Coordination polymer particles as potential drug delivery systems†
Inhar
Imaz
a,
Marta
Rubio-Martínez
a,
Lorena
García-Fernández
a,
Francisca
García
b,
Daniel
Ruiz-Molina
a,
Jordi
Hernando
c,
Victor
Puntes
a and
Daniel
Maspoch
*a
aCentro de Investigación en Nanociencia y Nanotecnología (ICN-CSIC) Campus UAB, 08193 Bellaterra, Spain. E-mail: daniel.maspoch.icn@uab.es
bDepartament de Química, Universitat Autònoma de Barcelona, Campus UAB, 08193-Bellaterra, Spain
cInstitut de biotecnologia i de biomedicina, Campus UAB, 08193-Bellaterra, Spain
First published on 20th May 2010
Abstract
Micro- and nanoscale coordination polymer particles can be used for encapsulating and delivering drugs. In vitro cancer cell cytotoxicity assays showed that these capsules readily release doxorubicin, which shows anticancer efficacy. The results from this work open up new avenues for metal–organic capsules to be used as potential drug delivery systems.
Micro- and nanomaterials able to encapsulate pharmaceutical agents have been actively explored as carriers for therapy to achieve a prolonged and better controlled drug administration.1 To date, the vast majority of carriers are based on dendrimers,2 liposomes,3 organic polymeric4 and inorganic particles.5 In this field, we have recently described a general method for encapsulating desired species into micro- and nanoscale coordination polymer particles6via a coordination polymerization followed by a fast precipitation,7 and have demonstrated its utility by entrapping organic dyes, quantum dots and magnetic nanoparticles into blue fluorescent spheres (hereafter referred to as Zn(bix)) created by connecting Zn2+ metal ions through 1,4-bis(imidazol-1-ylmethyl)benzene (bix) organic ligands. Owing to their small size and ability for entrapping a wide variety of substances, we believe that coordination polymer nanospheres also show promise for encapsulating drugs and thus being used as novel functional carriers for drug delivery.
Thus far, some advances have been made in using metal–organic frameworks (MOFs) as drug delivery systems.8–10 Férey's group first described promising adsorption and release properties of ibuprofen on bulk hybrid inorganic–organic solids,8 whereas Lin et al. have structured a Pt-based drug at the nanoscale by using it as one building block for creating the framework of a coordination polymer.9 Very recently, Horcajada, Gref and co-workers have also shown that porous crystalline nano-MOFs can adsorb and release several drugs, thus acting as potential non-toxic drug nanocarriers.10 Herein we wish to report an alternative general methodology for in situ encapsulating unmodified drugs into metal–organic frameworks in the form of micro- and nanoscale spherical particles (Fig. 1a). Doxorubicin (DOX), SN-38, camptothecin (CPT) and daunomycin (DAU) were chosen as archetypical drugs because of their current use for cancer therapy and fluorescence properties that facilitate their monitorization.11 The encapsulation of all these drugs, their release and initial in vitro cellular studies serve as excellent tests to preliminary illustrate the use of coordination polymer spheres as drug delivery systems.
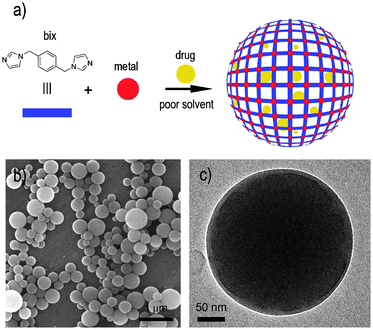 | ||
| Fig. 1 (a) Schematic illustration describing the encapsulation of drugs into metal–organic spheres created by the connection of metal ions, such as Zn2+, through multitopic organic ligands, such as bix. (b) SEM and (c) TEM images of a representative colloidal solution of DOX/Zn(bix) spheres. | ||
In a typical experiment, metal–organic Zn(bix) spheres with encapsulated DOX [DOX/Zn(bix)], SN-38 [SN-38/Zn(bix)], CPT [CPT/Zn(bix)] and DAU [DAU/Zn(bix)] were prepared by addition of an aqueous solution of Zn(NO3)2·6H2O to an ethanolic solution of bix containing the drug (c ∼3.3 × 10−3 M) under stirring at room temperature. The resulting spheres were then purified by centrifugation and washed several times with ethanol. This process was repeated until no fluorescence signal from free, non-encapsulated drug was detected in the supernatant solution. Fig. 1b and c show representative scanning (SEM) and transmission (TEM) electron microscopy images of the resulting coordination polymer spherical capsules, whose diameter was controlled from 100 to 1500 nm by adjusting the initial concentrations of the reactants.6
The entrapment of DOX, CPT, SN-38 and DAU into these spheres was confirmed by both absorption and fluorescence measurements, and encapsulation efficiencies up to 21% of the initial drug concentration were measured. In all cases, the fluorescence emission spectrum of the colloids matches that of free DOX, DAU, SN-38 and CPT (Fig. 2e–h), which results in blue [CPT/Zn(bix)], green [SN-38/Zn(bix)] and red [DOX/Zn(bix) and DAU/Zn(bix)] emitting spheres upon selective excitation of the drug (Fig. 2a–d). Furthermore, since bare Zn(bix) particles already show blue luminescence when excited at 355 nm, broadband fluorescence spectra are measured upon UV excitation, which show contributions from both the drug and Zn(bix) spheres emission (Fig. 2e–h).
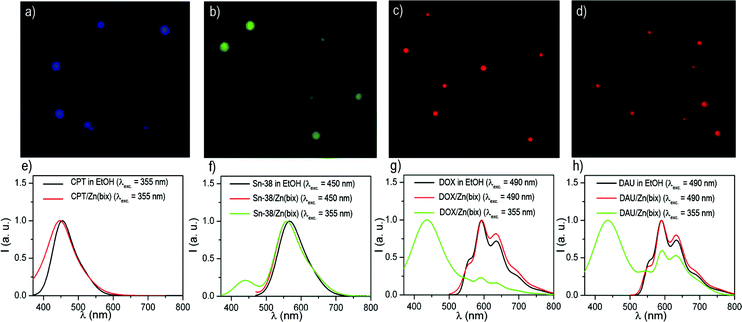 | ||
| Fig. 2 (a–d) Fluorescence optical microscope images of (a) CPT/Zn(bix), (b) SN-38/Zn(bix), (c) DOX/Zn(bix), and (d) DAU/Zn(bix) spheres. (e–h) Fluorescence emission spectra of (e) free CPT and CPT/Zn(bix) (collected at λexc = 355 nm), (f) free SN-38 and SN-38/Zn(bix) (collected at λexc = 450 and 355 nm), (g) free DOX and DOX/Zn(bix) (collected at λexc = 490 and 355 nm), and (h) free DAU and DAU/Zn(bix) (collected at λexc = 490 and 355 nm). | ||
To investigate the drug release from coordination polymer particles, we first prepared colloidal solutions of 300 ± 23 m in diameter DOX/Zn(bix) and SN-38/Zn(bix) spheres in phosphate buffered saline solution (PBS) at pH = 7.4, and the resulting colloids were placed in a dialysis bag (cut-off molecular weight: 3500) at 37 °C. Both dispersions were dialyzed against 100 mL of PBS for 48 hours. The DOX and SN-38 release profiles measured by fluorescence spectroscopy are depicted in Fig. 3a. At 37 °C, Zn(bix) spheres containing those drugs showed a fast release of ∼ 80% at 8 hours, followed by an additional release of ∼ 15% over the next 2 days. The DOX release from Zn(bix) spheres was also perceptive with naked eyes because the initial violet-pink colour of the colloid gradually changed to a white colour characteristic of bare Zn(bix) spheres.
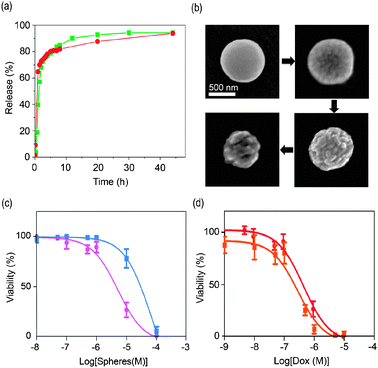 | ||
| Fig. 3 (a) In vitro release profile of DOX and SN-38 from DOX/Zn(bix) (dot, red) and SN-38/Zn(bix) (square, green) spheres incubated in pH 7.2 PBS at 37 °C. (b) SEM micrographs of DOX/Zn(bix) spheres taken at 1, 4, 8, and 24 hours, showing representative degradation in pH 7.4 PBS at 37 °C. (c and d) In vitro cytotoxicity assay curves after 24 h for HL60 cells obtained by plotting the cell viability percentage against the (c) Zn(bix) (square, blue) and DOX/Zn(bix) (dot, pink) concentration and (d) the DOX release from DOX/Zn(bix) spheres (dot, red) and DOX (square, orange) concentration. | ||
A number of factors can contribute to the release of DOX and SN-38 from coordination polymer particles, including desorption of drug adsorbed on the sphere surface, diffusion of drug through the coordination polymeric sphere, and erosion of the sphere. Similar to other encapsulating systems, the fast release can be attributed to both desorption and diffusion of drug as well as to the gradual erosion of Zn(bix) spheres in PBS for subsequent diffusion from the surface or through developing pores.12 To confirm the erosion of Zn(bix) spheres at 37 °C, a colloidal PBS solution of DOX/Zn(bix) spheres was maintained at this temperature and sequentially investigated by SEM and dynamic light scattering (DLS). Fig. 3b shows representative SEM images showing the time-dependent degradation of such spheres. As can be seen there, the surface of Zn(bix) spheres becomes gradually rougher and more cracked, reducing their overall volume with time, as also confirmed by DLS. Noticeably, such process was found to slow down at room temperature.
Our abilities to synthesize coordination polymer spheres for encapsulating drugs and the capacity to release them prompted us to preliminary evaluate their anticancer efficacies. We performed in vitro cytotoxicity assays on HL60 (Human promyelocytic leukemia cells) cell line with 24 h and 48 h of incubation (Fig. 3c). Treatment of HL60 with Zn(bix) and DOX/Zn(bix) spheres did not lead to any appreciable cell death after 24 h and 48 h of incubation at concentrations ranging from 0.01 µM to 0.5 µM. However, between 0.5 µM and 10 µM, some differences were observed between both types of spheres. In this range, the DOX from DOX/Zn(bix) spheres acts on the cells notably reducing the cell-viability down to 25% at 10 µM, whereas the Zn(bix) spheres gave a cell-viability close to 80%. Overall, DOX/Zn(bix) spheres gave a half maximal inhibitory concentration (IC50) of 5.2 µM and 4.5 µM after 24 h and 48 h of incubation, respectively, whereas the non-encapsulated Zn(bix) spheres had respective IC50 values of only 62.5 µM and 99.9 µM. These results demonstrate that at this range the Zn(bix) matrix has a low contribution on the cytotoxic effects, and confirms that delivered DOX from DOX/Zn(bix) spheres is the major responsible of their cytotoxic activity against HL60.
To further evaluate the cytotoxic effect of the DOX released from DOX/Zn(bix) spheres, HL60 cells were also treated with free DOX. Fig. 3d shows the dose response of free DOX and DOX from DOX/Zn(bix) spheres after 24 h of incubation.13 In both cases, an appreciable cell death was observed at concentrations ranging from 0.1 µM and 10 µM. Indeed, both free DOX and DOX from DOX/Zn(bix) spheres gave similar IC50 values of 0.3 µM and 0.4 µM, respectively. These results suggest that DOX/Zn(bix) spheres have similar cytotoxic effects against HL60 than free DOX. Once encapsulated, the DOX released from the metal–organic DOX/Zn(bix) spheres can induce the cell death in cancer cells.
In summary, the presented results show that coordination polymer micro- and nanospheres constitute a novel and promising type of materials to be used as functional matrices for encapsulating a large panel of drugs. We first demonstrate the release of drugs from coordination polymer capsules, and their potential anticancer efficacies in vitro. As coordination polymers with a broad range of structures and functionalities can be prepared, this approach could be generalized for obtaining metal–organic delivery systems with novel compositions and functionalities. Future work includes the in vivo studies of drug delivery and targeting of coordination polymer particles as well as the development of metal–organic capsules with novel properties and functionalities.
This work was supported by projects VALTEC08-2-003, MAT2009-13977-C03 and CTQ2009-07469. D. M. and I. I. thank the Ministerio de Ciencia y Tecnología for respective RyC and JdC contracts. M. R.-M. and L. G.-F. thank the Institut Català de Nanotecnologia for research fellowships. The authors thank the Servei de Microscopia of the UAB.
Notes and references
- (a) R. Langer, Nature, 1998, 392, 5 CAS; (b) T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818 CrossRef CAS.
- (a) J. M. J. Fréchet, J. Polym. Sci., Part A: Polym. Chem., 2003, 43, 3713 CrossRef; (b) S. Svenson and D. A. Tomalia, Adv. Drug Delivery Rev., 2005, 57, 2106 CrossRef CAS.
- (a) F. M. Muggia, J. D. Hainsworth, S. Jeffers, P. Miller, S. Groshen, M. Tan, L. Roman, B. Uziely, L. Muderspach, A. Garcia, A. Burnett, F. A. Greco, C. P. Morrow, L. J. Paradiso and L.-J. Liang, J. Clin. Oncol., 1997, 15, 987 CAS; (b) D. D. Lasic and D. Papahadjopoulos, Science, 1995, 267, 1275 CrossRef CAS.
- (a) J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329 CrossRef CAS; (b) B. J. Nehilla, P. G. Allen and T. A. Desai, ACS Nano, 2008, 2, 538 CrossRef CAS.
- (a) H. Otsuka, Y. Nagasaki and K. Kataoka, Adv. Drug Delivery Rev., 2003, 55, 403 CrossRef CAS; (b) I. Brigger, C. Dubernet and P. Couvreur, Adv. Drug Delivery Rev., 2002, 54, 631 CrossRef CAS.
- (a) I. Imaz, J. Hernando, D. Ruiz-Molina and D. Maspoch, Angew. Chem., Int. Ed., 2009, 48, 2325 CAS.
- (a) M. Oh and C. A. Mirkin, Nature, 2005, 438, 651 CrossRef CAS; (b) X. Sun, S. Dong and E. Wang, J. Am. Chem. Soc., 2005, 127, 13102 CrossRef CAS; (c) H. Maeda, M. Hasegawa, T. Hashimoto, T. Kakimoto, S. Nishio and T. Nakanishi, J. Am. Chem. Soc., 2006, 128, 10024 CrossRef CAS; (d) K. H. Park, K. Jang, S. U. Son and D. A. Sweigart, J. Am. Chem. Soc., 2006, 128, 8740 CrossRef CAS; (e) H. Wei, B. Li, Y. Du, S. Dong and E. Wang, Chem. Mater., 2007, 19, 2987 CrossRef CAS; (f) S. Jung and M. Oh, Angew. Chem., Int. Ed., 2008, 47, 2049 CrossRef CAS; (g) M. Oh and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 45, 5492; (h) Y.-M. Jeon, G. S. Armatas, J. Heo, M. G. Kanatzidis and C. A. Mirkin, Adv. Mater., 2008, 20, 2105 CrossRef CAS; (i) I. Imaz, D. Maspoch, C. Rodríguez-Blanco, J.-M. Pérez-Falcón, J. Campo and D. Ruiz-Molina, Angew. Chem., Int. Ed., 2008, 47, 1860.
- (a) P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974 CrossRef CAS; (b) P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regi, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774 CrossRef CAS.
- W. J. Rieter, K. M. Pott, K. M. L. Taylor and W. Lin, J. Am. Chem. Soc., 2008, 130, 11584 CrossRef CAS.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS.
- (a) M. Muggia, J. D. Hainsworth, S. Jeffers, P. Miller, S. Groshen, M. Tan, L. Roman, B. Uziely, L. Muderspach, A. Garcia, A. Burnett, F. A. Greco, C. P. Morrow, L. J. Paradiso and L.-J. Liang, J. Clin. Oncol., 1997, 15, 987 CAS; (b) H. L. Wong, R. Bendayan, A. M. Rauth, Y. Li and X. Y. Wu, Adv. Drug Delivery Rev., 2007, 59, 491 CrossRef CAS.
- (a) R. P. Batycky, J. Hanes, R. Langer and D. A. Edwards, J. Pharm. Sci., 1997, 86, 1464 CrossRef CAS; (b) N. Faisant, J. Siepmann and J. P. Benoit, Eur. J. Pharm. Sci., 2002, 15, 355 CrossRef CAS; (c) J. Wang, B. M. Wang and S. P. Schwendeman, J. Controlled Release, 2002, 82, 289 CrossRef CAS; (d) C. Berkland, K. Kim and D. W. Pack, Pharm. Res., 2003, 20, 1055 CrossRef CAS.
- The concentration of delivered DOX from DOX/Zn(bix) spheres was calculated according to a maximum encapsulation efficiency of 21% with respect to the initial DOX concentration used for the encapsulation process, and a release of 88% (for 24 h) of the encapsulated DOX, according to the in vitro delivery studies.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic and experimental procedures, SEM, TEM, fluorescence optical and DLS images, and in vitro cytotoxicity assay curves. See DOI: 10.1039/c003084h |
| This journal is © The Royal Society of Chemistry 2010 |
