 Open Access Article
Open Access ArticleSeparation enhanced methanol and dimethyl ether synthesis
Jasper
van Kampen
 *ab,
Jurriaan
Boon
*ab,
Jurriaan
Boon
 a and
Martin
van Sint Annaland
a and
Martin
van Sint Annaland
 b
b
aSustainable Technologies for Industrial Processes, TNO Energy Transition, P.O. Box 15, 1755 ZG Petten, The Netherlands. E-mail: jasper.vankampen@tno.nl
bChemical Process Intensification, TU/e, P.O. Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: j.v.kampen@tue.nl
First published on 23rd June 2021
Abstract
Separation enhanced reaction processes are promising process intensification strategies for carbon dioxide utilisation. In recent years, major improvements have been made in adsorption and membrane technology for the direct production of methanol and dimethyl ether from carbon dioxide rich feedstock and hydrogen. In situ water removal results in high single-pass conversions, thereby circumventing the disadvantages of conventional routes, such as the low carbon efficiency, energy intensive downstream separation and large recycles. In situ water removal by adsorption results in extremely high single-pass conversion and yield, especially in direct DME production. Membrane reactors allow for high single-pass conversion and yield, especially for methanol production. Here, we highlight recent advances in membrane and adsorption-enhanced synthesis of methanol and DME.
Methanol and dimethyl ether (DME), the simplest ether and the dehydrated form of methanol, are valuable platform chemicals and synthetic fuels. They are expected to play an important role in the energy transition, where fossil-based fuels and chemicals have to be replaced by products from renewable feedstock, including switching to bio-based feedstock and the chemical recycling of carbon dioxide.1 However, the conventional production processes are limited starting from CO2, and therefore considered unattractive.2,3 As for many other industrial CO2 utilisation processes a main hurdle is the production and efficient handling of steam.1,4,5 Steam separation enhancement is shown to be a promising route for CO2 conversion.4 The concept of separation enhancement is based on Le Chatelier's principle, where an equilibrium limited reaction is shifted to enhance conversion by selectively removing reaction products, and is mainly utilised for various processes and products considering CO2 separation.6,7 The recent review and outlook by van Kampen et al. (2019) addressed the opportunities of adsorptive and membrane reactors for CO2 utilisation processes, discussing the advantages and the future developments for both technologies. Crucial aspects discussed are the hydrothermal stability of the membranes and their permselectivity, whereas high temperature working capacities and heat management are crucial aspects for reactive steam adsorption processes.4
Thermal stability of polymer membranes limits their temperature of operation, requiring more active low temperature catalysis, a topic which also gained a lot of attention in the recent years. While zeolite membranes have been shown to outperform the other membrane types in steam permeance and selectivity at higher temperatures, their stability, mainly associated to defects, remains a point of attention. Recently Li et al. (2020) have created a defect-free zeolite (NaA) membrane and have shown its performance in a membrane reactor for CO2 conversion to methanol.8 Indeed, Fig. 1 illustrates the important progress made in membrane reactors for methanol synthesis from 2004 (zeolite), to 2015 (polymer), to 2020 (zeolite).8–10 Gallucci et al. (2004) have shown good performance of a zeolite membrane reactor. The CO2 conversion was higher than for a traditional reactor at similar conditions, but the improvement remained modest with a yield of 8.7%.9 In the CARENA project (2011–2015) polymer membranes have been developed and tested for their eased production and lower costs compared to zeolite membranes. Although limited by lower operating temperatures and therefore reduced catalyst activity, thermodynamic equilibria for traditional methanol synthesis have been exceeded by measured yields up to 30%.10 In Fig. 1 also the maximum single-pass methanol yield is shown. A fundamental limit exists in the maximum methanol yield that can be achieved using separation enhancement, because even though the synthesis of methanol from CO is also equilibrium limited, it does not produce water. Therefore, even by removing all water and achieving full CO2 conversion, a mixture of methanol and CO will remain, of which the composition is dictated by thermodynamics. Ongoing research should lead to improving cost efficiency, focusing on the large scale production of defect-free zeolite membranes, improved selectivity and permeance of cheaper polymer membranes, and improved operation and system integration.
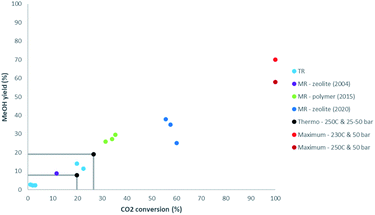 | ||
| Fig. 1 Comparison of CO2 conversion and methanol yield for membrane reactors (MR) with results for traditional reactors (TR). TR: light blue.9,11 MR (2004): purple.9 MR (2015): green.10 MR (2020): dark blue.8 Thermodynamic equilibrium at 250 °C and 25–50 bar: black. Theoretical maximum at 230–250 °C and 50 bar: red. | ||
In the direct synthesis of DME a maximum single-pass yield of 100% could theoretically be achieved by removal of all by-product water (Fig. 2). The developed NaA zeolite membrane reactor was also demonstrated in the direct production of DME from CO2, achieving a single-pass conversion up to 73% and a DME yield up to 54.5%.11 In parallel, sorption enhanced technology for the production of DME has been developed and scaled up in recent years.4,12–18 Interestingly, the most promising materials for high temperature steam adsorption are again LTA-type zeolites. LTA zeolites, and specifically 3A, are highly selective for water due to size exclusion by the limited pore size.14 Whereas the preferential permeation of polar molecules (water and methanol) is observed for various zeolitic membranes (FAU and MFI) and can be explained by a preferential adsorption mechanism rather than a size exclusion mechanism.4 The recent experimental validation of pressure swing regeneration in sorption enhanced DME synthesis (SEDMES), already demonstrating a factor four increase in productivity, has opened up further process optimisation by cycle design.13,15 For sorption enhanced DME synthesis from CO2, conversions up to 99% and DME yields of up to 82% have been reported.4 Adsorption technologies are very well suited for steam level reductions as low as trace amounts, thereby maximising CO2 conversion. The product distribution consists of DME and some remaining, unconverted CO. As illustrated by the methanol synthesis, an equilibrium between methanol and CO remains even at dry conditions and consequently for conversion of the remaining CO to DME even deeper drying is required. Although the concept of sorption enhanced methanol synthesis has been demonstrated by several authors, the performances are limited, obtaining more CO than methanol, and more research is required to assses the suitability of the process.19,20 Ongoing research, ultimately improving the cost efficiency, focuses on cycle design, i.a. faster cycling, improved operating conditions, closely linked to the system integration.
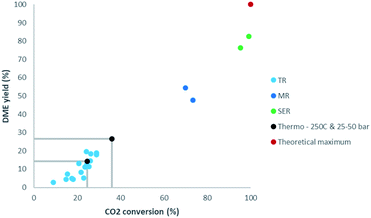 | ||
| Fig. 2 Comparison of CO2 conversion and DME yield for sorption enhanced reactors (SER) and membrane reactors (MR) with results for traditional reactors (TR). TR: light blue.11 MR: dark blue.11 SER: green.4,15 Thermodynamic equilibrium at 250 °C and 25–50 bar: black. Theoretical maximum: red. | ||
In conclusion, recent developments in separation enhanced reaction processes using membranes and adsorbents, have proven their potential for carbon dioxide conversion to methanol and DME. Both technologies outperform traditional reactor concepts, achieving single-pass conversions far beyond equilibrium. In situ water removal by adsorption allows deep drying and results in extremely high single-pass conversion and yield, especially in DME production. Membrane reactors allow for high single-pass conversion and yield, especially for methanol production. Techno-economic and life cycle analyses of the various, suitable process intensifications, such as adsorption and membranes processes, but also condensation and absorption processes for methanol synthesis,21–24 would allow for the appropriate reactor selection for a specific case.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Mrs M. Saric is kindly acknowledged for the fruitful discussions on membrane and adsorption-enhanced reactors.References
- G. Centi and S. Perathoner, Opportunities and prospects in the chemical recycling of carbon dioxide to fuels, Catal. Today, 2009, 148, 191–205 CrossRef CAS.
- V. Dieterich, A. Buttler, A. Hanel, H. Spliethoff and S. Fendt, Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: a review, Energy Environ. Sci., 2020, 13, 3207–3252 RSC.
- R. J. Detz, J. N. H. Reek and B. C. C. van der Zwaan, The future of solar fuels: when could they become competitive?, Energy Environ. Sci., 2018, 11, 1653–1669 RSC.
- J. van Kampen, J. Boon, F. van Berkel, J. Vente and M. van Sint Annaland, Steam separation enhanced reactions: review and outlook, Chem. Eng. J., 2019, 374, 1286–1303 CrossRef CAS.
- A. Katelhon, R. Meys, S. Deutz, S. Suh and A. Bardow, Climate change mitigation potential of carbon capture and utilization in the chemical industry, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef CAS PubMed.
- A. E. Rodrigues, L. M. Madeira, Y.-J. Wu and R. Faria, Sorption Enhanced Reaction Processes, World Scientific, 2017 Search PubMed.
- Sorption Enhancement of Chemical Processes, ed J. C. Abanades, J. Boon, P. Cobden, K. Coenen, D. S. M. Constantino, R. P. V. Faria, J. R. Fernández, F. Gallucci, M. C. Iliuta, A. E. Rodrigues, E. van Dijk, M. van Sint Annaland and A. Lemonidou, Academic Press, 1st edn, 2017, vol. 51 Search PubMed.
- H. Li, C. Qiu, S. Ren, Q. Dong, S. Zhang, F. Zhou, X. Liang, J. Wang, S. Li and M. Yu, Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels, Science, 2020, 367, 667–671 CrossRef CAS PubMed.
- F. Gallucci, L. Paturzo and A. Basile, An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor, Chem. Eng. Process., 2004, 43, 1029–1036 CrossRef CAS.
- CARENA, Catalytic membrane reactor based on new materials for C1–C4 valorization, Petten, 2011 Search PubMed.
- H. Li, S. Ren, S. Zhang, S. Padinjarekutt, B. Sengupta, X. Liang, S. Li and M. Yu, The high-yield direct synthesis of dimethyl ether from CO2 and H2 in a dry reaction environment, J. Mater. Chem. A, 2021, 9, 2678–2682 RSC.
- J. van Kampen, J. Boon, J. Vente and M. van Sint Annaland, Sorption enhanced dimethyl ether synthesis for high efficiency carbon conversion: modelling and cycle design, J. CO2 Util., 2020, 37, 295–308 CrossRef CAS.
- J. van Kampen, S. Booneveld, J. Boon, J. Vente and M. van Sint Annaland, Experimental validation of pressure swing regeneration for faster cycling in sorption enhanced dimethyl ether synthesis, Chem. Commun., 2020, 56, 13540–13542 RSC.
- J. van Kampen, J. Boon and M. van Sint Annaland, Steam adsorption on molecular sieve 3A for sorption enhanced reaction processes, Adsorption, 2021, 27, 577–589 CrossRef CAS.
- J. van Kampen, J. Boon, J. Vente and M. van Sint Annaland, Sorption enhanced dimethyl ether synthesis under industrially relevant conditions: experimental validation of pressure swing regeneration, React. Chem. Eng., 2021, 6, 244–257 RSC.
- J. Boon, J. van Kampen, R. Hoogendoorn, S. Tanase, F. P. F. van Berkel and M. van Sint Annaland, Reversible deactivation of γ-alumina by steam in the gas-phase dehydration of methanol to dimethyl ether, Catal. Commun., 2019, 119, 22–27 CrossRef CAS.
- D. Liuzzi, C. Peinado, M. A. Peña, J. van Kampen, J. Boon and S. Rojas, Increasing dimethyl ether production from biomass-derived syngas via sorption enhanced dimethyl ether synthesis, Sustainable Energy Fuels, 2020, 4, 5674–5681 RSC.
- S. Guffanti, C. G. Visconti, J. van Kampen, J. Boon and G. Groppi, Reactor modelling and design for sorption enhanced dimethyl ether synthesis, Chem. Eng. J., 2021, 404, 126573–126585 CrossRef CAS.
- J. Terreni, M. Trottmann, T. Franken, A. Heel and A. Borgschulte, Sorption-Enhanced Methanol Synthesis, Energy Technol., 2019, 7, 1801093–1801101 CrossRef.
- P. Maksimov, A. Laari, V. Ruuskanen, T. Koiranen and J. Ahola, Methanol synthesis through sorption enhanced carbon dioxide hydrogenation, Chem. Eng. J., 2021, 418, 129290–129303 CrossRef CAS.
- J. G. van Bennekom, R. H. Venderbosch, J. G. M. Winkelman, E. Wilbers, D. Assink, K. P. J. Lemmens and H. J. Heeres, Methanol synthesis beyond chemical equilibrium, Chem. Eng. Sci., 2013, 87, 204–208 CrossRef CAS.
- M. J. Bos and D. W. F. Brilman, A novel condensation reactor for efficient CO2 to methanol conversion for storage of renewable electric energy, Chem. Eng. J., 2015, 278, 527–532 CrossRef CAS.
- K. R. Westerterp, M. Kuczynski and C. H. M. Kamphuis, The synthesis of methanol in a reactor system with interstage product removal, Process Engineering and Design, 1989, 28, 763–771 CAS.
- J. Reichert, S. Maerten, K. Meltzer, A. Tremel, M. Baldauf, P. Wasserscheid and J. Albert, Shifting the equilibrium of methanol synthesis from CO2 by in situ absorption using ionic liquid media, Sustainable Energy Fuels, 2019, 3, 3399–3405 RSC.
| This journal is © The Royal Society of Chemistry 2021 |
