A six-carbazole-decorated cyclophosphazene as a host with high triplet energy to realize efficient delayed-fluorescence OLEDs†
Takuro
Nishimoto
a,
Takuma
Yasuda
*abc,
Sae Youn
Lee
a,
Ryosuke
Kondo
a and
Chihaya
Adachi
*ab
aDepartment of Applied Chemistry, Center for Organic Photonics and Electronics Research (OPERA), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: yasuda@cstf.kyushu-u.ac.jp; adachi@cstf.kyushu-u.ac.jp; Fax: +81 92-802-6921
bInternational Institute for Carbon Neutral Energy Research (WPI-I2CNER), Kyushu University, Japan
cPRESTO, Japan Science and Technology Agency (JST), Chiyoda-ku, Tokyo 102-0076, Japan
First published on 26th September 2013
Abstract
A high triplet energy (ET) host material, hexakis(9H-carbazol-9-yl)cyclotriphosphazene (PzCz), is used in high-efficiency organic light-emitting diodes (OLEDs). PzCz (ET = 3.00 eV) functions as an effective host for thermally activated delayed fluorescence (TADF) molecules. Highest external electroluminescence quantum efficiencies over 15% and 18% are achieved for blue-green and green TADF-OLEDs, respectively.
Organic light-emitting diodes (OLEDs) based on phosphorescent heavy-metal complexes can harvest both singlet (S1) and triplet (T1) excitons for light emission, providing the opportunity to realize internal electroluminescence (EL) quantum efficiencies of close to 100%.1 This makes OLEDs promising candidates for next-generation flat-panel displays and general lighting products.2 Although phosphorescent OLEDs currently have huge superiority in quantum efficiencies, the price and rarity of phosphorescent metal complexes including precious metals like iridium and platinum limit their low-cost, long-term mass production. Moreover, phosphorescent OLEDs still suffer from severe efficiency roll-off at high current densities caused by triplet–triplet or triplet–polaron exciton annihilation.3 Recently, we have successfully exploited a novel promising mechanism to achieve highly efficient EL, that is, thermally activated delayed fluorescence (TADF)4,5 featuring purely organic luminescent molecules. The TADF luminophores possess a very small energy gap between S1 and T1 excited states, which allows efficient up-conversion of electro-generated T1 population to the emissive S1 state. OLEDs employing a green TADF emitter have exhibited a high external EL quantum efficiency (ηext) of up to ∼19%,4 far exceeding the theoretical limit for conventional fluorescent OLEDs. Recently, a similar singlet harvesting mechanism to achieve efficient light emission has also been proposed for Cu(I) complexes.6
In TADF-OLEDs, the emitters are usually dispersed in a suitable host matrix at a relatively low concentration to avoid concentration quenching and/or triplet–triplet annihilation and guarantee highly efficient EL.3–7 Therefore, it is desirable to develop host materials that have the following features: (i) sufficiently higher triplet energy (ET) than the TADF emitter to prevent reverse energy transfer from the guest to the host, regardless of the emitting color, (ii) suitably aligned HOMO and LUMO levels to ensure effective charge injection from the adjacent layers, (iii) larger HOMO–LUMO energy gap (Eg) than that of the guest to facilitate direct charge trapping on the dopant emitter, (iv) bipolar charge transport properties for balanced hole and electron densities in the emitting layer, and (v) high morphological stability and film-forming ability. Therefore, a host for blue TADF luminophores requires an ET level higher than 2.9 eV.5b Obviously, selective modulation of the energy levels of the host materials is needed to achieve high-performance full-color TADF-OLEDs. As we know, carbazole intrinsically possesses a high ET of about 3.0 eV and excellent hole-transporting ability. In addition, its simple structure offers many options to introduce building blocks onto the carbazole unit through facile chemical approaches. In this regard, numerous carbazole-based high ET host materials7 substituted with electron-accepting moieties (e.g., phosphine oxide,8 oxadiazole,9 benzimidazole,10 triazole,11 triazine,12 and pyridine13) to render bipolar charge transport properties have been developed.
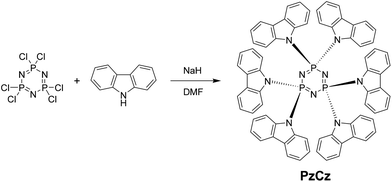
In this communication, we fabricate a six-carbazole-decorated cyclotriphosphazene host material, PzCz (Scheme 1), for the enhancement of device performance. The inorganic cyclotriphosphazene core has a rigid planar structure and is known to exhibit excellent chemical and thermal stability.14 Very recently, phenyl and phenoxy-functionalized cyclophosphazene derivatives have been reported.15 Here we present a comprehensive investigation of the structural, thermal, and photophysical properties of the organic–inorganic hybrid PzCz, and demonstrate its applicability as a host for TADF-OLEDs. By combining organic carbazole and inorganic cyclophosphazene constituents, a promising host material that possesses a high triplet energy, excellent thermal stability, and bipolar transport properties can be produced.
PzCz was synthesized from hexachlorocyclotriphosphazene (P3N3Cl6) in 54% yield using a one-pot nucleophilic substitution procedure (Scheme 1), and was subsequently purified by vacuum train sublimation. Detailed experimental procedures and characterization data are described in the ESI.† The structure of PzCz was further confirmed by single-crystal X-ray analysis. As can be seen from Fig. 1, six carbazolyl units are arranged above and below the central P3N3 ring because of the quasi-tetrahedral geometry of the P atoms. The interplanar angles between the outer carbazolyl units and the P3N3 plane are 59–78°. This molecular geometry is beneficial for the reduction of π-conjugation between the outer carbazolyl units and the central P3N3 core, leading to the high ET of PzCz (see below).
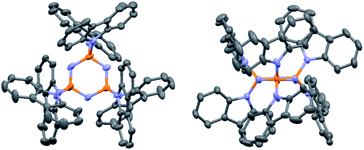 | ||
| Fig. 1 ORTEP diagram of PzCz. Hydrogen atoms are omitted for clarity. Atom color code: C, gray; N, blue; P, orange. | ||
The thermal stability of PzCz was examined by thermogravimetric analysis and differential scanning calorimetry (DSC) under a nitrogen atmosphere. PzCz exhibited excellent thermal stability with a 5% weight-loss decomposition temperature (Td) as high as 474 °C (ESI†). This value is considerably higher than those of conventional host materials, such as 1,3-bis(carbazol-9-yl)benzene16 (mCP, Td ∼ 370 °C) and 4,4′-bis(carbazol-9-yl)biphenyl (CBP, Td ∼ 440 °C), because of the molecular rigidity of PzCz. In addition, no noticeable signal corresponding to crystallization, glass transition, or melting was observed in the DSC curves of PzCz during repeated heating and cooling cycles in the temperature range of 0–400 °C, implying its intrinsic amorphous nature, desirable for optoelectronic device applications. The high quality and stable solid-state morphology of vacuum-deposited thin films of PzCz were ascertained by AFM images, which revealed a root-mean-square surface roughness of less than 0.6 nm (ESI†). No aggregation or crystallization was observed in the PzCz thin film, whereas a CBP film readily crystallized because of its rather low glass-transition temperature (Tg) at 62 °C. The HOMO energy level of PzCz in the thin film was determined to be −6.4 eV by photoelectron yield spectroscopy (ESI†). The LUMO energy level of PzCz, deduced from the obtained HOMO level and optical energy gap (Eg = 3.9 eV), was −2.5 eV.
Fig. 2 illustrates the absorption and photoluminescence (PL) spectra of PzCz in CH2Cl2 solution and a vacuum-deposited thin film. PzCz exhibits a strong absorption peak at 287 nm and a weak one at 315 nm, which are assigned to π–π* and n–π* transitions, respectively, for the cyclophosphazene-linked carbazolyl units. PL emission from PzCz in solution is observed in the UV region with a peak at 332 nm. The PL emission peak of PzCz in the solid state is red-shifted relative to that in dilute solution, suggesting the existence of intermolecular fragmental interactions to some degree in the excited state. The low-temperature phosphorescence spectrum of PzCz shows the highest-energy 0–0 transition band at 414 nm (Fig. 2), corresponding to the ET of 3.00 eV. This value is higher than those of mCP (ET = 2.90 eV)16 and CBP (ET = 2.66 eV),17 as a consequence of the prevention of π-conjugation with the P3N3 core. In addition, it is noted that the ET values of PzCz and mCP are high enough to host a newly synthesized blue-green TADF luminophor, 2,5-bis(carbazol-9-yl)-1,4-dicyanobenzene (CzTPN, ET = 2.65 eV) and to prevent triplet energy back transfer, as discussed later.
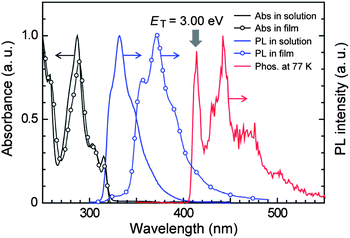 | ||
| Fig. 2 Absorption and PL spectra of PzCz in CH2Cl2 solution and vacuum-deposited thin films at room temperature, and phosphorescence (Phos.) spectrum in CH2Cl2 glass recorded at 77 K. | ||
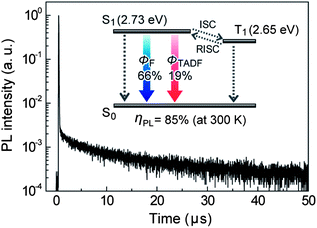
To confirm the applicability of PzCz as a host material for TADF-OLEDs, we investigated the photophysical properties of codeposited thin films of CzTPN doped in PzCz, with an optimum doping concentration of 3 wt%. As displayed in Fig. 3, the transient PL decay curve of the CzTPN:PzCz film measured under a nitrogen atmosphere clearly exhibits both a nano-second-scale prompt component (with a lifetime of τ = 34 ns) and a micro-second-scale delayed component (τ = 11.3 μs) at room temperature (300 K), and can be fitted by a biexponential model. The prompt and delayed decay components are ascribed to fluorescence and TADF emission, respectively.4,5 A high overall PL quantum yield (ηPL) of 85 ± 2% including a TADF component (ΦTADF) of approximately 19 ± 2% was attained for the 3 wt%-CzTPN:PzCz film under a nitrogen atmosphere at room temperature, which is comparable to that of a 3wt%-CzTPN:mCP film (ηPL = 87 ± 2%). According to the energy-level diagram depicted in Fig. 3, CzTPN possesses a very small energy gap (ΔEST) of ca. 80 meV between S1 and T1 excited states (ESI†), which can enhance thermal T1 → S1 up-conversion (i.e., reverse intersystem crossing (RISC)), thus leading to efficient TADF emission under oxygen-free conditions. It is also conceivable that the prevention of endothermic back energy transfer from the CzTPN dopant (ET = 2.65 eV) to the PzCz (or mCP) host with a higher ET contributes to the high PL quantum efficiencies. Under ambient air, however, the overall ηPL of the 3 wt%-CzTPN:PzCz film decreased to 66 ± 2% through nonradiative deactivation by excited energy transfer to triplet oxygen.
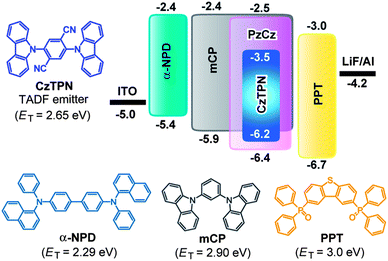
Using the highly luminescent CzTPN:host TADF system as an emitting layer (EML), blue-green OLEDs were fabricated with the following configuration: ITO/α-NPD (35 nm)/mCP (10 nm)/3 wt%-CzTPN:host (20 nm)/PPT (40 nm)/LiF (0.8 nm)/Al (80 nm). The chemical structures and energy levels of the materials used in the devices are presented in Fig. 4. We employed 4,4′-bis[N-(1-naphthyl)-N-phenyl]biphenyl diamine (α-NPD) as a hole-transporting layer (HTL) and 2,8-bis(diphenylphosphoryl)dibenzo[b,d]thiophene18 (PPT) as a hole-blocking and electron-transporting layer (ETL). A thin layer (10 nm) of mCP with a high ET was inserted to suppress triplet exciton quenching of CzTPN at the EML/HTL interface in the devices.
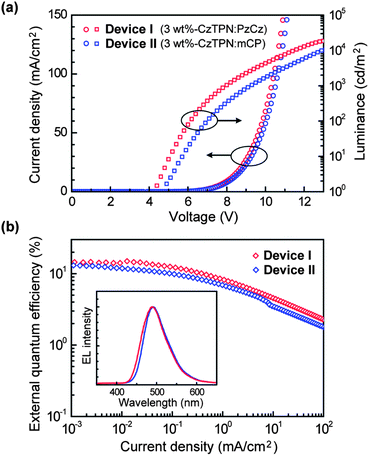
Fig. 5 shows current density–voltage–luminance (J–V–L) characteristics and quantum efficiency versus current density curves of the OLEDs, and the device performances are also summarized in Table 1. It has been found that the current density and luminance of Device I (the PzCz-hosted device) are higher than those of Device II (the mCP-hosted device) at the same driving voltage (Fig. 5a). Device I has a lower turn-on voltage (Von) of 4.2 V than that of Device II. More importantly, Device I with a PzCz host displays excellent device performance with a maximum current efficiency (ηc) of 37.2 cd A−1 and power efficiency (ηp) of 24.4 lm W−1, corresponding to a maximum ηext of 15.0% (photon/electron), which is higher than that of Device II over the whole current density range (Fig. 5b). At a practical brightness of 100 cd m−2, Device I still shows a high ηext of 10.1%. Both devices exhibit blue-green emission only from CzTPN with Commission Internationale de l'Éclairage (CIE) coordinates of (0.18, 0.45) for Device I and (0.17, 0.40) for Device II, indicating that the triplet excitons are well confined on the CzTPN emitter. We also motivated to test the suitability of PzCz as a host for a green TADF-OLED (Device III) with the same device configuration: ITO/α-NPD (35 nm)/mCP (10 nm)/3 wt%-4CzIPN:PzCz (20 nm)/PPT (40 nm)/LiF (0.8 nm)/Al (80 nm), containing 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene4 (4CzIPN, ET = 2.55 eV) as a green TADF dopant. The performance of Device III is listed in Table 1 (see also ESI†). Not only a high luminance of up to 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cd m−2, but also a considerably high ηext of 18.2% was achieved by incorporating a green-emitting 4CzTPN:PzCz codeposited film as the EML in Device III. The CIE coordinates of Device III were (0.20, 0.54).
200 cd m−2, but also a considerably high ηext of 18.2% was achieved by incorporating a green-emitting 4CzTPN:PzCz codeposited film as the EML in Device III. The CIE coordinates of Device III were (0.20, 0.54).
| Device | λ EL (nm) | V on (V) | L max (cd m−2) | η c (cd A−1) | η p (lm W−1) | η ext (%) |
|---|---|---|---|---|---|---|
| a Device configuration: ITO/α-NPD (35 nm)/mCP (10 nm)/3 wt%-CzTPN:host (PzCz for Device I; mCP for Device II, 20 nm)/PPT (40 nm)/LiF (0.8 nm)/Al (80 nm); for Device III, ITO/α-NPD (35 nm)/mCP (10 nm)/3 wt%-4CzIPN:PzCz (20 nm)/PPT (40 nm)/LiF (0.8 nm)/Al (80 nm). Abbreviations: λEL = EL emission maximum; Von = turn-on voltage at 1 cd m−2; Lmax = maximum luminance; ηc = maximum current efficiency; ηp = maximum power efficiency; ηext = maximum external EL quantum efficiency (at L > 1 cd m−2). | ||||||
| I | 494 | 4.2 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
37.2 | 24.4 | 15.0 |
| II | 491 | 4.8 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
27.1 | 16.7 | 11.9 |
| III | 506 | 4.5 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
60.2 | 38.6 | 18.2 |
In general, there are two primary mechanisms of exciton formation and light emission in OLEDs; i.e., host-to-dopant energy transfer and direct charge carrier recombination on the dopant molecules.16b,19,20 In the direct recombination mechanism, excitons are formed by the sequential trapping of holes and electrons on the dopant emitter, which will be a favorable way to achieve high efficiency in TADF-OLEDs, because energy loss during the host-to-dopant energy transfer process can be minimized. In Device I, since the HOMO level of the CzTPN dopant is shallower than that of the PzCz host (Fig. 4), CzTPN exciton formation would predominantly occur via direct charge carrier recombination. Conversely, in Device II, CzTPN exciton formation occurs through host-to-dopant energy transfer because holes are preferentially injected into the mCP host rather than the guest dopant, as a result of the deeper-lying HOMO level of CzTPN compared with that of the mCP host. Accordingly, the notable increase in the EL efficiency of Device I can be explained by the difference in the aforementioned exciton formation mechanisms governed by the difference between the HOMO levels of the PzCz and mCP hosts.
To better understand the hole injection/transport behavior in the CzTPN:host layer, we fabricated hole-only devices (HODs) with the structure: ITO/α-NPD (20 nm)/x wt%-CzTPN:host (60 nm; x = 0, 3)/α-NPD (20 nm)/Al (80 nm). Fig. 6 shows the J–V characteristics of the HODs. As for the HODs, holes injected from the anode into the α-NPD layer should be the exclusive type of charge carriers due to a large electron-injection barrier (ca. 1.8 eV) at the opposite cathode/α-NPD interface. The hole current density is strongly affected by the energy barrier for hole injection and transport properties. Therefore, the much larger hole current density of the mCP-based HOD in comparison with the PzCz-based one is attributed to its lower energy barrier at the α-NPD/host interface. It should be noted that for the PzCz-based HODs, the hole current density increases considerably with increasing the doping concentration of CzTPN (Fig. 6) because direct hole trapping on the CzTPN molecules facilitates the hole injection process at the α-NPD/host interface.20 In contrast, the hole current density of the mCP-based HODs remained unchanged over the whole voltage range even when 3 wt% CzTPN was doped into the host, indicating that holes are predominantly transported by the mCP layer.
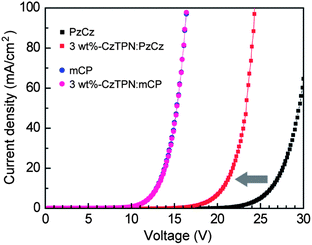 | ||
| Fig. 6 Current density–voltage (J–V) curves for hole-only devices with the structure: ITO/α-NPD (20 nm)/x wt%-CzTPN:host (60 nm; x = 0, 3)/α-NPD (20 nm)/Al (80 nm). | ||
Electron-only devices (EODs) based on PzCz and mCP were also fabricated to compare the electron and hole densities in the EML (ESI†). It is found that PzCz exhibits a higher electron current density compared to that of mCP in the EODs, which implies that the electron injection/transport properties are improved by incorporating the electron-withdrawing cyclophosphazene unit. In addition, there is no large difference between the electron and hole current densities in the 3 wt%-CzTPN:PzCz-based devices. Therefore, it is expected that PzCz possesses bipolar charge-transport capability, and can thus balance holes and electrons inside the EML in TADF-OLEDs.
In summary, we have designed a six-carbazole-decorated cyclophosphazene derivative, PzCz, as a new host material for TADF-OLEDs. PzCz retains a high triplet energy of 3.00 eV as the carbazolyl subunits. By employing PzCz as the host and CzTPN or 4CzIPN as the guest TADF emitter, we have successfully constructed high-performance blue-green and green TADF-OLEDs with maximum external EL quantum efficiencies as high as 15.0% and 18.2%, respectively. The obtained EL efficiency is one of the highest reported for TADF-OLEDs,4,5 and is comparable to those of phosphorescent OLEDs based on iridium complexes. Our results unambiguously demonstrate that a design strategy combining organic carbazole and inorganic cyclophosphazene constituents provides an effective molecular scaffold to produce thermally stable host materials with a high ET for next generation TADF-OLEDs. The electron-donating carbazole and electron-withdrawing cyclophosphazene subunits in PzCz cooperate with each other to realize fine control of charge carrier injection and transport in devices.
This work was supported by a Grant-in-Aid for the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST) from the Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid for Precursory Research for Embryonic Science and Technology (PRESTO) (no. 10111) from JST, and a Grant-in-Aid for Young Scientist (A) (no. 25708032). T.Y. is grateful for financial support from Kyushu University Interdisciplinary Programs in Education and Projects in Research Development. C.A. and T.Y. gratefully acknowledge the support of the International Institute for Carbon Neutral Energy Research (WPI-I2CNER), sponsored by MEXT, Japan.
References
- (a) M. A. Baldo, D. F. O'Brien, Y. You, A. A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS; (b) C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, J. Appl. Phys., 2001, 90, 5048 CrossRef CAS; (c) C. Adachi, M. A. Baldo, S. R. Forrest and M. E. Thompson, Appl. Phys. Lett., 2000, 77, 904 CrossRef CAS; (d) L. Xiao, S.-J. Su, Y. Agata, H. Lan and J. Kido, Adv. Mater., 2009, 21, 1271 CrossRef CAS.
- B. W. D'Andrade and S. R. Forrest, Adv. Mater., 2004, 16, 1585 CrossRef.
- (a) S. Reineke, K. Walzer and K. Leo, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125328 CrossRef; (b) M. A. Baldo, C. Adachi and S. R. Forrest, Phys. Rev. B: Condens. Matter, 2000, 62, 10967 CrossRef CAS.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234 CrossRef CAS PubMed.
- (a) A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Appl. Phys. Lett., 2011, 98, 083302 CrossRef; (b) S. Y. Lee, T. Yasuda, H. Nomura and C. Adachi, Appl. Phys. Lett., 2012, 101, 093306 CrossRef; (c) T. Nakagawa, S.-Y. Ku, K.-T. Wong and C. Adachi, Chem. Commun., 2012, 48, 9580 RSC; (d) Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706 CrossRef CAS PubMed; (e) G. Méhes, H. Nomura, Q. Zhang, T. Nakagawa and C. Adachi, Angew. Chem., Int. Ed., 2012, 51, 11311 CrossRef PubMed; (f) H. Tanaka, K. Shizu, H. Miyazaki and C. Adachi, Chem. Commun., 2012, 48, 11392 RSC; (g) J. Li, T. Nakagawa, Q. Zhang, H. Nomura, H. Miyazaki and C. Adachi, Adv. Mater., 2013, 25, 3319 CrossRef CAS PubMed; (h) K. Sato, K. Shizu, K. Yoshimura, A. Kawada, H. Miyazaki and C. Adachi, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 110, 247401 Search PubMed; (i) J. Lee, K. Shizu, H. Tanaka, H. Nomura, T. Yasuda and C. Adachi, J. Mater. Chem. C, 2013, 1, 4599 RSC.
- (a) J. C. Deaton, S. C. Switalski, D. Y. Kondakov, R. H. Young, T. D. Pawlik, D. J. Giesen, S. B. Harkins, A. J. M. Miller, S. F. Mickenberg and J. C. Peters, J. Am. Chem. Soc., 2010, 132, 9499 CrossRef CAS PubMed; (b) R. Czerwieniec, J. Yu and H. Yersin, Inorg. Chem., 2011, 50, 8293 CrossRef CAS PubMed; (c) H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622 CrossRef CAS; (d) Q. Zhang, T. Komino, S. Huang, S. Matsunami, K. Goushi and C. Adachi, Adv. Funct. Mater., 2012, 22, 2327 CrossRef CAS; (e) R. Czerwieniec, K. Kowalski and H. Yersin, Dalton Trans., 2013, 42, 9826 RSC.
- For recent reviews on OLEDs and host materials, see; (a) L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Gong and J. Kido, Adv. Mater., 2011, 23, 926 CrossRef CAS PubMed; (b) A. Chaskar, H.-F. Chen and K.-T. Wong, Adv. Mater., 2011, 23, 3876 CrossRef CAS PubMed; (c) Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943 RSC; (d) K. S. Yook and J. Y. Lee, Adv. Mater., 2012, 24, 3169 CrossRef CAS PubMed; (e) S. O. Jeon and J. Y. Lee, J. Mater. Chem., 2012, 22, 4233 RSC.
- (a) S. O. Jeon, K. S. Yook, C. W. Joo and J. Y. Lee, Adv. Funct. Mater., 2009, 19, 3644 CrossRef CAS; (b) S. O. Jeon, K. S. Yook, C. W. Joo and J. Y. Lee, Adv. Mater., 2010, 22, 1872 CrossRef CAS PubMed; (c) H.-H. Chou and C.-H. Cheng, Adv. Mater., 2010, 22, 2468 CrossRef CAS PubMed; (d) J. Ding, Q. Wang, L. Zhao, D. Ma, L. Wang, X. Jing and F. Wang, J. Mater. Chem., 2010, 20, 8126 RSC; (e) S. O. Jeon, S. E. Jang, H. S. Son and J. Y. Lee, Adv. Mater., 2011, 23, 1436 CrossRef CAS PubMed; (f) Y. J. Cho and J. Y. Lee, Chem.–Eur. J., 2011, 17, 11415 CrossRef CAS PubMed; (g) H. Liu, G. Cheng, D. Hu, F. Shen, Y. Lv, G. Sun, B. Yang, P. Lu and Y. Ma, Adv. Funct. Mater., 2012, 22, 2830 CrossRef CAS; (h) C. Fan, F. Zhao, P. Gan, S. Yang, T. Liu, C. Zhong, D. Ma, J. Qin and C. Yang, Chem.–Eur. J., 2012, 18, 5510 CrossRef CAS PubMed; (i) H. Sasabe, N. Toyota, H. Nakanishi, T. Ishizaka, Y.-J. Pu and J. Kido, Adv. Mater., 2012, 24, 3212 CrossRef CAS PubMed; (j) W. Yang, Z. Zhang, C. Han, Z. Zhang, H. Xu, P. Yan, Y. Shao and S. Liu, Chem. Commun., 2013, 49, 2822 RSC; (k) A. Wada, T. Yasuda, Q. Zhang, Y. S. Yang, I. Takasu, S. Enomoto and C. Adachi, J. Mater. Chem. C, 2013, 1, 2404 RSC.
- (a) Y. Tao, Q. Wang, C. Yang, Q. Wang, Z. Zhang, T. Zou, J. Qin and D. Ma, Angew. Chem., Int. Ed., 2008, 47, 8104 CrossRef CAS PubMed; (b) Y. Tao, Q. Wang, C. Yang, C. Zhong, K. Zhang, J. Qin and D. Ma, Adv. Funct. Mater., 2010, 20, 304 CrossRef CAS; (c) S. Gong, Q. Fu, W. Zeng, C. Zhong, C. Yang, D. Ma and J. Qin, Chem. Mater., 2012, 24, 3120 CrossRef CAS.
- (a) C.-H. Chen, W.-S. Huang, M.-Y. Lai, W.-C. Tsao, J. T. Lin, Y.-H. Wu, T.-H. Ke, L.-Y. Chen and C.-C. Wu, Adv. Funct. Mater., 2009, 19, 2661 CrossRef CAS; (b) S. Takizawa, V. A. Montes and P. Anzenbacher Jr, Chem. Mater., 2009, 21, 2452 CrossRef CAS; (c) S. Gong, Y. Zhao, C. Yang, C. Zhong, J. Qin and D. Ma, J. Phys. Chem. C, 2010, 114, 5193 CrossRef CAS; (d) H. Huang, X. Yang, B. Pan, L. Wang, J. Chen, D. Ma and C. Yang, J. Mater. Chem., 2012, 22, 13223 RSC; (e) H. Huang, Y. Wang, B. Pan, X. Yang, L. Wang, J. Chen, D. Ma and C. Yang, Chem.–Eur. J., 2013, 19, 1828 CrossRef CAS PubMed.
- J. H. Kim, D. Y. Yoon, J. W. Kim and J.-J. Kim, Synth. Met., 2007, 157, 743 CrossRef CAS.
- (a) H. Inomata, K. Goushi, T. Masuko, T. Konno, T. Imai, H. Sasabe, J. J. Brown and C. Adachi, Chem. Mater., 2004, 16, 1285 CrossRef CAS; (b) K. S. Son, M. Yahiro, T. Imai, H. Yoshizaki and C. Adachi, Chem. Mater., 2008, 20, 4439 CrossRef CAS; (c) M. M. Rothmann, S. Haneder, E. Da Como, C. Lennartz, C. Schildknecht and P. Strohriegl, Chem. Mater., 2010, 22, 2403 CrossRef CAS; (d) C.-H. Chang, M.-C. Kuo, W.-C. Lin, Y.-T. Chen, K.-T. Wong, S.-H. Chou, E. Mondal, R. C. Kwong, S. Xia, T. Nakagawa and C. Adachi, J. Mater. Chem., 2012, 22, 3832 RSC.
- (a) E. L. Williams, K. Haavisto, J. Li and G. E. Jabbour, Adv. Mater., 2007, 19, 197 CrossRef CAS; (b) S.-J. Su, H. Sasabe, T. Takeda and J. Kido, Chem. Mater., 2008, 20, 1691 CrossRef CAS; (c) S.-J. Su, C. Cai and J. Kido, Chem. Mater., 2011, 23, 274 CrossRef CAS; (d) S.-J. Su, C. Cai and J. Kido, J. Mater. Chem., 2012, 22, 3447 RSC.
- (a) Phosphazenes: A Worldwide Insight, ed. M. Gleria and R. De Jaeger, Nova Science Publishers, 2004 Search PubMed; (b) J.-P. Majoral and A.-M. Caminade, Chem. Rev., 1999, 99, 845 CrossRef CAS PubMed; (c) C. W. Allen, Inorg. Chim. Acta, 2011, 372, 32 CrossRef CAS; (d) V. Chandrasekhar and B. Murugesapandian, Acc. Chem. Res., 2009, 42, 1047 CrossRef CAS PubMed; (e) K. Inoue and T. Itaya, Bull. Chem. Soc. Jpn., 2001, 74, 1381 CrossRef CAS.
- (a) P. Schrögel, M. Hoping, W. Kowalsky, A. Hunze, G. Wagenblast, C. Lennartz and P. Strohriegl, Chem. Mater., 2011, 23, 4947 CrossRef; (b) M. S. Soh, S. A. G. Santamaria, E. L. Williams, M. Pérez-Morales, H. J. Bolink and A. Sellinger, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 531 CrossRef CAS.
- (a) V. Adamovich, J. Brooks, A. Tamayo, A. M. Alexander, P. I. Djurovich, B. W. D'Andrade, C. Adachi, S. R. Forrest and M. E. Thompson, New J. Chem., 2002, 26, 1171 RSC; (b) R. J. Holmes, S. R. Forrest, Y.-J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Appl. Phys. Lett., 2003, 82, 2422 CrossRef CAS.
- (a) M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4 CrossRef CAS; (b) K. Goushi, R. Kwong, J. J. Brown, H. Sasabe and C. Adachi, J. Appl. Phys., 2004, 95, 7798 CrossRef CAS.
- X. Cai, A. B. Padmaperuma, L. S. Sapochak, P. A. Vecchi and P. E. Burrows, Appl. Phys. Lett., 2008, 92, 083308 CrossRef.
- (a) C. Adachi, R. C. Kwong, P. Djurovich, V. Adamovich, M. A. Baldo, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 2001, 79, 2082 CrossRef CAS; (b) H. Yersin, Top. Curr. Chem., 2004, 241, 1 CrossRef CAS.
- (a) R. J. Holmes, B. W. D'Andrade, S. R. Forrest, X. Ren, J. Li and M. E. Thompson, Appl. Phys. Lett., 2003, 83, 3818 CrossRef CAS; (b) X. Ren, J. Li, R. J. Holmes, P. I. Djurovich, S. R. Forrest and M. E. Thompson, Chem. Mater., 2004, 16, 4743 CrossRef CAS; (c) S. Ye, Y. Liu, C. Di, H. Xi, W. Wu, Y. Wen, K. Lu, C. Da, Y. Liu and G. Yu, Chem. Mater., 2009, 21, 1333 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, additional data and spectra. CCDC 954020. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3mh00079f |
| This journal is © The Royal Society of Chemistry 2014 |