Open Access Article
Open Access ArticleRed tides in the Galician rías: historical overview, ecological impact, and future monitoring strategies†
Rodríguez
F.
 *ab,
Escalera
L.
*ab,
Escalera
L.
 a,
Reguera
B.
a,
Nogueira
E.
a,
Reguera
B.
a,
Nogueira
E.
 a,
Bode
A.
a,
Bode
A.
 c,
Ruiz-Villarreal
M.
c,
Ruiz-Villarreal
M.
 c,
Rossignoli
A. E.
d,
Ben-Gigirey
B.
c,
Rossignoli
A. E.
d,
Ben-Gigirey
B.
 b,
Rey
V.
b and
Fraga
S.
e
b,
Rey
V.
b and
Fraga
S.
e
aCentro Nacional Instituto Español de Oceanografía (IEO-CSIC), Centro Oceanográfico de Vigo (COV), 36390 Vigo, Spain. E-mail: francisco.rodriguez@ieo.csic.es
bEuropean Union Reference Laboratory for Monitoring of Marine Biotoxins (AESAN), Citexvi, Campus Universitario de Vigo, 36310 Vigo, Spain
cCentro Nacional Instituto Español de Oceanografía (IEO-CSIC), Centro Oceanográfico de A Coruña (COAC), 15001 A Coruña, Spain
dCentro de Investigacións Mariñas (CIMA), 36620 Vilanova de Arousa, Spain
ePraza Mestra Manuela 1, 36340 Nigrán, Spain
First published on 7th November 2023
Abstract
The Galician rías (NW Iberia, Spain) are coastal embayments at the northern boundary of the Canary Current upwelling system. Their favourable conditions for phytoplankton growth turn them into a suitable area for the development of aquaculture activities and a site of most of the national shellfish production. Phytoplankton blooms, a natural phenomenon inside the rías, under certain conditions eventually lead to seawater discolourations (colloquially known as “red tides”). Because of their transient nature, available records derive mainly from opportunistic samplings or casual observations, and are scattered in the literature. As a rule of thumb, red tides in the NW Iberian Peninsula are of non-toxic nature and are not systematically monitored. However, in recent years striking exceptions such as those of the toxic dinoflagellate Alexandrium minutum, a producer of paralytic shellfish toxins, have been registered. The present study goes through a historical overview of red tides in the Galician rías, describing their colouring, responsible organisms, seasonal and geographical occurrence, and their association with other features (harmful algal blooms, biotoxins and shellfish harvesting closures, bioluminescence, etc.), ending with social challenges and proposals for improving the monitoring of red tides in the future.
Environmental significanceHigh biomass microalgal blooms causing seawater discolorations (also termed “red tides”) are often perceived as a potential threat for human health and coastal resources. Knowledge about their nature and consequences appears particularly relevant in productive coastal areas with intense aquaculture and other human activities. This review compiles the available knowledge on these events in an upwelling coastal system (NW Iberia, Spain), detailing the responsible organisms and their effects, which can be generalised to other similar areas in the world. |
1 Introduction
High biomass phytoplankton blooms (>106 cells L−1) are the base of marine food webs and support of pelagic fisheries.1 Some of these blooms are formed by flagellates which may aggregate at the sea surface and cause water discolourations known as red tides. These are typically dominated by a single species and despite the “red” term their colour and appearance are diverse and depend on the pigment composition and abundance of the phytoplankton species.2 Red tides are global phenomena in open ocean and nearshore waters, reported in all kinds of marine systems from equatorial to polar areas (e.g.3–7). Most red tides are harmless, but some may eventually become high biomass harmful algal blooms (HABs). These, conceived as any microalgal proliferation posing a threat to human health and coastal commodities, involve a large variety of microalgal genus/species with different environmental requirements and noxious effects.8 Harmfulness is not an intrinsic character of a species and will be largely determined by the magnitude of the accumulated biomass, the site-specific coastal morphology, hydrodynamics and the exposed resources. For example, blooms of spiny silicoflagellates and diatoms may cause gill damage to wild and farmed salmon.9 High density blooms of practically any species may become noxious if (i) grazing pressures are low and (ii) abrupt environmental changes in physico-chemical conditions (hypoxia, hyperoxygenation, and mucilage secretion) take place from microalgal metabolic processes.10 An exemplary case is the dinoflagellate Noctiluca scintillans, one of the most widespread and cited red tide species.3Harmless red tides of N. scintillans in well flushed systems may cause social alarm if public education on this issue is lacking. The fate of an event of the same magnitude in enclosed systems with aquaculture activities will have more serious consequences, e.g. fish mortality, if large amounts of ammonium from decaying cell vacuoles are released in the water.11
Some microalgal species produce potent toxins which are accumulated by filter feeding bivalves and transferred through the food web. Even at low cell densities (102–104 cells L−1) they are responsible for shellfish poisoning syndromes (amnesic (ASP), diarrhetic (DSP), paralytic (PSP), and neurotoxic (NSP) shellfish poisoning) affecting consumers of the contaminated shellfish. These low biomass HABs of toxic species cause lengthy harvesting bans and are among the most damaging for the shellfish industry. In the popular language any HAB is designated as a red tide, but here the term will be used in its strict sense, i.e., discoloured waters due to high densities of phytoplankton (∼106 cells L−1).
The Northwestern Iberian coast (hereinafter referred to as “NW Iberia”) (42–43°N) lies at the northern limit of the Canary Current upwelling system12–14 (Fig. 1). Upwelling in NW Iberia is seasonal due to the latitudinal shift of the Azores High-Icelandic Low pressure system.15 During spring-summer, the predominance of northeasterly winds promotes upwelling of cold, salty and nutrient-rich Eastern North Atlantic Central Water (ENACW) on the shelf. In contrast, the predominance of southwesterly winds during autumn–winter promotes downwelling. There, several flooded tectonic valleys known as “rías” are sites of intensive shellfish aquaculture, which coexist with port facilities (shipyards, overseas and artisanal fisheries) and the tourism industry.16–18 The development and composition of phytoplankton populations in the Galician rías are strongly influenced by climatic and meteorological conditions, with a prominent role of the seasonal variability of wind patterns. In fact, wind-driven circulation combined with site-specific coastal morphology has been identified as the major abiotic factor behind the alternance between different oceanographical regimes.19–22 Hydrodynamically, the rías behave as partially mixed embayments with a positive two-layered residual circulation pattern (outflow at the surface and inflow at the bottom layer). This pattern is reinforced under the spin-up phase of upwelling pulses but may be reversed under downwelling.23,24 Upwelling, nutrient runoff and remineralisation25–28 at the sediment–water interface within the rías and adjacent shelf (nutrient-trap29,30) support a high productivity at different trophic levels.
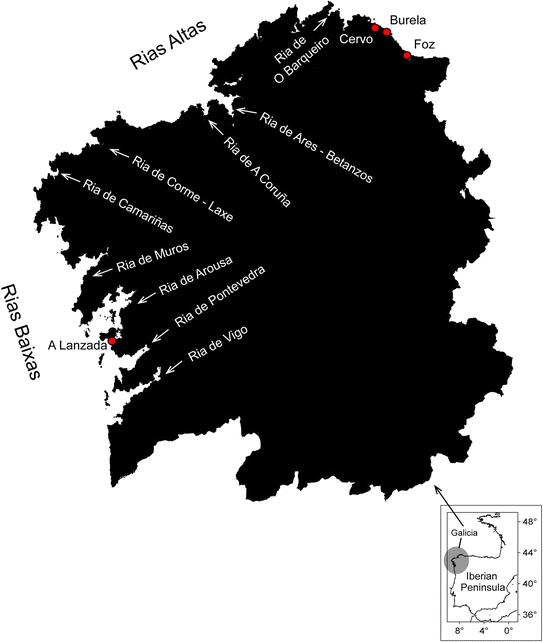 | ||
| Fig. 1 Map of NW Iberian Peninsula, Spain, including the name of the Galician rías and other coastal locations (red dots), where red tide events have been reported (full details provided in ESI Table S1†). | ||
Privileged natural conditions of the Galician rías allowed the initiation and expansion, in the second part of the 20th century, of a vigorous shellfish industry (mainly raft-cultivated mussels, but also oyster farming; cockle and scallop cultivation on the seabed and raised intertidal beds for oyster growth), which nowadays accounts for most of the national, around 50% of the European and 11% of the world mussel's production.31,32
The oceanography and plankton communities in the rías have been thoroughly characterised since the 1970s,30,33–35 after a landmark event in 1976. That year, a PSP outbreak affected over 176 people from several European countries, after eating mussels traced back to the southern Galician rías (the “Rías Baixas”). The impact of this episode, which crossed international borders and caused significant losses to the shellfish industry, prompted the urgent establishment of a monitoring programme. Plankton-borne shellfish intoxications were probably a cryptic sanitary problem before the 1970s, but the 1976 outbreak made health and fisheries policy-makers aware of the problem. Since then, very few human intoxications have been reported (or at least known and published in the literature). Weekly controls at regional monitoring programmes were carried out first by the Spanish Oceanographic Institute, and since the 1990s by the Technological Institute for the Control of the Marine Environment of Galicia (INTECMAR, Xunta de Galicia)36 contributed to it. All cases of human illness (according to mass mediaa,b and the scientific literature37,38) were caused by seafood consumption outside official safety controls.
As a rule of thumb, red tides in Galicia are harmless and toxic events are caused by low biomass blooms of toxic dinoflagellates. Exceptionally, some of these have formed toxic red tides (see Section 2.4).
The triggering factors of different HAB events are species and site specific, and a multidisciplinary approach is required to unveil the mechanisms underlying bloom development, maintenance, advection and decay. In that sense, a large body of knowledge on the characterization and population dynamics of toxic species in the rías has been generated in the last few decades.21,28,39–41
Low biomass HABs of different shellfish-toxin producers are the main threat to shellfish exploitation in the rías. Dinoflagellates from the genus Dinophysis responsible for DSP are the most frequent issue. Endemic blooms (102 < 105 cell L−1) of D. acuminata are coupled to the upwelling season (spring-summer) and start as soon as spring thermohaline stratification develops.42 Other species, e.g. D. acuta, with a shorter seasonality (late summer) may contribute to the DSP outbreaks in years with positive temperature anomalies during dry summers.43
Other toxins related to local shellfish poisonings, such as ASP and PSP, are associated respectively with: (i) diatoms of the genus Pseudo-nitzschia (INTECMAR, http://www.intecmar.gal/ and (ii) dinoflagellates (Gymnodinium catenatum and Alexandrium minutum). Blooms of Pseudo-nitzschia spp. may reach high densities (>106 cells L−1) in late spring in subsurface layers. These diatoms are known to form cryptic thin layers in the pycnocline region which often escape detection with conventional bottle sampling .27 Finally, it is worth noting that ichthyotoxic microalgal blooms have seldom been reported in Galicia,44,45 though a recent review suggests these could represent frequent and unexplored local events.45
All toxic species/genera listed above, with a predominance of dinoflagellates, grow within different environmental windows between spring and autumn. Exchanges between rías and shelf waters are the main physical control for the dilution (with upwelling) or accumulation (downwelling) into the rías, except A. minutum (with preference for small embayments with freshwater inputs).19 Wind reversals to downwelling circulation advects surface shelf waters into the rías; downward currents push diatoms down and select dinoflagellates resulting in a risk scenario for initiation or intensification of toxic HABs.
Persistent downwelling at the end of the upwelling season (autumn) leads to maximal accumulation and dominance of dinoflagellates, enriched by additional alongshore transport of southern HAB populations. This mechanism of autumn HAB formation, equivocally called “the red tide assemblage”,14 led to the most dramatic toxic outbreak in the Galician Rías Baixas in 1976. Persistent downwelling is a well-known mechanism that generates red tides in other regions like the coastal waters off Callao (Perú)46 and Walvis Bay (Namibia), both sites of permanent upwelling located at the core of the Humboldt and Benguela systems, respectively. Dinoflagellate blooms develop in these systems during late summer and early autumn, with massive red tides (>107 cells L−1; up to 100 µg Chl a L−1) following accumulation near the coast under downwelling conditions.47 Persistent downwelling leads to bloom decay and low-oxygen events that kill fish and benthic organisms,48 affecting fisheries recruitment (reported since the early 2000s49). Degradation of the accumulated debris in anoxic sediments by sulphur-oxidising bacteria and production of poisonous hydrogen sulphide and methane gases over extensive shelf areas may follow.50,51 The blackening effects of hydrogen sulphide on the ships' paintwork are colloquially known in Perú as “El Pintor de Callao” (The Callao Painter). In contrast, harmless concentrations (2–3 orders of magnitude lower) of Tripos furca, T. fusus and other large dinoflagellates are observed under the same physical process in coastal areas, such as NW Iberia, where upwelling is seasonal.43,52,53
In this review a comprehensive overview of red tides in Galicia is presented to address the following questions: (i) how many red tides (and which types) have been recorded? (ii) which are the responsible groups/species? (iii) have red tides been increasing in recent years? (iv) do they cause social alarm (i.e. harmful vs. non-harmful episodes)? and if so, (v) which actions could be proposed to reduce this alarm?
To that aim, we reviewed the available literature on red tides both in scientific and public forums from the pioneering studies in the early 20th century until present. Then, available records of red tides have been listed based on their colours, the responsible organisms and their seasonal and geographical occurrence. Finally, their association with any other features and impacts (HABs, biotoxin production and shellfish harvesting closures, bioluminescence, etc.) is detailed, ending with social challenges and proposals for the future.
2 Red tides in Galicia: historic overview
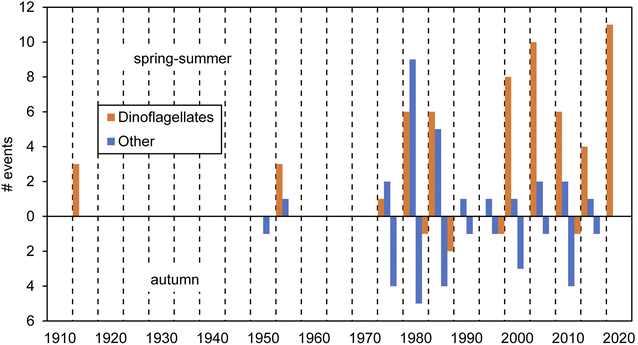
Red tides in Galicia have been known in the literature since the beginning of the 20th century. These events have been caused, to our knowledge, by at least 23 planktonic species (mostly dinoflagellates; Table 1). The available data on cell abundances show that red tides are often associated with densities around 106 cells L−1. Only N. scintillans, considerably larger than any other species in Table 1, causes red tides with numbers down to 104 cells L−1. Overall, we compiled a total of 112 red tide observations based on multiple sources: scientific literature, newspapers and other reported information, as detailed in ESI Table S1.† Past events and causative organisms were confirmed by various sources when available and recent events were backed by laboratory observations. Two thirds of those red tides were reported for the spring-summer season and the rest in the autumn (Fig. 2). Considering the qualitative nature of the dataset, the frequency of red tide occurrence in the last few decades seems constant, with records between 1980s–1990s vs. 2000s–2010s pretty similar. However, a relative augmentation in those associated with dinoflagellates vs. other groups has been noticed since the 1980s. In the next sections we detail the information about these events across different historical periods.| Group | Organism | Red tide concentrations (cells l−1) | |
|---|---|---|---|
| NW Iberian Peninsula | Other areas | ||
| a Margalef (1956). b Crawford (1989). c Wagey et al. (1998). d Nogueira et al. (2022). e Sacilotto et al. (2023). f Gómez-Fermín et al. (1996). g Graham (1943). h Crespo et al. (2007). i Figueroa et al. (2009). j Rodríguez F. (pers.obs., Miñor River, RV, 2023). k Jiménez et al. (1992). l Arévalo et al. (2006). m Harrison et al. (2011). n Khan et al. (1997). o Prego et al. (1998). p Sweeney (1975). q Sansón et al. (2005). r Walker & Pitcher (1991). s Lee et al. (1998). t Figueiras & Niell (1987). u Kopuz et al. (2014). v F. Rodríguez (pers. obs., Areamilla, Cangas 2021). | |||
| Ciliates | Mesodinium rubrum | Up to 106a | Up to 106b |
| Dinoflagellates | Alexandrium affine | — | 2 × 106c |
| A. minutum | 9–166 × 106d | — | |
| A. tamarense | 1 × 106e | — | |
| Amphidinium sp. | — | — | |
| Gonyaulax diacantha | 5 × 106a | — | |
| G. cf. polygramma | — | 3.4 × 106p | |
| G. spinifera | 3 × 106a | — | |
| Gymnodinium catenatum | 2.9 × 108f | Up to 106g | |
| Karenia cf. mikimotoi | 3 × 106h | — | |
| Kryptoperidinium triquetrum | 1.5 × 105i; 2.6 × 106j | — | |
| Lepidodinium chlorophorum | 70 × 106k | — | |
| Lingulodinium polyedra | 2.2 × 106a; 2.1 × 106l | 1.4–22.2 × 106p | |
| Noctiluca scintillans | 7.3 × 106v | 4.8 × 104–3 × 106m; 6.8× 106u | |
| Prorocentrum cordatum | 10.6 × 106h | — | |
| P. micans | 0.8–12 × 106p | 4.7 × 106s | |
| P. rostratum | — | — | |
| P. triestinum | — | — | |
| Tripos furca | — | 1.3 × 106r;4 × 106s | |
| Euglenophytes | Eutreptiella sp. | — | 1.8–3 × 106q |
| Raphidophytes | Heterosigma akashiwo | 4 × 107t | 12 × 107n |
| Silicoflagellates | Apedinella sp. | — | — |
| Dictyocha speculum | 2 × 105o | — | |
2.1. Early 20th century
In the beginning of the past century, Ramón Sobrino mentioned that red tides were not recent phenomena but natural events known from remote times.54 Seawater discolourations were appreciated by fishermen, who historically associated reddish waters with renewal of sea richness and natural seasonal cycles, similar to those in terrestrial environments, providing abundant food for fish, in particular for the precious sardines.cIn fact, the oldest description that could fit an account of red tides in the region appeared in an 18th century treaty about sardine fishing in Galicia:
“The sardine is one of those fish that move in schools, not feeding on the mud washed away by rivers and deposited in the bottom of the Rías, but with the unctuous part from these waters coming from terrestrial salts that floats and overruns […]. These gross and unctuous particles are found in abundance in the inlets and shelters of the Rías, which are like backwaters where torrents lay down the substance they brought from the lands, and that they cannot carry outside the coast”. [Translated from the original in Spanish].55
Cornide55 did not mention seawater discolouration explicitly and may have referred to any kind of suspended matter, but his words cited a natural periodic phenomenon that could well describe the accumulation of phytoplankton in sheltered areas. In fact, a link between sardines and red tides was hypothesised by Sobrino,54 as explained afterwards.
The earliest plankton reports in the region were elaborated by pioneer naturalists. The first unambiguous mention of red tides in Galicia dates from 1903 by Dr Roque Carús Falcón, a naturalist from Vilagarcía de Arousa, author of the first dinoflagellate illustrations in Spain.56 His work “Los misterios de la Naturaleza, investigaciones sobre el micro-plankton de la Ría de Arosa” (The mysteries of Nature, investigations about the micro-plankton in the Ría de Arosa) was an amateur report published in a context of remarkable scientific delay in Spain.57 There, he discussed about marine cyanobacteria (“Oscilariaceae” in the original), of the genus Trichodesmium:
“…that at certain times of the year, especially in late spring, appears in large masses like sawdust over vast surfaces of the sea, constituting the aquatic inflorescences, of a reddish-brown colour, which can also be caused by nodularia, rivularia (sic) and other genera. Here we frequently see a form that appears to be of the aforementioned genus, in groups of usually ashen filaments, straight and of unequal sizes, like bundles of blunt reeds.” [Translated from the original in Spanish].57
By the 1910s little or nothing was known about red tides in Galicia and when a recently built pier in the port of Vigo collapsed in 1915, red tides were suspected as a causative agent.58 Eduardo Cabello (engineer director of the Board of Public Works of Vigo Port Authorities) took advantage of the fact that in summer 1916 the Oceanographic Commission had been established in Vigo (chaired by the naturalist Odón de Buen, to carry out marine studies in the Ría de Vigo), to ask for data that could contribute to explain the collapse. Odón de Buen, founding Director of the Spanish Institute of Oceanography (IEO) in 1914,59 organised the first oceanographic cruise in Galicia (on board the Hernán Cortés, an old gunboat of the Spanish Navy). He welcomed with great interest Cabello's request with the following questions:
“(1) Analytical chemical composition, the most complete possible, of the sea water, taken in different places of this bay. (2) Origin of the phenomenon of the red seawater discolouration in the final days of the year, with such an intensity, at times and places, that makes the divers' work impossible. A phenomenon known in the locality with the name of “the sea purge”, without scientific studies until the date to determine its origin. (3) If the same colour the mud has in certain places of the bay is due to the same cause.” [Translated from the original in Spanish].58
During that first cruise they found a red tide that Odón de Buen described as follows:
“During this summer we have had the opportunity to study the reddish mass that, on certain days, invades the lower estuaries, colouring the water ochre in large areas […] The phenomenon is very interesting, it was brought to attention years ago […] The reddish water appears as if full of a faint dust; as if it had in suspension a powdered mineral […] Fishermen say that the sea is purging and relate that coloured mass to emigrations and even to the procreation of the sardine.” [Translated from the original in Spanish].60
A few lines later, de Buen offered this explanation:
“At first glance, one could believe that it was a microscopic algae similar to the one that colours the waters of the Red Sea or the one that has been observed in other areas of the Atlantic; the microscope has revealed that the author of that ochraceous colour is a proto-organism of the group of radiolarians, tiny animals of temperate seas […] On warm days their production is enormous in the open sea, and the tides and currents take them to the estuaries, penetrate […] and accumulate in the inner part, where they die; their organic matter rots and communicates the putrefaction to a large number of other plankton beings, which are mixed with the radiolarians […] Such intense masses of radiolarians have not, until now, been reported in the seas of Europe”.60
The conclusions of these samplings were that radiolarians were the cause of the red tide in 1916, and also, that the sedimentation of their proliferations gave rise to putrid sludge that released hydrogen sulphide gas (responsible for the bad smell in the waters when the organisms are in the water column). Odón de Buen pointed out that this would be the chemical agent causing the decomposition of the cement in the transversal pier, due to the formation of lime sulphate when it reacts with seawater, as referred later by Cabello.61
At present we know that red tides do not collapse infrastructures and the likely cause of the pier collapse was a cement defect; this curious episode should be seen with benevolence due to the historical circumstances. In fact, the importance given by the authorities to the scientific results of that cruise facilitated in 1917 the foundation of the headquarters of the Spanish Oceanographic Institute in Vigo.
After the findings about red tides by Odón de Buen, a series of articles were published in newspapers and magazines referring to this subject. Shortly before, Ramón Sobrino had also published an article in the local press: “Curious Phenomenon. The colour of the Estuary”.62 There, Sobrino reported the results of his own observations on seawater samples (supposedly) from a red tide that someone had provided him. In addition, he identified the haptophyte Phaeocystis pouchetii as the cause of the discolouration (“the sea purge”) and Noctiluca miliaris (=N. scintillans) as responsible for the “burning sea” that usually accompanied the red tides.
Therefore, the radiolarians mentioned by de Buen aroused the curiosity of Sobrino, who wished to contrast the different conclusions he had reached before. A year after, in summer 1917, Sobrino got himself samples from a red tide in the port of Marín (Ría de Pontevedra). Under the light microscope he identified unambiguously the dinoflagellate Gonyaulax polyedra (=Lingulodinium polyedra). His morphological description was very complete and illustrated54 (Fig. 3), while that of de Buen did not show any graphic evidence about radiolarians. Sobrino also mentioned the emission of light when samples were collected at night and shaken.
 | ||
| Fig. 3 Illustration of Lingulodinium polyedra in Sobrino (1918) and painting of its red tide in 1917 in the Ría de Pontevedra (by his brother, Carlos Sobrino). | ||
At that time, it was already known that bioluminescence was produced by multiple species of dinoflagellates, corroborating these organisms as responsible for both red tides (=“the sea purge”) and bioluminescence (=“burning sea”). Regarding the local expression of “the sea purge”, Sobrino54 explained its origins too:
“Sailors and other people of the sea usually say that the sea is purging, when a curious phenomenon occurs […] which consists of the colour, more or less reddish or bloody, that suddenly appears in the water […] some sailors believe that this unique marine phenomenon is a need that the sea feels to clean itself, in order to later produce its inexhaustible richness…”.
Sailors took advantage too of the “burning sea” (popularly known as “mar de ardora” or “ardentía”) to track fish as described below:
“[…] the sailors who are dedicated to sardine fishing use to fish “a la ardora”; a procedure that consists of causing clupeids to pack or entangle into the net towards which they are directed producing noises from the edge, after their presence in the waters is betrayed by a sudden phosphorescence produced in the surface layers while swimming confusedly.”
Such a procedure was known from ancient times as discussed in the dictionary of fishing gear by Rodríguez-Santamaría.63 Sobrino54 speculated and suggested the hypothesis of a link between winds, red tides and the local summer sardine sales. In his favour, Sobrino went back to 1886 when Albert I Prince of Monaco visited A Coruña, in the northern Rías Altas, interested in sardine fishing in Galicia. In those years, the sardine stock in French waters collapsed, and Albert I of Monaco wrote:64
“…for ten to fifteen years the progressive decline of the sardine on those coasts [western France] has led to fear of its disappearance […] Concerned about this question […] in 1886 during my scientific campaign in “L'Hirondelle”, I stopped in A Coruña, the most active centre of sardine landings in Spain. I thought I would get some information, useful examples or at least as much study material as possible, for delivery to competent hands”.
Prince Albert I described that on August 19 1886, the night was dark; they were sailing near the coast of A Coruña when they observed “clouds” of phosphorescence in the sea. The next morning the docks showed a frenetic activity and a small group of boats at the entrance of the harbour informed them:
“…the previous night a shoal of sardines had arrived causing the mysterious lights [bioluminescence] that had been glimpsed from the boat. They had witnessed, without knowing it, the capture of that “living manna”.
These sentences represent the first mention in Galicia, to the best of our knowledge, about bioluminescence. The Prince's expedition obtained viscera and whole sardines from that fishery. Pouchet and de Guerne65 analysed them and described the following: “The main interest of the viscera from A Coruña lies in the extraordinary abundance of peridineans that fill them. They belong to two main types: Peridinium divergens and P. polyedricum.” (=Protoperidinium divergens and L. polyedra respectively).
They calculated a minimum of 20 × 106 dinoflagellates in the intestine of each sardine concluding that, according to the circumstances, sardines had a mixed diet including copepods, eggs of small crustaceans, diatoms, etc. Sobrino focused mainly on the dinoflagellates to elaborate his hypothesis of a potential relationship between red tides and sardines. Phytoplankton is the basis of marine ecosystems and red tides are a phenomenon coinciding with sardine catches in summer, but Sobrino54 believed in more than just an “accidental coincidence”. This confusion was remarked by Fernando de Buen,66 son of Odón de Buen, who took a kind of “scientific revenge” after the lost dispute by his father with Sobrino about red tides. Nevertheless, in spite of being barely thirty, Sobrino was “the first Spanish author who wrote sensibly about it [red tides]”, according to the words of our next protagonist.33
2.2. Margalef's studies in the 1950s
The development of modern oceanography studies in the rías, including phytoplankton, did not start until the 1950s. The harsh socio-economic and political context in Spain after the civil war, with poor means and scarce funding for science in general, and the practical absence of aquaculture activities other than traditional fisheries until the 1960s and 1970s would be the main reasons.Then, in the 1950s the oceanographer Ramón Margalef, following a brief note,67 published what can be considered the first systematic studies of their kind in Galicia, focused on the phytoplankton from the Ría de Vigo.33,40,68,69 Among those, based on summer samplings of red tides in August and September 1955, he described the oceanography and the organisms associated with red tides.40 Margalef found that the still abundant dinoflagellate Gonyaulax polyedra (=L. polyedra) was not the single causative agent of red tides. He also pointed out other dinoflagellates, such as Gonyaulax diacantha, G. spinifera, Ceratium furca (=Tripos furca), as well as the ciliate Mesodinium, as responsible for these events. In that work, dinoflagellate concentrations in red waters ranged between 5 and 8 × 106 cells L−1.
Margalef observed that red tides produced by dinoflagellates varied from “rusty”, “olive” tones to more “bloody” ones in the case of L. polyedra. The most vivid red-coloured patches were those produced by the ciliate Mesodinium which he qualified as “degenerate”. “Don't think badly of the ciliate”, he used to write this expression because those red tides were associated with the final stages of the microplankton succession he described. Regarding the examination of the water discolouration, Margalef40 mentioned:
“…sea surface properties seem to be different in and outside the patches […] It looks smoother (lower surface tension?) and frequently accumulates foam […] following the patches from a boat is like trying to map a set of clouds crossing it in an aeroplane […] In general terms, it can be said that they only affect the upper 5 metres and, possibly, the lower limit of most patches coincides with the greatest thermal discontinuity layer […] which is between 2 and 3 metres deep.” [Translated from the original in Spanish].
A previous study33 reported for the first time the planktonic succession in the Galician Rías. These authors stated a three-stage process of around 3 months. In the first stage, small diatoms appeared, followed by larger diatoms with some dinoflagellates, and eventually in the third stage of the succession, there was a predominance of dinoflagellates, which was also a function of temperature and manifested more strongly in summer. Red tides would be the culmination of the third stage (dominance of dinoflagellates), provided by the water column stability in the studied Ría de Vigo that was maintained for an abnormally long period (at least 15 days). Regarding the expression “purge of the sea”, Margalef40 commented: “…we prefer the name ‘hematothalasia’ introduced by Sobrino” [Translated from the original in Spanish].
2.3. Modern times: from the 1976 paralytic shellfish poisoning (PSP) outbreak to date
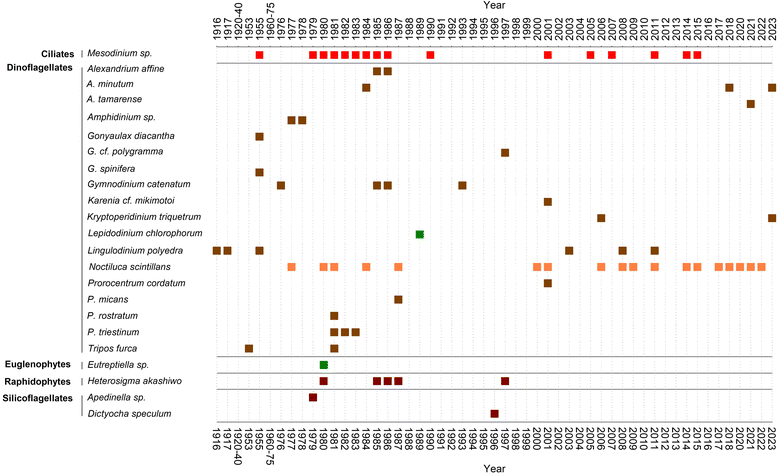
In 1976 a bloom of the dinoflagellate G. catenatum, accompanied by reddish-brown discolouration, was the suspected agent of a paralytic shellfish poisoning outbreak that affected mussel consumers in Spain and other European countries.70 The socioeconomic impact of this toxic red tide prompted the establishment of a marine biotoxins monitoring system in parallel with an intensification of harmful phytoplankton studies in the area.71,72 The increased sampling efforts served as a stepping-stone to gather new and more focused information about red tide organisms. In this line, Fraga73 compiled the only published summary, to our knowledge, about red tides in the region between 1916 and 1987.Fraga's list was updated in this review with further events until 2023 (ESI Table S1†). The list of causative organisms and their temporal distribution are summarised in Fig. 4. In addition to dinoflagellates, the list includes a diversity of protists from other groups, such as euglenophytes (Eutreptiella), silicoflagellates (Apedinella), raphidophytes (Heterosigma akashiwo), and above all the ciliate genus Mesodinium. To the best of our knowledge, none of these blooms were associated with public health issues, harm to marine organisms and/or poor environmental quality. Among the dinoflagellates, Prorocentrum (P. triestinum and P. rostratum), Alexandrium affine and Gonyaulax (G. spinifera and G. diacantha) stood out until the 1990s as the species commonly associated with red tides.
It is worth drawing attention to the fact that practically none of the dinoflagellates mentioned as red tide agents by Margalef in the 1950s caused further red tides in the subsequent decades. The exception is L. polyedra though its blooms have been reported from the Rías Altas74 (north of Cape Finisterre) but are no longer observed in the Rías Baixas of Vigo or Pontevedra.
Regarding dinoflagellate non-toxic red tides, Jiménez75 described a water discolouration in Ría de Pontevedra and the causative agent tentatively classified as Gyrodinium aureolum. However, a careful examination of their results, with a detailed morphological characterisation and the remark of an unusual emerald-green colour, agrees with those in recent reports of Lepidodinium chlorophorum from, e.g., the Bay of Biscay.76
In some inner areas of the Galician Rías, freshwater discharges at the head of these embayments promote recurrent dinoflagellate blooms. That was the case with brown water discolourations by the estuary of the Miñor River (that flows into Baiona Bay, Ría de Vigo), caused by Kryptoperidinium triquetrum77 (formerly K. foliaceum) in 2006 (ref. 78) and 2023 (ESI Table S1†).
Nowadays, predominant red tides appear to be those of the dinoflagellate N. scintillans and the ciliate Mesodinium, mainly in spring and summer months (Fig. 4 and Table S1†). However, given the absence of systematic records, these data are only valid to provide a qualitative account for red tides in the Galician Rías.
The genus Mesodinium represents a case of special interest, since red species from that genus (M. rubrum and M. major) constitute the only known prey supporting sustained growth of Dinophysis spp. in laboratory cultures.41,79 In fact, M. rubrum and M. major represent a species complex with eight subclades.80 During the annual cycle in the Galician rías, both the “medusa” form of M. major and the smaller “M. rubrum-like” cells are observed, suggesting that several genetic subclades coexist in the area (Fig. 5). Some species of the genus Dinophysis with acquired phototrophy (e.g. D. acuminata and D. acuta) produce lipophilic toxins (okadaic acid, dinophysistoxin 2 and their esters). These toxins are accumulated by filter-feeding bivalves and transferred through the food web, causing a gastroenteritis syndrome known as Diarrhetic Shellfish Poisoning (DSP). Dinophysis events, responsible for the most prolonged closures in the extractive activity of the Galician Rías, have been particularly intense over the last two decades.36
However, gastrointestinal disorders due to the consumption of shellfish with DSP toxins seem to have occurred in Spain since ancient times, as emphasised in the following lines about mussels:
“Mijillones [sic], or muscles of the Catalans and Mahoneses, are considered an exquisite seafood, although there is the suspicion that they sometimes produce severe colics, due to their indigestible meat. This symptom, which is true, must not be attributed to the fact that the mijillon is always of poor quality, but to those which cause indigestions due to particular circumstances”.81 [Translated from the original in Spanish].
Dinoflagellates D. acuminata and D. acuta are obligate mixotrophs which perform photosynthesis with plastids stolen (kleptoplastids) from their ciliate prey Mesodinium (M. rubrum and M. major), which in turn steal the chloroplasts of tiny microalgae from the Cryptophyceae group. The temporary chloroplasts or “kleptoplasts” of Dinophysis degrade over time,82 and need to be periodically replaced by new ones through feeding of its specific prey. Therefore, a red tide of Mesodinium represents an important nutritional source for Dinophysis to develop its populations and then, potentially produce a DSP episode. It is important to emphasise that Dinophysis has never produced red tides in Galicia, this being a phenomenon rarely observed in other areas (e.g. southern Brazil).83
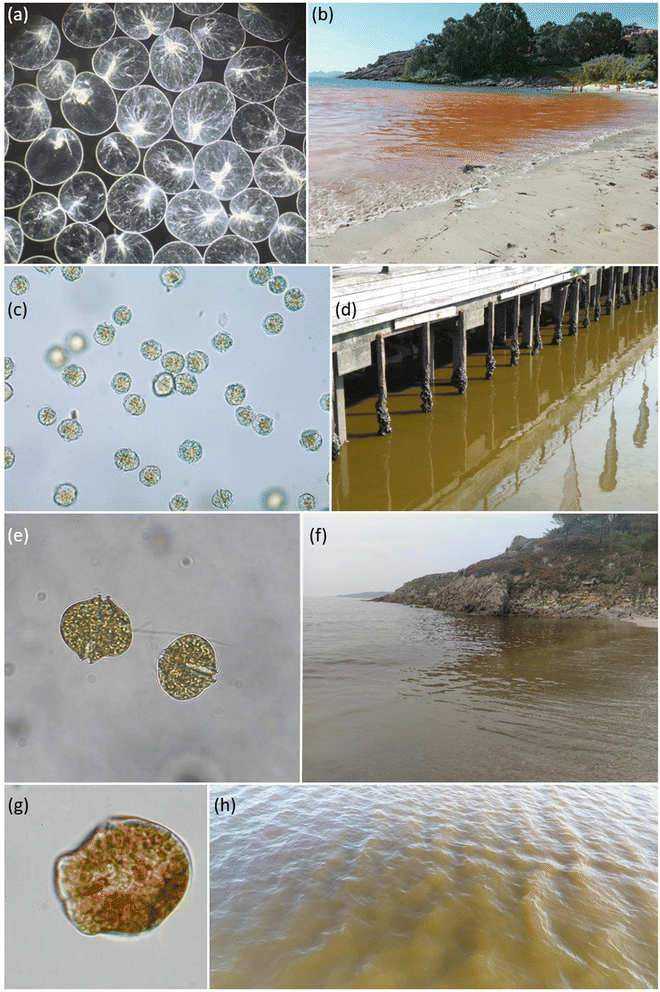
Mesodinium red tides are very common in the rías. One of these happened to occur in July 2007 by the Oceanographic Center of Vigo (IEO-CSIC), in the southern mouth of Ría de Vigo. This red tide was also observed nearby during the following days (Fig. 5). In spring and summer 2011 and 2014, respectively, two new red tides of Mesodinium were observed again from the IEO-CSIC. Red tides of Mesodinium display a vivid red colour, although the perception of its hue varies greatly according to the lighting of the day. Curiously, some decades ago, in the days when the Massó canning and whale factory was still active in Cangas (northern margin of Ría de Vigo), these red tides were misinterpreted as blood from the captured cetaceans.
Currently, Mesodinium species are not specifically monitored around the world except for a few places (e.g. REPHY, the time series from the French national phytoplankton monitoring network).84 The time series from the IEO-CSIC monthly sampling Radiales, which the Oceanographic Center of A Coruña, in the Galician North coast, started in 1989,85 shows that Mesodinium is regularly recorded in shelf waters off A Coruña in moderate concentrations (up to 8000 cells L−1) well below the typical red tide values for this species (Table 1). Likely, cell numbers would be much higher at coastal sites and, particularly, inside the rías.86
Regarding N. scintillans, its red tides have also been very common in the last few decades along the Galician coast, being reported almost every summer (Fig. 4 and ESI Table S1†). Some examples of those are the episodes in 2006 in Ría de Pontevedra, Muros, Arousa and in open beaches from northern Galicia. Also, in Cabo Silleiro (Baiona, Ría de Vigo), in 2008, and many other recent occasions both in the Rías de Pontevedra and Vigo, and further north in the coast of Lugo (Cantabrian Sea) in 2014.
Red tides of N. scintillans are characterised, in Galicia, by an orange colour and oily appearance in surface waters (Fig. 6). Only the red form of N. scintillans occurs in this area, the green variety with a photosynthetic endosymbiont being restricted to warmer seas, e.g. in southeast Asia.3Noctiluca scintillans is a non-toxic heterotrophic dinoflagellate, although its diet can include dinoflagellates that can be toxic and act (at least such possibility exists) as a vector of toxins in the marine food web.87 In addition to its potential role as a toxin vector, the grazing pressure of N. scintillans on toxic microalgal populations should be considered in population dynamic studies of these species, as also suggested by these and other authors. For instance, Frangópulos et al.,88 using N. scintillans grazing rates on A. minutum from Ría de Vigo, indicated its regulatory role in the growth of this PST producing species.
Moreover, hypoxia and ammonia released by declining starved populations in areas with restricted circulation have been associated with stress and increased mortality in wild and caged fish.89 Neither Mesodinium nor N. scintillans poses a risk for beachgoers, although prudence advises not to bathe in the water: some people may suffer from eczema or dermatitis precisely because of the ammonium. In the case of N. scintillans, a bad smell has also been noticed by beachgoers.
This organism is well known for its bioluminescence, and in recent years the reports about red tides and the visual spectacle of this property in summer nights have drawn the attention of the traditional and social media, thanks to the easy capture and distribution of pictures by any citizen equipped with a smartphone. Thus, N. scintillans has become automatically associated with these phenomena in the Galician ías, but it must be noted that in the growth season, especially during summer, dinoflagellate assemblages include several bioluminescent species.
The fact that red tides by N. scintillans are appealing and related to these stronger episodes cannot lead us to forget the fact that traditional local fisheries profited from the “burning sea” produced by the movements of fish schools to localise and capture them during the night. This happens on many occasions during that season, not only when orange patches of N. scintillans are discerned. Nevertheless, to the extent of our knowledge, seawater discolourations associated with bioluminescence caused by other dinoflagellates were unreported until 2021,90 except for the account made by Sobrino54 on L. polyedra.
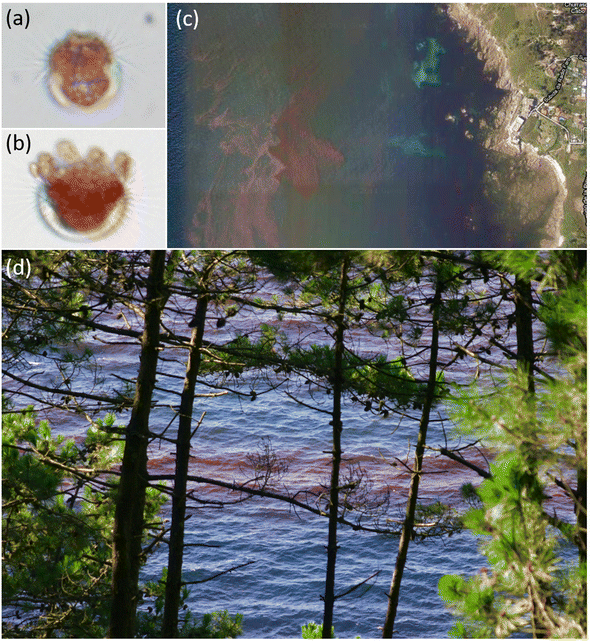
In summer 2021, intense bioluminescence episodes were continuously reported in several beaches in the inner part of the northern Ría de Corme-Laxe. Seawater samples collected by midnight at Rebordelo beach during an astounding bioluminescent episode showed that the microalgal population was dominated by Alexandrium tamarense.90 During daylight, brown patches were also observed in surface seawater (as shown in Fig. 6, also at Rebordelo beach), A. tamarense being the responsible organism for both phenomena. Not a single N. scintillans cell could be observed in the seawater samples collected; secondary species found were A. minutum (non-bioluminescent) with minor contributions of L. polyedra.90
These results provided the first report of bioluminescence due to a bloom of A. tamarense in Galicia. Nevertheless, this is not new in the area. The “mar de ardora” is taken as a common phenomenon by locals (F. Rodríguez pers. obs.). It is very likely that similar bioluminescent episodes are also linked with A. tamarense, at least in neighbouring northern locations such as Carnota (Ría de Muros; F. Rodríguez pers. obs., August 2023) with the largest Galician beach, which also has small riverine inputs beneficial for the seasonal development of populations from this estuarine species. Taking advantage of these bioluminescent episodes, reports of associated red tides are more easily retrieved and facilitate their opportunistic sampling.
2.4. Toxic red tides in Galicia
Regarding the northern Rías Altas, only a toxic red tide in spring 1984 in the Ría de Ares-Betanzos was reported,91 due to A. minutum originally cited as Gonyaulax tamarensis.2 Ever since and until 2018, the only red tides associated with toxic species in the southern Rías Baixas were those of L. polyedra and G. catenatum.Nowadays, L. polyedra are found to reach red tide densities (over 1 × 106 cells L−1) only sporadically in the Rías Altas (Ares-Betanzos), especially during summer.97 That was the case in 2003, when the presence of yessotoxins was associated with L. polyedra for the first time in Galicia.74 Moreover, the abundance of L. polyedra cysts in the sediments of that ría has been known for long.98 In summer 2008 additional red tides of this species were reported in other Galician Rías Altas99 (ESI Table S1†). Despite the intense discolourations caused by L. polyedra in the Rias of Vigo and Pontevedra during the first half of the 20th century,40,54 blooms of this species are no longer reported. Notwithstanding, L. polyedra cysts dominate the extant dinoflagellate cyst assemblages in the inner ría de Vigo.100 Although YTX maxima, associated in Galicia mainly with L. polyedra, have been reported in late summer, especially in the ría de Ares, yessotoxin levels have always been detected well below regulatory levels in the Galician monitoring and no closures of shellfish harvesting areas due to YTX have been necessary.36
Gymnodinium catenatum produces paralytic shellfish toxins (PSTs), a group of toxins which comprises various naturally occurring neurotoxic alkaloids that induce the PSP syndrome in humans.99 PSTs are taken up by plankton feeders, either directly (i.e. bivalve molluscs) or through several trophic levels. The ingestion of PST-contaminated shellfish can cause numbness, tingling of the lips, and in serious cases, death from respiratory arrest and cardiovascular shock. To protect human health EU Regulation (EC) N° 853/2004 (ref. 105) sets maximum PST concentrations of 800 µg STX·diHCl equiv. Kg−1 shellfish meat in bivalve molluscs, echinoderms, tunicates and marine gastropods. The toxin profiles of shellfish samples associated with the 2005 G. catenatum bloom in Galicia were variable and included several or all of the following PSTs: dcGTX2,3, C1,2, dcSTX, GTX5, C3,4, GTX6 and dcNEO.106,107
The PSTs analysis of 45 A. minutum strains isolated from the 2018 red tide110 revealed a toxic profile including gonyautoxins GTX1, GTX2, GTX3 and GTX4, with no other PSTs detected. Preliminary analyses during the red tide of A. minutum in 2023 have rendered an identical profile, with the dominance of GTX1 and GTX4 in field samples (V. Rey, pers. obs.). Within the Alexandrium genera, some species-specific toxin markers were identified for the A. minutum group,111 referring to the tendency to primarily or exclusively produce gonyautoxins. The results of the evaluation of PSTs in tissues from marine fauna (invertebrates and fish) collected during the episode in the port of Vigo,110 revealed that these were present not only in bivalve molluscs, but also in mullets, mackerels, starfish, ascidians and squids, with levels ranging from 16 to 3437 µg STX·diHCl equiv. Kg−1. A toxic profile dominated by one or more of these four GTXs, plus low saxitoxin levels, and sometimes very low/trace neosaxitoxin concentrations, is typical from Galician bivalve molluscs contaminated during A. minutum blooms.110
2.5. New tools for red tide detection
In Galicia there is an effective monitoring system operated by the Galician agency INTECMAR (Xunta de Galicia), which guarantees an adequate tracking of harmful algae species and toxicity for safeguarding human health.36 Red tides sometimes appear in areas away from national monitoring stations, usually located near aquaculture sites and therefore new methodologies for red tide detection and tracking are necessary. Novel monitoring techniques, especially with autonomous devices like drones, are being demonstrated to acquire phytoplankton data and characterise their high temporal and spatial variability.109 Therefore, we expect an improvement of our capacity of in situ monitoring of red tides with these new technologies. Additionally, in the 21st century, with the development of widely used new technologies, citizen science offers a new source of information for researchers. In our case, coastal areas with intense anthropogenic activities including tourism are particularly suited for these initiatives that could enhance our knowledge about the occurrence and distribution of red tides, to also explore their occasional relationship with HABs. At present, there are no initiatives of this kind other than an alert form for red tides reporting on the VGOHAB website (IEO-CSIC, Vigo) (https://vgohab.com/). In France, projects like PHENOMER (Ifremer) embraced in 2013 the citizen participation in monitoring phytoplankton seawater discolourations.112 Citizen alerts between 2013 and 2015 allowed sampling 74 seawater discolourations both within and outside the perimeter covered by REPHY, the national program for routine monitoring. Examples like this illustrate the opportunity of considering citizen science as a complement for monitoring systems. This approach, with its own advantages and drawbacks, provides not only an interesting tool for research projects but also an invaluable benefit associated with educational purposes and the involvement of the society in environmental issues.Since most of the toxic episodes in Galicia are caused by low-biomass subsurface blooms of Dinophysis spp., there are not many remote sensing studies of red tides or potentially toxic species in Galicia. A few studies with full resolution MODIS113 and MERIS114 satellite data, some using a regionally specific chlorophyll a algorithm,114 were able to follow surface chlorophyll in the rías, although they missed the subsurface chlorophyll maxima in upwelled waters.114
Some of these studies discuss the potential of full resolution MERIS images with regional specific algorithms to follow the potentially toxic Pseudo-nitzschia spp.114,115 Anyway, the red tides we review in this contribution are events with high biomass species colouring the water, so they could potentially be studied by using satellite ocean colour, as demonstrated in other areas in the world. In this regard, the detection and discrimination of red tide species depends on the spectral resolution of the satellite and the bio-optical properties of the species (see a recent review in Gernez et al.116).
Moreover, the usual short spatial scale of red tide patches requires high spatial resolution. Therefore, the limitations in the spatial resolution of ocean colour satellites have also hampered remote sensing studies of red tides in Galicia. The European Copernicus programme and the new high spatial resolution Sentinel-2A/B satellites, which additionally have improved spectral resolution, constitute a suitable tool to evaluate the onset of phytoplankton blooms and seawater discolourations if combined with phytoplankton in situ data including characterisation of their bio-optical characteristics, as recently reported in the SW and NW Iberian coastal waters for Lingulodinium polyedra,117N. scintillans and Alexandrium spp.90 The advent in recent years of these instruments, which combine high spatial and moderate spectral resolution, will contribute to the development of applications for red tide and HAB monitoring and early warning for aquaculture management purposes.
State of the art numerical models have shown to describe the variability of oceanographic conditions in response to upwelling–downwelling cycles, the interplay of different physical forces (wind events, tides, river runoff, etc.) and the potential advection of HAB populations.103,108,118 In the case of red tides, numerical models can simulate the factors that can play a role in red tide development such as local oceanographic conditions associated with stratification due to warming or riverine input, nutrient dynamics, near bottom currents that might resuspend cysts and physical advection that can transport populations.
There are still limitations in the capacity of state of the art models for simulating trophic interactions, phytoplankton species succession, mixotrophy, etc., which can be determinant in red tides from dinoflagellates. Nonetheless, numerical models combined with in situ data and satellite imagery can provide further insights into these events as well as early warning of HABs and red tide events as shown by some EU research projects in the last decade (e.g. ASIMUTH, PRIMROSE, Sen2Coast, etc.109,117,119).
4 Conclusion
General trends about red tides in Galicia can be described only at a qualitative level based on multiple sources due to the lack of systematic targeted samplings. However, our review provided some answers to the questions posed in the introduction:(i) How many red tides (and which types) have been recorded in the historic Galician time series? Since the early 20th century to date, a total of 112 red tide, mostly non-toxic, events have been reported in Galicia.
(ii) Which are the responsible group/species? Galician red tides were associated with up to 23 microplankton species, mostly dinoflagellates, but also with ciliates and other groups.
(iii) Have red tides been increasing in recent years? Red tides occur every year, particularly in summer. In the last four decades the number of observations stands rather constant, but a relative increase in red tides caused by dinoflagellates (mainly N. scintillans) has been reported. Many red tides in Galicia have been reported when and where there is retention and calm conditions that result in increased stratification and higher temperatures.19,40 Since it is possible that periods of increased temperature in the rías could become more frequent and long under the present climate change scenario, a potential impact of global warming could be the increase of red tides, although uncertainty exists in potential future temperature changes especially in this area influenced by seasonal upwelling (e.g.26). Moreover, exceptional toxic red tides due to A. minutum in the last few years and an increasing number of observations could challenge the paradigm stating that red tides in Galicia are usually of a non-toxic nature and therefore their monitoring should be intensified. While a systematic review of the different environmental factors identified as potential causes of red tides in Galicia is advisable, it must be stressed that most red tide events have specific triggering factors (like cyst resuspension, local retention101,108 nutrient and salinity-driven stratification from river outflow), including biological ones (life cycles and trophic interactions between species120,121), besides general trends related to water column stability and warming.
(iv) Do they cause social alarm? Notwithstanding the fact that red tides in Galicia are mostly caused by non-toxic species, press coverage and social media reports provide evidence of the rapid concern, both amongst the public and amongst fishermen, raised by press and social media coverage. This phenomenon is not restricted to certain coastal embayments but can take place at any coastal location given the appropriate environmental conditions.
(v) What actions could be proposed to reduce social alarm? Rapid identification of potential risks (e.g. toxicity) and species would help to manage coastal activities and increase social awareness of the planktonic ecological processes. Science dissemination to share available knowledge to the general public is also relevant in this context.
Finally, given that current trends indicate that toxic red tides could become a new normal in Galicia, a more systematic tracking would be advisable. This would be challenging given their sporadic and transient nature. Further advances in the understanding of red tides require the compilation of events with identification of the species involved combined with analysis of information from different sources. We hope that the present review will favour detailed studies integrating numerical tools, in situ observations and satellite imagery for the analysis of local oceanographic conditions, possible resuspension of cysts, advection of populations and trophic interactions between species. Consequently, observations from several sources (e.g. the monitoring program, novel technologies, citizen science and remote sensing techniques) could significantly enhance our knowledge on their spatio-temporal distribution, responsible species, dynamics and effects on aquatic ecosystems. All that information combined with numerical models could be relevant for early warning systems for forecasting the development of potential HABs and red tides in the Galician rías.
5 Online resources
a. Several sources (“Faro de Vigo” and “La Voz de Galicia”): https://www.lavozdegalicia.es/noticia/carballo/cee/2016/05/27/familia-hospital-tras-comer-mejillones-diez-veces-toxina-permitida-consumo/00031464383569220334941.htm; https://www.farodevigo.es/arousa/2014/08/07/personas-intoxicadas-consumir-mejillon-comprado-17146653.html; https://www.lavozdegalicia.es/noticia/somosmar/2015/07/08/seis-familias-intoxican-tras-comer-marisco-zonas-cerradas/0003_201507G8P31991.htm;b. DXSP, Dirección Xeral de Saúde Pública (2014). Primeira notificación de intoxicación pola biotoxina ASP en Galicia. Venres epidemiolóxico. Folla quincenal de información epidemiológica de Galicia 3 (16). https://www.sergas.es/Saude-publica/Documents/1590/Venres_Epidemioloxico_vol3_n16_20140801.pdf (accessed June 12, 2023).
c. Faro de Vigo (https://www.farodevigo.es/hemeroteca/; “Hematotalasia”, July 14, 2000, by Méndez-Ferrín, X. L.): “I remember an old story. It was in the past, when the seafaring people were more subject to the authority of the clergy than today. One day of Holy Friday, against everything that religion dictates, a certain person went out in command of his boat from the port of Bueu, to launch some pieces of “xeito” (*). Later on, while fishing, the sailor on board notices that a blow from the sea knocks and throws a red patch of water into the boat. Frightened, he shouts: – Skipper, there is blood on the bow! The heretic looks at his ship dyed red. He thinks it's God's punishment for fishing on Holy Friday. He asks for forgiveness and orders to turn back to the “Banda do Río” (**) and go to the holy offices, all weighted down, cap in hand.” [Translated from the original in Galician].
(*) Artisanal fishing gear that consists of a drift gill net targeting sardine.
(**) Refers to “Banda do Río” beach, in Bueu.
d. INTECMAR: http://www.intecmar.gal/ (accessed on June 23, 2023) and http://www.intecmar.gal/informacion/biotoxinas/EstadoZonas/Informes.aspx?sm=a2 (weekly reports, accessed April 26-May 19, 2023).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding was provided by the EU INTERREG Atlantic Area project PRIMROSE (EAPA 182/2016), BIOTOX (PID2021-125643OB-C22; Proyectos de generación de conocimiento 2021, MICINN), TOXEMER (PGIDIT-CIMA 21/01) and GPC (IN607B2023/11). This is a contribution to SCOR WG #165 MixONET, supported by grant OCE-214035 (NSF to SCOR) and contributions from SCOR National Committees. We thank Covadonga Salgado and Juan Blanco for helpful comments and suggestions on a previous version of the manuscript.References
- J. F. Tweddle, M. Gubbins and B. E. Scott, Should phytoplankton be a key consideration for marine management?, Mar. Policy, 2018, 97, 1–9 CrossRef.
- B. Reguera, L. Escalera, Y. Pazos and Á. Moroño, in La Ría de Vigo: una aproximación integral al ecosistema marino de la Ría de Vigo, Instituto de Estudios Vigueses, 2008, pp. 153–199 Search PubMed.
- P. J. Harrison, K. Furuya, P. M. Glibert, J. Xu, H. Liu, K. Yin, J. H. Lee, D. M. Anderson, R. Gowen and A. Al-Azri, Geographical distribution of red and green Noctiluca scintillans, Chin. J. Oceanol. Limnol., 2011, 29, 807–831 CrossRef.
- D. J. McLeod, G. M. Hallegraeff, G. W. Hosie and A. J. Richardson, Climate-driven range expansion of the red-tide dinoflagellate Noctiluca scintillans into the Southern Ocean, J. Plankton Res., 2012, 34, 332–337 CrossRef.
- Q. Zheng and V. V. Klemas, in Comprehensive Remote Sensing, ed. S. Liang, Elsevier, Oxford, 2018, vol. 8, pp. 89–120 Search PubMed.
- C. C. Davis, Gymnodinium brevis sp. nov., a cause of discolored water and animal mortality in the Gulf of Mexico, Bot. Gaz., 1948, 109, 358–360 CrossRef.
- M. Vandersea, P. Tester, K. Holderied, D. Hondolero, S. Kibler, K. Powell, S. Baird, A. Doroff, D. Dugan and A. Meredith, An extraordinary Karenia mikimotoi “beer tide” in Kachemak Bay Alaska, Harmful Algae, 2020, 92, 101706 CrossRef CAS PubMed.
- D. M. Anderson, A. D. Cembella and G. M. Hallegraeff, Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management, Ann. Rev. Mar. Sci., 2012, 4, 143–176 CrossRef PubMed.
- S. Esenkulova, C. Neville, E. DiCicco and I. Pearsall, Indications that algal blooms may affect wild salmon in a similar way as farmed salmon, Harmful Algae, 2022, 118, 102310 CrossRef PubMed.
- A. Ross Brown, M. K. S. Lilley, J. Shutler, C. Widdicombe, P. Rooks, A. McEvoy, R. Torres, Y. Artioli, G. Rawle, J. Homyard, C. R. Tyler and C. Lowe, Harmful Algal Blooms and their impacts on shellfish mariculture follow regionally distinct patterns of water circulation in the western English Channel during the 2018 heatwave, Harmful Algae, 2022, 111, 102166 CrossRef CAS PubMed.
- T. Okaichi, Identification of ammonia as the toxic principle of red tide of Noctiluca miliaris, Bull. Plankton Soc. Japan, 1976, 23, 75–80 Search PubMed.
- J. Arístegui, E. D. Barton, X. A. Álvarez-Salgado, A. M. P. Santos, F. G. Figueiras, S. Kifani, S. Hernández-León, E. Mason, E. Machú and H. Demarcq, Sub-regional ecosystem variability in the Canary Current upwelling, Prog. Oceanogr., 2009, 83, 33–48 CrossRef.
- F. Fraga, Upwelling off the Galician Coast, Northwest Spain, American Geophysical Union, Washington D.C., 1981 Search PubMed.
- X. A. Álvarez-Salgado, F. G. Figueiras, M. L. Villarino and Y. Pazos, Hydrodynamic and chemical conditions during onset of a red-tide assemblage in an estuarine upwelling ecosystem, Mar. Biol., 1998, 130, 509–519 CrossRef.
- W. S. Wooster, A. Bakun and D. McLain, The seasonal upwelling cycle along the eastern boundary of the North Atlantic, J. Mar. Res., 1976, 34, 131–141 Search PubMed.
- A. González-Garcés, F. Vilas-Martín and X. A. Álvarez-Salgado, La ría de Vigo. Una aproximación integral al ecosistema marino de la Ría de Vigo, Instituto de Estudios Vigueses, Vigo, 2009 Search PubMed.
- U. Labarta and M. J. Fernández-Reiriz, The Galician mussel industry: Innovation and changes in the last forty years, Ocean Coast. Manag., 2019, 167, 208–218 CrossRef.
- S. Villasante, A. Tubío, I. Gianelli, P. Pita and A. García-Allut, Ever changing times: sustainability transformations of Galician small-scale fisheries, Front. Mar. Sci., 2021, 8, 1006 Search PubMed.
- I. Bravo, S. Fraga, R. I. Figueroa, Y. Pazos, A. Massanet and I. Ramilo, Bloom dynamics and life cycle strategies of two toxic dinoflagellates in a coastal upwelling system (NW Iberian Peninsula), Deep-Sea Res. I: Oceanogr. Res. Pap., 2010, 57, 222–234 CrossRef CAS.
- P. P. Beca-Carretero, J. Otero, P. E. Land, S. Groom and X. A. Álvarez-Salgado, Seasonal and inter-annual variability of net primary production in the NW Iberian margin (1998–2016) in relation to wind stress and sea surface temperature, Prog. Oceanogr., 2019, 178, 102135 CrossRef.
- S. Fraga, D. M. Anderson, I. Bravo, B. Reguera, K. A. Steidinger and C. M. Yentsch, Influence of upwelling relaxation on dinoflagellates and shellfish toxicity in Ría de Vigo, Spain, Estuar. Coast. Shelf Sci., 1988, 27, 349–361 CrossRef.
- G. H. Tilstone, F. G. Figueiras and F. Fraga, Upwelling-downwelling sequences in the generation of red tides in a coastal upwelling system, Mar. Ecol. Prog. Ser., 1994, 112, 241–253 CrossRef.
- M. Gilcoto, J. L. Largier, E. D. Barton, S. Piedracoba, R. Torres, R. Grana, F. Alonso-Pérez, N. Villacieros-Robineau and F. de la Granda, Rapid response to coastal upwelling in a semi enclosed bay, Geophys. Res. Lett., 2017, 44, 2388–2397 CrossRef.
- R. Prego, A. W. Dale, M. DeCastro, M. Gómez-Gesteira, J. J. Taboada, P. Montero, M. Ruiz-Villareal and V. Pérez-Villar, Hydrography of the Pontevedra Ria: Intra-annual spatial and temporal variability in a Galician coastal system (NW Spain), J. Geophys. Res., 2001, 106, 19845–19858 CrossRef.
- E. Broullón, M. López-Mozos, B. Reguera, P. Choucino, M. D. Doval, B. Fernández-Castro, M. Gilcoto, E. Nogueira, C. Souto and B. Mouriño-Carballido, Thin layers of phytoplankton and Harmful Algae events in a coastal upwelling system, Prog. Oceanogr., 2020, 189, 102449 CrossRef.
- A. Bode, M. Álvarez, L. M. García García, M. Á. Louro, M. Nieto-Cid, M. Ruíz-Villarreal and M. M. Varela, Climate and local hydrography underlie recent regime shifts in plankton communities off Galicia (NW Spain), Oceans, 2020, 1, 181–197 CrossRef.
- L. Velo-Suárez, S. González-Gil, P. Gentien, M. Lunven, C. Bechemin, L. Fernand, R. Raine and B. Reguera, Thin layers of Pseudo-nitzschia spp. and the fate of Dinophysis acuminata during an upwelling-downwelling cycle in a Galician Ria, Limnol. Oceanogr., 2008, 53, 1816–1834 CrossRef.
- S. Fraga and A. Bakun, in Toxic Phytoplankton Blooms in the Sea. Proceedings of the Fifth International Conference on Toxic Marine Phytoplankton, ed. T. J. Smayda and Y. Shimizu, Elsevier, Newport, Rhode Island, U.S.A., 1993, pp. 59–65 Search PubMed.
- F. G. Figueiras, I. G. Teixeira, M. Froján, D. Zúñiga, B. Arbones and C. G. Castro, Seasonal variability in the microbial plankton community in a semienclosed bay affected by upwelling: the role of a nutrient trap, Front. Mar. Sci., 2020, 7, 578042 CrossRef.
- F. Figueiras and F. X. Niell, Distribución estacional y espacial del fitoplancton en la ría de Pontevedra (NO de España), Invest. Pesq., 1987, 52, 293–320 Search PubMed.
- Spanish Aquaculture Business Association, Aquaculture in Spain, accessed December 1, 2022.
- L. Avdelas, E. Avdic‐Mravlje, A. C. Borges Marques, S. Cano, J. J. Capelle, N. Carvalho, M. Cozzolino, J. Dennis, T. Ellis and J. M. Fernández Polanco, The decline of mussel aquaculture in the European Union: causes, economic impacts and opportunities, Rev. Aquac., 2021, 13, 91–118 CrossRef.
- R. Margalef, M. Durán and F. Saiz, El fitoplancton de la ría de Vigo de enero de 1953 a marzo de 1954, Invest. Pesq., 1955, 2, 85–129 Search PubMed.
- M. Varela, R. Prego and Y. Pazos, Vertical biogenic particle flux in a western Galician ria (NW Iberian Peninsula), Mar. Ecol. Prog. Ser., 2004, 260, 17–32 CrossRef.
- A. Bode and M. Varela, Primary production and phytoplankton in three Galician Rias Altas (NW Spain): Seasonal and spatial variability, Sci. Mar., 1998, 62, 319–330 CAS.
- J. Blanco, F. Arévalo, J. Correa and Á. Moroño, Lipophilic toxins in Galicia (NW Spain) between 2014 and 2017: Incidence on the main molluscan species and analysis of the monitoring efficiency, Toxins, 2019, 11, 612 CrossRef CAS PubMed.
- E. Bresnan, F. Arévalo, C. Belin, M. A. Branco, A. D. Cembella, D. Clarke, J. Correa, K. Davidson, M. Dhanji-Rapkova and R. F. Lozano, Diversity and regional distribution of harmful algal events along the Atlantic margin of Europe, Harmful Algae, 2021, 102, 101976 CrossRef CAS PubMed.
- Y. Pazos, F. Arévalo, J. Correa and C. Salgado, in 17th International Conference on Harmful Algae, Florianápolis Brazil, 2016, pp. 9–13 Search PubMed.
- X. A. Álvarez-Salgado, U. Labarta, M. J. Fernández-Reiriz, F. Figueiras, G. Rosón, S. Piedracoba, R. Filgueira and J. Cabanas, Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe), Harmful Algae, 2008, 7, 849–855 CrossRef.
- R. Margalef, Estructura y dinámica de la “purga de mar” en la ría de Vigo, Invest. Pesq., 1956, 5, 113–134 Search PubMed.
- B. Reguera, L. Velo-Suarez, R. Raine and M. G. Park, Harmful Dinophysis species: A review, Harmful Algae, 2012, 14, 87–106 CrossRef.
- P. A. Díaz, B. Reguera, M. Ruiz-Villarreal, Y. Pazos, L. Velo-Suárez, H. Berger and M. Sourisseau, Climate Variability and Oceanographic Settings Associated with Interannual Variability in the Initiation of Dinophysis acuminata Blooms, Mar. Drugs, 2013, 11, 2964–2981 CrossRef PubMed.
- P. A. Díaz, M. Ruiz-Villarreal, Y. Pazos, T. Moita and B. Reguera, Climate variability and Dinophysis acuta blooms in an upwelling system, Harmful Algae, 2016, 53, 145–159 CrossRef PubMed.
- F. G. Figueiras and F. X. Niell, Composición del fitoplancton de la ría de Pontevedra (NO de España), Invest. Pesq., 1987, 51, 371–409 Search PubMed.
- R. Prego, R. Carballeira, Y. Pazos and R. Bao, Oceanographical Context of the First Bloom of the Silicoflagellate Octactis speculum (Ehrenberg) Recorded to Cause Salmon Mortality in a Galician Ria: Was This Bloom a Rare Event in the Iberian Coast?, Toxins, 2023, 15, 435 CrossRef CAS PubMed.
- S. Sánchez and E. Delgado, Mareas rojas en el área del Callao (12° S) 1980-1995, Inf. Progr., 1996, 44, 19–37 Search PubMed.
- G. C. Pitcher, F. G. Figueiras, B. M. Hickey and M. T. Moita, The physical oceanography of upwelling systems and the development of harmful algal blooms, Prog. Oceanogr., 2010, 85, 5–32 CrossRef CAS PubMed.
- G. Pitcher and A. Cockcroft, Low Oxygen, Rock Lobster Strandings and PSP, HAN, 1998, pp. 1–3 Search PubMed.
- J. D. F. Gilchrist, An enquiry into fluctuations in fish supply on the South African coast, Mar. Biol. Rep., 1914, 2, 8–35 Search PubMed.
- K.-C. Emeis, V. Brüchert, B. Currie, R. Endler, T. Ferdelman, A. Kiessling, T. Leipe, K. Noli-Peard, U. Struck and T. Vogt, Shallow gas in shelf sediments of the Namibian coastal upwelling ecosystem, Cont. Shelf Res., 2004, 24, 627–642 CrossRef.
- J. Tarazona, D. Gutiérrez, C. Paredes and A. Indacochea, Overview and challenges of marine biodiversity research in Peru, Gayana, 2003, 67, 206–231 Search PubMed.
- B. Crespo, I. Teixeira, F. Figueiras and C. G. Castro, Microplankton composition off NW Iberia at the end of the upwelling season: source areas of harmful dinoflagellate blooms, Mar. Ecol. Prog. Ser., 2008, 355, 31–43 CrossRef CAS.
- Y. Pazos, F. G. Figueiras, X. A. Alvarez-Salgado and G. Rosón, in Harmful Marine Algal Blooms, ed. P. Lassus, G. Arzul, E. Erard-Le Denn, P. Gentien and C.Marcaillou- Le Baut , Lavoisier, Paris, 1995, pp. 651–656 Search PubMed.
- R. Sobrino, La purga del mar ó hematotalasia, Mem. Real Soc. Esp. Hist. Nat., 1918, 10, 407–458 Search PubMed.
- J. Cornide, Memoria sobre la pesca de sardina en las Costas de Galicia, Joachin Ibarra, 1774 Search PubMed.
- F. Gómez, Historia de las investigaciones sobre dinoflagelados marinos en España, Llull: Rev. Soc. Esp. Hist. Cien. Tec., 2006, 29, 307–330 Search PubMed.
- R. Carús Falcón, Los misterios de la Naturaleza. Investigaciones sobre el micro-plankton de la Ría de Arosa, Imprenta Viuda de Ferrer e Hijo, La Coruña, 1903 Search PubMed.
- B. Bruna, Efemérides del Puerto de Vigo, Archivo General del Puerto de Vigo. Autoridad Portuaria deVigo, 2017 Search PubMed.
- G. Parrilla-Barrera, Odón de Buen: Forerunner of Spanish Oceanography, Oceanography, 2005, 18, 128–135 CrossRef.
- O. de Buen, Trabajos españoles de oceanografía: campaña del Hernán Cortés este verano, Bol. Inst. Pesca., 1916, 3, 2–9 Search PubMed.
- E. Cabello, Memoria sobre el estado de las obras del puerto de Vigo a 31 de diciembre de 1926.—Vigo: Junta de Obras del Puerto, 1927 Search PubMed.
- R. Sobrino, El color de la Ría. La Correspondencia Gallega, Diario de Pontevedra, 1916, n° 8.006, 7-VII-1916 Search PubMed.
- B. Rodríguez-Santamaría, Diccionario de artes de pesca de España y sus posesiones, Madrid, Sucesores de Rivadeneyra, 1923 Search PubMed.
- A. I. Prince of Monaco, La pêche de la sardine sur les côtes d'Espagne, Rev. Sci., 1887, 17, 513–519 Search PubMed.
- G. Pouchet and J. de Guerne, Sur la nourriture de la sardine, C. R. Hebd. Seances Acad. Sci., 1887, 104, 712–715 Search PubMed.
- F. De Buen, Sobre la coloración roja del agua en las Rías bajas y la biología de la sardina, Bol. R. Soc. Esp. Hist. Nat., 1918, 18, 327–331 Search PubMed.
- R. Margalef, Estudio sumario del fitoplancton de la ría de Vigo (1948-1950), Bol. Inst. Esp. Oceanogr., 1952, 47 Search PubMed.
- M. Durán, F. Saiz, M. López-Benito and R. Margalef, El fitoplancton de la ría de Vigo, de abril de 1954 a junio de 1955, Invest. Pesq., 1956, 4, 67–95 Search PubMed.
- R. Margalef and M. Duran, Microplancton de Vigo, de Octubre de 1951 a Septiembre de 1952, Publ. Inst. Biol. Apl., 1953, 13, 5–78 Search PubMed.
- M. Estrada, F. J. Sánchez and S. Fraga, Gymnodinium catenatum (Graham) en las rías gallega (NO de España), Invest. Pesq., 1984, 48, 31–40 Search PubMed.
- B. Reguera, Las Mareas Rojas, Bol. Agropec., 1991, 21, 4–13 Search PubMed.
- J. Mariño, J. Maneiro and J. Blanco, in Harmful Algae, ed. B. Reguera, J. Blanco, M. L. Fernández and T. Wyatt, IOC of UNESCO - Xunta de Galicia, Santiago de Compostela, 1998, pp. 229–232 Search PubMed.
- S. Fraga, Las Purgas de mar en las Rías Bajas gallegas, Semin. Estud. Galegos, 1989, 4, 95–109 Search PubMed.
- F. Arévalo, Y. Pazos, J. Correa, C. Salgado, Á. Moroño, B. Paz and J. M. Franco, Presented in Part at the Proceedings of the 5th International Conference on Molluscan Shellfish Safety, Galway, Ireland, 2006 Search PubMed.
- C. Jiménez, F. X. Niell, F. Figueiras, V. Clavero, P. Algarra and J. Buela, Green mass aggregations of Gyrodinium cf. aureolum Hulburt in the Ria of Pontevedra (north-west Spain), J. Plankton Res., 1992, 14, 705–720 CrossRef.
- P. Roux, R. Siano, P. Souchu, K. Collin, A. Schmitt, S. Manach, M. Retho, O. Pierre-Duplessix, L. Marchand and S. Colliec-Jouault, Spatio-temporal dynamics and biogeochemical properties of green seawater discolorations caused by the marine dinoflagellate Lepidodinium chlorophorum along southern Brittany coast, Estuarine, Coastal Shelf Sci., 2022, 275, 107950 CrossRef CAS.
- U. Tillmann, S. Wietkamp, J. Kretschmann, J. Chacón and M. Gottschling, Spatial fragmentation in the distribution of diatom endosymbionts from the taxonomically clarified dinophyte Kryptoperidinium triquetrum (= Kryptoperidinium foliaceum, Peridiniales), Sci. Rep., 2023, 13, 8593 CrossRef CAS PubMed.
- R. I. Figueroa, I. Bravo, S. Fraga, E. Garcés and G. Llaveria, The life history and cell cycle of Kryptoperidinium foliaceum, a dinoflagellate with two eukaryotic nuclei, Protist, 2009, 160, 285–300 CrossRef PubMed.
- K. Drumm, A. Norlin, M. Kim, A. Altenburger and P. Juel Hansen, Physiological responses of Mesodinium major to irradiance, prey concentration and prey starvation, J. Eukaryotic Microbiol., 2021, 68, e12854 CrossRef CAS PubMed.
- M. D. Johnson, D. J. Beaudoin, A. Laza-Martinez, S. T. Dyhrman, E. Fensin, S. Lin, A. Merculief, S. Nagai, M. Pompeu and O. Setälä, The genetic diversity of Mesodinium and associated cryptophytes, Front. Microbiol., 2016, 7, 2017 Search PubMed.
- M. de la Paz Graells, Manual práctico de piscicultura o prontuario para servir de guía al piscicultor en España y a los empleados de la administración pública en nuestras aguas dulces y saladas, Cámara de Su Magestad y de su Real Casa, Madrid, 1864 Search PubMed.
- M. G. Park, J. S. Park, M. Kim and W. Yih, Plastid dynamics during survival of Dinophysis caudata without its ciliate prey, J. Phycol., 2008, 44, 1154–1163 CrossRef CAS PubMed.
- L. Mafra Jr, P. Nolli, L. Mota, C. Domit, M. Soeth, L. Luz, B. Sobrinho, J. Leal and M. Di Domenico, Multi-species okadaic acid contamination and human poisoning during a massive bloom of Dinophysis acuminata complex in southern Brazil, Harmful Algae, 2019, 89, 101662 CrossRef CAS PubMed.
- REPHY, Reseau d'observation et de surveillance du phytoplancton et de l'hydrologie dans les eaux littorales, REPHY : la surveillance du phytoplancton et des phycotoxines - Unité Littoral, (ifremer.fr), accessed 05/07/2023.
- L. Valdés, A. Bode, M. Latasa, E. Nogueira, R. Somavilla, M. M. Varela, C. González-Pola and G. Casas, Three decades of continuous ocean observations in North Atlantic Spanish waters: The RADIALES time series project, context, achievements and challenges, Prog. Oceanogr., 2021, 198, 102671 CrossRef.
- P. A. Díaz, M. Ruiz-Villarreal, B. Mouriño-Carballido, C. Fernández-Pena, P. Riobó and B. Reguera, Fine scale physical-biological interactions during a shift from relaxation to upwelling with a focus on Dinophysis acuminata and its potential ciliate prey, Prog. Oceanogr., 2019, 175, 309–327 CrossRef.
- L. Escalera, Y. Pazos, A. Moroño and B. Reguera, Noctiluca scintillans may act as a vector of toxigenic microalgae, Harmful Algae, 2007, 6, 317–320 CrossRef.
- M. Frangópulos, E. Spyrakos and C. Guisande, Ingestion and clearance rates of the red Noctiluca scintillans fed on the toxic dinoflagellate Alexandrium minutum (Halim), Harmful Algae, 2011, 10, 304–309 CrossRef.
- P. Rameshkumar, P. S. Thirumalaiselvan, M. Raman, L. Remya, R. Jayakumar, M. Sakthivel, G. Tamilmani, M. Sankar, K. K. Anikuttan, N. N. Menon, R. Saravanan, T. T. Ravikumar, I. Narasimapallavan, N. Krishnaveni, V. Muniasamy, S. M. Batcha and A. Gopalakrishnan, Monitoring of Harmful Algal Bloom (HAB) of Noctiluca scintillans (Macartney) along the Gulf of Mannar, India using in-situ and satellite observations and its impact on wild and maricultured finfishes, Mar. Pollut. Bull., 2023, 188, 114611 CrossRef CAS PubMed.
- A. M. Sacilotto Detoni, G. Navarro, J. L. Garrido, F. Rodríguez, J. Hernández-Urcera and I. Caballero, Mapping dinoflagellate blooms (Noctiluca and Alexandrium) in aquaculture production areas in the NW Iberian Peninsula with the Sentinel-2/3 satellites, Sci. Total Environ., 2023, 868, 161579 CrossRef PubMed.
- J. Blanco, J. Mariño and M. J. Campos, in Toxic Dinoflagellates, ed. D. M. Anderson, A. W. White and D. G. Baden, Elsevier, New York, 1985, pp. 79–84 Search PubMed.
- S. F. Ferreiro, N. Vilariño, C. Carrera, M. C. Louzao, A. G. Cantalapiedra, G. Santamarina, J. M. Cifuentes, A. C. Vieira and L. M. Botana, Subacute cardiotoxicity of yessotoxin: In vitro and in vivo Studies, Chem. Res. Toxicol., 2016, 29, 981–990 Search PubMed.
- S. F. Ferreiro, N. Vilarino, C. Carrera, M. C. Louzao, G. Santamarina, A. G. Cantalapiedra, J. M. Cifuentes, A. C. Vieira and L. M. Botana, Subacute immunotoxicity of the marine phycotoxin yessotoxin in rats, Toxicon, 2017, 129, 74–80 CrossRef CAS PubMed.
- A. Martín-López, J. J. Gallardo-Rodríguez, A. Sánchez-Mirón, F. García-Camacho and E. Molina-Grima, Cytotoxicity of yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4, Toxicon, 2012, 60, 1049–1056 CrossRef PubMed.
- European Commission Regulation (EC), No 2002/225/EC of the Commission Decision of 15 March 2002 laying down detailed rules for the implementation of Council Directive 91/492/EEC as regards the maximum levels and the methods of analysis of certain marine biotoxins in bivalve molluscs, echinoderms, tunicates and marine gastropods (Text with EEA relevance) (notified under document number C(2002) 1001), Off. J. Eur. Communities, 2002, 75, 62 Search PubMed.
- European Commission Regulation (EU), No 786/2013 of 16 August 2013 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards the permitted limits of yessotoxins in live bivalve molluscs (Text with EEA relevance), Off. J. Eur. Communities, 2013, 75, 14 Search PubMed.
- Y. Pazos and A. Moroño, in Avances y Tendencias en Fitoplancton Tóxico y Biotoxinas. IX Reunión Ibérica sobre Fitoplancton Tóxico y Biotoxinas, ed. J. Gilabert, Cartagena, 2007, pp. 13–28 Search PubMed.
- J. Blanco, Quistes de dinoflagelados de las costas de Galicia. I. Dinoflagelados Gonyaulacoides, Sci. Mar., 1989, 53, 785–796 Search PubMed.
- N. Ospina-Álvarez, R. Prego, I. Álvarez, M. DeCastro, M. T. Álvarez-Ossorio, Y. Pazos, M. Campos, P. Bernárdez, C. García-Soto and M. Gómez-Gesteira, Oceanographical patterns during a summer upwelling–downwelling event in the Northern Galician Rias: Comparison with the whole Ria system (NW of Iberian Peninsula), Cont. Shelf Res., 2010, 30, 1362–1372 CrossRef.
- I. García-Moreiras, S. V. Costas, S. García-Gil and C. M. Sobrino, Organic-walled dinoflagellate cyst assemblages in surface sediments of the Ría de Vigo (Atlantic margin of NW Iberia) in relation to environmental gradients, Mar. Micropaleontol., 2023, 180, 102217 CrossRef.
- R. Prego, Flows and budgets of nutrient salts and organic carbon in relation to a red tide in the Ria of Vigo (NW Spain), Mar. Ecol. Prog. Ser., 1992, 79, 289–302 CrossRef CAS.
- E. Gómez- Fermín, F. G. Figueiras, B. Arbones and M. L. Villarino, Short time scale development of a Gymnodinium catenatum population in the Ría de Vigo (NW Spain), J. Phycol., 1996, 32, 212–221 CrossRef.
- M. Ruiz-Villarreal, L. M. García-García, M. Cobas, P. A. Díaz and B. Reguera, Modelling the hydrodynamic conditions associated with Dinophysis blooms in Galicia (NW Spain), Harmful Algae, 2016, 53, 40–52 CrossRef PubMed.
- B. G. Crespo, F. G. Figueiras, P. Porras and I. G. Teixeira, Downwelling and dominance of autochthonous dinoflagellates in the NW Iberian margin: The example of the Ría de Vigo, Harmful Algae, 2006, 5, 770–781 CrossRef CAS.
- European Commission Regulation (EC), No 853/2004 of the European parliament and of the Council of 29 April 2004 laying down specific hygiene rules for the hygiene of foodstuffs, Off. J. Eur. Communities, 2004, 75, 55 Search PubMed.
- B. Ben-Gigirey, M. Rodriguez-Velasco, A. Villar-Gonzalez and L. Botana, Influence of the sample toxic profile on the suitability of a high performance liquid chromatography method for official paralytic shellfish toxins control, J. Chromatogr. A, 2007, 1140, 78–87 CrossRef CAS PubMed.
- B. Ben-Gigirey, M. Rodríguez-Velasco, A. Otero, J. Vieites and A. Cabado, A comparative study for PSP toxins quantification by using MBA and HPLC official methods in shellfish, Toxicon, 2012, 60, 864–873 CrossRef CAS PubMed.
- E. Nogueira, I. Bravo, P. Montero, P. Díaz-Tapia, S. Calvo, B. Ben-Gigirey, R. I. Figueroa, J. Garrido, I. Ramilo and N. Lluch, HABs in coastal upwelling systems: Insights from an exceptional red tide of the toxigenic dinoflagellate Alexandrium minutum, Ecol. Indic., 2022, 137, 108790 CrossRef CAS.
- M. Ruiz-Villarreal, M. Sourisseau, P. Anderson, C. Cusack, P. Neira, J. Silke, F. Rodriguez, B. Ben-Gigirey, C. Whyte and S. Giraudeau-Potel, Novel methodologies for providing in situ data to HAB early warning systems in the European Atlantic Area: the PRIMROSE experience, Front. Mar. Sci., 2022, 9, 791329 CrossRef.
- B. Ben-Gigirey, A. E. Rossignoli, P. Riobó and F. Rodríguez, First report of paralytic shellfish toxins in marine invertebrates and fish in Spain, Toxins, 2020, 12, 723 CrossRef CAS PubMed.
- D. M. Anderson, T. J. Alpermann, A. D. Cembella, Y. Collos, E. Masseret and M. Montresor, The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health, Harmful Algae, 2012, 14, 10–35 CrossRef PubMed.
- R. Siano, A. Chapelle, V. Antoine, E. Michel-Guillou, F. Rigaut-Jalabert, L. Guillou, H. Hegaret, A. Leynaert and A. Curd, Citizen participation in monitoring phytoplankton seawater discolorations, Mar. Policy, 2020, 117, 103039 CrossRef.
- P. A. Díaz, M. Ruiz-Villarreal, L. Velo-Suárez, I. Ramilo, P. Gentien, M. Lunven, L. Fernand, R. Robin and B. Reguera, Tidal and wind-event variability and the distribution of two groups of Pseudo-nitzschia species in an upwelling-influenced Ría, Deep-Sea Res. I: Oceanogr. Res. Pap., 2014, 101, 163–179 CrossRef.
- E. Spyrakos, L. G. Vilas, J. M. Torres-Palenzuela and E. D. Barton, Remote sensing chlorophyll a of optically complex waters (rias Baixas, NW Spain): Application of a regionally specific chlorophyll a algorithm for MERIS full resolution data during an upwelling cycle, Remote Sens. Environ., 2011, 115, 2471–2485 CrossRef.
- J. M. Torres-Palenzuela, L. González-Vilas, F. M. Bellas, E. Garet, Á. González-Fernández and E. Spyrakos, Pseudo-nitzschia Blooms in a Coastal Upwelling System: Remote Sensing Detection, Toxicity and Environmental Variables, Water, 2019, 11, 1954 CrossRef.
- P. Gernez, M. L. Zoffoli, T. Lacour, T. H. Fariñas, G. Navarro, I. Caballero and T. Harmel, The many shades of red tides: Sentinel-2 optical types of highly-concentrated harmful algal blooms, Remote Sens. Environ., 2023, 287, 113486 CrossRef.
- I. Caballero, R. Fernández, O. M. Escalante, L. Mamán and G. Navarro, New capabilities of Sentinel-2A/B satellites combined with in situ data for monitoring small harmful algal blooms in complex coastal waters, Sci. Rep., 2020, 10, 1–14 CrossRef PubMed.
- M. Bedington, L. M. García-García, M. Sourisseau and M. Ruiz-Villarreal, Assessing the Performance and Application of Operational Lagrangian Transport HAB Forecasting Systems, Front. Mar. Sci., 2022, 9, 749071 CrossRef.
- J. Maguire, C. Cusack, M. Ruiz-Villarreal, J. Silke, D. McElligott and K. Davidson, Applied simulations and integrated modelling for the understanding of toxic and harmful algal blooms (ASIMUTH): Integrated HAB forecast systems for Europe's Atlantic Arc, Harmful Algae, 2016, 53, 160–166 CrossRef PubMed.
- M. Busch, D. Caron and S. Moorthi, Growth and grazing control of the dinoflagellate Lingulodinium polyedrum in a natural plankton community, Mar. Ecol. Prog. Ser., 2019, 611, 45–58 CrossRef CAS.
- T. Wyatt and A. Zingone, Population dynamics of red tide dinoflagellates, Deep Sea Res. Part II Top. Stud. Oceanogr., 2014, 101, 231–236 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3em00296a |
| This journal is © The Royal Society of Chemistry 2024 |