Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cu2(OH)PO4/reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of 2,4-dichlorophenol under infrared light irradiation†
Chenyang Zhangab,
Zhen Dub,
Ruyi Zhoub,
Peng Xud,
Xinghua Dongbc,
Yanyan Fu*f,
Qing Wange,
Chunjian Su*a,
Liang Yan *bc and
Zhanjun Gu
*bc and
Zhanjun Gu *bc
*bc
aCollege of Mechanical and Electronic Engineering, Shandong University of Science and Technology, Qingdao 266590, P. R. China. E-mail: suchunjian2008@163.com
bCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, P. R. China. E-mail: yanliang@ihep.ac.cn; zjgu@ihep.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 101408, P. R. China
dCAS Key Laboratory of Standardization and Measurement for Nanotechnology, National Center for Nanoscience and Technology, Beijing 100190, P. R. China
eSchool of Material Science and Engineering, Shandong University of Science and Technology, Qingdao 266590, P. R. China
fState Key Lab of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Changning Road 865, Shanghai 200050, P. R. China. E-mail: fuyy@mail.sim.ac.cn
First published on 17th January 2018
Abstract
Sparked by the growing environmental crises, photocatalytic degradation of chlorophenols with inexhaustible solar energy is expected to be converted into actual applications. Here, we report the preparation of the nanocomposite of Cu2(OH)PO4 and reduced graphene oxide (Cu2(OH)PO4/rGO) through a one-step hydrothermal method and examined its infrared-light photocatalytic activity in the degradation of 2,4-dichlorophenol (2,4-DCP). As evidenced by the absorption spectra and the degradation of 2,4-DCP, Cu2(OH)PO4/rGO exhibited enhanced infrared light-driven photocatalytic activity compared to pure Cu2(OH)PO4 and was very stable even after repeated cycling. More importantly, the introduction of hydrogen peroxide (H2O2) could combine the photocatalytic and photo-Fenton effects into one reaction system and maximize the infrared light photocatalytic efficiency. Typically, the rate constant of Cu2(OH)PO4/rGO and H2O2 was more than 6.25 times higher than that of only Cu2(OH)PO4/rGO, and almost 10 times greater than the value for pure Cu2(OH)PO4. Further, a plausible mechanism for the enhanced photocatalytic properties of Cu2(OH)PO4/rGO has been discussed. These findings may help the development of novel hybrid photocatalysts with enhanced infrared light photocatalytic activity for applications in the treatment of chlorophenol-contaminated wastewater.
Introduction
Pesticides are chemical substances widely used in horticulture, forestry and public health – and,1–4 of course, in agriculture where the unwanted pests that carry or transmit diseases can be repelled and killed.5 Over the past decades, tremendous problems, such as water contamination and overall ecological degradation, have been caused by the misuse and over-use of pesticides.6–8 For example, chlorophenols readily bio-accumulate in the human body, and subsequently cause disturbances in the structure of cellular bilayer phospholipids, finally causing carcinogenic effects.9 Therefore, a large number of methods have been developed to remove chlorophenols from water, including adsorption,10 biological degradation11 and electrochemical degradation.12 However, adsorption merely concentrates chlorophenols, but does not degrade them into less toxic compounds. Biological treatment suffers from the drawbacks of slow reaction rate and the need for strict control of suitable pH and temperature. For these reasons, an effective technique needs to be proposed for the removal of chlorophenols from different water systems.Alternatively, semiconductor nanomaterials have been emerging as efficient photocatalysts for the degradation of chlorophenols, such as TiO2 in the ultraviolet range (<400 nm)13 and Ag3PO4 in the visible range (400–800 nm).14 For the optimized use of solar energy, efficient and stable photocatalysts that are capable of harvesting infrared light, which accounts for ca. 50% of solar energy, are required. Much effort has been under way so far to tentatively seek the efficient photocatalysts, including Bi2WO6 (ref. 15) and WS2,16 for the degradation of organic pollutants, but not chlorophenols, under infrared irradiation. The limitation of photocatalysts for pesticide photocatalytic degradation under infrared light is essentially due to the insufficient photocatalytic activity that results from charge-carrier recombination as well as the low-photon energy of infrared light, and the inhibition of charge transfer because of the mismatched band energy alignment between each other. To overcome the above limitation, graphitic carbon nitride coupled with upconversion nanoparticles can extend the activity towards the infrared region for the photodegradation of chlorophenols.17 However, the efficiency of this photocatalyst is rather low due to the narrow absorption band of light at 980 nm. Therefore, infrared light responsive photocatalysts for the degradation of chlorophenols are still being actively pursued.
Herein, we synthesized the infrared-light active nanocomposites composing of copper hydroxide phosphate (Cu2(OH)PO4) and reduced graphene oxide (rGO) by a one-step hydrothermal method (Cu2(OH)PO4/rGO) (Scheme 1). Cu2(OH)PO4, which consists of CuO4(OH)2 octahedron and CuO4(OH) trigonal bipyramid, has been considered as a promising photocatalyst for the degradation of organic pollutants under visible light.18,19 With the presence of the distorted polyhedrons in the crystal structure, Cu2(OH)PO4 is even responsive to infrared light and hence displays photocatalytic activity in the infrared range.20 In previous studies, it has been reported that the generated electrons at CuO4(OH) trigonal bipyramids under infrared light irradiation (forming CuIII sites) can be transferred to the neighboring CuO4(OH)2 octahedra (forming CuI sites).20,21 Subsequently, the produced CuIII sites are responsible for oxidizing chlorophenols (Fig. 1, route 1). Nevertheless, Cu2(OH)PO4, as an infrared-activated photocatalyst, suffers from the fast recombination of photogenerated electron–hole pairs. To overcome this limitation, graphene with the high-surface area and electrical conductivity should act as an avenue for driving photogenerated carriers away from the surface of Cu2(OH)PO4,22,23 facilitating more efficient generation of CuIII sites which are applied to degrade chlorophenols and, as a result, become CuII sites (Fig. 1, route 2). Moreover, to fully use the advantage of this photocatalyst, we use hydrogen peroxide (H2O2) as an electron acceptor to react irreversibly with the produced CuI sites (that is, photo-Fenton reaction, similar to CuI-induced Fenton reaction24) to further enhance the separation efficiency of photogenerated electron–hole pairs; while the produced CuI can effectively promote the generation of highly active hydroxyl radicals (HO˙) which are capable of oxidizing chlorophenols, and return to CuII sites. More importantly, these two processes can complete the full photocatalytic circle and be occurred repeatedly, resulting in the combination of the photocatalytic and photo-Fenton effects in one reaction system and finally maximizing the photocatalytic activity of Cu2(OH)PO4/rGO for the mineralization of chlorophenols to CO2 and H2O. As expected, our results show that, compared to pure Cu2(OH)PO4, the as-prepared Cu2(OH)PO4/rGO exhibits remarkably enhanced photocatalytic activity for the degradation of 2,4-dichlorophenol (2,4-DCP, a typical type of chlorophenols) under infrared light irradiation (>800 nm). Typically, the photocatalytic rate constant of Cu2(OH)PO4/rGO and H2O2 is almost 10 times higher than that of only Cu2(OH)PO4 under the same condition. In addition, there is no appreciable loss of photocatalytic activity after repeated cycles, and the morphology and structure of Cu2(OH)PO4/rGO remain nearly unchanged. Finally, mechanism of enhanced photocatalysis under infrared light is further proposed and discussed in detailed.
 | ||
| Fig. 1 Schematic diagram for the photocatalytic mechanism of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO under infrared light irradiation. | ||
Experimental
Materials and chemicals
Graphite (99%) and 2,4-dichlorophenol (2,4-DCP, 97%) were purchased from Alfa Aesar. Chemical reagents including disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O, 98%), copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 99%) and sodium hydroxide (NaOH, 96%) were obtained from Aladdin Chemical Co. Potassium permanganate (KMnO4, 99%), hydrogen peroxide (H2O2, 30 wt%) and concentrated sulfuric acid (H2SO4, 98 wt%) were obtained from Beijing Chemical Co. Terephthalic acid (TA, 99%) was purchased from Sigma-Aldrich. HUVECs (human umbilical vein endothelial cells) and cell counting kit-8 (CCK-8) were acquired from Wuhan Boster Biological Technology Ltd (Wuhan, China). Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS) and Penicillin–Streptomycin Solution were purchased from Gibco (Shanghai, china). All of the reagents were analytical grade and were used without further purification. In addition, deionized water was used in the whole experimental process.Synthesis of nanocomposites with different mass ratios of Cu2(OH)PO4 to graphene oxide
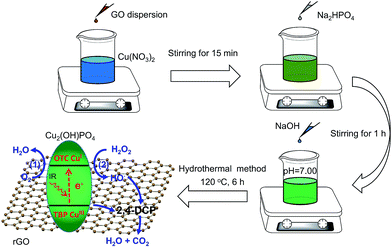
A series of nanocomposites of Cu2(OH)PO4 and rGO with various mass ratios of graphene oxide (GO) were synthesized by a simple one-step hydrothermal method (Cu2(OH)PO/rGO).20 Briefly, 4 mL of Cu(NO3)2 solution (1.0 M) and an appropriate amount of GO dispersion (2.0 mg mL−1, ESI†) were mixed into 20 mL of deionized water under constantly stirring for 15 min. Then, 2 mL of Na2HPO4 solution (1.0 M) was added to the above mixture. After stirring for another 1 h, the pH value of the obtained mixture was adjusted to ∼7.00 through gradually adding NaOH aqueous solution. The resulting suspension was then transferred into a 45 mL sealed teflon-scaled autoclave and kept at 120 °C for 6 h. After naturally cooling to room temperature, the products were collected by centrifuging, washed with deionized water several times, and finally dried overnight in a freezer dryer for further use. According to the mass ratios of Cu2(OH)PO4 and GO in the original mixtures, nanocomposites were labelled as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.001, 1
0.001, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.002, 1
0.002, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005, 1
0.005, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, 1
0.01, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02, 1
0.02, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 and 1
0.05 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, respectively.
0.1, respectively.
Characterization
Transmission electron microscopy (TEM) images were obtained on a JEM-2100 microscope at an acceleration voltage of 200 kV. Morphologies of samples were characterized using scanning electron microscopes (FE-SEM, S-4800, an acceleration voltage of 10 kV, Hitachi High-technologies, Japan) with an energy-dispersive X-ray (EDX) acceleration voltage analyzer. X-ray diffraction (XRD) patterns was obtained from a Bruker D8 Advance X-ray diffractometer (Bruker, USA) with Cu-Kα radiation (λ = 1.5406 Å) at a scanning rate of 10° min−1 and a scanning range from 10° to 90°. X-ray photoelectron spectroscopy (XPS) measurement was carried out with an ESCALab220i-XL spectrometer using a twin-anode Al-Kα X-ray source (1486.6 eV). Micro-Raman spectra were achieved from a Raman Spectroscope (Renishaw inVia plus, United Kingdom) under ambient conditions with 514 nm excitation from an argon ion laser. Ultraviolet-visible (UV-vis) data were acquired with a U-3900 spectrophotometer (Hitachi, Ltd., Japan). Ultraviolet-visible-infrared (UV-vis-IR) diffuse reflectance spectra were recorded at room temperature on an Agilent Cary 500 UV-vis-IR Spectrometer equipped with an integrating sphere using BaSO4 as a reference. The photoluminescence spectra were obtained using a Horiba Jobin Yvon FluoroLog3 spectrometer.Photocatalytic performance measurement
The photocatalytic activity of Cu2(OH)PO4/rGO was evaluated by the degradation of 2,4-DCP in the presence and absence of H2O2 under infrared light irradiation. In addition, the photocatalytic activity of Cu2(OH)PO4 and sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 to 2,4-DCP was explored under visible light irradiation. Typically, 60 mL mixture of sample 1
0.005 to 2,4-DCP was explored under visible light irradiation. Typically, 60 mL mixture of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 (60 mg) and 2,4-DCP (30 μg mL−1) was firstly stirred in the dark for 100 min to achieve an adsorption/desorption equilibrium between the photocatalyst and 2,4-DCP. Afterwards, an appropriate amount of H2O2 aqueous solution was added into the obtained mixture prior to photo-irradiation if necessary. Then, the mixture was irradiated by infrared light (>800 nm) using a 300 W xenon lamp installed an 800 nm cut-off filter, where the transmission spectrum of the cut-off filter was shown in Fig. S1.† Then, 1.5 mL of mixture was collected at varied irradiation time, centrifuged, and finally analyzed by a UV-3900 UV-vis spectrophotometer to determine the concentration of 2,4-DCP in the absence of H2O2 or by a Multi TOC Analyzer (2100, Analytik Jena AG Corporation) to detect total organic carbon (TOC) in the presence of H2O2. The photodegradation of 2,4-DCP by other Cu2(OH)PO/rGO were also performed under the similar condition. In addition, the stability of Cu2(OH)PO/rGO was studied by a separated photodegradation experiment which repeatedly re-employed the used samples for the next cycle under the identical conditions. After each photocatalytic process, Cu2(OH)PO/rGO was recovered by centrifugation, washed with deionized water, and then dried in the freezer dryer under vacuum for 24 h before until the subsequent reaction cycle.
0.005 (60 mg) and 2,4-DCP (30 μg mL−1) was firstly stirred in the dark for 100 min to achieve an adsorption/desorption equilibrium between the photocatalyst and 2,4-DCP. Afterwards, an appropriate amount of H2O2 aqueous solution was added into the obtained mixture prior to photo-irradiation if necessary. Then, the mixture was irradiated by infrared light (>800 nm) using a 300 W xenon lamp installed an 800 nm cut-off filter, where the transmission spectrum of the cut-off filter was shown in Fig. S1.† Then, 1.5 mL of mixture was collected at varied irradiation time, centrifuged, and finally analyzed by a UV-3900 UV-vis spectrophotometer to determine the concentration of 2,4-DCP in the absence of H2O2 or by a Multi TOC Analyzer (2100, Analytik Jena AG Corporation) to detect total organic carbon (TOC) in the presence of H2O2. The photodegradation of 2,4-DCP by other Cu2(OH)PO/rGO were also performed under the similar condition. In addition, the stability of Cu2(OH)PO/rGO was studied by a separated photodegradation experiment which repeatedly re-employed the used samples for the next cycle under the identical conditions. After each photocatalytic process, Cu2(OH)PO/rGO was recovered by centrifugation, washed with deionized water, and then dried in the freezer dryer under vacuum for 24 h before until the subsequent reaction cycle.
Detection of hydroxyl radical
The generation of HO˙ by nanocomposites under infrared light irradiation was evaluated using terephthalic acid (TA).25 The concentration of hydroxyl radical (HO˙) is determined via monitoring the fluorescence of 2-hydroxy terephthalic acid (TAOH, the maximum fluorescence peak at 435 nm) which is formed from the reaction of HO˙ with TA. Taking sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 as an example, five groups were obtained: group I (TA and infrared light); group II (TA, H2O2 and infrared light); group III (sample 1
0.005 as an example, five groups were obtained: group I (TA and infrared light); group II (TA, H2O2 and infrared light); group III (sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 and infrared light); group IV (TA, sample 1
0.005 and infrared light); group IV (TA, sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 and infrared light); group V (TA, H2O2, sample 1
0.005 and infrared light); group V (TA, H2O2, sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 and infrared light). The final working concentrations were 50 μg mL−1, 100 μM and 500 μM for sample 1
0.005 and infrared light). The final working concentrations were 50 μg mL−1, 100 μM and 500 μM for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005, H2O2 and TA, respectively. After infrared light irradiation, the changes at the 435 nm fluorescence emission peak were recorded.
0.005, H2O2 and TA, respectively. After infrared light irradiation, the changes at the 435 nm fluorescence emission peak were recorded.
Cytotoxicity assay for Cu2(OH)PO4/rGO nanocomposite
The cytotoxicity of Cu2(OH)PO4/rGO nanocomposites with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 ratio to HUVECs (human umbilical vein endothelial cells) was assessed by using the CCK-8 assay. HUVECs were placed in 96-well plates at a density of 8 × 103 cells per well, where HUVECs were maintained in the DMEM medium with 10% FBS, 1% penicillin/streptomycin for 24 h at 37 °C in 5% CO2. Then, the cells were incubated with different concentrations of sample 1
0.005 ratio to HUVECs (human umbilical vein endothelial cells) was assessed by using the CCK-8 assay. HUVECs were placed in 96-well plates at a density of 8 × 103 cells per well, where HUVECs were maintained in the DMEM medium with 10% FBS, 1% penicillin/streptomycin for 24 h at 37 °C in 5% CO2. Then, the cells were incubated with different concentrations of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 (5, 10, 20, 40, 60, 80, and 90 μg mL−1). After incubation for another 24 h, the cell medium was removed and replaced with 100 μL of fresh culture medium containing 10 μL of CCK-8 solution per well for detecting the cell viability. After 1 h of incubation, cell viability on HUVECs was assayed by measuring the absorbance at 450 nm using a microplate reader (Thermo Scientific, Multiscan MNK3).
0.005 (5, 10, 20, 40, 60, 80, and 90 μg mL−1). After incubation for another 24 h, the cell medium was removed and replaced with 100 μL of fresh culture medium containing 10 μL of CCK-8 solution per well for detecting the cell viability. After 1 h of incubation, cell viability on HUVECs was assayed by measuring the absorbance at 450 nm using a microplate reader (Thermo Scientific, Multiscan MNK3).
Results and discussion
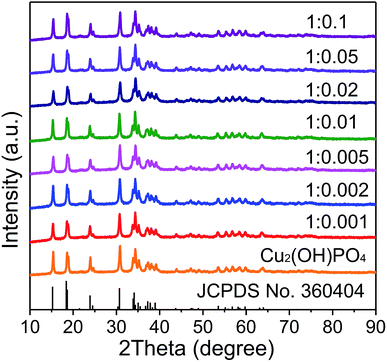
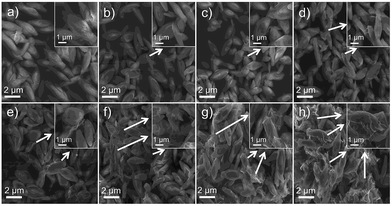
XRD patterns of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO with different mass ratios of Cu2(OH)PO4 and GO nanosheets with the thickness of ∼1.2 nm (Fig. S2†) are displayed and compared in Fig. 2. According to JCPDS card no. 360404, all the diffraction peaks are associated with the orthorhombic phase of Cu2(OH)PO4.26 Moreover, it is clear that the position of the diffraction peaks of Cu2(OH)PO4/rGO basically keep unchanged, which indicates that the crystalline structure of Cu2(OH)PO4 is not affected after the introduction of GO. Additionally, no stacking-related (002) diffraction peaks of graphene (at ∼26° for graphite and ∼13° for graphite oxide) are detected, suggesting that the dispersion of graphene is probably close to the single-sheet level in all the nanocomposites.27The morphology of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO were characterized by SEM and TEM measurement. As shown in Fig. 3, pure Cu2(OH)PO4 are ellipsoid-shaped with an average length of 3–4 μm and an aspect ratio of ∼3, and there are ravines on their surface that are short and narrow. In contrast, with the addition of GO, Cu2(OH)PO4 are tightly encapsulated by (rGO) nanosheets, which is consistent with the TEM images shown in Fig. S3.† This indicates the presence of strong van der Waals force between graphene and Cu2(OH)PO4.28 In addition, on increasing the mass ratio of GO, more Cu2(OH)PO4 is encapsulated by graphene, exhibiting more obviously crinkled and rough textures on the surface of Cu2(OH)PO4/rGO. Remarkably, graphene nanosheets not only are adsorbed onto the surface of Cu2(OH)PO4/rGO tightly, but also are connected or even overlapped between the adjacent microcrystals, building interconnected conductive pathways for electron transfer.
The structural and chemical information of Cu2(OH)PO4/rGO was further studied using Raman spectroscopy and XPS measurement. Raman spectra of GO, rGO, pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO are displayed in Fig. S4a.† From Raman spectra of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO, vibration peak at ∼972.5 cm−1 is the characteristics of Cu2(OH)PO4. Raman spectra of rGO exhibits two characteristic peaks corresponding to D band at around 1350.4 cm−1 (involving the disorder and defect) and G band at about 1599.6 cm−1 (involving first order scattering of the tangential stretching phonon mode), respectively.29,30 In comparison to rGO, Cu2(OH)PO4/rGO also shows the typical features of graphene with the presence of D band and G band, indicating the successful combination of Cu2(OH)PO4 with rGO. Interestingly, it can be obviously found from Fig. S4b† that, on progressively increasing the proportion of GO, there is significant red-shift in the G band; while the D band firstly blue-shifts to 1361.14 cm−1 from samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.001 to 1
0.001 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005, and then red-shifts to 1351.95 cm−1 from samples 1
0.005, and then red-shifts to 1351.95 cm−1 from samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 to 1
0.005 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. This indicates the presence of van der Waals interaction alone with charge transfer between rGO and Cu2(OH)PO4, and the interaction is the strongest for sample 1
0.1. This indicates the presence of van der Waals interaction alone with charge transfer between rGO and Cu2(OH)PO4, and the interaction is the strongest for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005.31 Moreover, the D/G intensity ratios of Cu2(OH)PO4/rGO are larger than that of GO (ID/IG = 0.806), which suggests a decrease in the average size of the sp2 domains upon reduction of GO, as well as an increase of edge planes and the degree of disorder.32 The full-scale XPS spectra of Cu2(OH)PO4/rGO shown in Fig. 4a and S5† reveal the presence of P, O, and Cu elements, which is consistent with the EDS results shown in Fig. S6.† For the XPS spectra of pure Cu2(OH)PO4, two main peaks are observed at about 936.61 and 955.86 eV, which can be attributed to Cu 2p3/2 and Cu 2p1/2 of copper ions, respectively (Fig. 4b).33 More importantly, the peak position of Cu 2p3/2 in the Cu2(OH)PO4/rGO firstly decreases from 935.76 eV to 935.46 eV as the mass ratio increases from 1
0.005.31 Moreover, the D/G intensity ratios of Cu2(OH)PO4/rGO are larger than that of GO (ID/IG = 0.806), which suggests a decrease in the average size of the sp2 domains upon reduction of GO, as well as an increase of edge planes and the degree of disorder.32 The full-scale XPS spectra of Cu2(OH)PO4/rGO shown in Fig. 4a and S5† reveal the presence of P, O, and Cu elements, which is consistent with the EDS results shown in Fig. S6.† For the XPS spectra of pure Cu2(OH)PO4, two main peaks are observed at about 936.61 and 955.86 eV, which can be attributed to Cu 2p3/2 and Cu 2p1/2 of copper ions, respectively (Fig. 4b).33 More importantly, the peak position of Cu 2p3/2 in the Cu2(OH)PO4/rGO firstly decreases from 935.76 eV to 935.46 eV as the mass ratio increases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.001 to 1
0.001 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005, and then increases to 936.56 eV on further increasing the proportion of GO. This may be due to the screening effect via charge transfer between rGO and pure Cu2(OH)PO4.34 With low mass ratio of GO, the electron of rGO can effectively transfer to pure Cu2(OH)PO4, causing the shift of both Cu 2p3/2 and Cu 2p1/2 towards lower binding energy; whereas, above the mass ratio of 1
0.005, and then increases to 936.56 eV on further increasing the proportion of GO. This may be due to the screening effect via charge transfer between rGO and pure Cu2(OH)PO4.34 With low mass ratio of GO, the electron of rGO can effectively transfer to pure Cu2(OH)PO4, causing the shift of both Cu 2p3/2 and Cu 2p1/2 towards lower binding energy; whereas, above the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005, rGO begins to aggregate, thereby leading to inhibit electron transfer. Therefore, we predict that sample 1
0.005, rGO begins to aggregate, thereby leading to inhibit electron transfer. Therefore, we predict that sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 may be the optimal mass ratio for the photocatalytic degradation of chlorophenols. Furthermore, the C 1s XPS spectra of GO and Cu2(OH)PO4/rGO are shown in the Fig. 4c and d. For GO, three different peaks located at 284.70, 286.78 and 287.84 eV can be obtained, corresponding to C
0.005 may be the optimal mass ratio for the photocatalytic degradation of chlorophenols. Furthermore, the C 1s XPS spectra of GO and Cu2(OH)PO4/rGO are shown in the Fig. 4c and d. For GO, three different peaks located at 284.70, 286.78 and 287.84 eV can be obtained, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–OH, and C
C, C–OH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.35,36 There is also three peaks at the same position for Cu2(OH)PO4/rGO (ESI, Fig. S7†); while the peak areas of C–OH and C
O bonds, respectively.35,36 There is also three peaks at the same position for Cu2(OH)PO4/rGO (ESI, Fig. S7†); while the peak areas of C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O significantly decrease compared to GO, suggesting the oxygen functional groups are removed during the hydrothermal treatment. This is good agreement with the result obtained by the Raman spectroscopy. These demonstrate that graphene is successfully combined with Cu2(OH)PO4 and simultaneously is reduced effectively during the hydrothermal reaction. Therefore, this structure of Cu2(OH)PO4/rGO can enhance their photocatalytic activity under infrared light via promoting charge separation of photocarriers.37,38
O significantly decrease compared to GO, suggesting the oxygen functional groups are removed during the hydrothermal treatment. This is good agreement with the result obtained by the Raman spectroscopy. These demonstrate that graphene is successfully combined with Cu2(OH)PO4 and simultaneously is reduced effectively during the hydrothermal reaction. Therefore, this structure of Cu2(OH)PO4/rGO can enhance their photocatalytic activity under infrared light via promoting charge separation of photocarriers.37,38
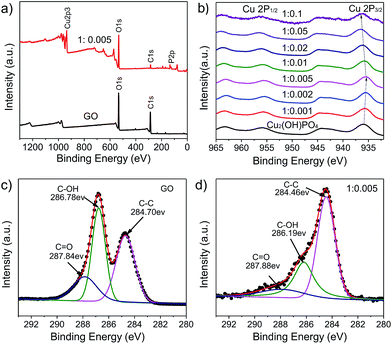 | ||
Fig. 4 (a) Full-scale XPS spectra of GO and sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005. (b) Cu 2p XPS spectra of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO. (c) C 1s XPS spectrum of GO. (d) C 1s XPS spectrum of sample 1 0.005. (b) Cu 2p XPS spectra of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO. (c) C 1s XPS spectrum of GO. (d) C 1s XPS spectrum of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005. 0.005. | ||
The optical properties of pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO are investigated by UV-vis-NIR diffuse reflectance spectroscopy (DRS). As shown in Fig. S8a,† pure Cu2(OH)PO4 displays a strong absorption in the infrared region, which can be further fitted with four Gaussian peaks centered at 662, 777, 965 and 1237 nm (1.55 eV, 1.32 eV, 1.06 eV and 0.88 eV, respectively, Fig. S8b†). According to the previous report, the absorption peak at ∼777 nm is mainly attributed to the 2Eg–B1g transition for CuII sites that exists in axially elongated CuO4(OH)2 octahedra, and the absorption peaks located at ∼662, 965, and 1237 nm are largely associated with d–d transitions for CuII sites that exist in axially compressed CuO4(OH) trigonal bipyramids.20,39,40 On the introduction of GO into Cu2(OH)PO4, Cu2(OH)PO4/rGO exhibits enhanced optical absorption in infrared region over pure Cu2(OH)PO4, with red-shift in the maximum absorption peak. (Fig. S8c, d and S9†) Note that the color of Cu2(OH)PO4/rGO becomes much darker with the increasing mass ratio of GO. Remarkably, when mass ratio of GO reaches up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, the absorption peak in the infrared region gradually decreases because that the excessive graphene is capable of shielding the infrared light and inhibits the photo absorption by Cu2(OH)PO4.41,42
0.01, the absorption peak in the infrared region gradually decreases because that the excessive graphene is capable of shielding the infrared light and inhibits the photo absorption by Cu2(OH)PO4.41,42
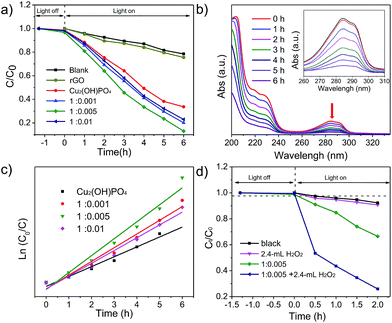
To evaluate the photocatalytic properties of Cu2(OH)PO4/rGO under infrared light (λ > 800 nm), 2,4-DCP, which is fairly stable under solar light irradiation, is used as a model pollutant. To minimize the loss of 2,4-DCP by evaporation, the temperature of the solution was maintained at 20–25 °C during infrared light irradiation. Fig. 5a and S10a† shows time profiles of Ct/C0 under infrared light irradiation in the presence of Cu2(OH)PO4/rGO, where Ct is the concentration of 2,4-DCP at the irradiation time of t and C0 is the concentration in the adsorption equilibrium of the photocatalysts before photo-irradiation. The results show that almost no photolysis is observed without photocatalysts after 6 h of infrared light irradiation. 2,4-DCP is slightly degraded in the presence of Cu2(OH)PO4; while the degradation is remarkably accelerated with Cu2(OH)PO4/rGO. On progressively increasing the proportion of GO, the degradation rate initially increases for Cu2(OH)PO4/rGO, and then declines. Among all the nanocomposites, sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 shows the highest degradation rate (87.1%) after irradiation with infrared light for 6 h, which is rather higher than that of pure Cu2(OH)PO4 (66.4%). This enhancement clearly indicates effective recombination suppression, which can be attributed to the van der Waal heterojunction between Cu2(OH)PO4 and rGO. This is consistent with the results of fluorescence spectra of pure Cu2(OH)PO4, 1
0.005 shows the highest degradation rate (87.1%) after irradiation with infrared light for 6 h, which is rather higher than that of pure Cu2(OH)PO4 (66.4%). This enhancement clearly indicates effective recombination suppression, which can be attributed to the van der Waal heterojunction between Cu2(OH)PO4 and rGO. This is consistent with the results of fluorescence spectra of pure Cu2(OH)PO4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.001, 1
0.001, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 and 1
0.005 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, which is shown in Fig. S11.† However, from samples 1
0.1, which is shown in Fig. S11.† However, from samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 to 1
0.01 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, the degradation rate decreases to 78.5% after 6 h of infrared light irradiation. This is largely attributed to the fact that the excessive graphene shields Cu2(OH)PO4, preventing absorption and blocking electron transfer between Cu2(OH)PO4 and graphene, as demonstrated by the results of XPS, Raman spectrum and DRS measurements. It is worth noting that these values are still higher than that of pure Cu2(OH)PO4. Fig. 5b shows the evaluation of the absorption spectra of 2,4-DCP with reaction time in the presence of sample 1
0.1, the degradation rate decreases to 78.5% after 6 h of infrared light irradiation. This is largely attributed to the fact that the excessive graphene shields Cu2(OH)PO4, preventing absorption and blocking electron transfer between Cu2(OH)PO4 and graphene, as demonstrated by the results of XPS, Raman spectrum and DRS measurements. It is worth noting that these values are still higher than that of pure Cu2(OH)PO4. Fig. 5b shows the evaluation of the absorption spectra of 2,4-DCP with reaction time in the presence of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005.
0.005.
The pseudo-first order kinetic model is then used for the determination of the photocatalytic degradation rate constant (k, h−1) which is expressed by eqn (1):43,44
| ln(C0/Ct) = kt, | (1) |
Fig. 5c and S10b† depict the ln(C0/Ct) versus t for pure Cu2(OH)PO4 and Cu2(OH)PO4/rGO. Pure Cu2(OH)PO4 presents an apparent photocatalytic rate constant of 0.190 h−1 under the irradiation of infrared light. However, the photocatalytic rate constants of Cu2(OH)PO4/rGO for 2,4-DCP are higher than that of pure Cu2(OH)PO4 under the same condition. Among these Cu2(OH)PO4/rGO with various mass ratios, sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 shows the highest photocatalytic rate constant, which is 1.72 times than that of pure Cu2(OH)PO4. The photocatalytic rate constant of other samples is summarized in Table S1.†
0.005 shows the highest photocatalytic rate constant, which is 1.72 times than that of pure Cu2(OH)PO4. The photocatalytic rate constant of other samples is summarized in Table S1.†
The above results clearly demonstrate that graphene plays a significant role in the enhanced infrared light photocatalytic activity. A tentative photocatalytic mechanism of Cu2(OH)PO4/rGO for degradation of 2,4-DCP under infrared light irradiation is proposed and schematically illustrated in Fig. 1. Similar to pure Cu2(OH)PO4,20 copper atoms of Cu2(OH)PO4/rGO have two nonequivalent crystallographic sites, including octahedral sites (rGO-OCT CuII) and trigonal bipyramidal sites (rGO-TBP CuII). In this system, the distorted polyhedrons can lead to a net dipole moment in such units, facilitating electron transfer from rGO-TBP CuII to neighboring rGO-OCT CuII. Benefiting from the strong absorption in infrared region, Cu2(OH)PO4/rGO can be excited by infrared light and therefore generate electrons (forming rGO-OCT CuI sites at the CuO4(OH)2 octahedra) and holes (forming rGO-TBP CuIII sites at the CuO4(OH) trigonal bipyramids). Then, by taking advantage of the high-specific area surface and electrical conductivity, graphene nanosheets can provide more sites to absorb 2,4-DCP and promote photogenerated holes transfer from rGO-TBP CuIII to 2,4-DCP, which is responsible for degrading 2,4-DCP. In parallel, rGO-OCT CuI may drive the half-reaction of oxygen reduction. The main processes in the photocatalytic degradation of 2,4-DCP could be summarized as follows:
| rGO-TBP CuII + hv → rGO-TBP CuIII + e− | (2) |
| rGO-OCT CuII + e− → rGO-OCT CuI | (3) |
| rGO-TBP CuIII + 2,4-DCP → rGO-TBP CuII + H2O + CO2 + H+ + Cl− | (4) |
| rGO-OCT CuI + H+ + O2 → rGO-OCT CuII + H2O | (5) |
Furthermore, the introduction of H2O2 as a sacrificial electron acceptor can maximize the photocatalytic efficiency for the degradation of 2,4-DCP with Cu2(OH)PO4/rGO through the combination of the photocatalytic and photo-Fenton effects into one reaction system. Taking sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 as an example, it is found that the photocatalytic activity is gradually improved on the increase of the amount of H2O2 (Fig. S12†). As shown in Fig. 5d, when 2.4 mL H2O2 is added, the photodegradation efficiency of 2,4-DCP increases from 33.6% to 74.1% after 2 h of infrared light irradiation. According to the kinetic curves in Fig. S13,† the photocatalytic rate constant of sample 1
0.005 as an example, it is found that the photocatalytic activity is gradually improved on the increase of the amount of H2O2 (Fig. S12†). As shown in Fig. 5d, when 2.4 mL H2O2 is added, the photodegradation efficiency of 2,4-DCP increases from 33.6% to 74.1% after 2 h of infrared light irradiation. According to the kinetic curves in Fig. S13,† the photocatalytic rate constant of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 and 2.4 mL H2O2 is more than 6.25 times higher than the corresponding value for only sample 1
0.005 and 2.4 mL H2O2 is more than 6.25 times higher than the corresponding value for only sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005, and almost 10 times greater than the value for pure Cu2(OH)PO4. These results confirm that the addition of H2O2 as a sacrificial acceptor can remarkably enhance the photocatalytic efficiency of Cu2(OH)PO4/rGO for the degradation of 2,4-DCP under infrared light irradiation. In addition, it is found from Fig. S14† that the photocatalytic properties of sample 1
0.005, and almost 10 times greater than the value for pure Cu2(OH)PO4. These results confirm that the addition of H2O2 as a sacrificial acceptor can remarkably enhance the photocatalytic efficiency of Cu2(OH)PO4/rGO for the degradation of 2,4-DCP under infrared light irradiation. In addition, it is found from Fig. S14† that the photocatalytic properties of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 is not much better than pure Cu2(OH)PO4 under visible light irradiation. At the same time, sample 1
0.005 is not much better than pure Cu2(OH)PO4 under visible light irradiation. At the same time, sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 shows the higher degradation rate (87.1%) after irradiation with infrared light for 6 h relative to the degradation rate (54.5%) with visible light irradiation, which it is possible that the redox potential of 2,4-DCP is not match the band position of Cu2(OH)PO4 corresponding to visible light.
0.005 shows the higher degradation rate (87.1%) after irradiation with infrared light for 6 h relative to the degradation rate (54.5%) with visible light irradiation, which it is possible that the redox potential of 2,4-DCP is not match the band position of Cu2(OH)PO4 corresponding to visible light.
The mechanism of photocatalytic degradation of 2,4-DCP by Cu2(OH)PO4/rGO with H2O2 as the sacrificial electron acceptor is proposed as follows:
| rGO-OCT CuI + H2O2 → rGO-OCT CuII + HO˙ + OH− | (6) |
| H+ + OH− → H2O | (7) |
| 2,4-DCP + HO˙ → H2O + CO2 + H+ + Cl− | (8) |
It is generally accepted that H2O2 is a better sacrificial electron acceptor than O2.45 Therefore, in the presence of H2O2, rGO-OCT CuI produced under infrared light irradiation can react irreversibly with H2O2 to (a) further improve the electron–hole separation and (b) the effective generation of powerful reactive species HO˙ radicals via the photo-Fenton reaction, which can further oxidize 2,4-DCP during the photocatalytic degradation reaction,46 finally returning to CuII sites. In addition to reaction (4), these two processes complete the full photocatalytic circle and are occurred repeatedly, finally maximizing the photocatalytic efficiency for the mineralization of 2,4-DCP to CO2 and H2O. This is the unique advantage of these Cu2(OH)PO4/rGO as a novel infrared-light-active photocatalyst. As shown in Fig. S15,† it can be easily seen that sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 is capable of catalyzing H2O2 efficiently to generate HO˙ only when irradiated with infrared light. The amount of the generated HO˙ for sample 1
0.005 is capable of catalyzing H2O2 efficiently to generate HO˙ only when irradiated with infrared light. The amount of the generated HO˙ for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 under infrared light irradiation is over 7 times than the control groups. Remarkably, there is no generation of HO˙ without the irradiation of infrared light. These demonstrate that the infrared light photocatalytic activity is further significantly enhanced when the photocatalytic and photo-Fenton effects are combined in one reaction system.
0.005 under infrared light irradiation is over 7 times than the control groups. Remarkably, there is no generation of HO˙ without the irradiation of infrared light. These demonstrate that the infrared light photocatalytic activity is further significantly enhanced when the photocatalytic and photo-Fenton effects are combined in one reaction system.
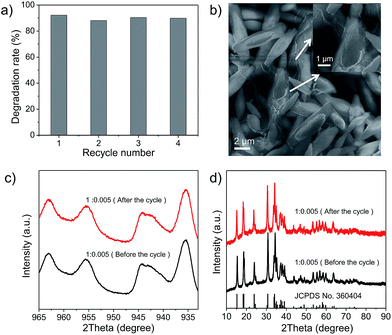
Finally, the stability of Cu2(OH)PO4/rGO was evaluated using cyclic experiments. Fig. 6a shows the degradation of 2,4-DCP for four runs of reactions. The photocatalytic efficiency of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 does not decrease even after several cycles. This indicates that sample 1
0.005 does not decrease even after several cycles. This indicates that sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 can be efficiently recycled and reused for repeated cycles without appreciable loss of activity. SEM images and XPS spectra of sample 1
0.005 can be efficiently recycled and reused for repeated cycles without appreciable loss of activity. SEM images and XPS spectra of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 before the photocatalytic reaction and after the photocatalytic reaction cycles are presented in Fig. 6b and c, respectively. No differences have been found, either in the morphology of sample 1
0.005 before the photocatalytic reaction and after the photocatalytic reaction cycles are presented in Fig. 6b and c, respectively. No differences have been found, either in the morphology of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 or in their chemical structure after photocatalytic degradation. Furthermore, XRD patterns shown in Fig. 6d indicate that the crystal structures of sample 1
0.005 or in their chemical structure after photocatalytic degradation. Furthermore, XRD patterns shown in Fig. 6d indicate that the crystal structures of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 remained the same after repeated photocatalytic cycling. At the same time, SEM images, XPS spectra and XRD patterns shown in Fig. S16–S18,† respectively arrested that other Cu2(OH)PO4/rGO also possess nice photocatalytic stability. In addition, in order to evaluate the biosecurity of Cu2(OH)PO4/rGO nanocomposites, the cytotoxicity of sample 1
0.005 remained the same after repeated photocatalytic cycling. At the same time, SEM images, XPS spectra and XRD patterns shown in Fig. S16–S18,† respectively arrested that other Cu2(OH)PO4/rGO also possess nice photocatalytic stability. In addition, in order to evaluate the biosecurity of Cu2(OH)PO4/rGO nanocomposites, the cytotoxicity of sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 was assessed to HUVECs (human umbilical vein endothelial cells) by the CCK-8 assay. It can be seen from Fig. S19† that no obvious cytotoxicity is induced in human umbilical vein endothelial cells, even at high concentrations up to 90 μg mL−1, which exhibits Cu2(OH)PO4/rGO nanocomposites have good biosecurity.
0.005 was assessed to HUVECs (human umbilical vein endothelial cells) by the CCK-8 assay. It can be seen from Fig. S19† that no obvious cytotoxicity is induced in human umbilical vein endothelial cells, even at high concentrations up to 90 μg mL−1, which exhibits Cu2(OH)PO4/rGO nanocomposites have good biosecurity.
Conclusion
In summary, Cu2(OH)PO4/rGO nanocomposites with different mass ratios of GO are successfully synthesized through a one-step hydrothermal method. By coupling with graphene, the photocatalytic activity of pure Cu2(OH)PO4 is remarkably enhanced under infrared light irradiation. With an optimal mass ratio of 0.5 wt% graphene oxide, the highest rate of 2,4-DCP degradation is achieved due to the effective hybridization between Cu2(OH)PO4. Moreover, the introduction of H2O2 as the sacrificial electron acceptor can maximize the infrared light photocatalytic activity via the combination of the photocatalytic and photo-Fenton effects into one reaction system. In this system, the reaction of H2O2 with rGO-OCT CuI sites, as well as the coupling of graphene with Cu2(OH)PO4, can improve the separation and transportation of photogenerated electrons and holes; while the generated HO˙ via the photo-Fenton reaction can further oxidize 2,4-DCP. These are the unique advantages of Cu2(OH)PO4/rGO as a novel infrared-light-active photocatalyst. Typically, the photocatalytic rate constant of Cu2(OH)PO4/rGO and H2O2 is ∼6.25 times higher than the corresponding value for only Cu2(OH)PO4/rGO, and ∼10 times greater than the value for pure Cu2(OH)PO4. Moreover, Cu2(OH)PO4/rGO is very stable after many photocatalytic cycles and can be reused without significant loss of photocatalytic activity. In addition, a possible decomposition mechanism for the degradation of 2,4-DCP is further proposed. This work may help the development of a new strategy to search stable and effective photocatalysts with high infrared light photocatalytic activity and bring the promise to fulfilment of actual applications in the treatment of non-biodegradable chlorophenols with lower costs and non-secondary pollution to the environment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Basic Research Programs of China (2016YFA0201600 and 2015CB932104), National Natural Science Foundation of China (No. 31571015, 11621505, 11435002 and 21320102003) and Youth Innovation Promotion Association CAS (2013007 and 2015190).Notes and references
- C. Gonçalves and M. F. Alpendurada, Talanta, 2005, 65, 1179–1189 CrossRef PubMed.
- J. Regueiro, M. Llompart, C. Garcia-Jares, J. C. Garcia-Monteagudo and R. Cela, J. Chromatogr. A, 2008, 1190, 27–38 CrossRef CAS PubMed.
- V. K. Gupta, I. Ali, Suhas and V. K. Saini, J. Colloid Interface Sci., 2006, 299, 556–563 CrossRef CAS PubMed.
- M. C. Alavanja, J. A. Hoppin and F. Kamel, Annu. Rev. Public Health, 2004, 25, 155–197 CrossRef PubMed.
- Y. Xiang, M. Wang, X. Sun, D. Cai and Z. Wu, ACS Sustainable Chem. Eng., 2014, 2, 918–924 CrossRef CAS.
- S. Malato and A. Agüera, in Pesticide Decontamination and Detoxification, American Chemical Society, 2003, vol. 863, ch. 9, pp. 113–126 Search PubMed.
- Z. Gerstl, A. Nasser and U. Mingelgrin, J. Agric. Food Chem., 1998, 46, 3803–3809 CrossRef CAS.
- M. G. Mogul, H. Akin, N. Hasirci, D. J. Trantolo, J. D. Gresser and D. L. Wise, Resour., Conserv. Recycl., 1996, 16, 289–320 CrossRef.
- U. D. Patel and S. Suresh, J. Hazard. Mater., 2007, 147, 431–438 CrossRef CAS PubMed.
- H. Ding, X. Li, J. Wang, X. Zhang and C. Chen, J. Environ. Sci., 2016, 43, 187–198 CrossRef PubMed.
- J. Hou, F. Liu, N. Wu, J. Ju and B. Yu, J. Nanobiotechnol., 2016, 14, 5 CrossRef PubMed.
- J. De Coster, W. Vanherck, L. Appels and R. Dewil, J. Environ. Manage., 2017, 190, 61–71 CrossRef CAS PubMed.
- C. Castañeda, F. Tzompantzi, R. Gómez and H. Rojas, J. Chem. Technol. Biotechnol., 2016, 91, 2170–2178 CrossRef.
- Y. Ren, Q. Zhao, X. Li, W. Xiong, M. Tade and L. Liu, J. Nanopart. Res., 2014, 16, 2532 CrossRef.
- J. Tian, Y. Sang, G. Yu, H. Jiang, X. Mu and H. Liu, Adv. Mater., 2013, 25, 5075–5080 CrossRef CAS PubMed.
- Y. Sang, Z. Zhao, M. Zhao, P. Hao, Y. Leng and H. Liu, Adv. Mater., 2015, 27, 363–369 CrossRef CAS PubMed.
- M.-Z. Huang, B. Yuan, L. Dai and M.-L. Fu, J. Colloid Interface Sci., 2015, 460, 264–272 CrossRef CAS PubMed.
- L. Ji and R. Yu, Asia-Pacific Energy Equipment Engineering Research Conference, Beijing, April 2015, pp. 224–228 Search PubMed.
- C. Chen, Y. Zhou, N. Wang, L. Cheng and H. Ding, RSC Adv., 2015, 5, 95523–95531 RSC.
- G. Wang, B. Huang, X. Ma, Z. Wang, X. Qin, X. Zhang, Y. Dai and M.-H. Whangbo, Angew. Chem., Int. Ed., 2013, 52, 4810–4813 CrossRef CAS PubMed.
- Z. Li, Y. Dai, X. Ma, Y. Zhu and B. Huang, Phys. Chem. Chem. Phys., 2014, 16, 3267–3273 RSC.
- L. Yan, Y. B. Zheng, F. Zhao, S. Li, X. Gao, B. Xu, P. S. Weiss and Y. Zhao, Chem. Soc. Rev., 2012, 41, 97–114 RSC.
- D. Deng, K. S. Novoselov, Q. Fu, N. Zheng, Z. Tian and X. Bao, Nat. Nanotechnol., 2016, 11, 218–230 CrossRef CAS PubMed.
- A. D. Bokare and W. Choi, J. Hazard. Mater., 2014, 275, 121–135 CrossRef CAS PubMed.
- Y. H. Kim, Y. J. Hong, K. Y. Baik, G. C. Kwon, J. J. Choi, G. S. Cho, H. S. Uhm, D. Y. Kim and E. H. Choi, Plasma Chem. Plasma Process., 2014, 34, 457–472 CrossRef CAS.
- Y. Zhan, H. Li and Y. Chen, J. Hazard. Mater., 2010, 180, 481–485 CrossRef CAS PubMed.
- T. Ramanathan, A. A. Abdala, S. Stankovich, D. A. Dikin, M. Herrera Alonso, R. D. Piner, D. H. Adamson, H. C. Schniepp, X. Chen, R. S. Ruoff, S. T. Nguyen, I. A. Aksay, R. K. Prud'Homme and L. C. Brinson, Nat. Nanotechnol., 2008, 3, 327–331 CrossRef CAS PubMed.
- D. Pierucci, H. Henck, C. H. Naylor, H. Sediri, E. Lhuillier, A. Balan, J. E. Rault, Y. J. Dappe, F. Bertran, P. L. Fèvre, A. T. C. Johnson and A. Ouerghi, Sci. Rep., 2016, 6, 26656 CrossRef CAS PubMed.
- S. Han, L. Hu, Z. Liang, S. Wageh, A. A. Al-Ghamdi, Y. Chen and X. Fang, Adv. Funct. Mater., 2014, 24, 5719–5727 CrossRef CAS.
- E. J. Heller, Y. Yang, L. Kocia, W. Chen, S. Fang, M. Borunda and E. Kaxiras, ACS Nano, 2016, 10, 2803–2818 CrossRef CAS PubMed.
- K. Singh, A. Ohlan, V. H. Pham, B. R. S. Varshney, J. Jang, S. H. Hur, W. M. Choi, M. Kumar, S. K. Dhawan, B.-S. Kong and J. S. Chung, Nanoscale, 2013, 5, 2411–2420 RSC.
- T. Som, G. V. Troppenz, R. Wendt, M. Wollgarten, J. Rappich, F. Emmerling and K. Rademann, ChemSusChem, 2014, 7, 854–865 CrossRef CAS PubMed.
- B. Bajaj, H. I. Joh, S. M. Jo, G. Kaur, A. Sharma, M. Tomar, V. Gupta and S. Lee, J. Mater. Chem. B, 2016, 4, 229–236 RSC.
- J. S. Corneille, J.-W. He and D. W. Goodman, Surf. Sci., 1994, 306, 269–278 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- T. Xing, Y. Zheng, L. H. Li, B. C. C. Cowie, D. Gunzelmann, S. Z. Qiao, S. Huang and Y. Chen, ACS Nano, 2014, 8, 6856–6862 CrossRef CAS PubMed.
- Y.-C. Yang, L. Xu, W.-Q. Huang, C.-Y. Luo, G.-F. Huang and P. Peng, J. Phys. Chem. C, 2015, 119, 19095–19104 CAS.
- X. Pan, M.-Q. Yang, Z.-R. Tang and Y.-J. Xu, J. Phys. Chem. C, 2014, 118, 27325–27335 CAS.
- A. Lin, B. H. Kim, D. S. Moon, Y. Chung and W.-T. Han, Opt. Express, 2007, 15, 3665 CrossRef CAS PubMed.
- X. Peng, M. Li and C. K. Chan, J. Phys. Chem. C, 2015, 119, 4684–4693 CAS.
- T. Xian, H. Yang, L. Di, J. Ma, H. Zhang and J. Dai, Nanoscale Res. Lett., 2014, 9, 327 CrossRef PubMed.
- C. Han, M.-Q. Yang, N. Zhang and Y.-J. Xu, J. Mater. Chem. A, 2014, 2, 19156–19166 CAS.
- J. Sun, Y. Fu, P. Xiong, X. Sun, B. Xu and X. Wang, RSC Adv., 2013, 3, 22490–22497 RSC.
- S. Das and S. Jana, Environ. Sci.: Nano, 2017, 4, 596–603 RSC.
- C. C. Wong and W. Chu, Environ. Sci. Technol., 2003, 37, 2310–2316 CrossRef CAS PubMed.
- A. N. Pham, G. Xing, C. J. Miller and T. D. Waite, J. Catal., 2013, 301, 54–64 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S16 and Table S1. See DOI: 10.1039/c7ra12684k |
| This journal is © The Royal Society of Chemistry 2018 |