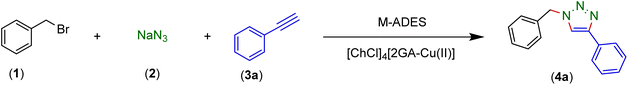Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel Cu(II) acidic deep eutectic solvent as an efficient and green multifunctional catalytic solvent system in base-free conditions to synthesize 1,4-disubstituted 1,2,3-triazoles†
Nastaran Bagherzadeh,
Mohammad Amiri and
Ali Reza Sardarian *
*
Chemistry Department, College of Sciences, Shiraz University, Shiraz, Iran. E-mail: sardarian@shirazu.ac.ir; Fax: +98 7136460788; Tel: +98 7136137107
First published on 14th December 2023
Abstract
A novel green Cu(II)-acidic deep eutectic solvent (Cu(II)-ADES) bearing copper salt, choline chloride, and gallic acid ([ChCl]4[2GA-Cu(II)]) was synthesized and thoroughly specified by physicochemical approaches such as FT-IR, EDX, XRD, Mapping, ICP, and UV-Vis analyses and physicochemical properties. After the detection of authentic data, the central composite design (CCD) was utilized to accomplish the pertaining tests and develop the optimum condition, and, in the following, [ChCl]4[2GA-Cu(II)] was applied as a green multifunctional catalytic solvent system in reducing agent-free and base-free condition for the three-component click reaction from sodium azide, alkyl, allyl, ester, and benzyl halide, and terminal alkyne made from amines and caprolactam as a cyclic amide to furnish a successful new library of 1,4-disubstituted 1,2,3-triazoles with a yield of up to 98%. The Cu(II)-ADES is stable and can comfortably be recovered and reused without a considerable decline in its acting for seven cycles. This triazole synthesizing methodology is distinguished via its atom economy, operational facility, short reaction times, diverse substrate scope, and high performance for large-scale synthesis of the desired products including: –CN and –NO2 as efficient functional groups.
1. Introduction
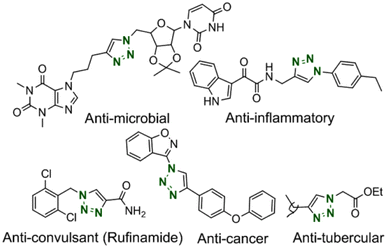
Heterocyclic 1,2,3-triazoles are a privileged category of nitrogenous heterocyclic compounds attracting a high extent of consideration in industrial and academic areas. These critical structures have found an extensive range of applications in chemical biology,1 organic synthesis,2 supramolecular chemistry,3 materials science,4 and pharmaceuticals chiefly offered for their anti-tumor, anti-microbial, anti-viral, anti-cancer, anti-tubercular, anti-inflammatory.5 Some of the substantial scaffolds are exhibited in Fig. 1. Furthermore, these compounds have beneficial properties like hydrogen bonding ability, excellent chemical stability, intense dipole moment and π-stacking interaction, and aromatic character.6 Accordingly, the establishment of mild and convenient methodologies have been encouraged for synthesizing these compounds.The most popular method was reported by Sharpless's laboratories, as the factual pioneers in this theme through the Cu(I)-catalyzed Huisgen cycloaddition, which is well known as Cu-catalyzed azide–alkyne cycloaddition (CuAAC).7
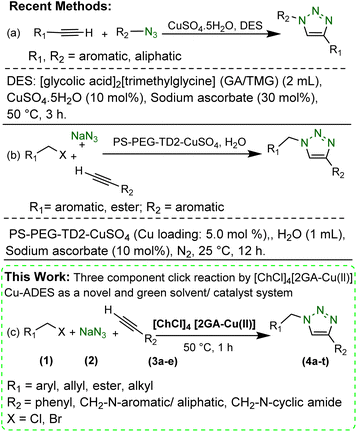
There are many reports in which the regioselective production of 1,4-disubstituted 1,2,3-triazoles has been carried out using the CuAAC strategy.8–11 There are several negative aspects in most of these reported methodologies, such as (a) utilization of non-reusable catalysts, (b) application of hazardous volatile organic solvents that are significantly problematic for the environment, (c) using prefabricated organic azides that are unstable, explosive, commercially unavailable, and (d) demanding various additives when Cu(II) catalysts are utilized (e.g. large amount of reducing agent).10,11 Thus, many efforts have been taken to develop new CuAAC sustainable chemical protocols for the production of 1,4-disubstituted 1,2,3-triazoles using reusable catalysts and green solvents.11a,b,12,13,14 Among these methods, it can be pointed, for instance, to Giofrè et al.12 (Scheme 1a) and Uozumi et al.13 (Scheme 1b). Nonetheless, these methodologies have their merits as using deep eutectic solvents (DESs) or water as green solvent and reusable heterogeneous Cu catalysts however include one or several weak points as the application of (a) large amounts of sodium ascorbate for in situ reduction of Cu(II) to Cu(I), and (b) expensive and multistep synthesized Cu(II) heterogeneous catalyst. Consequently, there is still demand for developing new economical, benign, and simple handling methodologies for the preparation of the 1,2,3-triazoles.
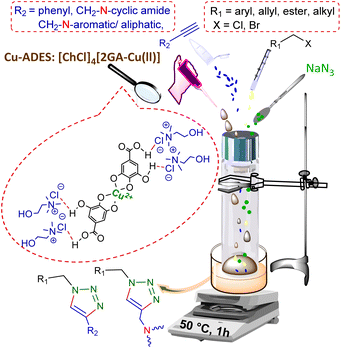
Fascinating features of DESs as a specific type of ionic liquids (ILs) with their distinct superiorities versus ILs,15 such as simplicity in preparation of ideal atom economy synthesis, non-toxicity, non-volatility, non-flammability, easy recyclability, thermal stability, and most importantly, susceptibility to dissolve polar and nonpolar reagents,16,17 motivate us to design a deep eutectic solvent as a novel dual solvent/catalyst for the CuAAC reaction.
Thus, we report the manufacture and characterization of a novel copper acidic deep eutectic solvent (Cu-ADES), bearing choline chloride (ChCl), gallic acid (GA), and CuCl2, which are non-toxic, inexpensive, and available chemicals. Then this DES ([ChCl]4[2GA-Cu(II)]) is applied as an efficient, reusable, and sustainable dual solvent and catalyst system in the base-free three-component click reaction NaN3, benzyl, alkyl halides, and terminal alkynes constructed of amine and cyclic amide resources as abundant azide precursors for the regioselective synthesis of novel 1,4-disubstituted 1,2,3-triazole frameworks, Scheme 1c.
2. Results and discussion
In this work, the [2GA-Cu(II)] complex was formed by mixing GA with CuCl2·2H2O with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in an aqueous solution at room temperature in pH 7.5.18,19 Then the desired novel DES was formed as a dark brown liquid system using the previous procedure reported.20 A possible illustration of coordination between copper ion (Cu2+) and gallic acid (GA), in order to prepare Cu complex and interactions between [2GA-Cu(II)] complex and choline chloride (ChCl) in the structure of the desired Cu(II)-AEDS exhibited in Scheme 2.
1 in an aqueous solution at room temperature in pH 7.5.18,19 Then the desired novel DES was formed as a dark brown liquid system using the previous procedure reported.20 A possible illustration of coordination between copper ion (Cu2+) and gallic acid (GA), in order to prepare Cu complex and interactions between [2GA-Cu(II)] complex and choline chloride (ChCl) in the structure of the desired Cu(II)-AEDS exhibited in Scheme 2.
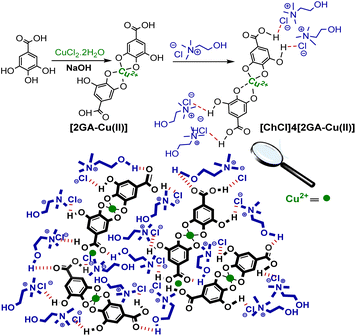 | ||
| Scheme 2 The suggested illustration of molecular structure of [2GA-Cu(II)] complex and also possible interactions between [2GA-Cu(II)] complex and choline chloride (ChCl). | ||
2.1 Characterization of the [2GA-Cu(II)] complex and [ChCl]4[2GA-Cu(II)] as Cu(II)-AEDS
Several physicochemical measurements were accomplished to illustrate the specifications of the [2GA-Cu(II)] complex and [ChCl]4[2GA-Cu(II)] as Cu(II)-ADES. The prepared [2GA-Cu(II)] and Cu(II)-ADES were identified by Energy-dispersive X-ray (EDX), Mapping, XRD pattern, Fourier transform infrared (FTIR), ICP (Inductively coupled plasma), and Ultraviolet-Visible spectroscopy (UV-Vis) analyses. As shown in Fig. 2a, the XRD spectrum of [2GA-Cu(II)] showed a close resemblance to the diffraction spectrum reported in the literature for this complex,18,19 specifically at 2θ = 10.1°, 13.2°, 20.1°, 28.0°, 31.1°, and 42.8°, and is an indication for successful production of the related copper complex.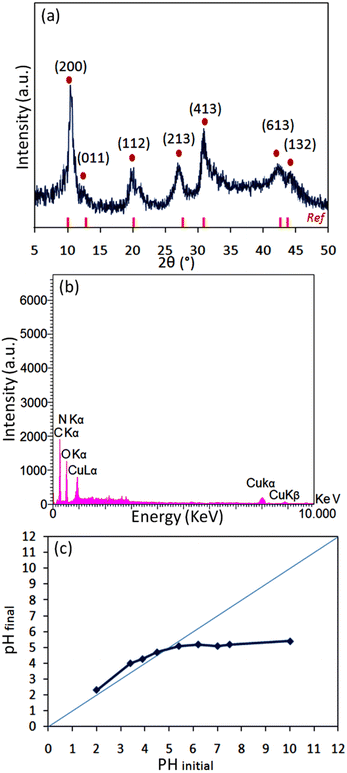 | ||
| Fig. 2 (a) Powder X-ray diffraction of [2GA-Cu(II)]; (b) EDX analysis of [2GA-Cu(II)]; (c) pHpzc determination graph of [2GA-Cu(II)]. | ||
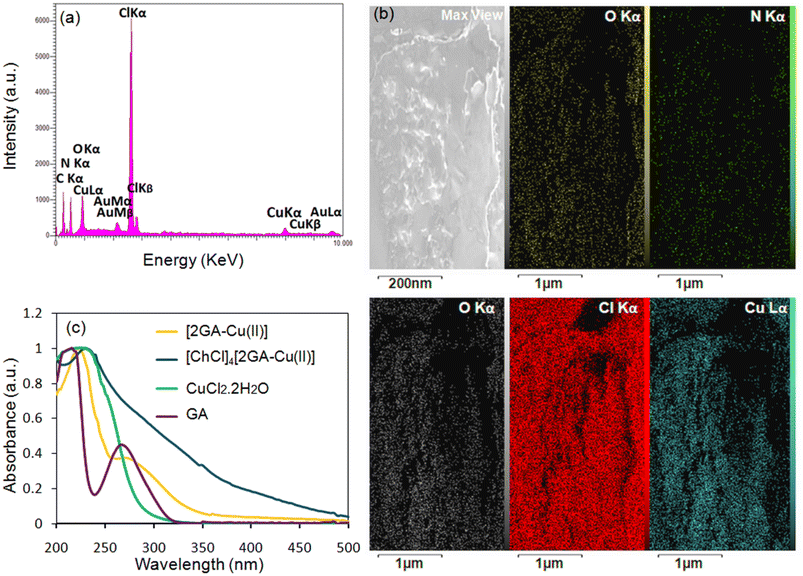
EDX, as a helpful technique for the determination of constituent atomic components of a compound, was applied on [2GA-Cu(II)]. The EDX spectrum revealed the presence of C, N, O, and Cu elements and also the absence of Cl element in the structure of [2GA-Cu(II)] (Fig. 2b) and therefore confirms the synthesis of this metal-phenolic network.
The negative surface charge of [2GA-Cu(II)] was proved via the pHpzc value (Fig. 2c). This analysis was performed with the method recorded by Angkawijaya et al.21 in earlier reports. A series of 5 mL NaCl solutions (0.1 M) in covered vials were provided at an adjusted pH of 2 to 10. Afterward, in each vial, 15 mg of the present DES was added and allowed to be mixed in a shaking incubator with 200 rpm at 30 °C for 48 h. After passing the desired time, the final pH was measured for each sample with a pH meter. Then, the related pHfinal was plotted against the pHinitial for each case, and the intersection of the pHinitial = pHfinal linear graph with the experimental curve was distinguished as pHpzc, which is equal to 4.61. The dissociation of several proton (H+) ions from the functional groups –COOH and –OH cause the surface charge [2GA-Cu(II)] to be negative, which is given the fact that the pH of a solution is higher than pHpzc. But while a series of [2GA-Cu(II)] particles at pH ≥ 6 have a negative surface charge, may some of their functional groups stay protonated. Since the type of interaction between the DES components has not been clearly defined in the literature so far, it is assumed that these protonated groups of GA take part in multiple possible interactions consisting of dipole–dipole H-bonding, electrostatic H-bonding, electrostatic interaction between charged components,20b also H-bonding of the carbonyl group of GA and the hydroxyl group of choline chloride when it interacts with choline chloride to form a eutectic mixture.
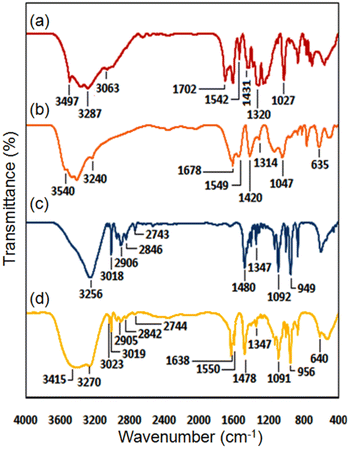
The following investigation was done by FT-IR analysis for the characterization of functional groups in [2GA-Cu(II)], and [ChCl]4[2GA-Cu(II)] (Cu(II)-ADES) and the FT-IR spectra of the parent components gallic acid (GA) and choline chloride (ChCl) were also given for comparison, which can be observed in the Fig. 3a–d and Table 1. As presented in (Fig. 3a, b, and d), the characteristic peaks of gallic acid can be observed in the FT-IR spectra of [2GA-Cu(II)] and [ChCl]4[2GA-Cu(II)], especially the acidic index peaks (2500–3600 cm−1), aromatic stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1540–1550 cm−1), C
C (1540–1550 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (1635–1700 cm−1), bending vibrations of C–O (1420–1480 cm−1), and C–O bonds stretching peaks in the region of 1020–1090 cm−1. Further, in the [2GA-Cu(II)] chromatogram, several band shifts can be perceived as a shift was observed from 1431 cm−1 in GA to 1420 cm−1 in [2GA-Cu(II)] of the C–O vibration stretching band. The peak shifts related to phenol-C–O vibration in the GA spectrum at 1027 and 1250 cm−1, respectively, to 1047 and 1140 cm−1 in the [2GA-Cu(II)] spectrum have been observed. The band shifts for these groups indicate a change in the bond length, which can be attributed to the coordination between Cu and GA. Moreover, the vibration of the Cu–O group at 635 cm−1 appears in the [2GA-Cu(II)] spectrum, confirming the participation of the Cu–O group in the formation of [2GA-Cu(II)] and is explicitly observed in the M-ADES spectrum (Fig. 3d) that elucidates the stability of Cu–O band formation in all synthetic steps.21 In continue, the incorporation of ChCl to the [2GA-Cu(II)] and formation of eutectic brown liquid of Cu(II)-ADES was confirmed by the shift of the (OH)–hydroxyl and (OH)–carboxyl stretching bands to lower wavenumbers in the Cu(II)-ADES compared to those in the gallic acid due to formation of hydrogen bonding of these functional groups and several possible interactions, in addition to shifting in the (OH)–alcohol band (at ChCl) and C
O stretching (1635–1700 cm−1), bending vibrations of C–O (1420–1480 cm−1), and C–O bonds stretching peaks in the region of 1020–1090 cm−1. Further, in the [2GA-Cu(II)] chromatogram, several band shifts can be perceived as a shift was observed from 1431 cm−1 in GA to 1420 cm−1 in [2GA-Cu(II)] of the C–O vibration stretching band. The peak shifts related to phenol-C–O vibration in the GA spectrum at 1027 and 1250 cm−1, respectively, to 1047 and 1140 cm−1 in the [2GA-Cu(II)] spectrum have been observed. The band shifts for these groups indicate a change in the bond length, which can be attributed to the coordination between Cu and GA. Moreover, the vibration of the Cu–O group at 635 cm−1 appears in the [2GA-Cu(II)] spectrum, confirming the participation of the Cu–O group in the formation of [2GA-Cu(II)] and is explicitly observed in the M-ADES spectrum (Fig. 3d) that elucidates the stability of Cu–O band formation in all synthetic steps.21 In continue, the incorporation of ChCl to the [2GA-Cu(II)] and formation of eutectic brown liquid of Cu(II)-ADES was confirmed by the shift of the (OH)–hydroxyl and (OH)–carboxyl stretching bands to lower wavenumbers in the Cu(II)-ADES compared to those in the gallic acid due to formation of hydrogen bonding of these functional groups and several possible interactions, in addition to shifting in the (OH)–alcohol band (at ChCl) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (at GA) from 3256 to 3270, and 1702 to 1638 in Cu(II)-ADES, respectively (Fig. 3d and Table 1). As well as peak shift at 949 cm−1 in the ChCl spectrum to 956 cm−1 [ChCl]4[2GA-Cu(II)] spectrum is related to the C–N stretching in the ammonium structure. Also, the presence of bands at the round of 2740–3020 cm−1 specified the attendance of (–CH2–), (–CH3) symmetric and asymmetric stretching vibrations in the choline chloride and Cu(II)-ADES22 (Fig. 3c and d). Eventually, the comparison study of FT-IR spectra of choline chloride, [2GA-Cu(II)], and [ChCl]4[2GA-Cu(II)] demonstrate that Cu(II)-ADES formation does not lead to the establishment of any new functional groups in the final mixture.
O stretching (at GA) from 3256 to 3270, and 1702 to 1638 in Cu(II)-ADES, respectively (Fig. 3d and Table 1). As well as peak shift at 949 cm−1 in the ChCl spectrum to 956 cm−1 [ChCl]4[2GA-Cu(II)] spectrum is related to the C–N stretching in the ammonium structure. Also, the presence of bands at the round of 2740–3020 cm−1 specified the attendance of (–CH2–), (–CH3) symmetric and asymmetric stretching vibrations in the choline chloride and Cu(II)-ADES22 (Fig. 3c and d). Eventually, the comparison study of FT-IR spectra of choline chloride, [2GA-Cu(II)], and [ChCl]4[2GA-Cu(II)] demonstrate that Cu(II)-ADES formation does not lead to the establishment of any new functional groups in the final mixture.
| GAa | FT-IR spectra (cm−1) | Types of bond vibration of functional groups | ||
|---|---|---|---|---|
| GA-Cu(II) | ChClb | [ChCl]4[2GA-Cu(II)] | ||
| a Gallic acid.b Choline chloride. | ||||
| 1027, 1250 | 1047, 1140 | 1092 | 1091 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (C–O) (C–O) |
| 1320 | 1314 | 1347 | 1347 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (OH)-bending (OH)-bending |
| 1431 | 1420 | 1480 | 1478 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (C–O) stretching (C–O) stretching |
| 1542 | 1549 | — | 1550 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (C (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching C) stretching |
| 1702 | 1678 | — | 1638 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (C (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching O) stretching |
| 3287 | 3240 | — | — | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (OH)–carboxyl (OH)–carboxyl |
| — | — | — | Overlap in 3270 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (OH)–carboxyl with hydrogen bonding (OH)–carboxyl with hydrogen bonding |
| 3497 | 3540 | — | — | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (OH)–hydroxyl (OH)–hydroxyl |
| — | — | — | 3415 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (OH)–hydroxyl with hydrogen bonding (OH)–hydroxyl with hydrogen bonding |
| — | — | 3256 | 3270 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (OH)–alcohol (OH)–alcohol |
| — | — | 949 | 956 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (C–N) stretching-ammonium structure (C–N) stretching-ammonium structure |
| 3063 | Overlap in 3050 | — | 3023 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (CH) stretching (CH) stretching |
| — | — | 2743–3018 | 2744–3019 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (–CH2–), (–CH3) stretching (–CH2–), (–CH3) stretching |
| — | 635 | — | 640 | ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) (Cu–O) (Cu–O) |
In addition, the EDX pattern and mapping analyses of [ChCl]4[2GA-Cu(II)] were taken and illustrated precisely the presence of all constructing elements in the novel catalytic solvent structure, especially Cl and the Cu elements (Fig. 4a and b). Accordingly, this analysis provides a further sign for the production of Cu(II)-ADES, as a novel multifunctional catalytic solvent system, in which Cu(II) is responsible for the Lewis acidity property of this system.
The UV-Visible absorption spectra of CuCl2·2H2O, GA, [2GA-Cu(II)], and [ChCl]4[2GA-Cu(II)] were studied by UV-Vis spectrophotometer (Fig. 4c). The presence of the eminent absorption band at around 250 nm can be attributed to π → π* transitions from the full orbital of the phenolic oxygen to the d empty orbital of Cu(II) ions, indicating the prosperous coordination of Cu(II) ion and the ligand.23 Also, this peak in 2GA-Cu(II) and [ChCl]4[2GA-Cu(II)] spectra confirm that the valence of copper after immobilization on the gallic acid and finally in the related prepared Cu(II)-ADES remains +2 and has not changed (Fig. 4c).
2.2 Physicochemical properties of [ChCl]4[2GA-Cu(II)] DES
| Physicochemical properties | ||||||
|---|---|---|---|---|---|---|
| Density (ρ, g cm−3) | Viscosity (μ, mPa s) | Ionic conductivity (k, mS cm−1) | Refractive indexb (nD) | Melting point (°C) | pHb | |
a Salt = ChCl, HBD = [ChCl]4[2GA-Cu(II)], molar ratio of salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD = 4 HBD = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b In solution (0.0025 M) of deionized water at 16.5 °C. 1.b In solution (0.0025 M) of deionized water at 16.5 °C. |
||||||
| Data | 1.31 | 3144 (25 °C) | 1.87 (25 °C) | 1.3439 | 8 | 5.32 |
| 1788 (50 °C) | ||||||
Finally, evaluating the exact content of decorated Cu particles in the [ChCl]4[2GA-Cu(II)] ADES structure was taken through inductively coupled plasma atomic emission spectrometry (ICP-MS) analysis. As a result, the loading Cu particles were measured to be 9 mol% in 2 mL of ADES.
2.3 Evaluation of the effect [ChCl]4[2GA-Cu(II)] as a M-ADES in the synthesis of 1,4-disubstituted 1,2,3-triazoles
For exploring the effect of the novel synthesized Cu(II)-ADES as a multifunctional catalytic solvent system in the synthesis of 1,2,3-triazoles, the one-pot three-component click reaction benzyl bromide (1), sodium azide (2), and phenylacetylene (3a) were elected as the model reaction in selected reaction condition (catalyst (5 mol%), ADES (2 mL), temperature (55 °C), time (0.5 h)) to provide 1-benzyl-4-phenyl-1H-1,2,3-triazole (4a). The results, which have been registered in (Table 3), a range of catalysts, and various ADESs were employed, and all experiments were accomplished using stoichiometric amounts of the starting materials under atmospheric conditions.| Entry | Catalyst | ADES/(mL; ρ (g cm−3)) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: benzyl bromide (1 mmol), sodium azide (1.5 mmol), phenylacetylene (1.2 mmol), catalyst (5 mol%), ADES (2 mL), temperature (55 °C), time (0.5 h).b Isolated yield. | |||
| 1 | CuCl2 | [ChCl]2[GA]/(2; 1.14) | 80 |
| 2 | Ni(OAc)2 | [ChCl]2[GA]/(2; 1.14) | 70 |
| 3 | ZnCl2 | [ChCl]2[GA]/(2; 1.14) | 68 |
| 4 | None | [ChCl]2[GA]/(2; 1.14) | 55 |
| 5 | CuCl2 | None | 22 |
| 6 | None | None | Trace |
| 7 | None | [ChCl]4[2GA-Cu(II)]/(2; 1.31) | 90 |
| 8 | None | [ChCl]4[2GA-Ni(II)]/(2; 1.37) | 75 |
| 9 | None | [ChCl]4[2GA-Zn(II)]/(2; 1.34) | 74 |
To begin the reaction optimization process, the model reaction was run in the presence of Ni(II), Cu(II), and Zn(II) salts separately, and [ChCl]2[GA] as an ADES (Table 3, entries 1–3), which the best efficiency was observed with CuCl2 as Lewis acid (Table 3, entry 1). The reaction was then performed in the presence of [ChCl]2[GA] and the absence of any Lewis acid (Table 3, entry 4) to disclose the demand of the reaction to Cu(II) species. The model reaction resulted in just 22% yield of the title product when the reaction was performed in the presence of CuCl2 without using [ChCl]2[GA] (Table 3, entry 5). Concerning the results gained from previous responses for the increase of ADES [ChCl]2[GA] performance and avoidance of direct use of metal salts and running the reaction in the heterogeneous media, we established a novel type of M-ADES as [ChCl]4[2GA-Cu(II)] afterward, the model reaction was carried out in the presence of [ChCl]4[2GA-Cu(II)] (Table 3, entry 7). It is clear that the present reactivity systems, compared to entry 1, approximately created no significant change in the desired product yield, Albeit the acceptable conversion was attained by executing the model reaction in the presence of [ChCl]4[2GA-Cu(II)] without the utilization of any common solvents (Table 3, entry 7). Due to the excellent result obtained subsequently, the performance of [ChCl]4[2GA-Cu(II)] was evaluated as a solvent-catalytic system and compared with other M-ADESs prepared from ChCl and [2GA-Ni(II)], and [2GA-Zn(II)] complexes (Table 3, entries 8 and 9). The results of these experiments revealed the supremacy of [ChCl]4[2GA-Cu(II)] over the other M-ADESs synthesized (Table 3, entry 7). Thereupon, by discovering the best catalytic solvent system ([ChCl]4[2GA-Cu(II)]) for the synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, we used the response surface methodology (RSM) for optimization. RSM is a method to evaluate the optimal situations in a multivariate system to achieve a maximum response rate. The most significant benefits of RSM can be included: (a) reduction in the number of tests and less time-consuming and save costs, (b) increase the accuracy and repeatability of tests, and (c) investigate the effect of different factors on each other and changing tendencies. Accordingly, to optimize the reaction conditions, RSM was used. To design experiments, changes in the input variables are made and then, the number of changes in the output response through the software is checked.25
2.4 Experimental design by central composite design (CCD)
Therefore, after determining the effectiveness of [ChCl]4[2GA-Cu(II)] M-ADES (Table 3, entry 7), a central composite design (CCD) was applied to investigate the effects of experiential factors and their significant interactions exhibited in the experiments.26 So, a CCD was utilized for three parameters to design experiments. As summarized in Table 4, three-level of three independent process variables such as [ChCl]4[2GA-Cu(II)] (mL), temperature, and time were selected by Box–Behnken. Next, to design production by choosing the target amount of factors and the highest yield of the desired product, also introducing two repetitions from the central point as the input into the Design-Expert software (version 13) eventually designed for us fourteen experiments as the output (Table 5).| Variables | Unit | Symbol coded | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Time | h | A | 0.75 | 1 | 1.25 |
| [ChCl]4[2GA-Cu(II)] | mL | B | 1 | 2 | 3 |
| Temperature | °C | C | 40 | 50 | 60 |
| Run | A: time (h) | B: [ChCl]4[2GA-Cu(II)] (mL)/(mol% of Cu) | C: temperature (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzyl bromide (1 mmol), sodium azide (1.5 mmol), phenylacetylene (1.2 mmol).b Isolated yield. | ||||
| 1 | 0.75 | 2/9 | 40 | 82 |
| 2 | 1 | 3/13.5 | 60 | 84 |
| 3 | 0.75 | 1/4.5 | 50 | 70 |
| 4 | 0.75 | 3/13.5 | 50 | 86 |
| 5 | 1 | 2/9 | 50 | 98 |
| 6 | 1 | 1/4.5 | 60 | 75 |
| 7 | 0.75 | 2/9 | 60 | 88 |
| 8 | 1.25 | 2/9 | 60 | 95 |
| 9 | 1 | 3/13.5 | 40 | 82 |
| 10 | 1 | 1/13.5 | 40 | 69 |
| 11 | 1.25 | 1/4.5 | 50 | 78 |
| 12 | 1 | 2/9 | 50 | 98 |
| 13 | 1.25 | 2/9 | 40 | 89 |
| 14 | 1.25 | 3/13.5 | 50 | 89 |
Experiments were performed, and the desired product efficiency was calculated in each of the conditions; the fit of the results obtained in the laboratory scale with the results predicted by the software (adjusted R2 = 0.9959, sequential p-value < 0.0001) is shown in Fig. 6. The best yield for the model reaction was obtained in [ChCl]4[2GA-Cu(II)] (2 mL), time (1 h), and temperature 50 °C (entries 5 and 12) and was considered as the best reaction condition.
In fact, ramps are preferred to visualize the optimization results by researchers, as shown in Fig. 7 these are a sample of numerical optimization for maximizing desired product production. Accordingly, by accepting the values of the factors to the laboratory conditions and performing the test, no exceptional differences were found between experimental data and predicted data by Design-Expert software, and can be reliably expressed simulation results. As a result, the conditions time (1 h), [ChCl]4[2GA-Cu(II)] (2 mL), 50 °C with two repetitions entries were recorded as optimal conditions for the continuing process. Due to the excellent result observed from the [ChCl]4[2GA-Cu(II)] catalytic solvent system to enhance the scope of reactants and the synthesis of novel high-performance triazole compounds, at first, we decided to synthesize a series of terminal alkynes of aromatic/aliphatic amines and caprolactam. In this way, we applied an impressive K2CO3-promulgated method for the N-alkynation of 5H-dibenzo[b,f]azepine and N-ethylaniline (3b and 3d) as well as N-alkynation of diphenylamine and caprolactam (3c and 3e) by NaNH2 as a strong base by propargyl bromide in dry N,N-dimethylformamide (DMF) at room temperature for 4 to 5 hours to procuring desired compounds in good yields (Table S1 in ESI,† 3b–e).
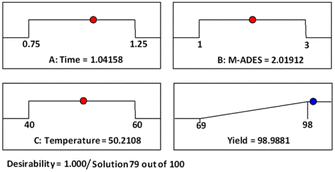 | ||
| Fig. 7 Adjusted numerical optimization for maximizing the desired product production by Design-Expert software. | ||
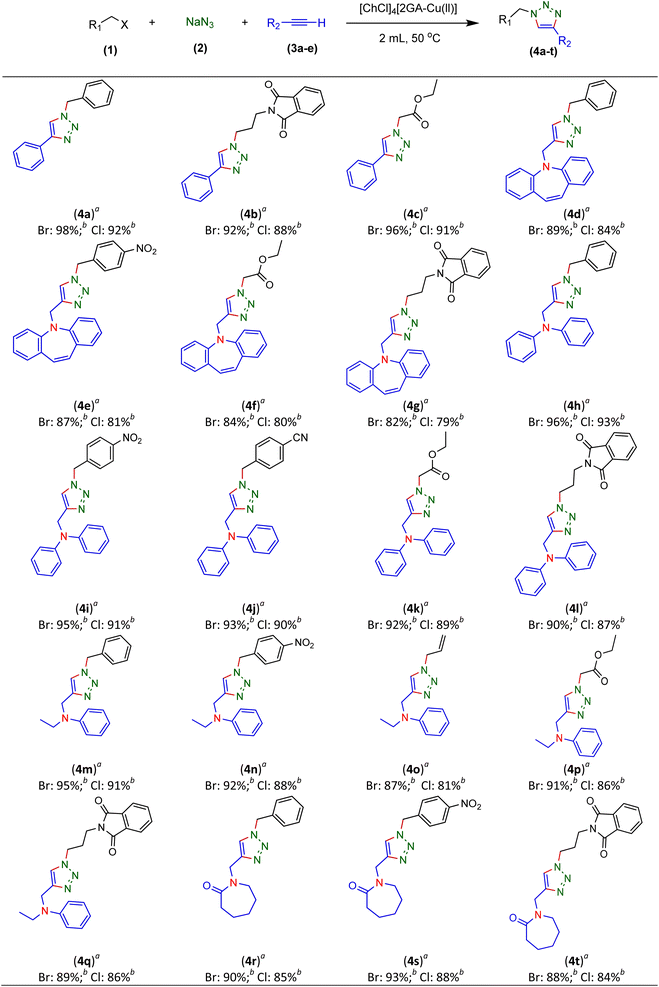
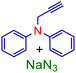
Therefore, phenylacetylene (3a) and synthesized terminal N-alkynes (3b–e) in the presence of NaN3 and aryl/alkyl/allyl and ester halides provided the novel various 1,4-disubstituted 1,2,3-triazoles preparation under the optimized reaction conditions (M-ADES: 2 mL, time: 1 h, temperature: 50 °C) in the present [ChCl]4[2GA-Cu(II)] M-ADES as solvent/catalyst system (Table 6 and Scheme 3).
With the optimized conditions in hand and various types of terminal alkynes, for the finding of the potential scope and versatility of reagents for this CuAAC one-pot three-component procedure, under very favorable situations, different types of benzyl, allyl, alkyl, and ester halides were used as the substrates for the production of 1,4-disubstituted 1,2,3-triazoles which the outcomes are rendered in Table 6. In all cases, expected triazoles 4a–t were acquired in high yields (up to 98%) after an ordinary purification process exhibiting an extensive scope and high endurance of functional groups in the reaction media. We first treated structurally diverse halides with phenylacetylene and NaN3, which reacted with excellent efficiency (Table 6, 4a–4c in 92–98% yields), as bromine halides behaved very excellent, while the idem triazoles 4a–4c were generated in 88–91% yields with the less active chlorine halides. Afterward, to exhibit reaction generality, the terminal N-alkynes derived (Table S1 in ESI,† 3b–e) were investigated to give all the desired triazoles (Table 6, 4d–t) in good to high yields regardless of different functional groups. On the other hand, ester and allyl functional groups sustained allowed us to acquire the desired products (4c, f, k, o, and p), which can be benefited as scaffolds for complex structures. Besides, the existence of benzyl halides with electron-withdrawing (–NO2, –CN) groups (4e, i, j, and n) led to better efficiency in comparison with the propyl-isoindoline-1,3-dione group (4b, f, k, o, and p) (Table 6).
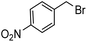
Notwithstanding the above impressive results, the utilization of the presented efficacious strategy was examined for the preparation of N-((1-(4-nitrobenzyl)-1H-1,2,3-triazole-4-yl)methyl)-N-phenylaniline and 4-((4-((diphenylamino)methyl)-1H-1,2,3-triazole 1-yl)methyl)benzonitrile in several scales-up (1, 10, and 50 mmol) comprising a nitro/nitrile group that can be transformed into an amine functional group with high performance in organic synthesis and pharmaceutical industries also as appropriate functional groups in other organic reactions. Furthermore, diphenylamine derivatives and concatenation of cyclic triazole into diphenylamine would modify the physicochemical functions and acquire applications in the scope of biology and material science.27 It is noteworthy that the registered outputs in Table 7 manifested the supreme capacity and ability of [ChCl]4[2GA-Cu(II)] to be applied in chemical industrial manufacturers in the future.
| Substrates | Aryl halide | Amount of aryl halide (mmol) | ADES (mL) | Yieldd (%) |
|---|---|---|---|---|
| a Reaction conditions: N-phenyl-N-(prop-2-yn-1-yl)aniline (1.2 mmol), sodium azide (1.5 mmol).b Reaction conditions: N-phenyl-N-(prop-2-yn-1-yl)aniline (12.5 mmol), sodium azide (15.5 mmol).c Reaction conditions: N-phenyl-N-(prop-2-yn-1-yl)aniline (62.5 mmol), sodium azide (77.5 mmol).d Isolated yield. | ||||
 |
 |
1 | 2 | 95a |
| 10 | 5 | 92b | ||
| 50 | 15 | 89c | ||
 |
1 | 2 | 93a | |
| 10 | 5 | 90b | ||
| 50 | 15 | 88c | ||
To prove the Cu(II)-ADES performance in this process, we have recorded in Fig. 8 the UV-Vis spectra for fresh [ChCl]4[2GA-Cu(ll)] and CuCl2 treated with NaN3 to ascertain confirmation of the oxidation state of Cu(II) to Cu(I) by sodium azide in the present protocol. In both UV-Vis spectra, the corresponding absorptions of Cu(I) in 300–425 nm wavelength and maximum intensity in wavelength of 270 nm appeared, which demonstrates Cu(I) is present in both spectra, according to the results collected from the UV absorption spectrum was determined that after treatment with NaN3, Cu(II) gets reduced to Cu(I) state.28
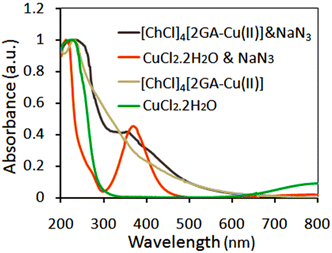 | ||
| Fig. 8 UV-Vis normalized spectra of CuCl2 & NaN3, [ChCl]4[2GA-Cu(II)] & NaN3, [ChCl]4[2GA-Cu(II)], and CuCl2·2H2O in deionized water (0.0005 M). | ||
It is not possible to present the reaction mechanism in detail according to the complexity and novelty of the catalytic effect of DESs, but considering the experiments done and the prior reports in the literature, a plausible mechanism has been proposed for the above-mentioned construction of 1,4-disubstituted 1,2,3-triazoles of aryl alkyl amines via CuAAC without using an extra reducing agent, as has been illustrated in Scheme 4. In primary, reducing Cu(II) to Cu(I) of catalyst will occur by sodium azide, as one of the required starting materials to provide A.26 Then, the acetylene-activated complex B is formed through the interaction with the hydrogen bond acceptors groups of DES to loss a proton in the absence of base and provide the copper complex C.12 The nucleophilic attack on C by the in situ generated organic azide is led to the intermediate D, which undergoes an intermolecular cycloaddition reaction to give the isomer E.29 Protonolysis of F, which is formed in turn via the rearrangement reaction in E, creates the desire triazole H and regenerates Cu(I) species.
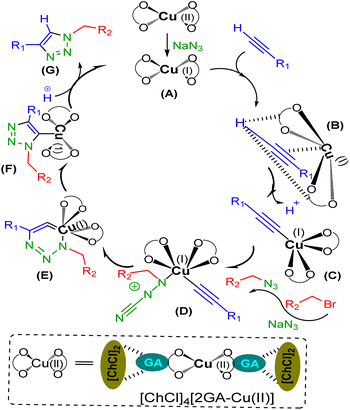 | ||
| Scheme 4 Proposed mechanism of one-pot CuAAC reaction using [ChCl]4[2GA-Cu(II)] for the regioselective formation of 1,4-disubstituted 1,2,3-triazoles. | ||
Confidently, the durability and recyclability of the catalytic systems are very significant in terms of being eco-friendly and economically at the industrial level and drafting efficient and green synthetic processes due to lessening detrimental environmental and cost-effectiveness impacts. For this purpose, we evaluated these factors of [ChCl]4[2GA-Cu(II)] as a solvent/catalyst system in the optimized reaction conditions, as shown in Fig. 9.
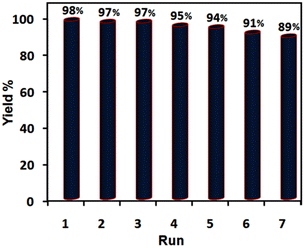 | ||
| Fig. 9 Evaluating recyclability and reusability of [ChCl]4[2GA-Cu(II)] in the synthesis of 1-benzyl-4-phenyl-1H-triazole under the optimized conditions. | ||
Therefore, in the [ChCl]4[2GA-Cu(II)] recycling experiment, after completing the reaction in each round, diethyl ether (2 × 15 mL) was added to the reaction media to extract all remaining precursors and products. Then [ChCl]4[2GA-Cu(II)] dissolved in 10 mL water and was simply recovered by extraction with ethyl acetate. Finally, Cu-ADES was dehumidified by evaporation of the organic layer under vacuum conditions at 70 °C for 1 h and then reused in the next run. According to the related outputs illustrated in Fig. 9 an insignificant decrease in the intended product yield after seven successive cycles. So, these data indicate that the catalytic activity of [ChCl]4[2GACu(II)] has been maintained after seven consecutive cycles.
3. Experimental section
All the information is perfectly presented in the ESI file.†4. Conclusions
In summary, a novel and green three-component metal acidic deep eutectic solvent, Cu(II)-ADES, bearing copper salt, gallic acid, and choline chloride, was synthesized and fully identified by physicochemical techniques as a solvent/catalyst system. This benign and cost-effective M-ADES exhibited excellent reactivity when it was served for creating a novel library of 1,4-disubstituted 1,2,3-triazoles via three-component click reactions in base-free and reducing agent-free conditions from a variety of benzyl, allyl, ester, and alkyl halides, sodium azide, and terminal alkynes, which mainly were manufactured of various aromatic and aliphatic amines as well as caprolactam. This catalyst/solvent system, [ChCl]4[2GA-Cu(II)], was also recyclable and reusable without considerable loss in its activity after seven consecutive runs.Consequently, herein, in addition to the establishment of a novel family of DES, it has been possible to find a synthetic methodology using this M-ADES which satisfies most of the economic and environmental aspects in the green chemical industry by including these reliable features: (a) inexpensive, reusable, and ecological friendliness multifunctional M-ADES in base-free condition, (b) easily accessible aryl/alkyl amines and cyclic amide as highly beneficial precursors for terminal alkynes, and ultimately, (c) development of practical extensive scale-up synthesis to procure 1,4-disubstituted 1,2,3-triazoles with nitro, nitrile, allyl, and ester functional groups that will be useful for subsequent synthetic applications.
Author contributions
Nastaran Bagherzadeh: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing-original draft, visualization. Mohammad Amiri: investigation, resources. Ali Reza Sardarian: conceptualization, project administration, resources, funding acquisition, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to sincerely appreciate the financial support of the research council of Shiraz University.References
- J. J. Yang, W. W. Yu, L. L. Hu, W. J. Liu, X. H. Lin, W. Wang, Q. Zhang, P. L. Wang, S. W. Tang, X. Wang and M. Liu, J. Med. Chem., 2019, 63, 569–590 CrossRef PubMed
.
-
(a) J. Das, S. Dey and T. Pathak, J. Org. Chem., 2019, 84, 15437–15447 CrossRef CAS PubMed
; (b) I. L. Wong, X. Zhu, K. F. Chan, M. C. Law, A. M. Lo, X. Hu, L. M. Chow and T. H. Chan, J. Med. Chem., 2018, 61, 9931–9951 CrossRef CAS PubMed
; (c) A. Dondoni, Org. Biomol. Chem., 2010, 8, 3366–3385 RSC
; (d) N. Agouram, E. M. E. Hadrami and A. Bentama, Molecules, 2021, 26, 2937 CrossRef CAS PubMed
; (e) X. Gu, S. Guo and G. Wang, Polym. Eng. Sci., 2020, 60, 3270–3280 CrossRef CAS
; (f) S. Yamasaki, Y. Kamon, L. Xu and A. Hashidzume, Polym, 2021, 13, 1627 CrossRef CAS PubMed
; (g) K. Bozorov, J. Zhao and H. A. Aisa, Bioorg. Med. Chem., 2019, 27, 3511–3531 CrossRef CAS PubMed
.
-
(a) L. Sun, Y. Li, Z. Liang, J. Yu and R. Xu, Dalton Trans., 2012, 41, 12790–12796 RSC
; (b) X. J. Wang, P. Z. Li, Y. Chen, Q. Zhang, H. Zhang, X. X. Chan, R. Ganguly, Y. Li, J. Jiang and Y. Zhao, Sci. Rep., 2013, 3, 1–5 Search PubMed
.
-
(a) H. Singh, J. Sindhu and J. M. Khurana, J. Lumin., 2015, 158, 340–350 CrossRef CAS
; (b) G. O. Resende, S. F. Teixeira, I. F. Figueiredo, A. A. Godoy, D. J. F. Lougon, B. A. Cotrim and F. C. D. Souza, Int. J. Electrochem., 2019, 2019, 12 Search PubMed
.
-
(a) P. K. Bangalore, S. K. Vagolu, R. K. Bollikanda, D. K. Veeragoni, P. C. Choudante, S. Misra, D. Sriram, B. Sridhar and S. Kantevari, J. Nat. Prod., 2019, 83, 26–35 CrossRef
; (b) S. Zhang, Z. Xu, C. Gao, Q. C. Ren, L. Chang, Z. S. Lv and L. S. Feng, Eur. J. Med. Chem., 2017, 138, 501–513 CrossRef CAS
; (c) B. Zhang, Eur. J. Med. Chem., 2019, 168, 357–372 CrossRef CAS
; (d) N. Ashwini, M. Garg, C. D. Mohan, J. E. Fuchs, S. Rangappa, S. Anusha, T. R. Swaroop, K. S. Rakesh, D. Kanojia, V. Madan and A. Bender, Bioorg. Med. Chem., 2015, 23, 6157–6165 CrossRef CAS PubMed
.
-
(a) F. Naaz, M. P. Pallavi, S. Shafi, N. Mulakayala, M. S. Yar and H. S. Kumar, Bioorg. Chem., 2018, 81, 1–20 CrossRef CAS PubMed
; (b) R. R. Ruddarraju, A. C. Murugulla, R. Kotla, M. C. B. Tirumalasetty, R. Wudayagiri, S. Donthabakthuni, R. Maroju, K. Baburao and L. S. Parasa, Eur. J. Med. Chem., 2016, 123, 379–396 CrossRef CAS PubMed
; (c) G. F. Maria de Lourdes, L. C. Pinheiro, O. A. Santos-Filho, M. D. Peçanha, C. Q. Sacramento, V. Machado, V. F. Ferreira, T. M. L. Souza and N. Boechat, Med. Chem. Res., 2014, 23, 1501–1511 CrossRef
.
-
(a) J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302–1315 RSC
; (b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. A. Sharpless, Angew. Chem., Int. Ed., 2002, 141, 2596–2599 CrossRef
.
- F. Llgen and B. König, Green Chem., 2009, 11, 848–854 RSC
.
- C. Vidal and J. García-Álvarez, Green Chem., 2014, 16, 3515–3521 RSC
.
- J. E. De La Cerda-Pedro, S. Rojas-Lima, R. Santillan and H. López-Ruiz, J. Mex. Chem. Soc., 2015, 59, 130–136 CAS
.
-
(a) G. Vilé, G. Di Liberto, S. Tosoni, A. Sivo, V. Ruta, M. Nachtegaal, A. H. Clark, S. Agnoli, Y. Zou, A. Savateev, M. Antonietti and G. Pacchioni, ACS Catal., 2022, 12, 2947–2958 CrossRef
; (b) J. Héron and D. Balcells, ACS Catal., 2022, 12, 4744–4753 CrossRef
; (c) A. K. Feldman, B. Colasson and V. V. Fokin, Org. Lett., 2004, 6, 3897–3899 CrossRef CAS PubMed
; (d) G. Acquaah-Harrison, S. Zhou, J. V. Hines and S. C. Bergmeier, J. Comb. Chem., 2010, 12, 491–496 CrossRef CAS
.
- S. V. Giofrè, M. Tiecco, A. Ferlazzo, R. Romeo, G. Ciancaleoni, R. Germani and D. Iannazzo, Eur. J. Org Chem., 2021, 2021, 4777–4789 CrossRef
.
- S. Pan, S. Yan, T. Osako and Y. Uozumi, ACS Sustainable Chem. Eng., 2017, 5, 10722–10734 CrossRef CAS
.
-
(a) C. P. Kaushik, R. Luxmi, M. Kumar, D. Singh, K. Kumar and A. Pahwa, Synth. Commun., 2019, 49, 118–128 CrossRef CAS
; (b) P. Kalra, R. Kaur, G. Singh, H. Singh, G. singh, G. Kaur and J. Singh, J. Organomet. Chem., 2021, 994, 121846 CrossRef
; (c) M. Emami, R. Bikas, N. Noshiranzadeh, A. Kozakiewicz and T. Lis, ACS Omega, 2020, 5, 13344–13357 CrossRef CAS PubMed
.
-
(a) R. D. Rogers, Science, 2003, 302, 792–793 CrossRef PubMed
; (b) D. Zhao, Y. Liao and Z. Zhang, Clean: Soil, Air, Water, 2007, 35, 42–48 CAS
; (c) D. Coleman and N. Gathergood, Chem. Soc. Rev., 2010, 39, 600–637 RSC
.
-
(a) N. Bagherzadeh, A. R. Sardarian and I. Dindarloo Inaloo, New J. Chem., 2021, 45, 11852–11858 RSC
; (b) A. J. Maneffa, A. B. Harrison, S. J. Radford, A. S. Whitehouse, J. H. Clark and A. S. Matharu, ChemistryOpen, 2020, 9, 550–558 CrossRef
; (c) A. Gutiérrez-Hernández, A. Richaud, L. Chacón-García, C. J. Cortés-García, F. Méndez and C. A. Contreras-Celedón, J. Org. Chem., 2021, 86, 223–234 CrossRef
; (d) Y. Riadi, O. Ouerghi, A. Kaiba and P. Guionneau, J. Mol. Liq., 2021, 323, 115011 CrossRef CAS
; (e) Y. Ni, Z. Bi, H. Su and L. Yan, Green Chem., 2019, 21, 1075–1079 RSC
.
-
(a) K. A. Omar and R. Sadeghi, J. Mol. Liq., 2022, 360, 119524 CrossRef CAS
; (b) M. Tiecco, A. Grillo, E. Mosconi, W. Kaiser, T. Del Giacco and R. Germani, J. Mol. Liq., 2022, 364, 120043 CrossRef CAS
; (c) J. Liu, X. Li and K. H. Row, J. Mol. Liq., 2022, 362, 119654 CrossRef CAS
; (d) Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC
.
- S. P. Santoso, V. Bundjaja, A. E. Angkawijaya, C. Gunarto, A. Woo Go, M. Yuliana, P. L. Tran-Nguyen, C. W. Hsieh and Y. H. Ju, Sci. Rep., 2021, 11, 12021 CrossRef CAS PubMed
.
-
(a) A. E. Fazary, E. Hernowo, A. E. Angkawijaya, T. C. Chou, C. H. Lin, M. Taha and Y. H. Ju, J. Solution Chem., 2011, 40, 1965–1986 CrossRef CAS
; (b) K. Chen and D. Xue, CrystEngComm, 2012, 14, 8068–8075 RSC
; (c) B. Azhar, A. E. Angkawijaya, S. P. Santoso, C. Gunarto, A. Ayucitra, A. Woo Go, P. L. Tran-Nguyen, S. Ismadji and Y. H. Ju, Sci. Rep., 2020, 10, 19212 CrossRef CAS PubMed
.
-
(a) Z. Maugeri and P. D. de María, RSC Adv., 2012, 2, 421–425 RSC
; (b) Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC
.
- M. Gijare, S. Chaudhari, S. Ekar and A. Garje, J. Anal. Sci. Technol., 2021, 12, 1–10 CrossRef
.
- T. Aissaoui, Pharm. Anal. Acta, 2015, 6, 2153–2435 Search PubMed
.
- H. R. Ong, M. M. R. Khan, R. Ramli, Y. Du, S. Xi and R. M. Yunus, RSC Adv., 2015, 5, 24544–24549 RSC
.
-
(a) A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed
; (b) A. P. Abbott, R. C. Harris and K. S. Ryder, J. Phys. Chem. B, 2007, 111, 4910–4913 CrossRef CAS PubMed
.
-
(a) A. L. García-Cabeza, L. P. Ray, R. Marín-Barrios, M. J. Ortega, F. J. Moreno-Dorado, F. M. Guerra and G. M. Massanet, Org. Process Res. Dev., 2015, 19, 1662–1666 CrossRef
; (b) J. Fan, C. Yi, X. Lan and B. Yang, Org. Process Res. Dev., 2013, 17, 368–374 CrossRef CAS
.
- A. Asfaram, M. Ghaedi, S. Agarwal, I. Tyagi and V. K. Gupta, RSC Adv., 2015, 5, 18438–18450 RSC
.
-
(a) B. Baumeister and S. Matile, Chem. Commun., 2000, 913–914 RSC
; (b) B. Baumeister, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2000, 39, 1955–1958 CrossRef CAS
; (c) H. K. Ulbrich, A. Luxenburger, P. Prech, E. E. Eriksson, O. Soehnlein, P. Rotzius, L. Lindbom and G. A. Dannhardt, J. Med. Chem., 2006, 49, 5988–5999 CrossRef CAS PubMed
; (d) P. Rajakumar, C. Satheeshkumar and S. Raja, Tetrahedron Lett., 2010, 51, 5167–5172 CrossRef CAS
; (e) K. Ohta, Y. Chiba, T. Ogawa and Y. Endo, Bioorg. Med. Chem. Lett., 2008, 18, 5050–5053 CrossRef CAS PubMed
.
-
(a) S. Mohammed, A. K. Padala, B. A. Dar, B. Singh, B. Sreedhar, R. A. Vishwakarma and S. B. Bharate, Tetrahedron, 2012, 68, 8156–8162 CrossRef CAS
; (b) S. Hamad, G. K. Podagatlapalli, S. P. Tewari and S. V. Rao, Pramana, 2014, 82, 331–337 CrossRef CAS
.
- A. Francis and R. J. S. Carey, Advanced Organic Chemistry, 2007, pp. 629–711, ISBN: 978-0-387-44897-8 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06570g |
| This journal is © The Royal Society of Chemistry 2023 |