Nanoarchitectonics: what's coming next after nanotechnology?
Katsuhiko
Ariga
 ab
ab
aWPI Research Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: ARIGA.Katsuhiko@nims.go.jp
bGraduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8561, Japan
First published on 9th March 2021
Abstract
In science and technology today, the crucial importance of the regulation of nanoscale objects and structures is well recognized. The production of functional material systems using nanoscale units can be achieved via the fusion of nanotechnology with the other research disciplines. This task is a part of the emerging concept of nanoarchitectonics, which is a concept moving beyond the area of nanotechnology. The concept of nanoarchitectonics is supposed to involve the architecting of functional materials using nanoscale units based on the principles of nanotechnology. In this focus article, the essences of nanotechnology and nanoarchitectonics are first explained, together with their historical backgrounds. Then, several examples of material production based on the concept of nanoarchitectonics are introduced via several approaches: (i) from atomic switches to neuromorphic networks; (ii) from atomic nanostructure control to environmental and energy applications; (iii) from interfacial processes to devices; and (iv) from biomolecular assemblies to life science. Finally, perspectives relating to the final goals of the nanoarchitectonics approach are discussed.
Nanotechnology as a game changer
It is said that the concept of nanotechnology was initiated by Richard Feynman through his lecture entitled “There's Plenty of Room at the Bottom”.1 Objects confined within nanoscale regions often exhibit properties much different from those in the bulk phase, as typically seen in the emergence of various quantum effects. Different ways of creating nanoscale arrangements of atoms and molecules can create limitless varieties of materials, as recognized in a wide range of scientific fields. For example, a single atom, carbon, can form the totally different materials charcoal, diamond, graphene (graphite), and carbon nanotubes through different arrangements and connections of carbon atoms. Different sequences of only four molecular units, adenosine, thymine, guanosine, and cytosine, in DNA can generate most biological activities. Including information widely known in other research fields, compositional and structural regulation in nanoscale regions is crucial for physics, chemistry, and biology applications.Although many scientific principles were discovered and novel material systems were developed in the 20th century, the scale-focused concept of nanotechnology was further emphasized as a result of technological developments in the microscopy field, such as scanning tunnelling microscopy, atomic force microscopy, and advanced electron microscopy, allowing the direct observation of nanoscale objects.2 The application of these technologies was not limited to simple observation: the manipulation of nanoscale objects, and even single atoms, was demonstrated. As well as the rapid development of various nanoscale observation and micro/nanofabrication techniques, related methodologies for microscope-based fabrication gained much attention, especially in the 1990s.3 These scientific areas of discovery can be regarded as representative nanoscale research horizons.
The regulation of nanoscale components and structures is also important in materials science. This recognition was emphasized historically by the US governmental program “The National Nanotechnology Initiative”, advocated by former president Bill Clinton in 2000. Nanotechnology is not only an innovative scientific concept, but it can also be a game changer in materials science for societal applications. In current research developments relating to societal benefits in energy,4 environmental,5 and biomedical6 fields, the crucial importance of the regulation of nanoscale objects and structures is well recognized, in addition to the traditionally well-documented areas of organic chemistry,7 materials science,8 and supramolecular chemistry.9
Nanoarchitectonics: what's coming next after nanotechnology?
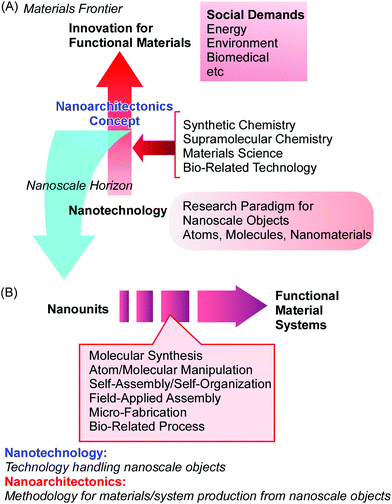
The nanotechnology concept has opened up ways to observe, analyse, and manipulate nanoscale objects. The production of functional materials with nanoscale components should follow new research avenues that have been initiated due to the development of nanotechnology. In order to fabricate materials with high performance, functional materials are desirably produced with the feature of nanoscale structure control. Ideally, such materials are synthesized rationally from nanoscale units. This type of bottom-up approach to produce functional materials from molecular units was conceptually proposed as molecular nanotechnology by K. Eric Drexler.10 In his book titled “Engines of Creation: The Coming Era of Nanotechnology”, a nanoscale assembler was proposed as an ideal machine or system that could replicate and/or produce materials through molecular nanotechnology. Instead of conceptual machine systems, the production of functional material systems using nanoscale units can be achieved via the fusion of nanotechnology with other research disciplines. This task is the focus of an emerging concept, nanoarchitectonics (Fig. 1A),11 a concept moving beyond the area of nanotechnology.In parallel with the rapid progress made in the field of nanotechnology, various scientific research fields for assembling and synthesizing materials have undergone continuous developments and seen successful advances, such as synthetic chemistry,12 supramolecular assembly,13 materials processing,14 and bio-related technology.15 The scientific principles of these achievements are capable of being used to contribute to the architecting of materials from nanoscale units. To match the great effects seen from the proposal of nanotechnology, a novel concept should be proposed to combine these research fields with nanotechnology for this new era (the 21st century). This is the concept of nanoarchitectonics, which was initially proposed by Masakazu Aono16 in 2000 at the 1st International Symposium on Nanoarchitectonics Using Suprainteractions in Tsukuba, Japan. This concept can be the next game-changer moving beyond nanotechnology.
The nanoarchitectonics concept is supposed to involve the architecting of functional materials using nanoscale units based on the principles of nanotechnology. The production of functional materials via nanoarchitectonics approaches is done through the combination and selection of several unit processes, including molecular synthesis, atom/molecular manipulation, self-assembly/self-organization, field-applied assembly, micro-fabrication, and bio-related processes (Fig. 1B).17 Because these processes can be used widely, regardless of the material or purpose, nanoarchitectonics approaches should be applicable to a wide ranges of targets, from physical devices18 to biomedical uses.19 Both nanotechnology and nanoarchitectonics are broad comprehensive concepts. Therefore, there is much overlap and many common issues between them. The development of some functional nanostructures and their functional performances can be ascribed to both nanotechnology and nanoarchitectonics. However, their very basic natures and definitions are different: nanotechnology is technology for handling nanoscale objects; and nanoarchitectonics is a methodology for the production of materials/systems from nanoscale objects.
The possibility for the inclusion of multiple processes in materials production can make nanoarchitectonics much different from simple self-assembly processes. Self-assembly processes are usually based on equilibrium and thus often produce symmetrical and uniform structures. The inclusion of non-equilibrium aspects in basic equilibrium processes is advantageous for the formation of more advanced structures with asymmetrical and hierarchical features. For example, the simple self-assembly of cationic and anionic species results in charge-compensated aggregates, but layer-by-layer assemblies formed via the combination of equilibrium electrostatic assemblies and non-equilibrium sequential deposition can provide layered organizations with intentionally designed layer sequences and thicknesses.20
So-called bottom-up assembly basically involves self-assembly (a non-energy-consuming process) and self-organization (an energy-consuming process). Unlike this, nanoarchitectonics more comprehensively includes fabrication processes, such as top-down-type nanofabrication and manipulation. For the development of functional material systems, the merging of bottom-up and top-down processes is said to be important. The nanoarchitectonics concept satisfies this kind of demand, unlike conventional bottom-up assembly.
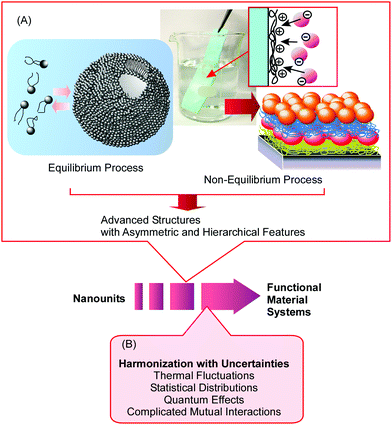
Nanoarchitectonics approaches can utilize non-equilibrium processes together with conventional equilibrium assemblies with limitless combinations and selections. Therefore, nanoarchitectonics methods are advantageous for producing asymmetric and hierarchical material structures from various nanounits (Fig. 2A).21 As seen in many advanced functional material systems in artificial structures22 and naturally occurring matter,23 vectorial flows of electrons, energy, and information and efficient sequences of events are always key. The nanoarchitectonics approach can be used to fabricate these asymmetrical and hierarchical material structures and to satisfy material production demands for societal necessities.
 | ||
| Fig. 2 The features of nanoarchitectonics strategies: (A) the inclusion of equilibrium and non-equilibrium processes for obtaining advanced structures with asymmetrical and hierarchical aspects; and (B) the harmonization of various uncertainties in nanoscale regions. Reproduced from ref. 21 with permission. Copyright: 2020, Wiley-VCH. | ||
Because nanoarchitectonics approaches rely on material/molecular interactions on the nanoscale, physico-chemical features specific to nanoscale regions make indispensable contributions to material fabrication. Unlike material interactions in larger size regions, certain uncertainties, such as thermal fluctuations, statistical distributions, quantum effects, and complicated mutual interactions, cannot be avoided during nanoscale processes.24 Therefore, the effects of multiple interactions are often different from those expected from a simple summation. Rather than being summed, the effects of independent interactions are harmonized in nanoscale regions. Therefore, nanoarchitectonics processes for component assembly involve the harmonization of various causes and effects (Fig. 2B).25 This situation may be similar to material systems in biology, where most of the biomolecules work in certain harmony under thermal fluctuations. Nanoarchitectonics approaches are potentially capable of producing functional materials with bio-like flexibility and softness.26
Nanoarchitectonics for functional materials
As mentioned above, nanoarchitectonics-type approaches for functional material systems can be applied to various research targets. In this section, functional material fabrication processes from nanoscale units for several applications are exemplified.From atomic switches to neuromorphic networks
The manipulation of atoms and molecules is a basic-level process in nanoarchitectonics approaches. Atom manipulation using an STM tip, relocating single atoms into artificial nanoscale words and cartoons, was demonstrated to reveal the high capabilities of scanning probe technologies. Recently, related approaches were applied to reactions including molecular manipulation. Kawai et al. demonstrated the debromination of graphene nanoribbons and substitution with a fullerene molecule using an STM tip (Fig. 3).27 The removal of a bromine atom using the STM tip resulted in a radical on the graphene nanoribbons. A fullerene C60 molecule was picked up by the STM tip and brought closer to the radical. Then, the C60 molecule was covalently added site-specifically to the graphene nanoribbons. This tip-mediated reaction procedure may go against the common-sense rules of organic synthesis in solution or on solid surfaces. Reaction sites can be determined on the nanoscale-level based on actions with the STM tip, and the traditional rules of organic chemistry do not have to be obeyed. This methodology would be one of the most-bottom-level processes in the nanoarchitectonics approach.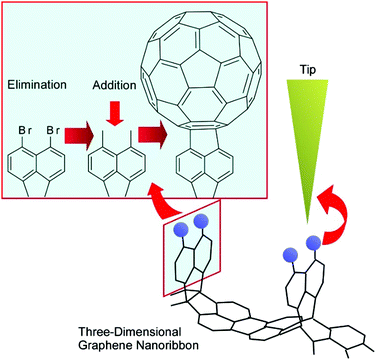 | ||
| Fig. 3 The debromination of graphene nanoribbons and substitution with a fullerene molecule using an STM tip. | ||
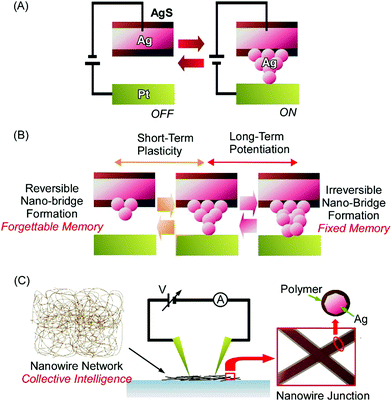
Atom-/molecular-level manipulation can be applied to architect advanced devices. A representative approach from this research area is the fabrication of atomic switches, as seen in the various types of atomic switches developed by Aono and co-workers.28 In a typical example, the atomic switch nanoarchitecture is composed of a Pt electrode and Ag2S electrode positioned at an interval of ca. 1 nm (Fig. 4A).29 Chemical processes, such as redox, atomic diffusion, and atomic aggregation, lead to device-like switching operations. The supply of electrons from the Pt electrode to the Ag2S electrode through a tunnelling current upon the application of a negative bias to the former electrode results in the formation of Ag atoms from Ag ions via reduction. The diffusion and aggregation of several of these produced Ag atoms forms an electrically connected bridge between the two electrodes. The formation of a conductive Ag bridge can turn on the atomic switch device. The reverse application of a positive bias voltage to the Pt electrode oxidized Ag atoms into Ag ions, resulting in the dissolution of the conductive bridge, which is accompanied by the switch turning off.
These actions are based on chemical atomic processes and not on simple electrode contact. This feature can lead to advanced operations beyond simple on/off switching using atomic switch mechanisms. For example, artificial synaptic operations, such as short-term plasticity and long-term potentiation, can be achieved, as reported by Ohno, Hasegawa, and co-workers (Fig. 4B).30 Based on intervals of input pulses, the architecting of reversible and irreversible nanobridge formation can result in memory-like short-term plasticity and long-term potentiation. The application of low-frequency pulses at intervals of 20 s caused the reversible formation and dissolution of a conductive Ag bridge between the electrodes. This operation corresponds to short-term plasticity. In contrast, the repetition of an electrical input with short 2 s intervals resulted in the formation of a stable long-life conductive bridge, retaining the switched-on status. This is the realization of long-term potentiation operation. Therefore, nanoarchitectonics involving atomic bridges across a nanoscale gap space between electrodes can mimic bio-like advanced functions.
Huge numbers of atomic switch units can be integrated into entangled network structures forming a nanowire assembly.31 Diaz-Alvarez, Nakayama, and co-workers recently fabricated a neuromorphic network device based upon nanoarchitectures of randomly self-assembled Ag nanowires (Fig. 4C).32 The formation of numerous atomic switches in nanowire networks via nanoarchitectonics may even lead to collective intelligence. After an integrated network of Ag nanowires was fabricated, the nanowires were coated with polyvinylpyrrolidone as an insulator layer. The junction points at the crossing of two nanowires worked as resistive switches. The formation of Ag filaments between the Ag nanowires was induced through Ag-ion transport within the polyvinylpyrrolidone layer upon the application of a large electric field. Multiscale interactions between switchable junctions revealed that the specific behaviour of the architected neuromorphic network included a collective memory response, distinct low and high network conductance states, and reconfiguration dynamics. The latter aspect in particular corresponds to synaptic plasticity in the brain for resilience and adaptation. Temporal relations in the active network were changed upon shifts in voltages connectivity due to stochastic fluctuations at the single-junction scale accompanied by hierarchical collective dynamics. The observed results could provide important insights for training and learning with respect to information processing systems. Kuncic and co-workers applied graph theory to neuromorphic networks architected from Ag nanowires.33 The neuromorphic networks may have similar natures to those of biological systems, such as C. elegans, and they are much different from random networks and grid networks. The results can have useful implications for mimicking cognitive properties.
From nanostructure control to energy and environmental applications
Energy and environmental applications, which are in high demand in society, are mostly supported by various catalytic processes, including material conversion,34 redox reactions,35 and photocatalysis.36 Nanoscale structural regulation, such as atomic arrangements and molecular configurations, can determine catalytic activities and selectivities, and well-explored macroscale catalytic processes can produce bulk-size materials and practical levels of energy. Therefore, catalyst-related atom-/molecular-level nanoarchitectonics can be connected with macroscopic methods for environmental and energy applications.37As seen in examples such as single atom catalysis and lattice-controlled catalysis, atomic-level arrangement can drastically regulate catalytic capabilities.38 Suitably nanoarchitected surfaces of naturally abundant materials can exhibit catalytic abilities comparable to those observed with precious metal catalysts. Abe and co-workers demonstrated that facet-regulated CuO materials showed high catalytic activity for NO reduction in the presence of CO (Fig. 5).39 The CuO catalyst materials were prepared from the simple chemicals CuCl2 and urea. Although the synthesis is very simple, the nanoarchitectured catalyst contained assembled structures of two-dimensional pieces with active-facet control. The corresponding catalytic activity of abundant CuO was actually better than Pt nanoparticles and comparable to Rh nanoparticles. The prepared CuO thin single crystals with developed {001} facets had high catalytic activity toward NO reduction though surface-specific interactions and reactions. CO molecules initially adsorbed on the {001} facet surface and reacted with outermost oxygen to form oxygen vacancies. The formed oxygen vacancies played the role of reaction centers for oxygen removal from NOx. This sequence is possible only on the CuO{001} facet. The too-tight binding of CO molecules to the {100} surface, with the formation of stable carbonate (CO3), suppressed the formation of reaction centers for NO reduction. Control of the crystalline facets can make abundant CuO materials highly active catalysts.
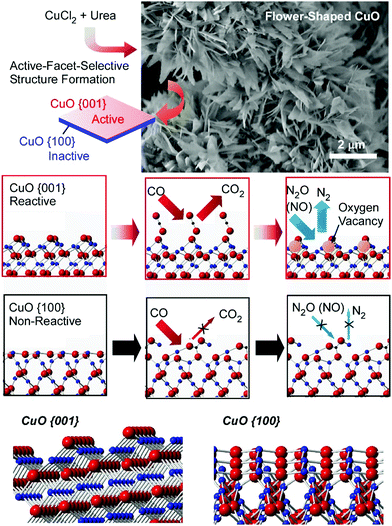 | ||
| Fig. 5 The fabrication and catalytic performance of facet-regulated CuO materials with catalytic activity for NO reduction in the presence of CO. Reproduced from ref. 39 with permission. Copyright: 2014, Wiley-VCH. | ||
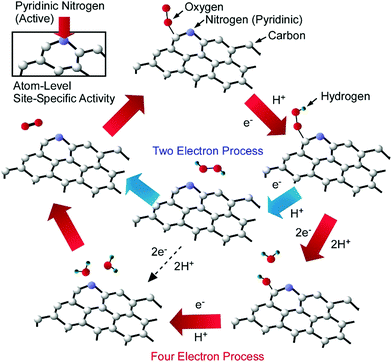
Kondo, Nakamura, and co-workers successfully specified the active site for the oxygen reduction reaction via nitrogen-doped carbon using model catalysts.40 The oxygen reduction reaction is one of the key processes in fuel cell applications. High electrocatalytic performance during the oxygen reduction reaction is observed with the use of nitrogen-doped carbon, but the actual active site for the oxygen reduction reaction was not fully clear. They clarified the active site for the oxygen reduction reaction using the model catalyst of highly oriented pyrolytic graphite with controlled nitrogen-doping sites (Fig. 6). Nitrogen doping at pyridinic sites in particular resulted in highly active performance, implying the nanoarchitectonics importance of atom-level site-specific doping. According to the characterized results, the reaction mechanism follows a process in which an oxygen molecule is initially adsorbed on a carbon atom next to pyridinic nitrogen. After the protonation of the adsorbed oxygen, further processes obey either a four-electron mechanism or a two- + two-electron mechanism. The other two protons attach to the two oxygen atoms, leading to O–OH bond formation and then OH formation in the four-electron mechanism. The addition of protons results in H2O. On the other hand, H2O2 is produced via the two- + two-electron mechanism. The reduction of H2O2 by two protons finally generates H2O. In both cases, carbon atoms next to pyridinic nitrogen are the key reaction sites onto which oxygen molecules adsorb in the initial step during the oxygen reduction reaction. Such atomic level understanding of catalytic sites is crucially important for the design of catalysts with better performance.
Nanoarchitectured materials have sometimes been used for artificial photosynthetic processes, including CO2 fixation and water splitting.41 Ever since the photocatalytic basics of artificial photosynthesis were initiated by Honda and Fujishima almost half a century ago,42 various material systems with a wide range of compositions and structures have been investigated for this application. Nanoarchitectonics concepts can introduce useful elements to this hot research field. As summarized in a recent review article by Maeda and Mallouk,43 two-dimensional nanosheets of metal oxides and related materials have intrinsically advantageous features for these applications. Nanosheets, as anisotropic single crystals, possess thicknesses of 1–2 nm and lateral sizes of a few micrometers. Their nanoarchitectured structural features of shortened photo-generated electron–hole pair diffusion lengths and decreased recombination probabilities are advantageous for heterogeneous photocatalyst applications.
The control of atomic compositional arrangements within two-dimensional and related materials can be used to effectively modify their properties, as discussed in a recent review article by Rao and Pramoda.44 The chemical doping of graphene with heteroatoms such as nitrogen and boron is an effective way to regulate their band gaps to generate useful properties. Borocarbonitrides, BxCyNz, with various compositional nanoarchitectures can become well-tuned materials lying between insulative boron nitride and conductive graphene. These compositionally architected materials have been used for several energy-related applications, such as in fuel cells and supercapacitors. Some borocarbonitrides exhibited catalytic activities in the hydrogen evolution reaction comparable to those of Pt catalysts.
The high potential of metal cluster catalysts with precise sizes and compositions for energy and environmental usages is expected. However, their production, with high structural precision and in acceptable quantities, is not always easy. Nanoarchitectonics production with ultraprecise regulation, such as single-atom-level size controls, is desired, as summarized in a recent review article by Imaoka and Yamamoto.45 They proposed the usage of dendrimers with basicity gradients as templates for metal cluster syntheses (Fig. 7). In the usage of phenylazomethine dendrimers with intramolecular basicity gradients, significant differences between the binding constants of metal ions with basic sites in every core generation of the dendrimers made it possible to form metal clusters with precise numbers of atoms.
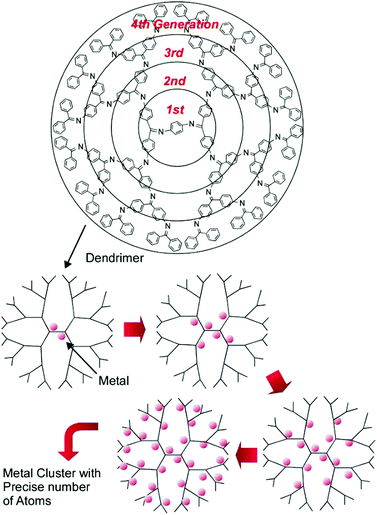 | ||
| Fig. 7 Dendrimers with basicity gradients as templates for metal cluster syntheses with precise control of the number of atoms. | ||
Determining factors for catalytic performance are not limited to structures on atomic and molecular levels. Microscopic morphologies also have significant effects on catalyst performance. The optimization of the microscopic structures of catalyst bodies often leads to the minimization of the required amounts of precious metal catalysts. For example, open-mouthed hollow Pt microcapsules (ca. 10 μm scale) have much more efficient catalytic activity than conventional Pt particle catalysts (Fig. 8).46 These open-mouthed catalyst capsules were fabricated though the nanofilm assembly (deposition) of Pt metal on a microscopic template polymer sphere. The removal of the polymer sphere via combustion induced the formation of open-mouthed structures. The open-mouthed architecture is beneficial for material diffusion, even in capsule agglomeration states. The free diffusion of CO molecules inside the capsules though their open mouths led to the facile contact of CO with Pt catalytic sites located on the inside surfaces of the microcapsules. The enhanced catalytic performance toward CO oxidation of the open-mouthed hollow Pt microcapsules mainly originates from simple structural factors, e.g., the open-mouthed structure and hollow inside, allowing the availability of active catalytic sites both inside and outside. In particular, the interior surface sites are basically free from negative effects as a result of particle agglomeration at higher temperatures. Even though the microcapsules fused into agglomerates during reactions at high temperature, the activity of the Pt catalyst at interior surfaces can be maintained.
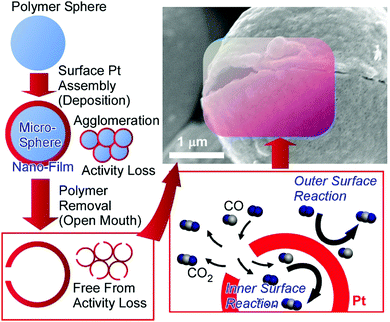 | ||
| Fig. 8 The fabrication and catalytic action of open-mouthed hollow Pt microcapsules with enhanced catalytic performance toward CO oxidation. Reproduced from ref. 46 with permission. Copyright: 2010, American Chemical Society. | ||
Nanoarchitectonics when applied to nanoporous materials and regular coordination structures, as seen in mesoporous materials,47 metal–organic frameworks,48 and coordination polymers,49 can make a good contribution to the energy and environmental fields. These materials are sometimes converted to nanoporous carbon materials for better performance. Recently, Henzie, Yamauchi, Na, and co-workers reported the facile fabrication of flexible microsupercapacitors using nanocarbon materials derived from zeolitic imidazolate framework (ZIF-8) via a simple electrophoretic process.50 The nanoarchitectured microsupercapacitors exhibited excellent electrochemical performance. Together with high simplicity and convenience, this method would be a nice approach for fabricating miniaturized flexible power suppliers.
From interfacial processes to devices
Interfacial media often become environments appropriate for nanoarchitectonics processes.51 In particular, solid interfaces, including gas-solid interfaces and liquid–solid interfaces, are essential media for basic research into atomic/molecular processes52 and device fabrication.53 Unlike solid surfaces, nonsolid–liquid interfaces, such as air–water interfaces and liquid–liquid interfaces, still remain unexplored, although some traditional methodologies, such as the Langmuir–Blodgett (LB) method,54 have been continuously used. The LB method for thin-film preparation is based on molecular self-assembly and a non-equilibrium process driven by external energy. Therefore, LB fabrication might be regarded as one of the representative nanoarchitectonics processes according to its features, as shown in Fig. 2.Molecules and nanomaterials at liquid interfaces have high freedom of motion. In fluidic interfacial environments, nanoarchitectures with huge varieties of shapes can be formed even from simple zero-dimensional objects such as fullerenes.55 Molecular interactions, such as hydrogen bonding between water-soluble molecules and water-insoluble molecules, are significantly emphasized at air–water interfaces.56 The flexible nature of molecular assemblies at air–water interfaces is advantageous for the nanoarchitecturing of complicated receptor structures from simple functional amphiphiles (Fig. 9).57 The conformational flexibility of the receptor molecules enables us to delicately tune receptor structures, making adjustments for guest structures, upon the lateral mechanical compression of the receptor monolayer.58 Precise discrimination between nucleic acid bases can be optimized via receptor tuning at the air–water interface.59 Similar procedures make it possible to switch the enantioselectivity of amino-acid recognition with certain pressure dependency.60 The latter process can be regarded as a new mode of molecular recognition based on molecular tuning (Fig. 10).61
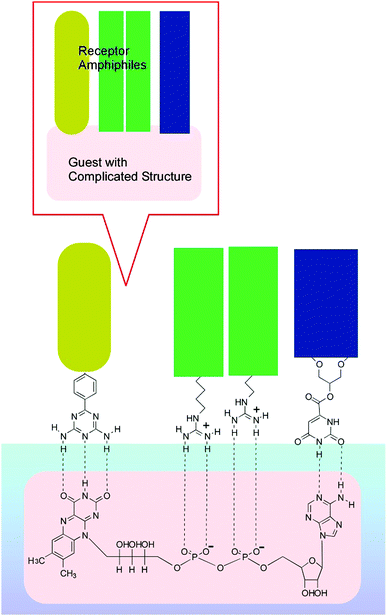 | ||
| Fig. 9 An example of nanoarchitectonics for obtaining complicated receptor structures from simple functional amphiphiles at the air–water interface. | ||
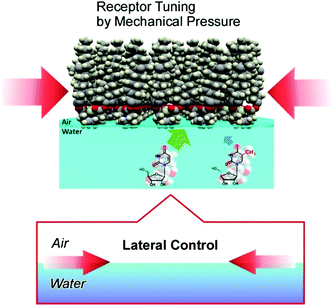 | ||
| Fig. 10 Precise discrimination between aqueous guest molecules through molecular recognition via molecular tuning. | ||
Liquid interfaces, such as air–water interfaces, have huge anisotropic structural dimensions and flexibility. The macroscopic size and motional flexibility in the lateral direction can be coupled with nanoscale molecular motion.62 Therefore, the handling of molecular machines via macroscopic hand-like motion can become possible at air–water interfaces.63 Successful examples can be seen in the control of guest capture and release upon macroscopic motion in the range of tens of centimeters,64 as well as in the regulation of molecular rotors,65 molecular pliers,66 molecular flappers,67 and molecular cars.68 Recently, Adachi et al. demonstrated submarine emission as a novel type of molecular emission control (Fig. 11).69 Double-paddled binuclear PtII complexes, as submarine molecules, were first spread on the water surface. Without applying any surface pressure, the chromophore part stayed under the highly polar water, and emission from the complex was quenched. Applying lateral pressure caused an orientational change of the double-paddled binuclear PtII complex, accompanied by the floating of the chromophore part up to the air phase with a low dielectric constant. The latter change induced a significant increase in the phosphorescence emission of the complex. Like a submarine, lighting-up resulted from the emergence of the molecule from the water phase.
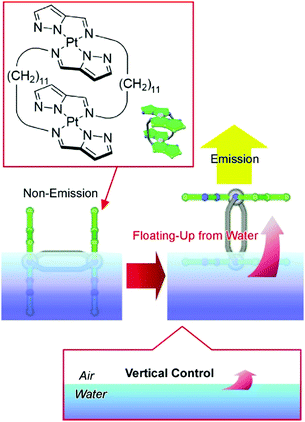 | ||
| Fig. 11 Submarine emission as a novel type of molecular emission control via floating the chromophore part up to the air phase from the water phase. | ||
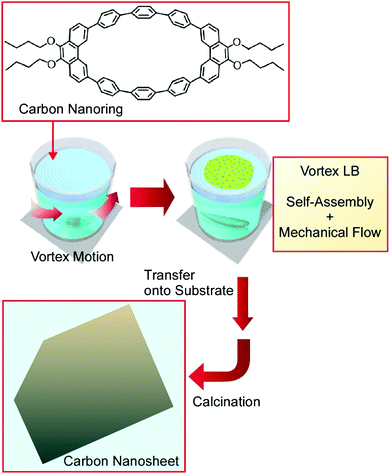
Nanoarchitectonics involving bottom-up-type materials, going from molecular carbon nanorings to two-dimensional carbon nanosheets was demonstrated at the air–water interface using a novel thin film fabrication technique known as the vortex Langmuir–Blodgett (v-LB) method (Fig. 12).70 Conventional LB processes are done carefully under vibration-free static conditions. Unlike the traditional LB method, the v-LB method utilizes continuous vortex-flow-induced assembly forces for molecular association in addition to the intrinsic self-assembly nature of the molecules themselves. Therefore, the assembly process becomes a combination of equilibrated molecular self-assembly and an energy-consuming process. π-Conjugated anisotropic macrocyclic carbon nanoring molecules, 9,9′,10,10′-tetra-butoxy-cyclo[6]-paraphenylene-[2]-3,6-phenanthrenylene, were spread on the surface of water in the presence of a vortex flow. The formed ultrathin film was transferred as uniform supramolecular nanosheets with a thickness of ca. 10 nm onto a solid surface. A calcination process converted the supramolecular nanosheets to carbon nanosheets with the maintenance of the thin-film morphology accompanied by the conversion of their electric nature from insulating to conducting. Nitrogen doping was accomplished simply via mixing with pyridine in the initial stage. Similarly uniform ultrathin nanosheets of nitrogen-doped nanocarbon were obtained after the calcination process, with a further enhancement in conductivity. The prepared materials and this nanoarchitectonics strategy to bridge molecular design and nanomaterials would be useful for certain elements for energy applications, such as catalysts for the oxygen reduction reaction in high-performance fuel cells.
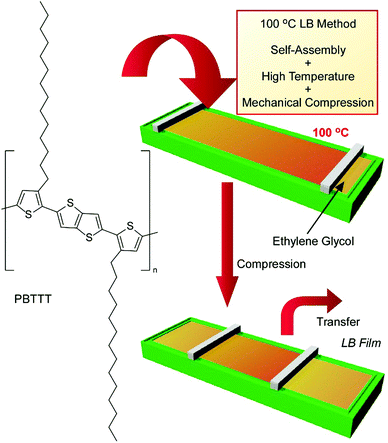
The conventional LB method is usually conducted at room temperature, such as 20 °C, because disturbances due to water evaporation have to be avoided. However, processes at room temperature are not always advantageous for preparing uniform and oriented films from materials with the tendency to aggregate easily. Instead of water, which is used in the conventional LB process, an inert and low-vapor-pressure liquid, ethylene glycol, was used as the subphase for LB processes at ultra-high temperatures of up to 100 °C in recently reported work by Ito et al. (Fig. 13).71 The proposed 100 °C LB method was applied to nanoarchitect high-quality films of the semicrystalline polymer poly[2,5-bis(3-tetradecylthiophen-2-yl)thieno(3,2-b)thiophene] (PBTTT), which works as a good polymeric semiconductor in its well-aligned state but undergoes undesirable aggregation during conventional film preparation methods. This high-temperature LB method includes molecular self-assembly, energy-consuming mechanical compression, and system heating. The polymeric semiconductor PBTTT was spread homogeneously on ethylene glycol at high temperature, and further compression of the thin films on high-temperature (80 to 100 °C) ethylene glycol induced the uniaxial alignment of polymer chains, resulting in high dichroic ratios and good mobility. Because the subphase for this new methodology can be expanded to various non-aqueous liquids, such as ionic liquids and other diol compounds, various temperatures and available film components can be considered in appropriate combinations. The limitations of the old-fashioned LB method, such as the water subphase and lipid-like film components, are removed upon using this new high-temperature LB method.
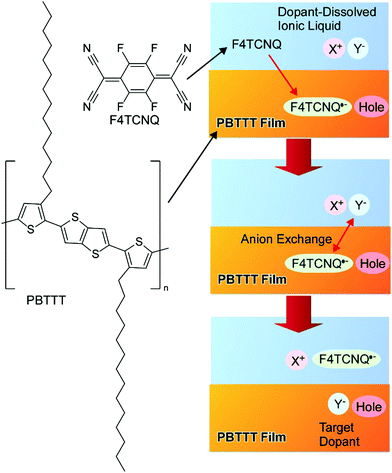
Yamashita, Watanabe, and co-workers recently reported a powerful nanoarchitectonics doping approach using an anion exchange method (Fig. 14).72 In order to obtain optimized matching between the electron affinity and ionization potential during the chemical doping of the polymeric semiconductor (PBTTT), conventional p-type dopant small anions were replaced as the initial dopants by a second anion supplied by an ionic liquid. The limitation of the redox potential as per Marcus theory can be surpassed through the inclusion of the optimized anions into a conventional binary donor/acceptor system. The doping efficiency possibly becomes 100%, with one dopant entity per unit charge. The stability of the doped structure was enhanced accompanied by the achievement of excellent transport properties. The proposed doping nanoarchitectonics methodology can significantly contribute to the development of advanced molecular electronics.
From biomolecular assemblies to life science
Living creatures can be regarded as the most successful examples of nanoarchitectonics because highly functional systems are architected from molecular-scale units. Biomolecules are mostly capable of forming self-assembled structures through specific interactions, such as hydrogen bonding. Therefore, biomolecules themselves and their mimics can be attractive component nanounits for nanoarchitectonics processes.73 Peptides are probably the most prominent structural units for forming assembled structures through main-chain hydrogen bonding in several patterns with side-chain interactions having a certain level of variety. Instead of using long peptide chains and/or integrated peptide chains in proteins, nanoarchitectonics approaches utilizing rather shorter peptides and amino acids have been proposed, as seen in several recent review articles.In a recent review article by Roy and Govindaraju, a related concept, molecular architectonics, was proposed, through which novel properties and functionality are obtained on the basis of the design of molecular assemblies.74 As one series of examples, conjugate molecules are formed between arylenediimides (naphthalenedimide and perylenediimide) and peptides and amino acids. Assembles can be architected through balanced interactions, including hydrogen bonding and interactions between aromatic rings. The accumulated structures of semiconductive arylenediimide molecules have the potential to exhibit good charge-carrier mobilities and fluorescence properties. Yan and co-workers developed various nanoarchitectonics motifs mainly using shorter peptide units such as dipeptides.75 Although the component units are rather simple, their assembled structures have sufficient variety, and their composites with other components exhibited various functionalities. Peptide nanoarchitectonics are a versatile platform for various biomedical applications, such as magnetic resonance imaging and tumour photodynamic therapy. Mihara and co-workers investigated nanoarchitectonics assemblies of oligopeptides, such as a de novo designed peptide, Ac-EYEYKYEYKY-NH2, for biomedical applications.76 Assemblies of these peptides provide environments similar to natural extracellular matrices, which are useful for cell cultures in tissue engineering. The used peptide molecules could form hydrogel materials and were also used for controlled drug release.
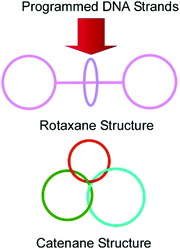
DNA has a rather limited variety of structural units (basically four kinds of nucleobases) compared with peptides. However, sequences from the four unit-structures have high variety. In addition, the strict definition of base-pairing allows us to anticipate how assembled DNA structures form their sequences. Therefore, it can be said that DNA nanoarchitectonics is accomplished on the basis of molecular programming. This concept is typically realized in DNA origami technology,77 and some examples of this show dynamic functions, like molecular machines. As described in a recent review article by Liang, Komiyama, and co-workers, huge varieties of DNA-/RNA-based molecular machines with various structural designs and functions have been demonstrated.78 As basic examples, interlocked nanoarchitectures such as rings, catenanes, rotaxanes, and their integrated structures can be constructed through DNA programming (Fig. 15). More advanced objects, including molecular shuttles, molecular transporters, molecular walkers, molecular pumps, nano- and micro-robots, and logic gates, have been demonstrated based on DNA/RNA structures. Furthermore, these DNA machineries have been applied to some functions such as sensing and catalysis.
Materials nanoarchitectonics has been efficiently used for cell cultures and the control of cell fates. One of the most successful examples is cell sheet technology. As described in a recent review by Kobayashi and Okano,79 cell sheets as two-dimensional cell assemblies can be fabricated through a nanoarchitectonics approach involving poly(N-isopropylacrylamide)-grafted surfaces (Fig. 16). Cell adhesion forces on the poly(N-isopropylacrylamide)-grafted surface can be drastically modified based on temperature. Cell cultures reach confluency on the poly(N-isopropylacrylamide)-grafted surface at 37 °C, and the formed cell sheet can be detached from the surface via decreasing the temperature to 20 °C. Because surface modifications of grafted polymers are highly possible, cultures and assemblies, even with heterotypic cells, could be obtained on surfaces with spatially controlled micropatterns. In addition, transplantation in clinical settings and the formation of three-dimensional tissue samples were also demonstrated with the aid of additional nanoarchitectonics factors, such as gelatine hydrogels and membranes. Further challenges relating to these cell architectonics strategies would be additional engineering for creating three-dimensional functional organs. Polymer-chain-level surface nanoarchitectonics can be converted to organ-level cell architectonics.
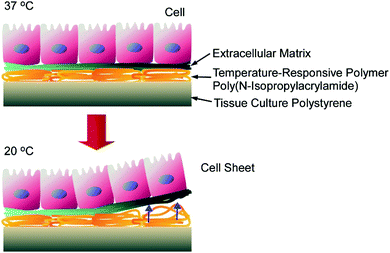 | ||
| Fig. 16 Cell sheets as two-dimensional cell assemblies fabricated through nanoarchitectonics involving poly(N-isopropylacrylamide)-grafted surfaces. | ||
The delicate control of cell interactions with a surface can lead to the regulation of cell elongation, cell proliferation, and cell differentiation. Surface nanoarchitectonics can become an important way to control these life events. Very recently, Song et al. experimentally demonstrated useful surface modifications with fullerene nanowhiskers to enhance the self-renewal and multipotency capabilities of human mesenchymal stem cells (Fig. 17).80 The one-dimensional nanocarbon material, fullerene nanowhiskers, was first prepared through a nanoarchitectonics process at a liquid–liquid interface, and the aligned structures were transferred onto a glass plate via the LB technique. The growth behaviours of human mesenchymal stem cells, such as orientation, spreading, and focal adhesion, were significantly influenced through contact with the aligned fullerene nanowhiskers. In addition, both cell proliferation and the long-term retention of multipotency are achieved in the case of human mesenchymal stem cells. The partially hydrophobic nature of the surface covered with fullerene nanowhiskers probably induced appropriate affinity between the stem cells and the nanoarchitectured surface. Focal adhesion became suitable for cell contractility with the nucleus localization of yes-associated protein. This situation resulted in the retention of multipotency. The proposed nanoarchitectonics method for stem cell control shows various additional advantages, such as high proliferation, good cell-shape control, biocompatibility, large-area cultures, low-cost, and simplicity. Therefore, significant contributions to stem cell technology can be expected.
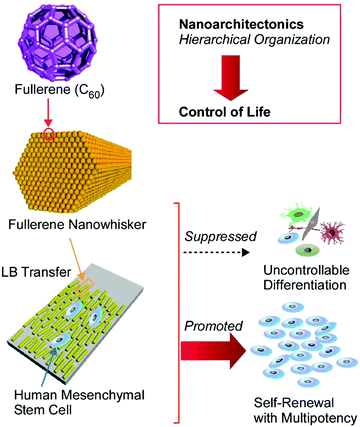 | ||
| Fig. 17 Surface modification with fullerene nanowhiskers for enhancing the self-renewal and multipotency capabilities of human mesenchymal stem cells. | ||
As a thinking-outside-the-box approach, Minami et al. demonstrated a solid-substrate-free cell culture system using an interfacial environment between a fluorocarbon liquid and aqueous liquid.81 An interfacial environment without mechanical stress from a solid surface significantly affects the differentiation of cultured cells. Further applications of this liquid–liquid cell culture system to human mesenchymal stem cells were demonstrated by Jia et al. (Fig. 18).82 At the interface between perfluorocarbon and the aqueous phase, a monolayer nanosheet of co-presented fibronectin was spontaneous formed. The cultured human mesenchymal stem cells underwent weak interactions with the formed protein nanosheet. These two objects undergo mutual interactions. Transformation of the planar protein nanosheet to a fibrous structure happened together with the neurodifferentiation of the stem cells. This neurodifferentiation can be induced without using expensive liquid factors for the induction of cell differentiation such as cytokines. This solid-free culture system with differentiation capabilities may contribute to practical stem cell technology.
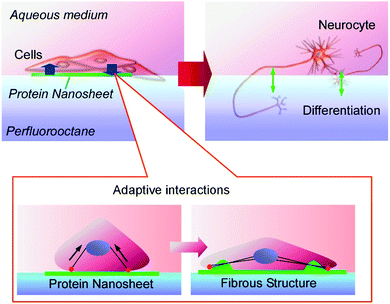 | ||
| Fig. 18 The neurodifferentiation of stem cells induced by the transformation of a planar protein nanosheet to a fibrous structure at the interface between perfluorocarbon and an aqueous phase. | ||
Perspectives
The scale-focused concept of nanotechnology has developed together with drastic progress in the area of observation/analysis technology aimed at small objects such as atoms and molecules. Nanotechnology is not only an innovative scientific concept, but it can also be a game changer in materials science to address societal demands. In science and technology today, the crucial importance of the regulation of nanoscale objects and structures is well recognized. The concept of nanotechnology has opened up ways to observe, analyse, and manipulate nanoscale objects, which should be followed by the production of functional materials with nanoscale components. The production of functional material systems using nanoscale units can be achieved via the fusion of nanotechnology with other research disciplines. This task is the focus of an emerging concept, nanoarchitectonics, as an area of interest moving beyond the nanotechnology field.In this focus article, several examples of the production of materials from nanoscale units have been introduced from the perspective of nanoarchitectonics. Architecting functional structures even from atoms and molecules results in surprisingly advanced functions. As exemplified above, basic atom responses, including redox reactions, diffusion, and aggregation, can lead to device functions ranging from simple switches to synapse-/brain-like neuromorphic devices. The construction of atomic arrangements and their related assembled structures can lead to various efficient catalytic processes, including material conversion, redox reactions, and photocatalysis. These nanoarchitectured catalysts are widely utilized for energy and environmental applications. Nanoarchitectonics fabrication in interfacial media can lead to the formation of organized structures of functional molecules in two-dimensional thin films. This feature is advantageous for the preparation of devices. Biomolecules such as peptides and DNA are highly capable of forming assembled structures through several specific interactions, such as hydrogen bonding. Therefore, these biomolecules can be powerful components in nanoarchitectonics methods for creating functional material systems. Nanoarchitectonics processes with biomolecules have been used for various applications from molecular-level machines to biomedical approaches, including tissue engineering.
As seen in these examples, the targets of nanoarchitectonics approaches are found to exist across a wide scale range, from the atomic level to visual size. The target objects are plotted in Fig. 19 as function of their size and complexity. The nanoarchitectonics concept has the ability to bridge nanoscale science and visual size materials. This transient size region (from the nanoscale to the submicron scale) actually corresponds to the scale region between non-living matter and living creatures.83 On the nanoscale and slightly larger scales, atoms, molecules, and materials are purely physicochemical objects. However, these physicochemical objects are architected and integrated into living creatures in the submicrometer and micrometer regions. Increases in the complexities of organized systems in the nanoarchitectonics size region results in life-like higher functioning with autonomous action. Therefore, the final goal of nanoarchitectonics approaches would be the creation of living-creature-like functional material systems from simple nanoscale objects.84 Nanoarchitectonics could be the future of nanoscale science, following nanotechnology, with the goal of obtaining life-like sophisticated systems.
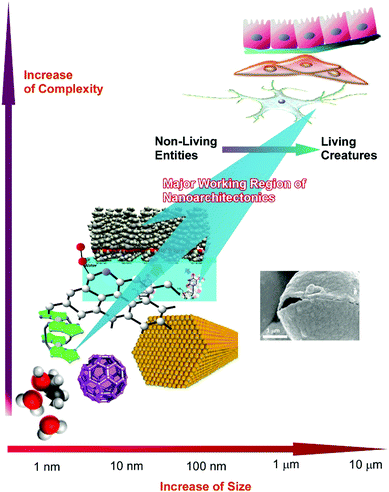 | ||
| Fig. 19 Nanoarchitectonics approaches across a wide scale range, from the atomic level to visual size, which may be able to convert purely physicochemical objects into living creatures. | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by JSPS KAKENHI Grant Number JP16H06518 (Coordination Asymmetry), JP20H00392, and JP20H00316.Notes and references
- R. P. Feynman, Eng. Sci., 1960, 23, 32 Search PubMed; M. Roukes, Sci. Am., 2001, 285, 48 CrossRef CAS PubMed.
- P. Jelínek, J. Phys. Condens. Matter, 2017, 29, 343002 CrossRef CAS PubMed; A. Shiotari, Y. Sugimoto and H. Kamio, Phys. Rev. Mater., 2019, 3, 093001(R) CrossRef; T. Shimizu, D. Lungerich, J. Stuckner, M. Murayama, K. Harano and E. Nakamura, Bull. Chem. Soc. Jpn., 2020, 93, 1079 CrossRef; K. Kamei, T. Shimizu, K. Harano and E. Nakamura, Bull. Chem. Soc. Jpn., 2020, 93, 1603 CrossRef; B. Ding, Z. Li, G. Xu, H. Li, Z. Hou, E. Liu, X. Xi, F. Xu, Y. Yao and W. Wang, Nano Lett., 2020, 20, 868 CrossRef PubMed.
- D. Eigler and E. K. Schweizer, Nature, 1990, 344, 524 CrossRef CAS; D. H. Huang, H. Uchida and M. Aono, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 1994, 12, 2429 CrossRef; L. Bartels, G. Meyer and K. H. Rieder, Phys. Rev. Lett., 1997, 79, 697 CrossRef; F. Tao and P. A. Crozier, Chem. Rev., 2016, 116, 3487 CrossRef PubMed.
- J. Cherusseri, N. Choudhary, K. S. Kumar, Y. Jung and J. Thomas, Nanoscale Horiz., 2019, 4, 840 RSC; B. Li, H. Xu, Y. Ma and S. Yang, Nanoscale Horiz., 2019, 4, 77 RSC; Q. Wang and K. Domen, Chem. Rev., 2020, 120, 919 CrossRef CAS PubMed; D. Luo, R. Su, W. Zhang, Q. Gong and R. Zhu, Nat. Rev. Mater., 2020, 5, 44 CrossRef; S.-Y. Bae, J. Mahmood, I.-Y. Jeon and J.-B. Baek, Nanoscale Horiz., 2020, 5, 43 RSC; E. Cha, M. Patel, S. Bhoyate, V. Prasad and W. Choi, Nanoscale Horiz., 2020, 5, 808 RSC.
- M. F. Hochella Jr., D. W. Mogk, J. Ranville, I. C. Allen, G. W. Luther, L. C. Marr, B. P. McGrail, M. Murayama, N. P. Qafoku, K. M. Rosso, N. Sahai, P. A. Schroeder, P. Vikesland, P. Westerhoff and Y. Yang, Science, 2019, 363, 1414 CrossRef CAS; G. Sai-Anand, A. Sivanesan, M. R. Benzigar, G. Singh, A.-I. Gopalan, A. V. Baskar, H. Ilbeygi, K. Ramadass, V. Kambala and A. Vinu, Bull. Chem. Soc. Jpn., 2019, 92, 216 CrossRef; X. Zhan, C. Si, J. Zhou and Z. Sun, Nanoscale Horiz., 2020, 5, 235 RSC; M. Rebber, C. Willa and D. Koziej, Nanoscale Horiz., 2020, 5, 431 RSC.
- J. Chen, H. Meng, Y. Tian, R. Yang, D. Du, Z. Li, L. Qu and Y. Lin, Nanoscale Horiz., 2019, 4, 321 RSC; K. D. Patel, R. K. Singh and H.-W. Kim, Mater. Horiz., 2019, 6, 434 RSC; J. L. Paris and M. Vallet-Regí, Bull. Chem. Soc. Jpn., 2020, 93, 220 CrossRef CAS; E. Yuba, J. Mater. Chem. B, 2020, 8, 1093 RSC; H. Arora, M. Ramesh, K. Rajasekhar and T. Govindaraju, Bull. Chem. Soc. Jpn., 2020, 93, 507 CrossRef; H. Cabral, H. Kinoh and K. Kataoka, Acc. Chem. Res., 2020, 53, 2765 CrossRef PubMed; H. Li, X. Yan, D. Kong, R. Jin, C. Sun, D. Du, Y. Lin and G. Lu, Nanoscale Horiz., 2020, 5, 218 RSC; P. Wang, T. Kim, M. Harada, C. Contag, X. Huang and B. R. Smith, Nanoscale Horiz., 2020, 5, 628 RSC.
- G. Povie, Y. Segawa, T. Nishihara, Y. Miyauchi and K. Itami, Science, 2017, 356, 172 CrossRef CAS PubMed; Z. Sun, K. Ikemoto, T. M. Fukunaga, T. Koretsune, R. Arita, S. Sato and H. Isobe, Science, 2019, 363, 151 CrossRef PubMed; W. Muramatsu, T. Hattori and H. Yamamoto, Bull. Chem. Soc. Jpn., 2020, 93, 759 CrossRef; T. Okamoto, C. P. Yu, C. Mitsui, M. Yamagishi, H. Ishii and J. Takeya, J. Am. Chem. Soc., 2020, 142, 9083 CrossRef PubMed; Y. Ran, Y.g Guo and Y. Liu, Mater. Horiz., 2020, 7, 1955 RSC.
- P. Luo, F. Zhuge, Q. Zhang, Y. Chen, L. Lv, Y. Huang, H. Li and T. Zhai, Nanoscale Horiz., 2019, 4, 26 RSC; K. Akagi, Bull. Chem. Soc. Jpn., 2019, 92, 1509 CrossRef CAS; S. Horike, S. S. Nagarkar, T. Ogawa and S. Kitagawa, Angew. Chem., Int. Ed., 2020, 59, 6652 CrossRef PubMed; Y. Li, X. Li, H. Zhanga and Q. Xiang, Nanoscale Horiz., 2020, 5, 765 RSC; H. Tao, N. Lavoine, F. Jiang, J. Tang and N. Lin, Nanoscale Horiz., 2020, 5, 607 RSC.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed; H. Masai and J. Terao, Bull. Chem. Soc. Jpn., 2019, 92, 529 CrossRef; S. Datta, Y. Kato, S. Higashiharaguchi, K. Aratsu, A. Isobe, T. Saito, D. D. Prabhu, Y. Kitamoto, M. J. Hollamby, A. J. Smith, R. Dalgliesh, N. Mahmoudi, L. Pesce, C. Perego, G. M. Pavan and S. Yagai, Nature, 2020, 583, 400 CrossRef PubMed; J. Li, J. Wang, H. Li, N. Song, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2020, 49, 1144 RSC; L. S. Manni, W.-K. Fong and R. Mezzenga, Nanoscale Horiz., 2020, 5, 914 RSC.
- K. Eric Drexler, Nanosystems: Molecular Machinery, Manufacturing, and Computation, Wiley, New York, 1992 Search PubMed.
- K. Ariga, Q. Ji, W. Nakanishi, J. P. Hill and M. Aono, Mater. Horiz., 2015, 2, 406 RSC; K. Ariga, K. Minami, M. Ebara and J. Nakanishi, Polym. J., 2016, 48, 371 CrossRef CAS.
- S. Rej, Y. Ano and N. Chatani, Chem. Rev., 2020, 120, 1788 CrossRef CAS PubMed; X. Xu, K. Müllen and A. Narita, Bull. Chem. Soc. Jpn., 2020, 93, 490 CrossRef; Y. Ogiwara and N. Sakai, Angew. Chem., Int. Ed., 2020, 59, 574 CrossRef PubMed.
- T. Haino, Polym. J., 2019, 51, 303 CrossRef CAS; T. Akiyama, Bull. Chem. Soc. Jpn., 2019, 92, 1181 CrossRef; Y. Domoto, M. Abe, T. Kikuchi and M. Fujita, Angew. Chem., Int. Ed., 2020, 59, 3450 CrossRef PubMed; Z. Yang, Y. Dai, L. Shan, Z. Shen, Z. Wang, B. C. Yung, O. Jacobson, Y. Liu, W. Tang, S. Wang, L. Lin, G. Niu, P. Huang and X. Chen, Nanoscale Horiz., 2019, 4, 426 RSC.
- W. Gao, H. Ota, D. Kiriya, K. Takei and A. Javey, Acc. Chem. Res., 2019, 52, 523 CrossRef CAS PubMed; Y. Watanabe, H. Sasabe and J. Kido, Bull. Chem. Soc. Jpn., 2019, 92, 716 CrossRef; V. Amendola, D. Amans, Y. Ishikawa, N. Koshizaki, S. Scirè, G. Compagnini, S. Reichenberger and S. Barcikowski, Chem. – Eur. J., 2020, 26, 9206 CrossRef PubMed.
- Y. Tobimatsu and M. Schuetz, Curr. Opin. Biotechnol., 2019, 56, 75 CrossRef CAS PubMed; J. Guo, T. Suma, J. J. Richardson and H. Ejima, ACS Biomater. Sci. Eng., 2019, 5, 5578 CrossRef PubMed; K. Tanaka and K. Vong, Bull. Chem. Soc. Jpn., 2020, 93, 1275 CrossRef; Y. Sun and Z. Guo, Nanoscale Horiz., 2019, 4, 52 RSC.
- M. Aono, Sci. Technol. Adv. Mater., 2011, 12, 040301 CrossRef CAS PubMed; K. Ariga, Q. Ji, J. P. Hill, Y. Bando and M. Aono, NPG Asia Mater., 2012, 4, e17 CrossRef; K. Ariga, M. Nishikawa, T. Mori, J. Takeya, L. K. Shrestha and J. P. Hill, Sci. Technol. Adv. Mater., 2019, 20, 51 CrossRef.
- K. Ariga, J. Li, J. Fei, Q. Ji and J. P. Hill, Adv. Mater., 2016, 28, 1251–1286 CrossRef CAS PubMed; K. Ariga and L. K. Shrestha, APL Mater., 2019, 7, 120903 CrossRef; K. Ariga, Trends Chem., 2020, 2, 779 CrossRef.
- S. Ishihara, J. Labuta, W. Van Rossom, D. Ishikawa, K. Minami, J. P. Hill and K. Ariga, Phys. Chem. Chem. Phys., 2014, 16, 9713 RSC; K. Ariga, S. Watanabe, T. Mori and J. Takeya, NPG Asia Mater., 2018, 10, 90 CrossRef CAS; K. Ariga, M. Ito, T. Mori, S. Watanabe and J. Takeya, Nano Today, 2019, 28, 100762 CrossRef.
- W. Nakanishi, K. Minami, L. K. Shrestha, Q. Ji, J. P. Hill and K. Ariga, Nano Today, 2014, 9, 378 CrossRef CAS; K. Ariga, Q. Ji, T. Mori, M. Naito, Y. Yamauchi, H. Abe and J. P. Hill, Chem. Soc. Rev., 2013, 42, 6322 RSC; K. Ariga, D. T. Leong and T. Mori, Adv. Funct. Mater., 2018, 28, 1702905 CrossRef; S. Dutta, J. Kim, P.-H. Hsieh, Y.-S. Hsu, Y. Y. Kaneti, F.-K. Shieh, Y. Yamauchi and K. C.-W. Wu, Small Methods, 2019, 3, 1900213 CrossRef.
- G. Rydzek, Q. Ji, M. Li, P. Schaaf, J. P. Hill, F. Boulmedais and K. Ariga, Nano Today, 2015, 10, 138 CrossRef CAS; K. Ariga, E. Ahn, M. Park and B.-S. Kim, Chem. – Asian J., 2019, 14, 2553 CrossRef PubMed; D. Jeon, H. Kim, C. Lee, Y. Han, M. Gu, B.-S. Kim and J. Ryu, ACS Appl. Mater. Interfaces, 2017, 9, 40151 CrossRef PubMed; H. Kim, Y. You, D. Kang, D. Jeon, S. Bae, Y. Shin, J. Lee, J. Lee and J. Ryu, Adv. Funct. Mater., 2019, 29, 1906407 CrossRef; M. Park, M. Gu and B.-S. Kim, ACS Nano, 2020, 14, 6812 CrossRef PubMed; M. Gu and B.-S. Kim, Acc. Chem. Res., 2021, 54, 57 CrossRef PubMed.
- K. Ariga, X. Jia, J. Song, J. P. Hill, D. T. Leong, Y. Jia and J. Li, Angew. Chem., Int. Ed., 2020, 59, 15424 CrossRef CAS PubMed.
- D.-L. Jiang and T. Aida, Nature, 1997, 388, 454 CrossRef CAS; T. Kato, K. Strakova, J. García-Calvo, N. Sakai and S. Matile, Bull. Chem. Soc. Jpn., 2020, 93, 1401 CrossRef.
- Y. Umena, K. Kawakami, J.-R. Shen and N. Kamiya, Nature, 2011, 473, 55 CrossRef CAS PubMed; H. Kandori, Bull. Chem. Soc. Jpn., 2020, 93, 76 CrossRef; M. Giacomello, A. Pyakurel, C. Glytsou and L. Scorrano, Nat. Rev. Mol. Cell Biol., 2020, 21, 204 CrossRef PubMed.
- K. Ariga, X. Jia, J. Song, J. P. Hill, D. T. Leong, Y. Jia and J. Li, Angew. Chem., Int. Ed., 2020, 59, 15269 CrossRef PubMed.
- K. Ariga, T. Mori, T. Kitao and T. Uemura, Adv. Mater., 2020, 32, 1905657 CrossRef CAS PubMed.
- K. Ariga, Small Sci., 2021, 1, 2000032 CrossRef.
- S. Kawai, O. Krejčí, T. Nishiuchi, K. Sahara, T. Kodama, R. Pawlak, E. Meyer, T. Kubo and A. S. Foster, Sci. Adv., 2020, 6, eaay8913 CrossRef CAS PubMed.
- R. Waser and M. Aono, Nat. Mater., 2007, 6, 833 CrossRef CAS PubMed; T. Hasegawa, K. Terabe, T. Tsuruoka and M. Aono, Adv. Mater., 2012, 24, 252 CrossRef PubMed.
- K. Terabe, T. Hasegawa, T. Nakayama and M. Aono, Nature, 2005, 433, 47 CrossRef CAS PubMed.
- T. Ohno, T. Hasegawa, T. Tsuruoka, K. Terabe, J. K. Gimzewski and M. Aono, Nat. Mater., 2011, 10, 591 CrossRef CAS PubMed.
- A. V. Avizienis, H. O. Sillin, C. Martin-Olmos, H. H. Shieh, M. Aono, A. Z. Stieg and J. K. Gimzewski, PLoS One, 2012, 7, e42772 CrossRef CAS PubMed.
- A. Diaz-Alvarez, R. Higuchi1, P. Sanz-Leon, I. Marcus, Y. Shingaya, A. Z. Stieg, J. K. Gimzewski, Z. Kuncic and T. Nakayama, Sci. Rep., 2019, 9, 14920 CrossRef PubMed.
- A. Loeffler, R. Zhu, J. Hochstetter, M. Li, K. Fu, A. Diaz-Alvarez, T. Nakayama, J. M. Shine and Z. Kuncic, Front. Neurosci., 2020, 14, 184 CrossRef PubMed.
- L. Han, S. Cai, M. Gao, J. Hasegawa, P. Wang, J. Zhang, L. Shi and D. Zhang, Chem. Rev., 2019, 119, 10916 CrossRef CAS PubMed; A. Glotov, A. Stavitskaya, Y. Chudakov, E. Ivanov, W. Huang, V. Vinokurov, A. Zolotukhina, A. Maximov, E. Karakhanov and Y. Lvov, Bull. Chem. Soc. Jpn., 2019, 92, 61 CrossRef; L. K. Mannepalli, C. Gadipelly, G. Deshmukh, P. Likhar and S. Pottabathula, Bull. Chem. Soc. Jpn., 2020, 93, 355 CrossRef; K.-J. Jiao, Y.-K. Xing, Q.-L. Yang, H. Qiu and T.-S. Mei, Acc. Chem. Res., 2020, 53, 300 CrossRef PubMed.
- T. Adachi, Y. Kitazumi, O. Shirai and K. Kano, Catalysts, 2020, 10, 236 CrossRef CAS; K. Mashima, Bull. Chem. Soc. Jpn., 2020, 93, 799 CrossRef; S. Fa, M. Yamamoto, H. Nishihara, R. Sakamoto, K. Kamiya, Y. Nishina and T. Ogoshi, Chem. Sci., 2020, 11, 5866 RSC.
- M. Wen, G. Li, H. Liu, J. Chen, T. An and H. Yamashita, Environ. Sci.: Nano, 2019, 6, 1006 RSC; P. Verma, Y. Kuwahara, K. Mori and H. Yamashita, Bull. Chem. Soc. Jpn., 2019, 92, 19 CrossRef CAS; Z.-j. Wang, H. Song, H. Liu and J. Ye, Angew. Chem., Int. Ed., 2020, 59, 8016 CrossRef PubMed; A. Akhundi, A. Badiei, G. M. Ziarani, A. Habibi-Yangjeh, M. J. Munoz-Batista and R. Luque, Mol. Catal., 2020, 488, 110902 CrossRef.
- H. Abe, J. Liu and K. Ariga, Mater. Today, 2016, 19, 12 CrossRef CAS; H. Wang, S. Yin, K. Eid, Y. Li, Y. Xu, X. Li, H. Xue and L. Wang, ACS Sustainable Chem. Eng., 2018, 6, 11768 CrossRef; F. Wu, K. Eid, A. M. Abdullah, W. Niu, C. Wang, Y. Lan, A. A. Elzatahry and G. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 31309 CrossRef.
- Y. Wang, J. Mao, X. Meng, L. Yu, D. Deng and X. Bao, Chem. Rev., 2019, 119, 1806 CrossRef CAS PubMed; A. Beniya and S. Higashi, Nat. Catal., 2019, 2, 590 CrossRef.
- F. M. Auxilia, S. Ishihara, S. Mandal, T. Tanabe, G. Saravanan, G. V. Ramesh, N. Umezawa, T. Hara, Y. Xu, S. Hishita, Y. Yamauchi, D. Arivuoli, J. P. Hill, K. Ariga and H. Abe, Adv. Mater., 2014, 26, 4481 CrossRef CAS PubMed.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361 CrossRef CAS PubMed.
- A. Miyoshi, S. Nishioka and K. Maeda, Chem. – Eur. J., 2018, 24, 18204 CrossRef CAS PubMed; N. Roy, N. Suzuki, C. Terashima and A. Fujishima, Bull. Chem. Soc. Jpn., 2019, 92, 178 CrossRef; D. Zhao, Z. Zhuang, X. Cao, C. Zhang, Q. Peng, C. Chen and Y. Li, Chem. Soc. Rev., 2020, 49, 2215 RSC.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- K. Maeda and T. E. Mallouk, Bull. Chem. Soc. Jpn., 2019, 92, 38 CrossRef CAS.
- C. N. R. Rao and K. Pramoda, Bull. Chem. Soc. Jpn., 2019, 92, 441 CrossRef CAS.
- T. Imaoka and K. Yamamoto, Bull. Chem. Soc. Jpn., 2019, 92, 941 CrossRef CAS.
- S. Mandal, M. Sathish, G. Saravanan, K. K. R. Datta, Q. Ji, J. P. Hill, H. Abe, I. Honma and K. Ariga, J. Am. Chem. Soc., 2010, 132, 14415 CrossRef CAS PubMed.
- E. Doustkhah, J. Lin, S. Rostamnia, C. Len, R. Luque, X. Luo, Y. Bando, K. C.-W. Wu, J. Kim, Y. Yamauchi and Y. Ide, Chem. – Eur. J., 2019, 25, 1614 CrossRef CAS PubMed; B. Singh, J. Na, M. Konarova, T. Wakihara, Y. Yamauchi, C. Salomon and M. B. Gawande, Bull. Chem. Soc. Jpn., 2020, 93, 1459 CrossRef; J. A. Costa, R. A. de Jesus, D. O. Santos, J. F. Mano, L. P. C. Romao and C. M. Paranhos, Microporous Mesoporous Mater., 2020, 29, 109698 CrossRef.
- M. Safaei, M. M. Foroughi, N. Ebrahimpoor, S. Jahani, A. Omidi and M. Khatami, TrAC, Trends Anal. Chem., 2019, 118, 401 CrossRef CAS; D. Salinas-Torres, A. Nozaki, M. Navlani-García, Y. Kuwahara, K. Mori and H. Yamashita, Bull. Chem. Soc. Jpn., 2020, 93, 438 CrossRef; C. Zhu, H. Wang and C. Guan, Nanoscale Horiz., 2020, 5, 1188 RSC.
- A. Azhar, Y. Li, Z. Cai, M. B. Zakaria, M. K. Masud, Md. S. A. Hossain, J. Kim, W. Zhang, J. Na, Y. Yamauchi and M. Hu, Bull. Chem. Soc. Jpn., 2019, 92, 875 CrossRef CAS; C. V. Nguyen, W.-H. Chiang and K. C.-W. Wu, Bull. Chem. Soc. Jpn., 2019, 92, 1430 CrossRef.
- Y. Li, J. Henzie, T. Park, J. Wang, C. Young, H. Xie, J. W. Yi, J. Li, M. Kim, J. Kim, Y. Yamauchi and J. Na, Bull. Chem. Soc. Jpn., 2020, 93, 176 CrossRef CAS.
- K. Ariga, M. V. Lee, T. Mori, X.-Y. Yu and J. P. Hill, Adv. Colloid Interface Sci., 2010, 154, 20 CrossRef CAS PubMed; J. M. Giussi, M. L. Cortez, W. A. Marmisollé and O. Azzaroni, Chem. Soc. Rev., 2019, 48, 814 RSC; K. Ariga, M. Ishii and T. Mori, Curr. Opin. Colloid Interface Sci., 2019, 44, 1 CrossRef.
- K. Tahara, A. Noguchi, R. Nakayama, E. Ghijsens, S. De Feyter and Y. Tobe, Angew. Chem., Int. Ed., 2019, 58, 7733 CrossRef CAS PubMed; X.-Y. Wang, X. Yao, A. Narita and K. Müllen, Acc. Chem. Res., 2019, 52, 2491 CrossRef PubMed.
- A. Yamamura, S. Watanabe, M. Uno, M. Mitani, C. Mitsui, J. Tsurumi, N. Isahaya, Y. Kanaoka, T. Okamoto and J. Takeya, Sci. Adv., 2018, 4, eaao5758 CrossRef CAS PubMed; C.-Y. Chen, C.-M. Wang and W.-S. Liao, Bull. Chem. Soc. Jpn., 2019, 92, 600 CrossRef.
- K. Ariga, Y. Yamauchi, T. Mori and J. P. Hill, Adv. Mater., 2013, 25, 6477 CrossRef CAS PubMed; T. Yunoki, Y. Kimura and A. Fujimori, Bull. Chem. Soc. Jpn., 2019, 92, 1662 CrossRef; K. Ariga, Langmuir, 2020, 36, 7158 CrossRef PubMed; M. Gabaji, J. Médard, A. Hemmerle, J. Pinson and J. P. Michel, Langmuir, 2020, 36, 2534 CrossRef PubMed.
- K. Miyazawa, J. Nanosci. Nanotechnol., 2009, 9, 41 CrossRef CAS PubMed; L. K. Shrestha, Q. Ji, T. Mori, K. Miyazawa, Y. Yamauchi, J. P. Hill and K. Ariga, Chem. – Asian J., 2013, 8, 1662 CrossRef PubMed.
- K. Ariga and T. Kunitake, Acc. Chem. Res., 1998, 31, 371 CrossRef CAS; K. Ariga and Phys Chem, Chem. Phys., 2020, 22, 24856 Search PubMed.
- K. Ariga, H. Ito, J. P. Hill and H. Tsukube, Chem. Soc. Rev., 2012, 41, 5800 RSC.
- K. Sakakibara, L. A. Joyce, T. Mori, T. Fujisawa, S. H. Shabbir, J. P. Hill, E. V. Anslyn and K. Ariga, Angew. Chem., Int. Ed., 2012, 51, 9643 CrossRef CAS PubMed; K. Ariga, M. Ishii and T. Mori, Chem. – Eur. J., 2020, 26, 6461 CrossRef PubMed.
- T. Mori, K. Okamoto, H. Endo, J. P. Hill, S. Shinoda, M. Matsukura, H. Tsukube, Y. Suzuki, Y. Kanekiyo and K. Ariga, J. Am. Chem. Soc., 2010, 132, 12868 CrossRef CAS.
- T. Michinobu, S. Shinoda, T. Nakanishi, J. P. Hill, K. Fujii, T. N. Player, H. Tsukube and K. Ariga, J. Am. Chem. Soc., 2006, 128, 14478 CrossRef CAS PubMed.
- K. Ariga, ChemNanoMat, 2020, 6, 870 CrossRef CAS.
- K. Ariga, T. Mori and J. P. Hill, Adv. Mater., 2012, 24, 158 CrossRef CAS.
- K. Ariga, T. Mori and J. Li, Langmuir, 2019, 35, 3585 CrossRef CAS PubMed; K. Ariga, Chem. Sci., 2020, 11, 10594 RSC.
- K. Ariga, Y. Terasaka, D. Sakai, H. Tsuji and J. Kikuchi, J. Am. Chem. Soc., 2000, 122, 7835 CrossRef CAS; K. Ariga, T. Nakanishi, Y. Terasaka, H. Tsuji, D. Sakai and J. Kikuchi, Langmuir, 2005, 21, 976 CrossRef PubMed.
- T. Mori, H. Komatsu, N. Sakamoto, K. Suzuki, J. P. Hill, M. Matsumoto, H. Sakai, K. Ariga and W. Nakanishi, Phys. Chem. Chem. Phys., 2018, 20, 3073 RSC; T. Mori, H. Chin, K. Kawashima, H. Ngo, N.-J. Cho, W. Nakanishi, J. P. Hill and K. Ariga, ACS Nano, 2019, 13, 2410 Search PubMed.
- D. Ishikawa, T. Mori, Y. Yonamine, W. Nakanishi, D. Cheung, J. P. Hill and K. Ariga, Angew. Chem., Int. Ed., 2015, 54, 8988 CrossRef CAS PubMed; T. Mori, D. Ishikawa, Y. Yonamine, Y. Fujii, J. P. Hill, I. Ichinose, K. Ariga and W. Nakanishi, ChemPhysChem, 2017, 18, 1470 CrossRef PubMed.
- W. Nakanishi, S. Saito, N. Sakamoto, A. Kashiwagi, S. Yamaguchi, H. Sakai and K. Ariga, Chem. – Asian J., 2019, 14, 2869 CrossRef CAS PubMed.
- M. Ishii, T. Mori, W. Nakanishi, J. P. Hill, H. Sakai and K. Ariga, ACS Nano, 2020, 14, 13294 CrossRef PubMed.
- J. Adachi, T. Mori, R. Inoue, M. Naito, N. H.-T. Le, S. Kawamorita, J. P. Hill, T. Naota and K. Ariga, Chem. – Asian J., 2020, 15, 406 CrossRef CAS PubMed.
- T. Mori, H. Tanaka, A. Dalui, N. Mitoma, K. Suzuki, M. Matsumoto, N. Aggarwal, A. Patnaik, S. Acharya, L. K. Shrestha, H. Sakamoto, K. Itami and K. Ariga, Angew. Chem., Int. Ed., 2018, 57, 9679 CrossRef CAS PubMed.
- M. Ito, Y. Yamashita, Y. Tsuneda, T. Mori, J. Takeya, S. Watanabe and K. Ariga, ACS Appl. Mater. Interfaces, 2020, 12, 56522 CrossRef PubMed.
- Y. Yamashita, J. Tsurumi, M. Ohno, R. Fujimoto, S. Kumagai, T. Kurosawa, T. Okamoto, J. Takeya and S. Watanabe, Nature, 2019, 572, 634 CrossRef CAS PubMed.
- Q. Zou, K. Liu, M. Abbas and X. Yan, Adv. Mater., 2016, 28, 1031 CrossRef CAS PubMed; J. Liu, H. Zhou, W. Yang and K. Ariga, Acc. Chem. Res., 2020, 53, 644 CrossRef PubMed.
- B. Roy and T. Govindaraju, Bull. Chem. Soc. Jpn., 2019, 92, 1883 CrossRef CAS.
- L. Zhao, Q. Zou and X. Yan, Bull. Chem. Soc. Jpn., 2019, 92, 70 CrossRef CAS.
- K. Fukunaga, H. Tsutsumi and H. Mihara, Bull. Chem. Soc. Jpn., 2019, 92, 391 CrossRef CAS.
- M. Endo and H. Sugiyama, Molecules, 2018, 23, 1766 CrossRef CAS PubMed; S. Mishra, Y. Feng, M. Endo and H. Sugiyama, ChemBioChem, 2020, 21, 33 CrossRef; Y. Dong, C. Yao, Y. Zhu, L. Yang, D. Luo and D. Yang, Chem. Rev., 2020, 120(17), 9420 CrossRef PubMed; H. Bila, E. E. Kurisinkal and M. M. C. Bastings, Biomater. Sci., 2019, 7, 53 RSC.
- X. Liang, L. Li, J. Tang, M. Komiyama and K. Ariga, Bull. Chem. Soc. Jpn., 2020, 93, 581 CrossRef CAS.
- J. Kobayashi and T. Okano, Bull. Chem. Soc. Jpn., 2019, 92, 817 CrossRef CAS.
- J. Song, X. Jia, K. Minami, J. P. Hill, J. Nakanishi, L. K. Shrestha and K. Ariga, ACS Appl. Nano Mater., 2020, 3, 6497 CrossRef CAS.
- K. Minami, T. Mori, W. Nakanishi, N. Shigi, J. Nakanishi, J. P. Hill, M. Komiyama and K. Ariga, ACS Appl. Mater. Interfaces, 2017, 9, 30553 CrossRef CAS PubMed; K. Ariga, X. Jia, J. Song, C.-T. Hsieh and S.-h. Hsu, ChemNanoMat, 2019, 5, 692 CrossRef.
- X. Jia, K. Minami, K. Uto, A. C. Chang, J. P. Hill, T. Ueki, J. Nakanishi and K. Ariga, Small, 2019, 15, 1804640 CrossRef CAS PubMed; X. Jia, K. Minami, K. Uto, A. C. Chang, J. P. Hill, J. Nakanishi and K. Ariga, Adv. Mater., 2020, 32, 1905942 CrossRef PubMed.
- K. Ariga, Mater. Chem. Front., 2017, 1, 208 RSC.
- K. Ariga and Y. Yamauchi, Chem. – Asian J., 2020, 15, 718 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |