A multifunctional Fenton nanoagent for microenvironment-selective anti-biofilm and anti-inflammatory therapy†
Yuqing
Li
a,
Weijun
Xiu
a,
Kaili
Yang
a,
Qirui
Wen
a,
Lihui
Yuwen
 *a,
Zichao
Luo
*a,
Zichao
Luo
 b,
Xiaogang
Liu
b,
Dongliang
Yang
c,
Xiaoji
Xie
b,
Xiaogang
Liu
b,
Dongliang
Yang
c,
Xiaoji
Xie
 d and
Lianhui
Wang
d and
Lianhui
Wang
 *a
*a
aKey Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM) & Jiangsu National Synergetic Innovation Centre for Advanced Materials (SICAM), Nanjing University of Posts and Telecommunications, Nanjing 210023, China. E-mail: iamlhyuwen@njupt.edu.cn; iamlhwang@njupt.edu.cn
bDepartment of Chemistry, National University of Singapore, Singapore 117543, Singapore
cInstitute of Advanced Materials (IAM), School of Physical and Mathematical Sciences, Nanjing Tech University, Nanjing 211800, China
dInstitute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Centre for Advanced Materials (SICAM), Nanjing Tech University, Nanjing 211800, China
First published on 23rd January 2021
Abstract
Bacterial biofilm infections are intractable to traditional antibiotic treatment and usually cause persistent inflammation. Chemodynamic therapy (CDT) based on the Fenton reaction has recently emerged as a promising anti-biofilm strategy. However, the therapeutic efficacy of current Fenton agents often suffers from inefficient Fenton activity and lacks anti-inflammatory capability. Herein, FePS3 nanosheets (NSs) are explored for the first time as novel microenvironment-selective therapeutic nanoagents for bacterial biofilm infections with both self-enhanced Fenton activity for an anti-biofilm effect and reactive oxygen species (ROS) scavenging properties for an anti-inflammatory effect. In biofilms with acidic microenvironments, FePS3 NSs release Fe2+ to generate toxic ROS by Fenton reaction and reductive [P2S6]4− to enhance the Fenton activity by reducing Fe3+ to Fe2+. In the surrounding normal tissues with neutral pH, FePS3 NSs scavenge ROS by reductive [P2S6]4− with an anti-inflammatory effect. This work demonstrates multifunctional Fenton nanoagents with microenvironment-selective ROS generation and elimination properties for effective treatment of bacterial biofilm infections with both anti-biofilm and anti-inflammatory effects.
New conceptsFenton agents catalytically convert hydrogen peroxide (H2O2) to highly oxidative hydroxyl radicals (˙OH) for effective bacteria-killing without common drug-resistance issues. However, their therapeutic efficacy for bacterial biofilm infections is usually confronted with two challenges: (i) the low iron redox cycling efficiency from Fe3+ to Fe2+ limits the Fenton reaction activity and ˙OH formation, resulting in inefficient biofilm eradication. (ii) Current Fenton agents lack microenvironment-selective reactive oxygen species (ROS) regulation capability, which restrains their use only for ROS generation to kill bacteria in biofilms rather than ROS elimination to mitigate inflammation in normal tissues. Herein, a novel multifunctional Fenton nanoagent (FePS3 nanosheets, FePS3 NSs) is firstly demonstrated to possess both self-enhanced Fenton activity in biofilm infected tissues with acidic pH and ROS scavenging properties in normal tissues with neutral pH. The microenvironment-selective ROS regulation properties of FePS3 NSs originates from the integration of Fenton-active Fe cations and reductive [P2S6]4− anions. Overall, this work not only offers an effective therapeutic method for the treatment of bacterial biofilm infections with both anti-biofilm and anti-inflammatory effects, but also inspires the rational design of multifunctional Fenton nanoagents by integrating Fenton-active cations and reductive anions. |
Bacterial infections have emerged as ever-growing threats to human health, and many recalcitrant bacterial infections have been proven to correlate with the formation of biofilms, such as lung infections in cystic fibrosis, implant-related infections, chronic wounds, dental caries, and so on.1–3 A bacterial biofilm is an aggregate of bacterial populations encapsulated in the extracellular polymeric substance (EPS) matrix, which can protect bacteria from the attack of host immune defenses and cause high resistance to antibiotics.4–6 Furthermore, persistent inflammation is often caused by bacterial biofilm infection, which can damage the surrounding normal tissues and hamper the recovery of infected tissues.7–9 To date, the effective treatment of bacterial biofilm infections is still highly desired, especially a method with both anti-biofilm and anti-inflammatory effects.
CDT based on Fenton/Fenton-like reactions has recently been considered a promising therapeutic strategy for the treatment of cancer and bacterial infections.10–14 Fenton agents catalytically convert hydrogen peroxide (H2O2) to highly oxidative hydroxyl radicals (˙OH), which irreversibly damage various biomolecules (proteins, lipids, DNA, etc.) and effectively kill drug-resistant cancer cells or bacteria.10,15–20 Various Fenton agents have been used to kill bacteria both in planktonic form and in biofilms.21–24 However, the anti-biofilm effect of Fenton agents is limited by the inefficient iron redox cycling and ˙OH production, because the reduction of the as-formed Fe3+ to Fe2+ by H2O2 is much slower than the oxidation of Fe2+.25–27 Although chelating agents or reductive agents can improve the iron redox cycling during Fenton reaction, they are complicated and unstable for biological use.28–30 On the other hand, common Fenton agents lack the anti-inflammatory capability to scavenge excessive ROS accumulated in the normal tissues around biofilms.31,32 Hence, Fenton agents with enhanced iron redox cycling and ROS scavenging activity are highly desired to treat bacterial biofilm infections.
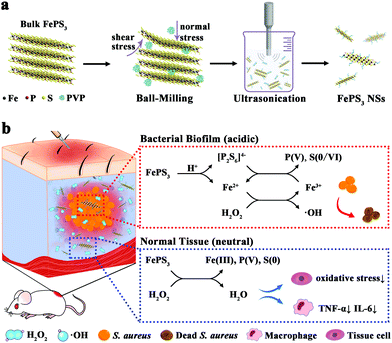
In bacterial biofilm infected tissues, interactions between the bacteria and the host result in unique biofilm microenvironments.9,33,34 The respiratory burst of abundant phagocytes around bacterial biofilms results in high levels of ROS (e.g., H2O2) in biofilm infected tissues.35,36 Besides, bacteria in oxygen-limited regions of the biofilm conduct anaerobic fermentation along with the accumulation of organic acids, which causes a more acidic microenvironment in biofilm infected tissues compared with neutral normal tissues.37,38 By utilizing the biofilm microenvironment as a stimulus, responsive nanoagents have shown selective anti-biofilm outcomes with diminished side effects to normal tissues.39–46 Inspired by these reports, we expect that FePS3 NSs have potential as microenvironment-selective therapeutic agents for biofilm infections based on the existence of both Fenton-active Fe cations (Fe2+/Fe3+) for bacterial killing and reductive anions ([P2S6]4−) for ROS scavenging.
FePS3 NSs were prepared from bulk FePS3 by ball-milling and subsequent ultrasonic exfoliation (Scheme 1). FePS3 NSs exhibit acid-responsive dissociation with the release of Fe2+ and [P2S6]4− while being relatively stable under neutral conditions. In bacterial biofilm infected tissues with acidic pH, the released Fe2+ further converts H2O2 to ˙OH through Fenton reaction, and the [P2S6]4− can reduce the as-formed Fe3+ to Fe2+ with accelerated iron redox cycling and enhance the Fenton activity. In contrast, in normal tissues with neutral pH, FePS3 NSs show antioxidative activity and can effectively scavenge H2O2 or ˙OH by the reductive [P2S6]4− through redox reaction. Experimental results show that the microenvironment-selective ROS regulation properties of FePS3 NSs enable simultaneous anti-biofilm and anti-inflammatory therapy for bacterial biofilm infections.
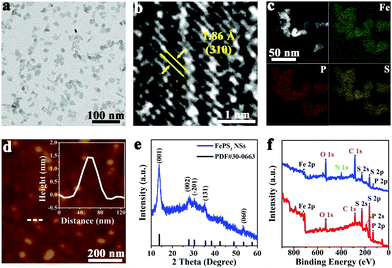
Bulk FePS3 was synthesized by high temperature solid state reaction using Fe, P, and S powders.47 The scanning electron microscopy (SEM) image (Fig. S1a, ESI†) shows clear layered morphology of bulk FePS3. The X-ray diffraction (XRD) pattern (Fig. S1b, ESI†) further confirms the successful synthesis of bulk FePS3. FePS3 NSs were obtained by ball-milling and subsequent liquid ultrasonication of the bulk FePS3 with the assistance of poly(vinyl pyrrolidone) (PVP). The transmission electron microscopy (TEM) image (Fig. 1a) and the statistical analysis (Fig. S2a, ESI†) illustrate that FePS3 NSs have uniform morphology with an average size of ∼15 nm. Dynamic light scattering (DLS) analysis shows that the hydrodynamic diameter of FePS3 NSs is around 24 nm (Fig. S2b, ESI†). As the high-resolution TEM (HRTEM) image shows in Fig. 1b, the crystal lattice space of 1.86 Å can be ascribed to the (310) plane of FePS3. High-angle annular dark field scanning TEM (HAADF-STEM) image and energy dispersive spectroscopy (EDS) elemental mapping images (Fig. 1c) show the homogeneous element distribution of Fe, P, and S in FePS3 NSs. Atomic force microscopy (AFM) measurement (Fig. 1d and Fig. S2c, ESI†) indicates the average thickness of FePS3 NSs to be ∼2.1 nm, suggesting that FePS3 NSs have 2–3 single layers.48 Besides, FePS3 NSs have negative charges on the surface with a zeta potential of about −10 mV (Fig. S2d, ESI†).
As the XRD pattern shows in Fig. 1e, the diffraction peaks of FePS3 NSs located at 13.8° and 27.8° can be ascribed to (001) and (002) planes of FePS3, respectively. Fig. S3a (ESI†) shows that FePS3 layers are formed by [P2S6]4− units and Fe ions, and then weakly bond with each other through van der Waals forces.49 The Raman spectrum of FePS3 NSs (Fig. S3b, ESI†) exhibits a peak at 385 cm−1 (A(2)1g), which represents P–P bond stretching and the symmetric stretching vibration of the P–S bond in the [P2S6]4− unit. Raman peaks at 215 cm−1 (E(1)g), 275 cm−1 (E(2)g), and 588 cm−1 (E(3)g) originate from the tangential vibration of the P–P bond, which suggests the inplane vibrations of the [P2S6]4− unit.48 Fourier transform infrared (FT-IR) spectra in Fig. S3c (ESI†) show the asymmetric stretching vibration of the P–S bond (572 cm−1) and the P–P vibration (442 cm−1) of FePS3.50,51 IR bands at 1642 cm−1 and 1428 cm−1 belong to the stretching vibration of amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the C–H bending vibration, respectively, suggesting the presence of PVP on FePS3 NSs.
O and the C–H bending vibration, respectively, suggesting the presence of PVP on FePS3 NSs.
FePS3 NSs were further characterized by using X-ray photoelectron spectroscopy (XPS). XPS survey spectra show that FePS3 NSs and bulk FePS3 have similar binding energy peaks of the main elements, while the N 1s peak for FePS3 NSs can be ascribed to the surface adsorbed PVP (Fig. 1f). As the high-resolution XPS spectra of Fe 2p show in Fig. S4a (ESI†), the binding energies of 2p3/2 and 2p1/2 orbitals of Fe(II) are 709.3 eV and 722.9 eV, respectively.52 And the peaks near 712.0 eV and 725.6 eV belong to the 2p3/2 and 2p1/2 orbitals of Fe(III), indicating the coexistence of Fe(II) and Fe(III) on the surface of bulk FePS3 and FePS3 NSs, similar to the reported results.47 Also, the main P 2p peaks located at 131.1 eV (2p3/2) and 132.0 eV (2p1/2) represent the intrinsic valence state of +4 for P in FePS3. Additional peaks at 132.8 eV and 133.7 eV for P 2p suggest partial oxidation of P, which may originate from the preparation process (Fig. S4b, ESI†).53 Besides, no obvious difference of S 2p binding energy peaks can be observed between FePS3 NSs and bulk FePS3 (Fig. S4c, ESI†). Moreover, the peaks at 188.7 eV and 225.6 eV represent the P 2s and S 2s orbitals of FePS3, respectively; whereas the C 1s peak (284.6 eV) and O 1s peak (531.6 eV) may originate from the test environment (Fig. 1f).
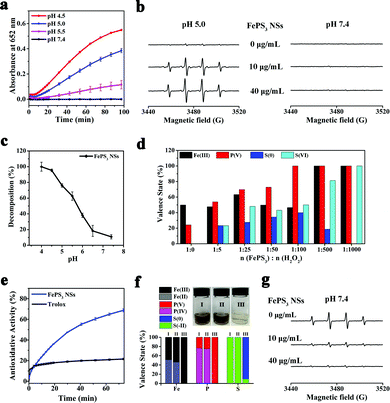
The existence of Fe(II) in FePS3 NSs indicates their potential Fenton activity, whereas the [P2S6]4− may possess reducibility due to the low valence states of P and S (+4 and −2, respectively). First of all, 3,3′,5,5′-tetramethylbenzidine (TMB) was used as the substrate to carry on the colorimetric assay of Fenton activity.54 As shown in Fig. 2a, the characteristic absorbance at 652 nm increases significantly with the decrease of pH, while no obvious change is observed at pH 7.4, indicating that FePS3 NSs exhibit Fenton activity only under acidic conditions. Moreover, the generation of ˙OH in these reactions was further detected by electron paramagnetic resonance (EPR) by using 5,5-dimethyl-1-pyridine N-oxide (DMPO) as the spin trap.55Fig. 2b shows obvious DMPO/˙OH signals for FePS3 NSs + H2O2 mixture at pH 5.0, while no characteristic signal appears at pH 7.4, which further verifies the acid-responsive Fenton activity of FePS3 NSs.
The time-dependent absorbance of FePS3 NSs (at 375 nm) was monitored under different pH conditions, and the corresponding dissolution percentage was calculated based on the decrease of absorbance over time (Fig. S5 and S6, ESI†). As shown in Fig. 2c, FePS3 NSs show little dissolution under neutral conditions, while they can completely dissolve under acidic conditions. As previously reported, FePS3 is formed based on the ionic bonds between Fe cations and [P2S6]4−, which may influence their dissociation at different pH conditions.49,53 Besides, XPS analysis (Fig. S7, ESI†) demonstrates no obvious change on the valence states of the elements in FePS3 NSs whether dispersed in aqueous solutions with different pH conditions (5.0 or 7.4), suggesting that the decreased absorbance of FePS3 NSs is related to the dissociation of FePS3 rather than the oxidative decomposition by the dissolved oxygen. These results reveal that FePS3 NSs may dissolve and release Fe2+ cations and [P2S6]4− anions in response to the acidic environment while keeping stable under neutral conditions.
The acid-responsive Fe2+ release can account for the acid-responsive Fenton activity of FePS3 NSs (eqn (1) and (2)), while the [P2S6]4− also exerts an essential function in Fenton reaction. XPS analysis was performed to investigate the changes in the valence states of Fe/P/S in FePS3 NSs after reaction with different amounts of H2O2 at pH 5.0. As Fig. 2d and Fig. S8 (ESI†) show, when the amount of H2O2 added is less than 100 eq. of FePS3, the decrease of Fe(II) and increase of Fe(III) is limited. At the same time, the oxidation of P(IV) and S(−II) gradually increases. Once the [P2S6]4− anions are completely consumed, the conversion of Fe(II) to Fe(III) begins. Also, all the FePS3 + H2O2 mixtures carry on Fenton reaction, whether the oxidation of [P2S6]4− is complete (Fig. S9, ESI†), indicating that [P2S6]4− can reduce the as-formed Fe3+ to Fe2+ during the Fenton reaction until depletion (eqn (3) and (4)). Thus, [P2S6]4− may participate in the iron redox cycling in the Fenton reaction, which can enhance the Fenton activity of FePS3 NSs.
| 2FePS3 → 2Fe2+ + [P2S6]4− | (1) |
| Fe2+ + H2O2 → Fe3+ + ˙OH + OH− | (2) |
| [P2S6]4− + 14Fe3+ + 8H2O → 14Fe2+ + 2PO43− + 6S + 16H+ | (3) |
| [P2S6]4− + 50Fe3+ + 32H2O → 50Fe2+ + 2PO43− + 6SO42− + 64H+ | (4) |
| FePS3 + 4H2O2 → Fe3+ + PO43− + 3S + 4H2O | (5) |
Under neutral condition, FePS3 NSs have neglectable Fenton activity due to the limited release of Fe2+ and acid-dependent Fenton activity, while the reductive [P2S6]4− potentially endows FePS3 NSs with ROS scavenging ability. Thus, 2-phenyl-4,4,5,5-tetramethylimidazolinium-3-oxo-1-oxo (PTIO) radicals (PTIO˙) and 2,2′-biazinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals (ABTS+˙) were used to test the antioxidative activity of FePS3 NSs.56,57 As Fig. 2e shows, the PTIO˙ scavenging capability of FePS3 NSs is about 3.2 times as high as that of a typical antioxidant (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid, Trolox). Similarly, FePS3 NSs also exhibit significant scavenging ability towards ABTS+˙, which is about 2.4 times that of Trolox (Fig. S10, ESI†). These results indicate the excellent antioxidative activity of FePS3 NSs. Moreover, the scavenging ability of FePS3 NSs for typical ROS (H2O2 and ˙OH) in a biological system was further studied.58 As Fig. 2f illustrates, the addition of H2O2 into the FePS3 NS solution (pH 7.4) leads to significant colour fading without the generation of oxygen bubbles or ˙OH (Fig. 2a and b). Corresponding XPS analysis shows that the Fe(II), P(IV) and S(−II) in FePS3 NSs were oxidized to form Fe(III), P(V) and S(0) (Fig. 2f and Fig. S11, ESI†), which suggests that FePS3 NSs may eliminate H2O2 through redox reaction as shown in eqn (5). In addition, ˙OH was generated via the Fe2+/H2O2 system to investigate the ˙OH scavenging capability of FePS3 NSs. EPR results show that the intensity of the DMPO/˙OH signal dramatically decreases with the addition of FePS3 NSs, indicating the elimination of ˙OH (Fig. 2g). Salicylic acid (SA), a ˙OH specific probe with a characteristic absorption peak at 510 nm after reaction with ˙OH, was also utilized.59 The absorbance of the SA + Fe2+/H2O2 + FePS3 NSs mixture is much lower than that of the SA + Fe2+/H2O2 mixture, and the calculated elimination percentage of ˙OH rises gradually with increasing concentrations of FePS3 NSs, revealing their ˙OH scavenging properties (Fig. S12, ESI†). Hence, FePS3 NSs possess antioxidative activity to effectively scavenge H2O2 and ˙OH in neutral conditions.
The acid-responsive self-enhanced Fenton activity of FePS3 NSs was further examined for the treatment of both planktonic bacteria and bacterial biofilms. The bacterial growth curves of Staphylococcus aureus (S. aureus) treated with FePS3 NSs show a significant bacteriostatic effect either without or with H2O2 (Fig. S13a and b, ESI†). Also, the bacterial growth inhibition effect of FePS3 NSs + H2O2 is much higher than that of FePS3 NSs, indicating enhanced antibacterial activity of FePS3 NSs via Fenton reaction. On the other hand, the inactivation efficiency of FePS3 NSs (50 μg mL−1) against planktonic S. aureus reached 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (∼99.99%) and 6.2
log (∼99.99%) and 6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
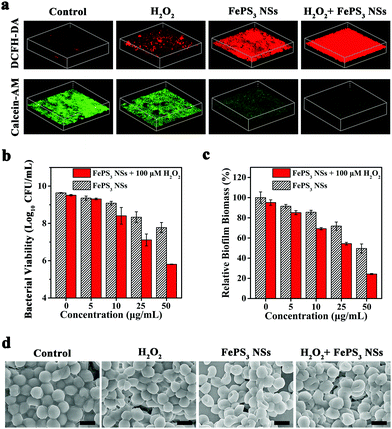
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (∼99.9999%) without and with H2O2 (100 μM), respectively, which reveals the good antibacterial activity of FePS3 NSs (Fig. S13c and d, ESI†).60–63 Moreover, S. aureus biofilms were further challenged by FePS3 NSs to investigate the in vitro anti-biofilm effect. Extra H2O2 (100 μM) was introduced to mimic the in vivo biofilm microenvironment.64,65 The ROS level in biofilms was measured using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA).64,66,67 As shown in the 3D confocal laser scanning microscopy (CLSM) images, the fluorescence intensity of the S. aureus biofilms treated by FePS3 NSs + H2O2 is much higher than those treated by H2O2 or FePS3 NSs (Fig. 3a and Fig. S14a, ESI†), indicating the generation of ˙OH caused by Fenton-active FePS3 NSs. Moreover, calcein acetoxymethyl ester (calcein-AM) was used to stain live bacteria in S. aureus biofilms. Among them, biofilms treated by FePS3 NSs + H2O2 show the lowest fluorescence intensity (Fig. 3a and Fig. S14b, ESI†), demonstrating their excellent bacterial killing effect in biofilms. As shown in Fig. 3b, the bacterial inactivation efficiency of H2O2 (100 μM) alone is only 0.13
log (∼99.9999%) without and with H2O2 (100 μM), respectively, which reveals the good antibacterial activity of FePS3 NSs (Fig. S13c and d, ESI†).60–63 Moreover, S. aureus biofilms were further challenged by FePS3 NSs to investigate the in vitro anti-biofilm effect. Extra H2O2 (100 μM) was introduced to mimic the in vivo biofilm microenvironment.64,65 The ROS level in biofilms was measured using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA).64,66,67 As shown in the 3D confocal laser scanning microscopy (CLSM) images, the fluorescence intensity of the S. aureus biofilms treated by FePS3 NSs + H2O2 is much higher than those treated by H2O2 or FePS3 NSs (Fig. 3a and Fig. S14a, ESI†), indicating the generation of ˙OH caused by Fenton-active FePS3 NSs. Moreover, calcein acetoxymethyl ester (calcein-AM) was used to stain live bacteria in S. aureus biofilms. Among them, biofilms treated by FePS3 NSs + H2O2 show the lowest fluorescence intensity (Fig. 3a and Fig. S14b, ESI†), demonstrating their excellent bacterial killing effect in biofilms. As shown in Fig. 3b, the bacterial inactivation efficiency of H2O2 (100 μM) alone is only 0.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (∼25.89%), while that of FePS3 NSs with lower concentration (27 μM, 5 μg mL−1) is 0.28
log (∼25.89%), while that of FePS3 NSs with lower concentration (27 μM, 5 μg mL−1) is 0.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (∼47.64%), indicating the better anti-biofilm effect of FePS3 NSs compared with H2O2. Besides, the colony-forming units (CFU) number of bacteria in biofilms treated by FePS3 NSs (50 μg mL−1) with H2O2 (100 μM) decreased by about 4
log (∼47.64%), indicating the better anti-biofilm effect of FePS3 NSs compared with H2O2. Besides, the colony-forming units (CFU) number of bacteria in biofilms treated by FePS3 NSs (50 μg mL−1) with H2O2 (100 μM) decreased by about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (∼99.99%) and the CFU number of the group treated by only FePS3 NSs (50 μg mL−1) decreased by less than 2
log (∼99.99%) and the CFU number of the group treated by only FePS3 NSs (50 μg mL−1) decreased by less than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (∼98.62%), which revealed the excellent sterilization of ˙OH generated via FePS3 NSs through Fenton reaction. In addition, crystal violet staining was conducted after different treatments to evaluate the total biofilm biomass including bacteria and EPS. Compared with the sole treatment of H2O2 or FePS3 NSs, the S. aureus biofilms treated by H2O2 + FePS3 NSs show much less biomass (Fig. S15, ESI†). As the quantitative analysis shows in Fig. 3c, the relative biofilm biomass is reduced to about 24% after the treatment of FePS3 NSs (50 μg mL−1) with the presence of H2O2 (100 μM), much lower than that of H2O2 (∼95%) or FePS3 NSs (∼50%). The morphology of the biofilms after different treatments was studied by SEM images (Fig. 3d). S. aureus inside biofilms without treatment presents sphere-like morphology with smooth and intact cell walls, while the bacterial surface becomes distorted and wrinkled in the FePS3 NSs + H2O2 group. These results demonstrate that FePS3 NSs have excellent anti-biofilm effect in response to the acid and H2O2 in the biofilm microenvironment by self-enhanced Fenton activity.
log (∼98.62%), which revealed the excellent sterilization of ˙OH generated via FePS3 NSs through Fenton reaction. In addition, crystal violet staining was conducted after different treatments to evaluate the total biofilm biomass including bacteria and EPS. Compared with the sole treatment of H2O2 or FePS3 NSs, the S. aureus biofilms treated by H2O2 + FePS3 NSs show much less biomass (Fig. S15, ESI†). As the quantitative analysis shows in Fig. 3c, the relative biofilm biomass is reduced to about 24% after the treatment of FePS3 NSs (50 μg mL−1) with the presence of H2O2 (100 μM), much lower than that of H2O2 (∼95%) or FePS3 NSs (∼50%). The morphology of the biofilms after different treatments was studied by SEM images (Fig. 3d). S. aureus inside biofilms without treatment presents sphere-like morphology with smooth and intact cell walls, while the bacterial surface becomes distorted and wrinkled in the FePS3 NSs + H2O2 group. These results demonstrate that FePS3 NSs have excellent anti-biofilm effect in response to the acid and H2O2 in the biofilm microenvironment by self-enhanced Fenton activity.
The cytotoxicity of FePS3 NSs was first studied using murine fibroblast (NIH-3T3) cells. After incubation with FePS3 NSs at the concentration up to 80 μg mL−1, no obvious decrease in the viability of NIH-3T3 cells can be observed (Fig. S16a, ESI†). Besides, FePS3 NSs exhibit low hemolysis effect, indicating their good blood compatibility (Fig. S16b, ESI†). Furthermore, the in vivo toxicity of FePS3 NSs was assessed in healthy mice by tail vein injection (∼1 mg kg−1). The body weights of the mice injected with FePS3 NSs have no noticeable difference from those injected with saline (0.85% NaCl) (Fig. S16c, ESI†). Hematoxylin and eosin (H&E) staining images show that the major organs from these mice have no observable damage post-injection for 21 days (Fig. S16d, ESI†). Meanwhile, there are no abnormal results in both hematology assay and liver/kidney function markers (Fig. S17, ESI†). Therefore, FePS3 NSs have negligible toxicity to mice at the dose used in this work.
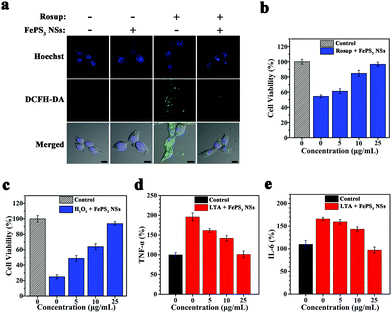
To evaluate the in vitro ROS scavenging properties of FePS3 NSs, NIH-3T3 cells were pretreated with Rosup to induce intracellular ROS generation, and then treated with FePS3 NSs.59,68 The fluorescence intensity of NIH-3T3 cells stained with DCFH-DA decreases significantly upon the addition of FePS3 NSs (Fig. 4a and Fig. S18, ESI†), suggesting the lowered intracellular ROS level.69 The cell viability of NIH-3T3 cells treated by Rosup decreased to 55% due to the oxidative stress (Fig. 4b). In comparison, the viability of NIH-3T3 cells stimulated by Rosup and followed by FePS3 NS treatment remained up to 95%, which indicates the cell protection effect of FePS3 NSs from excessive ROS. Besides, when H2O2 was used to induce oxidative damage to NIH-3T3 cells, the pretreatment by FePS3 NSs can also efficiently protect them from oxidative stress (Fig. 4c). These results suggest that FePS3 NSs are an effective ROS scavenger for the relief of oxidative stress. On the other hand, since ROS can act as a secondary cellular messenger for inflammatory cytokine signalling, the anti-inflammatory effect of FePS3 NSs by ROS scavenging was further studied.70,71 Lipoteichoic acid (LTA) was employed to stimulate murine macrophage (RAW264.7) cells to induce an in vitro inflammatory response. After the treatment, tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were tested as inflammation markers. Pretreatment with FePS3 NSs significantly reduced both the TNF-α level and IL-6 level (Fig. 4d and e), suggesting reduced inflammation. Hence, FePS3 NSs may mitigate the oxidative stress and inflammation in normal tissues by scavenging excessive ROS.
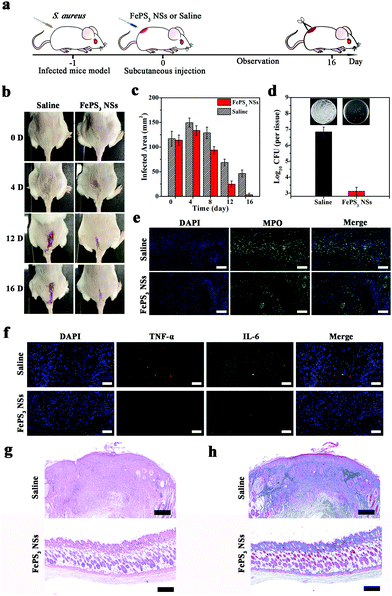
Considering the difference of pH conditions between biofilm infected tissues and normal tissues, FePS3 NSs are expected to have microenvironment-selective anti-biofilm and anti-inflammatory effects. The in vivo therapeutic efficacy of FePS3 NSs was studied on S. aureus biofilm infected mice models (Fig. 5a). One day after infection, FePS3 NSs (∼0.5 mg kg−1) and saline (control) were in situ injected into the abscesses of infected mice, respectively. As illustrated in Fig. 5b and c, the infected areas of the mice treated with FePS3 NSs are much smaller than that of the mice treated by saline. At the 16th-day post-treatment with FePS3 NSs, the abscesses and wound beds of mice almost disappear, which indicates that FePS3 NSs can effectively facilitate the recovery of S. aureus biofilm infected tissues. As shown in Fig. 5d, the number of live S. aureus in the infected tissues was quantified, and the bacterial inactivation efficiency of FePS3 NSs is about 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (99.98%), suggesting an excellent in vivo anti-biofilm effect of FePS3 NSs. Moreover, neutrophils in the infected tissues were detected by immunofluorescence staining of myeloperoxidase (MPO).72,73 Strong fluorescence can be clearly observed in the infected tissues of the saline group, while that of the FePS3 NS group is remarkably reduced, indicating fewer neutrophils infiltration and reduced inflammation (Fig. 5e). Meanwhile, the expressions of typical pro-inflammatory cytokines (TNF-α and IL-6) in the infected tissues treated by FePS3 NSs show a significant reduction, which further confirms the in vivo anti-inflammatory effect of FePS3 NSs (Fig. 5f). The therapeutic efficiency of FePS3 NSs towards S. aureus biofilm infected mice was also evaluated by histological analysis. As the H&E staining images (Fig. 5g) show, the infiltration of plenty of inflammatory cells indicates that the intensive inflammation still remained in the infected tissues of the saline group. In contrast, fewer inflammatory cells and a more intact epidermis layer can be observed in the FePS3 NS group. Besides, Masson's trichrome staining images (Fig. 5h) for the FePS3 NS group show better collagen fiber formation in the infected tissues. These results demonstrate that FePS3 NSs exhibit excellent anti-biofilm and anti-inflammatory efficacy in the S. aureus biofilm infected mice.
log (99.98%), suggesting an excellent in vivo anti-biofilm effect of FePS3 NSs. Moreover, neutrophils in the infected tissues were detected by immunofluorescence staining of myeloperoxidase (MPO).72,73 Strong fluorescence can be clearly observed in the infected tissues of the saline group, while that of the FePS3 NS group is remarkably reduced, indicating fewer neutrophils infiltration and reduced inflammation (Fig. 5e). Meanwhile, the expressions of typical pro-inflammatory cytokines (TNF-α and IL-6) in the infected tissues treated by FePS3 NSs show a significant reduction, which further confirms the in vivo anti-inflammatory effect of FePS3 NSs (Fig. 5f). The therapeutic efficiency of FePS3 NSs towards S. aureus biofilm infected mice was also evaluated by histological analysis. As the H&E staining images (Fig. 5g) show, the infiltration of plenty of inflammatory cells indicates that the intensive inflammation still remained in the infected tissues of the saline group. In contrast, fewer inflammatory cells and a more intact epidermis layer can be observed in the FePS3 NS group. Besides, Masson's trichrome staining images (Fig. 5h) for the FePS3 NS group show better collagen fiber formation in the infected tissues. These results demonstrate that FePS3 NSs exhibit excellent anti-biofilm and anti-inflammatory efficacy in the S. aureus biofilm infected mice.
Conclusions
In summary, FePS3 NSs possess both self-enhanced Fenton activity under acidic conditions and ROS scavenging properties under neutral conditions. As a pH-responsive multifunctional Fenton nanoagent, FePS3 NSs exhibit microenvironment-selective anti-biofilm and anti-inflammatory effects against bacterial biofilm infections. In bacterial biofilms with an acidic microenvironment, FePS3 NSs can release Fe2+ and react with H2O2 by Fenton reaction to generate Fe3+ and highly oxidative ˙OH for bacteria-killing in biofilms; while the [P2S6]4− can reduce the as-formed Fe3+ to Fe2+, which significantly enhances the therapeutic effect of the Fenton reaction by promoting iron redox cycling. In normal tissues with neutral pH, FePS3 NSs exhibit effective ROS scavenging properties via the reductive [P2S6]4−, which can relieve oxidative stress and exert an anti-inflammation effect. Both in vitro and in vivo experimental results demonstrate that FePS3 NSs can simultaneously eradicate bacterial biofilms and mitigate tissue inflammation, and achieve excellent therapeutic efficacy for bacterial biofilm infections. Besides, FePS3 NSs show good biocompatibility with neglectable toxicity to mice. Overall, this work demonstrates that the integration of Fe cations and reductive anions in one Fenton agent makes FePS3 NSs a kind of novel pH-responsive therapeutic nanoagent with self-enhanced Fenton activity and ROS scavenging properties, facilitating the microenvironment-selective anti-biofilm and anti-inflammatory therapy for bacterial biofilm infections.Authors’ contributions
L. Wang and L. Yuwen conceived the idea and supervised the study. Y. Li carried out the experiments, analysed the results, and wrote the manuscript. W. Xiu, K. Yang, Q. Wen and Z. Luo helped with the experimental characterization and the results discussion. D. Yang supported the operation of animal experiments. X. Liu and X. Xie contributed to the scientific discussion of the manuscript. All authors discussed, commented and agreed on the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2017YFA0205302), the Natural Science Foundation of Jiangsu Province (BK20191382), the Key Research and Development Program of Jiangsu (BE2018732), the Natural Science Key Fund for Colleges and Universities in Jiangsu Province (17KJA430011), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, YX030003).References
- J. W. Costerton, P. S. Stewart and E. P. Greenberg, Science, 1999, 284, 1318 Search PubMed.
- R. A. Fisher, B. Gollan and S. Helaine, Nat. Rev. Microbiol., 2017, 15, 453–464 CrossRef CAS.
- H. Flemming, J. Wingender, U. Szewzyk, P. Steinberg, S. A. Rice and S. Kjelleberg, Nat. Rev. Microbiol., 2016, 14, 563–575 Search PubMed.
- F. Sun, F. Qu, Y. Ling, P. Mao, P. Xia, H. Chen and D. Zhou, Future Microbiol., 2013, 8, 877–886 CrossRef CAS.
- N. Høiby, T. Bjarnsholt, M. Givskov, S. Molin and O. Ciofu, Int. J. Antimicrob. Agents, 2010, 35, 322–332 CrossRef.
- H.-C. Flemming and J. Wingender, Nat. Rev. Microbiol., 2010, 8, 623–633 CrossRef CAS.
- T. Bjarnsholt, M. Alhede, M. Alhede, S. R. Eickhardt-Sorensen, C. Moser, M. Kuhl, P. O. Jensen and N. Hoiby, Trends Microbiol., 2013, 21, 466–474 CrossRef CAS.
- N. Hoiby, T. Bjarnsholt, C. Moser, G. L. Bassi, T. Coenye, G. Donelli, L. Hall-Stoodley, V. Hola, C. Imbert, K. Kirketerp-Moller, D. Lebeaux, A. Oliver, A. J. Ullmann, C. Williams, E. S. G. f. Biofilms and Z. Consulting External Expert Werner, Clin. Microbiol. Infect., 2015, 21, 1–25 CrossRef.
- P. S. Stewart and M. J. Franklin, Nat. Rev. Microbiol., 2008, 6, 199–210 CrossRef CAS.
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS.
- H. Ranji-Burachaloo, P. A. Gurr, D. E. Dunstan and G. G. Qiao, ACS Nano, 2018, 12, 11819–11837 CrossRef CAS.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Nat. Rev. Microbiol., 2013, 11, 371–384 CrossRef CAS.
- X. Ji, Y. Kang, J. Ouyang, Y. Chen, D. Artzi, X. Zeng, Y. Xiao, C. Feng, B. Qi, N. Y. Kim, P. E. Saw, N. Kong, O. C. Farokhzad and W. Tao, Adv. Sci., 2019, 6, 1901211 CrossRef CAS.
- N. Kong, X. Ji, J. Wang, X. Sun, G. Chen, T. Fan, W. Liang, H. Zhang, A. Xie, O. C. Farokhzad and W. Tao, Nano Lett., 2020, 20, 3943–3955 CrossRef CAS.
- E. Linley, S. P. Denyer, G. McDonnell, C. Simons and J. Y. Maillard, J. Antimicrob. Chemother., 2012, 67, 1589–1596 CrossRef CAS.
- J. A. Imlay and S. Linn, Science, 1988, 240, 1302–1309 CrossRef CAS.
- J. Tamarit, E. Cabiscol and J. Ros, J. Biol. Chem., 1998, 273, 3027–3032 CrossRef CAS.
- F. C. Fang, Nat. Rev. Microbiol., 2004, 2, 820–832 CrossRef CAS.
- J. Shan, X. Li, K. Yang, W. Xiu, Q. Wen, Y. Zhang, L. Yuwen, L. Weng, Z. Teng and L. Wang, ACS Nano, 2019, 13, 13797–13808 CrossRef CAS.
- Z. He, X. Huang, C. Wang, X. Li, Y. Liu, Z. Zhou, S. Wang, F. Zhang, Z. Wang, O. Jacobson, J. J. Zhu, G. Yu, Y. Dai and X. Chen, Angew. Chem., Int. Ed., 2019, 58, 8752–8756 CrossRef CAS.
- M. Song, Y. Cheng, Y. Tian, C. Chu, C. Zhang, Z. Lu, X. Chen, X. Pang and G. Liu, Adv. Funct. Mater., 2020, 2003587, DOI:10.1002/adfm.202003587.
- S. C. Park, N. H. Kim, W. Yang, J. W. Nah, M. K. Jang and D. Lee, J. Controlled Release, 2016, 221, 37–47 CrossRef CAS.
- X. Cheng, S. Zhang, H. Liu, H. Chen, J. Zhou, Z. Chen, X. Zhou, Z. Xie, Q. Kuang and L. Zheng, ACS Appl. Mater. Interfaces, 2020, 12, 36996–37005 CrossRef CAS.
- L. Wang, Y. Miao, M. Lu, Z. Shan, S. Lu, J. Hou, Q. Yang, X. Liang, T. Zhou, D. Curry, K. Oakes and X. Zhang, Chem. Commun., 2017, 53, 5862–5865 RSC.
- A. D. Bokare and W. Choi, J. Hazard. Mater., 2014, 275, 121–135 CrossRef CAS.
- H. Dong, C. Sans, W. Li and Z. Qiang, Sep. Purif. Technol., 2016, 171, 144–150 CrossRef CAS.
- S. Zhang, M. Sun, T. Hedtke, A. Deshmukh, X. Zhou, S. Weon, M. Elimelech and J. H. Kim, Environ. Sci. Technol., 2020, 54, 10868–10875 CrossRef CAS.
- B. Shen, C. Dong, J. Ji, M. Xing and J. Zhang, Chin. Chem. Lett., 2019, 30, 2205–2210 CrossRef CAS.
- H. Sun, G. Xie, D. He and L. Zhang, Appl. Catal., B, 2020, 267, 118383 CrossRef CAS.
- P. Zhao, Z. Tang, X. Chen, Z. He, X. He, M. Zhang, Y. Liu, D. Ren, K. Zhaoe and W. Bu, Mater. Horiz., 2019, 6, 369–374 RSC.
- P. Poprac, K. Jomova, M. Simunkova, V. Kollar, C. J. Rhodes and M. Valko, Trends Pharmacol. Sci., 2017, 38, 592–607 CrossRef CAS.
- T. P. A. Devasagayam, J. C. Tilak, K. K. Boloor, K. Sane, S. Ghaskadbi and R. Lele, J. Assoc. Physicians India, 2004, 52, 794–804 CAS.
- A. Scalise, A. Bianchi, C. Tartaglione, E. Bolletta, M. Pierangeli, M. Torresetti, M. Marazzi and G. Di Benedetto, Semin. Vasc. Surg., 2015, 28, 151–159 CrossRef CAS.
- W. Xiu, J. Shan, K. Yang, H. Xiao, L. Yuwen and L. Wang, View, 2020, 20200065, DOI:10.1002/viw.20200065.
- L. Chen and Y.-m. Wen, Int. J. Oral Sci., 2011, 3, 66–73 CrossRef CAS.
- P. O. Jensen, M. Kolpen, K. N. Kragh and M. Kuhl, Acta Pathol., Microbiol. Immunol. Scand., 2017, 125, 276–288 CrossRef CAS.
- S. Sivakanesan and E. A. Dawes, J. Gen. Microbiol., 1980, 118, 143–157 Search PubMed.
- D. S. Benoit and H. Koo, Nanomedicine, 2016, 11, 873–879 CrossRef CAS.
- W. Xiu, S. Gan, Q. Wen, Q. Qiu, S. Dai, H. Dong, Q. Li, L. Yuwen, L. Weng, Z. Teng, Y. Mou and L. Wang, Research, 2020, 9426453, DOI:10.34133/2020/9426453.
- D. S. Benoit and H. Koo, Nanomedicine, 2016, 11, 873–879 CrossRef CAS.
- Q. Deng, P. Sun, L. Zhang, Z. Liu, H. Wang, J. Ren and X. Qu, Adv. Funct. Mater., 2019, 29, 1903018 CrossRef.
- J. Wu, F. Li, X. Hu, J. Lu, X. Sun, J. Gao and D. Ling, ACS Cent. Sci., 2019, 5, 1366–1376 CrossRef CAS.
- L. Su, Y. Li, Y. Liu, R. Ma, Y. Liu, F. Huang, Y. An, Y. Ren, H. C. Mei, H. J. Busscher and L. Shi, Adv. Funct. Mater., 2020, 30, 2000537 CrossRef CAS.
- D. Hu, Y. Deng, F. Jia, Q. Jin and J. Ji, ACS Nano, 2020, 14, 347–359 CrossRef CAS.
- Y. Shi, J. Yin, Q. Peng, X. Lv, Q. Li, D. Yang, X. Song, W. Wang and X. Dong, Biomater. Sci., 2020, 8, 6093–6099 RSC.
- X. Lv, J. Zhang, D. Yang, J. Shao, W. Wang, Q. Zhang and X. Dong, J. Mater. Chem. B, 2020, 8, 10700–10711 RSC.
- D. Mukherjee, P. M. Austeria and S. Sampath, ACS Energy Lett., 2016, 1, 367–372 Search PubMed.
- Z. Cheng, T. A. Shifa, F. Wang, Y. Gao, P. He, K. Zhang, C. Jiang, Q. Liu and J. He, Adv. Mater., 2018, 30, 1707433 CrossRef.
- F. Wang, T. A. Shifa, P. Yu, P. He, Y. Liu, F. Wang, Z. Wang, X. Zhan, X. Lou, F. Xia and J. He, Adv. Funct. Mater., 2018, 28, 1802151 CrossRef.
- P. A. Joy and S. Vasudevan, J. Phys. Chem. Solids, 1993, 54, 343–348 CrossRef CAS.
- N. Ismail, A. A. El-Meligi, Y. M. Temerkb and M. Madian, Int. J. Hydrogen Energy, 2010, 35, 7827–7834 CrossRef CAS.
- W. Zhu, W. Gan, Z. Muhammad, C. Wang, C. Wu, H. Liu, D. Liu, K. Zhang, Q. He, H. Jiang, X. Zheng, Z. Sun, S. Chen and L. Song, Chem. Commun., 2018, 54, 4481–4484 RSC.
- R. Gusmão, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2019, 58, 9326–9337 CrossRef.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- W. Zhang, S. Hu, J. J. Yin, W. He, W. Lu, M. Ma, N. Gu and Y. Zhang, J. Am. Chem. Soc., 2016, 138, 5860–5865 CrossRef CAS.
- X. Li, J. Agric. Food Chem., 2017, 65, 6288–6297 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radical Biol. Med., 1999, 26, 1231–1237 CrossRef CAS.
- A. Karakoti, S. Singh, J. M. Dowding, S. Seal and W. T. Self, Chem. Soc. Rev., 2010, 39, 4422–4432 RSC.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. Lin, X. Wang, J. Wu, S. Lia and H. Wei, Chem. Sci., 2018, 9, 2927–2933 RSC.
- Y. Qiao, X. Liu, B. Li, Y. Han, Y. Zheng, K. W. K. Yeung, C. Li, Z. Cui, Y. Liang, Z. Li, S. Zhu, X. Wang and S. Wu, Nat. Commun., 2020, 11, 4446 CrossRef CAS.
- J. Ouyang, X. Ji, X. Zhang, C. Feng, Z. Tang, N. Kong, A. Xie, J. Wang, X. Sui, L. Deng, Y. Liu, J. S. Kim, Y. Cao and W. Tao, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 28667–28677 CrossRef CAS.
- J. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Y. Liang, Z. Li, S. Zhu, Y. Zheng, K. W. K. Yeung, X. Wang and S. Wu, Nat. Commun., 2019, 10, 4490 CrossRef.
- L. Tan, J. Li, X. Liu, Z. Cui, X. Yang, K. W. K. Yeung, H. Pan, Y. Zheng, X. Wang and S. Wu, Small, 2018, 14, 1703197 CrossRef.
- Z. Wang, K. Dong, Z. Liu, Y. Zhang, Z. Chen, H. Sun, J. Ren and X. Qu, Biomaterials, 2017, 113, 145–157 CrossRef CAS.
- Z. Chen, Z. Wang, J. Ren and X. Qu, Acc. Chem. Res., 2018, 51, 789–799 CrossRef CAS.
- C. Zhang, W. Bu, D. Ni, S. Zhang, Q. Li, Z. Yao, J. Zhang, H. Yao, Z. Wang and J. Shi, Angew. Chem., Int. Ed., 2016, 55, 2101–2106 CrossRef CAS.
- J. Shan, K. Yang, W. Xiu, Q. Qiu, S. Dai, L. Yuwen, L. Weng, Z. Teng and L. Wang, Small, 2020, 16, 2001099 CrossRef CAS.
- Y. Huang, Z. Liu, C. Liu, E. Ju, Y. Zhang, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2016, 55, 6646–6650 CrossRef CAS.
- Z. Zhang, W. Sang, L. Xie, W. Li, B. Li, J. Li, H. Tian, Z. Yuan, Q. Zhao and Y. Dai, Angew. Chem., Int. Ed., 2021, 60, 1967–1975 Search PubMed.
- L. Shan, W. Fan, W. Wang, W. Tang, Z. Yang, Z. Wang, Y. Liu, Z. Shen, Y. Dai, S. Cheng, O. Jacobson, K. Zhai, J. Hu, Y. Ma, D. O. Kiesewetter, G. Gao and X. Chen, ACS Nano, 2019, 13, 8903–8916 CrossRef CAS.
- F. Vatansever1, W. C. M. A. d. Melo, P. Avci, D. Vecchio, M. Sadasivam, A. Gupta, R. Chandran, M. Karimi, N. A. Parizotto, R. Yin, G. P. Tegos and M. R. Hamblin, FEMS Microbiol. Rev., 2013, 37, 955–989 CrossRef.
- J. Ouyang, M. Wen, W. Chen, Y. Tan, Z. Liu, Q. Xu, K. Zeng, L. Deng and Y.-N. Liu, Chem. Commun., 2019, 55, 4877–4880 RSC.
- P. P. Bradley, D. A. Priebat, R. D. Christensen and G. Rothstein, J. Invest. Dermatol., 1982, 78, 206–209 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0mh01921f |
| This journal is © The Royal Society of Chemistry 2021 |