS, the BiSb sample appeared to contain a pure Bi layer as the bottom layer and a 1
Sb ratio in the BiSb alloy. Combining the results from XRD, EDS, and XPS, the BiSb sample appeared to contain a pure Bi layer as the bottom layer and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiSb alloy layer as the top layer with the amount of Bi in the Bi layer and that in the BiSb layer to be approximately 1
1 BiSb alloy layer as the top layer with the amount of Bi in the Bi layer and that in the BiSb layer to be approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The formation of a Bi layer as the bottom layer could not be avoided because the onset potential for the reduction of Bi3+ to Bi0 is significantly more positive than that of Sb3+ to Sb0 in the tartrate solutions used in this study (Fig. S8†). This means that when the deposition of BiSb was accomplished using a deposition potential that is negative enough to reduce both Bi3+ and Sb3+, the overpotential applied for Bi deposition is significantly larger than that for Sb deposition. Thus, Bi was deposited first before Bi and Sb are co-deposited with the 1
1. The formation of a Bi layer as the bottom layer could not be avoided because the onset potential for the reduction of Bi3+ to Bi0 is significantly more positive than that of Sb3+ to Sb0 in the tartrate solutions used in this study (Fig. S8†). This means that when the deposition of BiSb was accomplished using a deposition potential that is negative enough to reduce both Bi3+ and Sb3+, the overpotential applied for Bi deposition is significantly larger than that for Sb deposition. Thus, Bi was deposited first before Bi and Sb are co-deposited with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Since XPS confirmed that the top layer is a pure 1
1 ratio. Since XPS confirmed that the top layer is a pure 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiSb alloy, the fact that the BiSb electrode contains Bi as the bottom layer did not affect our goal of comparing the ENRR performances of Bi, Sb, and BiSb.
1 BiSb alloy, the fact that the BiSb electrode contains Bi as the bottom layer did not affect our goal of comparing the ENRR performances of Bi, Sb, and BiSb.
3.2 ENRR performance of Bi, Sb, and BiSb as regular electrodes and pseudo-GDEs
As we mentioned earlier, one of the major goals of the current study is to examine whether the low solubility of N2 gas in aqueous electrolytes (e.g. 1.4 mM N2 at 20 °C)16 is one of the main reasons for low FEs for NH3 production and whether the use of a GDE, which can directly utilize the gas phase N2 as the reactant, can enhance the FE for NH3 production. Our Bi, Sb, and BiSb electrodes deposited on the carbon felt substrate allow us to use the same electrodes as a regular electrode and as a pseudo-GDE as explained in Scheme 1.Scheme 1a shows the case where these electrodes are used as regular electrodes. In this set-up, 15N2 is provided by a 15N2-purging needle immersed in the electrolyte. The solution is pre-saturated with 15N2 and 15N2 is also constantly purged during the reaction with a gas flow rate of 100 mL min−1. The catalyst coating on the carbon felt form the catalyst/electrolyte interface and the catalysts will utilize dissolved 15N2 for the ENRR. Scheme 1b shows the case where these electrodes are used as a pseudo-GDE. In this case, the 15N2-purging needle is placed in the middle of the carbon felt electrode. The 15N2 gas comes out from the multiple side openings of the needle, expelling the solution to create a gas pocket within the carbon felt electrode. By adjusting the rate of 15N2 gas coming out from the openings of the needle (50–200 mL min−1), an optimal three-phase interface among the 15N2 gas pocket, solid catalyst on the carbon fibers, and liquid electrolyte that contains water as the hydrogen source can be created.
The pseudo-GDE used in this study is the same as a true GDE in that it allows the catalyst to directly utilize gas phase N2 for the ENRR by creating the three-phase interface.37 However, unlike the preparation of a true GDE,37 no alteration for the substrate structure, catalyst preparation, and cell configuration was necessary for the conversion of a regular electrode to a pseudo-GDE in our study. Thus, its use in comparison with the use of the same electrode as a regular electrode allowed us to directly assess the change in the ENRR performances caused only by the use of gas phase N2versus dissolved N2.
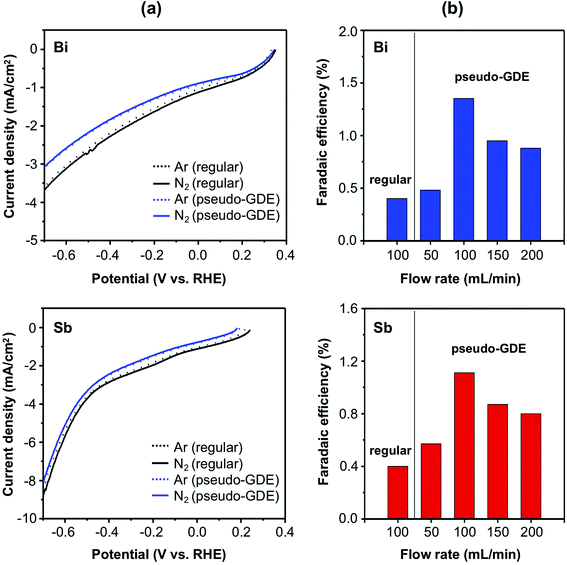
The ENRR performance of the Bi electrode as a regular electrode was first investigated by comparing LSVs obtained in 0.5 M borate (pH 9.3) saturated with either Ar or 15N2 (Fig. 3a, top). When the electrolyte was saturated with Ar, the hydrogen evolution reaction (HER) was the only reduction reaction that could occur. The reason why the LSV showed cathodic current even before the potential reaches the thermodynamic onset potential for the HER (0 V vs. RHE) is due to the high surface area carbon felt electrode creating high charging current when sweeping the potential. Since the charging current profile is not completely flat, it is rather difficult to know the exact onset potential for the HER. When the electrolyte is saturated with 15N2, little change in current density was observed in the LSV. This does not necessarily mean that Bi cannot conduct the ENRR because if Bi performs the ENRR using a fraction of electrons that would otherwise be used for the HER, the total current density would not change. Thus, in order to examine whether or not the ENRR occurs on the Bi electrode, a constant potential reduction and product analysis was necessary.
When the ENRR was performed at a constant potential of −0.6 V vs. RHE, a FE of 0.4% for NH3 production was observed (Fig. 3b, top). The Bi electrode shows a negligible ENRR activity at potentials more positive than −0.6 vs. RHE (Table S4†).
Next, the ENRR performance of the Bi electrode as a pseudo-GDE was investigated using the setup shown in Scheme 1b. The LSVs obtained with a gas flowing rate of 100 mL min−1 is shown in Fig. 3a, top. The LSV of Bi as a pseudo-GDE obtained with Ar shows a slight decrease in the current density compared with that of Bi as a regular electrode. This is because purging Ar within the carbon felt electrode created a gas pocket inside the carbon felt electrode, decreasing the electrode surface area that is in contact with the electrolyte. This decreases the electrode area that can perform the HER.
When 15N2 was purged instead of Ar in the pseudo-GDE, a similar decrease in the current density was observed in the LSV due to the same reason. Since the major reaction that occurs on the electrode is the HER, the decrease in the electrode/electrolyte area resulted in the decrease in the total current density even if the FE for the ENRR may increase. The LSVs obtained with various flow rates of 15N2 (50–200 mL min−1) can be found in Fig. S9,† where a gradual decrease in the total current density is shown with an increase in the N2 flow rate.
We performed the ENRR at a constant potential of −0.6 V vs. RHE using Bi as a pseudo-GDE with various flow rates of N2 and quantified the produced NH3 (Fig. 3b, top). The highest FE for NH3 production (1.3%) was obtained with the 15N2 flow rate of 100 mL min−1, and this FE is approximately three times higher than that obtained with Bi as a regular electrode. When the flow rate is lower than 100 mL min−1, it appeared that not a sufficient 15N2 pocket was developed to convert the Bi electrode to an effective pseudo-GDE. On the other hand, when the flow rate is higher than the optimal rate, the supply of 15N2 is no longer a limiting factor for the ENRR. It appears that an unnecessarily high flow rate can disrupt the formation of an optimal three-phase interfacial structure in our pseudo-GDE system.
Our result demonstrated that the use of a pseudo-GDE can certainly enhance the FE for NH3 production. However, the FE for NH3 is still low, suggesting that the low solubility of N2 is not the most critical limiting factor for the low FE for NH3 production when a Bi metal layer with an intrinsically limiting ENRR catalytic property is used as the electrode.
The Sb electrodes were investigated using the same procedures (Fig. 3, bottom and Fig. S9b†). Again, the LSVs obtained with Ar and 15N2 look comparable and the LSV obtained using Sb as a pseudo-GDE shows a slight decrease in the current density compared with that obtained with Sb as a regular electrode (Fig. 3a, bottom). When the ENRR was performed at a constant potential of −0.6 V vs. RHE, the FE for 15NH3 production was 0.4% as a regular electrode and 1.1% as a pseudo-GDE with the N2 flow rate of 100 mL min−1 (Fig. 3b, bottom), showing that the use of a GDE can enhance the ENRR performance of Sb by approximately three times. When the Sb electrode was used as a pseudo-GDE with various 15N2 flow rate, the FE obtained with the flow rate of 100 mL min−1 was the highest as in the case of Bi. The catalytic behaviors of Sb are comparable to those of Bi. However, Sb appears to be slightly inferior to Bi in terms of FE for NH3 production.
There may be a concern that the carbon felt used as the substrate may contain nitrogen species, which can be reduced to NH3 during the ENRR, and falsely increase the FE for NH3. We examined that the N1s region of the XPS spectrum of the carbon felt and confirmed the absence of nitrogen on the carbon felt surface (Fig. S10†). More importantly, we note that our FEs for NH3 were calculated based only on the amount of 15NH3 detected, and 14NH3 was not counted as the N2 reduction product. Thus, even if the nitrogen species in the carbon felt were reduced to 14NH3, it would not affect our FE results. We also performed the ENRR with 15N2 using a bare carbon felt electrode at −0.6 V vs. RHE and detected no 15NH3. This confirms that all 15NH3 detected in our experiments was truly from the reduction of 15N2 by Bi or Sb.
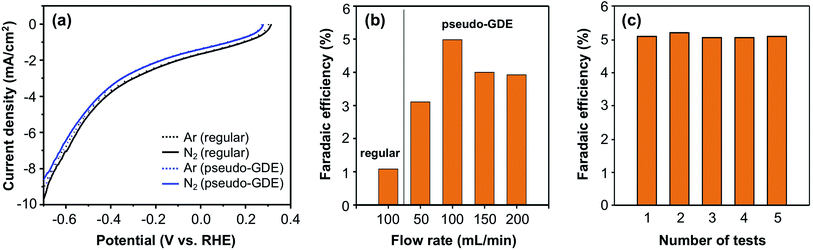
The ENRR performance of BiSb was examined using the same procedures (Fig. 4a and S9c†). Fig. 4a shows the LSVs of the BiSb electrode as a regular electrode and as a pseudo-GDE obtained in 0.5 borate (pH 9.3). As in the case of Bi and Sb, no noticeable difference in the LSVs was observed when the solution was purged with Ar or 15N2. Also, as in the case of Bi and Sb, the current density obtained using the BiSb electrode as a pseudo-GDE was slightly lower than the current density obtained using BiSb as a regular electrode.
When a constant potential ENRR was performed at −0.6 V vs. RHE, the BiSb electrode showed a considerably higher FE for 15NH3 production (Fig. 4b) than those obtained with the Bi and Sb electrodes. When used as a regular electrode, the FE for 15NH3 production by BiSb was 1.1%, which is more than a two-fold increase from those by Bi and Sb electrodes. When used as a pseudo-GDE, the FE for NH3 reaches 5.0% at a 15N2 flow rate of 100 mL min−1, which is more than a four-fold increase from those of Bi or Sb alone (as in the case of Bi and Sb, the highest FE was obtained when the N2 flow rate was 100 mL min−1). We also note that the enhancement in FE observed by the use of a pseudo-GDE is the greatest for BiSb; the supply of 15N2 can be a more limiting factor for a better ENRR catalyst. We conducted the constant potential ENRR four more times using the same BiSb pseudo-GDE (Fig. 4c) and obtained the same level of FE for NH3, which confirms the stability of the BiSb electrode.
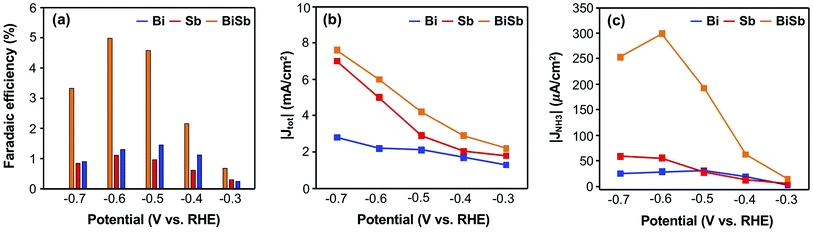
In Fig. 5a, the FEs for NH3 production by Bi, Sb, and BiSb as pseudo-GDEs are compared at various potentials when the 15N2 flow rate is fixed as 100 mL min−1. The FEs for NH3 production by BiSb are significantly higher than those by Bi and Sb at all potentials and is at a maximum at −0.6 V vs. RHE. We also compared the total current densities of the three pseudo-GDEs obtained at various constant potentials. We note that the current density is as important as the FE as the rate of NH3 production is governed by both factors. If the increase in FE is accompanied by a decrease in current density, no net gain may be obtained in terms of the production rate and yield of NH3. Our results show that the total current densities generated by the BiSb electrode during the constant potential ENRR at all potentials are also higher than those by Bi and Sb (Fig. 5b). Owing to the combination of enhanced FEs for NH3 production and enhanced current densities, the partial current densities for NH3 production by BiSb, which directly affect the production rate of NH3, are much higher than those of Bi and Sb (Fig. 5c). The production rates of NH3 by Bi, Sb and BiSb used as a pseudo-GDE are summarized in Table 1. The original J–t plots of Bi, Sb and BiSb obtained from the constant potential ENRR at various potentials are shown in Fig. S11.†
| Potential V vs. RHE | Production rate (μg cm−2 h−1) | ||
|---|---|---|---|
| Bi | Sb | BiSb | |
| −0.3 | 0.73 | 1.21 | 3.31 |
| −0.4 | 4.26 | 2.80 | 14.00 |
| −0.5 | 6.88 | 6.23 | 43.05 |
| −0.6 | 6.40 | 12.42 | 66.90 |
| −0.7 | 5.64 | 13.16 | 56.66 |
It is worth mentioning that while the FE values for NH3 production reported in this study may seem low compared with FE values reported in a few recent studies,6–9 the partial current density achieved by BiSb for NH3 production is considerable (Fig. 5c) because the total current density generated by BiSb is high (e.g., ∼6 mA cm−2 at −0.6 V vs. RHE) (Fig. 5b). The robustness of the BiSb alloy catalyst, which makes it possible to electrodeposit the catalyst with no delicate nanoscale morphology control or defect control, can be a practical advantage of BiSb.
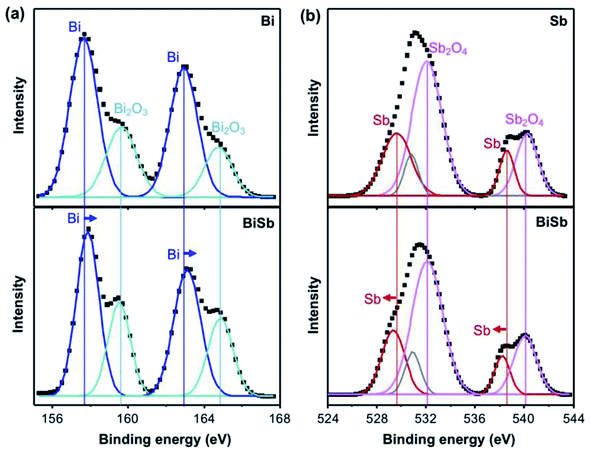
Our result that BiSb exhibits a considerably better ENRR performance for the ENRR is intriguing considering that BiSb is a solid solution of Bi and Sb, which at first does not seem to possess any unique features not owned by Bi or Sb. In order to investigate the reason for the enhanced ENRR of BiSb, we examined the XPS spectra of Bi, Sb, BiSb, which revealed surprising results. Fig. 6a shows the Bi 4f peaks of Bi and BiSb. Since Bi is not a noble metal, its surface is oxidized to Bi2O3. Thus, the Bi 4f region of the Bi electrode shows Bi peaks from Bi metal and also from a surface Bi2O3 layer.38,39 We note that the ENRR results we obtained were not affected by the presence of the Bi2O3 layer because the surface oxide layer was electrochemically pre-reduced to Bi prior to the ENRR (see the Methods section). The Bi2O3 detected by XPS was formed after the ENRR experiment when Bi was exposed to the air before the XPS measurements, which was unavoidable. The Bi 4f region of BiSb also shows Bi peaks from Bi metal and Bi2O3. However, the positions of the Bi peaks of BiSb are shifted to a higher binding energy compared with the positions of the Bi peaks of Bi, suggesting that Bi in BiSb bears a partial positive charge. The Bi2O3 peaks of BiSb do not show the same shift compared with the Bi2O3 peaks of Bi, meaning that the shifts of the Bi peaks of BiSb are not due to any systematic error but truly due to an intrinsic difference in the charge of Bi in Bi and BiSb.
Fig. 6b shows the Sb 3d peaks of Sb and BiSb. Since Sb is not a noble metal, its surface is also oxidized. Thus, the Sb 3d region of the Sb electrode shows peaks from Sb metal and a surface Sb2O4 layer.39–42 Again, since we pre-reduced the catalyst surface before the ENRR, our ENRR results were not affected by the surface oxide layer, and the Sb2O4 layer formed in the air after the ENRR is irrelevant to the ENRR performance. The Sb 3d region of the BiSb electrode also shows peaks from Sb and Sb2O4. This time, while the positions of the Sb2O4 remain the same, the Sb peaks of BiSb are clearly shifted to lower binding energy, meaning that Sb in BiSb possess a partial negative charge.
The XPS results indicate that Bi in BiSb bears a slightly positive charge and Sb in BiSb bears a slightly negative charge. The polarity between Bi and Sb in BiSb is unexpected as the electronegativities of Bi and Sb are very similar (2.02 for Bi and 2.05 for Sb).43 Nonetheless, the partial positive charge on Bi and the partial negative charge on Sb created by the formation of a BiSb alloy is most likely the key to explain the enhanced ENRR performances of BiSb compared with Bi and Sb metals that bear no charges. Thus, we turned to computational studies to confirm the charge distribution in BiSb and compare the surface properties and thermodynamics of Bi, Sb, and BiSb relevant to the ENRR.
3.3 Computational results
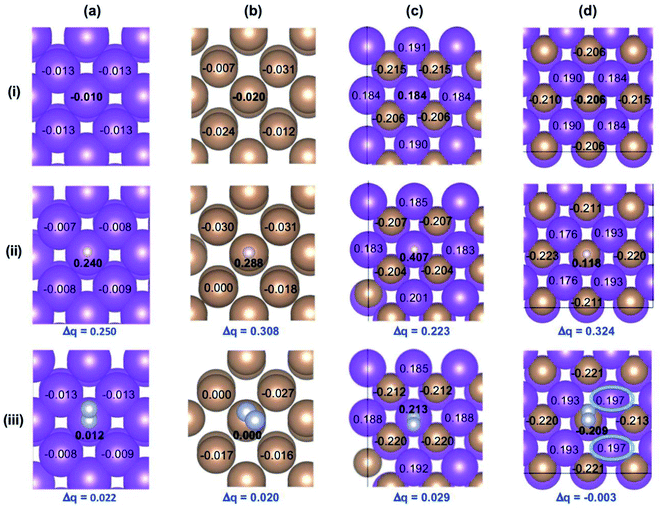
The charge of atoms on the catalyst surface can affect the binding strength and charge transfer between the catalyst surface and reactant species. Thus, we first performed Bader charge analysis on the (012) planes of Bi, Sb, and BiSb to examine whether the alloy formation can affect the charge distribution on Bi and Sb atoms. The reason for selecting the (012) plane is described in the method section, and the analysis results are summarized in Fig. 7. Fig. 7(i) shows that all atoms on the Bi and Sb surfaces contain slightly negative charges. However, when Bi and Sb form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 alloy, Bi atoms develop distinctive positive charges while Sb atoms develop distinctive negative charges. These results agree well with the experimental results obtained from the XPS analysis.
1 alloy, Bi atoms develop distinctive positive charges while Sb atoms develop distinctive negative charges. These results agree well with the experimental results obtained from the XPS analysis.
Next, we examined how the charges on the Bi, Sb and BiSb surfaces affect the adsorption of H, which is the critical step for the HER, the major competing reaction with the ENRR. Fig. 7(ii) shows the charges on Bi, Sb, Bi in BiSb, and Sb in BiSb after the adsorption of H. A greater change in charge at the binding site before and after H adsorption means a greater amount of charge transferred from the metal site to H and a stronger adsorption of H. The results show that adsorption strength of H is in the order of Sb in BiSb > Sb > Bi > Bi in BiSb. When we examined the changes of the charges on the catalyst surfaces after N2 adsorption, an opposite trend was observed. Fig. 7(iii) shows that change in the charge before and after N2 adsorption, which is equivalent to the adsorption strength of N2, is in the order of Bi in BiSb > Bi > Sb > Sb in BiSb.
The greatest amount of charge transferred from Bi in BiSb to N2 can be explained by considering the interaction of both the lone pair orbital and π* orbital of N2 with the metal atoms. Unlike H, N2 has lone pair electrons that can be donated to the metal binding site to form a sigma bond. Thus, Bi in BiSb that bears positive charges and can act as a Lewis acid, forms a stronger sigma bond with N2 compared with Bi, Sb, or Sb in BiSb that possess negative charges. The stronger sigma bond between Bi in BiSb and N2 in turn allows for a stronger π back bonding between them so that a greater amount of charge can be transferred from Bi in BiSb to the π* orbital of N2, which will more effectively activate the triple bond in N2. The Sb in BiSb shows negligible change in the charge before and after N2 adsorption. However, it is worth noting that the Bi atoms next to Sb show a noticeable increase in positive charge after N2 adsorption on Sb (blue circles in Fig. 7d(iii)), meaning that charge transfer occurs from neighboring Bi atoms to N2 adsorbed on Sb via the orbital interaction between Bi and Sb. In this case, the adsorption strength of N2 on Sb in BiSb may be stronger than that predicted by considering the change in charge only on the Sb binding site. In fact, ΔG for N2 adsorption discussed below suggests that N2 adsorption on Sb in BiSb is as favorable as that on Bi in BiSb. After N2 adsorption on Sb, the positive charges accumulated on the neighboring Bi atoms may further promote the adsorption of N2 on these Bi atoms.
The results obtained from the Bader charge analysis indicates that between Bi and Sb in BiSb, Bi is the more active site for the ENRR; the positive charges on Bi developed by the formation of a BiSb alloy makes the Bi sites more favorable for N2 adsorption but less favorable for H adsorption than Bi or Sb alone. Thus, it appears that the Bi site in BiSb is responsible for BiSb achieving a higher FE for NH3 production than Bi or Sb alone.
To further analyze the catalytic difference caused by the formation of a BiSb alloy, we calculated the Gibbs free energy of each elemental step involved with the ENRR. According to a literature survey there are two mechanisms: dissociative and associative (Fig. S12†).44,45 In the dissociative pathway, the triple bond in N2 is broken first, and the resulting two N atoms adsorb on two different catalytic sites (Fig. S12a†). Since N2 adsorbs very weakly on Bi, Sb, and BiSb, it is highly improbable that the triple bond in N2 is cleaved during the initial adsorption step. Hence, the energetically unfavorable dissociative pathway was not considered in our calculations. In the associative mechanism there are also two pathways, the alternate pathway and the distal pathway (Fig. S12b and c†).26,44–46 The Gibbs free energy for the elemental steps of alternate and distal pathways are provided in Tables S5 and S6.† The configurations of N2 and other intermediate species on the catalyst surfaces are shown in Fig. S3–S6.† Our results show that the alternate pathway is thermodynamically more favored than the distal pathway for all catalysts investigated in this study and that the formation of the intermediate species NNH, which is the common step for both pathways, is the potential determining step (PDS) for the ENRR for all catalysts (Tables S5 and S6†). Table 2 summarizes the ΔG for N2 adsorption, ΔG for the PDS of the ENRR (ΔGENRRPDS), and ΔG for the PDS of the HER (ΔGHERPDS), which is the adsorption of H, for all catalysts. The rest of the ENRR steps are thermodynamically more favorable than the N2 adsorption and the formation of NNH.
| Site | ΔG for N2 adsorption | ΔGENRRPDS | ΔGHERPDS |
|---|---|---|---|
| Bi | 0.098 | 1.912 | 0.627 |
| Bi in BiSb | 0.020 | 2.020 | 0.680 |
| Sb in BiSb | 0.021 | 1.968 | 0.460 |
| Sb | 0.111 | 1.964 | 0.521 |
The results in Table 2 revealed that the formation of BiSb does not result in a decrease in ΔGENRRPDS. Instead, the formation of BiSb makes the N2 adsorption on BiSb more favorable than that on Bi or Sb. This makes N2 adsorption more facile and increases the N2 coverage on BiSb, enhancing the FE for the ENRR. The comparison of ΔGHERPDS values revealed another effect of forming BiSb; the HER becomes less favorable on Bi in BiSb than on Bi alone while the HER becomes more favorable on Sb in BiSb than on Sb alone, which agrees well with the results obtained from the Bader charge analysis. Combining all results, it can be concluded that the formation of BiSb facilitates N2 adsorption on the Bi site and also makes the HER less favorable on the Bi site, thus enhancing the ENRR on the Bi site in BiSb. This also means that Bi in BiSb is the active site for ENRR and is what makes BiSb a better ENRR catalyst than Bi or Sb. On the other hand, by forming BiSb, the Sb site becomes more effective for the HER. However, since HER is the dominant reaction anyway on Bi or Sb alone, the formation of a BiSb alloy, which makes 50% of its surface (i.e., Bi sites) more catalytic for the ENRR can increase the FE for the ENRR.
4. Conclusions
In summary, we prepared Bi, Sb, and a BiSb alloy as uniform coating layers on a high surface area carbon felt electrode so that any difference observed in their ENRR performances could solely be due to the difference in their compositions. The resulting electrodes served as both regular electrodes and pseudo-GDEs for us to investigate the difference in using dissolved N2 and gas phase N2 as the reactant. Our experimental results showed that the formation of a BiSb alloy can considerably enhance the ENRR performance in terms of both FE and the rate for NH3 production compared with pure Bi or Sb. Also, the enhancement in the FE for NH3 production caused by using the electrode as a pseudo-GDE was greatest for BiSb. Computational investigations revealed that the formation of a BiSb alloy resulted in the development of a partial positive charge on Bi in BiSb and a partial negative charge on Sb in BiSb, which was experimentally confirmed by XPS results. The Bader charge analysis showed that the partial charges developed in BiSb allowed for stronger N2 adsorption on the BiSb surface than the surface of Bi or Sb. Also, from the Gibbs free energy calculations for the elemental steps involved with the ENRR and HER, it was demonstrated that Bi in BiSb makes the PDS of the HER (i.e. the adsorption of H) more difficult than pure Bi or Sb, thus enhancing the ENRR. In other words, Bi in BiSb is the more active site for the ENRR and makes BiSb a better ENRR catalyst than Bi or Sb.Our combined experimental and computational study suggests that producing an alloy where one metal type develops a partial positive and the other develops a partial negative charge can be an effective general strategy to increase the strength of N2 adsorption on a metal catalyst surface. We have recently demonstrated that maintaining positively charged Bi3+ on a Bi metal catalyst during the ENRR by the formation of surface layers (e.g. BiPO4, Bi(PO4)1−x(VO4)x) that are more difficult to reduce than Bi2O3 is an effective strategy to enhance the FE for the ENRR.9 However, Bi3+ in any compound must be eventually reduced to Bi0 when a more reductive bias is applied to increase the rate of NH3 production. However, BiSb is an alloy composed of Bi and Sb metal atoms that cannot be further reduced. Thus, the partial positive charge developed on Bi in BiSb cannot be further reduced under the reductive bias conditions and can promote N2 adsorption even in the high overpotential region. In the same manner, other metal ENRR catalysts can perform better when forming alloys if the formation of alloys results in the development of partial charges that can enhance N2 adsorption or formation of ENRR intermediates.
Conflicts of interest
The authors declare no competing interests.Acknowledgements
The experimental study of the work was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy through Grant DE-SC0008707. The computational study of the work was supported by the Nancy and Stephen Grand Technion Energy Program, the Ministry of Science and Technology (MOST) of Israel, and the COST Action 18234 supported by COST (European Cooperation in Science and Technology). Y. J. Jang acknowledges financial support by the National Research Foundation of Korea (NRF) grant funded by the Korea (MSIT) (No. 2021R1A4A3027878, No. 2021R1F1A1063146) and the research fund of Hanyang University (HY-202000000000520). The authors thank Prof. Daniel C. Fredrickson for his help with initial Bader charge analysis.References
- A. Klerke, C. H. Christensen, J. K. Nørskov and T. Vegge, Ammonia for hydrogen storage: challenges and opportunities, J. Mater. Chem., 2008, 18, 2304–2310 RSC.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, How a century of ammonia synthesis changed the world, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- V. Smil, Detonator of the population explosion, Nature, 1999, 400, 415 CrossRef CAS.
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Beyond fossil fuel–driven nitrogen transformations, Science, 2018, 360, eaar6611 CrossRef.
- B. H. R. Suryanto, H.-L. Du, D. Wang, A. N. Simonov and D. R. MacFarlane, Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia, Nat. Catalysis., 2019, 2, 290–296 CrossRef CAS.
- Y. C. Hao, Y. Guo, L. W. Chen, M. Shu, X. Y. Wang, T. A. Bu, W. Y. Gao, N. Zhang, X. Su, X. Feng, J. W. Zhou, B. Wang, C. W. Hu, A. X. Yin, R. Si, Y. W. Zhang and C. H. Yan, Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water, Nat. Catal., 2019, 2, 448–456 CrossRef CAS.
- Y. Wang, M.-M. Shi, D. Bao, F.-l. Meng, Q. Zhang, Y.-T. Zhou, K.-H. Liu, Y. Zhang, J.-z. Wang, Z.-w. Chen, D.-p. Liu, Z. Jiang, M. Luo, L. Gu, Q.-h. Zhang, X.-z. Cao, Y. Yao, M.-H. Shao, Y. Zhang, X.-B. Zhang, J. G. Chen, J.-M. Yan and Q. Jiang, Generating Defect-Rich Bismuth for Enhancing the Rate of Nitrogen Electroreduction to Ammonia, Angew. Chem. Int. Edit., 2019, 58, 9464–9469 CrossRef CAS PubMed.
- L. Li, C. Tang, B. Xia, H. Jin, Y. Zheng and S.-Z. Qiao, Two-Dimensional Mosaic Bismuth Nanosheets for Highly Selective Ambient Electrocatalytic Nitrogen Reduction, ACS Catal., 2019, 9, 2902–2908 CrossRef CAS.
- Y. J. Jang and K.-S. Choi, Enabling electrochemical N2 reduction to NH3 in the low overpotential region using non-noble metal Bi electrodes via surface composition modification, J. Mater. Chem. A., 2020, 8, 13842–13851 RSC.
- S. Trasatti, Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions, J. Electroanal. Chem., 1972, 39, 163–184 CrossRef CAS.
- J. P. Issi, Low Temperature Transport Properties of Group V Semimetals, Aust. J. Phys., 1979, 32, 585–628 CrossRef CAS.
- A. R. Singh, B. A. Rohr, J. A. Schwalbe, M. Cargnello, K. Chan, T. F. Jaramillo, I. Chorkendorff and J. K. Nørskov, Electrochemical Ammonia Synthesis—The Selectivity Challenge, ACS Catal., 2017, 7, 706–709 CrossRef CAS.
- L. Di, X. Chen, Y.-T. Liu, J. Yu and B. Ding, Sb2S3 nanoparticles anchored on SnO2 nanofibers: a high-performance hybrid electrocatalyst toward ammonia synthesis under ambient conditions, Chem. Commun., 2019, 55, 13892–13895 RSC.
- M. Bat-Erdene, G. Xu, M. Batmunkh, A. S. R. Bati, J. J. White, M. J. Nine, D. Losic, Y. Chen, Y. Wang, T. Ma and J. G. Shapter, Surface oxidized two-dimensional antimonene nanosheets for electrochemical ammonia synthesis under ambient conditions, J. Mater. Chem. A, 2020, 8, 4735–4739 RSC.
- X. Liu, H. Jang, P. Li, J. Wang, Q. Qin, M. G. Kim, G. Li and J. Cho, Antimony-Based Composites Loaded on Phosphorus-Doped Carbon for Boosting Faradaic Efficiency of the Electrochemical Nitrogen Reduction Reaction, Angew. Chem. Int. Edit., 2019, 58, 13329–13334 CrossRef CAS.
- N. I. Kolev, Solubility of O2, N2, H2, and CO2 in water. in, Multiphase Flow Dynamics, Springer, Berlin, 1st edn, 2011 Search PubMed.
- S. Z. Andersen, V. Čolić, S. Yang, J. A. Schwalbe, A. C. Nielander, J. M. McEnaney, K. Enemark-Rasmussen, J. G. Baker, A. R. Singh, B. A. Rohr, M. J. Statt, S. J. Blair, S. Mezzavilla, J. Kibsgaard, P. C. K. Vesborg, M. Cargnello, S. F. Bent, T. F. Jaramillo, I. E. L. Stephens, J. K. Nørskov and I. Chorkendorff, A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements, Nature, 2019, 570, 504–508 CrossRef CAS PubMed.
- G. W. Watt and J. D. Chrisp, Spectrophotometric Method for Determination of Hydrazine, Anal. Chem., 1952, 24, 2006–2008 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- G. Kresse and D. Joubert, From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. S. L. J. Kohn, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- Y. Lin, L. Yang, H. Jiang, Y. Zhang, Y. Bo, P. Liu, S. Chen, B. Xiang, G. Li, J. Jiang, Y. Xiong and L. Song, Sulfur Atomically Doped Bismuth Nanobelt Driven by Electrochemical Self-Reconstruction for Boosted Electrocatalysis, J. Phys. Chem. Lett., 2020, 11, 1746–1752 CrossRef CAS.
- Y.-C. Hao, Y. Guo, L.-W. Chen, M. Shu, X.-Y. Wang, T.-A. Bu, W.-Y. Gao, N. Zhang, X. Su, X. Feng, J.-W. Zhou, B. Wang, C.-W. Hu, A.-X. Yin, R. Si, Y.-W. Zhang and C.-H. Yan, Promoting Nitrogen Electroreduction to Ammonia with Bismuth Nanocrystals and Potassium Cations in Water, Nat. Catal., 2019, 2, 448–456 CrossRef CAS.
- Y. Wang, M. M. Shi, D. Bao, F. L. Meng, Q. Zhang, Y. T. Zhou, K. H. Liu, Y. Zhang, J. Z. Wang, Z. W. Chen, D.-P. Liu, Z. Jiang, M. Luo, L. Gu, Q.-H. Zhang, X.-Z. Cao, Y. Yao, M.-H. Shao, Y. Zhang, X.-B. Zhang, J. G. Chen, J.-M. Yan and Q. Jiang, Generating Defect-Rich Bismuth for Enhancing the Rate of Nitrogen Electroreduction to Ammonia, Angew. Chem., Int. Ed., 2019, 58, 9464–9469 CrossRef CAS PubMed.
- Y. Yang, J. Li, K. Chen, Q. J. Chen and Y. Feng, Catalytic Performance of Two-Dimensional Bismuth Tuned by Defect Engineering for Nitrogen Reduction Reaction, J. Phys. Chem. C, 2020, 124(36), 19563–19570 CrossRef CAS.
- Y. Nie, M. Rahman, D. Wang, C. Wang and G. Guo, Strain induced topological phase transitions in monolayer honeycomb structures of group-V binary compounds, Sci. Rep., 2015, 5, 1–6 CAS.
- C. S. Barrett, The structure of bismuth at low temperatures, Aust. J. Phys., 1960, 13, 209–222 CrossRef CAS.
- L. Li, C. Tang, B. Xia, H. Jin, Y. Zheng and S.-Z. Qiao, Two-Dimensional Mosaic Bismuth Nanosheets for Highly Selective Ambient Electrocatalytic Nitrogen Reduction, ACS Catal., 2019, 9, 2902–2908 CrossRef CAS.
- R. Zhang, L. Ji, W. Kong, H. Wang, R. Zhao, H. Chen, T. Li, B. Li, Y. Luo and X. Sun, Electrocatalytic N2-to-NH3 conversion with high faradaic efficiency enabled using a Bi nanosheet array, Chem. Commun., 2019, 55(36), 5263–5266 RSC.
- E. Skulason, T. Bligaard, S. Gudmundsdottir, F. Studt, J. Rossmeisl, F. Abild-Pedersen, T. Vegge, H. Jonsson and J. K. Norskov, A Theoretical Evaluation of Possible Transition Metal Electro-Catalysts for N2 Reduction, Phys. Chem. Chem. Phys., 2012, 14, 1235–1245 RSC.
- J. H. Montoya, C. Tsai, A. Vojvodic and J. K. Norskov, The Challenge of Electrochemical Ammonia Synthesis: A New Perspective on the Role of Nitrogen Scaling Relations, ChemSusChem, 2015, 8, 2180–2186 CrossRef CAS.
- G. Henkelman, A. Arnaldsson and H. Jónsson, A fast and robust algorithm for Bader decomposition of charge density, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- J. Berger, B. Christ and J. Troschke, Lattice Parameter Study in the Bi1-xSbx Solid-solution System, Cryst. Res. Technol., 1982, 17, 1233–1239 CrossRef.
- D. Higgins, C. Hahn, C. Xiang, T. F. Jaramillo and A. Z. Weber, Gas-Diffusion Electrodes for Carbon Dioxide Reduction: A New Paradigm, ACS Energy Lett., 2019, 4, 317–324 CrossRef CAS.
- V. S. Dharmadhikari, S. R. Sainkar, S. Badrinarayan and A. Goswami, J. Electron Spectrosc. Relat. Phenom., 1982, 25, 181–189 CrossRef CAS.
- L. Escobar-Alarcón, J. Morales-Mendez, D. Solís-Casados, S. Romero, M. Fernández and E. Haro-Poniatowski, J. Phys.: Conf. Ser., 2015, 582, 012013 CrossRef.
- F. Garbassi, XPS and AES study of antimony oxides, Surf. Interface Anal., 1980, 2, 165–169 CrossRef CAS.
- R. Izquierdo, E. Sacher and A. Yelon, X-ray photoelectron spectra of antimony oxides, Appl. Surf. Sci., 1989, 40, 175–177 CrossRef CAS.
- T. A. Evans and K.-S. Choi, Electrochemical Synthesis and Investigation of Stoichiometric, Phase-Pure CoSb2O6 and MnSb2O6 Electrodes for the Oxygen Evolution Reaction in Acidic Media, ACS Appl. Energy Mater., 2020, 3, 5563–5571 CrossRef CAS.
- L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, New York, 3rd edn, 1960 Search PubMed.
- R. Zhao, H. Xie, L. Chang, X. Zhang, X. Zhu, X. Tong, T. Wang, Y. Luo, P. Wei, Z. Wang and X. Sun, Recent Progress in the Electrochemical Ammonia Synthesis under Ambient Conditions, EnergyChem, 2019, 1, 100011 CrossRef.
- X. Cui, C. Tang and Q. Zhang, A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions, Adv. Energy Mater., 2018, 8, 1800369 CrossRef.
- H.-R. Zhu, Y.-L. Hu, S.-H. Wei and D.-Y. Hua, Single-Metal Atom Anchored on Boron Monolayer (β12) as an Electrocatalyst for Nitrogen Reduction into Ammonia at Ambient Conditions: A First-Principles Study, J. Phys. Chem. C, 2019, 123, 4274–4281 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta05327b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |