Liquid marble for gas sensing
Junfei
Tian
,
Tina
Arbatan
,
Xu
Li
and
Wei
Shen
*
Australian Pulp and Paper Institute, Department of Chemical Engineering, Monash University, Wellington Rd, Clayton, Vic. 3800, Australia. E-mail: wei.shen@eng.monash.edu.au; Tel: +61 3 99053447
First published on 20th May 2010
Abstract
The porous and superhydrophobic shell of a liquid marble prevents contact of its liquid core with outside surfaces, but allows gas transport. Liquid marble can therefore be used to sense gas or emit gas. Liquid marbles loaded with different indicators can simultaneously sense different gases via different mechanisms.
Since the phenomenon of liquid marble was reported by Quéré and Aussillous,1 there has been a flourish of studies of this phenomenon on the fundamental physics and physical chemistry criteria of marble formation.2–5 The liquid marble phenomenon is generally understood as being a manifestation of superhydrophobicity; hydrophobic particles are in contact with a liquid which does not wet the particles; liquid marble may be made by rolling a liquid drop over a hydrophobic powder bed, and particles picked up by the liquid drop form a loosely packed and porous shell encapsulating the drop.5 The hydrophobic liquid marble shell prevents any direct contact between the liquid core and surfaces outside the marble shell, allowing liquid marbles to assume a near spherical shape and sit stably on a supporting surface, solid or liquid. Recently, liquid marble made using solid particles that have a <90° contact angle with the liquid has been reported.6 Nguyen7 studied the shell morphology of liquid marbles using confocal microscopy and obtained a more detailed picture of the porous and multilayer structure of the liquid marble shell: smaller particles tend to be in direct contact with the liquid core and larger particles make up the outer layers, which are more porous and rough. Such a structure makes the liquid marble shell stretchable; it enables a liquid marble to withstand a certain level of mechanical impact and deformation.
The interesting phenomenon of liquid marble has attracted a growing number of application studies exploiting the properties of the system. Hapgood et al.8 reported X-ray tomography images showing the hollow structure of dried liquid marbles. Their study explored the drying of the liquid core of a liquid marble to create hollow marble shells for pharmaceutical applications. Zhang et al. reported the use of liquid marble for DNA amplification using PCR microfluidic devices.9 This application took advantage of the low liquid transport friction and non-contamination nature of liquid marble. Zhao et al.10 have also shown that liquid marbles formed using hydrophobic Fe3O4 nanoparticles can be manipulated and transported using magnetic fields.
Among the many properties of liquid marble, its porous shell structure attracts our attention. The porous and superhydrophobic marble shell prevents direct contact between the marble liquid core and any surface outside of the marble, but allows the transport of gas or vapour. This property intuitively enables liquid marble to be used as a gas or vapour sensor. When a liquid marble is formed with an appropriate indicator solution and placed onto the surface of a gas or vapour emitting solid or liquid, the indicator solution is at an intimately close distance from the gas or vapour emitting source while having no direct contact with the source. This makes the liquid marble an efficient sensor for gases or vapours of interest. In this communication we describe the use of liquid marbles as colorimetric and fluorescent gas sensors for ammonia and hydrochloric acid gases. The quantitative aspects of the liquid marble sensors are also studied using paper-based colorimetric measurements.
In a qualitative NH3 sensing experiment, four groups of liquid marbles were made using water (MilliQ, 18 MΩ), a phenolphthalein solution (AR, BDH), a 0.1 M CoCl2·2H2O solution (AR, Analar) and a 0.1 M CuCl2·2H2O solution (AR, Analar); they were all shelled with Teflon powder (Sigma-Aldrich). When those liquid marbles were exposed to NH3 gas emitted from an NH4·OH solution (6.25%, diluted from 25% ammonia solution (AR, Analar)), the colour changes of the liquid marbles rapidly developed (Fig. 1). The rapid colour change of the indicators inside the liquid marbles is an indication that the porous liquid marble shell presents low resistance to gas transport through the shell; liquid marbles can, therefore, be used as effective gas sensors with appropriate indicators.
 | ||
| Fig. 1 (a) Four groups of liquid marbles containing water, phenolphthalein, CoCl2, and CuCl2 solutions, respectively, were placed from left to right in an “M” pattern. (b) The colour changes of the indicators in these liquid marbles after exposure to ammonia gas. | ||
Ammonia sensing from an aqueous NH3 emitting source using liquid marbles was also carried out. A liquid marble loaded with phenolphthalein indicator was placed onto the surface of an NH4OH solution; rapid colour changes of liquid marbles were observed as expected. To confirm that the colour change of the indicator is caused by the ammonia gas transport across the marble shell and not by any direct liquid contact between the indicator solution and the ammonia solution, we further made two liquid marbles, one contained NH4OH solution and the other contained NaOH solution. These two liquid marbles were placed onto two Petri dishes filled with the phenolphthalein indicator solution. Whilst the NH4OH liquid marble caused immediate colour change of the indicator solution, the NaOH liquid marble did not cause any colour change, therefore confirming that the indicator colour change was caused by ammonia gas transport across the marble shell and not by liquid contact.
Among the liquid marbles made of the three different indicators, the colour change of the phenolphthalein indicator gradually faded and disappeared in 2 h as the NH3 dissolved in the indicator solution evaporated. On the other hand, the transition metal salt indicators provided more permanent colour changes after reacting with NH3 and forming stable complexes11 (Fig. 1). The complex tetraamminediaquacopper(II) ions, for example, have a stability constant of Kstab = 1012.11 The use of different indicators to achieve different colour changes also implies that liquid marbles made using different but chemically specific indicators can potentially be used to detect different gaseous species. Conversely, this phenomenon also promises the possible use of liquid marbles to detect chemical species in aqueous media using gas(es) as an indicator(s).
Fluorescent pH indicator was found to have a special advantage when used as the core solution of liquid marbles for gas sensing. We used liquid marbles made of an aqueous solution of 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) (10 mg L−1, Sigma-Aldrich) and Teflon powder to elucidate the gas detection using fluorescent indicator. HPTS is a strongly water soluble and pH dependent fluorescent dye with a pKa of ∼7.3.12 HPTS has a pH dependent fluorescent colour change from blue (405 nm) at pH ≤ 6 to green (centered at 450 nm) at pH ≥ 8.12 HPTS liquid marbles show no specific colour under ambient lighting before and after exposure to HCl vapour (Fig. 2(a)), but show a green fluorescence under UV light (Fig. 2(b)).
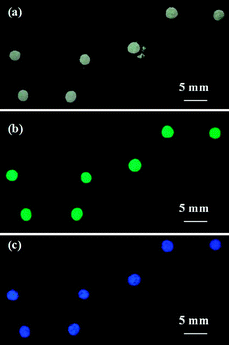 | ||
| Fig. 2 (a) Liquid marbles containing HPTS indicator under ambient lighting before and after exposure to HCl vapour; (b) liquid marbles containing the HPTS indicator under UV light before exposure to HCl vapour; (c) liquid marbles containing HPTS under UV light after exposure to HCl vapour. | ||
This property of the fluorescent indicator allows it to be distinguished from colorimetric indicators. When HPTS liquid marbles were exposed to HCl vapour, HPTS showed a clear fluorescent colour change which could be observed under UV light (Fig. 2(c)). This result has an implication that different detection mechanisms (e.g. colorimetry and fluorescence) can be employed to carry out simultaneous detections of different vapours or gases. Liquid marble shells provide a superhydrophobic separating layer which effectively prevents the indicator solutions in different marbles from contacting one another, allowing the indicators to report independent colour changes.
Here we further present an application of gas sensing using liquid marble in the work-place in the printing industry. Flexography is a dominant process in the printing of a wide range of packaging materials, including corrugated boxes and polymer films.13,14 Water-based ink formulations for flexographic printing are widely used for the polymer film printing. A successful commercial water-based formulation is based on the acrylic resin system; inks formulated using the acrylic resin system are a stable pigment suspension under elevated pH (8.0–9.5) which is provided by the dissociation of water in the presence of ammonia and low molecular weight amines. The drying of the ink after printing is triggered by the evaporation of ammonia and low molecular weight amines. The acrylic resin suspension system coagulates as the pH decreases to below 7,15 providing adhesion of the dried ink to the substrate. However, the release of ammonia and low molecular weight amine during ink drying is a serious source of air pollution in the printing industry.
Liquid marble made of a CoCl2 solution and Teflon powder was used to detect the ammonia and amine released from the flexographic ink, since Co(II) ion17 can form stable complexes with low molecular weight primary, secondary and some tertiary amines. Five hundred micrograms of acrylic resin based flexographic ink (TMI, USA) was placed into a Petri dish (ϕ = 10 cm); a smaller Petri dish (ϕ = 5 cm) carrying a CoCl2 liquid marble (formed with 0.1 M CoCl2 solution) was also placed into the larger Petri dish which was then capped. The liquid marble changed colour (Fig. 3) within 7 min.
 | ||
| Fig. 3 A liquid marble of CoCl2 shelled with Teflon powder changed colour after being exposed to the vapour of water-based flexographic ink (left). A fresh CoCl2 liquid marble (right) was placed in the same Petri dish for comparison. | ||
To visually compare the colour change, a fresh CoCl2 liquid marble was placed near the liquid marble that had been exposed to the ink vapour (Fig. 3). The unambiguous colour development of the liquid marble exposed to the ink vapour, therefore, demonstrates the potential of using liquid marble for practical vapour emission detection.
The potential to use liquid marble for quantitative gas sensing was also explored. Our aim in this study was to establish correlations between the colorimetric sensing signal of a gaseous analyte and its concentration in aqueous samples so as to prove the concept of quantitative gas sensing using liquid marble. Ammonia was used as the gaseous chemical species of interest. The experimental arrangement was made so that a NH4OH solution was encapsulated inside liquid marbles and a quantitative colorimetric signal was collected from outside the liquid marbles.
Ammonia solutions of different concentrations (25%, 12.5%, 6.25%, 3.13% and 1.56%) were used to form liquid marbles. Liquid drops of the same size (4 μL) were generated using a Hamilton syringe and a gauge 30 needle to form liquid marbles. A paper-based gas sensing method was used to detect NH3 released from the marbles. Circular paper discs (ϕ = 2 mm) were produced from Whatman No. 4 filter paper using a disc punching device (Facit 4070, Sweden). A 600 nL aliquot of 0.1 M CuCl2 solution was introduced onto each paper disc and allowed to dry. Five so-treated paper discs were then fixed onto sticky tape, which was placed in a Petri dish to form one gas sensing device. The five indicator-loaded paper discs allowed five replicate colorimetric measurements to be made. Five liquid marbles containing NH4OH solution of a specific concentration were introduced into each of the above paper-based gas sensing devices. The device was then capped to allow the controlled exposure of the indicator-loaded paper discs to NH3 for 10 min. Circular movement of the sensing device was provided to allow the free rolling of the liquid marbles in the Petri dish. At the end of the 10 min period, liquid marbles were removed from the Petri dish.
Colour changes of the paper discs were quantified using a desktop scanner (EPSON perfection 2450 PHOTO) and the software (Adobe PhotoShop 7.0) following the procedure we reported previously.16Fig. 4(a) and (b) show the visual appreciation of the colour changes of the paper discs; Fig. 4(c) shows the calibration curve of the colorimetric measurements. The quantitative results so obtained are encouraging.
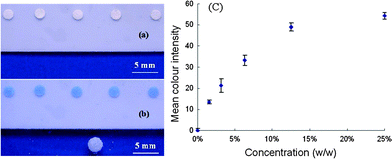 | ||
| Fig. 4 (a) Paper discs loaded with the CuCl2 indicator; (b) colour change of the indicator-treated paper discs after the controlled exposure to NH3; (c) color intensity vs. concentration of the NH4OH solutions. Colour intensity values were obtained using the software Adobe PhotoShop 7.0. | ||
In conclusion, the porous structure of the liquid marble shell offers a realistic means to use liquid marble to sense gaseous and vaporous components in an environment. The careful selection of hydrophobic powdered materials to form liquid marbles may eliminate chemical reactions of gas and vapour with the liquid marble shell. We used Teflon powder to form liquid marble for NH3 and HCl sensing; Teflon has an excellent chemical resistance to these gases, any chemical interference by Teflon is, therefore, negligible.18
Liquid marble enables several chemical sensing design possibilities to be exploited. While a liquid marble loaded with an indicator solution can be used as a gas or vapour sensor, a liquid marble loaded with one (or several) water dissolvable gas(es) can be used as a gas emitter. In this sensor design, the gas is used as an indicator to indicate the presence of the chemical species of interest in the environment. Fluorescent and colorimetric reactions can be used to further enhance the capabilities of gas sensing.
The ability to use liquid marble to produce quantitative chemical sensing is particularly attractive and worth pursuing. It offers a less expensive and more flexible gas sensing option to the current main stream gas sensing instruments which use a hydrophobic and gas permeable polymeric membrane to separate gas for indirect electrochemical detection.19
Australian Research Council funding through projects DP1094179 and LP0989823 is gratefully acknowledged. JT, TA, XL would like to thank Monash University and the Faculty of Engineering for their postgraduate scholarships.
Notes and references
- P. Aussillous and D. Quéré, Nature, 2001, 411, 924 CrossRef CAS.
- E. Bormashenko, Y. Bormashenko and A. Musin, J. Colloid Interface Sci., 2009, 333, 419 CrossRef CAS.
- E. Bormashenko, Y. Bormashenko, A. Musin and Z. Barkay, ChemPhysChem, 2009, 10, 654 CrossRef CAS.
- L. Gao and T. J. McCarthy, Langmuir, 2007, 23, 10445 CrossRef CAS.
- P. Aussillous and D. Quéré, Proc. R. Soc. London, Ser. A, 2006, 462, 973 CrossRef CAS.
- M. Dandan and H. Y. Erbil, Langmuir, 2009, 25, 8362 CrossRef CAS.
- T. H. Nguyen, Masters Thesis, Monash University, 2009 Search PubMed.
- K. P. Hapgood, L. Farber and J. N. Michaels, Powder Technol., 2009, 188, 248 CrossRef CAS.
- C. Zhang, J. Xu, W. Ma and W. Zheng, Biotechnol. Adv., 2006, 24, 243 CrossRef CAS.
- Y. Zhao, J. Fang, H. Wang, X. Wang and T. Lin, Adv. Mater., 2010, 22, 707 CrossRef CAS.
- T. Jackson, Chemistry Ideas, Heinemann Education Publishers, Bath, 2nd edn, 2000, p. 296 Search PubMed.
- C. C. Overly, K. D. Lee, E. Berthiaume and P. J. Hollenbeck, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 3156 CAS.
- J. M. Adams and P. A. Dolin, Printing Technology, Delmar, New York, 5th edn, 2002, p. 352 Search PubMed.
- R. H. Leach and R. J. Pierce, The Printing Ink Manual, Kluwer Adademic Publishers, Dordrecht, 5th edn, 1999, p. 547 Search PubMed.
- P. Laden, Chemistry and Technology of Water Based Inks, Chapman & Hall, London, 1997, p. 198 Search PubMed.
- X. Li, J. Tian and W. Shen, Anal. Bioanal. Chem., 2010, 396, 495 CrossRef CAS.
- G. J. Van Driel, W. L. Driessen and J. Reedijk, Inorg. Chem., 1985, 24, 2919 CrossRef CAS.
- http://www.vp-scientific.com/Chemical_Resistance_Chart.htm .
- D. A. Skoog, F. J. Holler and T. A Nieman, Principle of Instrumental Analysis, Harcourt Brace & Co., Orlando, 5th edn, 1998, 607 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
