Open Access Article
Open Access ArticleA new frontier towards the development of efficient SPEEK polymer membranes for PEM fuel cell applications: a review
Mayetu
Segale
a,
Tumelo
Seadira
 *a,
Rudzani
Sigwadi
a,
Touhami
Mokrani
a and
Gabriel
Summers
b
*a,
Rudzani
Sigwadi
a,
Touhami
Mokrani
a and
Gabriel
Summers
b
aDepartment of Chemical and Materials Engineering (CSET), University of South Africa (Science Campus), Private Bag X6, Florida Park, Roodepoort 1709, South Africa. E-mail: tumelo.seadira@gmail.com
bDepartment of Chemistry (CSET), University of South Africa (Science Campus), Private Bag X6, Florida Park, Roodepoort 1709, South Africa
First published on 28th August 2024
Abstract
Proton exchange membrane fuel cells (PEMFCs) have gained popularity over the last decade as a potential clean energy source for electric vehicles and portable electronic devices. Nafion is commonly used as a membrane material but suffers from high methanol crossover and cost. These drawbacks negatively influence the widespread commercial application of PEMFCs. Currently, the focus is on developing high-performance, low-cost PEMs to replace Nafion membranes. Sulfonated poly-ether-ketone-ether (SPEEK) has been identified as a promising alternative PEM in fuel cell applications due to its advantageous properties, such as low cost, mechanical and chemical stability, and ease of preparation and operation. The main purpose of this review is to demonstrate the benefits of SPEEK-based composite membranes over Nafion® by mixing the SPEEK material with fluorinated polymers, hydrocarbon polymers, carbon-based materials, metal oxide materials, etc. The ion-exchange capacity and proton conductivity of SPEEK polymers with different fillers are highlighted. SPEEK-based composite membranes are far more suitable for PEMFC and DMFC applications because SPEEK polymers are produced in an environmentally friendly manner. This critical review guides researchers in developing processes to maximise the properties of SPEEK-based membranes for fuel cell applications.
1. Introduction
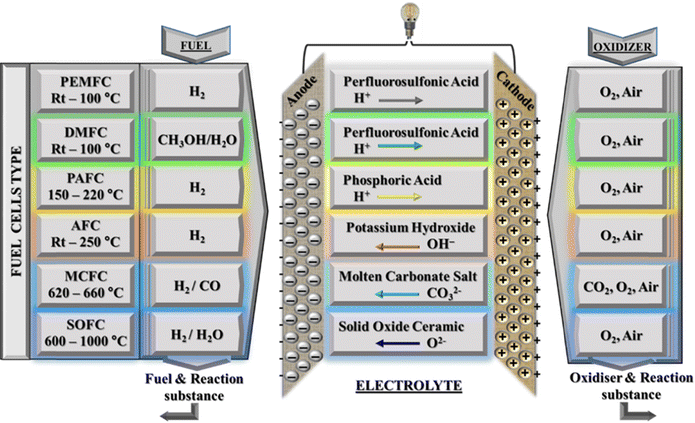
For decades, fossil fuels have been considered the main sources of the increased pollution levels in the environment due to the emission of toxic greenhouse gases such as COx, NOx, and SOx upon their combustion.1–3 Furthermore, there are growing concerns about the long-term viability of fossil fuels which are expected to run out sooner rather than later. As a result, sustainable, renewable, and environmentally friendly fuels will emerge sooner or later.To address these issues, extensive research and development are being conducted to identify alternative sources of electricity that are efficient, renewable, and environmentally friendly. Fuel cells are one of many technologies that will enable future sustainable hydrogen, carbon-free cycles, and a circular economy.4,5 Over the last two decades, fuel cell applications have grown in popularity in engines, and stationary and portable power sources.6,7 Mohammed et al.,8 described a fuel cell as an electrochemical device that converts the chemical energy of a fuel (the reactant) such as methanol, ethanol or ethylene glycol into electrical energy without any fuel combustion. The fuel is directly oxidised, producing electricity, heat, and water vapour. The electrochemical reactions within the fuel cell are explained as follows: when hydrogen passes through the anode, it is converted into hydrogen ions, and electrons are released, which travel through an external circuit before reaching the cathode to produce an electrical current.9 The membrane electrode assembly (MEA) is the primary component of the fuel cell, consisting of a gas diffusion layer, catalyst, and electrolyte (membrane). Protons migrate through the electrolyte to the cathode, where they unite with oxygen and electrons to produce water and heat. Fuel cell technologies are characterized by the nature of electrolyte they use. The electrolyte is an important part of a fuel cell as it defines the properties and the operating conditions of the fuel cell.10,11 There are six distinct types of fuel cells namely, (i) alkaline fuel cells (AFCs), (ii) direct methanol fuel cells (DMFCs), (iii) molten carbonate fuel cells (MCFCs), (iv) phosphoric acid fuel cells (PAFCs), (v) proton exchange membrane fuel cells (PEMFCs) and (vi)) solid oxide fuel cells (SOFCs), and each operates under different reaction conditions and uses different electrolytes. Out of the six fuel cells, two fuel cells (i.e. hydrogen fuel cells (H2-FCs) and direct methanol fuel cells (DMFCs)) use a polymeric membrane as the electrolyte, while others are based on the electrochemical principles. The components, fuel types and performance characteristics of the various types of fuel cells are presented in Fig. 1.
Among the fuel cells listed above, proton exchange membrane fuel cell (PEMFC) technology is a major area of global research interest.12 Their high energy density and efficiency, along with their potential for low emissions, make them a promising clean energy technology. The proton exchange membrane (PEM) acts as a barrier to the fuel gas between the electrodes, transferring protons from the anode to the cathode of the PEMFC. The reactions that occur in the PEMFC are as follows.
Anode side:
| 2H2 → 4H+ + 4e− | (1) |
Cathode side:
| O2 + 4e− + 4H+ → 2H2O | (2) |
Overall cell reaction:
| 2H2 + O2 → 2H2O + heat + electrical energy | (3) |
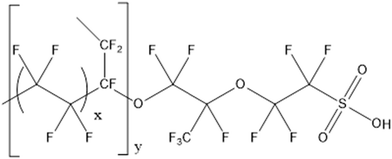
For the anodic reaction, hydrogen flows through the gas diffusion layer before dissociating into two electrons and two protons in the catalyst layer (eqn (1)). The two protons pass through the PEM to reach the catalyst layer at the cathode, and the two electrons pass through the external circuit to the cathode.13,14 Similar to the anodic reaction, which results in the production of heat and water, the cathodic reaction occurs when air enters the catalyst layer through the gas diffusion layer and reacts with the two electrons and two protons (eqn (2)). The most common membranes used in PEM fuel cells are fluorinated membranes. The perfluorosulfonic acid (PFSA) polymers known as Nafion membranes are the most common type. Sulfonated polymers, such as Nafion, with perfluorinated backbones and sulfonated side-chains, are the most widely used membrane for PEM cells because they function well at temperatures below 100 °C. The perfluoroether in Nafion is responsible for the chemical stability, while sulfonated sidechains aggregate and facilitate hydration.15 Due to its high ionic conductivity (approximately 0.1 S cm−1 when fully hydrated), as well as its thermal and chemical stability, the Nafion membrane has been chosen as a standard for polymeric electrolyte fuel cells.16 However, Nafion membranes have several drawbacks, including a decrease in ionic conductivity and low humidity at high temperatures, which makes commercialization difficult. For these reasons, other proton conducting membranes with either partially fluorinated or hydrocarbon-based polymers containing ionic transfer sites have been developed to improve fuel cell performance. The chemical structure of Nafion is shown in Fig. 2.
In this context, many studies have been conducted to develop PEMs with improved performance characteristics such as low cost, ease of synthesis, good thermal and mechanical stability, and eco-friendliness. A partially fluorinated PEM can be created by synthesizing block copolymers, one of which is a fluoropolymer. Partially fluorinated membranes, like fluorinated membranes, have demonstrated high proton conductivity. However, they are costly and cannot be referred to as low cost due to the use of expensive fluorinated materials. Furthermore, commercialization of these materials has been hampered by high costs and a scarcity of the trifluorostyrene monomer.16
Non-fluorinated membranes, made from polymer materials with aromatic structures and functional groups in either the polymeric backbone or side groups, are being used in the proton exchange membranes (PEMs) in place of perfluorinated membranes.
One major advantage of hydrocarbon membranes is that it is simple to design the polymeric structure to have the right characteristics for fuel cell applications.17 Various types of monomers are used to control the reaction conditions when preparing hydrocarbon membranes with excellent properties. Moreover, the cost of the monomers used in the production of hydrocarbon-based polymers is comparatively lower than that of fluorinated membranes, which is a major benefit for commercialization.15,18 In general, hydrocarbon-based polymers have polar groups and a carbon backbone, and they have high water uptake over a wide temperature range. Despite having increased proton conductivity and poor dimensional stability in membranes, water channel formation occurs.19 Rigid sites, such as aromatic structures, are incorporated directly into the polymer backbone to improve membrane stability and properties. The aromatic rings provide rigidity, which leads to thermal and mechanical stability. As a result, a variety of hydrocarbon-based polymers, including poly-ether ketones (PEK), poly(arylene ethers), poly(ether ether ketone) (PEEK), polyesters, and polyimides (PIs), have been actively researched and developed for use in fuel cell applications.20–23 With a wide variety of alternative aromatic polymers to choose from, poly(ether ether ketone) (PEEK) appears to have the best potential as a PEM for fuel cell application. PEEK polymers are semicrystalline thermoplastic polymers with ether and ketone chemical properties. This polymer has a well-balanced blend of excellent mechanical properties, low cost and superior thermo-oxidative stability.22 The PEEK polymer has an aromatic, nonfluorinated backbone with 1,4-disubstituted phenyl groups separated by ether (–O–) and carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) linkages (as shown in Fig. 3), making it a high-performance thermostable engineering polymer.24
O) linkages (as shown in Fig. 3), making it a high-performance thermostable engineering polymer.24
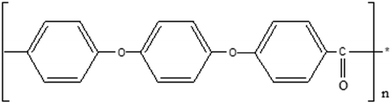 | ||
| Fig. 3 General structure of PEEK.24 | ||
This polymer's inherent hydrophobicity is typically overcome through chemical modification of the polymer chains. Sulfonic acid functionalities are easily incorporated onto the aromatic backbone of PEEK. Sulfonated poly(ether ether ketone) (SPEEK) is a semi-crystalline, amorphous polymer that exhibits high chemical and thermal stabilities due to the presence of aromatic rings.25 It is made by polymerizing different monomers using the following synthesis techniques: (i) displacement reaction; (ii) nickel-catalyzed coupling polymerization; (iv) ring-opening polymerization involving monomers with sulfonic acid groups; and (iii) Friedel–Crafts acylation.26 The degree of sulfonation (DS) has a strong influence on the properties of SPEEK, which can be controlled by adjusting the reaction conditions (reaction temperature, acid concentration, and sulfonation time). SPEEK demonstrated desirable chemical durability at low DS, with greater dimensional, mechanical, and thermal stability than Nafion but lower water uptake and proton conductivity.27 At higher DS, membrane swelling in aqueous solutions promotes the formation of interconnected channels of hydrophilic clusters. This resulted in high proton conductivity similar to Nafion, but with undesirable mechanical properties, excessive dimensional swelling, fuel permeability, and consequently low durability. Many studies have been conducted to develop modified SPEEK membranes in order to improve the fuel cell performance.28,29
The intensive research activities in the development of modified SPEEK membranes, particularly for fuel cell applications, are critical for evaluating progress in this specific research field. With considerable efforts made to enhance SPEEK membranes, this review concentrates on the development of PEMs based on sulfonated poly(ether ether ketone) (SPEEK) polymers. The physicochemical properties and characteristics of pure sulfonated poly(ether ether ketone) (SPEEK) membranes are discussed. The article also discusses strategies for improving the performance of the SPEEK matrix membrane. The results for various types of modified SPEEK membranes are summarised and analysed. This paper concludes with the challenges and opportunities encountered during the development of SPEEK-based membranes for fuel cell applications.
2. Mechanism of proton conduction in PEMs
Proton conduction is the most important factor to consider when assessing membranes for possible fuel cell applications. In PEMFC operation, the membrane must ensure the systematic movement of water and ions, rejection of electrons, and dissociation of reactant gases. Sufficient hydration levels of PEM are critical for maintaining high proton conductivity during fuel cell operation. Two major mechanisms can hydrate proton transfer at the molecular level: electro-osmotic drag (vehicle) and proton hopping (Grotthuss).30A schematic design of the Grotthus and vehicular mechanisms is shown in Fig. 4.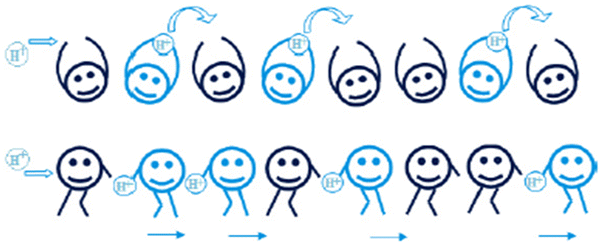 | ||
| Fig. 4 A schematic representation of the Grotthus and vehicular mechanisms.31 | ||
In the proton hopping (Grotthus) mechanism, protons move from one hydrolyzed ion (SO3−, H3O+) to another via polymeric matrices. The activation energy required for proton conductivity to occur for this proton hopping mechanism is 0.1–0.4 eV. Protons are drawn from the hydronium ions by more adjacent water molecules, and the cycle is repeated. In this mechanism, the ion area forms a specific hydrophilic cluster that expands with water. As a result, protons will undergo percolation mechanisms. The Grotthus mechanism contributes to the conductivity of a perfluorinated sulfonic acid membrane like Nafion.32
The ion exchange capacity (IEC) value affects Grotthus-type transfer because it represents the number of ionizable groups loaded into the fuel cell membrane. Electro-osmotic drag (vehicle) in the membrane transports hydrogen ions (H3O+) throughout the aqueous medium. As a result, water and methanol molecules act as proton transporters in the polymeric membrane. The activation energy is required to initiate proton conductivity Eact > 0.5 eV.33 In this mechanism, hydrated protons (hydronium ions) permeate an aqueous medium due to electrochemical differences. Protons bind to vehicles such as water or methanol before diffusing into the medium to form cationic complexes such as H3O+, H5O2+, H9O4+, and CH3OH2+. The presence of free volumes in the polymeric chain of proton exchange membranes is critical to the vehicular mechanism. Water aids proton conductivity in PEMs by influencing size, stability, production, clusters and ion route connection. Under aqueous conditions, as cluster size increases, proton conductivity increases in proportion to humidity. The proton conductivity of polymeric membranes at high temperatures and low relative humidity could be enhanced by choosing inorganic additives using this mechanism.34
3. Sulfonation of PEEK
Poly(ether ether ketone) polymers are chemically resistant, and thermally and mechanically stable. However, due to their completely hydrophobic segments, they cannot be used directly in fuel cell applications.29 As a result, adding a sulfonic acid group to a PEEK polymer improves its hydrophilicity, solubility in polar solvents, and ion exchange capacity. At different degrees of sulfonation, the sulfonated form (SPEEK) is soluble in different solvents. The solubility of the SPEEK membrane is reported as follows: a sulfonation degree (DS) of about 30% makes it soluble only in hot DMF, DMAc, DMSO, and NMP; a DS of 40–70% makes it soluble at room temperature in DMF, DMAc, DMSO, and NMP; a DS of above 70% makes it soluble in methanol; and a DS of 100% makes it soluble in hot water.35 Three synthetic methods can be used to prepare sulfonated poly(ether ether ketone):(a) Direct sulfonated monomer copolymerization reactions with suitable monomers.
(b) Direct post-polymerization sulfonation reactions with poly(ether ether ketone).
(c) Sulfonation reactions of poly(ether ether ketone)s to introduce the sulfonate group pendant into the polymer chains.
3.1. Direct sulfonated monomer copolymerization reactions with suitable monomers
A specific sulfonated dihalogenated diaryl ketone monomer or sulfonated bisphenol derivative can be directly copolymerized with a suitable dihalogenated diaryl ether monomer unit to produce random (statistical) sulfonated poly(ether ether ketone).36 Through careful control of the reaction stoichiometry, the sulfonate group can be introduced regiospecifically along the polymer backbone. This is achieved through the step-growth copolymerization reaction utilising sulfonated monomers.37 Electrophilic aromatic substitution reactions with various sulfonating agents can be used to produce sulfonated dihalogenated diaryl ketone or bisphenol monomer derivatives.38 Nguyen et al.39 synthesised a sulfonated di-halogenated diaryl ketone monomer by treating 4,4′-difluorobenzophenone with 25.3% fuming sulfuric acid, resulting in 100% yield and high purity disodium-3,3′-disulfate-4,4′-difluorobenzophenone (Fig. 5).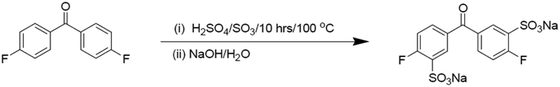 | ||
| Fig. 5 The reaction formula of a sulfonated di-halogenated diaryl ketone monomer.39 | ||
A series of sulfonated poly(ether ether ketone) were produced by the base-catalysed nucleophilic aromatic polycondensation reaction of 4,4′-difluorobenzophenone and pure sulfonated monomer, disodium-3,3′-disulfate-4,4′-difluorobenzophenone with hexa-fluoro isopropylidene diphenol (Fig. 6).40 High molecular weight polymers that exhibit thermal stability up to 260 °C were produced. The resulting sulfonated poly(ether ether ketone)s were used as proton exchange membranes in fuel cells.
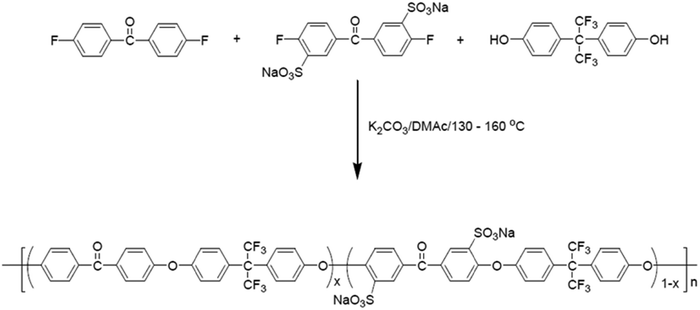 | ||
| Fig. 6 Reaction mechanism for the preparation of sulfonated poly(ether ether ketone) via base catalyzed nucleophilic aromatic polycondensation.40 | ||
3.2. Direct post-polymerization sulfonation reactions with poly(ether ether ketone)
Poly(ether ether ketone)s are highly effective polymers that are insoluble in the vast majority of organic solvents. Incorporating the sulfonic acid group along the polymer backbone of poly(ether ether ketone) reduces crystallinity and increases polymer solubility.41 Direct sulfonation of poly(ether ether ketone)s using different sulfonating agents is not region-specific due to the lack of control over the degree and site of sulfonation during the sulfonation process.41 Furthermore, polymer degradation and numerous side reactions have been observed. The electrophilic substitution reaction mechanism is used in the sulfonation of poly(ether ether ketone)s by sulfonating agents such as sulfuric acid (H2SO4), as shown in Fig. 7. The ether linkage activates the polymer chain's phenyl rings for electrophilic substitution reactions, and the sulfonating group is introduced into the polymer chain's hydroquinone segment.42,43 One sulfonic acid group is typically added per unit because the carbonyl group attracts electrons, which lowers the electron density of the other aromatic rings.41,44 However, disulfonation reactions are possible at higher temperatures or for longer reaction times. Sulfonation reactions with poly(ether ether ketone)s are typically carried out in the presence of sulfonating agents like chlorosulfonic acid or sulfuric acid.45 The reaction time, temperature, and acid concentration all influence sulfonation with sulfuric acid. In order to create polymers with different levels of sulfonation, Daud et al.,46 prepared sulfonated poly(ether ether ketone) from Victrex and 95–97% concentrated sulfuric acid and chlorosulfuric acid at room temperature to avoid PEEK polymer degradation and cross-linking reactions. The reaction was performed over a range of reaction times and a degree of sulfonation of 80% was reported. In another study, Muthu Lakshmi et al.,47 investigated the effect of temperature and reaction duration on the degree of sulphonation of Gatone, as well as the characteristics of sulphonated polymers. Sulphonation was performed at 35–50 °C for 3–5 hours. The degree of sulphonation was between 50–80%. The sulfonated poly(ether ether ketone) derivatives were used in fuel cell and electrodialysis processes as electrochemical devices.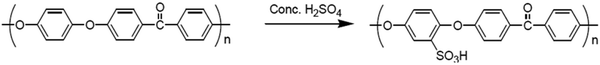 | ||
| Fig. 7 Sulfonation reactions of Gatone poly(ether ether ketone).47 | ||
To make it easier to incorporate the sulfonic acid group pendant into the polymer chain, standard organic reactions are commonly used to functionalize poly(ether ether ketone)s. Reactive sites in the polymer chain can be added either directly along the polymer backbone or by incorporating a suitable functional group pendant to the chain prior to the polymer precursor's sulfonation functionalization reaction.
Xu et al.,42 used dihydroxy functionalized poly(ether ether ketone)s as substrates to synthesise a series of new sulfonated poly(ether ether ketone)s. The base catalyzed nucleophilic aromatic substitution polymerization method was used to create the corresponding dihydroxynaphthalene based poly(ether ether ketone) derivative. Sulfonated poly(ether ether ketone) was produced by the base-catalyzed nucleophilic reaction of dihydroxynaphthalene-based poly(ether ether ketone) with 1,4-butane sulfone (Fig. 8). The resulting sulfonated poly(ether ether ketone) derivative exhibited high proton conductivity in DMFC applications.
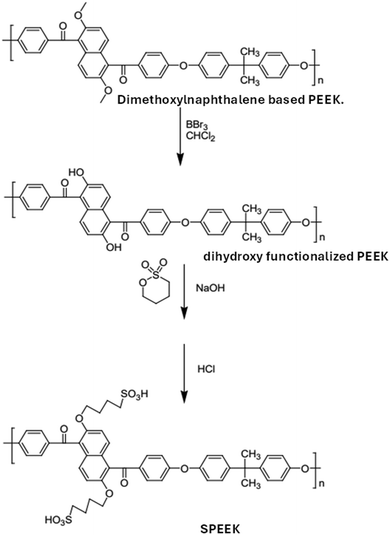 | ||
| Fig. 8 Nucleophilic reaction of dihydroxynaphthalene-based poly(ether ether ketone) with 1,4-butane sulfone.42 | ||
Another synthetic method for creating sulfonated poly(ether ether ketone)s with the sulfonic acid group pendant added to the polymer chain was created by Tsai et al.48 In order to produce pristine sulfonated poly(ether ether ketone), poly(ether ether ketone) was first treated with concentrated sulfuric acid. This was done by treating the resulting sulfonated poly(ether ether ketone) with 1,1′-carbonyl-diimidazole (CDI). Novel main-chain and side-chain sulfonated poly(ether ether ketone) with enhanced nano-phase separation morphology was formed after reaction with 2-aminoethanesulfonic acid (see Fig. 9). The addition of the new sulfonated group pendant to the polymer chain resulted in a well-defined nano-phase separation morphology and improved the properties of the proton exchange membrane in DMFC applications.
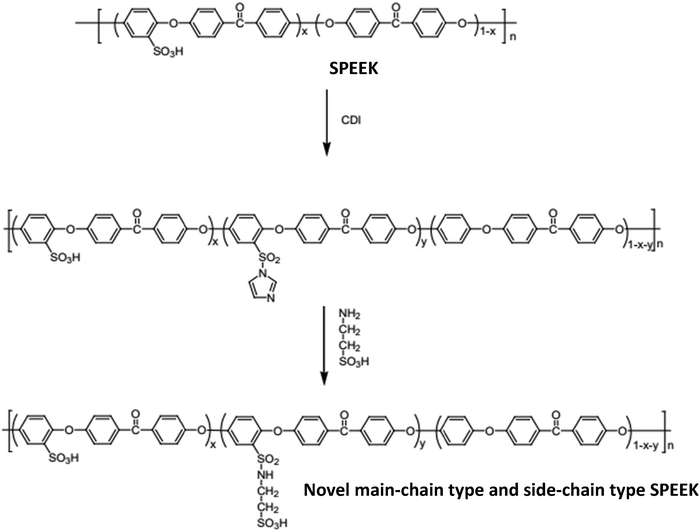 | ||
| Fig. 9 Sulfonated poly(ether ether ketone) synthesis route using 1,1′-carbonyl-diimidazole (CDI).48 | ||
The sulfonic acid group can also be added to the poly(ether ether ketone) chain by the thiol–ene reaction, which involves the following steps:
(i) The synthesis of a poly(ether ether ketone) precursor derivative with an unsaturation site attached to the polymer chain.
(ii) The pendant site of unsaturation reacts with a mercapto compound containing a sulfonate group using the classic thiol–ene reaction.
Li et al.,49 employed the thiol–ene method to synthesise poly(aryl ether ketone) ionomers with pendant sulfonic acid groups in the polymer backbone, as illustrated in Fig. 10. Quantitative yields of sulfonated poly(ether ether ketone) were obtained by treating the propenyl derivative of poly(ether ether ketone) with sodium 3-mercapto-1-propane sulfonate and AIBN in NMP/DMSO. Sulfonated poly(ether ether ketone)s were utilised as polymeric membrane substrates for fuel cell technology.
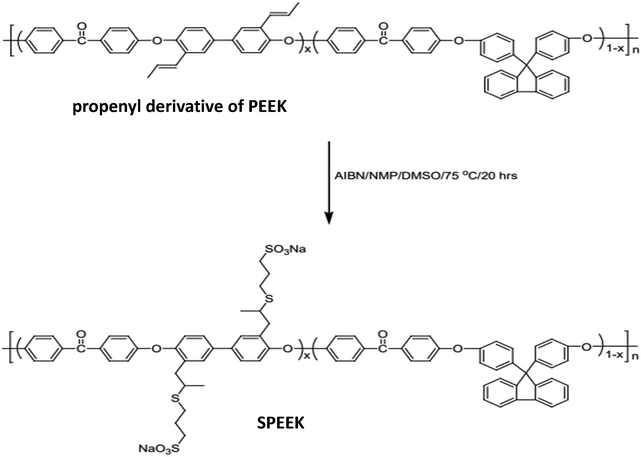 | ||
| Fig. 10 Synthesis of sulfonated poly(ether ether ketone) using the thiol–ene method.49 | ||
4. SPEEK modification methods
SPEEK polymer sulfonation is highly dependent on reaction conditions such as reaction time, temperature, and inert atmosphere. If the conditions are not properly maintained, the degree of sulfonation (DS) will either increase or decrease. The higher the DS of the SPEEK material, the more it swells at high temperatures before dissolving in water. Higher DS has always been associated with superior ion exchange capacity (IEC) and proton conductivity. Consequently, a range of modification techniques, including blending and cross-linking, have been researched to create effective SPEEK membranes.504.1. Membrane crosslinking
Xiang et al.51 used a combination of cross-linking agents comprising trimethylolpropane triacrylate (TMPTA), polyester acrylate, 2-(2-ethoxy-ethoxy)ethyl acrylate (EOEOA), and 1,6-hexandiol diacrylate (HDDA) to introduce EB cross-linking in the SPEEK structure. When different EB irradiation samples dosed at 6 kGy min−1 were used, the degree of cross-linking and the density of the structure were directly influenced by the exposure dose. Higher EB irradiation doses resulted in greater thermomechanical and dimensional stability. They discovered that cross-linked polymer membranes have a greater cluster transition temperature than the Nafion® 117 membrane, implying that cross-linked membranes may be more beneficial in high temperature fuel cells than Nafion® 117 membranes. SAXS revealed ionic sites were not deactivated by the cross-linking reaction, but rather increased proton conductivity occurred, especially at higher temperatures (90 °C). Moreover, the greater proton conductivity and dimensional stability at 80 °C and under fully humidified conditions allowed for the achievement of the maximum power density of 0.225 W cm−2 at a higher EB irradiation dosage (200 kGy). Xiaomin et al.52 synthesised 1,6-bis(4-vinylphenyl)hexane (BVPH), an unhydrolyzable cross-linker, to cross-link SPEEK membranes by EB irradiation at room temperature in order to address challenges with dimensional stability, mechanical strength, and methanol crossover. A higher degree of cross-linking was achieved by adding the cross-linking agent (BVPH) at varying content ranges of 5–15 wt% at a constant irradiation dose of 350 kGy and dose rate of 6 kGy min−1. Cross-linked membranes containing 15% BVHP outperformed pristine SPEEK membranes in terms of dimensional and chemical stability, as well as mechanical strength. Additionally, SPEEK containing 15% BVPH showed the enhanced oxidative resistance and tensile strength of 93 MPa (dry) and 38 h (3% H2O2, 2 ppm Fe2+, 80 °C). However, due to increased hydrophobicity and decreased water sorption and active ionic sites, the proton conductivity of cross-linked SPEEK was slightly reduced.
Although there are many photoinitiators and crosslinking agents on the market, they can be highly unstable or very costly.54 Chemical crosslinking can decrease the efficiency of polymer chain packing, leading to an increase in gas permeability and potential modifications in the properties of membranes.55 Consequently, most researchers have focused their attention on adding the photoinitiator and/or suitable crosslinking sites to the polymer backbone. The UV-crosslinked hybrid SPEEK membrane, which is combined with a biodegradable polymer, reduces the polymer chains' elasticity by forming a denser network. Ramly et al.,56 studied SPEEK with methylcellulose (MC) and UV radiation, using benzoin ethyl ether (BEE) as a photoinitiator. Increased hydrophilicity was achieved through radiation-induced demethylation, chain cleavage, acid group formation, and carbonyl in MC. After the non-crosslinked membrane was crosslinked with BEE under UV light for 30 minutes, the proton conductivity at 30 °C increased from 0.004 S cm−1 to 0.008 S cm−1. The UV membrane improved dimensional stability after crosslinking because of its denser structure. Teruel-Juanes et al.,57 carried out the crosslinking reaction first by UV irradiating polystyrene–ethylene–butylene block copolymers (SEBS) with DVB, as opposed to first performing the sulfonation and then the crosslinking. This was followed by a post-sulfonation of the hardened membranes in trimethylsilyl chlorosulfonate solutions in 1,2-dichloroethane (DCE). The dielectric relaxation spectrum (Fig. 11) revealed two main relaxations that corresponded to the glass transitions of the ethylene–butylene (EB) and styrene (S) blocks, as well as sub-Tg intramolecular non-cooperative dielectric relaxation. In addition to having an impact on the fragilities of both styrene (S) and ethylene–butylene (EB) blocks, the photo-crosslinking and post-sulfonation processes also have an effect on the entire dielectric relaxation spectrum. They concluded that the behaviour of the membranes can be estimated and reengineered based on modifications to the desired cell performance thanks to a correlation found between relaxation processes and membrane performance in H2/O2-PEM single cells.
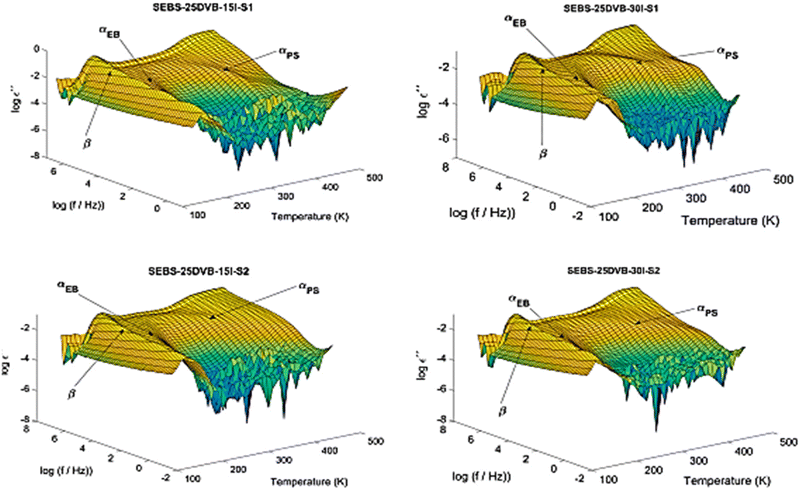 | ||
| Fig. 11 Dielectric relaxation spectrum.57 | ||
Polyatomic alcohols such as glycerol, ethylene glycol, and meso-erythrite can be used as crosslinking agents to increase or maintain the SPEEK membrane's flexibility (Fig. 12). This is due to the fact that the sulfone bond created by thermal crosslinking between two sulfonic acid groups is less flexible than the sulfonic ester bond created by condensation with polyatomic alcohol. Conductivity is enhanced by the flexibility of the macromolecular chains, which enables them to align into hydrophilic and hydrophobic domains.58 Kumari et al.,59 reported on the effect of polyatomic alcohol linker length. They used ethylene glycol (PEG) with different molecular weights (MW Da−1: 200 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and measured the effect of the molecular weight on the membrane's final properties. The authors reported that PEG 400 could form many small hydrophilic and hydrophobic clusters, which were more feasible than larger clusters formed by PEG with a MW less than 600. They made this discovery using atomic force microscopy (AFM) and small-angle X-ray scatting (SAXS). The results also demonstrated that there was an optimal linker length for stacking macromolecular chains into hydrophobic and hydrophilic domains.
000) and measured the effect of the molecular weight on the membrane's final properties. The authors reported that PEG 400 could form many small hydrophilic and hydrophobic clusters, which were more feasible than larger clusters formed by PEG with a MW less than 600. They made this discovery using atomic force microscopy (AFM) and small-angle X-ray scatting (SAXS). The results also demonstrated that there was an optimal linker length for stacking macromolecular chains into hydrophobic and hydrophilic domains.
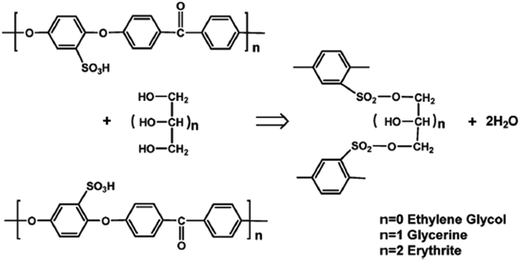 | ||
| Fig. 12 Reaction scheme of SPEEK cross-linked with polyatomic alcohol.42 | ||
The diol crosslinking agents' flexibility was also examined by Gupta et al. in a different study.60 To crosslink the SPEEK membrane, cyclohexane di-methanol (CDM) was utilised as the stiff crosslinking reagent and PEG (MW 200) as the flexible crosslinking reagent. According to their report, the ideal ratio of polymer to crosslinker was determined to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after conducting conductivity and water uptake experiments with various crosslinking agent ratios. Moreover, membranes crosslinked with the stiff CDM exhibited inferior properties in comparison to those crosslinked with the flexible crosslinker PEG. Therefore, the crosslinkers' flexibility is another factor to consider when crosslinking SAP.
1 after conducting conductivity and water uptake experiments with various crosslinking agent ratios. Moreover, membranes crosslinked with the stiff CDM exhibited inferior properties in comparison to those crosslinked with the flexible crosslinker PEG. Therefore, the crosslinkers' flexibility is another factor to consider when crosslinking SAP.
4.2. SPEEK blend polymer membrane
Blending is a simple method for defining and adjusting phase separation in the microstructure of homopolymers, provided the second polymer is completely compatible with the primary polymer.61 Hydrogen bonds and ionic interactions, which are the most common physical interactions between polymers, can be used to reinforce blend membranes.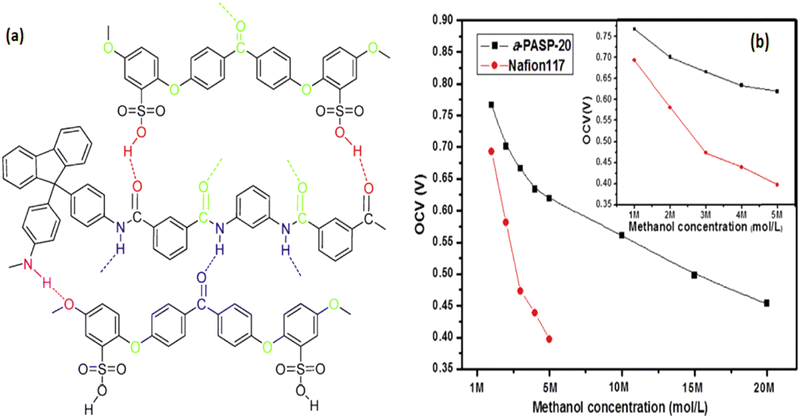 | ||
| Fig. 13 (a) Expected hydrogen bonding between the blend of sulfonated poly(ether ether ketone) with fully aromatic polyamide (fa-PA), and (b) OCV of the fa-PASP-20 blend and Nafion® 117 membranes at different methanol concentrations.65 | ||
Haragirimana et al.,66 created a synergistic effect in SPEEK and SPAES by using acid–acid blending and sulfone bridges between sulfonic acid groups and electron-rich phenyl units. In their study, they fabricated a series of PEEK/SPAES polymer blends through a three-component system. Ductile and dense membranes were successfully fabricated through simple solution mixing and casting due to the excellent compatibility and fine dispersion of both copolymers inside the membrane. The incorporation of SPAES into SPEEK had a significant positive effect on the control of membrane water-swelling behaviour and oxidative stability, particularly at high temperatures. This resulted from the interfacial interactions (π–π interactions) and strong hydrogen bond formation between the SPAES and SPEEK chains.
In another fascinating study, Nayak et al.,69 combined non-fluorinated blend membranes and SPEEK with fluorinated blend membranes. Sulfonated poly(ether-ether-ketone)/poly(vinylidene fluoride-co-hexafluoro propylene)/silica (SPEEK/PVdF–HFP/SiO2) composite proton exchange membranes were developed for fuel cell applications. The SiO2 (7.5 wt%) polymer membrane of SPEEK (80 wt%)/PVdF–HFP (20 wt%) demonstrated the highest proton conductivity value of 8 × 10−2 S cm−1. Additionally, a maximum power density of 1.5 mW m−2 was reported. According to this study, a different PEM may be possible if SiO2 is added to polymer composite membranes.
A high ionic conduction sulfonated poly(ether ether ketone)/poly(vinylidene fluoride) (SPEEK/PVDF) blend membrane doped with boron phosphate (BP) was developed by Cali et al.70 SPEEK/PVDF/10BP had the highest current density (0.4 A cm−2) and power density (0.242 W cm−2) at 0.6 V. The proton conductivity of the SPEEK/PVDF/10BP sample was measured at 80 °C to be 39 mS cm−1. The addition of both the boron phosphate and the SPEEK/PVDF mix membrane resulted in promising results for future fuel cell operations.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) was found to have a proton conductivity of 0.070 S cm−1. A blend of SPEEK and sulfonated poly(phthalazinone ether sulfone ketone) (SPPESK) was developed by Liu et al.72 Excellent water absorption and a low swelling ratio are features of the reported SPPESK/SPEEK membrane. At 80 °C, the blend membrane's proton conductivity was reported to be 0.212 S cm−1.
30) was found to have a proton conductivity of 0.070 S cm−1. A blend of SPEEK and sulfonated poly(phthalazinone ether sulfone ketone) (SPPESK) was developed by Liu et al.72 Excellent water absorption and a low swelling ratio are features of the reported SPPESK/SPEEK membrane. At 80 °C, the blend membrane's proton conductivity was reported to be 0.212 S cm−1.
4.3. The modification of SPEEK membranes with other polymers
In most polymers, carbon atoms are covalently bound to other elements like hydrogen, oxygen, or nitrogen. These organic molecules can be thought of as polymers. Hence, combining a polymer with SPEEK can result in the formation of acid–base interactions or hydrogen bonds between polymer chains, which can drastically alter the mixture's characteristics. SPEEK combined with other polymers is a technique commonly utilized in composite membrane manufacture, offering excellent proton conductivity and acceptable mechanical qualities. Blends of SPEEK with various polymers, including polyacrylonitrile (PAN),73 polybenzimidazole (PBI),74 poly(ether sulfone) (PES),75 polyimide (PI),76 polyphenylene oxide (PPO),77 polytetrafluorethylene (PTFE),78 vinylidene fluoride,79 polyvinylpyrrolidone (PVP)80 and poly(tungstophosphoric acid (TPA).81 According to Peng et al., the performance of a SPEEK membrane can be enhanced by altering its microstructure using either dibutyl phthalate (DBP) porogen or Nafion resin, which is applied as a layer over polypropylene (PP). It has been reported that a modified membrane structure consisting of the SPEEK membrane coated with polydopamine (PDA) layers improves the mechanical strength and selectivity. All of these methods improve performance and point to the use of composite SPEEK membranes in PEMFC applications.Recently, phosphonate membranes have received increased attention as potential replacements for PEMFC applications. Phosphorylated polysulfone (PPSU-As) in the acid form with degrees of phosphonation (DP) of 0.4, 0.75, and 0.96 was successfully prepared and blended by Abu-Thabit et al.,82 using SPEEK with a DS of 0.75. The phosphoryl group (–PO3H2) could form strong hydrogen bonds with acidic SPEEK polymers, reducing swelling while sacrificing minimal proton conductivity. When compared to pure SPEEK, the blended SPEEK/PPSU membrane demonstrated lower methanol permeability, increased mechanical strength, and water uptake without sacrificing proton conductivity. The blend membrane (30PPSU-A-0.96) performed better in terms of proton conductivity than the pristine SPEEK membrane. The maximum proton conductivity of 0.124 S cm−1 was reached at 120 °C, where this performance was sustained. Sultan et al.,83 created a novel hybrid membrane poly(trimellitic anhydride chloride-co-4,4′-methylenedianiline) (SPEEK/PTCMA) with PTCMA loadings ranging from 10% to 50% with a DS of 53%. SPEEK/PTCMA (50 wt%) exhibited lower water uptake of 11% at room temperature, because of the acid–base interaction of amine and sulfonated groups. At 90 °C, the composite membrane SPEEK/PTCMA (20 wt%) demonstrated higher proton conductivity of 0.004 S cm−1. The addition of PTCMA improved the proton conductivity because the nitrogen atom of PTCMA can be protonated and contribute to the proton transfer. Overall, the findings demonstrated that the proton conductivity value decreased as the PTCMA content increased. In turn, this lowers the amount of sulfonic acid groups in the composites, increasing their crystallinity and thereby decreasing water uptake, a crucial stage in proton transfer.
Han et al.,84 created SPEEK/PBI composite membranes by dissolving the two polymers in DMAC before casting the membrane. The interaction of the –NH groups in PBI and the –SO3 groups in SPEEK results in the formation of a three-dimensional network polymer structure that is advantageous for proton transport. The PEM demonstrated excellent proton conductivity performance, with a value of 0.14 S cm−1 at 80 °C, comparable to Nafion 117 (0.142 S cm−1). The permeability of methanol is also as low as 2.38 × 10−8 cm2 s−1, which is much lower than that of Nafion. Aside from mechanical properties, thermal stability is also important. This kind of polymer membrane design is successful and close to being used in DMFCs. Wei et al.,76 proposed a PI/SPEEK/PI nanofiber composite membrane with a sandwich structure and simple fabrication processes. The formation of an acid-rich layer and the solid support of PI nanofibers on the SPEEK matrix resulted in significantly improved proton performance. Due to the acid–base interaction between tertiary amide groups and sulfonated groups, the novel hybrid membranes PI/SPEEK with PI loadings of 3% and PI/SPEEK/PI with PI loadings of 1.5% had a lower methanol permeability than SPEEK membranes. The sandwiched membranes demonstrated excellent conductivity of 0.178 S cm−1 at 60 °C, which is noticeably higher than that of the neat SPEEK membrane. The fuel cell's performance can reach 0.152 W cm−2. The swelling ratio and water uptake of the PI/SPEEK nanofiber composite membrane are 24.3% and 50.8%, respectively, at 60 °C and 100% RH, demonstrating the sandwiched PEM's excellent dimensional stability. The excellent results of the polymers indicate that the PI/SPEEK membrane is a promising candidate for commercial PEM with balanced proton conductivity, stability, and durability. The sandwich-structure membrane concept can also be applied in other areas such as in vanadium redox flow batteries, gas separation membranes, and so on.
Another promising membrane with strong chemical resistance and high hydrophilicity is chitosan. The combination of SPEEK and the natural polymer chitosan was suggested by Hidayati et al.85 Chitosan has low methanol permeability and good conductivity and was treated with SPEEK to eliminate hydroxyl and amine groups. The SPEEK/chitosan composite membrane showed enhanced methanol permeability of 2.46 × 10−6 cm2 S−1 at room temperature when compared to pristine chitosan. The SPEEK/chitosan IEC values are higher, resulting in high proton conductivity. It was reported that SPEEK/chitosan produced contrasting results for DMFC, implying that more research is needed.
5. Modification of SPEEK membranes with inorganic materials
SPEEK polymer modification with inorganic materials such as silica, clays, metal oxides, HPA, carbon nanotubes, and others has recently been investigated in fuel cell applications. The incorporation of inorganic substances into PEMs is known to improve proton conductivity, mechanical strength, and composite membrane durability.86 While simultaneously enhancing the mechanical and thermal stabilities of the composites, inorganic elements can reduce methanol crossover and excessive water swelling.87 Various types of additives in SPEEK, such as graphene, silica, metal oxides, heteropolyacids (HPAs), carbon nanotubes, metal organic frameworks (MOFs) and clay will be thoroughly discussed within these subtopics. The influence of different additives on the SPEEK matrix, and the impact on SPEEK performance with a focus on fuel cells are shown in Table 1.| Additive type | Temperature (°C) | IEC (meq g−1) | Water uptake (%) | Proton conductivity (S cm−1) | Power density (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|
| SiO2@CNT | 25 | — | 43 | 4.1 × 10−2 | — | 88 |
| SWCNT–fly ash | 90 | 1.59 | 27.3 | 3.4 × 10−2 | 672 | 89 |
| SsCNT-5 | 90 | 2.19 | 43.85 | 4.31 × 10−2 | — | 90 |
| CCNF | 80 | — | 32.3 | 5.6 × 10−2 | — | 91 |
| β-CD–DHNTS/HPW | — | 1.04 | 30 | 9.0 × 10−2 | — | 92 |
| Cs–HPAs | 80 | — | 40 | 2.25 × 10−3 | 247 | 93 |
| Pt–Cs2.5H0.5PW12O40 | 60 | 1.96 | 46 | 6.82 × 10−2 | — | 94 |
| PEOS/PWA/SiO2 | 100 | — | — | 6.25 × 10−3 | 25 | 95 |
| Cs–TPA | 80 | 1.5 | 37 | 1.3 × 10−1 | — | 96 |
| Pd–GO–L-Tyr | — | 2.05 | 50.6 | 2.56 × 10−3 | — | 97 |
| PANI–GO | — | 1.83 | 40 | 8.4 × 10−3 | 13.51 | 98 |
| SPBI/PrSGO | 90 | 2.02 | — | 1.7 × 10−1 | 820 | 99 |
| SPVdF–HFP–SiO2 | 90 | 1.83 | 36.5 | 7.9 × 10−2 | 110 | 100 |
| HPW@KMSNs | 60 | — | 31.5 | 2.43 × 10−1 | — | 101 |
| PVA/TEOS | 80 | 2.02 | 76 | 8.1 × 10−2 | 336 | 102 |
| IL/SHMO | 200 | — | — | 4.6 × 10−3 | — | 88 |
| Bentonite/clesite30 | 80 | — | 18.4 | 1.24 × 10−5 | — | 103 |
| fGO/halloysite | — | 0.35 | 4.7 × 10−4 | 72.2 | 104 | |
| SiO2–montmorillonite | 100 | — | 25 | 1.58 × 10−1 | 105 | |
| BaZro3 | 90 | 1.96 | 41.5 | 3.12 × 10−1 | 183 | 106 |
| Al–CeZrO4/HPW | 80 | 1.65 | 8.1 | 1.3 × 10−3 | 1001 | 107 |
| NBO | 90 | 1.80 | 38.4 | 2.9 × 10−2 | 601 | 108 |
| ZCO | 90 | 1.46 | 20.3 | 2.0 × 10−2 | — | 109 |
| HPW@ML | 60 | 1.54 | 50 | 1.36 × 10−1 | — | 97 |
| MOF-C-SO3H | 80 | 1.63 | 28.7 | 1.1 × 10−1 | 82 | 110 |
| Co-MOF-74/[IM2][H2PO4] | 120 | 6.5 | 90 | 2.6 × 10−2 | — | 111 |
| Cu-MOF | 80 | 2.46 | 36.7 | 7.1 × 10−2 | — | 112 |
| ZIF-8/CNT | 120 | 1.48 | 40.2 | 5.0 × 10−2 | — | 113 |
| ZIF-67 | 120 | 0.3 | 40 | 1.4 × 10−2 | 28 | 114 |
5.1. Carbon nanotubes as fillers for SPEEK membranes
Carbon nanotubes (CNTs) are one-dimensional tubular-like hexagonal graphene sheets formed by sp2 bonds between carbon atoms. CNTs have extremely high mechanical properties due to this bonding structure, which is stronger than the sp3 bonds found in diamond. CNTs can be single-walled (SWCNT) or multiwalled (MWCNT), with diameters ranging from 1 nm to more than 100 nm, as shown in Fig. 14. The rolling-up direction of the graphene sheet has a significant impact on the CNT's electrical conductivity. This is due to the fact that the chirality vector describes the hexagonal carbon atom lattice. However, due to their higher surface defects and lower electrical conductivity, MWCNTs are preferred over SWCNTs for use in PEMs.115 Due to their stiffness, low density, high aspect ratio, and optical qualities, as well as their exceptional tensile strength of roughly 63 GPa, which is 50 times stronger than steel, carbon nanotubes (CNTs) have received a lot of attention as a reinforcing material for polymers.116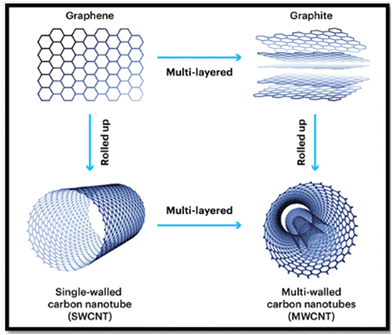 | ||
| Fig. 14 Single walled and multiwalled carbon nanotubes.116 | ||
Carbon nanotubes (CNTs) are a cutting-edge nanomaterial for the production of exceptional polymer composites. Recently, SPEEK has employed CNTs as fillers to address DS-dependent issues. Nonetheless, CNTs have a negative impact on proton conductivity because they are an electron conductor rather than a proton conductor, which may pose a significant risk of short-circuiting on PEMs in fuel cells. Cui et al.,88 successfully prepared silica-coated CNTs (SiO2@CNTs) by a simple sol–gel method, and subsequently applied them as a novel additive to SPEEK-based composite membranes, which are enhanced by silica's exceptional water retention and electronic shielding properties. Not only did the hydrophilic and insulated silica coating on the CNTs’ surface prevent short circuiting, but it also enhanced the CNTs’ interfacial contact with the SPEEK matrix, promoting uniform dispersion of the CNTs. Moreover, the methanol permeability of the SPEEK/SiO2@CNT composite membrane with a SiO2@CNT loading of 5% was nearly one order of magnitude lower at 4.22 × 10−8 cm2 s−1 as compared to the pure SPEEK membrane (3.42 × 10−7 cm2 s−1). At room temperature, the proton conductivity remained greater than 10−2 S cm−1. The obtained results demonstrate that SPEEK/SiO2@CNT membranes can be used as high-performance PEMs in direct methanol fuel cells. Sivasubramanian et al.,89 successfully synthesised sulfonated poly(ether ether ketone) (SPEEK)-based polymer nanocomposite membranes comprising single-walled carbon nanotubes (SWCNTs) and fly ash as inorganic fillers using the solution casting method. The degree of sulfonation in poly(ether ether ketone) was evaluated using proton nuclear magnetic resonance spectroscopy and found to be 64%. They investigated and analyzed the produced membranes’ physicochemical characteristics and potential for fuel cell applications. At 90 °C, the SP-CNT-FA-8 membrane had the maximum proton conductivity (3.4 × 10−2 S cm−1), whereas the pristine membrane had a conductivity of 3.1 × 10−2 S cm−1. Apart from their favourable proton conductivity, it was also reported that the electrolyte membranes demonstrated remarkable thermal and mechanical stability. These findings suggest that the composite membranes utilizing SPEEK, SWCNT, and fly ash could be promising electrolyte membrane options for fuel cell applications.
Gahlot et al.,90 used solution casting to create functionalized carbon nanotubes (f-CNT) that are aligned electrically with SPEEK. The CNTs were functionalized via carboxylation and sulfonation. During the membrane's drying process, the CNTs were aligned using a constant electric field of 500 V cm−1. To determine whether they have the potential for direct methanol fuel cell application, the proton conductivity and methanol crossover resistance were assessed at temperatures ranging from 30 °C to 90 °C. According to the findings, the addition of aligned carbon nanotubes reduces the permeability of methanol while increasing the ion-exchange capacity, water retention, and proton conductivity. The highest proton conductivity (4.31 × 10−2 S cm−1) was observed in the SsCNT-5 nanohybrid PEM, which exhibited a higher resistance to methanol crossover. As the concentration of s-CNTs in the SPEEK matrix increased, so did the storage modulus (Fig. 15). The S-sCNT-5 membrane had the highest modulus value of 2503 MPa, which is nearly 2.4 times higher than the SPEEK membrane. The increase in storage modulus of S-sCNT membranes indicates strong bonding due to the presence of a common sulfonic acid group in CNT and PEEK, as well as the effect of an electric field on CNT alignment in the SPEEK matrix. The electrically aligned functionalized CNT/SPEEK membranes outperformed the randomly aligned composite membranes.
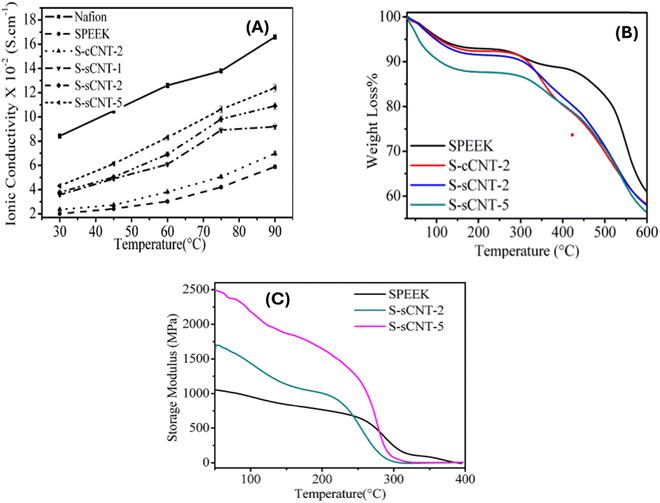 | ||
| Fig. 15 (A) Proton conductivity vs. temperature, (B) TGA thermographs and (C) DMA of SPEEK, S-sCNT-2, and S-sCNT-5 membranes.90 | ||
Zhao Guodong et al.,91 prepared a composite membrane for use in PEMs by incorporating continuous carbon nanofibers (CCNFs) into SPEEK. The CCNFs were evenly distributed in an electrolyte polymer membrane after being easily blended into the SPEEK matrix. The characterization of the composite membranes shows that all dense composite membranes have low methanol permeability, good proton conductivity, high mechanical performance, and excellent water swelling. The composite membrane containing 0.51 weight percent (wt%) CCNFs was fully hydrated and had a proton conductivity of 0.056 S cm−1 at room temperature. Moreover, 1.5 times the relative selectivity of a pure SPEEK membrane was observed in the hybrid membrane containing 2.52 weight percent CCNFs. These results showed that polyelectrolyte membranes for fuel cells with a CCNF support (SPEEK) are a promising option.
5.2. Heteropolyacids (HPAs) as fillers for SPEEK membranes
HPAs are highly conductive and thermally stable crystalline inorganic materials. HPA salts are composed of MOx polyhedra, where M represents polyatoms such as tungsten (W), molybdenum (Mo), niobium (Nb), tantalum (Ta), and vanadium (V), and x represents heteroatoms such as silicon (Si), phosphorus (P), iron (Fe), and cobalt (Co) through an oxygen atom coordination bridge.117 They are typically distinguished by Wells–Dawson, Keggin, or lacunar structural configurations (Fig. 16). Different salts with different structures and properties can be formed by changing the central metal ion and addenda atoms. HPAs are soluble in polar solvents, where they form the Keggin structure (XM12O40), which is a heteropolyacid anion structure with a condensation ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. The high acidity of HPAs is attributed to the polyanion's large size, which results in a low delocalized charge density. Chemical analysis, ion selective membranes, sensors, chemical cleaners, catalysts, and additives in fuel cell component materials are just a few of the applications for HPAs. Phosphotungstic acid (PWA) is one of the most promising inorganic additives for PEM composites due to its high proton conductivity and excellent thermal stability.
12. The high acidity of HPAs is attributed to the polyanion's large size, which results in a low delocalized charge density. Chemical analysis, ion selective membranes, sensors, chemical cleaners, catalysts, and additives in fuel cell component materials are just a few of the applications for HPAs. Phosphotungstic acid (PWA) is one of the most promising inorganic additives for PEM composites due to its high proton conductivity and excellent thermal stability.
HPA can be partially replaced with cesium (CsHPA) to increase its surface acidity.120 This substitution can maximise contact with the polymer matrix by decreasing the solubility of salt in water while increasing its surface area. Silica-based salts enhance the mechanical properties of the membrane while also improving its conductivity. Metal oxides (silica, titania), functional metal oxides,121 clay,122 aluminium phosphate (ALP)123 and zeolites124 have been used to modify the SPEEK membrane as a water retainer.
He et al.,92 successfully incorporated -cyclodextrin (-CD) onto halloysite nanotubes (HNTs) using polydopamine coating to make water-insoluble -CD-DHNTs, and subsequently SPEEK/-CD-DHNTs/HPW composite membranes were fabricated by traditional solution casting. It is reported that both HPW and β-CD-DHNTs were well dispersed in the SPEEK matrix because of the hydrogen bonding complexation between [PW12O40]3− and β-CD. The SPEEK/β-CD-DHNTs/HPW composite membranes’ proton conductivity increased with the increase in HPW content (0.090 S cm−1), reaching the maximum of ∼120% increase relative to that of the SPEEK membrane. Cs-HPAs were added to the SPEEK matrix by Oh et al.,93 to create composite membranes. The greatest power density values (245 and 247 mW cm−2) and enhanced conductivity of approximately 2.25 × 10−3 S cm−1 at 80 °C under 80% RH were demonstrated by these membranes. By embedding Cs2.5H0.5PW12O40 on Pt in a SPEEK matrix, Zhang et al.,125and Peighambardoust et al.,94 obtained nearly identical results. At 60 °C and 100% relative humidity, Zhang et al., obtained a proton conductivity of 5.3 × 10−2 S cm−1, while Peighambardoust et al. obtained approximately 6.82 × 10−2 S cm−1, which is thought to be higher than Nafion 117. Colicchio et al.,95 also investigated SPEEK, polyethoxysiloxane (PEOS), and PWA (H3PW12O40) with 20% silica (SiO2). According to the findings, this combination's proton conductivity is twice as high as pure SPEEK's at 90% relative humidity and 100 °C. Overall, the HPA-modified SPEEK membranes showed higher stability and increased proton conductivity values (6.25 × 10−3 S cm−1) when compared to the low values produced by a plain SPEEK membrane (2.21 × 10−3 S cm−1). Therefore, the HPA/SPEEK composite membranes are good candidates to replace Nafion-based membranes in PEM fuel cells due to their better proton conductivity and long-term stability.
Dogan et al.,96 created cesium salt of tungstophosphoric acid (Cs-TPA) particles by combining aqueous solutions of tungstophosphoric acid and cesium hydroxide, as well as Cs-TPA particles and sulfonated poly(ether ether ketone). They investigated the effects of Cs-TPA on SPEEK membranes in terms of SPEEK sulfonation degrees and Cs-TPA content. The composite membranes' performance was measured in terms of water uptake, ion exchange capacity, proton conductivity, chemical stability, hydrolytic stability, thermal stability, and methanol permeability. It was discovered that the Cs-TPA particles aggregated as the degree of sulfonation of SPEEK increased from 60 to 70%. SPEEK (DS: 60%)/Cs-TPA membrane with 10% Cs-TPA concentration reduced the methanol permeability to 4.7 × 10−7 cm2 s−1. At 80 °C and 100% RH, the membrane attained an acceptable proton conductivity of 1.3 × 10−1 S cm−1. They also discovered that weight loss at 900 °C increased with the inclusion of inorganic particles, as expected. The addition of Cs-TPA particles to the SPEEK/Cs-TPA based composite membranes increased their hydrolytic stability. The authors also discovered that SPEEK60/Cs-TPA composite membranes were more hydrolytically stable than SPEEK70/Cs-TPA composite membranes. SPEEK60 composite membranes had reduced permeability values for methanol, water vapor, and hydrogen compared to Nafion®.
5.3. Graphene as fillers for SPEEK membranes
Graphene, a two-dimensional sheet of carbon, has astounded the world with its fascinating unique chemical, physical, and thermal properties, opening the door to numerous applications.115 Due to its large surface area, which is highly valued in energy storage systems, graphene is primarily used as an electrode material in various electrochemical applications. Graphene is a carbon allotrope with a honeycomb lattice of sp2-hybridized two-dimensional monolayers.126 In comparison to graphite and carbon nanotubes (CNTs), graphene has a larger surface area (2629 m2 g−1) than CNTs (1315 m2 g−1), and is regarded as a fundamental building block for graphitic materials.127 Graphene also has excellent electronic properties, exhibiting a half integral quantum Hall effect even at room temperature.128,129 Graphene was extracted from graphite using a simple scotch tape method, and a Nobel Prize was awarded in 2010 for its discovery.130 Since then, many scientists have shifted their research focus to it, particularly in the areas of synthesis, functionalization, and application in various electrochemical devices such as fuel cells, solar cells, batteries, and ultra-capacitors.Das et al.,131 synthesised solution-cast palladium graphite oxide-grafted amino acid nanocomposites (Pd–GO–L-Tyr) in sulfonated poly(ether ether ketone) (SPEEK). The composite membrane exhibited enhanced proton conductivity when compared to the pristine SPEEK membrane, due to its increased hydrophilicity, surface wettability, and ion exchange capacity, which are attributed to the increased presence of hydroxy and carboxyl groups. The SPEEK/Pd–GO–L-Tyr membrane's high proton conductivity (2.56 mS cm−1) and low methanol crossover resulted in significantly higher selectivity (5.57 × 10 S cm−3 s) compared to the SPEEK membrane (4.8 × 102 S cm−3 s−1) and Nafion-117 membrane (2.78 × 103 S cm−3 s−1). Incorporating Pd–GO–L-Tyr into the SPEEK membrane matrix creates a physical barrier to prevent methanol crossover. With the above-mentioned factors, the authors concluded that composite membranes (Pd–GO–L-Tyr–SPEEK) are better candidates for DMFC applications, compared to the standard Nafion®117 membranes. Yogarathinam et al.,98 synthesised conductive polyaniline decorated graphene oxide (PANI–GO) and graphene oxide (GO), which they added to a sulfonated poly(ether ether ketone) (SPEEK) nanocomposite membrane to decrease methanol crossover. Surface morphology and crystallinity analysis verified the formation of PANI coated GO nanostructures (Fig. 17). The analysis of membrane topography and morphology verified that PANI–GO and GO were evenly distributed across the surface of the SPEEK membrane. With a water uptake of 40% and an ion exchange capacity of 1.74 meq g−1, the 0.1 wt% PANI–GO modified SPEEK nanocomposite membrane demonstrated the highest performance. The nanocomposite membranes' oxidative stability was also improved. The modified SPEEK membrane with 0.1 wt% PANI–GO had a lower methanol permeability of 4.33 × 10−7 cm2 s−1. The presence of acidic and hydrophilic groups in PANI and GO increased the proton conductivity of the PANI–GO modified SPEEK membrane. The selectivity of the PANI–GO modified SPEEK membrane was 1.94 × 104 S cm−3 s−1. The PANI–GO modified SPEEK membrane was discovered to be a potential material for DMFC applications.
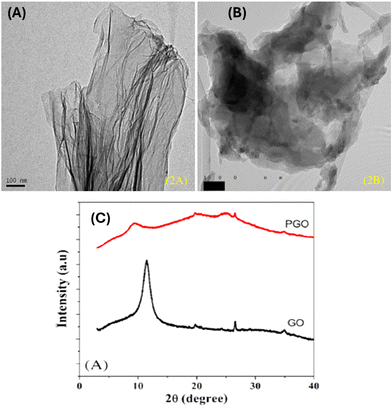 | ||
| Fig. 17 (A) A transmission electron microscopy (TEM) image showing the morphology of GO, (B) a TEM image showing the morphology of the PANI–GO nanocomposites and (C) X-ray diffractometer (XRD) patterns of the GO and PANI–GO nanocomposites.98 | ||
Maiti et al.,99 investigated a novel strategy for the advancement of proton exchange membranes by incorporating propylsulfonic acid-functionalized graphene oxide in crosslinked acid–base polymer blends and exploring its fuel cells applications. The molecular dynamics (MD) simulations were conducted at different PrSGO loadings for the SPEEK/SPBI, XSPEEK/SPBI, and cross-linked SPEEK/SPBI composite systems. After increasing the loading percentage of SPBI and PrSGO filler in the polymer matrix and cross-linking the polymer composites, the glass transition temperature (Tg) was increased. It was also reported that the mechanical, chemical, and thermal stability of the XSPEEK/SPBI/PrSGO nanocomposite membranes increased significantly with an increase in PrSGO loading, due to the strong interfacial interaction between PrSGO and the XSPEEK/SPBI matrix. The proton conductivity of the XSPEEK/SPBI/PrSGO nanocomposite membrane improved significantly up to 0.17 S cm−1 at 4 weight percent PrSGO loading at 100% relative humidity (RH) and 90 °C. Furthermore, at 100% RH, 80 °C, the XSPEEK/SPBI/PrSGO nanocomposite membrane demonstrated excellent fuel cell (FC) performance with a maximum power density of 0.82 W cm2. Due to the hygroscopic nature of PrSGO, the authors observed a higher number of sulfonic acid groups and excellent interaction between the acid functionalized fillers and the cross-linked SPEEK/SPBI-based matrix. The membranes' overall performance and other critical characteristics, including their proton conductivity, were enhanced by the addition of PrSGO nanofillers to the polymer matrix.
5.4. Silica as fillers for SPEEK membranes
The extensive research on silica-based nanoparticles is due to their lower cost, good mechanical, and water retention properties. However, due to their poor organic compatibility and non-conductive properties, SiO2 particles aggregate in the polymeric matrix and reduce the conductivity of PEMs.132 Higher silica loading in the membrane results in a significant dilution effect for the membrane's ion exchangeable groups.133 As a result, as the pure silica content increases, the membrane's ion exchange capacity decreases.134 However, numerous studies have been conducted to improve membrane IEC by functionalizing silica filler with sulfonic group derivatives. Optimal silica loading improves membrane strength.135 Higher silica content in the matrix, on the other hand, is detrimental to the mechanical properties of the polymer due to increased filler–filler interaction compared to filler–polymer interaction, which destroys membrane homogeneity and causes the membrane to become brittle. As a result, a perfect combination of inorganic material and membrane can result in nanocomposites with improved mechanical properties.136Martina et al.,100 used the solvent cast method to create sulfonated silica (S-SiO2) nanoparticles incorporated into a blend of sulfonated poly(vinylidene fluoride-co-hexafluoropropylene) (SPVdF–HFP) and sulfonated poly(ether ether ketone) (SPEEK). They claimed that incorporating S-SiO2 into SPEEK improved the polymer's water uptake, IEC, and mechanical properties. At 90 °C and 100% RH, sulfonated silica with an 80 wt% SPEEK–20 wt% SPVdF–HFP nanocomposite membrane demonstrated a maximum proton conductivity and current density of 7.9 × 10−2 S cm−1 and 354 mA cm−2, respectively. The enhanced proton conductivity is attributed to the presence of S-SiO2. The hydrophilic nature promotes the ion channels and swells the membrane which results in enhanced proton conductivity.
Meng et al.,101 investigated how amino-modified mesoporous silica nanospheres affected the properties of SPEEK/phosphotungstic acid (HPW). They state that while immobilising acids is an issue, adding acid proton carriers to a polymer matrix is an effective method for increasing proton conductivity. They discovered that adding HPW and aminated mesoporous silica nanoparticles (K-MSNs) to SPEEK enhanced the dimensional stability and proton conductivity. At a temperature of 60 °C and 1 wt% K-MSN loading, the composite membrane's proton conductivity was 243 mS cm−1, indicating that SPEEK/HPW/K-MSN composite membranes have significant potential in methanol fuel cell applications. Sahin et al. produced a blending polymer consisting of SPEEK, PVA, and tetraethyl orthosilicate (TEOS).102 The author demonstrated how adding PVA could increase the number of modifiable groups, which would enhance proton transport and improve oxidative and hydrolytic stability when TEOS is added. This finding was supported by the author's results, which showed that the SPEEK/PVA/TEOS blend outperformed the other samples (pure SPEEK, PVA, and SPEEK/PVA composite) in terms of oxidative and hydrolytic stability. The addition of TEOS increased hydrolytic stability while reducing the amount of –OH groups, which in turn improved water resistivity. Additionally, the membrane shows better cell performance values when compared to Nafion 117. The outcomes demonstrate how promising these membranes are as candidates for PEMFC applications.
In another study, Li et al.,88 successfully synthesised a composite membrane by dispersing ionic liquid (IL) in sulfonated hollow mesoporous organosilica (sHMO) into the SPEEK polymer backbone. A comparison study of various SPEEK/IL/sHMO-x (where x represents: 2.5, 5.0, 7.5 and 10 wt%) composite membranes and SPEEK/IL/HMO was conducted. The authors reported that the SPEEK/IL-30/sHMO-7.5 membrane had a conductivity of 1.13 mS cm−1 at 200 °C, which is twice that of the SPEEK/IL-30/HMO-7.5 (0.60 mS cm−1) under the same conditions. The improvement in the conductivity can be attributed to the addition of sHMO, which might have facilitated the formation of a continuous network or continuous pathway with IL. The organosilica sphere's surface exhibits a strong interaction between the hydroxyl group and IL, leading to significant IL retention. The IL loss study revealed that adding an organosilica sphere significantly reduced the membrane's IL loss. The anhydrous membrane is expected to be useful in PEMFCs under mild conditions.
5.5. Clay as fillers for SPEEK membranes
Clay is a common nanofiller used in many different applications. Natural and synthetic clays include talc, mica, layered double hydroxide (LDH), LAPONITE® (LAP), SAP, and montmorillonite (MMT). The structures of LAPONITE® clay, layered double hydroxide, and montmorillonite are depicted in Fig. 18.140 Compared to mica and talc, MMT, which has the chemical formula (Na,Ca)0.33(AlMg)2(Si4O10)(OH)2nH2O, has drawn a lot of attention and is used as a nanofiller in many applications, including fuel cells. MMT is a cation clay with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 crystal structure made up of one layer of octahedral aluminium hydroxide or magnesium hydroxide sheets and two interconnected tetrahedral silicon oxide sheets. LDH consists of a positively charged metal hydroxide brucite-type sheet with various anions and water inside galleries to counterbalance the charge.137 Apart from LDH, LAP is also a component of a synthetic clay that belongs to the 2
1 crystal structure made up of one layer of octahedral aluminium hydroxide or magnesium hydroxide sheets and two interconnected tetrahedral silicon oxide sheets. LDH consists of a positively charged metal hydroxide brucite-type sheet with various anions and water inside galleries to counterbalance the charge.137 Apart from LDH, LAP is also a component of a synthetic clay that belongs to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phyllosilicate structural group. The structure and composition of LAP, which has the chemical formula Na0.7(Si8Mg5.5Li0.3)O20(OH)4 are similar to those of natural clay hectorite minerals.138 LAP is made up of octahedral magnesium oxide and two parallel sheets of tetrahedral silica, resulting in two-dimensional layers. A single LAP layer is 25 nm in diameter and 1 nm thick, with positive charges on the edges and negative charges on the faces. Clay is readily available in nature and easily synthesised. It has a high ion-exchange capacity, chemical stability, and rheological properties. Clay's thin platelet structure contributes to its high aspect ratio.139 Due to its morphology, size, structure, and ionic nature, clay nanofiller performs exceptionally well as an electrolyte, particularly in fuel cell composite membranes.
1 phyllosilicate structural group. The structure and composition of LAP, which has the chemical formula Na0.7(Si8Mg5.5Li0.3)O20(OH)4 are similar to those of natural clay hectorite minerals.138 LAP is made up of octahedral magnesium oxide and two parallel sheets of tetrahedral silica, resulting in two-dimensional layers. A single LAP layer is 25 nm in diameter and 1 nm thick, with positive charges on the edges and negative charges on the faces. Clay is readily available in nature and easily synthesised. It has a high ion-exchange capacity, chemical stability, and rheological properties. Clay's thin platelet structure contributes to its high aspect ratio.139 Due to its morphology, size, structure, and ionic nature, clay nanofiller performs exceptionally well as an electrolyte, particularly in fuel cell composite membranes.
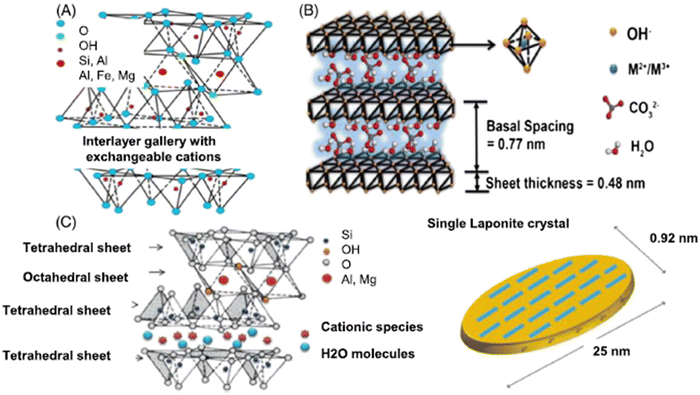 | ||
| Fig. 18 Structures of (A) montmorillonite (MMT), (B) layered double hydroxides (LDH), and (C) LAPONITE® (LAP) clays with a single LAPONITE® crystal.140 | ||
He et al.,141 compared the performance of unmodified clays (Na+–montmorillonite) (IC) with that of organ modified clays (I.44P (Na+–montmorillonite modified by I.24TL (Na+–montmorillonite modified by HOOC(CH2)17NH3+) (HC) and (CH3(CH2)17N(CH3)2+) (OC). When loading less than 10 wt% of HC, the SPEEK/HC membrane outperforms all other types of SPEEK/clay composite membranes in terms of overall performance and achieves higher selectivity than the pure SPEEK membrane. These SPEEK/HC composite membranes have been reported to have increased methanol permeability and proton conductivity. Combining the HC carboxylic acid group increased the HC bonding between membrane ion groups and the dispersibility due to higher proton conductivity without compromising membrane stability. For SPEEK/IC and SPEEK/OC hybrid membranes, proton conductivity simultaneously decreases with increasing filler content.
Kumar et al.,103 created sulfonated poly(ether ether ketone) (SPEEK) composites with bentonite and cloisite 30B nanoclays. The enhanced glass transition temperature and altered membrane morphology in the pristine SPEEK membrane (Fig. 19) indicated the presence of nanoclays. In comparison to the pristine membrane, the addition of 0.5 weight percent of bentonite and cloisite to SPEEK decreased the proton conductivity and water uptake. According to the author, this could be due to blocked ionic micro-structure channels caused by nano clay particles, which reduce ion exchange carriers. The addition of cloisite and bentonite to SPEEK polymer matrices limits the available nanometric channels for the migration of polar molecules such as hydrogen ions and water. Cloisite and bentonite layers’ increased rigidity complicates proton transport, which accounts for the decrease in conductivity. Gokulakrishnan et al.,104 synthesised membranes of functionalized graphene oxide (f-GO) nanocomposites at different concentrations and halloysite nano clay using dry phase inversion. The study discovered that the sulfonic acid group in SPEEK and silane functionalization of GO increased the ion exchange capacity from 0.22 to 0.35 meq g−1, which improved the proton conductivity. In comparison to the pure SPEEK membrane, which had a proton conductivity of 0.31 mS cm−1 and power density of 28 mW cm−2, the composite membrane, which contained 3 wt% halloysite nano clay and 2 wt% f-GO, maintained values of 0.47 mS cm−1 and 72.2 mW cm−2. The 2 wt% f-GO and 3 wt% SPEEK membranes with halloysite incorporation showed improved proton conductivity; these membranes are crucial for direct methanol fuel cell (DMFC) applications.
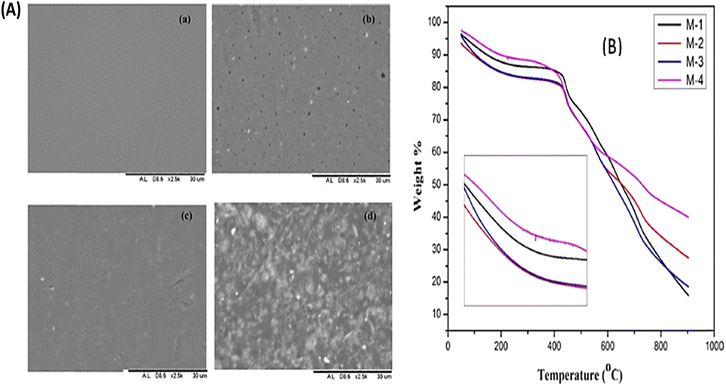 | ||
| Fig. 19 (A) SEM images of various membranes: (a) SPEEK, (b) SPEEK/bentonite, (c) SPEEK/cloisite and (d) SPEEK/bentonite/cloisite, (B) TGA thermograms of SPEEK (M-1), SPEEK/cloisite (M-2), SPEEK/bentonite (M-3) and SPEEK/cloisite/bentonite (M-4).103 | ||
To create composite membranes with different nanofiller contents, Charradi et al.,105 used a porous SiO2–montmorillonite heterostructured material packed with delaminated clay particles and a synthetic Mg–Al layered double hydroxide (LDH) exchanged with sulphate anions. The addition of Mg–Al LDH and SiO2–montmorillonite fillers to the SPEEK appears to improve the water retention and thermal stability of the resulting composite electrolyte membranes. At 120 °C and 100% relative humidity, Si–montmorillonite exhibited a higher proton conductivity (0.158 S cm−1) than both Mg–Al LDH and neat SPEEK (0.070 and 0.023 S cm−1, respectively). This could improve the performance of fuel cell membranes at high temperatures.
5.6. Metal oxide as fillers for SPEEK membranes
Interfacial interactions between membranes and catalysts, which constitute MEA components, are critical to the appropriate operation of fuel cells. This interfacial interaction is closely related to the catalyst and membrane structures, as well as the method used to prepare MEA.142 Metal oxides are classified into several types, including ZrO2, SiO2, Al2O3, and TiO2, each with its own set of properties. The conductivity of the membrane protons is typically increased when a metal oxide is added as an additive in composite polymers for a variety of reasons, such as the following:(1) Defects in the interface that arise when metal oxides occupy the polymer chamber and distance charge sheets are present.
(2) Metal oxide nanofillers are the predominant material used under amorphous conditions because they promote proton transport and increase the free volume within the polymer matrix.
(3) Increased ion dissociation in the polymer electrolyte membrane.
However, the PEM water intake and conductivity are affected by the properties and reactions of metal oxides such as strontium cerate, silica, titania, zeolite and zirconia. Because of their large aspect ratio and surface area, metal oxide nanofibers are superior to other additives for composite materials. The addition of Fe3O4 to PEEK, SPEEK, SPES and Nafion improved proton conductivity by promoting precise water hopping mechanisms. Furthermore, molybdenum oxide (MoO3) has good conductivity and physicochemical properties, making it suitable for use in energy-related fields.
Alumina, or aluminium oxide, is a common nanofiller in composites. Alumina has the chemical formula Al2O3 and can be found in a variety of minerals, including bauxite, diaspore (Al2O3H2O) and gibbsite (Al2O33H2O).143,144 Alumina can exist in various crystalline structures, but Al2O3 is the most thermodynamically stable. Its large surface area and high catalytic activity make it a good nanofiller. One of the metal oxide types that is frequently utilised as a nanofiller in fuel cells is silicon oxide, or silica (SiO2). Sol gel, microemulsion, fuming, and precipitation are a few of the techniques used to create nanoparticle silica. The hydrophilic characteristics of this metal oxide have been attributed to the development of siloxane and silanol groups on the SiO2 surface, which is a 3D network in structure. Surface-modified SiO2, which comes in a range of sizes (mesopores, spheres, fibres, and rods) has been employed as a nanofiller in fuel cell membranes. The common techniques for producing the silica/polymer composite are sol–gel, in situ polymerization, and straightforward blending. The most popular technique is simple blending because it is easy to control the parameters (sonicating time and temperature) needed to create high homogeneity of the polymer composite.145
Selvakumar et al.,106 created sulfonation PEEK membranes using the solvent casting technique of bariun zirconate (BaZrO3). The polymer electrolyte's proton conductivity was significantly enhanced at a high weight ratio of 6 wt% BaZrO3 filler, with 3.12 × 10−1 S cm−1 at 90 °C. They found that the values of proton conductivity rise with increasing temperature. Proton conduction occurs because BaZrO3 nanoparticles can dissolve protons from water in wet environments. The composite membrane exhibited a current density of 280 mA cm−2 and power density of 183 mW cm−2. The author concluded that the 94 wt% SPEEK 6 wt% BaZrO3 polymer composite membrane is a viable option for PEM fuel cell applications. Wang et al. have effectively synthesised a sulfonated poly(ether ether ketone) (SPEEK) nanocomposite membrane through the integration of phosphotungstic acid (HPW) and aluminium doped cerium-based oxides (Al–CeZrO4) into the SPEEK matrix.107 The addition of Al–CeZrO4 improved the chemical stability of the SPEEK membrane while maintaining conductivity, and the addition of HPW increased proton conduction via acid–base interactions. Comparing the SPEEK/Al–CeZrO4 nanocomposite membrane to the SPEEK/HPW nanocomposite membrane, there was a 15.5% increase in proton conductivity. Therefore, it is believed that Al–CeZrO4/HPW is a useful inorganic nanofiller for enhancing the chemical stability and proton conductivity of SPEEK membranes, and more research should be done on the hybrid composite membrane.
Gandhimathi et al.,108 developed sulfonation PEEK membranes by casting niobium oxide in a solvent. At a high weight ratio of 10% NBO filler, the polymer electrolyte's proton conductivity was dramatically increased to 2.9 × 10−2 S cm−1 at 90 °C, compared to 1.8 × 10−2 S cm−1 for the pure SPEEK membrane. The thermal stability of the composite membranes was also significantly enhanced by the impregnation of NBO. It was reported that the SP-NBO-10 nanocomposite membrane achieved a maximum power density of 601 mW cm−2, while the pristine membrane could only achieve a maximum of 497 mW cm−2. The increase in the trend of current density and power density of the composite membrane may be induced by the vehicular proton transport mechanism involved in the sulfonic acid-based ionomeric membrane that leads to adsorption and retention of more water molecules. Based on the electrochemical results the author concluded that the SP-NBO-10 polymer composite membrane is a feasible material for PEM fuel cell applications. Prathap et al.,109 successfully created a new set of polymer composite membranes employing a linear sulfonated poly(ether ether ketone) (SPEEK) polymer and zinc cobalt oxide (ZCO) as an inorganic filler, which were tested for fuel cell applications. SPEEK was created by directly sulfonating PEEK with concentrated sulfuric acid, then loading sufficient amounts of ZCO into it to form polymer composites. Proton nuclear magnetic resonance investigations demonstrated a 55% sulfonation of SPEEK, whereas XRD and morphological examinations confirmed the successful integration of inorganic fillers into the polymer matrix, as illustrated in Fig. 20. Additionally, the authors stated that at 30 °C, the pristine SPEEK membrane had a proton conductivity of 9 × 10−3 S cm−1, while the composite membranes loaded with 2.5 to 10 wt% of ZCO showed values in the range of 1.2 × 10−2–2 × 10−2 S cm−1. The membranes' measured ion exchange capacities fell between 1.26 and 1.46 meq g−1. The composite membranes demonstrated remarkable thermal stability up to 370 °C. Thus, the membranes created in this study have the potential to considerably contribute to the creation of new proton conducting SP-ZCO composite membranes for use in PEM fuel cells.
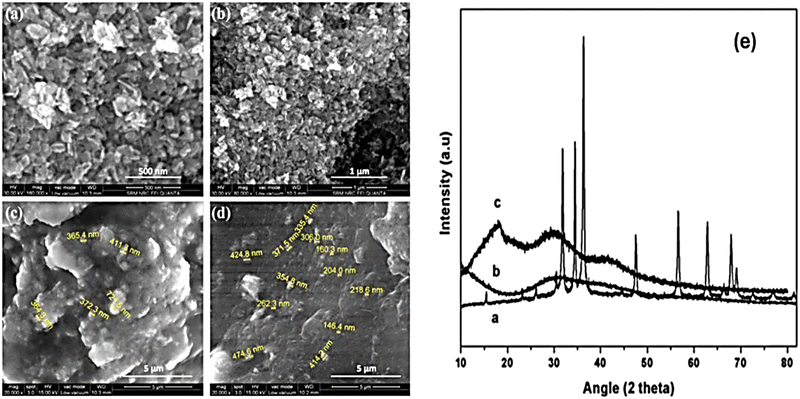 | ||
| Fig. 20 FESEM images of (a and b) ZCO, (c) SP-ZCO-5, and (d) SP-ZCO-10. (e) XRD spectra of the SPEEK and SP-SZO nanocomposites.109 | ||
5.7. Metal–organic frameworks (MOFs) as fillers for SPEEK membranes
Metal–organic frameworks (MOFs) are highly porous materials with a tunable pore size and chemical functionality. They are created by unifying metal ions or their clusters with various organic linkers. Compared to other additives, MOFs' organic linkers provide higher loading because of their improved compatibility with the organic polymers.146,147 The effective manipulation of MOFs' composition and pore size can be achieved through the appropriate choice of precursors, adjustment of synthesis conditions (reagent molar ratio, temperature, etc.), or post synthetic chemical modifications. The tunable functionality of MOFs, combined with their high porosity and surface area, makes them appealing for applications involving interactions with guest species.148 Zeolite imidazolate frameworks, or ZIFs, are a significant subclass of metal–organic frameworks (MOFs) that offer high surface area and thermal stability due to their structural similarities to zeolite and the structural diversity that MOFs provide through properties like pore size tunability and chemical functionality.149 For small molecules like hydrogen, the tiny pores at the entrance provide a molecular sieving effect, and the large internal cavities encourage quick diffusion.150,151 Several ZIF–polymer composite membranes containing ZIF-7, ZIF-8, ZIF-22, ZIF-90, ZIF-100, and other components have been proven to significantly improve hydrogen separation, but ZIF-8 has been successfully commercialised and widely used due to its higher stability and better resistance to acid and alkaline environments.152 MOFs are increasingly being used in electrochemical systems for clean energy applications, such as solar cells, fuel cells, hydrogen production and storage, supercapacitors, and lithium-ion batteries. Over the last decade, the chemistry of MOF compounds has received a lot of attention for its use in many fields of material chemistry, including gas storage and separation,153 biomedical applications,154 catalysis,155 and electro-optical devices.156Zhang et al.,97 developed a SPEEK/HPW@MIL membrane by combining amino-modified MIL-101(Cr) with HPW to form nano-hybrid membranes for PEMFC applications. HPW was anchored using hydrogen bonds to reduce leakage and improve overall compatibility. The SPEEK/HPW@MIL composite membrane's proton conductivity increased by 26% due to the effective anchoring effect of MIL-101(Cr)-NH2 on HPW and the hydrogen bond network with HPW and SPEEK. Huang et al. created sulfonated spindle-like carbon derived from a metal-organic framework, MOF-C-SO3H, which was employed as a filler for the SPEEK membrane.110 The obtained MOF-C-SO3H@SPEEK membrane exhibits improved properties as a PEM for DMFCs when 3 wt% MOF-C-SO3H is added. They also found that the MOF-C-SO3H@SPEEK membrane had higher proton conductivity and significantly lower methanol permeability than Nafion 115. According to their findings, the MOF-C-SO3H@SPEEK membrane's high performance was due in large part to its specific porous and sulfonated carbon structures. The effective dispersion of MOF-C-SO3H in the SPEEK matrix decreased the membrane's methanol permeability and swelling ratio while simultaneously enhancing proton transport and improving the membrane's proton conductivity. The authors reported a maximum power density of 83.91 mW cm−2, approximately 50% higher than that of Nafion 115. The superior stability of the MOF-C-SO3H@SPEEK membrane in contrast to Nafion 115 implies that it is a viable proton exchange membrane for fuel cells.
In another study, Sun et al.,111 used the solution casting method to incorporate Co-MOF-74/phosphate-4-phenylimidazole into SPEEK ternary composite membranes (Co-MOF-74/[IM2][H2PO4]/SPEEK). A minor agglomeration on the surface of the Co-MOF-74/[IM2][H2PO4]/SPEEK was observed. A ternary composite membrane with increased Co-MOF-74 contents and a gear-like structure was observed in the cross section of the prepared composite membrane using SEM. By means of hydrogen bonding, the metal organic framework (MOF) encapsulates the ionic liquid (IL) and reducing its loss, and consequently enhancing the proton conductivity of the Co-MOF-74/[IM2][H2PO4]/SPEEK membrane. The authors found that using 2.5 wt% Co-MOF-74/[IM2][H2PO4]/SPEEK resulted in a 25.96 mS cm−1 increase in proton conductivity at 120 °C, as well as a decrease in IL loss rate. Additionally, it was proposed that the Co-MOF-74/[IM2][H2PO4]/SPEEK ternary composite membrane could be used at temperatures up to 320 °C. These results suggest that encapsulating IL in the MOF enhances the thermal stability of SPEEK membranes in addition to increasing their proton conductivity.
Aparna et al.,112 also fabricated Cu-MOF anchored SPEEK and SPEEK/PI composite membranes for PEMFC applications. This membrane displayed high mechanical, thermal and physiochemical properties. Cu-MOF loading at 3 wt% resulted in a maximum proton conductivity of 0.0711 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1 and an IEC value of 2.35 meq g−1 with a water uptake of 38.18
cm−1 and an IEC value of 2.35 meq g−1 with a water uptake of 38.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) %. The experimental results of the prepared membranes revealed that they function as an efficient proton exchange membrane (PEM) for PEMFCs. Sun et al., successfully synthesized a novel two-dimensional (2D) zeolite structure ZIF8/CNT hybrid crosslinked network (ZCN) by an in situ growth procedure as shown in Fig. 21.113 The introduction of ZCN and SPEEK significantly improved the proton conductivity and inhibited methanol permeability. The proton conductivity of the SPEEK@ZCN composite membrane was reaching 50.24 mS cm−1 at 120 °C and 30% RH, which was 11.2 times that of the recast SPEEK membrane (4.50 mS cm−1) under the same conditions. Furthermore, it was discovered that the membrane's proton conductivity was greatly enhanced by the addition of two-dimensional fillers.
%. The experimental results of the prepared membranes revealed that they function as an efficient proton exchange membrane (PEM) for PEMFCs. Sun et al., successfully synthesized a novel two-dimensional (2D) zeolite structure ZIF8/CNT hybrid crosslinked network (ZCN) by an in situ growth procedure as shown in Fig. 21.113 The introduction of ZCN and SPEEK significantly improved the proton conductivity and inhibited methanol permeability. The proton conductivity of the SPEEK@ZCN composite membrane was reaching 50.24 mS cm−1 at 120 °C and 30% RH, which was 11.2 times that of the recast SPEEK membrane (4.50 mS cm−1) under the same conditions. Furthermore, it was discovered that the membrane's proton conductivity was greatly enhanced by the addition of two-dimensional fillers.
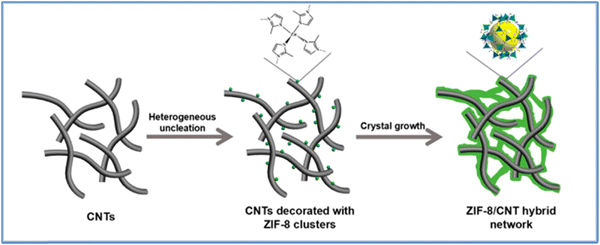 | ||
| Fig. 21 Schematic illustration of the synthesis process of ZCN through an in situ growth procedure.113 | ||
Barjola et al.,114 prepared nanocomposite membranes by mixing 1, 3, and 5 wt% (SPEEK-Z1, SPEEK-Z3, and SPEEK-Z5) cobalt-based zeolitic imidazolate frameworks (ZIF-67) with sulfonated poly(ether ether ketone) (SPEEK) via a casting method. The thermal stability and proton conductivity were greatly enhanced by the addition of 1 wt% ZIF-67 to SPEEK. A proton conductivity of 0.014 S cm−1 for the SPEEK-Z1 composite membrane was reported. The authors believe that an appropriate optimisation process is still necessary, even though the polymer electrolyte membrane fuel cell (PEMFC) performance experiments showed promising results for these membranes working at intermediate temperatures above 100 °C.
6. Conclusion and future perspectives
Without a doubt, the growing fuel cell market will provide a powerful driving force for increased research into non-fluorinated PEMs, which are less expensive and perform better than expensive Nafion® membranes. Polymers based on sulfonated poly(ether ether ketone) have the potential to be used as fuel cell electrolyte membranes. This review article examined the most current advancements in the design of various SPEEK-based electrolyte membranes for PEMFC and DMFC applications. Nevertheless, there are still certain issues with using SPEEK membranes in practical applications: (1) SPEEKs could not achieve the excellent performance of the C–F chemical bond of PFSA membranes without any modification; (2) excessive overall swelling and low thermal stability are always caused by the higher DS of SPEEK for higher proton conductivity; (3) cross-linked SPEEK membranes formed by covalent bonds may improve dimension and chemical stability, but they will also reduce proton conductivity. Other polymers and fillers should and have been introduced into the fabrication of SPEEK membranes. As a result, one of the primary goals of future research will be to design and prepare SPEEK membranes with an appropriate structure in the presence of other polymers and fillers. When compared to Nafion® membranes, SPEEK organic–inorganic composite membranes offer the best chances of superior performance. The addition of inorganic fillers may improve the mechanical and electrical properties of the membranes, making them more suitable for fuel cell applications. However, there are still some issues that require further investigation:(a) More hopping sites should be produced by the composite process to encourage the tendency of the proton conduction mechanisms towards the hopping mechanism, which will help to increase the methanol permeability and proton conductivity even at higher temperatures (preferably 120 °C).
(b) To strengthen the bond between the filler and polymer, it is crucial to choose the right inorganic filler and modify their interface.
(c) To understand the morphology and structure of PEMs, it is important to perform dynamic simulations using mathematics and computer software. This allows for the design of modifications to SPEEK and inorganic fillers, as well as optimisation of polymer and filler combinations.
The SPEEK composite membrane has significant advantages, including low methanol crossover and high proton exchange. The proton conductivity of almost all SPEEK-related composite materials was on the order of 10−2 S cm−1, which was adequate for them to be utilised as a membrane in a hydrogen–oxygen fuel cell. The impact of various metal oxides on the SPEEK matrix was also covered in this review, and it was concluded that SPEEK-based membranes are among the best polymer electrolytes for proton exchange in fuel cells. More investigation is needed to use the right inorganic particles and focus on the increase in affinity towards water-containing membranes, which increases proton conductivity. Regarding PEM development in the future, it is unrealistic to think that a single type of PEM will be able to satisfy all the needs for a large range of applications, including stationary, mobile, and automotive fuel cell applications. Research priorities will vary depending on the application goals, but it is more important for specialists in various fields to collaborate, including those working in the fields of physics, electrochemistry, polymers, composite materials, and simulation. As a result, we hope that this review will give a general overview of the developments surrounding SPEEK-based PEMs and offer some suggestions for the creation of high-performing non-fluorinated PEMs in the future.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.Author contributions
Mayetu Segale: conceptualization, writing – original draft, and writing – review & editing. Tumelo Seadira: conceptualization, supervision, and review & editing. Rudzani Sigwadi: conceptualization, supervision, and review & editing. Touhami Mokrani: supervision and writing – review & editing. Gabriel Summers: supervision and writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We would like to acknowledge that this work has been supported in part by the University of South Africa and the National Research Foundation (NRF) of South Africa. The opinions, findings and conclusions/recommendations expressed in this publication are those of the authors, and the NRF accepts no liability whatsoever in this regard.References
- P. Singh and D. Yadav, Link between air pollution and global climate change, in Global Climate Change, Elsevier, 2021, pp. 79–108 Search PubMed.
- R. Al Shaikh, A. Al-Othman, M. Tawalbeh, A. Shamayleh and P. Nancarrow, Development of MXene incorporated PVDF based membranes for an enhanced performance in higher temperature PEM fuel cells, Process Saf. Environ. Protect., 2024, 189, 985–994 CrossRef.
- A. Al-Othman, M. Tawalbeh, A. Ka’aki, I. Shomope and M. F. Hassan, Novel zirconium phosphate/MXene/ionic liquid membranes for PEM fuel cells operating up to 145 °C, Process Saf. Environ. Protect., 2024, 189, 1368–1378 CrossRef.
- A. A. Ali, A. Al-Othman and M. Tawalbeh, Exploring natural polymers for the development of proton exchange membranes in fuel cells, Process Saf. Environ. Protect., 2024, 189, 1379–1401 CrossRef.
- W. Nimir, A. Al-Othman and M. Tawalbeh, Unveiling zirconium phytate-heteropolyacids-ionic liquids membranes for PEM fuel cells applications up to 150 °C, Int. J. Hydrogen Energy, 2024 DOI:10.1016/j.ijhydene.2024.06.120.
- M. A. Abdelkareem, K. Elsaid, T. Wilberforce, M. Kamil, E. T. Sayed and A. Olabi, Environmental aspects of fuel cells: A review, Sci. Total Environ., 2021, 752, 141803 CrossRef.
- H. Nazir, N. Muthuswamy, C. Louis, S. Jose, J. Prakash, M. E. Buan, C. Flox, S. Chavan, X. Shi and P. Kauranen, Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities, Int. J. Hydrogen Energy, 2020, 45(53), 28217–28239 CrossRef.
- H. Mohammed, A. Al-Othman, P. Nancarrow, M. Tawalbeh and M. E. H. Assad, Direct hydrocarbon fuel cells: A promising technology for improving energy efficiency, Energy, 2019, 172, 207–219 CrossRef.
- T. A. Suter, K. Smith, J. Hack, L. Rasha, Z. Rana, G. M. A. Angel, P. R. Shearing, T. S. Miller and D. J. Brett, Engineering Catalyst Layers for Next-Generation Polymer Electrolyte Fuel Cells: A Review of Design, Materials, and Methods, Adv. Energy Mater., 2021, 11(37), 2101025 CrossRef.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research, Appl. Energy, 2011, 88(4), 981–1007 CrossRef.
- H. Zhang and P. K. Shen, Recent development of polymer electrolyte membranes for fuel cells, Chem. Rev., 2012, 112(5), 2780–2832 CrossRef.
- F. Xiao, Y. C. Wang, Z. P. Wu, G. Chen, F. Yang, S. Zhu, K. Siddharth, Z. Kong, A. Lu and J. C. Li, Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells, Adv. Mater., 2021, 33(50), 2006292 CrossRef.
- X. Gao, J. Chen, R. Xu, Z. Zhen, X. Zeng, X. Chen and L. Cui, Research progress and prospect of the materials of bipolar plates for proton exchange membrane fuel cells (PEMFCs), Int. J. Hydrogen Energy, 2024, 50, 711–743 CrossRef.
- B. Shabani, M. Hafttananian, S. Khamani, A. Ramiar and A. Ranjbar, Poisoning of proton exchange membrane fuel cells by contaminants and impurities: Review of mechanisms, effects, and mitigation strategies, J. Power Sources, 2019, 427, 21–48 CrossRef.
- E. Ogungbemi, O. Ijaodola, F. Khatib, T. Wilberforce, Z. El Hassan and J. Thompson, M. Ramadan, and A. Olabi, Fuel cell membranes–Pros and cons, Energy, 2019, 172, 155–172 CrossRef.
- D. J. Kim, M. J. Jo and S. Y. Nam, A review of polymer–nanocomposite electrolyte membranes for fuel cell application, J. Ind. Eng. Chem., 2015, 21, 36–52 CrossRef.
- G. G. Gagliardi, A. Ibrahim, D. Borello and A. El-Kharouf, Composite polymers development and application for polymer electrolyte membrane technologies—A review, Molecules, 2020, 25(7), 1712 CrossRef PubMed.
- J. Walkowiak-Kulikowska, J. Wolska and H. Koroniak, 10. Polymers application in proton exchange membranes for fuel cells (PEMFCs), Polym. Eng., 2017, 293–348 Search PubMed.
- C. H. Park, C. H. Lee, M. D. Guiver and Y. M. Lee, Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs), Prog. Polym. Sci., 2011, 36(11), 1443–1498 CrossRef.
- O. Ghita, E. James, R. Trimble and K. E. Evans, Physico-chemical behaviour of poly (ether ketone)(PEK) in high temperature laser sintering (HT-LS), J. Mater. Process. Technol., 2014, 214(4), 969–978 CrossRef.
- Z. Huang, J. Liu, Y. Liu, Y. Xu, R. Li, H. Hong, L. Shen, H. Lin and B.-Q. Liao, Enhanced permeability and antifouling performance of polyether sulfone (PES) membrane via elevating magnetic Ni@ MXene nanoparticles to upper layer in phase inversion process, J. Membr. Sci., 2021, 623, 119080 CrossRef.
- H. Luo, G. Vaivars and M. Mathe, Double cross-linked polyetheretherketone proton exchange membrane for fuel cell, Int. J. Hydrogen Energy, 2012, 37(7), 6148–6152 CrossRef.
- A. Kausar, Progression from polyimide to polyimide composite in proton-exchange membrane fuel cell: a review, Polym.-Plast. Technol. Eng., 2017, 56(13), 1375–1390 CrossRef.
- M. Gil, X. Ji, X. Li, H. Na, J. E. Hampsey and Y. Lu, Direct synthesis of sulfonated aromatic poly (ether ether ketone) proton exchange membranes for fuel cell applications, J. Membr. Sci., 2004, 234(1–2), 75–81 CrossRef.
- B. M. Mahimai, G. Sivasubramanian, K. Sekar, D. Kannaiyan and P. Deivanayagam, Sulfonated poly (ether ether ketone): efficient ion-exchange polymer electrolytes for fuel cell applications–a versatile review, Mater. Adv., 2022, 3(15), 6085–6095 RSC.
- N. D. Govinna, T. Keller, C. Schick and P. Cebe, Melt-electrospinning of poly (ether ether ketone) fibers to avoid sulfonation, Polymer, 2019, 171, 50–57 CrossRef.
- S. Sarirchi, S. Rowshanzamir and F. Mehri, Simultaneous improvement of ionic conductivity and oxidative stability of sulfonated poly (ether ether ketone) nanocomposite proton exchange membrane for fuel cell application, Int. J. Energy Res., 2020, 44(4), 2783–2800 CrossRef.
- R. R. RS, W. Rashmi, M. Khalid, W. Wong and J. Priyanka, Recent progress in the development of aromatic polymer-based proton exchange membranes for fuel cell applications, Polymers, 2020, 12(5), 1061 CrossRef.
- F. Beyraghi, S. H. Mirfarsi, S. Rowshanzamir, A. Karimi and M. J. Parnian, Optimal thermal treatment conditions for durability improvement of highly sulfonated poly (ether ether ketone) membrane for polymer electrolyte fuel cell applications, Int. J. Hydrogen Energy, 2020, 45(24), 13441–13458 CrossRef.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen and J. Wu, Thermal behaviour and kinetic study for woody biomass torrefaction and torrefied biomass pyrolysis by TGA, Biosyst. Eng., 2013, 116(4), 420–426 CrossRef.
- Z. Zakaria, N. Shaari, S. K. Kamarudin, R. Bahru and M. T. Musa, A review of progressive advanced polymer nanohybrid membrane in fuel cell application, Int. J. Energy Res., 2020, 44(11), 8255–8295 CrossRef.
- B. Gilois, F. Goujon, A. Fleury, A. Soldera and A. Ghoufi, Water nano-diffusion through the Nafion fuel cell membrane, J. Membr. Sci., 2020, 602, 117958 CrossRef.
- G. Wang, Z. Liu, C. Liu and W. Chen, Molecular Study of Nonequilibrium Transport Mechanism for Proton and Water in Porous Proton Exchange Membranes, Int. J. Energy Res., 2023, 2023(1), 1138198 Search PubMed.
- R. Haider, Y. Wen, Z.-F. Ma, D. P. Wilkinson, L. Zhang, X. Yuan, S. Song and J. Zhang, High temperature proton exchange membrane fuel cells: progress in advanced materials and key technologies, Chem. Soc. Rev., 2021, 50(2), 1138–1187 RSC.
- X. Dong, D. Lu, T. A. Harris and I. C. Escobar, Polymers and solvents used in membrane fabrication: a review focusing on sustainable membrane development, Membranes, 2021, 11(5), 309 CrossRef PubMed.
- K. M. Mukeba, Step-growth polymerization of perfluoro-vinyl ether, -cycloalkenes, and -acyclic alkenes with bisphenols containing variable polycyclic aromatic cores, Theses and dissertations, 2022, 5494, https://scholarsjunction.msstate.edu/td/5494.
- A. Bossion, K. V. Heifferon, L. Meabe, N. Zivic, D. Taton, J. L. Hedrick, T. E. Long and H. Sardon, Opportunities for organocatalysis in polymer synthesis via step-growth methods, Prog. Polym. Sci., 2019, 90, 164–210 CrossRef.
- Y. Yang, Y. Wang, M. Zhu, J. Zhao, D. Cai and H. Cao, Valorization of lignin for renewable non-isocyanate polyurethanes: a state-of-the-art review, Mater. Today Sustainability, 2023, 22, 100367 CrossRef.
- M. D. T. Nguyen, S. Yang and D. Kim, Pendant dual sulfonated poly (arylene ether ketone) proton exchange membranes for fuel cell application, J. Power Sources, 2016, 328, 355–363 CrossRef.
- H. Fulcrand, L. Rouméas, G. Billerach, C. Aouf and E. Dubreucq, Advances in Bio-based thermosetting polymers, Rec. Adv. Polyphenol. Res., 2019, 6, 285–334 Search PubMed.
- Y. Sun, S. Zhou, G. Qin, J. Guo, Q. Zhang, S. Li and S. Zhang, A chemical-induced crystallization strategy to fabricate poly (ether ether ketone) asymmetric membranes for organic solvent nanofiltration, J. Membr. Sci., 2021, 620, 118899 CrossRef.
- M. Xu, H. Xue, Q. Wang and L. Jia, Sulfonated poly (arylene ether) s based proton exchange membranes for fuel cells, Int. J. Hydrogen Energy, 2021, 46(62), 31727–31753 CrossRef.
- N. A. M. Nor, M. A. Mohamed and J. Jaafar, Modified sulfonated polyphenylsulfone proton exchange membrane with enhanced fuel cell performance: a review, J. Ind. Eng. Chem., 2022, 116, 32–59 CrossRef.
- P. Khomein, W. Ketelaars, T. Lap and G. Liu, Sulfonated aromatic polymer as a future proton exchange membrane: A review of sulfonation and crosslinking methods, Renewable Sustainable Energy Rev., 2021, 137, 110471 CrossRef.
- N. A. M. Harun, N. Shaari and N. F. H. N. Zaiman, A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly (ether ether ketone), Int. J. Energy Res., 2021, 45(14), 19671–19708 CrossRef.
- S. N. S. Sayed Daud, M. N. A. Mohd Norddin, J. Jaafar and R. Sudirman, High degree sulfonated poly (ether ether ketone) blend with polyvinylidene fluoride as a potential proton-conducting membrane fuel cell, High Perform. Polym., 2020, 32(1), 103–115 CrossRef.
- R. Muthu Lakshmi, V. Choudhary and I. Varma, Sulphonated poly (ether ether ketone): Synthesis and characterisation, J. Mater. Sci., 2005, 40, 629–636 CrossRef.
- S. Tsai, Introduction to composite materials, Routledge, 2018 Search PubMed.
- Z. Li, R. Yu, C. Liu, J. Zheng, J. Guo, T. A. Sherazi, S. Li and S. Zhang, Preparation and characterization of side-chain poly (aryl ether ketone) anion exchange membranes by superacid-catalyzed reaction, Polymer, 2021, 222, 123639 CrossRef.
- C. Y. Wong, W. Y. Wong, K. S. Loh, W. R. W. Daud, K. L. Lim, M. Khalid and R. Walvekar, Development of poly (vinyl alcohol)-based polymers as proton exchange membranes and challenges in fuel cell application: a review, Polym. Rev., 2020, 60(1), 171–202 CrossRef.
- Z. Xiang, H. Liu, P. Deng, M. Liu, Y. Yin and X. Ge, The effect of irradiation on morphology and properties of the PET/HDPE blends with trimethylol propane trimethacrylate (TMPTA), Polym. Bull., 2009, 63, 587–597 CrossRef.
- G. Xiaomin, L. Yonghua and L. Jinlong, Review on modification of sulfonated poly (-ether-ether-ketone) membranes used as proton exchange membranes, Mater. Sci., 2015, 21(4), 574–582 Search PubMed.
- S. Hayes, C. Boote, C. S. Kamma-Lorger, M. S. Rajan, J. Harris, E. Dooley, N. Hawksworth, J. Hiller, N. J. Terill and F. Hafezi, Riboflavin/UVA collagen cross-linking-induced changes in normal and keratoconus corneal stroma, PLoS one, 2011, 6(8), e22405 CrossRef PubMed.
- Y. Gao, K. Peng and S. Mitragotri, Covalently Crosslinked hydrogels via step-growth reactions: crosslinking chemistries, polymers, and clinical impact, Adv. Mater., 2021, 33(25), 2006362 CrossRef.
- S. Wu, J. Liang, Y. Shi, M. Huang, X. Bi, Z. Wang and J. Jin, Design of interchain hydrogen bond in polyimide membrane for improved gas selectivity and membrane stability, J. Membr. Sci., 2021, 618, 118659 CrossRef CAS.
- N. Ramly, N. Aini, N. Sahli, S. Aminuddin, M. Yahya and A. Ali, Dielectric behaviour of UV-crosslinked sulfonated poly (ether ether ketone) with methyl cellulose (SPEEK-MC) as proton exchange membrane, Int. J. Hydrogen Energy, 2017, 42(14), 9284–9292 CrossRef.
- R. Teruel-Juanes, B. Pascual-Jose, C. del Río, O. García and A. Ribes-Greus, Dielectric analysis of photocrosslinked and post-sulfonated styrene-ethylene-butylene-styrene block copolymer based membranes, React. Funct. Polym., 2020, 155, 104715 CrossRef.
- N. Meng, F. Lian and G. Cui, Macromolecular design of lithium conductive polymer as electrolyte for solid-state lithium batteries, Small, 2021, 17(3), 2005762 CrossRef.
- M. Kumari, H. S. Sodaye, D. Sen and R. C. Bindal, Properties and morphology studies of proton exchange membranes based on cross-linked sulfonated poly (ether ether ketone) for electrochemical application: effect of cross-linker chain length, Solid State Ionics, 2018, 316, 75–84 CrossRef.
- D. Gupta and V. Choudhary, Studies on novel heat treated sulfonated poly (ether ether ketone)[SPEEK]/diol membranes for fuel cell applications, Int. J. Hydrogen Energy, 2011, 36(14), 8525–8535 CrossRef.
- A. Ajitha and S. Thomas, Introduction: Polymer blends, thermodynamics, miscibility, phase separation, and compatibilization, in Compatibilization of polymer blends, Elsevier, 2020, pp. 1–29 Search PubMed.
- J. Liang, J. Ge, K. Wu, Q. Zhang, J. Wang and Z. Ye, Sulfonated polyaryletherketone with pendant benzimidazole groups for proton exchange membranes, J. Membr. Sci., 2020, 597, 117626 CrossRef.
- C. Simari, C. L. Vecchio, V. Baglio and I. Nicotera, Sulfonated polyethersulfone/polyetheretherketone blend as high performing and cost-effective electrolyte membrane for direct methanol fuel cells, Renewable Energy, 2020, 159, 336–345 CrossRef.
- K. Raja, M. Raja Pugalenthi and M. Ramesh Prabhu, Investigation on the sulfonated poly (ether ether ketone)/poly (amide-imide)/barium cerate-based nanocomposite membrane for proton exchange membrane fuel cells, Int. J. Energy Res., 2021, 45(6), 8564–8576 CrossRef.
- C. Li, Z. Yang, X. Liu, Y. Zhang, J. Dong, Q. Zhang and H. Cheng, Enhanced performance of sulfonated poly (ether ether ketone) membranes by blending fully aromatic polyamide for practical application in direct methanol fuel cells (DMFCs), Int. J. Hydrogen Energy, 2017, 42(47), 28567–28577 CrossRef.
- A. Haragirimana, P. B. Ingabire, Y. Liu, N. Li, Z. Hu and S. Chen, An effective strategy to enhance the performance of the proton exchange membranes based on sulfonated poly (ether ether ketone) s, Int. J. Hydrogen Energy, 2020, 45(16), 10017–10029 CrossRef.
- Z. Shang, R. Wycisk and P. Pintauro, Electrospun composite proton-exchange and anion-exchange membranes for fuel cells, Energies, 2021, 14(20), 6709 CrossRef.
- X. Qian, M. Ostwal, A. Asatekin, G. M. Geise, Z. P. Smith, W. A. Phillip, R. P. Lively and J. R. McCutcheon, A critical review and commentary on recent progress of additive manufacturing and its impact on membrane technology, J. Membr. Sci., 2022, 645, 120041 CrossRef CAS.
- J. K. Nayak, U. Shankar and K. Samal, Fabrication and development of SPEEK/PVdF-HFP/SiO2 proton exchange membrane for microbial fuel cell application, Chem. Eng. J. Adv., 2023, 14, 100459 CrossRef CAS.
- A. Çalı, A. Şahin and A. Irfan, Experimental Investigation of boron phosphate Incorporated speek/pvdf blend membrane for proton exchange membrane fuel cells, Int. J. Hydrogen Energy, 2022, 47(95), 40476–40490 CrossRef.
- D. Wang and C. J. Cornelius, Ionomer thermodynamic interrelationships associated with wettability, surface energy, swelling, and water transport, Eur. Polym. J., 2016, 85, 126–138 CrossRef CAS.
- Q. Liu, X. Li, S. Zhang, Z. Wang, Y. Chen, S. Zhou, C. Wang, K. Wu, J. Liu and Q. Mao, Novel sulfonated N-heterocyclic poly (aryl ether ketone ketone) s with pendant phenyl groups for proton exchange membrane performing enhanced oxidative stability and excellent fuel cell properties, J. Membr. Sci., 2022, 641, 119926 CrossRef CAS.
- G. Zhao, L. Shi, M. Zhang, B. Cheng, G. Yang and X. Zhuang, Self-assembly of metal-organic framework onto nanofibrous mats to enhance proton conductivity for proton exchange membrane, Int. J. Hydrogen Energy, 2021, 46(73), 36415–36423 CrossRef CAS.
- L. da Trindade, L. Zanchet, R. Dreon, J. Souza, M. Assis, E. Longo, E. Martini, A. Chiquito and F. Pontes, Microwave-assisted solvothermal preparation of Zr-BDC for modification of proton exchange membranes made of SPEEK/PBI blends, J. Mater. Sci., 2020, 55, 14938–14952 CrossRef CAS.
- S. N. S. S. Daud, M. M. Norddin, J. Jaafar, R. Sudirman, M. Othman and A. Ismail, Highly sulfonated poly (ether ether ketone) blend with hydrophobic polyether sulfone as an alternative electrolyte for proton exchange membrane fuel cell, Arabian J. Sci. Eng., 2021, 46, 6189–6205 CrossRef CAS.
- P. Wei, Y. Sui, X. Li, Q. Liu, B. Zhu, C. Cong, X. Meng and Q. Zhou, Sandwich-structure PI/SPEEK/PI proton exchange membrane developed for achieving the high durability on excellent proton conductivity and stability, J. Membr. Sci., 2022, 644, 120116 CrossRef CAS.
- F. Chu, X. Chu, T. Lv, Z. Chen, Y. Ren, S. Zhang, N. Yuan, B. Lin and J. Ding, Amphoteric membranes based on sulfonated polyether ether ketone and imidazolium-functionalized polyphenylene oxide for vanadium redox flow battery applications, ChemElectroChem, 2019, 6(19), 5041–5050 CrossRef CAS.
- X. Yang, H. Zhu, F. Jiang and X. Zhou, Notably enhanced proton conductivity by thermally-induced phase-separation transition of Nafion/Poly (vinylidene fluoride) blend membranes, J. Power Sources, 2020, 473, 228586 CrossRef CAS.
- G. Liu, W.-C. Tsen, S.-C. Jang, F. Hu, F. Zhong, H. Liu, G. Wang, S. Wen, G. Zheng and C. Gong, Mechanically robust and highly methanol-resistant sulfonated poly (ether ether ketone)/poly (vinylidene fluoride) nanofiber composite membranes for direct methanol fuel cells, J. Membr. Sci., 2019, 591, 117321 CrossRef CAS.
- M. Chamakh and A. I. Ayesh, Production and investigation of flexible nanofibers of sPEEK/PVP loaded with RuO2 nanoparticles, Mater. Des., 2021, 204, 109678 CrossRef CAS.
- H. Purnama, M. Mujiburohman, M. F. Hakim and N. Hidayati, Preparation and Characterisation of Composite Sulfonated Polyether Ether Ketone for Direct Methanol Fuel Cells, J. Phys.: Conf. Ser., 2019, 1295(1), 012048 CrossRef CAS.
- N. Y. Abu-Thabit, S. A. Ali, S. J. Zaidi and K. Mezghani, Novel sulfonated poly (ether ether ketone)/phosphonated polysulfone polymer blends for proton conducting membranes, J. Mater. Res., 2012, 27(15), 1958–1968 CrossRef CAS.
- A. Sultan, J. K. Adewole, A. Al-Ahmed, M. Nazal and S. J. Zaidi, Preparation and performance evaluation of speek/polyaniline composite membrane for direct methanol fuel cell, Int. Polym. Process., 2017, 32(1), 41–49 CrossRef CAS.
- M. Han, G. Zhang, M. Li, S. Wang, Z. Liu, H. Li, Y. Zhang, D. Xu, J. Wang and J. Ni, Sulfonated poly (ether ether ketone)/polybenzimidazole oligomer/epoxy resin composite membranes in situ polymerization for direct methanol fuel cell usages, J. Power Sources, 2011, 196(23), 9916–9923 CrossRef CAS.
- N. Hidayati, T. Harmoko, M. Mujiburohman and H. Purnama, Characterization of sPEEK/chitosan membrane for the direct methanol fuel cell, Exploring ReSources, Process and Design for Sustainable Urban Development, 2019, 2114(1), 060008 Search PubMed.
- X. Sun, S. C. Simonsen, T. Norby and A. Chatzitakis, Composite membranes for high temperature PEM fuel cells and electrolysers: a critical review, Membranes, 2019, 9(7), 83 CrossRef CAS PubMed.
- G. Liu, W.-C. Tsen, S.-C. Jang, F. Hu, F. Zhong, B. Zhang, J. Wang, H. Liu, G. Wang and S. Wen, Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells, J. Membr. Sci., 2020, 611, 118242 CrossRef CAS.
- Z. Li, Z. Guan, C. Wang, B. Quan and L. Zhao, Addition of modified hollow mesoporous organosilica in anhydrous SPEEK/IL composite membrane enhances its proton conductivity, J. Membr. Sci., 2021, 620, 118897 CrossRef CAS.
- G. Sivasubramanian, K. Hariharasubramanian, P. Deivanayagam and J. Ramaswamy, High-performance SPEEK/SWCNT/fly ash polymer electrolyte nanocomposite membranes for fuel cell applications, Polym. J., 2017, 49(10), 703–709 CrossRef CAS.
- S. Gahlot and V. Kulshrestha, Dramatic improvement in water retention and proton conductivity in electrically aligned functionalized CNT/SPEEK nanohybrid PEM, ACS Appl. Mater. Interfaces, 2015, 7(1), 264–272 CrossRef CAS PubMed.
- Y. Di, W. Yang, X. Li, Z. Zhao, M. Wang and J. Dai, Preparation and characterization of continuous carbon nanofiber-supported SPEEK composite membranes for fuel cell application, RSC Adv., 2014, 4(94), 52001–52007 RSC.
- S. He, Y. Ai, W. Dai, S. Zhai, H. Song and J. Lin, Composite membranes anchoring phosphotungstic acid by β-cyclodextrins modified halloysite nanotubes, Polym. Test., 2021, 100, 107246 CrossRef CAS.
- S. Oh, T. Yoshida, G. Kawamura, H. Muto, M. Sakai and A. Matsuda, Proton conductivity and fuel cell property of composite electrolyte consisting of Cs-substituted heteropoly acids and sulfonated poly (ether–ether ketone), J. Power Sources, 2010, 195(18), 5822–5828 CrossRef CAS.
- S. Peighambardoust, S. Rowshanzamir, M. Hosseini and M. Yazdanpour, Self-humidifying nanocomposite membranes based on sulfonated poly (ether ether ketone) and heteropolyacid supported Pt catalyst for fuel cells, Int. J. Hydrogen Energy, 2011, 36(17), 10940–10957 CrossRef CAS.
- I. Colicchio, F. Wen, H. Keul, U. Simon and M. Moeller, Sulfonated poly (ether ether ketone)–silica membranes doped with phosphotungstic acid. Morphology and proton conductivity, J. Membr. Sci., 2009, 326(1), 45–57 CrossRef CAS.
- H. Doğan, T. Y. Inan, E. Unveren and M. Kaya, Effect of cesium salt of tungstophosphoric acid (Cs-TPA) on the properties of sulfonated polyether ether ketone (SPEEK) composite membranes for fuel cell applications, Int. J. Hydrogen Energy, 2010, 35(15), 7784–7795 CrossRef.
- X. Zhang, H. Ma, T. Pei, R. Zhang and Y. Liu, Anchoring HPW by amino-modified MIL-101 (Cr) to improve the properties of SPEEK in proton exchange membranes, J. Appl. Polym. Sci., 2023, e53978 CrossRef CAS.
- L. T. Yogarathinam, J. Jaafar, A. F. Ismail, P. S. Goh, M. H. Bin Mohamed, M. F. Radzi Hanifah, A. Gangasalam and J. Peter, Polyaniline decorated graphene oxide on sulfonated poly (ether ether ketone) membrane for direct methanol fuel cells application, Polym. Adv. Technol., 2022, 33(1), 66–80 CrossRef CAS.
- T. K. Maiti, P. Dixit, J. Singh, N. Talapatra, M. Ray and S. Chattopadhyay, A novel strategy toward the advancement of proton exchange membranes through the incorporation of propylsulfonic acid-functionalized graphene oxide in crosslinked acid-base polymer blends, Int. J. Hydrogen Energy, 2023, 48(4), 1482–1500 CrossRef CAS.
- P. Martina, R. Gayathri, M. R. Pugalenthi, G. Cao, C. Liu and M. R. Prabhu, Nanosulfonated silica incorporated SPEEK/SPVdF-HFP polymer blend membrane for PEM fuel cell application, Ionics, 2020, 26, 3447–3458 CrossRef CAS.
- X. Meng, C. Li, J. Wen, H. Ye, C. Cong, Q. Zhou and L. Xu, The effect of amino-modified mesoporous silica nanospheres on properties of SPEEK/HPW@ Mesoporous Silica Nanoparticles proton exchange membrane, J. Chinese Chem. Soc., 2021, 68(7), 1197–1204 CrossRef CAS.
- A. Sahin, The development of Speek/Pva/Teos blend membrane for proton exchange membrane fuel cells, Electrochim. Acta, 2018, 271, 127–136 CrossRef CAS.
- V. Kumar, S. GokulaKrishnan, G. Arthanareeswaran, A. F. Ismail, J. Jaafar, D. B. Das and L. T. Yogarathinam, Cloisite-and bentonite-based stable nanocomposite membranes for enhancement of direct methanol fuel cell applications, Polym. Bull., 2023, 1–19 Search PubMed.
- S. Gokulakrishnan, V. Kumar, G. Arthanareeswaran, A. Ismail and J. Jaafar, Thermally stable nanoclay and functionalized graphene oxide integrated SPEEK nanocomposite membranes for direct methanol fuel cell application, Fuel, 2022, 329, 125407 CrossRef CAS.
- K. Charradi, Z. Ahmed, P. Aranda and R. Chtourou, Silica/montmorillonite nanoarchitectures and layered double hydroxide-SPEEK based composite membranes for fuel cells applications, Appl. Clay Sci., 2019, 174, 77–85 CrossRef CAS.
- K. Selvakumar, S. Rajendran and M. Ramesh Prabhu, Influence of barium zirconate on SPEEK-based polymer electrolytes for PEM fuel cell applications, Ionics, 2019, 25, 2243–2253 CrossRef.
- Y. Wang, J. You, Z. Cheng, K. Jiang, L. Zhang, W. Cai, Y.-Q. Liu and S. Li, A promising Al-CeZrO4/HPW-incorporated SPEEK composite membrane with improved proton conductivity and chemical stability for PEM fuel cells, High Perform. Polym., 2021, 33(3), 295–308 CrossRef.
- S. Gandhimathi, H. Krishnan and D. Paradesi, Development of proton-exchange polymer nanocomposite membranes for fuel cell applications, Polym. Polym. Compos., 2020, 28(7), 492–501 Search PubMed.
- M. Prathap, K. Poonkuzhali, M. M. Berlina, P. Hemalatha and D. Paradesi, Synthesis and characterization of sulfonated poly (ether ether ketone)/zinc cobalt oxide composite membranes for fuel cell applications, High Perform. Polym., 2020, 32(9), 984–991 CrossRef.
- H. Huang, Y. Ma, Z. Jiang and Z.-J. Jiang, Spindle-like MOFs-derived porous carbon filled sulfonated poly (ether ether ketone): A high performance proton exchange membrane for direct methanol fuel cells, J. Membr. Sci., 2021, 636, 119585 CrossRef.
- L. Sun, S. Qu, X. Lv, L. Ding, J. Duan and W. Wang, Sulfonated Poly Ether Ether Ketone Membranes Reinforced by Metal–Organic Frameworks/Ionic Liquids, ACS Appl. Polym. Mater., 2023, 5(12), 10081–10090 CrossRef.
- M. Aparna, P. Hemalatha, D. Paradesi and D. A. Raj, Design and development of copper trimesic acid anchored sPEEK/polyimide composite membranes for fuel cell applications, ChemistrySelect, 2023, 8(14), e202204584 CrossRef.
- H. Sun, B. Tang and P. Wu, Two-dimensional zeolitic imidazolate framework/carbon nanotube hybrid networks modified proton exchange membranes for improving transport properties, ACS Appl. Mater. Interfaces, 2017, 9(40), 35075–35085 CrossRef CAS PubMed.
- A. Barjola, J. L. Reyes-Rodríguez, O. Solorza-Feria, E. Giménez and V. Compan, Novel SPEEK-ZIF-67 proton exchange nanocomposite membrane for PEMFC application at intermediate temperatures, Ind. Eng. Chem. Res., 2021, 60(25), 9107–9118 CrossRef CAS.
- M. Taufiq Musa, N. Shaari and S. K. Kamarudin, Carbon nanotube, graphene oxide and montmorillonite as conductive fillers in polymer electrolyte membrane for fuel cell: an overview, Int. J. Energy Res., 2021, 45(2), 1309–1346 CrossRef CAS.
- N. Gupta, S. M. Gupta and S. Sharma, Carbon nanotubes: Synthesis, properties and engineering applications, Carbon Lett., 2019, 29, 419–447 CrossRef.
- N. C. Nqakala, Construction of an enzyme-free electrochemical sensor based on Ag-Fe2O3/POM/RGO novel nanocomposite for hydrogen peroxide detection, 2018 Search PubMed.
- N. Li, J. Liu, J. J. Liu, L. Z. Dong, S. L. Li, B. X. Dong, Y. H. Kan and Y. Q. Lan, Self-Assembly of a Phosphate-Centered Polyoxo-Titanium Cluster: Discovery of the Heteroatom Keggin Family, Angew. Chem., Int. Ed., 2019, 58(48), 17260–17264 CrossRef CAS PubMed.
- I. A. Weinstock, R. E. Schreiber and R. Neumann, Dioxygen in Polyoxometalate Mediated Reactions, Chem. Rev., 2017, 118(5), 2680–2717 CrossRef PubMed.
- O. Ponomareva, O. Matveeva, A. Nikiforov, I. Dobryakova, I. Kasyanov, A. Shkuropatov and I. Ivanova, Synthesis of butadiene from Formaldehyde and Propylene on Cesium Salts of Silicotungstic heteropoly Acid, Pet. Chem., 2021, 61(8), 916–924 CrossRef CAS.
- J. E. Sánchez-Velandia, H. G. Baldoví, A. Y. Sidorenko, J. A. Becerra and F. Martínez, Synthesis of heterocycles compounds from condensation of limonene with aldehydes using heteropolyacids supported on metal oxides, Mol. Catal., 2022, 528, 112511 CrossRef.
- N. Shaari, N. F. Raduwan, Y. N. Yusoff, N. A. M. Harun and N. F. H. N. Zaiman, Membrane and catalyst in direct methanol fuel cell and direct borohydride fuel cell application, in Renewable Energy Production and Distribution, Elsevier, 2023, vol. 2, pp. 409–458 Search PubMed.
- H. Mao, X. Li, F. Xu, Z. Xiao, W. Zhang and T. Meng, Vapour-phase selective O-methylation of catechol with methanol over metal phosphate catalysts, Catalysts, 2021, 11(5), 531 CrossRef CAS.
- G. Y. Ryu, H. Jae, K. J. Kim, H. Kim, S. Lee, Y. Jeon, D. Roh and W. S. Chi, Hollow Heteropoly Acid-Functionalized ZIF Composite Membrane for Proton Exchange Membrane Fuel Cells, ACS Appl. Energy Mater., 2023, 6(8), 4283–4296 CrossRef CAS.
- Y. Zhang, H. Zhang, C. Bi and X. Zhu, An inorganic/organic self-humidifying composite membranes for proton exchange membrane fuel cell application, Electrochim. Acta, 2008, 53(12), 4096–4103 CrossRef CAS.
- A. Ghosh, Synthesis of Graphene: Theory and Application, in Constraint Decision-Making Systems in Engineering, IGI Global, 2023, pp. 219–238 Search PubMed.
- L. E. Dizaji, Synthesis of new nano metal–organic frameworks with urea and thiourea ligands and investigation of their application in sensing, catalysis and removal of hazardous materials, Doctoral dissertation, University of Antwerp, 2022 Search PubMed.
- D. Collomb, P. Li and S. Bending, Frontiers of graphene-based Hall-effect sensors, J. Phys.: Condens. Matter, 2021, 33(24), 243002 CrossRef CAS.
- W. Guo, M. Zhang, Z. Xue, P. K. Chu, Y. Mei, Z. Tian and Z. Di, Extremely High Intrinsic Carrier Mobility and Quantum Hall Effect Of Single Crystalline Graphene Grown on Ge (110), Adv. Mater. Interfaces, 2023, 10(23), 2300482 CrossRef CAS.
- V. B. Mbayachi, E. Ndayiragije, T. Sammani, S. Taj and E. R. Mbuta, Graphene synthesis, characterization and its applications: A review, Res. Chem., 2021, 3, 100163 CAS.
- P. Das, B. Mandal and S. Gumma, L-tyrosine grafted palladium graphite oxide and sulfonated poly (ether ether ketone) based novel composite membrane for direct methanol fuel cell, Chem. Eng. J., 2021, 423, 130235 CrossRef CAS.
- J. Sun, D. Han, M. M. Mohideen, S. Li, C. Wang, P. Hu and Y. Liu, Constructing vertical proton transport channels in proton exchange membranes of fuel cells, Int. J. Hydrogen Energy, 2024, 50, 1456–1480 CrossRef.
- Z. Guo, J. Chen, J. J. Byun, R. Cai, M. Perez-Page, M. Sahoo, Z. Ji, S. J. Haigh and S. M. Holmes, High-performance polymer electrolyte membranes incorporated with 2D silica nanosheets in high-temperature proton exchange membrane fuel cells, J. Energy Chem., 2022, 64, 323–334 CrossRef CAS.
- M. Porozhnyy, S. Shkirskaya, D. Y. Butylskii, V. Dotsenko, E. Y. Safronova, A. Yaroslavtsev, S. Deabate, P. Huguet and V. Nikonenko, Physicochemical and electrochemical characterization of Nafion-type membranes with embedded silica nanoparticles: Effect of functionalization, Electrochimica Acta, 2021, 370, 137689 CrossRef CAS.
- A. E. E. Mohamednour, N. A. H. M. Nordin, M. R. Bilad, S. N. A. Shafie, S. M. Hizam and N. I. M. Nawi, Quantifying the impact of silica hydrophilicity and loading on membrane surface properties through response surface methodology, J. Mater. Sci., 2023, 58(35), 13974–13993 CrossRef CAS.
- N. Pal and M. Agarwal, Advances in materials process and separation mechanism of the membrane towards hydrogen separation, Int. J. Hydrogen Energy, 2021, 46(53), 27062–27087 CrossRef CAS.
- M. Mohapi, J. S. Sefadi, M. J. Mochane, S. I. Magagula and K. Lebelo, Effect of LDHs and other clays on polymer composite in adsorptive removal of contaminants: a review, Crystals, 2020, 10(11), 957 CrossRef CAS.
- S. Morariu and M. Teodorescu, LAPONITE®–A versatile component in hybrid materials for biomedical applications, Mem. Sci. Sect. Romanian Acad., 2020, 43, 141–155 Search PubMed.
- M. Dor, Y. Levi-Kalisman, R. J. Day-Stirrat, Y. Mishael and S. Emmanuel, Assembly of clay mineral platelets, tactoids, and aggregates: Effect of mineral structure and solution salinity, J. Colloid Interface Sci., 2020, 566, 163–170 CrossRef CAS PubMed.
- N. Shaari and S. K. Kamarudin, Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview, Int. J. Energy Res., 2019, 43(7), 2756–2794 CrossRef CAS.
- S. He, H. Jia, Y. Lin, H. Qian and J. Lin, Effect of clay modification on the structure and properties of sulfonated poly (ether ether ketone)/clay nanocomposites, Polym. Compos., 2016, 37(9), 2632–2638 CrossRef CAS.
- M. Chen, C. Zhao, F. Sun, J. Fan, H. Li and H. Wang, Research progress of catalyst layer and interlayer interface structures in membrane electrode assembly (MEA) for proton exchange membrane fuel cell (PEMFC) system, ETransportation, 2020, 5, 100075 CrossRef.
- A. Abyzov, Aluminum oxide and alumina ceramics (review). Part 1. Properties of Al 2 O 3 and commercial production of dispersed Al 2 O 3, Refract. Ind. Ceram., 2019, 60, 24–32 CrossRef.
- F. Liu, P. Dong, W. Lu and K. Sun, On formation of AlOC bonds at aluminum/polyamide joint interface, Appl. Surf. Sci., 2019, 466, 202–209 CrossRef CAS.
- A. Kamal, M. Ashmawy, A. M. Algazzar and A. H. Elsheikh, Fabrication techniques of polymeric nanocomposites: A comprehensive review, Proc. Inst. Mech. Eng., Part C: J. Mech. Eng. Sci., 2022, 236(9), 4843–4861 CrossRef CAS.
- V. Unnikrishnan, O. Zabihi, M. Ahmadi, Q. Li, P. Blanchard, A. Kiziltas and M. Naebe, Metal–organic framework structure–property relationships for high-performance multifunctional polymer nanocomposite applications, J. Mater. Chem. A, 2021, 9(8), 4348–4378 RSC.
- S. Yang, V. V. Karve, A. Justin, I. Kochetygov, J. Espin, M. Asgari, O. Trukhina, D. T. Sun, L. Peng and W. L. Queen, Enhancing MOF performance through the introduction of polymer guests, Coord. Chem. Rev., 2021, 427, 213525 CrossRef CAS.
- H. Nabipour, X. Wang, L. Song and Y. Hu, Metal-organic frameworks for flame retardant polymers application: A critical review, Composites, Part A, 2020, 139, 106113 CrossRef CAS.
- Z. Zheng, Z. Rong, H. L. Nguyen and O. M. Yaghi, Structural Chemistry of Zeolitic Imidazolate Frameworks, Inorg. Chem., 2023, 62(51), 20861–20873 CrossRef CAS PubMed.
- M. A. Little and A. I. Cooper, The chemistry of porous organic molecular materials, Adv. Funct. Mater., 2020, 30(41), 1909842 CrossRef CAS.
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. Mizrahi Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, MOF-based membranes for gas separations, Chem. Rev., 2020, 120(16), 8161–8266 CrossRef CAS PubMed.
- F. Yang, J. Wu, X. Zhu, T. Ge and R. Wang, Enhanced stability and hydrophobicity of LiX@ ZIF-8 composite synthesized environmental friendly for CO2 capture in highly humid flue gas, Chem. Eng. J., 2021, 410, 128322 CrossRef CAS.
- H. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang and B. Chen, Porous metal-organic frameworks for gas storage and separation: Status and challenges, EnergyChem, 2019, 1(1), 100006 CrossRef PubMed.
- J. Yang and Y. W. Yang, Metal–organic frameworks for biomedical applications, Small, 2020, 16(10), 1906846 CrossRef CAS PubMed.
- D. Li, H.-Q. Xu, L. Jiao and H.-L. Jiang, Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities, EnergyChem, 2019, 1(1), 100005 CrossRef.
- V. Siva and A. Murugan, A. samad Shameem, S. Athimoolam, and S.A. Bahadur, A new metal-organic hybrid material: Synthesis, structural, electro-optical properties and quantum chemical investigation, Opt. Mater., 2021, 121, 111616 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |