DOI:
10.1039/C9FO00872A
(Paper)
Food Funct., 2020,
11, 1006-1026
Volumetric, acoustic, viscometric, calorimetric and spectroscopic studies to elucidate the effects of citrate and tartrate based food preservatives on the solvation behaviors of acidic amino acids at different temperatures†
Received
25th April 2019
, Accepted 20th November 2019
First published on 20th November 2019
Abstract
The effects of trisodium citrate (TSC) and disodium tartrate (DST) based food preservatives on the hydration behaviors of the amino acids L-aspartic acid (ASP) and L-glutamic acid (GLU) have been studied using thermodynamic, transport, calorimetric and spectroscopic studies. The volumetric, acoustic and viscosity data suggest that hydrophilic–ionic/hydrophilic interactions are predominant in these systems. The calculated parameters are worthwhile for exploring the solutes as structure-breakers, and the solutes undergo pairwise interactions with the co-solutes. The sweetness of both amino acids decreases in the presence of the preservatives. The hydration number and solvation data suggest that these solutes are more hydrated in water. The dominance of dehydration effects in relation to TSC is observed from the positive enthalpy changes in calorimetry studies and the negative chemical shifts in 1H NMR studies.
1. Introduction
Food preservation is carried out to maintain the quality and physicochemical properties of raw food.1 Preservatives are generally additives, which enhance the life spans of foods and drinks through preventing microorganism growth.2 As legislation has restricted the use and the levels of some preservatives in different foods, these preservatives must be approved by food safety authorities.3 Organic acids (citric and tartaric acid) are traditional preservatives. Organic acids are added to foods as acidulants, flavorants, or preservatives, inactivating the growth of spoilage microorganisms and foodborne pathogens.4 Citric and tartaric acid show various biological activities. They exhibit metal ion complexing properties with amino acids and, thus, they are preferably used for some common applications in food and agricultural industries as sequestrants, antioxidants, etc. The use of disodium tartrate (DST) and trisodium citrate (TSC) as food additives has been approved by the European Food Safety Authority. They have been assigned with the E numbers 335 and 331, respectively, on the list of antioxidants. It has been mentioned in the literature that sodium salts of citrate and tartrate have been used as anticaking agents, flavour enhancers, etc.5,6 Salts are effective preservatives because they reduce the water activity of foods. As well as their food-related value, DST can extend the range of plant growth promoters and TSC can help in the production of soil amendments for organic crop production.
L-Glutamate and L-aspartate are key molecules in cellular metabolism. Dietary proteins are broken down in humans through digestion into amino acids, which act as metabolic fuel for other functional activities in the body.7 Casein, lactalbumin, Promine-D (soy protein), and wheat gluten contain significant amounts of racemized L-aspartic acid (ASP) and L-glutamic acid (GLU).8 GLU is an important nonessential amino acid that acts as a neurotransmitter. It is not considered to be an essential nutrient because it can be manufactured in the human body from simpler compounds. GLU is a building block in protein synthesis. This dicarboxylic amino acid is available in a wide variety of foods and is an important molecule for the metabolism of sugars and fats.9 It is also used to enhance the flavour of foods.10 ASP is used for the generation of adenosine triphosphate, which is a required fuel for cell activity.11 Water is ubiquitous in biochemical processes because its presence generates hydrophobic forces.12 The physicochemical properties of studied solutes have been used to provide insights into hydrophobicity, hydration properties and solute–solvent interactions.13 The use of citrate and tartrate based salts, along with other additives, not only makes flavours sharper but also improves the stability of food products in many cases. The present report aims to study the solvation and taste quality behaviors of acidic amino acids (ASP and GLU) in the presence of citrate (TSC) and tartrate (DST) food preservatives.
2. Experimental
2.1. Materials
All the chemicals used in the present study are of high-purity analytical grade. The purity of the studied chemicals is ≥99%. Details relating to the chemicals are given in Table 1. Before using the chemicals, they have all been dried in vacuo over anhydrous CaCl2.
Table 1 Sources and solubilities of the chemicals used
| S. no. |
Compound |
Structure |
Molar mass (g mol−1) |
Source |
Puritya |
CAS number |
Solubility in watera (mg ml−1) |
|
As provided by the supplier.
|
| 1 |
L-Aspartic acid |
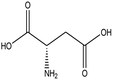
|
133.10 |
Siso Research Laboratories, India |
99% |
56–86–0 |
≈4.5 |
| 2 |
L-Glutamic acid |
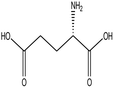
|
147.13 |
Siso Research Laboratories, India |
99% |
2419–94.5 |
≈7.5 |
| 3 |
Di-sodium tartrate |
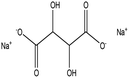
|
230.08 |
Central Drug House, India |
99% |
6132–24–7 |
≈290 |
| 4 |
Tri-sodium citrate |
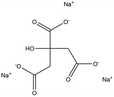
|
294.10 |
Siso Research Laboratories, India |
99% |
6132–04–3 |
≈92 |
2.2. Equipment and procedures
All stock solutions have been prepared using deionised, double-distilled and degassed water with a specific conductance less than 1.3 × 10−4 S m−1. The solutions were prepared on a mass basis using a Mettler balance (Model: AB265-S) with a precision of ±0.01 mg. The standard uncertainty in molality is ±2.8 × 10−4 mol kg−1.
2.2.1. Density and speed of sound.
The densities and speeds of sound were measured simultaneously using a vibrating-tube digital density and sound velocity meter (DSA 5000M, Anton Paar). The two-in-one instrument is designed with two cells, i.e., a density cell and a sound velocity cell. Both cells have a set temperature, adjusted using a built-in Peltier thermostat (PT-100) having an accuracy of ±0.001 K. The speed of sound is measured via a propagation time technique. Calibration adjustments were performed at 293.15 K with triple-distilled degassed water, and with dry air at atmospheric pressure. The uncertainties in the temperature, density and speed of sound measurements are 0.03 K, 0.06 kg m−3 and 0.6 m s−1, respectively.
2.2.2. Viscosity.
Viscosities, η, were determined using a suspended level Ubbelohde-type capillary viscometer having temperature stability within ±0.01 K (model: MC 31A Julabo/Germany). Flow time measurements were done using a stopwatch with a resolution of ±0.01 s. The viscometer has been calibrated with deionized, double-distilled and degassed water. The efflux time data for water was recorded at T = 288.15, 298.15, 308.15 and 318.15 K. Final efflux times are the means of at least six readings. The standard uncertainty in viscosity, u(η), on an average basis is ±0.012 mPa s. The reliability of the apparatus was checked through measuring the viscosities of aqueous solutions of glycine at T = 298.15 K, and the results agreed very well with the literature values.14 The viscosities of pure water have been taken from the literature.15
2.2.3. Enthalpy of dilution.
An isothermal titration calorimeter, (iTC200, MicroCal USA) was used to perform calorimetric titrations at 298.15 ± 0.005 K for the determination of enthalpy of dilution values. 100, 250, and 500 mM DST/TSC solutions were added to a sample cell with a capacity of 200 μl and the syringe was filled with 40 μl of 30 mM ASP/GLU. A total of 19 injections of 2 μl of ASP/GLU was titrated into the sample cell containing DST/TSC and the contents of the cell were stirred at a fixed speed of 500 rpm to ensure complete mixing. The time between successive injections was 120 s, which was managed using the software provided with the instrument. The standard uncertainty in the enthalpy values is ±0.5 kJ mol−1.
2.2.4. Chemical shift measurements.
1H NMR spectra were obtained using a Bruker spectrometer (AVANCE-III, HD 500 MHz) with a probe temperature of 300.15 K. The centre of the HDO signal (4.650 ppm) is considered as the internal reference for the other nuclei. NMR spectra of ASP and GLU in water and in the presence of aqueous solutions of DST and TSC (mB = 0.1–0.75 mol kg−1) have been studied in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) H2O–D2O solution. From these spectra, the chemical shift changes (Δδ) were recorded.
1 (w/w) H2O–D2O solution. From these spectra, the chemical shift changes (Δδ) were recorded.
3. Results and discussion
3.1. Volumetric measurements
3.1.1. Apparent molar Volumes, V2,ϕ.
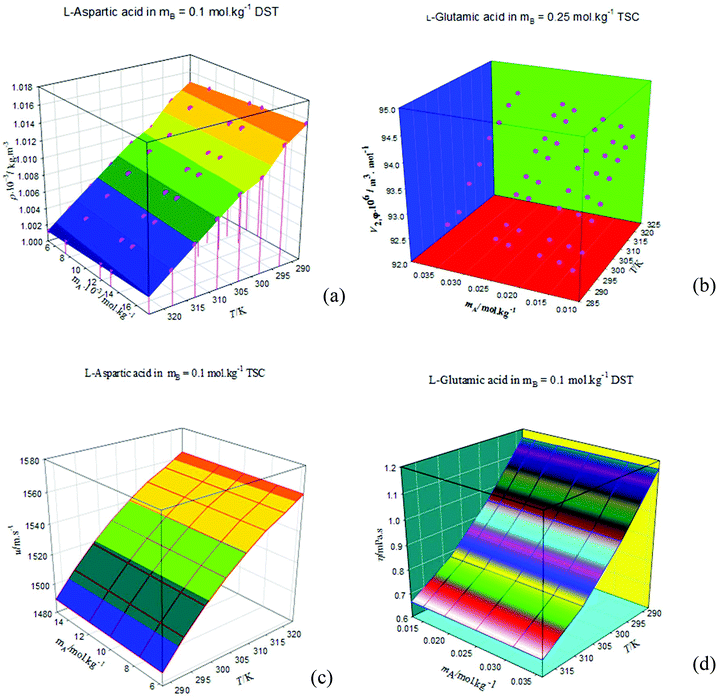
The densities, ρ, of the studied amino acids (ASP and GLU) as a function of molality (mA) in water, and in aqueous solutions of DST and TSC at mB (molality of DST/TSC) values of 0.10, 0.25, 0.50, and 0.75 mol kg−1 over a temperature range from 288.15 to 323.15 K are given in Table 2. The ρ values directly rise with the mA and mB values and decrease with T. The ρ values of aqueous DST and TSC solutions are in good agreement with the literature values,16–23 and a comparison is shown graphically in Fig. 1. The density values of aqueous solutions of ASP and GLU are also in line with the literature values9,24–28 and are represented in Fig. 2. The variations in the ρ values of ASP in aqueous DST solutions at different temperatures are shown in Fig. 3(a) (density, ρ, versus molality, mA, for ASP in DST at mB = 0.1 mol kg−1 as a function of temperature). In the present study, apparent molar volumes, V2,ϕ, have been calculated from the experimentally measured densities using the relation:| | | V2,ϕ = {M/ρ} − {(ρ − ρ0)/(mAρρ0)} | (1) |
where ρ and ρ0 are the densities of the solution and the solvent (H2O or H2O + DST/TSC), M is the molar mass, and mA is the molality of the solute. The V2,ϕ values are positive for the studied amino acids both in aqueous and in mixed aqueous solutions. The V2,ϕ values increase with the mA, mB and T values. The V2,ϕ values of the studied solutes in the absence, as well as in the presence, of aqueous solutions of DST and TSC at mB values of 0.10, 0.25, 0.50, and 0.75 mol kg−1 and at temperatures from 288.15 to 323.15 K are given in ESI Table S1.† A representative plot of V2,ϕversus mA for GLU in aqueous TSC solutions at mB = 0.25 mol kg−1 and different T values is shown in Fig. 3(b).
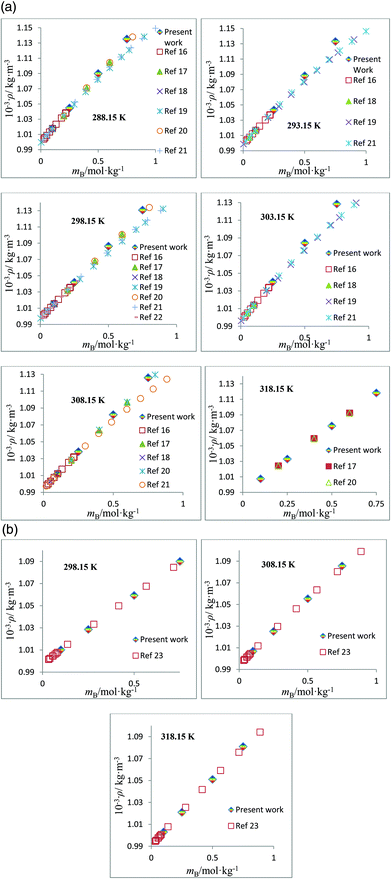 |
| | Fig. 1 (a) Plots of density, ρ, versus molality, mB, for aqueous solutions of TSC and comparisons with the literature values at different temperatures. (b) Plots of density, ρ, versus molality, mB, for aqueous solutions of DST and comparisons with the literature values at different temperatures. | |
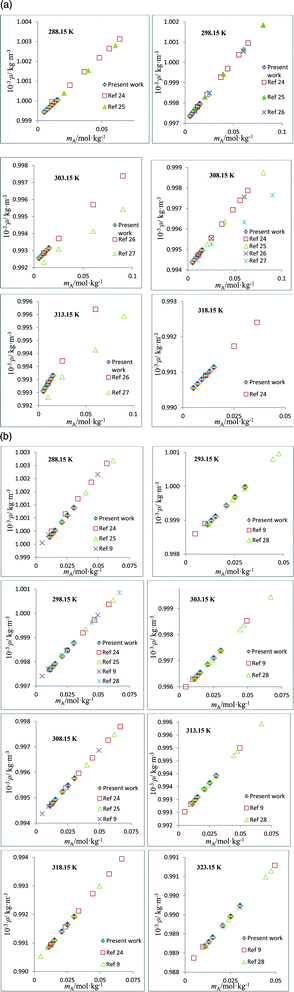 |
| | Fig. 2 (a) Plots of density, ρ, versus molality, mA, for aqueous solutions of ASP and comparisons with the literature values at different temperatures. (b) Plots of density, ρ, versus molality, mA, for aqueous solutions of GLU and comparisons with the literature values at different temperatures. | |
 |
| | Fig. 3 Representative plots of: (a) density, ρ, versus molality, mA, for L-aspartic acid in aqueous DST solutions (mB = 0.1 mol kg−1) as a function of temperature, T; (b) apparent molar volume, V2,ϕ, versus molality, mA, for L-glutamic acid in aqueous TSC solutions (mB = 0.25 mol kg−1) as a function of temperature, T; (c) speed of sound, u, versus molality, mA, for L-aspartic acid in aqueous TSC solutions (mB = 0.1 mol kg−1) as a function of temperature, T; and (d) viscosity, η, versus molality, mA, for L-glutamic acid in aqueous DST solutions (mB = 0.1 mol kg−1) as a function of temperature, T. | |
Table 2 Densities, ρ, of L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate at T values from 288.15 to 323.15 K and P = 101.3 kPa
|
m
A/mol kg−1 |
10−3·ρ/kg m−3 |
| 288.15 K |
293.15 K |
298.15 K |
303.15 K |
308.15 K |
313.15 K |
318.15 K |
323.15 K |
|
m
A is the molality of the solute in water or water + DST/TSC. mB is the molality of DST/TSC in water. Standard uncertainties, u, are u(T) = 0.03 K, u(P) = 0.5 kPa, u(m) = 2.8 × 10−4 mol kg−1, u(ρ) = 0.06 kg m−3. |
|
L-Aspartic acid in water |
| 0.00000 |
0.999102 |
0.998224 |
0.997047 |
0.995670 |
0.994040 |
0.992238 |
0.990220 |
0.988069 |
| 0.00517 |
0.999445 |
0.998547 |
0.997360 |
0.995990 |
0.994375 |
0.992558 |
0.990553 |
0.988378 |
| 0.00712 |
0.999563 |
0.998665 |
0.997477 |
0.996108 |
0.994492 |
0.992674 |
0.990669 |
0.988494 |
| 0.00921 |
0.999690 |
0.998791 |
0.997602 |
0.996232 |
0.994617 |
0.992799 |
0.990794 |
0.988618 |
| 0.01109 |
0.999803 |
0.998904 |
0.997715 |
0.996345 |
0.994729 |
0.992911 |
0.990905 |
0.988729 |
| 0.01300 |
0.999918 |
0.999018 |
0.997829 |
0.996460 |
0.994843 |
0.993024 |
0.991018 |
0.988842 |
| 0.01506 |
1.000042 |
0.999141 |
0.997952 |
0.996582 |
0.994965 |
0.993147 |
0.991140 |
0.988964 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.1 mol kg−1 |
| 0.00000 |
1.014500 |
1.012800 |
1.010169 |
1.008856 |
1.006932 |
1.004950 |
1.002974 |
1.000950 |
| 0.00502 |
1.014789 |
1.013089 |
1.010457 |
1.009144 |
1.007220 |
1.005238 |
1.003261 |
1.001237 |
| 0.00741 |
1.014926 |
1.013226 |
1.010594 |
1.009280 |
1.007356 |
1.005374 |
1.003398 |
1.001374 |
| 0.01180 |
1.015178 |
1.013477 |
1.010730 |
1.009531 |
1.007607 |
1.005624 |
1.003648 |
1.001624 |
| 0.01306 |
1.015249 |
1.013548 |
1.010844 |
1.009603 |
1.007678 |
1.005695 |
1.003719 |
1.001695 |
| 0.01732 |
1.015492 |
1.013791 |
1.010916 |
1.009845 |
1.007921 |
1.005938 |
1.003960 |
1.001936 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.25 mol kg−1 |
| 0.00000 |
1.032450 |
1.030457 |
1.028594 |
1.026540 |
1.025089 |
1.023450 |
1.020973 |
1.018500 |
| 0.00584 |
1.032778 |
1.030784 |
1.028920 |
1.026866 |
1.025415 |
1.023775 |
1.021298 |
1.018824 |
| 0.00817 |
1.032908 |
1.030914 |
1.029050 |
1.026995 |
1.025544 |
1.023904 |
1.021427 |
1.018953 |
| 0.01106 |
1.033069 |
1.031075 |
1.029211 |
1.027156 |
1.025704 |
1.024064 |
1.021586 |
1.019112 |
| 0.01337 |
1.033198 |
1.031203 |
1.029338 |
1.027283 |
1.025832 |
1.024191 |
1.021713 |
1.019239 |
| 0.01555 |
1.033319 |
1.031323 |
1.029459 |
1.027404 |
1.025952 |
1.024311 |
1.021833 |
1.019358 |
| 0.01667 |
1.033380 |
1.031385 |
1.029520 |
1.027465 |
1.026013 |
1.024372 |
1.021894 |
1.019419 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.50 mol kg−1 |
| 0.00000 |
1.063200 |
1.061500 |
1.059301 |
1.057450 |
1.055350 |
1.053240 |
1.050971 |
1.048200 |
| 0.00638 |
1.063544 |
1.061843 |
1.059644 |
1.057793 |
1.055692 |
1.053582 |
1.051313 |
1.048542 |
| 0.00782 |
1.063620 |
1.061920 |
1.059721 |
1.057870 |
1.055769 |
1.053659 |
1.051389 |
1.048619 |
| 0.00982 |
1.063726 |
1.062027 |
1.059828 |
1.057976 |
1.055876 |
1.053765 |
1.051495 |
1.048725 |
| 0.01307 |
1.063899 |
1.062200 |
1.060001 |
1.058150 |
1.056049 |
1.053938 |
1.051667 |
1.048897 |
| 0.01509 |
1.064006 |
1.062307 |
1.060109 |
1.058257 |
1.056156 |
1.054044 |
1.051774 |
1.049004 |
| 0.01539 |
1.064074 |
1.062375 |
1.060178 |
1.058325 |
1.056225 |
1.054113 |
1.051842 |
1.049072 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.75 mol kg−1 |
| 0.00000 |
1.093900 |
1.091750 |
1.090008 |
1.087450 |
1.085611 |
1.083560 |
1.080970 |
1.078900 |
| 0.00547 |
1.094183 |
1.092032 |
1.090289 |
1.087731 |
1.085892 |
1.083841 |
1.081250 |
1.079180 |
| 0.00739 |
1.094282 |
1.092131 |
1.090387 |
1.087829 |
1.085990 |
1.083938 |
1.081348 |
1.079278 |
| 0.01025 |
1.094429 |
1.092278 |
1.090532 |
1.087975 |
1.086135 |
1.084084 |
1.081493 |
1.079423 |
| 0.01347 |
1.094595 |
1.092443 |
1.090697 |
1.088138 |
1.086298 |
1.084247 |
1.081656 |
1.079585 |
| 0.01664 |
1.094758 |
1.092605 |
1.090856 |
1.088298 |
1.086458 |
1.084407 |
1.081816 |
1.079743 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.1 mol kg−1 |
| 0.00000 |
1.017603 |
1.01651 |
1.015194 |
1.013658 |
1.011929 |
1.010035 |
1.007541 |
1.005738 |
| 0.00576 |
1.017926 |
1.016832 |
1.015515 |
1.013979 |
1.012250 |
1.010356 |
1.007861 |
1.006058 |
| 0.00754 |
1.018025 |
1.016931 |
1.015614 |
1.014077 |
1.012348 |
1.010454 |
1.007960 |
1.006157 |
| 0.01002 |
1.018164 |
1.017069 |
1.015752 |
1.014214 |
1.012485 |
1.010592 |
1.008097 |
1.006294 |
| 0.01189 |
1.018267 |
1.017173 |
1.015855 |
1.014317 |
1.012588 |
1.010695 |
1.008200 |
1.006397 |
| 0.01520 |
1.018451 |
1.017357 |
1.016038 |
1.014500 |
1.012770 |
1.010878 |
1.008383 |
1.006579 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.25 mol kg−1 |
| 0.00000 |
1.044743 |
1.043390 |
1.041855 |
1.040130 |
1.038238 |
1.036425 |
1.033088 |
1.030213 |
| 0.00624 |
1.045084 |
1.043730 |
1.042194 |
1.040469 |
1.038576 |
1.036762 |
1.033425 |
1.030549 |
| 0.00784 |
1.045171 |
1.043816 |
1.042281 |
1.040555 |
1.038662 |
1.036848 |
1.033510 |
1.030635 |
| 0.01080 |
1.045332 |
1.043976 |
1.042441 |
1.040715 |
1.038821 |
1.037007 |
1.033668 |
1.030794 |
| 0.01208 |
1.045401 |
1.044045 |
1.042510 |
1.040784 |
1.038889 |
1.037075 |
1.033736 |
1.030862 |
| 0.01490 |
1.045554 |
1.044197 |
1.042663 |
1.040936 |
1.039040 |
1.037225 |
1.033886 |
1.031012 |
| 0.01825 |
1.045735 |
1.044377 |
1.042843 |
1.041116 |
1.039218 |
1.037403 |
1.034064 |
1.031189 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.50 mol kg−1 |
| 0.00000 |
1.089975 |
1.088190 |
1.086290 |
1.084250 |
1.082085 |
1.078250 |
1.075665 |
1.072453 |
| 0.00597 |
1.090285 |
1.088499 |
1.086598 |
1.084557 |
1.082393 |
1.078558 |
1.075973 |
1.072761 |
| 0.00809 |
1.090395 |
1.088608 |
1.086707 |
1.084666 |
1.082501 |
1.078666 |
1.076081 |
1.072869 |
| 0.00985 |
1.090485 |
1.088699 |
1.086797 |
1.084757 |
1.082591 |
1.078756 |
1.076171 |
1.072959 |
| 0.01284 |
1.090639 |
1.088852 |
1.086951 |
1.084910 |
1.082744 |
1.078909 |
1.076323 |
1.073111 |
| 0.01520 |
1.090760 |
1.088973 |
1.087071 |
1.085030 |
1.082863 |
1.079029 |
1.076443 |
1.073232 |
| 0.01810 |
1.090908 |
1.089120 |
1.087219 |
1.085177 |
1.083010 |
1.079176 |
1.076589 |
1.073378 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.75 mol kg−1 |
| 0.00000 |
1.135208 |
1.132990 |
1.130725 |
1.128370 |
1.125933 |
1.122547 |
1.118243 |
1.115480 |
| 0.00626 |
1.135513 |
1.133294 |
1.131029 |
1.128673 |
1.126237 |
1.122852 |
1.118548 |
1.115786 |
| 0.00788 |
1.135591 |
1.133373 |
1.131107 |
1.128752 |
1.126315 |
1.122930 |
1.118627 |
1.115864 |
| 0.01082 |
1.135733 |
1.133514 |
1.131249 |
1.128893 |
1.126457 |
1.123073 |
1.118769 |
1.116006 |
| 0.01180 |
1.135779 |
1.133562 |
1.131296 |
1.128940 |
1.126505 |
1.123120 |
1.118816 |
1.116052 |
| 0.01461 |
1.135914 |
1.133696 |
1.131431 |
1.129075 |
1.126640 |
1.123255 |
1.118952 |
1.116188 |
| 0.01732 |
1.136042 |
1.133825 |
1.131560 |
1.129204 |
1.126769 |
1.123384 |
1.119082 |
1.116318 |
|
L-Glutamic acid in water |
| 0.01104 |
0.999776 |
0.998875 |
0.997680 |
0.996307 |
0.994687 |
0.992865 |
0.990860 |
0.988682 |
| 0.01308 |
0.999894 |
0.998992 |
0.997796 |
0.996423 |
0.994802 |
0.992978 |
0.990973 |
0.988794 |
| 0.01522 |
1.000019 |
0.999115 |
0.997919 |
0.996544 |
0.994921 |
0.993096 |
0.991091 |
0.988912 |
| 0.02078 |
1.000341 |
0.999434 |
0.998235 |
0.996857 |
0.995233 |
0.993404 |
0.991399 |
0.989217 |
| 0.02517 |
1.000595 |
0.999686 |
0.998485 |
0.997104 |
0.995479 |
0.993645 |
0.991640 |
0.989457 |
| 0.03030 |
1.000890 |
0.999978 |
0.998775 |
0.997390 |
0.995765 |
0.993927 |
0.991922 |
0.988735 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.1 mol kg−1 |
| 0.01288 |
1.015222 |
1.013518 |
1.010883 |
1.009568 |
1.007642 |
1.005658 |
1.003681 |
1.001656 |
| 0.01523 |
1.015352 |
1.013648 |
1.011011 |
1.009697 |
1.007770 |
1.005787 |
1.003808 |
1.001782 |
| 0.01930 |
1.015577 |
1.013873 |
1.011235 |
1.009919 |
1.007992 |
1.006010 |
1.004028 |
1.002001 |
| 0.02496 |
1.015890 |
1.014184 |
1.011546 |
1.010228 |
1.008300 |
1.006318 |
1.004335 |
1.002307 |
| 0.03127 |
1.016238 |
1.014530 |
1.011890 |
1.010571 |
1.008642 |
1.006659 |
1.004674 |
1.002645 |
| 0.03591 |
1.016492 |
1.014782 |
1.012142 |
1.010819 |
1.008890 |
1.006909 |
1.004920 |
1.002892 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.25 mol kg−1 |
| 0.01410 |
1.033219 |
1.031223 |
1.029358 |
1.027302 |
1.025850 |
1.024208 |
1.021730 |
1.019255 |
| 0.01537 |
1.033286 |
1.031291 |
1.029426 |
1.027369 |
1.025917 |
1.024276 |
1.021797 |
1.019322 |
| 0.01930 |
1.033499 |
1.031503 |
1.029638 |
1.027580 |
1.026127 |
1.024485 |
1.022006 |
1.019531 |
| 0.02391 |
1.033747 |
1.031750 |
1.029885 |
1.027826 |
1.026372 |
1.024730 |
1.022250 |
1.019775 |
| 0.03000 |
1.034075 |
1.032075 |
1.030211 |
1.028150 |
1.026695 |
1.025053 |
1.022572 |
1.020096 |
| 0.03235 |
1.034200 |
1.032200 |
1.030334 |
1.028271 |
1.026819 |
1.025176 |
1.022695 |
1.020217 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.50 mol kg−1 |
| 0.01228 |
1.063847 |
1.062143 |
1.059940 |
1.058089 |
1.055985 |
1.053871 |
1.051601 |
1.048830 |
| 0.01354 |
1.063912 |
1.062208 |
1.060005 |
1.058153 |
1.056049 |
1.053935 |
1.051666 |
1.048892 |
| 0.01654 |
1.064069 |
1.062363 |
1.060160 |
1.058307 |
1.056202 |
1.054088 |
1.051818 |
1.049043 |
| 0.02018 |
1.064258 |
1.062551 |
1.060347 |
1.058492 |
1.056388 |
1.054274 |
1.052003 |
1.049227 |
| 0.02585 |
1.064553 |
1.062844 |
1.060638 |
1.058782 |
1.056675 |
1.054561 |
1.052290 |
1.049512 |
| 0.03703 |
1.065130 |
1.063412 |
1.061210 |
1.059350 |
1.057240 |
1.055124 |
1.052854 |
1.050070 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.75 mol kg−1 |
| 0.01159 |
1.094489 |
1.092337 |
1.090661 |
1.087995 |
1.086187 |
1.084131 |
1.081540 |
1.079468 |
| 0.01375 |
1.094597 |
1.092445 |
1.090767 |
1.088101 |
1.086294 |
1.084236 |
1.081645 |
1.079572 |
| 0.01765 |
1.094792 |
1.092640 |
1.090960 |
1.088293 |
1.086486 |
1.084426 |
1.081835 |
1.079760 |
| 0.01995 |
1.094907 |
1.092755 |
1.091073 |
1.088405 |
1.086599 |
1.084538 |
1.081945 |
1.079870 |
| 0.02676 |
1.095245 |
1.093095 |
1.091407 |
1.088740 |
1.086931 |
1.084867 |
1.082275 |
1.080198 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.1 mol kg−1 |
| 0.01247 |
1.018281 |
1.017186 |
1.015870 |
1.014333 |
1.012603 |
1.010708 |
1.008213 |
1.006409 |
| 0.01463 |
1.018396 |
1.017301 |
1.015986 |
1.014449 |
1.012719 |
1.010823 |
1.008329 |
1.006524 |
| 0.01634 |
1.018487 |
1.017392 |
1.016077 |
1.014541 |
1.012811 |
1.010913 |
1.008420 |
1.006615 |
| 0.02218 |
1.018800 |
1.017706 |
1.016391 |
1.014854 |
1.013124 |
1.011224 |
1.008729 |
1.006926 |
| 0.02857 |
1.019140 |
1.018044 |
1.016732 |
1.015194 |
1.013464 |
1.011563 |
1.009067 |
1.007265 |
| 0.03390 |
1.019423 |
1.018324 |
1.017015 |
1.015478 |
1.013747 |
1.011844 |
1.009348 |
1.007545 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.25 mol kg−1 |
| 0.01213 |
1.045383 |
1.044029 |
1.042489 |
1.040763 |
1.038868 |
1.037053 |
1.033716 |
1.030842 |
| 0.01438 |
1.045500 |
1.044146 |
1.042605 |
1.040879 |
1.038983 |
1.037169 |
1.033832 |
1.030956 |
| 0.01771 |
1.045674 |
1.044320 |
1.042778 |
1.041051 |
1.039155 |
1.037340 |
1.034000 |
1.031126 |
| 0.02308 |
1.045954 |
1.044600 |
1.043055 |
1.041328 |
1.039430 |
1.037615 |
1.034274 |
1.031400 |
| 0.02531 |
1.046069 |
1.044715 |
1.043170 |
1.041442 |
1.039542 |
1.037728 |
1.034386 |
1.031512 |
| 0.03401 |
1.046520 |
1.045167 |
1.043619 |
1.041888 |
1.039985 |
1.038168 |
1.034828 |
1.031953 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.50 mol kg−1 |
| 0.01104 |
1.090520 |
1.088735 |
1.086830 |
1.084790 |
1.082623 |
1.078787 |
1.076200 |
1.072989 |
| 0.01239 |
1.090585 |
1.088800 |
1.086896 |
1.084854 |
1.082687 |
1.078851 |
1.076265 |
1.073054 |
| 0.01547 |
1.090735 |
1.088950 |
1.087047 |
1.085003 |
1.082836 |
1.078999 |
1.076412 |
1.073202 |
| 0.02012 |
1.090960 |
1.089176 |
1.087272 |
1.085226 |
1.083059 |
1.079222 |
1.076634 |
1.073426 |
| 0.02521 |
1.091205 |
1.089423 |
1.087517 |
1.085470 |
1.083303 |
1.079465 |
1.076874 |
1.073670 |
| 0.03006 |
1.091438 |
1.089657 |
1.087749 |
1.085700 |
1.083535 |
1.079694 |
1.077100 |
1.073900 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.75 mol kg−1 |
| 0.01088 |
1.135703 |
1.133485 |
1.131216 |
1.128861 |
1.126424 |
1.123036 |
1.118734 |
1.115969 |
| 0.01408 |
1.135848 |
1.133629 |
1.131360 |
1.129005 |
1.126566 |
1.123180 |
1.118877 |
1.116112 |
| 0.01532 |
1.135903 |
1.133683 |
1.131415 |
1.129059 |
1.126620 |
1.123235 |
1.118932 |
1.116166 |
| 0.02000 |
1.136113 |
1.133892 |
1.131625 |
1.129267 |
1.126827 |
1.123443 |
1.119140 |
1.116373 |
| 0.02799 |
1.136469 |
1.134249 |
1.131980 |
1.129622 |
1.127179 |
1.123796 |
1.119494 |
1.116725 |
| 0.03107 |
1.136605 |
1.134385 |
1.132115 |
1.129756 |
1.127314 |
1.123930 |
1.119629 |
1.116855 |
3.1.2. Partial molar volumes, V2,ϕ0.
The V2,ϕ0 values have been evaluated by fitting the corresponding data to the given equation as follows:
The volumetric virial coefficient, i.e., Sv, is the experimental slope in the given equation. The V2,ϕ0 values and the standard deviation values are tabulated in Table 3. The V2,ϕ0 values for aqueous solutions of amino acids agree well with those reported in the literature (Table 3).24–30
Table 3 Partial molar volumes, V2,ϕ0, of L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate at T values from 288.15 to 323.15 K and P = 101.3 kPa
|
m
B/mol kg−1 |
106V2,ϕ0 m−3 mol−1 |
| |
288.15 K |
293.15 K |
298.15 K |
303.15 K |
308.15 K |
313.15 K |
318.15 K |
323.15 K |
|
Ref. 24.
Ref. 25.
Ref. 26.
Ref. 27.
Ref. 28.
Ref. 29.
Ref. 30; parentheses contain Sv (m3 kg mol−2) values; standard uncertainties, u, are u(T) = 0.03 K, u(p) = 0.5 kPa, u(m) = 2.8 × 10−4 mol kg−1.
|
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate |
| 0.0 |
71.73 ± 0.01 |
72.02 ± 0.04 |
72.35 ± 0.04 |
72.55 ± 0.03 |
72.74 ± 0.01 |
72.98 ± 0.01 |
73.25 ± 0.01 |
73.46 ± 0.02 |
| 71.8a, 72.21b |
(46.19 ± 5.10) |
72.30a, 73.14b, 73.34c, 73.33d |
74.13c, 74.13d |
72.78a, 74.17b, 75.04c, 75.03d |
76.01c, 76.01d |
73.23a |
(28.61 ± 2.80) |
| (45.37 ± 1.47) |
|
(42.67 ± 4.86) |
(34.31 ± 3.92) |
(31.06 ± 1.65) |
(26.65 ± 1.77) |
(28.96 ± 1.71) |
|
| 0.1 |
75.06 ± 0.03 |
75.19 ± 0.02 |
75.44 ± 0.01 |
75.50 ± 0.02 |
75.59 ± 0.02 |
75.62 ± 0.01 |
75.68 ± 0.02 |
75.75 ± 0.02 |
| (25.48 ± 2.70) |
(23.27 ± 2.25) |
(12.64 ± 1.18) |
(14.64 ± 2.36) |
(14.35 ± 1.97) |
(17.57 ± 1.30) |
(20.99 ± 2.31) |
(19.66 ± 2.38) |
| 0.25 |
76.04 ± 0.02 |
76.24 ± 0.03 |
76.48 ± 0.01 |
76.54 ± 0.01 |
76.65 ± 0.02 |
76.74 ± 0.02 |
76.85 ± 0.01 |
76.93 ± 0.01 |
| (26.40 ± 2.07) |
(25.89 ± 2.66) |
(19.64 ± 1.52) |
(23.10 ± 1.49) |
(20.60 ± 2.26) |
(25.38 ± 1.75) |
(26.12 ± 1.01) |
(32.56 ± 0.59) |
| 0.50 |
77.27 ± 0.03 |
77.39 ± 0.02 |
77.60 ± 0.03 |
77.69 ± 0.04 |
77.75 ± 0.02 |
77.79 ± 0.02 |
77.93 ± 0.03 |
77.98 ± 0.01 |
| (44.41 ± 3.76) |
(34.32 ± 2.84) |
(19.91 ± 3.35) |
(20.86 ± 4.10) |
(24.55 ± 2.27) |
(33.46 ± 2.14) |
(33.36 ± 3.21) |
(32.24 ± 1.61) |
| 0.75 |
78.46 ± 0.002 |
78.56 ± 0.02 |
78.71 ± 0.03 |
78.82 ± 0.01 |
78.90 ± 0.008 |
78.95 ± 0.03 |
79.11 ± 0.02 |
79.16 ± 0.01 |
| (4.23 ± 0.33) |
(11.50 ± 1.97) |
(27.31 ± 3.02) |
(24.49 ± 1.40) |
(28.81 ± 0.85) |
(31.10 ± 3.68) |
(28.16 ± 2.30) |
(38.43 ± 1.57) |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate |
| 0.1 |
76.51 ± 0.02 |
76.71 ± 0.01 |
76.92 ± 0.01 |
76.98 ± 0.03 |
77.02 ± 0.03 |
77.12 ± 0.01 |
77.22 ± 0.007 |
77.26 ± 0.01 |
| (22.38 ± 2.38) |
(17.03 ± 1.23) |
(16.05 ± 1.44) |
(24.68 ± 3.87) |
(28.24 ± 3.78) |
(15.76 ± 1.46) |
(18.43 ± 0.90) |
(23.00 ± 1.39) |
| 0.25 |
77.20 ± 0.005 |
77.40 ± 0.02 |
77.61 ± 0.01 |
77.72 ± 0.01 |
77.78 ± 0.03 |
77.95 ± 0.02 |
78.12 ± 0.05 |
78.18 ± 0.01 |
| (17.99 ± 0.52) |
(22.63 ± 2.29) |
(10.25 ± 1.13) |
(12.55 ± 0.90) |
(28.84 ± 2.86) |
(27.47 ± 2.32) |
(31.73 ± 4.68) |
(30.23 ± 1.34) |
| 0.50 |
78.26 ± 0.01 |
78.48 ± 0.01 |
78.71 ± 0.01 |
78.82 ± 0.02 |
78.84 ± 0.02 |
79.00 ± 0.02 |
79.05 ± 0.02 |
79.11 ± 0.02 |
| (22.48 ± 1.16) |
(20.19 ± 1.23) |
(13.71 ± 1.08) |
(15.64 ± 2.03) |
(25.68 ± 1.57) |
(21.00 ± 1.76) |
(27.61 ± 1.83) |
(28.47 ± 2.25) |
| 0.75 |
79.23 ± 0.02 |
79.42 ± 0.01 |
79.64 ± 0.02 |
79.75 ± 0.02 |
79.79 ± 0.02 |
79.81 ± 0.02 |
79.88 ± 0.01 |
79.94 ± 0.03 |
| (33.88 ± 2.43) |
(24.78 ± 1.52) |
(17.23 ± 1.73) |
(18.05 ± 1.71) |
(16.40 ± 2.20) |
(18.86 ± 2.20) |
(21.13 ± 1.20) |
(27.03 ± 3.29) |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate |
| 0.0 |
88.34 ± 0.01 |
88.78 ± 0.04 |
89.70 ± 0.02 |
90.01 ± 0.01 |
90.64 ± 0.03 |
91.19 ± 0.03 |
91.46 ± 0.03 |
91.90 ± 0.02 |
| 88.35a, 88.88b |
86.68e |
89.65a, 90.19b, 87.84e, 89f, 90.06f, 89.64g, 89.65g |
89.25e |
90.54a, 91.05b |
89.87e |
91.20a |
90.64e |
| (17.83 ± 0.51) |
(22.72 ± 2.42) |
(11.13 ± 1.08) |
(19.88 ± 0.86) |
(12.77 ± 1.80) |
(20.83 ± 1.91) |
(16.57 ± 1.49) |
(18.29 ± 1.26) |
| 0.1 |
90.37 ± 0.03 |
90.62 ± 0.01 |
91.10 ± 0.04 |
91.23 ± 0.02 |
91.46 ± 0.03 |
91.72 ± 0.03 |
91.86 ± 0.03 |
92.01 ± 0.06 |
| (16.23 ± 1.31) |
(18.16 ± 0.54) |
(14.97 ± 2.09) |
(19.55 ± 1.23) |
(19.40 ± 1.29) |
(13.04 ± 1.30) |
(21.12 ± 1.63) |
(23.46 ± 2.93) |
| 0.25 |
91.16 ± 0.03 |
91.32 ± 0.01 |
91.57 ± 0.03 |
91.73 ± 0.04 |
91.92 ± 0.03 |
92.17 ± 0.01 |
92.31 ± 0.02 |
92.52 ± 0.03 |
| (13.60 ± 1.74) |
(17.47 ± 0.62) |
(13.77 ± 1.55) |
(18.93 ± 2.12) |
(17.61 ± 1.49) |
(15.50 ± 0.58) |
(17.96 ± 1.29) |
(18.35 ± 1.96) |
| 0.50 |
91.59 ± 0.03 |
91.86 ± 0.03 |
92.35 ± 0.02 |
92.41 ± 0.06 |
92.75 ± 0.04 |
93.17 ± 0.01 |
93.33 ± 0.02 |
93.49 ± 0.07 |
| (14.53 ± 1.57) |
(20.06 ± 1.32) |
(11.94 ± 0.78) |
(19.37 ± 2.88) |
(18.96 ± 1.89) |
(13.09 ± 0.68) |
(13.05 ± 1.14) |
(21.08 ± 3.37) |
| 0.75 |
91.76 ± 0.01 |
92.05 ± 0.04 |
92.50 ± 0.04 |
92.71 ± 0.05 |
93.06 ± 0.01 |
93.56 ± 0.02 |
93.77 ± 0.03 |
93.95 ± 0.06 |
| (23.58 ± 1.05) |
(17.30 ± 3.03) |
(24.03 ± 3.00) |
(24.22 ± 4.05) |
(18.56 ± 0.63) |
(19.44 ± 1.54) |
(19.24 ± 2.84) |
(25.87 ± 4.95) |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate |
| 0.1 |
91.83 ± 0.07 |
92.00 ± 0.04 |
92.14 ± 0.02 |
92.25 ± 0.01 |
92.36 ± 0.01 |
92.50 ± 0.05 |
92.59 ± 0.03 |
92.87 ± 0.03 |
| (23.01 ± 3.57) |
(22.73 ± 2.30) |
(14.79 ± 1.09) |
(14.51 ± 0.75) |
(14.67 ± 0.54) |
(21.27 ± 2.74) |
(23.33 ± 1.76) |
(16.12 ± 1.57) |
| 0.25 |
92.28 ± 0.03 |
92.44 ± 0.03 |
92.90 ± 0.03 |
93.01 ± 0.03 |
93.28 ± 0.03 |
93.50 ± 0.02 |
93.60 ± 0.07 |
93.70 ± 0.06 |
| (16.13 ± 1.84) |
(13.40 ± 1.49) |
(12.20 ± 1.89) |
(15.75 ± 1.74) |
(19.07 ± 1.39) |
(16.54 ± 1.35) |
(23.30 ± 3.89) |
(23.59 ± 3.50) |
| 0.50 |
93.16 ± 0.03 |
93.35 ± 0.03 |
93.74 ± 0.02 |
93.83 ± 0.04 |
94.18 ± 0.03 |
94.40 ± 0.03 |
94.54 ± 0.03 |
94.80 ± 0.02 |
| (25.23 ± 2.01) |
(17.86 ± 1.69) |
(13.98 ± 1.20) |
(24.25 ± 2.27) |
(16.30 ± 2.00) |
(20.15 ± 1.60) |
(27.98 ± 1.56) |
(13.29 ± 1.05) |
| 0.75 |
94.09 ± 0.01 |
94.23 ± 0.05 |
94.65 ± 0.01 |
94.74 ± 0.03 |
94.86 ± 0.05 |
95.17 ± 0.01 |
95.30 ± 0.03 |
95.51 ± 0.04 |
| (16.50 ± 0.48) |
(17.87 ± 2.86) |
(11.21 ± 0.60) |
(15.94 ± 1.76) |
(21.36 ± 2.53) |
(14.19 ± 0.79) |
(15.59 ± 1.39) |
(21.00 ± 2.14) |
The V2,ϕ0 values of both amino acids increase with an increase in the concentrations of the studied salts and also with an increase in temperature. As the V2,ϕ0 values increase with an increase in molecular weight, the V2,ϕ0 values are higher in GLU than ASP, because of the extra –CH2 group. The V2,ϕ0 values for both ASP and GLU are higher in aqueous solutions of TSC than in DST.
3.1.3. Apparent specific volume, vϕ.
The apparent specific volume, vϕ, is calculated as follows
The apparent specific volume, vϕ, bears a relationship to taste quality in the order salty < sour < sweet < bitter and, probably, the entire range of human taste perception is confined to molecules with apparent specific volumes between 0.1 and 0.95 cm3 g−1.31 The vϕ values for the studied systems are given in Table S2.† In water, the vϕ values for ASP and GLU fall within the sweet taste range32 (0.52–0.71 cm3 g−1). ASP is sweeter than GLU in water. However, in aqueous solutions of DST and TSC, the vϕ values increase and, therefore, the sweetnesses of both ASP and GLU decrease as the mB values increase.
3.1.4. Partial molar volumes of transfer, ΔtrV2,ϕ0.
The ΔtrV2,ϕ0 values of the studied solutes, from water to aqueous solutions of DST and TSC, are calculated as follows:| | | ΔtrV2,ϕ0 = V2,ϕ0 (in aqueous solutions of DST/TSC) − V2,ϕ0 (in water) | (4) |
The ΔtrV2,ϕ0 values are positive for the studied amino acids and increase with the co-solute concentrations at all temperatures, as shown in Fig. 4(a, d, g and j). The ΔtrV2,ϕ0 values of ASP and GLU decrease with temperature. The ΔtrV2,ϕ0 values of both amino acids are greater in the case of TSC compared to DST, due to the high charge density.5 The magnitude of transfer is greater in the case of ASP compared to GLU.
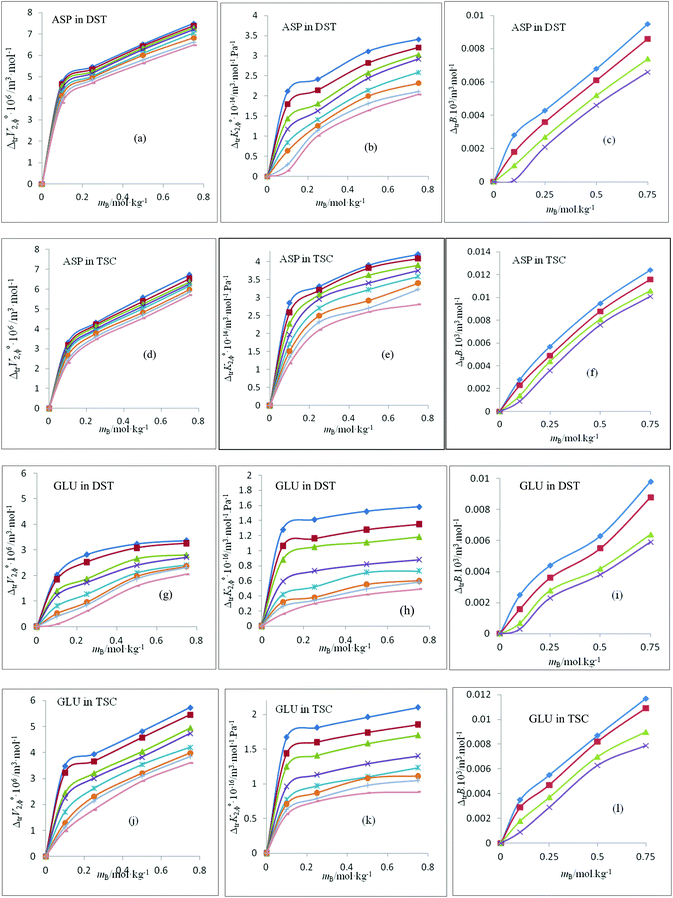 |
| | Fig. 4 (a, d, g and j) Partial molar volume of transfer values, ΔtrV2,ϕ0, of L-aspartic acid and L-glutamic acid vs. mB for aqueous solutions of di-sodium tartrate and tri-sodium citrate at T = (◆) 288.15 K; (■) 293.15 K; (▲) 298.15 K; (×) 303.15 K; (*) 308.15 K; (●) 313.15 K; (+) 318.15 K; and (−) 323.15 K. (b, e, h and k) partial molar isentropic compression of transfer values, ΔtrKs,2,ϕ0, of L-aspartic acid and L-glutamic acid vs. mB for aqueous solutions of di-sodium tartrate and tri-sodium citrate at T = (◆) 288.15 K; (■) 293.15 K; (▲) 298.15 K; (×) 303.15 K; (*) 308.15 K; (●) 313.15 K; (+) 318.15 K; and (−) 323.15 K. (c, f, i and l) Viscosity B-coefficient of transfer values, ΔtrB, of L-aspartic acid and L-glutamic acid vs. mB for aqueous solutions of di-sodium tartrate and tri-sodium citrate at T = (◆) 288.15 K; (■) 298.15 K; (▲) 308.15 K; and (×) 308.15 K. | |
The magnitude of ΔtrV2,ϕ0 initially increases sharply, and the increase is less at higher co-solute concentrations. A co-sphere overlap model33 is used to interpret these results. According to this model, hydrophilic (the –NH2 and –COOH parts of the solute)-ionic (the Na+ and −COO− parts of the co-solute) and hydrophilic (the –NH2 and –COOH groups of the solute)-hydrophilic (the –OH groups of the co-solute) interactions contribute positively, and hydrophobic (the alkyl groups of the solute)-hydrophilic (the –OH groups of the co-solute) and hydrophobic (the alkyl groups of the solute)-ionic (the Na+ and −COO− parts of the co-solute) interactions contribute negatively to the ΔtrV2,ϕ0 values. Therefore, in the present system, the positive ΔtrV2,ϕ0 values for these amino acids over the co-solute concentration range studied suggest that hydrophilic–ionic and hydrophilic–hydrophilic interactions dominate over hydrophobic–hydrophilic and hydrophobic–ionic interactions. Furthermore, the decrease in the magnitude of ΔtrV2,ϕ0 from ASP to GLU indicates the building up of hydrophobic–ionic interactions due to the extra –CH2 group. The additional –CH2 group exerts a destructive effect on the polar and charged group hydration spheres of the amino acids. The low ΔtrV2,ϕ0 values for GLU are in line with the observation that ΔtrV2,ϕ0 values decrease with an increase in the lengths of the alkyl side chains of amino acids.18
3.1.5. Hydration number, Nh.
The hydration number, Nh of the amino acids in water and in aqueous DST/TSC solutions can be calculated by using the following equation.34| | | Nh (in water) = Velect/(V0e−V0b) | (5) |
In the above equation, V0e = molar volume of electrostricted water and V0b = molar volume of bulk water/solvent. The (V0e − V0b) values are approximately −2.9 cm3 mol−1 at 288.15 K, −3.3 cm3 mol−1 at 298.15 K and −4.0 cm3 mol−1 at 308.15 K.35 The Velect values have been calculated from the corresponding V2,ϕ0 values using the equation:| | | Velect = V2,ϕ0 − V0int | (6) |
where the intrinsic molar volume, V0int, can be estimated from the crystallographic volume, Vcryst as follows:36| | | V0int = (0.7/0.634)Vcryst. | (7) |
The Nh data in Table S3† indicate that the dehydration of ASP and GLU increases with the co-solute molality. The Nh values for ASP are higher than for GLU and, also, the decreases in the Nh values with mB are larger in the case of ASP compared to GLU. TSC is found to be an effective preservative for dehydration processes, therefore, its use in the food industry is more preferred than DST.37 TSC has also been found to be a better food emulsifier than DST in the literature.5 TSC helps control the stickiness of some doughs through removing extra water and creating a firmer texture. Thus, it helps in the processing of baked foods.38,39 Therefore, the Nh data also indicate that TSC is a better food preservative than DST.
3.1.6. Partial molar expansion, E2,ϕ0.
To discuss the effects of temperature on the V2,ϕ0 values, the partial molar expansion values, E2,ϕ0 (E2,ϕ0 = (∂V2,ϕ0/∂T)P), along with the 2nd order derivatives, (∂2V2,ϕ0/∂T2)P, have been calculated by the method of least-squares using the following equation:where a, b and c are constants, and T is the temperature in Kelvin. The (∂V2,ϕ0/∂T)P values are positive for the studied solutes (Table S4†), except for ASP in TSC at mB = 0.75 mol kg−1 and at T = 323.15 K. The (∂V2,ϕ0/∂T)P values decrease with temperature, except for GLU in DST at mB = 0.25 mol kg−1 at higher temperatures and GLU in TSC at mB = 0.1 mol kg−1 at all temperatures. However, the (∂V2,ϕ0/∂T)P values do not follow any regular trend in relation to the mB values.
Hepler40 used a general thermodynamic equation, {(∂CP,20/∂P)T = −T(∂2V2,ϕ0/∂T2)P}, to determine the capacity of a solute to act as a structure-maker or a structure-breaker in solution. If the term (∂2V2,ϕ0/∂T2)P < 0, the solute is a structure-breaker and if (∂2V2,ϕ0/∂T2)P > 0, the solute is a structure-maker. The negative values of (∂2V2,ϕ0/∂T2)P in aqueous solutions of the co-solutes suggest that ASP and GLU act as structure-breaking solutes. However, at mB = 0.1 mol kg−1, GLU acts as a structure-maker in the case of TSC.
3.1.7. Interaction coefficients.
Interaction coefficients have been calculated using the McMillan–Mayer equation41 from the transfer volume, ΔtrV2,ϕ0, data for the studied solutes as follows:| | | ΔtrV2,ϕ0 = 2VAB·mB + 3VABB·mB2 + … | (9) |
where A stands for the solute and B for the co-solute, and VAB and VABB are the volumetric pair and triplet interaction coefficients, respectively (Table S5†). The higher magnitudes of VAB (positive values) in comparison to VABB (negative values, except for GLU in DST at T = 323.15 K) for the studied solutes at all temperatures suggest the predominance of pair-wise interactions. This suggests the dominance of hydrophilic–ionic/hydrophilic interactions, which again strengthens the above-mentioned views.
3.2. Acoustic measurements
3.2.1. Speed of sound, u.
The speed of sound, u, of amino acids (ASP and GLU) in water and in aqueous solutions of DST and TSC at mB = 0.10, 0.25, 0.50 and 0.75 mol kg−1 and at temperatures from 288.15 to 323.15 K are given in Table 4. The u values of water are in agreement with the literature values.42 The u values in aqueous solutions of DST and TSC match well with the available literature data,16–21,23 as seen in Fig. 5. The u values of ASP and GLU in water are in close agreement with the available literature data, as seen in Fig. 6.25–27 The u values increase with the mA, mB and T values, as is evident from the 3-D plot (Fig. 3(c)) of u versus mA for ASP at different temperatures in TSC at mB = 0.1 mol kg−1.
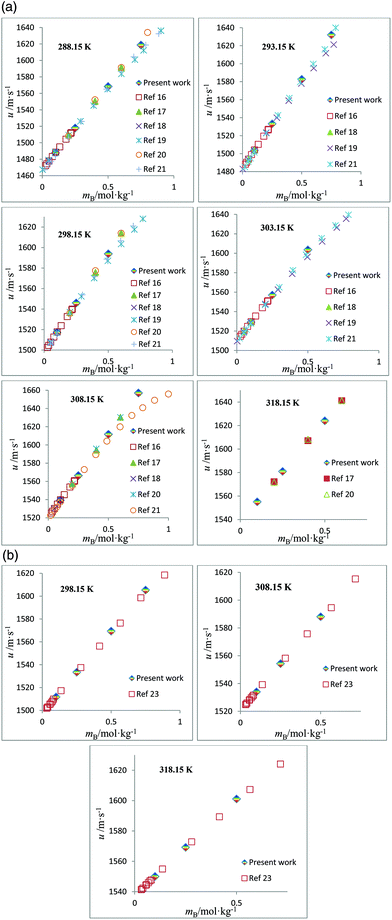 |
| | Fig. 5 (a) Plots of speed of sound values, u, versus molality, mB, for aqueous solutions of TSC and comparisons with the literature values at different temperatures. (b) Plots of speed of sound values, u, versus molality, mB, for aqueous solutions of DST and comparisons with the literature values at different temperatures. | |
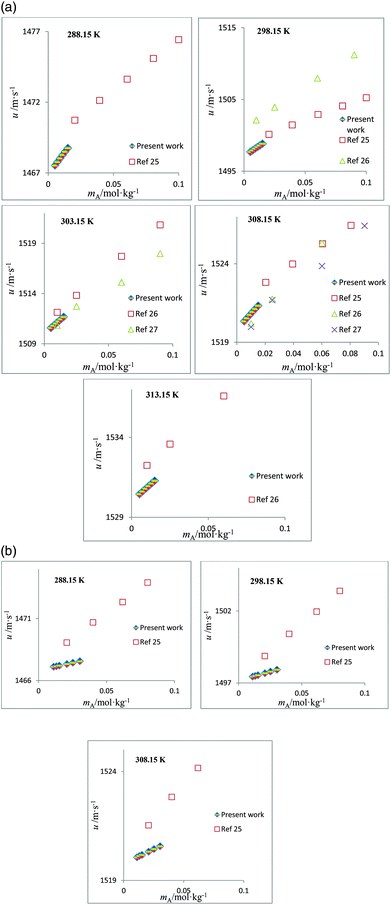 |
| | Fig. 6 (a) Plots of speed of sound values, u, versus molality, mA, for aqueous solutions of ASP and comparisons with the literature values at different temperatures. (b) Plots of speed of sound values, u, versus molality, mA, for aqueous solutions of GLU and comparisons with the literature values at different temperatures. | |
Table 4 The speed of sound values, u, of L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate at T values from 288.15 to 323.15 K and P = 101.3 kPa
|
m
A/mol kg−1 |
u/m sec−1 |
| 288.15 K |
293.15 K |
298.15 K |
303.15 K |
308.15 K |
313.15 K |
318.15 K |
323.15 K |
|
m
A is the molality of the solute in water or water + DST/TSC. mB is the molality of DST/TSC in water. Standard uncertainties, u, are u(T) = 0.03 K, u(P) = 0.5 kPa, u(m) = 2.8 × 10−4 mol kg−1, u(u) = 0.6 m s−1. |
|
L-Aspartic acid in water
|
| 0.00000 |
1466.83 |
1483.03 |
1497.10 |
1510.02 |
1519.83 |
1529.98 |
1536.40 |
1542.99 |
| 0.00517 |
1467.53 |
1483.68 |
1497.71 |
1510.59 |
1520.34 |
1530.45 |
1536.84 |
1543.38 |
| 0.00712 |
1467.78 |
1483.92 |
1497.94 |
1510.81 |
1520.54 |
1530.62 |
1536.99 |
1543.52 |
| 0.00921 |
1468.04 |
1484.17 |
1498.18 |
1511.04 |
1520.75 |
1530.80 |
1537.15 |
1543.67 |
| 0.01109 |
1468.28 |
1484.39 |
1498.40 |
1511.25 |
1520.94 |
1530.96 |
1537.30 |
1543.80 |
| 0.01300 |
1468.51 |
1484.62 |
1498.62 |
1511.46 |
1521.13 |
1531.12 |
1537.45 |
1543.94 |
| 0.01506 |
1468.76 |
1484.86 |
1498.85 |
1511.68 |
1521.33 |
1531.29 |
1537.60 |
1544.08 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.1 mol kg
−1
|
| 0.00000 |
1478.73 |
1494.57 |
1511.87 |
1522.45 |
1534.08 |
1543.75 |
1550.05 |
1557.45 |
| 0.00502 |
1479.25 |
1495.10 |
1512.40 |
1522.98 |
1534.59 |
1544.18 |
1550.48 |
1557.85 |
| 0.00741 |
1479.50 |
1495.34 |
1512.65 |
1523.23 |
1534.83 |
1544.38 |
1550.68 |
1558.04 |
| 0.01180 |
1479.95 |
1495.80 |
1513.28 |
1523.69 |
1535.27 |
1544.75 |
1551.05 |
1558.39 |
| 0.01306 |
1480.07 |
1495.93 |
1513.34 |
1523.82 |
1535.39 |
1544.85 |
1551.16 |
1558.48 |
| 0.01732 |
1480.51 |
1496.36 |
1514.04 |
1524.26 |
1535.81 |
1545.21 |
1551.51 |
1558.82 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.25 mol kg
−1
|
| 0.00000 |
1504.97 |
1519.22 |
1533.45 |
1544.85 |
1554.33 |
1561.75 |
1569.19 |
1577.89 |
| 0.00584 |
1505.61 |
1519.86 |
1534.09 |
1545.48 |
1554.91 |
1562.23 |
1569.64 |
1578.31 |
| 0.00817 |
1505.86 |
1520.11 |
1534.34 |
1545.73 |
1555.14 |
1562.42 |
1569.82 |
1578.47 |
| 0.01106 |
1506.17 |
1520.43 |
1534.65 |
1546.04 |
1555.42 |
1562.65 |
1570.03 |
1578.67 |
| 0.01337 |
1506.42 |
1520.67 |
1534.90 |
1546.28 |
1555.64 |
1562.83 |
1570.20 |
1578.82 |
| 0.01555 |
1506.65 |
1520.91 |
1535.13 |
1546.51 |
1555.85 |
1563.00 |
1570.36 |
1578.97 |
| 0.01667 |
1506.77 |
1521.03 |
1535.25 |
1546.63 |
1555.95 |
1563.08 |
1570.43 |
1579.04 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.50 mol kg
−1
|
| 0.00000 |
1549.36 |
1558.64 |
1569.41 |
1579.85 |
1588.09 |
1595.45 |
1601.10 |
1609.00 |
| 0.00638 |
1550.10 |
1559.37 |
1570.12 |
1580.54 |
1588.70 |
1595.96 |
1601.62 |
1609.47 |
| 0.00782 |
1550.27 |
1559.53 |
1570.28 |
1580.69 |
1588.84 |
1596.07 |
1601.74 |
1609.57 |
| 0.00982 |
1550.50 |
1559.76 |
1570.50 |
1580.89 |
1589.02 |
1596.22 |
1601.90 |
1609.72 |
| 0.01307 |
1550.87 |
1560.12 |
1570.85 |
1581.23 |
1589.33 |
1596.47 |
1602.16 |
1609.95 |
| 0.01509 |
1551.10 |
1560.35 |
1571.07 |
1581.44 |
1589.52 |
1596.62 |
1602.31 |
1610.09 |
| 0.01639 |
1551.24 |
1560.49 |
1571.20 |
1581.57 |
1589.63 |
1596.72 |
1602.41 |
1610.18 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.75 mol kg
−1
|
| 0.00000 |
1588.75 |
1597.00 |
1605.38 |
1615.75 |
1621.84 |
1628.04 |
1633.00 |
1641.75 |
| 0.00547 |
1589.45 |
1597.68 |
1606.03 |
1616.37 |
1622.42 |
1628.51 |
1633.50 |
1642.22 |
| 0.00739 |
1589.69 |
1597.92 |
1606.26 |
1616.58 |
1622.62 |
1628.67 |
1633.67 |
1642.38 |
| 0.01025 |
1590.05 |
1598.27 |
1606.59 |
1616.90 |
1622.91 |
1628.90 |
1633.92 |
1642.62 |
| 0.01347 |
1590.45 |
1598.66 |
1606.97 |
1617.25 |
1623.26 |
1629.16 |
1634.20 |
1642.89 |
| 0.01664 |
1590.84 |
1599.04 |
1607.33 |
1617.60 |
1623.57 |
1629.41 |
1634.48 |
1643.15 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at m
B
= 0.1 mol kg
−1
|
| 0.00000 |
1487.73 |
1503.57 |
1517.21 |
1529.07 |
1539.24 |
1548.45 |
1555.13 |
1562.78 |
| 0.00576 |
1488.28 |
1504.12 |
1517.76 |
1529.62 |
1539.76 |
1548.87 |
1555.53 |
1563.15 |
| 0.00754 |
1488.45 |
1504.29 |
1517.93 |
1529.79 |
1539.91 |
1549.00 |
1555.65 |
1563.27 |
| 0.01002 |
1488.69 |
1504.52 |
1518.16 |
1530.02 |
1540.13 |
1549.18 |
1555.82 |
1563.42 |
| 0.01189 |
1488.86 |
1504.70 |
1518.34 |
1530.19 |
1540.30 |
1549.31 |
1555.94 |
1563.54 |
| 0.0152 |
1489.17 |
1505.01 |
1518.65 |
1530.49 |
1540.59 |
1549.55 |
1556.16 |
1563.75 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at m
B
= 0.25 mol kg
−1
|
| 0.00000 |
1517.97 |
1533.22 |
1545.99 |
1557.02 |
1566.44 |
1574.85 |
1580.99 |
1587.00 |
| 0.00624 |
1518.60 |
1533.83 |
1546.57 |
1557.57 |
1566.95 |
1575.25 |
1581.36 |
1587.34 |
| 0.00784 |
1518.76 |
1533.98 |
1546.71 |
1557.71 |
1567.08 |
1575.35 |
1581.45 |
1587.42 |
| 0.01080 |
1519.05 |
1534.27 |
1546.98 |
1557.97 |
1567.31 |
1575.53 |
1581.62 |
1587.58 |
| 0.01208 |
1519.18 |
1534.39 |
1547.10 |
1558.08 |
1567.41 |
1575.61 |
1581.69 |
1587.65 |
| 0.01490 |
1519.46 |
1534.66 |
1547.35 |
1558.32 |
1567.63 |
1575.78 |
1581.85 |
1587.79 |
| 0.01825 |
1519.79 |
1534.97 |
1547.65 |
1558.61 |
1567.90 |
1575.99 |
1582.03 |
1587.96 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at m
B
= 0.50 mol kg
−1
|
| 0.00000 |
1568.36 |
1582.64 |
1593.94 |
1603.61 |
1611.76 |
1618.75 |
1624.10 |
1630.01 |
| 0.00597 |
1569.02 |
1583.27 |
1594.55 |
1604.20 |
1612.28 |
1619.16 |
1624.48 |
1630.34 |
| 0.00809 |
1569.26 |
1583.49 |
1594.77 |
1604.40 |
1612.46 |
1619.30 |
1624.61 |
1630.46 |
| 0.00985 |
1569.45 |
1583.67 |
1594.95 |
1604.57 |
1612.61 |
1619.42 |
1624.72 |
1630.56 |
| 0.01284 |
1569.77 |
1583.98 |
1595.26 |
1604.86 |
1612.85 |
1619.62 |
1624.91 |
1630.73 |
| 0.01520 |
1570.03 |
1584.22 |
1595.50 |
1605.08 |
1613.05 |
1619.77 |
1625.05 |
1630.86 |
| 0.0181 |
1570.34 |
1584.46 |
1595.78 |
1605.35 |
1613.28 |
1619.95 |
1625.22 |
1631.03 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at m
B
= 0.75 mol kg
−1
|
| 0.00000 |
1618.75 |
1632.06 |
1641.90 |
1650.20 |
1657.08 |
1662.45 |
1667.20 |
1673.45 |
| 0.00626 |
1619.55 |
1632.82 |
1642.62 |
1650.89 |
1657.68 |
1662.89 |
1667.60 |
1673.84 |
| 0.00788 |
1619.75 |
1633.01 |
1642.80 |
1651.06 |
1657.83 |
1663.00 |
1667.70 |
1673.94 |
| 0.01082 |
1620.12 |
1633.36 |
1643.13 |
1651.38 |
1658.10 |
1663.21 |
1667.89 |
1674.13 |
| 0.01180 |
1620.24 |
1633.47 |
1643.24 |
1651.48 |
1658.18 |
1663.28 |
1667.95 |
1674.19 |
| 0.01461 |
1620.59 |
1633.80 |
1643.55 |
1651.77 |
1658.44 |
1663.48 |
1668.13 |
1674.36 |
| 0.01732 |
1620.92 |
1634.12 |
1643.85 |
1652.05 |
1658.68 |
1663.67 |
1668.30 |
1674.53 |
|
L-Glutamic acid in water
|
| 0.01104 |
1467.12 |
1483.26 |
1497.44 |
1510.18 |
1520.06 |
1530.36 |
1536.68 |
1543.40 |
| 0.01308 |
1467.16 |
1483.31 |
1497.49 |
1510.23 |
1520.12 |
1530.42 |
1536.73 |
1543.45 |
| 0.01522 |
1467.21 |
1483.36 |
1497.54 |
1510.28 |
1520.17 |
1530.47 |
1536.79 |
1543.51 |
| 0.02078 |
1467.34 |
1483.49 |
1497.68 |
1510.43 |
1520.32 |
1530.62 |
1536.94 |
1543.66 |
| 0.02517 |
1467.45 |
1483.60 |
1497.79 |
1510.54 |
1520.44 |
1530.74 |
1537.06 |
1543.79 |
| 0.03030 |
1467.57 |
1483.72 |
1497.92 |
1510.68 |
1520.57 |
1530.88 |
1537.21 |
1543.93 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.1 mol kg
−1
|
| 0.01288 |
1479.08 |
1494.93 |
1512.24 |
1522.82 |
1534.46 |
1544.13 |
1550.43 |
1557.83 |
| 0.01523 |
1479.15 |
1495.00 |
1512.31 |
1522.89 |
1534.53 |
1544.20 |
1550.50 |
1557.91 |
| 0.01930 |
1479.27 |
1495.11 |
1512.43 |
1523.01 |
1534.65 |
1544.32 |
1550.63 |
1558.03 |
| 0.02496 |
1479.43 |
1495.28 |
1512.59 |
1523.18 |
1534.82 |
1544.49 |
1550.80 |
1558.20 |
| 0.03127 |
1479.61 |
1495.46 |
1512.78 |
1523.37 |
1535.01 |
1544.68 |
1550.99 |
1558.40 |
| 0.03591 |
1479.74 |
1495.60 |
1512.92 |
1523.52 |
1535.16 |
1544.82 |
1551.14 |
1558.55 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.25 mol kg
−1
|
| 0.01410 |
1505.42 |
1519.67 |
1533.90 |
1545.31 |
1554.79 |
1562.22 |
1569.66 |
1578.36 |
| 0.01930 |
1505.89 |
1520.15 |
1534.39 |
1545.80 |
1555.29 |
1562.71 |
1570.16 |
1578.86 |
| 0.02391 |
1506.10 |
1520.36 |
1534.60 |
1546.01 |
1555.50 |
1562.92 |
1570.37 |
1579.07 |
| 0.03000 |
1506.41 |
1520.67 |
1534.92 |
1546.33 |
1555.82 |
1563.25 |
1570.69 |
1579.40 |
| 0.03535 |
1506.52 |
1520.79 |
1535.03 |
1546.45 |
1555.94 |
1563.37 |
1570.82 |
1579.52 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.50 mol kg
−1
|
| 0.01104 |
1549.82 |
1559.11 |
1569.89 |
1580.33 |
1588.57 |
1595.94 |
1601.59 |
1609.49 |
| 0.01239 |
1549.87 |
1559.16 |
1569.94 |
1580.38 |
1588.62 |
1595.99 |
1601.64 |
1609.54 |
| 0.01547 |
1549.99 |
1559.28 |
1570.05 |
1580.50 |
1588.74 |
1596.11 |
1601.76 |
1609.67 |
| 0.02012 |
1550.13 |
1559.42 |
1570.20 |
1580.64 |
1588.89 |
1596.26 |
1601.91 |
1609.82 |
| 0.02521 |
1550.34 |
1559.64 |
1570.42 |
1580.87 |
1589.12 |
1596.49 |
1602.14 |
1610.05 |
| 0.03006 |
1550.78 |
1560.09 |
1570.86 |
1581.32 |
1589.57 |
1596.95 |
1602.60 |
1610.51 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at m
B
= 0.75 mol kg
−1
|
| 0.01159 |
1589.26 |
1597.51 |
1605.79 |
1616.32 |
1622.37 |
1628.57 |
1633.54 |
1642.29 |
| 0.01375 |
1589.35 |
1597.60 |
1605.89 |
1616.42 |
1622.47 |
1628.67 |
1633.64 |
1642.39 |
| 0.01765 |
1589.52 |
1597.78 |
1606.07 |
1616.60 |
1622.64 |
1628.86 |
1633.82 |
1642.58 |
| 0.01995 |
1589.63 |
1597.88 |
1606.17 |
1616.71 |
1622.75 |
1628.97 |
1633.93 |
1642.69 |
| 0.02676 |
1589.93 |
1598.18 |
1606.49 |
1617.02 |
1623.07 |
1629.29 |
1634.25 |
1643.01 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at m
B
= 0.1 mol kg
−1
|
| 0.01247 |
1488.11 |
1503.95 |
1517.59 |
1529.46 |
1539.63 |
1548.84 |
1555.52 |
1563.17 |
| 0.01463 |
1488.18 |
1504.02 |
1517.66 |
1529.52 |
1539.67 |
1548.91 |
1555.59 |
1563.23 |
| 0.01634 |
1488.23 |
1504.07 |
1517.72 |
1529.58 |
1539.75 |
1548.97 |
1555.65 |
1563.29 |
| 0.02218 |
1488.41 |
1504.26 |
1517.90 |
1529.76 |
1539.94 |
1549.16 |
1555.84 |
1563.47 |
| 0.02857 |
1488.62 |
1504.47 |
1518.11 |
1529.97 |
1540.14 |
1549.36 |
1556.05 |
1563.67 |
| 0.03390 |
1488.79 |
1504.64 |
1518.28 |
1530.14 |
1540.32 |
1549.54 |
1556.22 |
1563.84 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.25 mol kg−1 |
| 0.01213 |
1518.40 |
1533.65 |
1546.43 |
1557.46 |
1566.88 |
1575.30 |
1581.44 |
1587.45 |
| 0.01438 |
1518.48 |
1533.73 |
1546.52 |
1557.55 |
1566.97 |
1575.39 |
1581.53 |
1587.54 |
| 0.01771 |
1518.60 |
1533.86 |
1546.64 |
1557.67 |
1567.10 |
1575.51 |
1581.66 |
1587.66 |
| 0.02308 |
1518.79 |
1534.05 |
1546.84 |
1557.87 |
1567.31 |
1575.72 |
1581.86 |
1587.87 |
| 0.02531 |
1518.88 |
1534.13 |
1546.92 |
1557.96 |
1567.39 |
1575.80 |
1581.95 |
1587.96 |
| 0.03401 |
1519.19 |
1534.45 |
1547.24 |
1558.29 |
1567.73 |
1576.14 |
1582.28 |
1588.29 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.50 mol kg−1 |
| 0.01104 |
1568.85 |
1583.14 |
1594.44 |
1604.12 |
1612.26 |
1619.26 |
1624.61 |
1630.52 |
| 0.01239 |
1568.92 |
1583.20 |
1594.51 |
1604.18 |
1612.33 |
1619.32 |
1624.67 |
1630.58 |
| 0.01547 |
1569.06 |
1583.34 |
1594.65 |
1604.32 |
1612.47 |
1619.47 |
1624.82 |
1630.72 |
| 0.02012 |
1569.27 |
1583.55 |
1594.86 |
1604.54 |
1612.69 |
1619.69 |
1625.04 |
1630.94 |
| 0.02521 |
1569.51 |
1583.79 |
1595.10 |
1604.78 |
1612.93 |
1619.93 |
1625.28 |
1631.17 |
| 0.03006 |
1569.73 |
1583.96 |
1595.33 |
1605.02 |
1613.16 |
1620.16 |
1625.52 |
1631.40 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.75 mol kg−1 |
| 0.01088 |
1619.34 |
1632.66 |
1642.51 |
1650.81 |
1657.69 |
1663.06 |
1667.81 |
1674.06 |
| 0.01408 |
1619.52 |
1632.83 |
1642.69 |
1650.99 |
1657.87 |
1663.24 |
1667.99 |
1674.24 |
| 0.01532 |
1619.59 |
1632.90 |
1642.75 |
1651.06 |
1657.94 |
1663.31 |
1668.06 |
1674.31 |
| 0.02000 |
1619.85 |
1633.17 |
1643.02 |
1651.32 |
1658.21 |
1663.57 |
1668.32 |
1674.58 |
| 0.02799 |
1620.29 |
1633.61 |
1643.47 |
1651.78 |
1658.66 |
1664.03 |
1668.77 |
1675.03 |
| 0.03107 |
1620.47 |
1633.79 |
1643.64 |
1651.95 |
1658.84 |
1664.20 |
1668.95 |
1675.21 |
3.2.2. Apparent molar isentropic compression, Ks,2,ϕ.
The Ks,2,ϕ values for solutes in water and in aqueous solutions of DST and TSC at mB = 0.10, 0.25, 0.50, and 0.75 mol kg−1 and at temperatures from 288.15 to 323.15 K (given in Table S6†) have been calculated using the following relationship:| | | Ks,2,ϕ = (κsM/ρ) − {(κsoρ − κsρ0)/(mAρρ0)} | (10) |
The Ks,2,ϕ values are negative, and their magnitudes decrease with increases in the temperature and in the concentrations of both the solute and co-solute. ρ and ρ0, and κs and κso are the densities and isentropic compressibilities of the solution and solvent, respectively. The isentropic compressibilities (κs) were calculated from the measured speed of sound (u) as follows:
3.2.3. Partial molar isentropic compression, Ks,2,ϕ0.
The Ks,2,ϕ0 values have been calculated using the least squares fitting of the respective data to the given equation:| | | Ks,2,ϕ = Ks,2,ϕ0 + Skm. | (12) |
At infinite dilution, Ks,2,ϕ0, the apparent molar isentropic compression, and the partial molar isentropic compression, Ks,20, become the same. The Ks,2,ϕ0 values of the studied solutes in water agree well with the literature values.25–27,29,30 The Ks,2,ϕ0 values of the solutes are negative in water as well as in aqueous solutions of the co-solutes, which may be due to the hydration of the solutes, as the hydrated water molecules are already compressed and are thus less compressible than those present in the bulk. The magnitudes of the Ks,2,ϕ0 values decrease with temperature and with the co-solute concentrations (Table 5).
Table 5 Partial molar isentropic compression values, Ks,2,ϕ0, of L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate at T values from 288.15 to 323.15 K and P = 101.3 kPa
|
m
B/mol kg−1 |
1014·Ks,2,ϕ0/m3 mol−1 Pa−1 |
| 288.15 K |
293.15 K |
298.15 K |
303.15 K |
308.15 K |
313.15 K |
318.15 K |
323.15 K |
|
Ref. 25.
Ref. 26.
Ref. 27.
Ref. 29.
Ref. 30; parentheses contain Sk (m3 mol−2 kg Pa−1) values; standard uncertainties, u, are u(T) = 0.03 K, u(P) = 0.5 kPa, u(m) = 2.8 × 10−4 mol kg−1.
|
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate |
| 0 |
−7.51 ± 0.01 |
−7.09 ± 0.02 |
−6.63 ± 0.01 |
−6.26 ± 0.01 |
−5.60 ± 0.84 |
−4.50 ± 0.01 |
−4.11 ± 0.02 |
−3.64 ± 0.01 |
|
|
(11.45 ± 0.80) |
(16.68 ± 1.44) |
−3.61a, −6.67b, −7.14c |
(18.28 ± 0.88) |
−3.06 a |
(12.76 ± 0.52) |
(17.70 ± 2.30) |
(13.34 ± 0.63) |
|
|
|
|
(17.12 ± 0.28) |
−6.24b,c |
(19.59 ± 0.84) |
−4.53b,c |
|
|
|
|
|
|
|
|
−5.60b, −5.61c |
|
|
|
| 0.1 |
−5.39 ± 0.01 |
−5.29 ± 0.01 |
−5.19 ± 0.02 |
−5.08 ± 0.01 |
−4.75 ± 0.01 |
−3.86 ± 0.01 |
−3.81 ± 0.01 |
−3.50 ± 0.01 |
|
|
(7.39 ± 0.89) |
(7.88 ± 0.50) |
(7.62 ± 1.33) |
(6.25 ± 0.35) |
(7.20 ± 0.75) |
(8.42 ± 0.95) |
(7.48 ± 0.56) |
(8.02 ± 0.98) |
| 0.25 |
−5.09 ± 0.01 |
−4.94 ± 0.01 |
−4.82 ± 0.01 |
−4.63 ± 0.01 |
−4.18 ± 0.01 |
−3.23 ± 0.02 |
2.96 ± 0.01 |
−2.63 ± 0.01 |
|
|
(11.00 ± 0.42) |
(8.48 ± 0.57) |
(10.92 ± 0.44) |
(8.86 ± 0.84) |
(13.29 ± 0.45) |
(11.71 ± 1.23) |
(12.61 ± 0.69) |
(12.95 ± 0.88) |
| 0.50 |
−4.40 ± 0.01 |
−4.27 ± 0.01 |
−4.05 ± 0.01 |
−3.82 ± 0.01 |
−3.23 ± 0.01 |
−2.50 ± 0.01 |
−2.56 ± 0.01 |
−2.19 ± 0.01 |
|
|
(8.57 ± 0.66) |
(10.48 ± 1.03) |
(10.40 ± 1.22) |
(13.37 ± 0.64) |
(8.73 ± 0.86) |
(12.56 ± 1.16) |
(9.18 ± 0.56) |
(9.62 ± 0.58) |
| 0.75 |
−4.10 ± 0.01 |
−3.88 ± 0.01 |
−3.60 ± 0.01 |
−3.34 ± 0.01 |
−3.01 ± 0.01 |
−2.18 ± 0.01 |
−2.39 ± 0.01 |
−2.16 ± 0.01 |
|
|
(9.10 ± 0.50) |
(7.71 ± 0.36) |
(7.67 ± 0.74) |
(9.43 ± 0.55) |
(8.06 ± 0.33) |
(10.92 ± 0.25) |
(10.13 ± 0.81) |
(8.75 ± 0.43) |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate |
| 0.1 |
−4.66 ± 0.01 |
−4.50 ± 0.01 |
4.37 ± 0.01 |
−4.29 ± 0.01 |
−3.89 ± 0.01 |
−2.99 ± 0.01 |
−2.77 ± 0.01 |
−2.47 ± 0.01 |
|
|
(8.65 ± 0.43) |
(7.58 ± 1.05) |
(8.51 ± 0.54) |
(12.76 ± 0.81) |
(9.43 ± 0.61) |
(9.30 ± 0.98) |
(11.02 ± 1.23) |
(7.58 ± 1.05) |
| 0.25 |
−4.20 ± 0.01 |
−3.89 ± 0.01 |
−3.56 ± 0.01 |
−3.30 ± 0.01 |
−2.89 ± 0.01 |
−2.01 ± 0.01 |
−1.79 ± 0.02 |
−1.54 ± 0.01 |
|
|
(8.33 ± 1.12) |
(7.91 ± 0.96) |
(9.29 ± 0.72) |
(8.03 ± 0.55) |
(8.54 ± 0.62) |
(9.38 ± 0.80) |
(10.16 ± 1.24) |
(8.07 ± 0.52) |
| 0.50 |
−3.61 ± 0.01 |
−3.27 ± 0.01 |
−3.04 ± 0.02 |
−2.87 ± 0.02 |
−2.39 ± 0.02 |
−1.59 ± 0.02 |
−1.40 ± 0.02 |
−1.04 ± 0.01 |
|
|
(9.93 ± 0.74) |
(10.41 ± 0.81) |
(2.97 ± 1.65) |
(10.01 ± 1.44) |
(13.60 ± 1.70) |
(8.54 ± 1.65) |
(8.33 ± 1.46) |
(1.07 ± 0.37) |
| 0.75 |
−3.31 ± 0.01 |
−3.01 ± 0.01 |
−2.74 ± 0.01 |
−2.50 ± 0.01 |
−2.01 ± 0.01 |
−1.10 ± 0.01 |
−0.88 ± 0.01 |
−0.84 ± 0.01 |
|
|
(9.49 ± 0.62) |
(9.41 ± 0.30) |
(9.33 ± 0.69) |
(8.00 ± 0.59) |
(10.11 ± 1.13) |
(0.61 ± 0.39) |
(11.17 ± 0.72) |
(1.11 ± 0.37) |
|
10
16
·K
s,2,ϕ
0
/m
3
mol
−1
Pa
−1
|
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate |
| 0 |
−3.86 ± 0.01 |
−3.50 ± 0.01 |
−3.18 ± 0.01 |
−2.75 ± 0.02 |
−2.46 ± 0.01 |
−2.18 ± 0.01 |
−2.06 ± 0.02 |
−1.92 ± 0.01 |
|
|
−3.37a |
(5.81 ± 0.60) |
−3.20a, −3.18d, −3.92e |
(5.79 ± 1.08) |
−2.20a |
(6.80 ± 0.54) |
(6.58 ± 0.89) |
(8.64 ± 0.61) |
|
|
(5.87 ± 0.67) |
|
(6.25 ± 0.56) |
|
(7.16 ± 0.68) |
|
|
|
| 0.1 |
−2.58 ± 0.02 |
−2.44 ± 0.01 |
−2.30 ± 0.01 |
−2.16 ± 0.01 |
−2.03 ± 0.01 |
−1.85 ± 0.01 |
−1.80 ± 0.02 |
−1.71 ± 0.01 |
|
|
(4.57 ± 0.63) |
(4.63 ± 0.44) |
(4.42 ± 0.59) |
(4.91 ± 0.26) |
(5.26 ± 0.52) |
(4.91 ± 0.33) |
(4.81 ± 0.67) |
(5.47 ± 0.44) |
| 0.25 |
−2.45 ± 0.01 |
−2.34 ± 0.01 |
−2.13 ± 0.01 |
−2.02 ± 0.01 |
−1.94 ± 0.01 |
−1.80 ± 0.01 |
−1.72 ± 0.01 |
−1.60 ± 0.01 |
|
|
(4.13 ± 0.20) |
(4.10 ± 0.53) |
(4.86 ± 0.47) |
(3.95 ± 0.28) |
(4.30 ± 0.54) |
(6.42 ± 0.53) |
(4.91 ± 0.46) |
(4.51 ± 0.36) |
| 0.50 |
−2.33 ± 0.02 |
−2.23 ± 0.01 |
−2.07 ± 0.02 |
−1.93 ± 0.03 |
−1.75 ± 0.02 |
−1.63 ± 0.02 |
−1.57 ± 0.03 |
−1.46 ± 0.01 |
|
|
(4.55 ± 0.93) |
(4.83 ± 0.61) |
(5.03 ± 0.74) |
(5.28 ± 1.13) |
(4.76 ± 0.80) |
(5.48 ± 0.73) |
(4.85 ± 1.26) |
(5.25 ± 0.60) |
| 0.75 |
−2.28 ± 0.02 |
−2.16 ± 0.02 |
−2.00 ± 0.01 |
−1.87 ± 0.02 |
−1.73 ± 0.02 |
−1.58 ± 0.02 |
−1.47 ± 0.02 |
−1.40 ± 0.01 |
|
|
(6.44 ± 1.12) |
(6.33 ± 0.81) |
(5.86 ± 0.44) |
(6.13 ± 1.23) |
(6.46 ± 1.04) |
(7.82 ± 1.12) |
(6.15 ± 1.23) |
(6.94 ± 0.58) |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate |
| 0.1 |
−2.19 ± 0.02 |
−2.04 ± 0.02 |
−1.94 ± 0.02 |
−1.77 ± 0.02 |
−1.69 ± 0.01 |
−1.47 ± 0.02 |
−1.43 ± 0.01 |
−1.33 ± 0.02 |
|
|
(5.49 ± 0.67) |
(4.35 ± 0.71) |
(5.21 ± 0.96) |
(5.62 ± 0.74) |
(5.27 ± 0.59) |
(4.55 ± 0.66) |
(5.82 ± 0.61) |
(5.91 ± 0.80) |
| 0.25 |
−2.05 ± 0.02 |
−1.90 ± 0.02 |
−1.77 ± 0.01 |
−1.62 ± 0.02 |
−1.49 ± 0.02 |
−1.31 ± 0.02 |
−1.27 ± 0.02 |
−1.15 ± 0.01 |
|
|
(5.71 ± 0.68) |
(6.24 ± 1.06) |
(4.68 ± 0.56) |
(6.34 ± 0.87) |
(4.84 ± 0.91) |
(6.43 ± 0.91) |
(6.40 ± 0.87) |
(4.43 ± 0.62) |
| 0.50 |
−1.90 ± 0.01 |
−1.77 ± 0.01 |
−1.60 ± 0.02 |
−1.46 ± 0.01 |
−1.35 ± 0.01 |
−1.23 ± 0.02 |
−1.08 ± 0.01 |
−1.03 ± 0.01 |
|
|
(4.97 ± 0.71) |
(6.04 ± 0.54) |
(6.09 ± 0.83) |
(6.47 ± 0.56) |
(5.19 ± 0.66) |
(3.18 ± 1.05) |
(1.61 ± 0.43) |
(1.04 ± 0.16) |
| 0.75 |
−1.76 ± 0.02 |
−1.65 ± 0.01 |
−1.49 ± 0.01 |
−1.36 ± 0.01 |
−1.23 ± 0.02 |
−1.07 ± 0.01 |
−1.01 ± 0.01 |
−1.00 ± 0.01 |
|
|
(5.66 ± 0.89) |
(5.70 ± 0.67) |
(6.84 ± 0.68) |
(5.43 ± 0.60) |
(4.89 ± 0.81) |
(0.59 ± 0.12) |
(0.15 ± 0.01) |
(0.45 ± 0.22) |
3.2.4. Partial molar isentropic compression of transfer, ΔtrKs,2,ϕ0.
The partial molar isentropic compression of transfer values (ΔtrKs,2,ϕ0) were calculated using the following equation:| | | ΔtrKs,2,ϕ0 = Ks,2,ϕ0 (in aqueous solutions of DST/TSC) − Ks,2,ϕ0 (in water). | (13) |
The ΔtrKs,2,ϕ0 values are positive for all solutes; this suggests the predominance of hydrophilic–ionic/hydrophilic interactions and the strengthening of these interactions over the entire studied concentration range. The magnitudes decrease with an increase in temperature, as shown in Fig. 4(b, e, h and k). Due to interactions between the hydrophilic sites of the amino acids and the citrate and tartrate ions of the co-solutes, the less compressible water molecules in the hydration shells of the solute molecules come out in the bulk water, hence exhibiting positive ΔtrKs,2,ϕ0 values. Higher ΔtrKs,2,ϕ0 values are observed for the studied solutes in the case of TSC compared to DST.
3.3. Viscosity measurements
Viscosities have been determined for ASP and GLU in water and in aqueous solutions of DST and TSC, over an mB value range from 0.1–0.75 mol kg−1 and from T = 288.15 to 318.15 K, from flow time measurements using the following expression:where ρ is the density of the solution, t is the flow time, and A and B are viscometric constants (A = 0.0000669 mPa m3 kg−1 and B = 0.2244 mPa m3 s2 kg−1). The η values of TSC and GLU in water at 298.15 K matched well with the literature data (Fig. S1, ESI†).22,28 The η values increase with an increase in the concentration of amino acid in water and in aqueous solutions of DST/TSC, and decrease with temperature (Table 6). The variation in η values for solutions of GLU in aqueous DST at different temperatures are shown in Fig. 3(d) (viscosity, η, versus molality, mA, for GLU in DST at mB = 0.1 mol kg−1 as a function of temperature).
Table 6 Viscosities, η, of L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate at T values from 288.15 to 318.15 K and P = 101.3 kPa
|
η/mPa s |
|
m
A
|
T/K |
T/K |
T/K |
T/K |
| mol kg−1 |
288.15 |
298.15 |
308.15 |
318.15 |
|
m
A is the molality of the solute in water or water + DST/TSC (solvent). mB is the molality of DST/TSC in water. Standard uncertainties are u(T) = 0.03 K, u(P) = 0.5 kPa, u(m) = 2.8 × 10−4 mol kg−1 and u(η) = 0.012 mPa s. |
|
L-Aspartic acid in water |
|
|
η
0 = 1.1382 mPa s |
η
0 = 0.8904 mPa s |
η
0 = 0.7194 mPa s |
η
0 = 0.5963 mPa s |
| 0.00517 |
1.1390 |
0.8910 |
0.7197 |
0.5967 |
| 0.00712 |
1.1392 |
0.8912 |
0.7200 |
0.5968 |
| 0.00921 |
1.1395 |
0.8915 |
0.7202 |
0.5970 |
| 0.01109 |
1.1397 |
0.8917 |
0.7205 |
0.5972 |
| 0.01300 |
1.1400 |
0.8919 |
0.7206 |
0.5973 |
| 0.01506 |
1.1404 |
0.8921 |
0.7208 |
0.5975 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.10 mol kg−1 |
|
|
η
0 = 1.1631 mPa s |
η
0 = 0.9518 mPa s |
η
0 = 0.7783 mPa s |
η
0 = 0.6644 mPa s |
| 0.00502 |
1.1637 |
0.9525 |
0.7787 |
0.6647 |
| 0.00741 |
1.1640 |
0.9528 |
0.7790 |
0.6650 |
| 0.01180 |
1.1643 |
0.9529 |
0.7792 |
0.6651 |
| 0.01306 |
1.1646 |
0.9531 |
0.7794 |
0.6653 |
| 0.01732 |
1.1651 |
0.9534 |
0.7797 |
0.6655 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.25 mol kg−1 |
|
|
η
0 = 1.2425 mPa s |
η
0 = 1.0248 mPa s |
η
0 = 0.8544 mPa s |
η
0 = 0.7244 mPa s |
| 0.00584 |
1.2434 |
1.0255 |
0.8550 |
0.7247 |
| 0.00817 |
1.2436 |
1.0257 |
0.8553 |
0.7250 |
| 0.01106 |
1.2439 |
1.0260 |
0.8555 |
0.7253 |
| 0.01337 |
1.2442 |
1.0264 |
0.8556 |
0.7255 |
| 0.01555 |
1.2446 |
1.0266 |
0.8559 |
0.7256 |
| 0.01667 |
1.2451 |
1.0269 |
0.8561 |
0.7258 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.5 mol kg−1 |
|
|
η
0 = 1.4178 mPa s |
η
0 = 1.1996 mPa s |
η
0 = 0.9924 mPa s |
η
0 = 0.8446 mPa s |
| 0.00638 |
1.4186 |
1.2005 |
0.9931 |
0.8448 |
| 0.00782 |
1.4188 |
1.2008 |
0.9933 |
0.8453 |
| 0.00982 |
1.4193 |
1.2011 |
0.9936 |
0.8456 |
| 0.01307 |
1.4199 |
1.2013 |
0.9939 |
0.8460 |
| 0.01509 |
1.4203 |
1.2017 |
0.9942 |
0.8462 |
| 0.01639 |
1.4209 |
1.2021 |
0.9944 |
0.8465 |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate at mB= 0.75 mol kg−1 |
|
|
η
0 = 1.6087 mPa s |
η
0 = 1.3980 mPa s |
η
0 = 1.1562 mPa s |
η
0 = 0.9866 mPa s |
| 0.00547 |
1.6099 |
1.3990 |
1.1570 |
0.9873 |
| 0.00739 |
1.6102 |
1.3992 |
1.1573 |
0.9875 |
| 0.01025 |
1.6107 |
1.3999 |
1.1577 |
0.9878 |
| 0.01347 |
1.6111 |
1.4001 |
1.1580 |
0.9881 |
| 0.01664 |
1.6115 |
1.4005 |
1.1583 |
0.9885 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.1 mol kg−1 |
|
|
η
0 = 1.2165 mPa s |
η
0 = 0.9856 mPa s |
η
0 = 0.8023 mPa s |
η
0 = 0.6650 mPa s |
| 0.00576 |
1.2170 |
0.9862 |
0.8029 |
0.6655 |
| 0.00754 |
1.2172 |
0.9865 |
0.8031 |
0.6656 |
| 0.01002 |
1.2175 |
0.9868 |
0.8033 |
0.6658 |
| 0.01189 |
1.2178 |
0.9871 |
0.8035 |
0.6660 |
| 0.0152 |
1.2180 |
0.9874 |
0.8037 |
0.6662 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.25 mol kg−1 |
|
|
η
0 = 1.4206 mPa s |
η
0 = 1.1424 mPa s |
η
0 = 0.9460 mPa s |
η
0 = 0.7902 mPa s |
| 0.00624 |
1.4217 |
1.1431 |
0.9467 |
0.7906 |
| 0.00784 |
1.4220 |
1.1434 |
0.9469 |
0.7908 |
| 0.01080 |
1.4224 |
1.1438 |
0.9473 |
0.7912 |
| 0.01208 |
1.4228 |
1.1442 |
0.9475 |
0.7914 |
| 0.01490 |
1.4231 |
1.1445 |
0.9478 |
0.7917 |
| 0.01825 |
1.4234 |
1.1448 |
0.9480 |
0.7921 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.5 mol kg−1 |
|
|
η
0 = 1.7327 mPa s |
η
0 = 1.4619 mPa s |
η
0 = 1.2096 mPa s |
η
0 = 1.0194 mPa s |
| 0.00597 |
1.7340 |
1.4631 |
1.2103 |
1.0199 |
| 0.00809 |
1.7344 |
1.4634 |
1.2107 |
1.0204 |
| 0.00985 |
1.7347 |
1.4638 |
1.2111 |
1.0207 |
| 0.01284 |
1.7352 |
1.4640 |
1.2114 |
1.0209 |
| 0.01520 |
1.7356 |
1.4644 |
1.2118 |
1.0212 |
| 0.01810 |
1.7361 |
1.4649 |
1.2120 |
1.0215 |
|
L-Aspartic acid in an aqueous solution of tri-sodium citrate at mB= 0.75 mol kg−1 |
|
|
η
0 = 1.9928 mPa s |
η
0 = 1.7555 mPa s |
η
0 = 1.4983 mPa s |
η
0 = 1.2697 mPa s |
| 0.00626 |
1.9941 |
1.7569 |
1.4992 |
1.2703 |
| 0.00788 |
1.9946 |
1.7574 |
1.4999 |
1.2709 |
| 0.01082 |
1.9952 |
1.7578 |
1.5003 |
1.2712 |
| 0.01180 |
1.9958 |
1.7582 |
1.5007 |
1.2717 |
| 0.01461 |
1.9964 |
1.7587 |
1.5011 |
1.2720 |
|
L-Glutamic acid in water |
| 0.01104 |
1.1404 |
0.8921 |
0.7205 |
0.5974 |
| 0.01308 |
1.1407 |
0.8923 |
0.7209 |
0.5976 |
| 0.01522 |
1.1411 |
0.8927 |
0.7214 |
0.5978 |
| 0.02078 |
1.1417 |
0.8932 |
0.7218 |
0.5982 |
| 0.02517 |
1.1422 |
0.8937 |
0.7221 |
0.5986 |
| 0.03030 |
1.1429 |
0.8942 |
0.7225 |
0.5990 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.1 mol kg−1 |
| 0.01288 |
1.1646 |
0.9525 |
0.7789 |
0.6650 |
| 0.01523 |
1.1653 |
0.9534 |
0.7797 |
0.6655 |
| 0.01930 |
1.1661 |
0.9543 |
0.7803 |
0.6661 |
| 0.02496 |
1.1669 |
0.9549 |
0.7810 |
0.6666 |
| 0.03127 |
1.1676 |
0.9556 |
0.7815 |
0.6670 |
| 0.03591 |
1.1681 |
0.9564 |
0.7820 |
0.6675 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.25 mol kg−1 |
| 0.01410 |
1.2443 |
1.0262 |
0.8556 |
0.7250 |
| 0.01537 |
1.2450 |
1.0268 |
0.8563 |
0.7257 |
| 0.01930 |
1.2459 |
1.0275 |
0.8567 |
0.7262 |
| 0.02391 |
1.2464 |
1.0281 |
0.8571 |
0.7268 |
| 0.03000 |
1.2471 |
1.0287 |
0.8577 |
0.7273 |
| 0.03235 |
1.2478 |
1.0296 |
0.8583 |
0.7278 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.5 mol kg−1 |
| 0.01228 |
1.4199 |
1.2015 |
0.9935 |
0.8452 |
| 0.01354 |
1.4209 |
1.2023 |
0.9944 |
0.8462 |
| 0.01654 |
1.4217 |
1.2029 |
0.9952 |
0.8469 |
| 0.02018 |
1.4223 |
1.2034 |
0.9958 |
0.8475 |
| 0.02585 |
1.4230 |
1.2043 |
0.9963 |
0.8481 |
| 0.03703 |
1.4238 |
1.2052 |
0.9971 |
0.8488 |
|
L-Glutamic acid in an aqueous solution of di-sodium tartrate at mB= 0.75 mol kg−1 |
| 0.01159 |
1.6114 |
1.4004 |
1.1582 |
0.9877 |
| 0.01375 |
1.6125 |
1.4011 |
1.1588 |
0.9886 |
| 0.01765 |
1.6131 |
1.4017 |
1.1592 |
0.9895 |
| 0.01995 |
1.6139 |
1.4027 |
1.1602 |
0.9904 |
| 0.02676 |
1.6149 |
1.4036 |
1.1608 |
0.9908 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.1 mol kg−1 |
| 0.01247 |
1.2182 |
0.9874 |
0.8036 |
0.6660 |
| 0.01463 |
1.2188 |
0.9878 |
0.8039 |
0.6663 |
| 0.01634 |
1.2193 |
0.9882 |
0.8043 |
0.6667 |
| 0.02218 |
1.2198 |
0.9889 |
0.8050 |
0.6671 |
| 0.02857 |
1.2205 |
0.9893 |
0.8055 |
0.6676 |
| 0.03390 |
1.2212 |
0.9899 |
0.8060 |
0.6682 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.25 mol kg−1 |
| 0.01213 |
1.4232 |
1.1442 |
0.9474 |
0.7914 |
| 0.01438 |
1.4237 |
1.1446 |
0.9479 |
0.7919 |
| 0.01771 |
1.4243 |
1.1457 |
0.9484 |
0.7924 |
| 0.02308 |
1.4250 |
1.1462 |
0.9490 |
0.7929 |
| 0.02531 |
1.4257 |
1.1467 |
0.9497 |
0.7934 |
| 0.03401 |
1.4271 |
1.1475 |
0.9507 |
0.7938 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.5 mol kg−1 |
| 0.01104 |
1.7359 |
1.4646 |
1.2114 |
1.0211 |
| 0.01239 |
1.7362 |
1.4652 |
1.2122 |
1.0217 |
| 0.01547 |
1.7370 |
1.4659 |
1.2129 |
1.0223 |
| 0.02012 |
1.7381 |
1.4670 |
1.2138 |
1.0227 |
| 0.02521 |
1.7392 |
1.4677 |
1.2144 |
1.0239 |
| 0.03006 |
1.7410 |
1.4686 |
1.2154 |
1.0246 |
|
L-Glutamic acid in an aqueous solution of tri-sodium citrate at mB= 0.75 mol kg−1 |
| 0.01088 |
1.9955 |
1.7581 |
1.5003 |
1.2716 |
| 0.01408 |
1.9973 |
1.7596 |
1.5010 |
1.2726 |
| 0.01532 |
1.9978 |
1.7604 |
1.5017 |
1.2735 |
| 0.02000 |
1.9991 |
1.7614 |
1.5034 |
1.2740 |
| 0.02799 |
2.0009 |
1.7626 |
1.5050 |
1.2752 |
| 0.03107 |
2.0019 |
1.7636 |
1.5057 |
1.2766 |
3.3.1. Viscosity B-coefficient.
The relative viscosities are given bywhere, η and η0 are the viscosities of the solution and solvent, respectively. The B-coefficients were evaluated by fitting the ηr values to the Jones–Dole equation43via a least squares method as follows:where C is the molar concentration (calculated from the molality and density data). The viscosity B-coefficient or B value provides knowledge about the solvation behaviour of solutes. The B values of ASP and GLU in water at 298.15 K are in line with the literature values.14 The B values for the studied amino acids in water and in aqueous solutions of DST and TSC increase with temperature and with the molality of the co-solutes and they are given in Table S7.† The increase is greater in the case of TSC compared to DST. As the B-coefficient is directly dependent upon the size of the solute, the B value of GLU is slightly greater compared to ASP. Moreover, due to the greater ionic strength of TSC, the observed B values are larger compared to DST. The increase in the B values with the DST and TSC solution concentrations indicates a progressively more structured environment.
The derivative of the B-coefficient with respect to T (dB/dT) is a far better parameter for investigating the effects of additives on the structure of water, because it provides better information about the structure-making or structure-breaking behavior of the solute compared with the B-coefficient. A positive value of dB/dT indicates that a solute is a structure-breaker, whereas a negative value indicates that a solute is a structure-maker.44 The dB/dT values for ASP and GLU in water and in aqueous solutions of DST and TSC at different temperatures are given in Table S7.† The dB/dT values for ASP and GLU are positive in water and in aqueous DST and TSC solutions, which classifies these solutes as structure-breakers due to the dominating effects of the hydrophilic groups (–NH2 and –COOH) over the R-groups.
3.3.2. Viscosity B-coefficient of transfer, ΔtrB.
The viscosity B-coefficients of transfer, ΔtrB, from water to aqueous DST and TSC solutions have been calculated as follows:| | | ΔtrB = B-coefficient (in aqueous DST/TSC solution)-B- coefficient (in water). | (17) |
The B-coefficient values for the ASP and GLU amino acids in aqueous solutions of DST and TSC are higher than the values in water, which results in positive ΔtrB values. Plots of the ΔtrB values as a function of the DST and TSC molality are shown in Fig. 4(c, f, i, l). The transfer values increase with an increase in the concentration of DST/TSC. For both solutes, the ΔtrB values decrease with temperature in both aqueous DST and TSC solutions. The ΔtrB values have greater magnitudes in the case of TSC, which may again be attributed to the high charge density.
3.3.3. Ratio of the B-coefficient to the partial molar volume, B/V2,ϕ0.
The solvation can be judged by the ratio of the B-coefficient to the partial molar volume (B/V2,ϕ0). Unsolvated spherical species have B/V2,ϕ0 values ranging between 0 and 2.5.14 Solvated spherical species have B/V2,ϕ0 values higher than 2.5. In the present study, the lesser magnitudes of the B/V2,ϕ0 values of the studied amino acids in the presence of the co-solutes compared to in water indicate that these amino acids are more solvated in water (Table S7†).
3.4. Enthalpy measurements from calorimetry
The q values for ASP/GLU (30 mmol kg−1) in the absence, as well as in the presence, of aqueous solutions of DST and TSC over an mB range from 100–500 mmol kg−1 at T = 298.15 K have been measured using ITC. Positive q values for the solutes have been observed in water and in the presence of TSC/DST, whereas in some cases involving DST, negative q values have also been observed (Table 7). These values decrease with the molality of the solutes in water and increase in the presence of the co-solutes. The ΔdilH0 values (Table S8†) have been calculated by fitting the q and mA data to the following equations:| | | q = ΔdilH0 + mASw1 + mA2Sw2 + ... | (19) |
where Sw is the slope obtained via linear regression. In some cases, ΔdilH0 values have been obtained using high order polynomial fitting with the constants Sw1, Sw2, etc. Positive ΔdilH0 values, except for ASP in 100 mM DST and GLU in 100 and 250 mM DST, are observed for the studied amino acids in aqueous media and in the presence of the co-solutes. The positive ΔdilH0 values indicate the dominance of dehydration effects over hydrophilic–ionic/hydrophilic interactions. These dehydration effects encourage their use to prevent against microbial activity. Negative ΔdilH0 values indicate the predominance of hydrophilic–ionic/hydrophilic interactions. The magnitude of endothermicity for ASP/GLU is greater in the case of TSC than in the case of DST, which is in accordance with the greater dehydration effects of TSC compared to DST. This shows that hydrophilic–ionic/hydrophilic interactions are more predominant in the case of DST.
Table 7 Enthalpy of dilution values, q, of aqueous solutions of L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate at a T value of 298.15 K and P = 101.3 kPa
|
m
A/mmol kg−1 |
q/J mol−1 |
|
m
B = 0 mmol kg−1 |
m
B = 100 mmol kg−1 |
m
B = 250 mmol kg−1 |
m
B = 500 mmol kg−1 |
m
B = 100 mmol kg−1 |
m
B = 250 mmol kg−1 |
m
B = 500 mmol kg−1 |
| The standard uncertainties, u, are u(m) = 2.8·10−4 mol kg−1, u(q) = 0.5 kJ mol−1, u(T) = 0.03 K, and u(P) = 0.5 kPa. |
|
L-Aspartic acid in an aqueous solution of di-sodium tartrate |
L-Aspartic acid in an aqueous solution of tri-sodium citrate |
| 0.05897 |
2331.13 |
−445.97 |
1478.99 |
1412.85 |
2981.24 |
3639.50 |
5972.42 |
| 0.35207 |
1928.97 |
116.10 |
1553.81 |
1366.52 |
3057.06 |
3758.56 |
6016.78 |
| 0.64226 |
1120.55 |
607.86 |
1638.12 |
1309.33 |
3208.70 |
3843.82 |
6063.55 |
| 0.92955 |
707.04 |
961.64 |
1708.83 |
1264.60 |
3285.24 |
3932.01 |
6113.76 |
| 1.21394 |
538.85 |
1196.41 |
1772.46 |
1249.85 |
3345.96 |
3995.34 |
6156.42 |
| 1.49542 |
450.80 |
1354.93 |
1822.87 |
1227.90 |
3481.98 |
4032.45 |
6214.78 |
| 1.77400 |
393.72 |
1449.52 |
1871.82 |
1215.09 |
3571.76 |
4136.79 |
6251.32 |
| 2.04968 |
351.35 |
1514.27 |
1926.90 |
1244.20 |
3661.63 |
4165.05 |
6306.43 |
| 2.32246 |
317.49 |
1549.66 |
1986.88 |
1273.96 |
3736.21 |
4183.07 |
6347.57 |
| 2.59233 |
286.92 |
1587.42 |
2050.69 |
1335.26 |
3839.65 |
4259.48 |
6396.35 |
| 2.85929 |
263.99 |
1618.50 |
2123.13 |
1445.36 |
3905.29 |
4307.43 |
6452.41 |
| 3.12336 |
238.77 |
1655.39 |
2188.19 |
1518.47 |
3987.36 |
4361.31 |
6488.21 |
| 3.38452 |
228.07 |
1678.46 |
2299.31 |
1603.81 |
4084.03 |
4485.43 |
6537.78 |
| 3.64278 |
209.37 |
1677.83 |
2398.92 |
1708.21 |
4179.06 |
4562.62 |
6577.24 |
| 3.89813 |
198.41 |
1677.24 |
2456.84 |
1802.71 |
4256.64 |
4622.75 |
6627.50 |
| 4.15058 |
183.65 |
1697.28 |
2512.35 |
1915.64 |
4342.19 |
4726.03 |
6665.68 |
| 4.40013 |
168.35 |
1698.79 |
2561.23 |
2008.51 |
4432.38 |
4812.32 |
6705.08 |
| 4.64677 |
164.13 |
1698.79 |
2606.28 |
2090.75 |
4508.28 |
4868.91 |
6749.22 |
| 4.89051 |
154.10 |
1704.97 |
2703.05 |
2171.57 |
4565.64 |
4934.77 |
6798.33 |
|
L-Glutamic acid in an aqueous solution of Di-sodium tartrate |
L-Glutamic acid in an aqueous solution of tri-sodium citrate |
| 0.05897 |
397.36 |
−953.53 |
−868.11 |
345.92 |
1126.24 |
3303.28 |
4329.99 |
| 0.35207 |
339.42 |
−479.12 |
−816.65 |
423.73 |
1194.15 |
3343.50 |
4411.15 |
| 0.64226 |
274.18 |
−67.00 |
−772.47 |
469.41 |
1256.93 |
3391.24 |
4510.22 |
| 0.92955 |
251.38 |
145.23 |
−714.45 |
555.31 |
1314.97 |
3441.71 |
4593.43 |
| 1.21394 |
216.36 |
248.50 |
−662.62 |
644.28 |
1414.14 |
3491.56 |
4659.79 |
| 1.49542 |
179.98 |
306.75 |
−628.30 |
697.72 |
1465.35 |
3537.03 |
4727.20 |
| 1.77400 |
160.58 |
352.91 |
−592.38 |
753.07 |
1556.91 |
3589.88 |
4792.46 |
| 2.04968 |
141.98 |
394.64 |
−536.11 |
850.51 |
1635.86 |
3641.27 |
4837.82 |
| 2.32246 |
122.06 |
417.21 |
−502.91 |
902.46 |
1692.17 |
3696.22 |
4900.68 |
| 2.59233 |
105.23 |
431.29 |
−475.78 |
949.48 |
1768.96 |
3750.99 |
4945.44 |
| 2.85929 |
94.52 |
456.15 |
−437.47 |
999.15 |
1852.31 |
3807.83 |
5001.87 |
| 3.12336 |
72.25 |
487.66 |
−384.81 |
1045.31 |
1917.93 |
3849.69 |
5042.00 |
| 3.38452 |
58.34 |
490.60 |
−355.74 |
1094.73 |
1973.39 |
3908.89 |
5115.39 |
| 3.64278 |
42.74 |
493.88 |
−315.97 |
1190.22 |
2064.57 |
3953.95 |
5176.35 |
| 3.89813 |
36.68 |
478.74 |
−280.04 |
1237.70 |
2142.39 |
3998.64 |
5224.55 |
| 4.15058 |
28.84 |
499.50 |
−236.68 |
1275.55 |
2256.46 |
4032.62 |
5272.35 |
| 4.40013 |
19.63 |
501.58 |
−194.97 |
1326.44 |
2323.28 |
4079.62 |
5345.51 |
| 4.64677 |
10.21 |
504.97 |
−153.45 |
1382.78 |
2392.10 |
4130.85 |
5397.24 |
| 4.89051 |
4.70 |
504.22 |
−125.31 |
1448.78 |
2468.88 |
4180.52 |
5466.93 |
The ΔtrΔdilH0 values, i.e., the standard molar enthalpy of transfer, for the studied amino acids, from water to DST/TSC(aq.) are evaluated using the equation:
| | | ΔtrΔdilH0 = ΔdilH0 (in DST/TSC(aq.)) − ΔdilH0 (in water). | (20) |
Negative ΔtrΔdilH0 values for ASP in 100 mM TSC, ASP in DST and GLU in DST at all molalities, and positive ΔtrΔdilH0 values for ASP in 250 mM and 500 mM TSC and GLU in TSC at all molalities have been observed (Fig. S2, ESI†). The more positive magnitudes of the ΔtrΔdilH0 values in the case of GLU than ASP are due to the presence of an extra –CH2 (hydrophobic) group. The negative ΔtrΔdilH0 values suggest the dominance of hydrophilic-ionic/hydrophilic interactions and positive ΔtrΔdilH0 values may be due to the predominance of co-solute dehydration effects. Overall, the positive ΔtrΔdilH0 values in the case of TSC (greater dehydration effects) and the negative ΔtrΔdilH0 values in the case of DST coincide with the results obtained from the volumetric studies.
3.5. Analysis of NMR spectra
1H spectra have been recorded for ASP and GLU in the absence, as well as in the presence, of DST and TSC using a Bruker (AVANCE-III, HD 500 MHz) spectrometer at a probe temperature of 300.15 K. D2O was used for the deuterium lock and its signal at 4.650 ppm was taken as the internal reference for the other nuclei. The NMR spectra of ASP and GLU in water (mA = 0.01 and 0.02 mol kg−1) and in the presence of aqueous solutions of DST and TSC (mB = 0.75 mol kg−1) have been examined in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O–D2O solution. The changes in chemical shifts (δ) in ppm are summarized in Table 8.
1 H2O–D2O solution. The changes in chemical shifts (δ) in ppm are summarized in Table 8.
Table 8
1H data for L-aspartic acid and L-glutamic acid in water and in aqueous solutions of di-sodium tartrate and tri-sodium citrate
| T = triplet, Q = quartet, M = multiplet. |
|
In the 1H NMR spectra of ASP and GLU in aqueous solutions of DST and TSC (mB = 0.75 mol kg−1), the protons go upfield compared to in the D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O system (Fig. S3†). This shows that the hydrogen bonding of the solutes with water molecules decreases in the presence of DST/TSC owing to the dominance of dehydration effects over hydrophilic–ionic/hydrophilic interactions.
H2O system (Fig. S3†). This shows that the hydrogen bonding of the solutes with water molecules decreases in the presence of DST/TSC owing to the dominance of dehydration effects over hydrophilic–ionic/hydrophilic interactions.
4. Conclusions
Volumetric, acoustic, viscometric, calorimetric and NMR spectroscopic studies have been performed in the present study. The values of ΔtrV2,ϕ0, ΔtrK2,ϕ0 and ΔtrB suggest that hydrophilic–ionic/hydrophilic interactions dominate in the present study. The sweet tastes of both amino acids decrease in the presence of the studied preservatives. The values of the interaction parameter predict pairwise interactions between the amino acids and preservatives. Volumetric and viscometric results indicate that the amino acids behave as structure-breakers in both salt solutions, except in the case of GLU in TSC at mB = 0.1 mol kg−1. The hydration number and B/V2,ϕ0 values suggest that these amino acids are more solvated in water. Calorimetric and spectroscopic results support the fact that these preservatives stimulate the effects of dehydration on the studied amino acids.
Abbreviations
| GLU |
L-Glutamic acid |
| ASP |
L-Aspartic acid |
| DST | Disodium tartrate |
| TSC | Trisodium citrate |
| DSA | Density and sound velocity meter |
| ITC | Isothermal titration calorimetry |
| NMR | Nuclear magnetic resonance |
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
One of the authors (Aashima Beri) is grateful to the Department of Science and Technology, New Delhi for the award of an Inspire Fellowship. The authors also acknowledge the DST-PURSE scheme of the Department of Science and Technology, New Delhi and the UPE-Scheme of the University Grants Commission, New Delhi for research facilities.
References
- K. Jeevaratnam, M. Jamuna and A. S. Bawa, Biological preservation of foods-Bacteriocins of lactic acid bacteria, Indian J. Biotechnol., 2005, 4, 446–454 CAS.
-
W. C. Friezer, Contamination, Spoilage and Preservation of Yoghurt, AVI Publishers, U.S.A., 1989, pp. 13–29 Search PubMed.
-
L. Angiolillo, A. Conte and M. A. D. Nobile, Food Additives: Natural Preservatives. Reference Module in Food Science, in Encyclopedia of Food Safety, 2014, vol. 2, pp. 474–476 Search PubMed.
-
J. B. Gurtler and T. L. Mai, Preservatives | Traditional Preservatives-Organic Acids. Reference Module in Food Science, in Encyclopedia of Food Microbiology (2nd edn), 2014, pp. 119–130 Search PubMed.
- T. S. Banipal, H. Kaur, A. Kaur and P. K. Banipal, Effect of tartarate and citrate based food additives on the micellar properties of sodium dodecylsulfate for prospective use as food emulsifier, Food Chem., 2016, 190, 599–606 CrossRef CAS PubMed.
- J. S. L. Velez, A. R. Azzoni, P. De. Alcantara and P. Filho, Precipitation of lysozyme with sodium succinate, sodium tartrate and sodium citrate: solubility and osmotic second virial coefficient data, J. Chem. Thermodyn., 2017, 110, 25–32 CrossRef.
- S. A. A. Sajadi, Metal ion-binding properties of L-glutamic acid and L-aspartic acid, a comparative investigation, Nat. Sci., 2010, 2, 85–90 CAS.
- P. M. Masters and M. Friedman, Racemization of Amino Acids in Alkali-Treated Food Proteins, J. Agric. Food Chem., 1979, 27, 507–511 CrossRef CAS PubMed.
- Y. S. Nahum, A. Apelbla and E. Manzurola, Volumetric Properties of Aqueous Solutions of L-Glutamic Acid and Magnesium-L-Glutamate, J. Solution Chem., 2008, 37, 391–401 CrossRef.
- K. D. Umaley, G. B. Pethe and A. S. Aswar, Volumetric, Viscometric, Acoustical, and Optical Studies of Glutamic Acid in Aqueous Zinc and Copper Chloride Solutions, Russ. J. Phys. Chem. B, 2013, 7, 11–22 CrossRef CAS.
- M. Contineanu, I. Contineanu, A. Neacsu and S. Perisanu, A DSC Study on Gamma Irradiated Isomers of the Aspartic Acid, Rev. Chim. (Bucharest), 2010, 61, 774–777 CAS.
- A. K. Nain, R. Pal and P. Droliya, Study of (solute+solute) and (solute+solvent) interactions of homologous series of some α-amino acids in aqueous streptomycin sulphate solutions at different temperatures by using physicochemical methods, J. Chem. Thermodyn., 2016, 95, 77–98 CrossRef CAS.
- C. M. Romero and F. Negrete, Effect of Temperature on Partial Molar Volumes and Viscosities of Aqueous Solutions of α-Dl-Aminobutyric Acid, Dl-Norvaline and Dl-Norleucine, Phys. Chem. Liq., 2004, 42, 261–267 CrossRef CAS.
- H. Zhao, Viscosity B-coefficient and Standard Partial Molar Volume of Amino Acids and Their Role in Interpreting the Protein (Enzyme) Stabilization, Rev. Biophys. Chem., 2006, 122, 157–183 CrossRef CAS.
- J. Kestin, M. Sokolov and W. A. Wakeham, Viscosity of liquid water in the range −8 °C to 150 °C, J. Phys. Chem. Ref. Data, 1978, 7, 941–948 CrossRef CAS.
-
R. Sadeghi and F. Ziamajidi, Apparent Molar Volume and Isentropic Compressibility of Trisodium Citrate in Water and in Aqueous Solutions of Polyvinylpyrrolidone at T = (283.15 to 308.15) K, J. Chem. Eng. Data, 2007, 52, 1037–1044 Search PubMed.
- H. Kumar, Sheetal and S. K. Sharma, Volumetric and Acoustic Behavior of D ( + )-glucose and D (-)-fructose in Aqueous Trisodium Citrate Solutions at Different Temperatures, J. Solution Chem., 2016, 45, 1–27 CrossRef CAS.
- R. Sadeghi, R. Golabiazar and H. Shekaari, The Salting-Out Effect and Phase Separation in Aqueous Solutions of Tri-Sodium Citrate and 1-Butyl-3-Methylimidazolium Bromide, J. Chem. Thermodyn., 2010, 42, 441–453 CrossRef CAS.
- H. Kumar, K. Kaur, S. P. Kaur and M. Singla, Studies of Volumetric and Acoustic Properties of Trisodium Citrate and Tripotassium Citrate in Aqueous Solutions of N-Acetyl Glycine at Different Temperatures, J. Chem. Thermodyn., 2013, 59, 173–181 CrossRef CAS.
- H. Kumar, M. Singla and R. Jindal, Interactions of Glycine, L-Alanine and L-Valine with Aqueous Solutions of Trisodium Citrate at Different Temperatures: A Volumetric and Acoustic Approach, J. Chem. Thermodyn., 2013, 67, 170–180 CrossRef CAS.
- H. Kumar and C. Chadha, Densities, Sound Speed, and UV Absorption Studies of Trisodium Citrate and Tripotassium Citrate
in Aqueous Solutions of 1–Hexyl-3-methylimidazolium Chloride [C6mim][Cl], J. Chem. Eng. Data, 2014, 59, 4049–4061 CrossRef CAS.
- A. Salabat, L. Shamshiri and F. Sahrakar, Thermodynamic and transport properties of aqueous trisodium citrate system at 298.15 K, J. Mol. Liq., 2005, 118, 67–70 CrossRef CAS.
- M. T. Z. Moattar and S. Hosseinzadeh, Refractive Index, Viscosity, Density, and Speed of Sound of Aqueous Sodium Tartrate Solutions at Various Temperatures, J. Chem. Eng. Data, 2006, 51, 1190–1193 CrossRef.
- T. S. Banipal, K. Singh and P. K. Banipal, Volumetric Investigations on Interactions of Acidic/Basic Amino Acids with Sodium Acetate, Sodium Propionate and Sodium Butyrate in Aqueous Solutions, J. Solution Chem., 2007, 36, 1635–1667 CrossRef CAS.
- R. Gaba, A. Pal, D. Sharma, H. Kumar and A. Kumar, Molecular Interactions of Some Non-Essential Amino Acids in Aqueous Solutions of 1-Methylimidazolium Chloride at Different Temperatures, J. Mol. Liq., 2019, 279, 711–718 CrossRef CAS.
- M. A. Jamal, B. Naseem, M. K. Khosa, M. Muneer and J. H. Khan, Effect of Anionic Micellar Medium on Thermo Acoustical Parameters of Aspartic Acid and Serine Solutions, J. Mol. Liq., 2017, 237, 14–22 CrossRef CAS.
- M. A. Jamal, B. Naseem, J. H. Khan and I. Arif, Temperature Dependent Solution Properties of Amino Acids in Colloidal Solutions, J. Mol. Liq., 2019, 275, 105–115 CrossRef CAS.
- O. Iulian and A. Stefaniu, Volumetric and Viscometric Analyses for L-Glutamic Acid in NaCl Aqueous Solutions at Different Temperatures, J. Solution Chem., 2013, 42, 676–689 CrossRef CAS.
- Riyazuddeen and S. Afrin, Effect of NaCl and NaNO3 on the Partial Molar Volumes and Partial Molar Isentropic Compressibilities of Some Amino Acids at Several Different Temperatures (298.15–328.15) K, J. Solution Chem., 2012, 41, 1144–1155 CrossRef CAS.
- Riyazuddeen and T. Altamash, Study of Interactions of L-Histidine/L-Glutamic Acid/L-Tryptophan/Glycylglycine With KCl/KNO3 at Different Temperatures: 298.15, 303.15, 308.15, 313.15, 318.15, 323.15 K, Thermochim. Acta, 2010, 501, 72–77 CrossRef CAS.
- S. Shamil, G. G. Birch, M. Mathlouthi and M. N. Clifford, Apparent molar volumes and tastes of molecules with more than one sapophore, Chem. Senses, 1987, 12, 397–409 CrossRef CAS.
- M. B. Vranes, J. J. Panic, A. S. Tot and S. M. Papovic, The solvation properties and effect of D-fructose on taste behaviour of citrus aurantium active components in aqueous solutions, Food Funct., 2018, 9, 5569–5579 RSC.
-
R. W. Gurney, Ionic Processes in Solution, McGraw Hill, New York, vol. 3, 1953, ch. 1, pp. 1–20 Search PubMed.
- F. Shahidi and P. G. Farrell, Partial molar volumes of some α-aminocarboxylic acids in water, J. Chem. Soc., Faraday Trans., 1981, 77, 963–968 RSC.
- Z. Yan, J. Wang, K. W. Kong and J. Lu, Effect of temperature on volumetric and viscometric properties of some α-amino acids in aqueous calcium chloride solutions, Fluid Phase Equilib., 2004, 215, 143–150 CrossRef CAS.
- E. Berlin and M. J. Pallansch, Densities of several proteins and L-amino acids in the dry state, J. Phys. Chem., 1968, 72, 1887–1889 CrossRef CAS.
- C. K. Reddy, T. V. S. Sivapriya, U. A. Kumar and C. Ramalimgam, Optimization of Food Acidulant to Enhance the Organoleptic Property in Fruit Jellies, J. Food Process. Technol., 2016, 7 DOI:10.4172/2157-7110.1000635.
- T. Hutton, Sodium: Technological functions of salt in the manufacturing of food and drink products, Br. Food J., 2002, 104, 126–152 CrossRef.
-
T. P. Guinee and P. F. Fox, Salt in cheese: Physical, chemical and biological aspects, in Cheese: Chemistry, physics and microbiology, ed. P. F. Fox, P. L. H. McSweeney, T. M. Cogan and T. P. Guinee, 2004, Elsevier, Amsterdam, general aspects 3rd edn, vol. 1, pp. 207–259 Search PubMed.
- L. G. Hepler, Thermal Expansion and Structure in Water and Aqueous Solutions, Can. J. Chem., 1969, 47, 4613–4617 CrossRef CAS.
- W. G. McMillan and J. E. Mayor, The Statistical Thermodynamics of Multicomponent Systems, J. Chem. Phys., 1945, 13, 276–305 CrossRef CAS.
- M. Kikuchi, M. Sakurai and K. Nitta, Partial Molar Volumes and Isentropic Compressibilities of N-Acetyl Amino Acid Amides in Dilute Aqueous Solutions at (5, 15, 25, 35, and 45) °C, J. Chem. Eng. Data, 1996, 41, 1439–1445 CrossRef CAS.
- G. Jones and M. Dole, The Viscosity of Aqueous Solutions of Strong Electrolytes with Special Reference of Barium Chloride, J. Am. Chem. Soc., 1929, 51, 2950–2964 CrossRef CAS.
- T. S. Sarma and J. C. Ahluwalia, Experimental Studies on the Structure of Aqueous Solutions of Hydrophobic Solutes, Chem. Soc. Rev., 1973, 2, 203–232 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: DETAILS. See DOI: 10.1039/c9fo00872a |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) H2O–D2O solution. From these spectra, the chemical shift changes (Δδ) were recorded.
1 (w/w) H2O–D2O solution. From these spectra, the chemical shift changes (Δδ) were recorded.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O–D2O solution. The changes in chemical shifts (δ) in ppm are summarized in Table 8.
1 H2O–D2O solution. The changes in chemical shifts (δ) in ppm are summarized in Table 8.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O system (Fig. S3†). This shows that the hydrogen bonding of the solutes with water molecules decreases in the presence of DST/TSC owing to the dominance of dehydration effects over hydrophilic–ionic/hydrophilic interactions.
H2O system (Fig. S3†). This shows that the hydrogen bonding of the solutes with water molecules decreases in the presence of DST/TSC owing to the dominance of dehydration effects over hydrophilic–ionic/hydrophilic interactions.






