Palladium catalyzed direct allylation of azlactones with simple allylic alcohols in the absence of any activators†
Hui Zhouab,
Huameng Yanga,
Hongyu Yina,
Muwen Liuab,
Chungu Xia*a and
Gaoxi Jiang*ac
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, China. E-mail: cgxia@lzb.ac.cn; gxjiang2012@sinano.ac.cn; Fax: +86-512-62872775
bGraduate University of Chinese Academy of Sciences, Beijing, China
cSuzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, China
First published on 29th May 2014
Abstract
The first example of the readily scalable direct allylic alkylation of azlactones with simple allylic alcohols has been developed, which is catalyzed by Pd(PPh3)4 alone in the absence of any activators under neutral conditions.
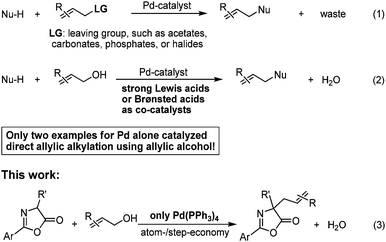
Palladium-catalyzed α-allylic alkylations of carbonyl compounds, namely Tsuji–Trost reaction, are powerful and widely used methodologies for C–C bond formation in the synthesis of pharmaceuticals, biologically active natural products, and materials.1 Generally, such reactions involve the allylic alkylation of nucleophiles with activated allylic alcohol derivatives as allylic species, for instance, carbonates, acetates, phosphates, amines and halides, which always generate stoichiometric waste (Scheme 1, eqn (1)).1 From the viewpoint of environmental issues and atom-/step-economy,2 the direct use of simple allylic alcohol itself as precursor of π-allyl fragment is much more attractive and practical for such kind of transformations, which gives only water as by-product. However, presumably suffering from the poor leaving capability of the hydroxyl group at allylic alcohol, extra activators are intrinsically required to activate in situ allylic alcohol for the success of allylation, including toxic inorganic acids,3 strong Lewis acids,4 and Brønsted acids5 (Scheme 1, eqn (2)). Despite great advances in Tsuji–Trost-type allylations have defined the current state of the art in C–C bond-forming reactions,1,3–5 the direct use of simple allylic alcohol as allylating reagents in the absence of any activating reagents has rarely been achieved.6 Herein, we report the first example of Pd(PPh3)4 alone catalyzed highly efficient allylation of azlactones with simple unactivated allylic alcohols in the absence of any activators under neutral conditions (Scheme 1, eqn (3)).7 It is noteworthy that the approach provides a practical access to quaternary allylic amino acids via hydrolysis of the allylated azlactones, which is of great potential in the synthesis of biologically active molecules.8
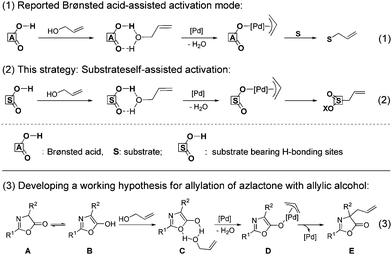
Inspired by the Pd/Brønsted acid co-catalyzed allylation, in which the hydrogen bonding between Brønsted acid and allylic alcohol played a critical role for the generation of π-allyl-Pd intermediate that propel the following allylic process5 (Scheme 2, eqn (1)), we reasoned that the extra addition of Brønsted acid might be not necessary if allylic alcohol can be activated by substrate self via hydrogen bonding (Scheme 2, eqn (2)). Upon this hypothesis, azlactone A is selected as the starting material owing to its tautomerization to 5-hydroxyl-oxazole B.9 The latter might react with allylic alcohol via hydrogen bonding (C) to expel the hydroxyl group, followed by insertion of Pd(0) catalyst for the generation of the key π-allyl-Pd intermediate D; then D gives the desired allylated adduct E (Scheme 2, eqn (3)).
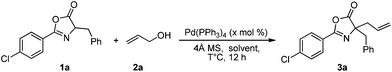
Gratifyingly, the treatment of azlactone 1a with two equiv. of allylic alcohol 2a in the presence of 5.0 mol% Pd(PPh3)4 and 4 Å molecular sieves (4 Å MS) in toluene at 60 °C for 12 h provided the allylated product 3a in almost quantitative yield smoothly (Table 1, entry 1). Remarkably, even reducing the Pd(PPh3)4 loading to 1.5 mol% didn't affect the yield (entry 2). Lowering the reaction temperature from 60 °C to 40 °C resulted in 75% yield (entry 3). A belief examination of the solvent effect revealed toluene as the solvent of choice. Changing the solvent to ethyl acetate (EA) and methyl-tert-butylether (MTBE) lowered the yield of 3a to 40% and 20%, respectively (entries 4 and 5).
| Entrya | Pd(PPh3)4 | T (°C) | Solvent | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), 4 Å MS (50 mg), solvent (1.0 mL).b Determined by 1H NMR analysis of the crude reaction mixture.c Isolated yield in the parenthesis. | ||||
| 1 | 5.0 mol% | 60 | Toluene | >99% (98%)c |
| 2 | 1.5 mol% | 60 | Toluene | >99% (97%)c |
| 3 | 1.5 mol% | 40 | Toluene | 75% |
| 4 | 1.5 mol% | 40 | EA | 40% |
| 5 | 1.5 mol% | 40 | MTBE | 20% |
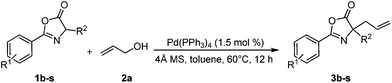
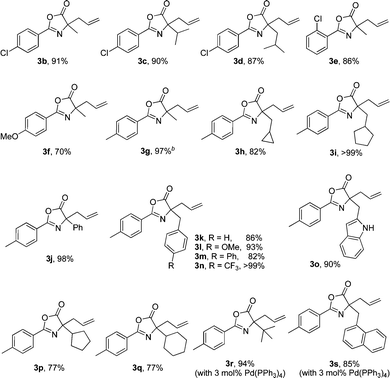
With the optimized reaction conditions in hand, our attention was turned to investigation of the substrate scopes with respect to azlactones and allylic alcohols. First, a series of azlactones were employed as the nucleophiles. Gratifyingly, the protocol is quite general and tolerates azlactones bearing a range of substitutes, including electron-withdrawing and electron-donating groups (Table 2). Besides 1a, treatment of azlactones 1b–d substituted with a chlorine atom at the para-position with 2a in the presence of 1.5 mol% Pd(PPh3)4 and 4 Å MS at 60 °C in toluene for 12 hours, the corresponding allylated adducts 3b–d efficiently formed in 91%, 90%, and 87% yield, respectively. Azlactone 1e, with a chlorine atom at the ortho-position, furnished 3e in 86% yield as well. Bearing the aryl group with electron-donating substituent, such as methoxyl and methyl group, 1f–j gave the desired products 3f–j in 70 ≥ 99% yield; even the reaction enlarged to two-gram scale, 3g was readily isolated in 97% yield. Substrates having different electronic properties of benzyl groups at the 4-position of the oxazolone ring could also undergo the direct allylation reaction smoothly, affording 3k–n in 82 ≥ 99% yield. Interestingly, the nucleophile (1o) derived from tryptophan with an unprotected N–H group was surprisingly well tolerated, resulting in the alkylated product 3o in 90% yield. Employing bulky substituted azlactones having 4-cyclopentyl (1p) and 4-cyclohexyl group (1q) under the mild reaction condition, the direct allylation processes proceeded in both 77% yield. Strikingly, the replacements of methyl (1g) with much more hindered tert-butyl (1r) and naphthalen-2-ylmethyl group (1s) are also applicable to the allylation reaction, and the reactions afforded 3r,s in 94% and 85% yield respectively, although a slightly increased Pd(PPh3)4-loading to 3.0 mol% were required for complete substrate conversions.
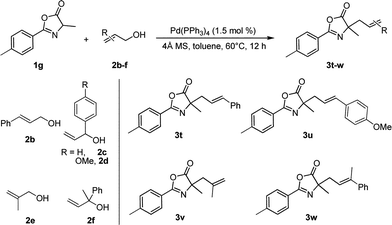
Importantly, we were pleased to find that the practical direct allylic alkylation reaction can be easily extended to a range of substituted allylic alcohols (Table 3). Consequently, the treatment of 1g with five substituted allylic alcohols 2b–f facilely led to allylated products 3t–w in 78–97% yields. It is noteworthy that the reactions of 1g with both 2b and 2c exclusively afforded 3t (entries 1 and 2) in similar yields, which indicate that both reactions proceeded via the same π-allyl-Pd intermediate.5 Remarkably, allylic alcohol 2f bearing highly steric hindrance is also suitable for the process, the reaction afforded 3w in 89% yield smoothly.
In conclusion, we have developed an efficient direct allylic alkylation of azlactones with simple allylic alcohols using Pd(PPh3)4 alone as catalyst under neutral conditions by substrate self-assisted activation strategy. The approach can be easily extended to gram scale in almost quantitative yield, which provides a practical synthetic approach to quaternary allylic amino acids.8 Further applications of the practical method, as well as the asymmetric variant of this reaction are under investigation in our laboratory and will be reported in due course.
Acknowledgements
Financial supports from Hundred Talent Program of Chinese Academy of Sciences (CAS) and the National Natural Science Foundation of China (21202175) are gratefully acknowledged.Notes and references
- For reviews, see: (a) J. Tsuji, Transition Metal Reagents and Catalysts, Wiley, New York, 2000 Search PubMed; (b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (c) U. Kazmaier, Curr. Org. Chem., 2003, 7, 317 CrossRef CAS; (d) W. Zhang and D. Liu, in Chiral Ferrocenes in Asymmetric Catalysis: Synthesis and Applications, ed. L.-X. Dai and X.-L. Hou, Wiley-VCH: Weinheim, 2010, ch. 14 Search PubMed; (e) D. Montserrat and P. Oscar, Acc. Chem. Res., 2010, 43, 312 CrossRef PubMed; (f) J. D. Weaver, A. Recio III, A. J. Grenning and J. A. Tunge, Chem. Rev., 2011, 111, 1846 CrossRef CAS PubMed; (g) B. M. Trost, Org. Process Res. Dev., 2012, 16, 185 CrossRef CAS PubMed; (h) B. M. Trost, Top. Organomet. Chem., 2013, 44, 1 CrossRef CAS For selected recent advances, see: ; (i) B. Alcaide, P. Almendros, T. M. del Campo and R. Rodríguez-Acebes, Adv. Synth. Catal., 2007, 349, 749 CrossRef CAS; (j) B. Alcaide, P. Almendros, T. M. del Campo, E. Soriano and J. L. Marco-Contelles, Chem.–Eur. J., 2009, 15, 1901 CrossRef CAS PubMed; (k) B. M. Trost, J. R. Miller and C. M. H. Jr, J. Am. Chem. Soc., 2011, 133, 8165 CrossRef CAS PubMed; (l) B. Alcaide, P. Almendros, J. M. Alonso, M. T. Quirós and P. Gadziński, Adv. Synth. Catal., 2011, 353, 1871 CrossRef CAS; (m) X. Zhao, D. Liu, H. Guo, Y. Liu and W. Zhang, J. Am. Chem. Soc., 2011, 133, 19354 CrossRef CAS PubMed; (n) J.-P. Chen, Q. Peng, B.-L. Lei, X.-L. Hou and Y.-D. Wu, J. Am. Chem. Soc., 2011, 133, 14180 CrossRef CAS PubMed; (o) B. M. Trost, D. A. Thaisrivongs and M. M. Hansmann, Angew. Chem., Int. Ed., 2012, 51, 11522 CrossRef CAS PubMed; (p) M. J. Bartlett, C. A. Turner and J. E. Harvey, Org. Lett., 2013, 15, 2430 CrossRef CAS PubMed.
- (a) B. M. Trost, Science, 1991, 254, 1471 CAS; (b) R. A. Sheldon, Green Chem., 2008, 10, 359 RSC; (c) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC.
- (a) X. Lu, L. Lu and J. Sun, J. Mol. Catal., 1987, 41, 245 CrossRef CAS; (b) X. Lu, X. Jiang and X. Tao, J. Organomet. Chem., 1988, 344, 109 CrossRef CAS; (c) R. Matsubara, K. Masuda, J. Nakano and S. Kobayashi, Chem. Commun., 2010, 46, 8662 RSC.
- (a) J. P. Takahara, Y. Masuyama and Y. Kurusu, J. Am. Chem. Soc., 1992, 114, 2577 CrossRef CAS; (b) T. Satoh, M. Ikeda, M. Miura and M. Nomura, J. Org. Chem., 1997, 62, 4877 CrossRef CAS; (c) S.-C. Yang and C.-W. Hung, J. Org. Chem., 1999, 64, 5000 CrossRef CAS; (d) S.-C. Yang and Y.-C. Tsai, Organometallics, 2001, 20, 763 CrossRef CAS; (e) M. Kimura, Y. Horino, R. Mukai, S. Tanaka and Y. Tamaru, J. Am. Chem. Soc., 2001, 123, 10401 CrossRef CAS; (f) S. Chandrasekhar, V. Jagadeshwar, B. Saritha and C. Narsihmulu, J. Org. Chem., 2005, 70, 6506 CrossRef CAS PubMed; (g) B. M. Trost and J. Quancard, J. Am. Chem. Soc., 2006, 128, 6314 CrossRef CAS PubMed; (h) Y. Tamaru and M. Kimura, Pure Appl. Chem., 2008, 80, 979 CrossRef CAS; (i) M. Kimura and Y. Tamaru, Mini-Rev. Org. Chem., 2009, 6, 392 CrossRef CAS; (j) Y.-X. Li, Q.-Q. Xu, L. Li, D. Wang, Y.-J. Chen and C.-J. Li, J. Am. Chem. Soc., 2013, 135, 12536 CrossRef CAS PubMed.
- (a) I. Kadota, A. Shibuya, Y. S. Gyoung and Y. Yamamoto, J. Am. Chem. Soc., 1998, 120, 10262 CrossRef CAS; (b) N. T. Patil and Y. Yamamoto, Tetrahedron Lett., 2004, 45, 3101 CrossRef CAS PubMed; (c) K. Manabe and S. Kobayashi, Org. Lett., 2003, 5, 3241 CrossRef CAS PubMed; (d) G. Jiang and B. List, Adv. Synth. Catal., 2011, 353, 1667 CrossRef CAS For direct asymmetric allylic alkylation with allylic alcohols by using chiral phosphoric acids, see: ; (e) G. Jiang and B. List, Angew. Chem., Int. Ed., 2011, 50, 9471 CrossRef CAS PubMed; (f) Z.-L. Tao, W.-Q. Zhang, D.-F. Chen, A. Adele and L.-Z. Gong, J. Am. Chem. Soc., 2013, 135, 9255 CrossRef CAS PubMed Another recent notable example for the direct allylation of α-branched aromatic aldehydes with allylic alcohols catalyzed by dual-catalytic strategy, see: ; (g) S. Krautwald, D. Sarlah, M. A. Schafroth and E. M. Carreira, Science, 2013, 340, 1065 CrossRef CAS PubMed Recently, Beller et al. reported a Pd-catalyzed carbonylation of allylic alcohol in the presence of TFA, in which in situ formation of trifluoroacetate was observed; see: Q. Liu, L. Wu, H. Jiao, X. Fang, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 8064 Search PubMed.
- So far, only two examples reported, see: (a) F. Ozawa, H. Okamoto, S. Kawagishi, S. Yamamoto, T. Minami and M. Yoshifuji, J. Am. Chem. Soc., 2002, 124, 10968 CrossRef CAS PubMed; (b) D. Banerjee, R. V. Jagadeesh, K. Junge, H. Junge and M. Beller, Angew. Chem., 2012, 124, 11724 (Angew. Chem., Int. Ed., 2012, 51, 11556) CrossRef.
- Very recently, Hartwig and Chen reported an example that is iridium-catalyzed diastereo- and enantioselective allylations of substituted 5H-oxazol-4-ones with acetates and carbonates under strong basic conditions, see: W. Chen and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 377 CrossRef CAS PubMed.
- (a) M. Sugiura, K. Hirano and S. Kobayashi, Org. Synth., 2006, 83, 170 CrossRef CAS; (b) Merck Index, Merck, Whitehouse Station, NJ, 14th edn, 2007, p. 6005; (c) D. Uraguchi, Y. Asai, Y. Seto and T. Ooi, Synlett, 2009, 4, 658 Search PubMed; (d) M. Hagel, D. Niu, T. S. Martin, M. P. Sheets, L. Qiao, H. Bernard, R. M. Karp, Z. Zhu, M. T. Labenski, P. Chaturvedi, M. Nacht, W. F. Westlin, R. C. Petter and J. Singh, Nat. Chem. Biol., 2011, 7, 22 CrossRef CAS PubMed; (e) C. L. Hugelshofer, K. T. Mellem and A. G. Myers, Org. Lett., 2013, 15, 3134 CrossRef CAS PubMed.
- (a) H. Gotthardt, R. Huisgen and H. O. Bayer, J. Am. Chem. Soc., 1970, 92, 4340 CrossRef CAS; (b) J. Christjohannes and H. Heinz, Helv. Chim. Acta, 1989, 72, 1639 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data for new compounds. See DOI: 10.1039/c4ra02477j |
| This journal is © The Royal Society of Chemistry 2014 |