 Open Access Article
Open Access ArticleRecent developments and sustainability in monitoring chlorine residuals for water quality control: a critical review†
Yohanz
Khor
ab,
A. R. Abdul
Aziz
 c and
Su Sin
Chong
c and
Su Sin
Chong
 *ab
*ab
aSchool of Energy and Chemical Engineering, Xiamen University Malaysia, Selangor Darul Ehsan, Malaysia. E-mail: susin.chong@xmu.edu.my
bCollege of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
cDepartment of Chemical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia
First published on 25th July 2024
Abstract
Clean and safe water is a vital resource for human life. To ensure that consumable water is bacteria-free, water treatment, including the widely used chlorination process, is performed. Free chlorine resulting from the chlorination process in consumable water is a dangerous analyte and it is one of the vital parameters in water quality monitoring. Global guidelines state that free chlorine in consumable water should be controlled at 0.2–5.0 mg L; deviations from this concentration range could cause consumers to suffer from dire health effects. To control the concentration within the said range, various methods for free chlorine monitoring have been developed in recent years, categorized into conventional, optical and electrochemical methods. However, limitations such as high cost and complexity of analysis prevent these conventional methods from meeting the “Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free and Deliverable to end users” criteria for diagnostic tests set by the World Health Organization. Paper-based methods are therefore introduced to replace the conventional methods in the hope of meeting the criteria. However, the paper-based methods are still confined to the lab scale and are highly dependent on chemicals for the detection of free chlorine. Therefore, the capabilities of carbon quantum dots are introduced as a suitable indicator for free chlorine measurement. Using carbon quantum dots as an indicator is recommended for the future development of sustainable portable paper-based sensors due to their excellent absorption and fluorescent properties; in addition, carbon quantum dots can be synthesized from natural resources.
| Yohanz Khor Yohanz Khor obtained his B.Sc. degrees in New Energy Science and Engineering from Xiamen University Malaysia in 2021. Currently, he is pursuing a master's degree at the same university in the School of Energy and Chemical Engineering. His research primarily focuses on the development, analysis, characterization, and applications of carbon-based quantum dots across various fields. |
| A. R. Abdul Aziz Ir Dr Abdul Aziz Abdul Raman is a professor in the Chemical Engineering Department, Faculty of Engineering, University of Malaya. Currently he is the Acting Registrar of the University. Previous positions held by him include Deputy Vice Chancellor (Student Affairs), Deputy Vice Chancellor (Development), and Dean of Faculty of Engineering. He has graduated more than 100 PhD and master's students and has published more than 300 articles in high impact journals and conference proceedings. His main research areas are green technology and advanced wastewater treatment. |
| Su Sin Chong Su Sin Chong received her PhD degree in engineering from University Malaya, Malaysia, in 2016. She subsequently joined the School of Mathematics, Computer Science & Engineering at City, University of London, United Kingdom, as a researcher. She currently works as an assistant professor at Xiamen University Malaysia, Department of Energy and Chemical Engineering. Her current research interests include water quality monitoring and the green synthesis and application of carbon-based quantum dots. Additionally, she is concurrently engaged in the design and development of fiber optic sensors. |
Sustainability spotlightBy maintaining appropriate chlorine levels, we can benefit sustainable water management by aiming to balance environmental, social, and economic aspects. This review paper summarizes existing research, providing a comprehensive overview of the topic. It assists researchers and practitioners in understanding the current state of knowledge, recent advancements, and gaps in the field. Additionally, this paper introduces the capabilities of carbon quantum dots (CQDs) as a suitable indicator for free chlorine measurement. Utilizing natural-based CQDs as an indicator is recommended for the future development of sustainable portable paper-based sensors due to their excellent absorption and fluorescent properties. In summary, the current review contributes to scientific progress, practical solutions, informed policies, and environmental stewardship. It empowers stakeholders to make informed decisions for a sustainable future. |
Introduction
Water and water resources are vital for maintaining a productive environment and ensuring a sufficient food supply for living things.1 Of the 326 million trillion gallons of water on Earth, less than 3% of it is freshwater and only 0.04% of it is readily usable.2 The National Academy of Sciences3 estimated that each person requires an average of 20 to 50 litres of clean safe water daily for various purposes that include drinking, cleaning and cooking. The functions of water in the human body include acting as a lubricant,4 regulating body temperature,5 exterminating dangerous toxins,6 and carrying nutrients to the entire body.7 Therefore, water is very important for the functioning of the human body. Consequently, clean safe water is a vital factor for human life and thus, through water treatments, members of society can obtain their share of clean safe water.To eliminate dangerous and toxic chemicals from water, multiple treatment procedures have been introduced, which are categorized into chemical, physical, biological and physico-chemical methods. Chemical treatment procedures involve the utilization of multiple chemical procedures to safely remove contaminants from water, namely pre-chlorination (algae and biological growth control), aeration (dissolved iron and manganese removal), and disinfection (pathogen killing using chlorination, ozonisation or ultra-violet (UV) light disinfection)8. On the other hand, physical treatment procedures are dependent on physical phenomena to complete the water treatment process. Common methods include sedimentation (utilizes gravity settling to separate particles from treatment water),9 filtration (pollutant removal based on particle size),10 and degasification (dissolved gas removal from treatment water).8 Conversely, biological treatment procedures utilize biodegradation to eliminate dissolved and suspended organic chemical components via the self-purification process of microbes.11 As for physico-chemical treatment, it utilizes chemical techniques to aid physical techniques for water treatment, hence being known as conventional treatment. Common steps in the physico–chemical treatment process include coagulation, chemical precipitation, flotation, membrane filtration, ion exchange, electrochemical treatment, and adsorption. Coagulation for water treatment neutralizes the charges of colloidal suspensions, which results in their destabilization, hence causing smaller particles to aggregate.12 Chemical precipitation is the process of reducing the concentration of heavy metals via the addition of chemicals.13 During flotation, dispersed liquids or solids are separated from the liquid phase via bubble attachment.14 Membrane filtration removes suspended solids and organic components as well as inorganic pollutants. Depending on the size of the particles, types of membrane filtration are selected, including reverse osmosis, ultrafiltration, and nanofiltration.12,13 Next, the ion exchange process exchanges ions from a resin with the ions in the treatment water to remove dissolved ionic contaminants.15 Furthermore, electrochemical treatment, such as electrodialysis, membrane electrolysis, and electrochemical precipitation, removes dissolved contaminants from the treatment water using electricity.16 Lastly, in the adsorption process, pollutants in treatment water (adsorbate) are physically and chemically bonded to the surface of an adsorbent, such as activated carbon, and are removed from the water.17 However, during the treatment process, errors might occur where excessive or insufficient treatment is performed, resulting in unsafe consumable water. Therefore, water quality monitoring plays a vital role in ensuring that consumable water is safe and clean.
Importance of water quality monitoring
Water quality monitoring provides important information that enables vital and reasonable decisions to be made on maintaining water quality, as well as early warnings for issues that may arise.18 Furthermore, water quality management can assist in controlling or monitoring the chemical constituents contained in the supply of water, particularly drinking water. In fact, compared to physical constituents, chemical constituents pose a higher health risk, especially for some chemicals that can cause health problems after prolonged exposure.19 Chemical elements and compounds regularly detected in water include chloride, fluoride, arsenic and iron. In most waters, chloride is commonly found and its amount is affected by leaching of domestic or industrial wastewater.20 Chloride, such as sodium chloride (NaCl), is a salt compound produced by combining chlorine (highly toxic) and a metal. Meanwhile, the presence of chlorine in water, especially treated water, is not due to nature but is actually added into water for chlorination.21 Chlorination is a process to kill bacteria, viruses and parasites in water through the addition of chlorine.21 The final and most important stage in the treatment of drinking water is disinfection, wherein chemicals are added to eradicate any bacteria, viruses, and parasites and stop the water from being recontaminated while it is being transported through pipes.22,23 Chlorine remains the main disinfectant used by the great majority of water treatment plants worldwide to protect the quality of their drinking water, even if there are other disinfection techniques such as ozonisation and UV disinfection. Moreover, the wastewater treatment procedure frequently employs chlorine to inactivate harmful bacteria and viruses before disposal.24 Therefore, the presence of chlorine in water, especially drinking water, is essential to prevent any dangerous microbes from spreading in water.In chlorination, two main types of chlorine, namely liquid sodium hypochlorite and gaseous chlorine, are widely utilized due to their low cost and high reliability. The effectiveness of chlorination is highly dependent on the production of hypochlorous acid (HClO) from the reaction between water and added chlorine (eqn (1)). Due to HClO being a weak acid, it partially dissociates in water to form hydrogen ions and hypochlorite ions (ClO−) (eqn (2)).
Cl2 + H2O ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) HClO + H+ + Cl− HClO + H+ + Cl− | (1) |
| HClO → ClO− + H+ | (2) |
Both HClO and ClO− are capable of oxidation, while the latter exhibits lower oxidative traits. Due to being neutrally charged, HClO can penetrate the bacterial cell wall and perform oxidation, thus resulting in a bactericidal effect caused by the destruction of the bacterial enzyme system. Although the process does not create any resulting harmful product, excessive HClO and ClO− in water are very dangerous when consumed, including carcinogenic effects on consumers. Hence, water quality monitoring plays an important role in ensuring that the concentration of chlorine in water is at a safe level for consumer usage.
Chlorine in water
There are three types of chlorine present in drinking/unprocessed/processed water, which are total chlorine, combined chlorine, and free chlorine. Free chlorine is the chlorine amount available contaminants sanitization whereas combined chlorine is the direct combination of contaminants with chlorine.25 Both HClO and ClO− are generalized as free chlorine. Total chlorine is defined as the total of combined chlorine and free chlorine. Among the three types of chlorine, free chlorine is very important for monitoring to ensure that there is a sufficient amount for water sanitization purposes.26 A specific and controlled range of free chlorine concentration in water was set by the World Health Organization (WHO) to prevent any excessive or deficient amounts of chlorine in treated water, thus ensuring that clean and safe drinking water is supplied to the public.Within the guidelines set by the WHO, the range of free chlorine concentration in drinking water is set at 0.2–5.0 mg L−1. Levels outside this range could have harmful effects on the health of consumers. In the case of deficiency, the water would be unsafe due to the lack of disinfection, thus causing waterborne diseases such as typhoid fever or dysentery.27 Furthermore, the lack of free chlorine in water could cause the growth of algae and even mosquito larvae.28 On the other hand, if the concentration of free chlorine residuals exceeds 5.0 mg L−1, it will cause the excessive formation of HClO and ClO−, which, if consumed, can cause bladder cancer.29 Furthermore, a high concentration of free chlorine will produce gases from the evaporation of water that is oversaturated with free chlorine, thus causing chlorine poisoning of the respiratory system.30 The effects of such poisoning include severe itchiness and discomfort as well as asthmatic conditions.31 In 1998, a water chlorination system malfunction occurred in a community pool in Rome, causing an incident where 282 people (including 134 children of age below 14) inhaled high concentrations of hydrogen chloride (14%) and sodium hypochlorite (33%). This incident caused the victims to suffer persistent respiratory symptoms and lung function impairment for up to a month.32,33 Hence, it is vital for water quality monitoring systems to function properly to ensure that the free chlorine concentration in water is within the safe range for consumption.
There are multiple methods utilized or developed by the water treatment industries or water distribution industries to detect or monitor free chlorine concentration in water, especially drinking water. For instance, commercialized sensors such as colour wheel test kits and digital colorimeters are commonly used. However, these sensors still have flaws that inhibit their efficiency or user-friendliness. Hence, this review is conducted to explore the sensors or methods available to detect free chlorine concentration in water and to pinpoint their strengths and limitations.
Methods for free chlorine monitoring in water
As mentioned, free chlorine monitoring in water is of upmost importance in preventing any deficient or excessive concentrations of free chlorine in water, especially in drinking water. Based on the standards/guidelines set by the WHO or national health authorities, free chlorine concentration in water, especially drinking water, should be monitored to ensure that there will be no adverse health effects on consumers. In this section, methods for free chlorine monitoring in water are briefly described. The methods are divided into conventional methods and lab-scale methods.Conventional methods for free chlorine monitoring in water
Conventional methods are methods that have been widely utilized or commercialized by members of society, including the N,N-diethyl-p-phenylenediamine (DPD) method which is currently used in various monitoring technologies, such as colour wheel test kits and digital colorimeters. Nevertheless, conventional methods are still optical-based methods that are dependent on the change in colour or fluorescence intensity. The analytical performance of the methods is summarized in Table 1.| Methods | Sensing mechanism | Sensing range (mg L−1) | Material used |
|---|---|---|---|
| OT oxidation method | When 1 mol of OT is added to an acidified sample that contains 1 mol of free chlorine, the mixture will immediately turn yellow, which hints at the production of holoquinone34 | 0–4.0 | OT |
| DPD colorimetric method | Free chlorine oxidizes DPD at a pH of 6.3–6.6 to form a magenta-coloured compound | 0.02–8.0 | DPD |
| Colour wheel test kit | Similar to the DPD colorimetric method | 0–3.4 | DPD |
| Digital colorimeter | Similar to the DPD colorimetric method | 0–2.5 | DPD |
| Iodometry/indirect iodometric titration method | Iodide is oxidized by free chlorine, which produces iodine. Iodine then dissolves in potassium iodide to form triiodide ions. Potassium triiodide is then titrated against sodium thiosulfate to form iodide again. The end point of the titration is determined using starch solution | 20–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Sodium thiosulfate |
| Potassium iodide | |||
| Starch solution | |||
| Luminescent ZnO quantum dot (QD) method | When free chlorine is added to the QD, hypochlorite takes the electron from the oxygen vacancy of the QD, thus giving rise to defective emission of the QD. | 0.002–0.200 | ZnO QD |
| Nitrogen and sulphur co-doped CDs | When free chlorine is added to the CD, hypochlorite takes the electron from the vacancy of the CD, thus giving rise to defective emission of the CD. | 0.0004–3.5 | Nitrogen and sulphur co-doped CDs |
| Amino-functionalized metal–organic framework (NH2-MIL-53(Al)) nanoplate-based energy transfer probe | ClO− ions of free chlorine shorten the distance between them and the amine groups in NH2-MIL-53(Al) nanoplates, thus leading to hydrogen bonding for energy migration from the nanoplates to ClO− ions, therefore causing fluorescence quenching of the nanoplates | 0.002–0.5 | NH2-MIL-53(Al) nanoplates |
| Bio-synthesized silver nanospheres | Both Hg2+ and ClO2− oxidize colloidal silver to Ag+ ions, thus causing quenching of the surface plasmon resonance (SPR) absorption peak | 0–8 | Piper nigrum extract |
| Ag+ solution | |||
| Fluorescence hypochlorite sensor | When ClO− reacts with the sensor, the oxidation of the sensor occurs and [2,2′-bithiophene]-5,5′-dicarbaldehyde is released from furan-2-carbohydrazide, thus causing the intramolecular fluorescence quenching to no longer function, resulting in the increase of fluorescence intensity of the sensor | 0–7.4 | [2,20-bithiophene]-5,50-dicarbaldehyde |
| Methanol | |||
| Furan-2-carbohydrazide | |||
| BSA functionalized BQDs | Due to the complex formed at the ground state, static quenching occurred and dynamic quenching occurred when it collided with an excited luminescent molecule, thus causing a decrease in photoluminescence intensity | 0–100 | BSA |
| Citric acid | |||
| Phenanthridine-based probe | When the probe reacted with OCl−, a new OCl− adduct species was formed via a favorable nucleophilic attack of OCl− on imine carbon. This is due to the reaction between the OCl− ion and the oxidized oxime group of PBO, which caused the emission of the probe to be partially reduced | 0–2.5 | PBC |
| Hydroxylamine hydrochloride | |||
| Triethylamine | |||
| Ethanol | |||
| Inkjet-printed silver electrodes | When free chlorine reacts with the silver electrode, an AgCl/Ag2O layer is formed over the electrode, which can be stripped. The silver is replated on the electrode via cathodic stripping voltammetry. The amount of silver oxidized is proportional to the free chlorine concentration | 1–100 | Ag/AgCl reference electrode |
| Platinum wire counter electrode | |||
| Polydopamine@ electrochemically reduced graphene oxide-modified electrode | Free chlorine reduces the functional group of the quinone structure of polydopamine to 5, 6-dihydroxylindole, thus increasing the PDA@ERGO/GC cathodic reduction peak current | 0.35–7.64 | PDA@ERGO/GC-modified electrode |
| Surfactant-modified Prussian blue (PB) electrochemical sensor | Catalytic reaction occurs where free chlorine reduces, thus increasing the cathodic current while reducing the anodic current | 0.009–10 | GCE/PB(BZTC) electrodes |
| Carbon black-based disposable sensor | The presence of free chlorine contributes to an increase in cathodic current | 0.05–200 | Carbon-black screen-printed electrodes |
| Britton–Robinson buffer | |||
| Pencil-drawn chemiresistive sensor | Free chlorine oxidizes the phenyl-capped aniline tetramer (PCAT) species, thus reducing the graphite-PCAT hybrid system resistance, and therefore increasing the detected current | 0.6–60 | Pencil-drawn chemiresistive sensor |
| Methods | Sensitivity | Accuracy (mg L−1) | Sensing condition | Strengths | Limitations |
|---|---|---|---|---|---|
| OT oxidation method | 0.3 mg L−1 | ±0.05 | pH < 1.8 | Low cost | Degradation of OT solution causes inaccurate readings |
| Very easy to use | Lacks precision and specificity | ||||
| OT is carcinogenic | |||||
| DPD colorimetric method | 0.0047 mg L−1 | ±0.05 | pH 6.3–6.6 | Easy to use | Suffers from interference by iodide ions |
| High selectivity among free and combined chlorines | |||||
| Colour wheel test kit | 0.2 mg L−1 | ±0.02 | Requires a light source for result observation | Accurate readings | Potential for user error |
| Low cost | Lack of calibration and standardization | ||||
| Digital colorimeter | 0.01 mg L−1 | ±0.03 | 0–50 °C | Highly accurate readings | High cost |
| <95% relative humidity | Fast results | Necessity of calibration with standards | |||
| Iodometry/indirect iodometric titration method | 2.5 mg L−1 | N/A | <20 °C | No special skills required | Temperature dependent |
| Low sensitivity | |||||
| Inability to distinguish between free and combined chlorine | |||||
| Can only detect chlorine levels above 1 mg L−1 | |||||
| Not specific to oxidants | |||||
| Luminescent ZnO quantum dot (QD) method | 0.001 mg L−1 | ±0.01 | pH > 4 | High selectivity and sensitivity | Requires scientific knowledge |
| Room temperature | Applicable to various types of water | Complicated synthesis | |||
| High pH stability | |||||
| Quick response time | |||||
| Nitrogen and sulphur co-doped CDs | 0.0001 mg L−1 | ±0.003 | Room temperature | Green | Requires scientific knowledge |
| pH > 4 | Low cost | Complicated synthesis | |||
| Simple | |||||
| Highly sensitive and selective | |||||
| Fast response time | |||||
| Amino-functionalized metal–organic framework (NH2-MIL-53(Al)) nanoplate-based energy transfer probe | 0.001 mg L−1 | ±0.007 | pH 7.4 | High water solubility and stability | Synthesis can only be done at low temperature |
| Room temperature | Wide detection range | Complicated synthesis | |||
| Fast response time | |||||
| High selectivity | |||||
| Bio-synthesized silver nanospheres | 0.074 mg L−1 | N/A | pH 4–5 for Hg2+ detection, pH 5–6 for ClO2− detection | Highly selective and sensitive | Requires scientific knowledge |
| High tolerance to a wide range of concentrations | |||||
| High stability of the sensing mechanism | |||||
| Cost effective and green synthesis method | |||||
| Fluorescence hypochlorite sensor | 0.218 mg L−1 | ±0.058 | pH 7 | Can be used in living organisms | No fluorescence increase if peroxides are present |
| Requires scientific knowledge | |||||
| BSA functionalized | 1.82 mg L−1 | N/A | In the presence of light | High selectivity | Require light to induce a reaction with hypochlorite ions |
| BQDs | Independent of the excitation wavelength | Require scientific knowledge | |||
| Phenanthridine-based probe | 4.12 × 10−4 mg L−1 | ±0.003 | pH 5–11 | Quick reaction time (10 s) | Requires scientific knowledge |
Better performance in a CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) environment 1, v/v) environment |
Low LOD | Requires a specific environment for better performance | |||
| High accuracy | |||||
| Inkjet-printed silver electrodes | 2 μA ppm−1 | ±0.12 | Room temperature | Disposable | Sensitivity lower than the DPD method |
| Atmospheric pressure | Simplicity | Require calibration and standardization | |||
| pH 8 | High reproducibility and sensitivity | ||||
| Reusability | |||||
| Polydopamine@ electrochemically reduced graphene oxide-modified electrode | 1.35 × 10−7 μA ppm−1 | ±0.01 | Room temperature | High electrocatalytic ability | Complicated synthesis and pH dependent |
| pH 7.2 | Good sensitivity, limit of detection, selectivity and stability | ||||
| Excellent range of recovery | |||||
| Surfactant-modified Prussian blue (PB) electrochemical sensor | 12 μA ppm−1 cm2 | ±0.02 | Room temperature | Wide detection range | Low reproducibility |
| pH < 6 | Low limit of detection | Requires calibrations | |||
| Carbon black-based disposable sensor | 0.36 μA ppm−1 | ±0.2 | Room temperature | Wide detection range | Difficult synthesis |
| pH 5 | Cost effective | Requires voltage supply | |||
| High sensitivity, selectivity and accuracy compared to the DPD method | |||||
| Pencil-drawn chemiresistive sensor | 0.005 μA ppm−1 | ±0.3 | N/A | Very low cost | Type of pencil may affect analysis results |
| Durable | |||||
| Resettable and reusable | |||||
| Resistant to fouling |
Orthotolidine (OT) method
Reported in 1913 by Ellms and Hauser, the first method to determine free chlorine involved the oxidation of OT in an acid solution using chlorine and other oxidants to produce a yellow complex mixture,35 where the higher the intensity of the colour, the more oxidants are present. However, due to factors such as OT being carcinogenic and the method's lack of precision, accuracy and specificity, the OT method was not adopted.36Fig. 1 shows the OT test kit produced by Orlab Instruments.37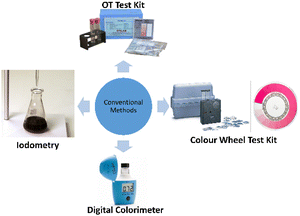 | ||
| Fig. 1 Conventional methods for free chlorine monitoring.37,41,43 | ||
DPD method
Next, one of the most common methods for free chlorine detection is spectrophotometry using DPD. The DPD method was first proposed in 1957 by Palin and despite suffering from interference by iodide ions, this method has the highest selectivity for free and combined chlorines. When DPD is oxidized by chlorine, two oxidation products are produced, which are Würster dye (primary product) and imine. Würster dye is a semi-quinoid cationic compound, which is magenta-coloured and has absorption maxima at 512 and 553 nm.38 By adding catalytic amounts of potassium iodide (KI) accompanied by a second absorbance reading, the DPD method can determine the total chlorine in the sample (both free and combined).39 This method has been integrated with both colour wheel test kits and digital colorimeters for free chlorine detection. Colour wheel test kits utilize a tablet or powder form of DPD, which will turn the water sample pink if chlorine is present. To measure the intensity of the colour change, a colour wheel is used to visually match the colour to a numerical free reading. Fig. 1 shows a picture of a colour wheel test kit commercialized by Hach.40 Colour wheel test kits can be used to measure both free chlorine and total chlorine via different chemicals included in the test kit. They are simpler and less expensive than digital meters and can detect chlorine in a range of 0–3.5 mg L−1. On the other hand, digital colorimeters, which are commonly used in developing countries, are the most accurate method for measuring free chlorine and total chlorine residual. Similar to colour wheel test kits, digital colorimeters use DPD powder or tablets to turn the water sample pink in a vial. The vial is then inserted into a meter, where the intensity of colour change is measured when a wavelength of light is emitted into the vial, thus determining and displaying the intensity of colour change digitally. Digital colorimeters can detect chlorine in a range of 0–4 mg L−1. Fig. 1 shows the digital colorimeter commercialized by Hanna Instruments (M) Sdn Bhd.41Iodometry method
Iodometry, also known as the indirect iodometric titration method, is one of the oldest chlorine detection methods, which involves titration of iodine detached in chemical reactions and is commonly used for determining chlorine exceeding 1 mg L−1.42 To determine chlorine, a small amount of KI is added to the sample. Chlorine in the sample will react with KI and produce triiodide (I3−). Then, the titration of the mixture with sodium thiosulfate is performed at a pH of the sample around 3–4 and at a temperature below 20 °C, with the addition of starch at the beginning as an end-point indicator. The titration is complete when the dark blue starch–iodide complex vanishes. Due to the method being temperature-dependent, its low sensitivity and its inability to distinguish between free and combined chlorine, iodometry is not commonly implemented.38 This method is illustrated in Fig. 1.43Lab-scale methods for free chlorine monitoring in water
Compared to conventional methods, lab-scale methods are still under development for industrial-scale utilization. However, the results of these methods are still very promising to prevail against the conventional methods. The methods are categorized based on the type of sensing method.Sensing methods
Multiple methods for free chlorine monitoring have been produced and some have even been commercialized to cater to both the industrial sector, including the water treatment industries, and the public sector that serves society at large. Due to the abundant methods available, they are further categorized based on their sensing procedures into both chemical methods and electrochemical methods. Optical methods focus on the technology that utilizes colour change or fluorescence for the detection and measurement of free chlorine in water. On the other hand, electrochemical methods utilize electrochemistry, such as the use of electrodes, to measure the concentration of free chlorine in water. Both methods are then further summarized in Table 1.Optical methods
There are only a few optical methods used to detect or monitor free chlorine in water. Optical methods are normally based on colorimetric tests, where the intensity of colour change or fluorescence is measured.Luminescent ZnO QDs
The following method describes the use of luminescent zinc oxide (ZnO) quantum dots (QDs). This method was proposed by Singh and Mehta,44 in which highly luminescent ZnO QDs are synthesized to act as an optical sensor for free chlorine detection. At 525 nm, the photoluminescence (PL) emission of ZnO QDs is adversely affected by free chlorine concentration. Due to its high electron gaining efficiency, electrons are taken by hypochlorite from the oxygen vacancies in ZnO QDs oxygen vacancy, which causes defective emission of the QDs. After testing with various metal ions and anions, it is found that this method shows high selectivity for free chlorine in water regardless of the ions present. The ions chosen for the test were environmentally relevant, such as Zn2+, Na+, Cu2+ and Fe3+. Hence, this method proves its high selectivity. Moreover, this method is proven to be applicable to various types of water due to its stability in water at pH higher than 4. In short, this method shows high sensitivity and selectivity, high pH stability, and quick response time, while the detection range is found to be around 0.05–0.7 μM. This method is illustrated in Fig. 2.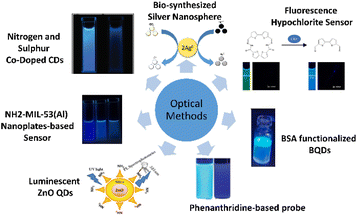 | ||
| Fig. 2 Optical methods for free chlorine monitoring.44–50 | ||
Nitrogen and sulphur co-doped carbon dots (CDs)
Xue et al. proposed a detection method using nitrogen and sulfur co-doped CDs.45 This method introduces CDs, co-doped with nitrogen and sulphur, which emit strong blue fluorescence that can quench when free chlorine is present. The mechanism behind this method is based on free chlorine destroying the surface passivation layer of the CDs, thus producing non-fluorescent ground state complexes, which lead to fluorescence quenching of CDs. The method shows a linear relationship between free chlorine concentration and the decreased fluorescence intensity of CDs. The detection range is measured to be from 0.01 to 100 μM with 91 to 106% recovery. It is concluded that this method is environmentally sustainable, low-cost, simple, and highly selective and sensitive, and has a fast response time. Fig. 2 shows the fluorescent carbon dots in the presence of free chlorine presented by Xue et al.45Amino-functionalized metal–organic framework nanoplate-based sensor
Lu et al.50 proposed an amino-functionalized metal–organic framework nanoplate-based energy transfer probe by creating fluorescent nanoplates for highly selective free chlorine analysis. Using urea as a modulator, one-step hydrothermal treatment of 2-amino-1,4-benzenedicarboxylic acid (NH2–H2BDC) and aluminium(III) chloride hexahydrate (AlCl3·6H2O) in water is performed to synthesize highly fluorescent NH2-MIL-53 (Al) (2-amino-1,4-benzenedicarboxylic acid connected to Al3+) nanoplates. The nanoplates are found to exhibit high stability and water solubility. After free chlorine is added, the nanoplates show significantly suppressed fluorescence, thus proving the method to be simple, fast and highly selective. This method exhibits a wide detection range of 0.05 to 15 μM, and unlike the conventional redox-based free chlorine fluorescent sensors, the nanoplates undermine the interference caused by strong oxidants like permanganate (MnO4−) and dichromate (Cr2O72−) ions. Fig. 2 shows an illustration of the sensor.Bio-synthesized silver nanospheres
As a means to detect both mercury and chlorite ions in aqueous solution, Vashisht et al.46 introduced bio-synthesized silver nanospheres produced from an aqueous extract of Piper nigrum, also known as black pepper, and silver ion solution. The absorption intensity of Ag NSs was quenched in the presence of mercury ions (Hg2+) among the cations and chlorite ions (ClO2−) among the anions. The limits of detection (LOD) of 7.47 μM and 1.11 μM were evaluated from the calibration curve for Hg2+ and ClO2−, respectively. Fig. 2 shows the illustration of the nanospheres.Fluorescence hypochlorite sensor
Kim et al.47 proposed a fluorescence sensor, which selectively detects hypochlorite in water. Using bithiophene and furan-carbohydrazide as the main precursors, the sensor was synthesized and exhibited enhanced fluorescence when reacted with hypochlorite in water. The LOD was determined to be 4.2 μM. Moreover, when tested with other anions, there was no enhanced fluorescence observed, hence proving the high selectivity of the sensor towards hypochlorite. To further test the capability of the sensor, Kim et al. applied it to a zebrafish to detect the hypochlorite inside the organism, which produced fulfilling results. The sensor is shown in Fig. 2.Bovine serum albumin (BSA) functionalized graphene quantum dots (BQDs)
Sharma et al. introduced another method based on BQDs,48 which decay upon reacting with hypochlorite ions, hence exhibiting weaker fluorescence. Synthesized from BSA and citric acid via a hydrothermal method, the highly hypochlorite-sensitive BQDs exhibit a LOD of 35.39 μM in aqueous medium. Fig. 2 shows an illustration of the BQDs.Phenanthridine-based probe
Proposed by Enbanathan et al.,50 an (E)-2-(40-(7,8,13,14-tetrahydrodibenzo[a,i] phenanthridin-5-yl)-[1,10-biphenyl]-4-yl) ethen-1-ol (PBO) sensor was synthesized using 40-(7,8,13,14-tetrahydrodibenzo [a,i] phenanthridin-5-yl)-[1,10-biphenyl]-4-carbaldehyde (PBC), hydroxylamine hydrochloride, and trimethylamine in ethanol solution. When reacted with OCl− ions, the fluorescence intensity of the sensitive PBO sensor reduced with a quick reaction time of 10 s and a low LOD of 8 nM. Moreover, the PBO sensor is selectively reactive towards OCl− in comparison to other anions. The illustration of the PBO probe is shown in Fig. 2.Electrochemical methods
On the other hand, electrochemical methods measure free chlorine using high precision readings gathered from the electrodes when applied in water.Inkjet-printed silver electrodes
Jović et al.51 introduced a detection method using inkjet-printed silver electrodes for the detection of free chlorine in water. This method presents free chlorine detection via linear sweep voltammetry (LSV) analysis on AgCl/Ag2O films produced over the inkjet-printed silver electrodes. The films are produced by a spontaneous reaction between the free chlorine in water and silver. It is found that there is a linear relationship between the free chlorine concentration in water and the number of films formed. This method demonstrates a detection range from 1 to 100 mg L−1 with a sensitivity of 30 μC L mg−1. The silver electrode used in this method is disposable and can be easily mass produced by inkjet printing. Fig. 3 shows the electrodes produced by Jović et al.51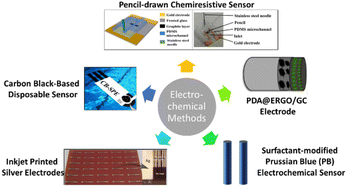 | ||
| Fig. 3 Electrochemical methods for free chlorine monitoring.51–56 | ||
Polydopamine@electrochemically reduced graphene oxide-modified (PDA@ERGO/GC) electrode
An electrocatalytic detection method of free chlorine via electropolymerization of dopamine on an ERGO (electrochemically reduced graphene oxide) surface was introduced by Kumar et al.52 The method proposed is based on a PDA@ERGO/GC electrode for electrochemical detection. Using cyclic voltammetry, the reduction of graphene oxide (GO) is performed to produce ERGO on a glassy carbon (GC) electrode. The ERGO/GC electrode surface is then electropolymerized for 30 cycles using dopamine, producing a polydopamine-modified electrode. Through characterization, the electrode is found to exhibit a high electrocatalytic ability, stability, sensitivity, selectivity, and limit of detection. The range of detection is found to be between 9.9 and 215.2 μM, while sensitivity is found to be 7.1 mA M−1. When tested in a swimming pool water sample, the method shows an excellent range of recovery, which is 102.4% to 103.0%. Fig. 3 shows the model of the proposed electrode.Surfactant-modified Prussian blue electrochemical sensor
Salazar et al.53 developed a surfactant-modified Prussian blue (PB) electrochemical sensor. The electrodeposition of PB onto a glassy carbon electrode (GCE) is assisted by a cationic surfactant, benzethonium (BZTC), which increased the amount of PB deposition and improved the electrochemical response of the sensor. Both cyclic voltammetry and amperometry are performed to measure the ability of the sensor to detect chlorine. The sensor exhibited a sensitivity of 12 μA ppm−1 cm−2, a detection range of 0.009–10 ppm, and a reproducibility of 4.2%. After testing in real applications, the results showed that the sensor is well-functioning and stable. Fig. 3 includes the illustration of the sensor.53,54Carbon black-based disposable sensor
Tomei et al.55 proposed a cost-effective and small screen-printed electrode, which was modified with a carbon black nanomaterial, resulting in the development of carbon black-based disposable sensor. Carbon black is utilized as a working electrode modifier to quantify chlorine at low applied potential to overcome the fouling issue. Moreover, through the drop casting method, the mass production of this sensor can be achieved due to the dispersion of the carbon black. This low-cost sensor has displayed a large detection range of 0.05–200 mg L−1 with high sensitivity, selectivity and accuracy compared to the DPD method. Fig. 3 shows a model illustration of the sensor.Pencil-drawn chemiresistive sensor
Fabricated by drawing with a pencil, a low-cost and simple, graphitic-film-based chemiresistive sensor was proposed by Hoque et al.56 to detect free chlorine in water. Mostly unaffected by species that theoretically cause interference during free chlorine measurements, the sensor can operate within the range of 1 to 10 ppm. This sensor exhibits high selectivity for oxidant species, including free chlorine during the functionalization of the pencil lines with the redox-active aniline oligomer (phenyl-capped aniline tetramer). Furthermore, the utilization of 9B grade pencils resulted in increased sensitivity of the sensor. Compared with electrochemical sensors, a reference electrode is not needed and the sensor can be operated endlessly online. Fig. 3 shows a graphical image (left) and real life image (right) of the sensor produced by Hoque et al.,56 respectively.Limitations of common free chlorine monitoring methods for household usage
Despite the availability of multiple methods for chlorine monitoring in water, there are limitations that make them unsuitable for household usage. Based on the methods mentioned above, they require complicated analysis to detect pollutants in water. Moreover, due to the high cost of materials required for testing, some of these methods are financially impractical for household usage. Furthermore, it can be seen that some methods require complicated synthesis and scientific knowledge or skills. Hence, these methods cannot be adapted for commercialization for the average household consumer.Hence, paper-based water quality sensors have been researched and developed throughout the years to fill the gap left by the existing conventional methods. The development of paper-based sensors drastically reduced the cost and complexity of chlorine monitoring for household water. Paper-based methods have the advantages of being inexpensive and producing quick results, all while maintaining a simple and portable process.57 However, in certain ways, these technologies fall short of typical laboratory tools, particularly in terms of sensitivity and detection limit. Recent advancements in paper-based technique testing address the restrictions while keeping the appealing aspects of these tests, such as a portable nanohybrid paper-based chemiresistive sensor introduced by Yen et al.58
Potential of paper-based free chlorine monitoring to meet the ASSURED criteria
By providing disposable, low-cost tools that can be used in remote settings, paper-based diagnostics have reformed the point-of-care approach for environmental and healthcare applications. The ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free, and Deliverable to End Users) requirements set by the WHO are met by these devices, which typically consist of biological, chemical, and microfluidic diagnostic components equipped on paper substrates. Affordability, as the first criterion, is the driver in the development of paper-based sensors. Affordable and cost-effective sensors are vital in environments that are resource-limited, given the poor state of the economies of many countries. Sensitivity is important to minimize or even avoid false negatives in tests, while specificity is vital to reduce or avoid any false positives. User-friendliness requires the sensors to be easy to use when testing and do not require any extensive user training or prior knowledge. Rapid and robust performance requires the sensors to be able to deliver results within a short period and withstand the supply chain without any extra storage or transport. Equipment-free refers to the ability of sensors to deliver results without needing any extra equipment. Finally, deliverable to end users pertains to the organizational structures and relationships in the supply chain for the purpose of delivering the product or technology to the end-users.59Paper-based diagnostics already meet the criteria of being affordable, equipment-free and deliverable to end users. Therefore, to meet all ASSURED criteria, extra functions must be integrated with them.60 The printing function can be combined with these devices, due to advances in the development of printed materials and in the field of printed electronics. Through automated read-out and communication of results, the device can be improved to meet the user-friendliness criterion. This also eliminates the external training or instrumentation required to perform the test successfully. Existing technologies from the printed electronics field, such as connectivity, displays, processors, sensors, and power supplies, can be combined to expand the capabilities of these devices.60 Examples of paper-based sensors are listed in the section below as well as the preparation method and sensing mechanism. The findings are then summarized in Table 2.
| Paper-based sensors | Sensor matrix material | Sensor indicator material | Sensing parameter | References |
|---|---|---|---|---|
| Nanohybrid paper-based chemiresistive sensor for free chlorine detection | • Whatman (type 1) filter paper | PEDOT:PSS and nanohybrid conductive ink (PEDOT:PSS/graphene) | Free chlorine | 58 |
| • Conductive silver paint (CSIP-998) | ||||
| • Protective layer (Vaseline Jelly Original) | ||||
| Hand-drawn, paper-based poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) sensor | • Whatman (type 542) filter paper | PEDOT:PSS solution | Free chlorine | 61 |
| • Silver (Ag) paste | ||||
| • Petroleum jelly (Vaseline® Jelly Original) | ||||
| Simultaneous monitoring μPAD for free chlorine, Cu2+ and Fe2+ | • Whatman (grade no. 41) filter paper | • DPC dissolved in a polymer formulation (250 mg TEHP, 150 mg PEG-400, and 50 mg PVB in 5 mL absolute ethanol) for Cu2+ detection | • Cu2+ | 62 |
| • Wax-based hydrophobic barriers | • TPTZ dissolved in a polymer formulation (similar to Cu2+) for Fe2+ detection | • Fe2+ | ||
| • Scotch tape | • OTO dissolved in a polymer formulation (30 g per L PVP in absolute ethanol) for free chlorine detection | • Free chlorine | ||
| Simultaneous detection paper test strip for free chlorine, hydrogen sulphide and formaldehyde | • Chromatography paper | • 0.10 M DPD for free chlorine detection | • Free chlorine | 63 |
| • Drawing paper | • 0.10 M NNDM for hydrogen sulphide detection | • Hydrogen sulphide | ||
| • Double-sided adhesive | • 0.05 M AHMT for formaldehyde detection | • Formaldehyde | ||
| • APTES (4% w/v) |
| Paper-based sensors | Sensing range (ppm) | Detection limit (LOD) (ppm) | Sensitivity | Selectivity | Accuracy | Response time (s) | References |
|---|---|---|---|---|---|---|---|
| Nanohybrid paper-based chemiresistive sensor for free chlorine detection | 0.1–500 | 0.18 | 0.043 | 50.0% | 89–96% | • 15 for 50–1000 ppm | 58 |
| • 300 for 0.1–50 ppm | |||||||
| Hand-drawn, paper-based poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) sensor | 0.5–500 | 0.5 | 0.044 | 53.3% | 86–90% | Less than 0.025 | 61 |
| Simultaneous monitoring μPAD for free chlorine, Cu2+ and Fe2+ | • 0.1–1.0 for Cu2+ | • 0.2 for Cu2+ | 0.053 | 89% | 40–80% | 0 (Instant) | 62 |
| • 0.1–0.5 for Fe2+ | • 0.1 for Fe2+ | ||||||
| • 0.2–5.0 for free chlorine | • 0.2 for free chlorine | ||||||
| Simultaneous detection paper test strip for free chlorine, hydrogen sulphide and formaldehyde | • 0.20–1.20 for free chlorine | • 0.08 for free chlorine | 0.065 | 95% | 96–100% | 300 | 63 |
| • 0.40–1.40 for hydrogen sulphide | • 0.14 for hydrogen sulphide | ||||||
| • 0.20–1.20 for formaldehyde | • 0.13 for formaldehyde |
Paper-based method
Nanohybrid paper-based chemiresistive sensor for free chlorine detection
A portable nanohybrid paper-based chemiresistive sensor that can be utilized with smartphones was introduced by Yen et al.58 to detect free chlorine ions. Using an easy and standardized coating method, the paper-based sensor is free-chlorine-sensitive at 0.1 to 500 ppm. The sensor provides a real-time display of the measured signals and results on a smartphone by integrating it with an electric readout system. The sensor's design is wax printed on filter paper, where the paper is placed on a hot plate to melt the wax, and then nanohybrid conductive ink is distributed on it as a sensing layer and left to dry at 80 °C. Conductive silver paint is applied to both sides of the sensing layer to serve as an electrode. A protective layer is then applied on the junctions of the sensor.Instruments utilized for the sensing tests includes a SEM (JSM-7610F, JEOL, Tokyo, Japan), LabVIEW software, a source meter (Keithley 2400, Tektronix, Beaverton, OR, USA), a built-in analog-to-digital converter (ATmega328), amplifier and electronics platform (Arduino Uno Rev3 SMD), Arduino software (IDE), and a mobile phone. Both paper-based chemiresistors fabricated using PEDOT:PSS and nanohybrid conductive composites are tested through the following tests:
(a) Surface morphologies via scanning electron microscopy (SEM).
(b) Free chlorine concentrations in sample solutions (0.1 to 1000 ppm) with recordings at intervals of 15, 120 and 300 s.
(c) Selectivity via dripping a drop of 1000 ppm testing solutions containing interference ions for 5 min at room temperature.
(d) Portability via integration of the sensor with a wireless module and signal-capturing system, which shows results on a smartphone in two modes, which are terminal mode (final measured free chlorine concentration) and graphic mode (resistance change and signal plots recorded in real time).
(e) A real life test via measuring samples gathered from various sources, such as swimming pool water and tap water. The results are compared with results obtained from commercial DPD-based colorimetric sensors.
To summarise the sensing mechanism, when free chlorine solution is introduced to the sensor, the aqueous hypochlorite oxidizes the thiophene in PEDOT to form a structure with a unsaturated polyol. The oxidized thiophene then eliminates the sulphur contaminants by forming sodium sulfate, thus decreasing the conductivity of the sensor. For samples with a low free chlorine concentration, the relative resistance change gradually increases until a significant response is obtained after 2 min. This is due to the PEDOT:PSS oxidation reaching a reaction-limited state at low hypochlorite concentrations (<50 mg L−1). This nanohybrid sensor is simple and inexpensive to fabricate, and thus it can be easily mass produced. Furthermore, it is portable and has a real-time display readily available on a mobile phone. It also has a wide concentration detection range (0.1–500 mg L−1) and a low LOD compared to commercial sensors (0.18 mg L−1). The volume of sample required for detection is small using this sensor. Nevertheless, this sensor is only applicable for small sample volumes. Moreover, PEDOT:PSS solution (sensor indicator material) is harmful if not handled properly.64 Other than that, this sensor requires heat and instrumentation for fabrication as well as stable input power during sample monitoring. In comparison to conventional methods, this sensor has shown a wider sensing range with a lower LOD and cost, as well as being mobile phone-integrated.
Hand-drawn, paper-based PEDOT:PSS sensor
Qin et al.61 proposed a paper-based electrochemical PEDOT: PSS sensor for free chlorine, which is produced via hand-drawing. Due to oxidation when exposed to free chlorine in water, the electrical resistivity of the PEDOT:PSS sensor increases, thus proving that the response of the sensor was represented by the relative change in electrical resistance. The fabrication of the said sensor can be accomplished at room temperature via hand-drawing, regardless of shapes and dimensions, thus needing no other equipment or instrumentation, and trained personnel or knowledge. PEDOT:PSS solution is drawn onto filter paper and dried naturally. Silver paste is then drawn on the PEDOT:PSS film and dried naturally. Petroleum jelly is spread on both sides of the filter paper to shield the silver contact. The sensor is conditioned in 10 ppm sodium hypochlorite solution. After the free chlorine detection tests, apart from being mechanically stable and reusable, the sensor is proven to exhibit a wide sensing range and high accuracy.For sensor characterization and sample analysis, instruments utilized include a field-emission SEM (SU-8000, Hitachi), semiconductor parameter analyzer (4200-SCS, Keithley), DPD-based colorimetric test kit (CN-70, Hach), tensile pulling tester (AG-X, Shimadzu), and multimeter (72-7730, TENMA). The sensor is characterized and analysed via the following tests:
(a) The surface morphologies of the PEDOT:PSS films and paper substrates via a field-emission SEM.
(b) Bending tests via a tensile pulling tester (compressed 1 cm at 1 mm s−1 and 0.05 Hz). The electrical resistance of the sensor is measured during bending via a semiconductor parameter analyser.
(c) Free chlorine concentration monitoring via sensor strips that are dipped into free chlorine solution of different concentrations (calibrated using the DPD-based colorimetric kit). A commercial multimeter is used to measure the resistance of the sensor before and after the test.
In free chlorine solution, thiophene in PEDOT is oxidized and its electrical resistivity increases due to the disruption of π–π conjugation in its chemical structures. For low free chlorine concentration (<5 mg L−1), a testing duration of longer than 5 min is required to obtain a significant response due to the reaction-limited state of PEDOT:PSS oxidation. This hand-drawn sensor is simple and cost-efficient to fabricate because it can be done at room temperature and no other instruments are required. Moreover, it is reusable and portable and has a wide sensing range. For measurement purposes, only a commercial multimeter is required. Nevertheless, similar to the previous nanohybrid sensor introduced by Yen et al.,58 it also uses PEDOT:PSS solution as the indicator, and thus this sensor should be handled properly to avoid any harm to the user. Furthermore, the delamination of the sensing material will increase the resistance of the sensor, thus increasing the error in the sample measurement. The bending stability (connected to the mechanical flexibility of the paper and the adhesion of PEDOT:PSS to the sensor) of the sensor can also have a minor effect on the change of resistance during sample measurement. In comparison to the conventional methods, this sensor has shown a wider sensing range with a lower cost. Moreover, this sensor is also reusable.
Simultaneous monitoring of free chlorine, Cu2+ and Fe2+ on μPAD
This two-layered μPAD was introduced by Huangfu et al.62 to simultaneously monitor three important analytes in drinking water, which are free chlorine, Cu2+ and Fe2+. Upon detection of the analytes, colour change that can be discerned by the naked eye will occur in the corresponding detection zones. Therefore, through the colour difference, the three analytes can then be simultaneously monitored and measured. Detection ranges for free chlorine, Cu2+ and Fe2+ can be measured to be 0.2–5.0 ppm, 0.1–1.0 ppm, and 0.1–0.5 ppm, respectively. This proves that the sensor has achieved higher selectivity and sensitivity than commercialized test strips. Fig. 4 shows the design of the μPAD.62 On the bottom layer, three hydrophilic detecting spots are placed in a triangle configuration, with 1,5-diphenylcarbazide (DPC), 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ), and OT solutions as the indicators for Cu2+, Fe2+, and free chlorine, respectively. On the top layer, a hydrophilic area consists of three channels with sample reservoirs corresponding to the detecting spots. In between the layers, scotch tape is used to attach the drive sample to the reservoir.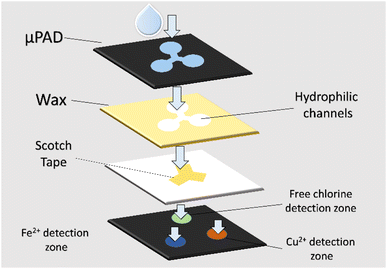 | ||
| Fig. 4 Design of simultaneous monitoring μPAD for free chlorine, Cu2+ and Fe2+.62 | ||
For sensor characterization, instruments utilized include a Titrator T50 (Mettler Toledo Co.), Xerox Phaser 8560DN color printer (Fuji, Japan), Epson V200 flatbed scanner, and commercialized portable instrument for free chlorine concentration measurement (HANNA Instruments). In the individual analyte detection parts for Fe2+ and Cu2+ detection, 100 μL of samples are included in the detection zones of the sensor. Filter paper is placed beneath the sensor for continuous drawing of the samples through the indicators. For free chlorine detection, 10 μL of sample is used for optimal results. Test paper imaging is done using a flatbed scanner, which images the sensor before and after exposure to the sample. Adobe Photoshop is then utilized to computerize the changes in colour, which is then defined as the total Euclidean distance (ED) viaeqn (3), where R, G and B are the red, blue and green intensity, respectively.
| ED = √((ΔR)2 + (ΔG)2 + (ΔB)2) | (3) |
For the detection of Cu2+, DPC (indicator) is oxidized to diphenylcarbazone (DPCO), and a chelate complex between DPCO and Cu(II) is formed. A purplish red colour is formed at the end of the test.65 For the detection of Fe2+, a complex of Fe (TPTZ)2+2 is formed and a dark blue colour is formed at the indicator.66 As for the detection of free chlorine, when OT is added to free chlorine, the mixture will immediately turn yellow, which hints at the production of holoquinone. Compared with the previous two paper-based sensors introduced by Yen et al.58 and Qin et al.,61 this portable and low-cost μPAD can detect three analytes simultaneously and has a fast response time. Moreover, it displays high selectivity and sensitivity and has a lower LOD when compared with commercial sensors. However, this μPAD has a narrow detection range of 0.2–5.0 mg L−1. Additionally, the OT solution used for the detection of free chlorine is hazardous and carcinogenic, and thus it should be handled with care. Furthermore, the colour formation for free chlorine detection fades significantly, and thus colorimetric observation should be done with focus. When compared with conventional methods, this μPAD offers a wider sensing range with a lower LOD and higher sensitivity at a lower cost.
Simultaneous detection of formaldehyde, hydrogen sulphide and free chlorine on paper test strips
Arsawiset and Teepoo63 developed functionalized paper test strips with 3-aminopropyltriethoxysilane (APTES) utilizing detection zones to immobilize specific chromogenic substrates, thus resulting in the simultaneous detection of three analytes, namely formaldehyde, hydrogen sulphide and free chlorine. After analysis, the results were sent for further analysis using a smartphone. The linear ranges of detection are found to be 0.20–1.20 mg L−1 for formaldehyde, 0.40–1.40 mg L−1 for hydrogen sulphide, and 0.20–1.20 mg L−1 for free chlorine. After the tests, it is also found that this method exhibits high selectivity, sensitivity, precision and accuracy and provides a portable format for analyte detection. 3 detection zones with chromatography paper as the base are stuck on drawing paper using double-sided adhesive. 2 μL 3-aminopropyltriethoxysilane (APTES) is placed on the detection zones and stored at 100 °C for 30 min. Then, the stored chromatography paper is washed with distilled water and then dried at 60 °C for 30 min. 4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole (AHMT), N,N-dimethyl-p-phenylenediamine (NNDM) and DPD solutions are used to detect formaldehyde, hydrogen sulfide and free chlorine, respectively.For sensor characterization, an Alcatel Shine Lite smartphone model 5080X (Shenzhen, China), UV-1601 UV-visible spectrophotometer (Shimadzu (Kyoto, Tokyo, Japan)), and X-ray diffractometer (XRD) are used. 2 mL of sample is added to each detection zone of the sensor. After the reaction is complete, the sensor is inserted into the detection box illuminated with white LED lamps. Using the Colour Picker application on the smartphone, the RGB intensities are used for quantitative detection.
For formaldehyde detection, AHMT reacts with formaldehyde to form purple tetrazine dye.67 For hydrogen sulphide analysis, by using ferric iron as the oxidising agent, hydrogen sulphide reacts with NNDM to form methylene blue dye.68 For free chlorine detection, free chlorine reacted with DPD to produce red Würster dye. Similar to the μPAD introduced by Huangfu et al.,62 this portable and cost-effective paper test strip can also detect three analytes simultaneously. Moreover, it shows high selectivity and a low LOD (0.08 mg L−1). However, this paper test strip has a narrow detection range of 0.2–1.2 mg L−1 and can only operate between 15 °C and 40 °C. Furthermore, it is also time and pH dependent for optimal results. According to Arsawiset and Teepoo,63 5 min are required for optimal results, and for optimal results of formaldehyde, hydrogen sulphide and free chlorine detection, samples of pH 6–7, 1–4, and 9–14 are required, respectively. DPD solution (free chlorine indicator) is also dangerous if not properly handled.69 Nevertheless, compared with conventional methods, this paper test strip displays a lower LOD and higher selectivity, and the fabrication cost is lower.
Similarities and differences between paper-based sensors
In terms of similarities, the four paper-based sensors are able to detect free chlorine in water and are portable. For the sensors introduced by Yen et al.58 and Qin et al.,61 they use PEDOT:PSS as the sensing material and the sensor designs are hand-drawn. Silver paint serves as sensor electrodes while petroleum jelly is used as the protective layer for both sensors. Moreover, both sensors utilize electrochemistry and use filter paper as the base paper of the sensor. On the other hand, for the sensors proposed by Huangfu et al.62 and Arsawiset and Teepoo,63 they are similar in the ability to detect three analytes simultaneously and they utilize colorimetry as the method of detection. Furthermore, in both sensors, the three detection zones are placed on a single base paper and colour change can be observed without any requirement for extra equipment.As for the differences, the PEDOT:PSS solution used by Yen et al.58 requires high temperature for drying (a hotplate is used), while the solution used by Qin et al.61 can be dried naturally.
Yen et al.58 devised their sensor via wax printing, while Qin et al.61 did not use any extra equipment for their sensor fabrication. The sensor introduced by Qin et al.61 requires conditioning in sodium hypochlorite before use and the filter paper used is Whatman Filter Paper Type 1. On the other hand, the sensor introduced by Yen et al.58 does not require any conditioning and uses Whatman Filter Paper Type 542 instead. For the other two sensors, other than free chlorine, the double-layered sensor devised by Huangfu et al.62 can also detect copper ions and iron(II) ions. Meanwhile, the single-layered sensor proposed by Arsawiset and Teepoo63 can detect formaldehyde and hydrogen sulphide instead. The sensor developed by Huangfu et al.62 uses Whatman Filter Paper Type 41 and does not require any conditioning before tests. Meanwhile, the sensor developed by Arsawiset and Teepoo63 uses drawing paper as the sensor base and chromatography paper as the detection zone base, as well as APTES solution as the conditioner. For the free chlorine detection indicator, Huangfu et al.62 used OT solution, while Arsawiset and Teepoo63 used DPD solution.
Potential of carbon quantum dots for free chlorine detection
Carbon quantum dots (CQDs) are nano-carbon materials with diameters less than 10 nm, which are a form of QD.70 They are also noted for having remarkable zero-dimensional characteristics and outstanding fluorescence properties.70 CQDs are gaining popularity due to their strong luminescence and water solubility.71 CQDs might be employed in a variety of applications due to their high water solubility, such as bath goods.72 Furthermore, electrocatalysis,73 bioimaging,74 solar cells,75 nanomedicine,76 chemical sensors,77 and light-emitting diodes (LEDs)78 are only a few of the applications of CQDs.79 Conventionally, CQDs may be made by surface functionalizing carbon nanoparticles with polymeric and organic compounds. Although these CQDs have a wide range of optical characteristics, they are less programmable.80To determine if a sample is a CQD, experts indicated that the carbohydrate must meet the requirements below:81
(i) Contains carbon (C), hydrogen (H), and oxygen (O) in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
(ii) Under hydrothermal conditions, the forms of O and C permit dehydration.
Hence, natural resources meet the requirements to synthesize CQDs. Natural resources such as sugar,82 lemon juice,83 and ginger84 can be used to prepare CQDs. Theoretically, the strong fluorescence of CQDs when tested in water samples will quench in the presence of free chlorine. This is due to the destruction of the surface passivation layer of the CQDs caused by free chlorine. Hence, non-fluorescent ground state complexes will be produced, which lead to the fluorescence quenching of the CQDs.44 Therefore, it can be said that the higher the concentration of free chlorine, the lower the fluorescence intensity of the CQDs. Furthermore, CQDs are expected to exhibit high selectivity for free chlorine when applied to water samples. This is due to the strong oxidative nature of free chlorine. Hence, unless there are oxidants with stronger oxidation capability, free chlorine would be the main cause of the fluorescence quenching of CQDs. Most natural water samples do not contain strong oxidants, thus suggesting that CQDs as a sensor for free chlorine will not be interfered with by other oxidants.85
Future prospects and challenges
Although multiple methods have been utilized and even commercialized by industries and the public sector, they still do not fully meet the ASSURED criteria of the WHO. For example, digital colorimeters that have been commercialized for years are very costly, and thus they do not meet the affordability criterion. Another example is colour wheel test kits that are sold in the market at low prices, but have the potential for user error, which defies the user-friendliness criterion. Moreover, the chemicals used in both of these sensors are dangerous if not handled properly. Therefore, paper-based sensors can be developed and introduced to replace the current conventional methods. However, the paper-based sensors currently in development are still facing some challenges in meeting the ASSURED criteria, especially in the user-friendliness aspect due to the complexity of the analysis of the results.In the next section, the challenges faced by paper-based sensors are explained with respect to their performance, practicability, and acceptance level by the public, as well as the costs and materials involved.
Performance
The performance of a sensor refers to the sensor's specifications, which quantify the ability of the sensor to deliver measurements to the user.86 Sensor performance includes the sensor's accuracy and sensitivity. Based on paper-based sensors developed to date, the accuracy seems to be lower than that of the conventional methods. For instance, the sensor introduced by Hu et al.87 demonstrates a certain accuracy in distinguishing some chemicals. Chemicals such as hydrazine and ethanolamine are very easy to distinguish, while other aqueous solutions and organic chemicals such as ethanol and methanol are harder to identify with sufficient accuracy. However, with modifications such as adding a LCR meter, the accuracy of the sensor has been found to improve.In terms of sensitivity, paper-based sensors are known to have limited sensitivity when compared with the conventional methods. This was shown by the sensor introduced by Hu et al.,87 where the sensitivity still cannot meet the criteria for water quality analysis. Furthermore, as the analyte flows across the paper, solution spreading or evaporation may occur, which will cause a decrease in local analyte concentration. However, through advances in fabrication and analytical methods, the sensitivity of paper-based sensors is improving.88 As an example, the sensor proposed by Tian et al.89 proves its high sensitivity for TNT sensing in groundwater samples by utilizing organic fluorescence dyes of different concentrations of TNT. As for the evaporation issue, Apilux et al.34 proposed that it can be solved via placing a porous paper substrate between polyester and vinyl plastic films or encapsulating the peripheral edges of paper using sticky tape.
Practicability
The feasibility of paper-based sensors for water quality analysis is still under consideration as they to be proposed and developed in laboratories. Compared to the conventional methods, which have been in use on an industrial scale since the late 19th century,90 the practicability of paper-based methods is often questioned and challenged. Nevertheless, at the laboratory stage, paper-based sensors are believed to be feasible, after some modifications to suit them for industrial use, due to their high selectivity and sensitivity, ease of use, and low cost.88Public acceptance level
When compared with conventional methods that have been implemented for more than a century, paper-based methods are a new technology in water quality analysis and still under development. Therefore, the public acceptance level would be low due to it being less-known among industries, and thus they remain in the laboratory stage. However, Liana et al.88 believe that through modifications and upgrades, paper-based methods will be feasible in the industrial sector, thus increasing the acceptance level of the public.Costs and materials
One of the aims of the research and development of paper-based methods is to ensure that they are cost-efficient for consumers to purchase, which can prove advantageous in the current COVID-19 pandemic situation. Normally during the selection of products, consumers will not just take note of the performance of the product as the cost is a major factor too. Sensors with high performance, such as digital colorimeters, are normally affordable only to industries and institutions due to the high price of the sensors. On the other hand, the public would buy low performance sensors such as colour wheel test kits due to their lower cost. Therefore, the idea of a low cost and high performance sensor would benefit not only industries and institutions, but also the public. Hence, the success of paper-based methods can be a big step towards ensuring clean and safe water for everyone. As an example, the portable nanohybrid paper-based sensor developed by Yen et al.58 is estimated to cost only $0.03 or RM0.13 to fabricate for one piece of the sensor. This would mark a great market advantage if commercialized due to its low cost and high performance. Furthermore, the materials that make up the sensor are also an important aspect to be taken note of by researchers. To ensure the user-friendliness of the sensors, researchers need to consider using light, cheap and sustainable materials for fabrication. This can result in a portable and safe device for the consumer to utilize without any worry or second thought. Moreover, during device disposal, the material ought to be eco-friendly to prevent any harmful effects on the surrounding environment, primarily pollution.Conclusion
Free chlorine is a harmful residual and one of the main concerns in water quality monitoring systems around the world. To prevent any deficient or excessive levels of free chlorine concentration in water, especially drinking water, sensors have been commercialized for use by the water treatment industries and households. More and more methods have also been developed to detect and monitor free chlorine concentration in water. However, limitations such as high cost, harmful chemicals and complicated analysis have kept most of the methods on hold from commercialization, for example the OT oxidation method and iodometry. Therefore, paper-based sensors are researched and developed to fill the gap left by these conventional methods. Being portable, cost-effective, high-performance, and user-friendly, paper-based methods are on the brink of replacing the conventional methods in the market. Nevertheless, the use of chemicals is still an issue of concern to prevent any possibility of leaching into water. Hence, a chemical-free, paper-based free chlorine sensor should be the focus of future studies for the safety of consumable water. Therefore, a CQD-based paper sensor is introduced as a green and novel idea for future research due to it being non-toxic and made from natural resources. Not only can such a sensor prevent any incident of leaching, but it can also meet the ASSURED criteria set by the WHO.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the Fundamental Research Grant Scheme (FRGS FRGS/1/2021/STG05/XMU/02/2) from the Ministry of Higher Education Malaysia and Xiamen University Malaysia Research Fund (XMUMRF/2019-C4/IENG/0023) from Xiamen University Malaysia, which financially supported this work.Notes and references
- Z. Kılıç, Int. J. Hydrol., 2020, 4, 239–241 CrossRef.
- A. R. Dansharif, Z. Abdulkadir, S. B. Abubakar, A. Ibrahim, I. Umar and M. A. Baballe, J. Math. Techn. Comput. Math., 2023, 2, 175–179 CrossRef.
- National Academy of Sciences, Safe Drinking Water is Essential: Why is Safe Water Essential?https://www.koshland-science-museum.org/water/new/en/Overview/Why-is-Safe-Water-Essential.html, 2016, accessed 14 October 2021.
- H. Göçerler, B. Pfeil, F. Franek, C. Bauer, E. Niculescu-Morzsa and S. Nehrer, Ind. Lubr. Tribol., 2019, 72, 31–37 CrossRef.
- L. E. Armstrong and E. C. Johnson, Nutrients, 2018, 10, 1928 CrossRef PubMed.
- B. M. Popkin, K. E. D'Anci and I. H. Rosenberg, Nutr. Rev., 2010, 68, 439–458 CrossRef PubMed.
- S. Bidaisee, Biomed. J. Sci. Tech. Res., 2018, 8, 17–20 Search PubMed.
- A. Saravanan, P. Senthil Kumar, S. Jeevanantham, S. Karishma, B. Tajsabreen, P. R. Yaashikaa and B. Reshma, Chemosphere, 2021, 280, 130595 CrossRef CAS PubMed.
- S. Samal, Powder Technol., 2020, 366, 43–51 CrossRef CAS.
- A. Ahmad, S. Rutten, L. de Waal, P. Vollaard, C. van Genuchten, H. Bruning, E. Cornelissen and A. van der Wal, Sep. Purif. Technol., 2020, 241, 116644 CrossRef CAS.
- K. GracePavithra, V. Jaikumar, P. S. Kumar and P. SundarRajan, J. Cleaner Prod., 2019, 228, 580–593 CrossRef CAS.
- F. Nyström, K. Nordqvist, I. Herrmann, A. Hedström and M. Viklander, Water Res., 2020, 182, 115919 CrossRef PubMed.
- L. K. Wang, D. A. Vaccari, Y. Li and N. K. Shammas, in Physicochemical Treatment Processes, ed. L. K. Wang, Y.-T. Hung and N. K. Shammas, Humana Press, Totowa, NJ, 2005, DOI:10.1385/1-59259-820-x:141, pp. 141–197.
- L. K. Wang, E. M. Fahey and Z. Wu, in Physicochemical Treatment Processes, ed. L. K. Wang, Y.-T. Hung and N. K. Shammas, Humana Press, Totowa, NJ, 2005, DOI:10.1385/1-59259-820-x:431, pp. 431–500.
- S. Vigneswaran, H. H. Ngo, D. S. Chaudhary and Y.-T. Hung, in Physicochemical Treatment Processes, ed. L. K. Wang, Y.-T. Hung and N. K. Shammas, Humana Press, Totowa, NJ, 2005, vol. 635, pp. 635–676, DOI:10.1385/1-59259-820-x.
- T. A. Kurniawan, G. Y. S. Chan, W.-H. Lo and S. Babel, Chem. Eng. J., 2006, 118, 83–98 CrossRef CAS.
- N. B. Singh, G. Nagpal, S. Agrawal and Rachna, Environ. Technol. Innovation, 2018, 11, 187–240 CrossRef.
- D. N. Myers, Why monitor water quality?https://water.usgs.gov/owq/WhyMonitorWaterQuality.pdf, 2014, accessed 17 October 2021.
- World Health Organization, Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum, World Health Organization, Geneva, 2017, Licence: CC BY-NC-SA 3.0 IGO Search PubMed.
- M. O. Dinka, in Water Challenges of an Urbanizing World, 2018, ch. 10, DOI:10.5772/intechopen.71352.
- A. D. Committee, 2017 Water Utility Disinfection Survey Report. 2018 Search PubMed.
- X. Ren and H. Chen, Sci. Total Environ., 2021, 752, 141223 CrossRef CAS PubMed.
- T. Li, Z. Wang, C. Wang, J. Huang and M. Zhou, Sci. Total Environ., 2022, 838, 156193 CrossRef CAS PubMed.
- M. A. Mazhar, N. A. Khan, S. Ahmed, A. H. Khan, A. Hussain, Rahisuddin, F. Changani, M. Yousefi, S. Ahmadi and V. Vambol, J. Cleaner Prod., 2020, 273, 123159 CrossRef CAS.
- Centres for Disease Control and Prevention (CDC), Water Disinfection with Chlorine and Chloramine, 2020, https://www.cdc.gov/healthywater/drinking/public/water_disinfection.html, accessed 30 September 2021.
- H.-Y. Jo, C. N. Tango and D.-H. Oh, Lwt, 2018, 92, 187–194 CrossRef CAS.
- Centres for Disease Control and Prevention (CDC), Free Chlorine Testing, 2020, https://www.cdc.gov/safewater/chlorine-residual-testing.html, accessed 30 September 2021.
- Minnesota Department of Health, Disinfection and Disinfection Byproducts, https://www.health.state.mn.us/communities/environment/water/factsheet/ddbp.html, 2019, accessed 4 October 2021 Search PubMed.
- C. DiLella and S. Mody, A major chlorine shortage is set to spoil summertime fun in the swimming pool, CNBC, 2021, https://www.cnbc.com/2021/04/30/a-major-chlorine-shortage-is-set-to-spoil-swimming-pool-fun-this-summer.html, accessed 4 October 2021 Search PubMed.
- Q. Liu, B. Liao, Y. Tian, Y. Chen, D. Luo, Y. Lin, H. Li and K.-J. Wang, Oncotarget, 2017, 8, 55467–55477 CrossRef PubMed.
- A. Morim and G. T. Guldner, Chlorine Gas Toxicity, in StatPearls [Internet], StatPearls Publishing, Florida, 2021 Search PubMed.
- J. Mastroianni, Testing for chlorine in drinking water, 2011, https://www.watertechonline.com/home/article/15535685/testing-for-chlorine-in-drinking-water, accessed 5 October 2021 Search PubMed.
- N. Agabiti, C. Ancona, F. Forastiere, A. Di Napoli, E. Lo Presti, G. M. Corbo, F. D’Orsi and C. A. Perucci, Occup. Environ. Med., 2001, 58, 399–404 CrossRef CAS PubMed.
- A. Apilux, W. Dungchai, W. Siangproh, N. Praphairaksit, C. S. Henry and O. Chailapakul, Anal. Chem., 2010, 82, 1727–1732 CrossRef CAS PubMed.
- G. Nikolov, E. Gieva, B. Nikolova and M. Marinov, Proceedings of the 2016 XXV International Scientific Conference Electronics (ET), Institute of Electrical and Electronics Engineers, Sozopol, 2016 Search PubMed.
- S. Saputro, K. Yoshimura, K. Takehara, S. Matsuoka and Narsito, in Chlorine Properties, Applications and Health Effects, Nova Science Publishers Inc., New York, 2011, pp. 259–274 Search PubMed.
- Orlab Instruments Pvt. Ltd, Residual Free chlorine (OT) Test Kit, 2019, http://www.orlabindia.com/residual-free-chlorine-ot-test-kit/, accessed 12 October 2021.
- M. Sargazi and M. Kaykhaii, Spectrochim. Acta, Part A, 2020, 227, 117672 CrossRef CAS PubMed.
- L. Moberg and B. Karlberg, Anal. Chim. Acta, 2000, 407, 127–133 CrossRef CAS.
- Hach, Free Chlorine Color Disc Test Kit, Model CN-66F, 2015, https://www.hach.com/free-chlorine-color-disc-test-kit-model-cn-66f/product?id=7640219520, accessed 12 October 2021.
- Hanna Instruments (M) Sdn Bhd, HI701 Free Chlorine Colorimeter – Checker® HC, 2017, http://www.hannamalaysia.com/index.php?ws=showproducts%26products_id=1693964, accessed 12 October 2021.
- M. A. Leonard, Endeavour, 1990, 14, 100 CrossRef.
- University of Illinois, Iodometric Titration, 2012, https://archives.library.illinois.edu/erec/UniversityArchives/1505050/ch106Brass/titration.htm, accessed 12 October 2021.
- K. Singh and S. K. Mehta, Analyst, 2016, 141, 2487–2492 RSC.
- M. Xue, L. Zhang, M. Zou, C. Lan, Z. Zhan and S. Zhao, Sens. Actuators, B, 2015, 219, 50–56 CrossRef CAS.
- D. Vashisht, S. Sangar, M. Kaur, E. Sharma, A. Vashisht, A. O. Ibhadon, S. Sharma, S. K. Mehta and K. Singh, Environ. Res., 2021, 197, 111142 CrossRef CAS PubMed.
- P. A. Kim, D. Choe, H. So, S. Park, B. Suh, S. Jeong, K.-T. Kim, C. Kim and R. G. Harrison, Spectrochim. Acta, Part A, 2021, 261, 120059 CrossRef CAS PubMed.
- E. Sharma, D. Vashisht, V. Thakur, A. Vashisht, S. K. Mehta and K. Singh, Mater. Chem. Phys., 2021, 273, 125088 CrossRef CAS.
- S. Enbanathan, S. Manickam, S. Munusamy, D. Jothi, S. Manoj Kumar and S. Kulathu Iyer, New J. Chem., 2022, 46, 6570–6576 RSC.
- T. Lu, L. Zhang, M. Sun, D. Deng, Y. Su and Y. Lv, Anal. Chem., 2016, 88, 3413–3420 CrossRef CAS PubMed.
- M. Jović, F. Cortés-Salazar, A. Lesch, V. Amstutz, H. Bi and H. H. Girault, J. Electroanal. Chem., 2015, 756, 171–178 CrossRef.
- D. R. Kumar, S. Kesavan, T. T. Nguyen, J. Hwang, C. Lamiel and J.-J. Shim, Sens. Actuators, B, 2017, 240, 818–828 CrossRef CAS.
- P. Salazar, M. Martín, F. J. García-García, J. L. González-Mora and A. R. González-Elipe, Sens. Actuators, B, 2015, 213, 116–123 CrossRef CAS.
- L. Yang, J. Wang, H. Lu and N. Hui, Mikrochim. Acta, 2021, 188, 25 CrossRef CAS PubMed.
- M. R. Tomei, F. Arduini, D. Neagu and D. Moscone, Talanta, 2018, 189, 262–267 CrossRef CAS PubMed.
- E. Hoque, L. H. H. Hsu, A. Aryasomayajula, P. R. Selvaganapathy and P. Kruse, IEEE Sens. Lett., 2017, 1, 1–4 Search PubMed.
- J. Mettakoonpitak, K. Boehle, S. Nantaphol, P. Teengam, J. A. Adkins, M. Srisa-Art and C. S. Henry, Electroanalysis, 2016, 28, 1420–1436 CrossRef CAS.
- Y. K. Yen, K. Y. Lee, C. Y. Lin, S. T. Zhang, C. W. Wang and T. Y. Liu, ACS Omega, 2020, 5, 25209–25215 CrossRef CAS PubMed.
- K. J. Land, D. I. Boeras, X. S. Chen, A. R. Ramsay and R. W. Peeling, Nat. Microbiol., 2019, 4, 46–54 CrossRef CAS PubMed.
- S. Smith, J. G. Korvink, D. Mager and K. Land, RSC Adv., 2018, 8, 34012–34034 RSC.
- Y. Qin, S. Pan, M. M. Howlader, R. Ghosh, N. X. Hu and M. J. Deen, Anal. Chem., 2016, 88, 10384–10389 CrossRef CAS PubMed.
- C. Huangfu, Y. Zhang, M. Jang and L. Feng, Sens. Actuators, B, 2019, 293, 350–356 CrossRef CAS.
- S. Arsawiset and S. Teepoo, Anal. Chim. Acta, 2020, 1118, 63–72 CrossRef CAS PubMed.
- J. Cameron and P. J. Skabara, Mater. Horiz., 2020, 7, 1759–1772 RSC.
- M. Ahmad, B. Zhang, J. Wang, J. Xu, K. Manzoor, S. Ahmad and S. Ikram, Int. J. Biol. Macromol., 2019, 136, 189–198 CrossRef CAS PubMed.
- A. A. Mohamed and A. A. Shalaby, Food Chem., 2019, 274, 360–367 CrossRef CAS PubMed.
- X. L. Guo, Y. Chen, H. L. Jiang, X. B. Qiu and D. L. Yu, Sensors, 2018, 18, 3141 CrossRef PubMed.
- R. R. Gaddam, S. Chambers, D. Murdoch, G. Shaw and M. Bhatia, J. Infect., 2017, 75, 293–300 CrossRef PubMed.
- Fisher Scientific Company, N, N-Diethyl-p-phenylenediamine; MSDS No. AC407390250, 2021, https://www.fishersci.com/store/msds?partNumber=AC407390250%26productDescription=N%2CN-DIETHYL-P-PHENYLENED+25GR%26vendorId=VN00032119%26countryCode=US%26language=en, accessed 12 January 2022.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921 RSC.
- M. Semeniuk, Z. Yi, V. Poursorkhabi, J. Tjong, S. Jaffer, Z. H. Lu and M. Sain, ACS Nano, 2019, 13, 6224–6255 CrossRef CAS PubMed.
- M. Farshbaf, S. Davaran, F. Rahimi, N. Annabi, R. Salehi and A. Akbarzadeh, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1331–1348 CrossRef CAS PubMed.
- L. Tian, Z. Li, P. Wang, X. Zhai, X. Wang and T. Li, J. Energy Chem., 2021, 55, 279–294 CrossRef CAS.
- M. A. Jhonsi, in State of the Art in Nano-Bioimaging, 2018, ch. 3, DOI:10.5772/intechopen.72723.
- Y. Meng, Y. Zhang, W. Sun, M. Wang, B. He, H. Chen and Q. Tang, Electrochim. Acta, 2017, 257, 259–266 CrossRef CAS.
- P. Devi, S. Saini and K. H. Kim, Biosens. Bioelectron., 2019, 141, 111158 CrossRef CAS PubMed.
- N. A. A. Nazri, N. H. Azeman, Y. Luo and A. A. A Bakar, Opt Laser. Technol., 2021, 139, 106928 CrossRef CAS.
- P. He, Y. Shi, T. Meng, T. Yuan, Y. Li, X. Li, Y. Zhang, L. Fan and S. Yang, Nanoscale, 2020, 12, 4826–4832 RSC.
- X. Wang, Y. Feng, P. Dong and J. Huang, Front. Chem., 2019, 7, 671 CrossRef CAS PubMed.
- G. E. LeCroy, S.-T. Yang, F. Yang, Y. Liu, K. A. S. Fernando, C. E. Bunker, Y. Hu, P. G. Luo and Y.-P. Sun, Coord. Chem. Rev., 2016, 320–321, 66–81 CrossRef CAS.
- B. T. Hoan, P. D. Tam and V.-H. Pham, J. Nanotechnol., 2019, 2019, 1–9 CrossRef.
- N. Papaioannou, A. Marinovic, N. Yoshizawa, A. E. Goode, M. Fay, A. Khlobystov, M. M. Titirici and A. Sapelkin, Sci. Rep., 2018, 8, 6559 CrossRef PubMed.
- M. He, J. Zhang, H. Wang, Y. Kong, Y. Xiao and W. Xu, Nanoscale Res. Lett., 2018, 13, 175 CrossRef PubMed.
- I. R. Isnaeni, R. Intan and M. Zakaria, J. Phys.: Conf. Ser., 2018, 985 Search PubMed.
- Y. Dong, G. Li, N. Zhou, R. Wang, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 8378–8382 CrossRef CAS PubMed.
- D. S. Bernstein, IEEE Control Syst., 2001, 21, 9–18 Search PubMed.
- J. Hu, C. T. Yew, X. Chen, S. Feng, Q. Yang, S. Wang, W. H. Wee, B. Pingguan-Murphy, T. J. Lu and F. Xu, Talanta, 2017, 165, 419–428 CrossRef CAS PubMed.
- D. D. Liana, B. Raguse, J. J. Gooding and E. Chow, Sensors, 2012, 12, 11505–11526 CrossRef CAS PubMed.
- X. Tian, H. Peng, Y. Li, C. Yang, Z. Zhou and Y. Wang, Sens. Actuators, B, 2017, 243, 1002–1009 CrossRef CAS.
- C. H. Connell, J. – Am. Water Works Assoc., 1947, 39, 209–218 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00188e |
| This journal is © The Royal Society of Chemistry 2024 |
