Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The volatile chemistry of orchid pollination
James
Perkins
a,
Tobias
Hayashi
a,
Rod
Peakall
ab,
Gavin R.
Flematti
b and
Björn
Bohman
 *abc
*abc
aResearch School of Biology, The Australian National University, Australia
bSchool of Molecular Sciences, The University of Western Australia, Australia
cDepartment of Plant Protection Biology, Swedish University of Agricultural Sciences, Sweden. E-mail: bjorn.bohman@slu.se
First published on 24th January 2023
Abstract
Covering: up to September 2022
Orchids are renowned not only for their diversity of floral forms, but also for their many and often highly specialised pollination strategies. Volatile semiochemicals play a crucial role in the attraction of a wide variety of insect pollinators of orchids. The compounds produced by orchid flowers are as diverse as the pollinators they attract, and here we summarise some of the chemical diversity found across orchid taxa and pollination strategies. We focus on compounds that have been experimentally demonstrated to underpin pollinator attraction. We also highlight the structural elucidation and synthesis of a select subset of important orchid pollinator attractants, and discuss the ecological significance of the discoveries, the gaps in our current knowledge of orchid pollination chemistry, and some opportunities for future research in this field.
1 Background
The orchid family (Orchidaceae) is one of the most species-rich and diverse of all plant families, with over 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 species.1 Orchids are also well known for the extraordinary diversity of their interactions with animal pollinators, making use of unusual and often highly specialized pollination strategies that go well beyond the typical exchange of nectar or pollen as food to pollinators for the transfer of gametes. Some orchids provide unusual rewards, such as perfumes to male euglossine bee pollinators, which collect them to attract mates.2 Many other orchids employ deceptive pollination strategies. For example, many ‘food deceptive’ orchids mimic the visual and olfactory signals of rewarding flowers, but do not provide a tangible reward. Others have evolved even more complex means to attract pollinators, such as mimicking sites for insects to lay eggs,3 or even the mimicry of insects themselves. Some insect-mimicking species emit alarm pheromones of aphids4 or honey bees5 to attract predators of those species as pollinators, and others emit aggregation pheromones to attract large swarms of potential pollinators.6 By far the most common insect-mimicry pollination strategy amongst orchids is ‘sexual deception’, whereby orchids mimic the sex pheromones and appearance of specific female insects to sexually lure conspecific males as pollinators. In these cases, pollination often occurs during attempted copulation with the flower, highlighting the extreme effectiveness of this bizarre trickery.7,8
000 species.1 Orchids are also well known for the extraordinary diversity of their interactions with animal pollinators, making use of unusual and often highly specialized pollination strategies that go well beyond the typical exchange of nectar or pollen as food to pollinators for the transfer of gametes. Some orchids provide unusual rewards, such as perfumes to male euglossine bee pollinators, which collect them to attract mates.2 Many other orchids employ deceptive pollination strategies. For example, many ‘food deceptive’ orchids mimic the visual and olfactory signals of rewarding flowers, but do not provide a tangible reward. Others have evolved even more complex means to attract pollinators, such as mimicking sites for insects to lay eggs,3 or even the mimicry of insects themselves. Some insect-mimicking species emit alarm pheromones of aphids4 or honey bees5 to attract predators of those species as pollinators, and others emit aggregation pheromones to attract large swarms of potential pollinators.6 By far the most common insect-mimicry pollination strategy amongst orchids is ‘sexual deception’, whereby orchids mimic the sex pheromones and appearance of specific female insects to sexually lure conspecific males as pollinators. In these cases, pollination often occurs during attempted copulation with the flower, highlighting the extreme effectiveness of this bizarre trickery.7,8
Whether it is by honest signalling of reward or by deception, orchid pollinator attraction is often dependent on volatile or semi-volatile compounds. Indeed, orchids are the most well-represented family in studies of plant volatiles, and are the source of many unusual natural products.9 Given the great diversity of orchids and their numerous chemically mediated pollinator interactions, orchids likely offer a treasure trove of natural products remaining to be discovered.
While much progress has been made towards cataloguing the diversity of known floral volatiles within orchids,9–12 relatively few studies have confirmed the ecological activity of the compounds, and fewer still have elucidated the chemical structures of potentially new-to-science compounds. When done systematically, such studies provide valuable insights into the diversity of compounds used by both plants and insects for communication, and aid our understanding of the evolution of plant–animal interactions.
In this review, we highlight the diversity of volatile and semi-volatile natural products from orchid flowers that may be relevant to pollination. We broadly follow the groupings established in prior reviews of plant volatiles,9,12,13 which class compounds based on their likely biosynthetic origin into three main groups – fatty acid derivatives, isoprenoids, and benzenoids. We prioritise compounds for which credible evidence for biological activity has been established. We regard experimental confirmation of pollinator attraction in field bioassays with reference compounds as the most compelling evidence for the biological activity of a compound, while we recognise that experiments with wind tunnels and/or olfactometers in the laboratory may also provide such evidence. Many studies report findings on the electrophysiological activity of the compounds extracted from orchids to one or more species of pollinator, based on coupled GC-Electroantennography Detection (GC-EAD), or less commonly via electroantennography (EAG) or GC-Single Sensillum Recordings (GC-SSR).14 While electrophysiological activity of a compound does not always translate to attractiveness in field experiments (see final remarks, 7.1), antennal electrophysiology is often critical for narrowing down the number of compounds of interest for further experiments in the field. Therefore, we highlight electrophysiological results where possible. Finally, we briefly explore the methods used to elucidate and synthetically prepare some orchid pollinator attractants, before discussing what we see as some exciting opportunities in this expansive field in the coming years.
2 Fatty acid derivatives
Fatty acid derivatives are often shorter in chain length than their precursor and may or may not retain a carbonyl moiety. They are formed by oxidative cleavage and/or decarboxylation of the fatty acid precursor.15 The biosynthesis of fatty acids from condensation of acetic acid (C2) units is well known and is catalysed by the enzyme fatty acid synthase. In animals and fungi, this is a single large multifunctional protein, however in plants and bacteria the individual steps are catalysed by an assembly of discrete enzymes, allowing for much more diversity in structure.16 These differences in biosynthesis make the plant and bacterial enzymes attractive targets for herbicides and antibacterials, respectively.17,182.1 Alkanes and alkenes
Alkanes and alkenes are biosynthesised from fatty acid intermediates through reduction to fatty aldehydes, followed by decarbonylation via aldehyde decarbonylase enzymes that have been found in both plants and insects.19,20 This pathway results in the production of mostly odd-numbered hydrocarbons from even-numbered fatty acid precursors. Unsaturation to form alkenes is achieved earlier in the biosynthetic sequence by desaturase enzymes acting on fatty acid derivatives, yielding predominantly (Z)-configured compounds in both plants and insects.21Unbranched saturated hydrocarbons (n-alkanes), and unsaturated hydrocarbons (alkenes), are important constituents of the cuticular waxy layer of plants, which has a critical primary function of protection against water loss, UV light, pathogens, and pests.22,23 Cuticular alkanes and alkenes are also widely found in insects, where they function as protection against desiccation and also as signalling molecules.24,25 Specific alkanes and alkenes or combinations are widely known as sex pheromones and mate recognition cues across a range of insect groups,26 including the Hymenopteran pollinators of some orchids. It is perhaps unsurprising, then, that alkanes and alkenes have been demonstrated to play key roles in the pollination of sexually deceptive orchids.
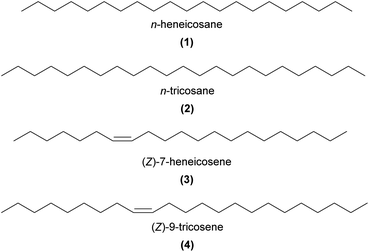
Using GC-EAD in combination with field bioassays, Schiestl et al.27,28 were the first to convincingly demonstrate female insect sex pheromone mimicry in sexually deceptive Ophrys orchids. The initial breakthrough was made in O. sphegodes, pollinated by sexually attracted male Andrena nigroaenea bees. A blend of 14 hydrocarbons (C19–C29, e.g.1 and 2) that had been found to be both EAD-active to the male bee pollinator and shared between the orchid labellum and female bees, were also sexually attractive in field bioassays.28 Later, in O. exaltata, a specific blend of EAD-active alkenes and n-alkanes was found to be attractive to male Colletes cunicularius bee pollinators, but alkenes were also attractive alone (e.g.3 and 4).29 In both orchid systems, alkene double bond configuration is pivotal for the specific bee pollinator attraction ((Z)-9, 11, and 12 in O. sphegodes, and (Z)-7 and 9 in O. exaltata but with the (Z)-7-alkenes being the most attractive).27–29
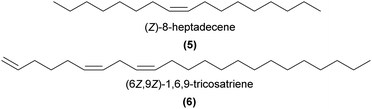
Alkanes and alkenes are now widely implicated as key components of the sex pheromone mimicry across bee-pollinated European Ophrys (see detailed reviews in ref. 7 and 30–32). In particular, C21–C33n-alkanes and specific alkenes, always in (Z) configuration, have been demonstrated to be EAD-active to the bee pollinators of multiple Ophrys species.27–29,33–37 Shorter chain hydrocarbons, (Z)-8-heptadecene (5) and n-pentadecane have been shown to be EAD-active in the wasp-pollinated O. insectifera, although their role in pollinator attraction remains unknown.38 Alkadienes, typically C25–C33 and all (Z), have also been detected in some Ophrys spp.,31,39,40 including some that are EAD-active,29 although their role in pollinator attraction is also yet to be demonstrated.
Perhaps one of the most unusual examples of hydrocarbon sex pheromone mimicry is found in the Australian orchid Pterostylis orbiculata, which attracts its male Mycomya sp. fungus gnat pollinator with a tri-unsaturated hydrocarbon.41 Laboratory bioassays with captive male gnats revealed that males responded to a GC-derived fraction containing two unsaturated hydrocarbons subsequently identified as (6Z,9Z)-1,6,9-tricosatriene (6) and (6Z,9Z)-6,9-tricosadiene. Field bioassays confirmed that male gnats were sexually attracted to a synthetic blend of five compounds in common between orchid labella and female gnats, comprising the two unsaturated hydrocarbons and three C21–C25n-alkanes. The triene alone was attractive, although gnats displayed weaker sexual behaviour than to the blend, and the blend without the triene was unattractive. In related species, (3Z,6Z,9Z)-3,6,9-tricosatriene (P. concava) and an unidentified tricosatriene and tricosatetraene (P. vittata) were detected, suggesting polyenes may be key to pollinator attraction in other Pterostylis species.41 Polyenes are not as common in nature as monoenes or dienes, but are well known as type II moth sex pheromones42 and have been occasionally reported in other insects43 and plants.42
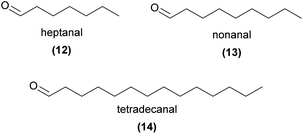
Alkenes are also components of the pollinator-attracting blend in the South American orchid Telipogon peruvianus, pollinated by male Eudejeania tachinid flies. Only pre-copulatory behaviour is observed at the orchid flower in this system,44 and a more complex mechanism than sex pheromone mimicry alone may be involved.45 Nonetheless, the EAD-active blend is dominated by (Z)-9-tricosene (4), along with other C20–C25 saturated and monounsaturated hydrocarbons and the aldehyde tetradecanal (14) in similar ratios to the female fly.45 Field bioassays with synthetic blends in both flower and female fly ratios elicited pre-copulatory behaviour from male flies, but attempted copulation was not observed.45
Alkenes and n-alkanes (C23–C31, alkenes in (Z)-9 configuration) also dominate the solvent extracts of another sexually deceptive South American orchid Maxillaria lineolata (previously Mormolyca ringens).46 Compared to fresh flowers, lower amounts of C25 and C27 (Z)-9-alkenes and (9Z)-9,17-octadecadienal were present in pollinated flowers that were unattractive to male (drone) bee pollinators. Solvent extracts elicited electrophysiological activity from antennae of male bees, although it is unknown which specific compounds are EAD-active.46
Alkenes have also been detected, but not yet shown to be biologically active, in a range of other non-sexually deceptive orchids (e.g. Maxillaria, Anacamptis, Gymnadenia, Serapias, Dactylorhiza, Himantoglossum, Platanthera, Neotinea47–49) suggesting alkene production in orchids is not unusual.
2.2 Alcohols
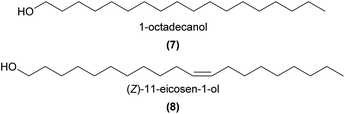
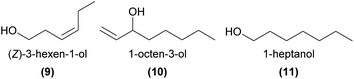
Several unbranched primary and secondary alcohols, and short chain unsaturated alcohols have been found across a variety of orchids, with evidence that some are involved in pollination. In the compelling case of Dendrobium sinense, which is pollinated by the predatory hornet Vespa bicolor, a synthetic mixture of EAD-active 1-octadecanol (7), 1-eicosanol, and (Z)-11-eicosen-1-ol (8), as well as 8 on its own, were attractive to the hornets in a flight cage and in Y-tube choice tests.5 This study was the first to report 8 as a plant volatile, and further noted that this molecule is a major component of the Asian and European honey bee alarm pheromones. These findings, coupled with the unusual observations of hornets ‘pouncing’ on the flowers and thus picking up pollinia, led the authors to conclude that the rewardless D. sinense mimics the honeybee alarm pheromone to deceptively attract its hornet pollinator.In another case of orchid pollination by predatory wasps, a synthetic mixture of EAD-active (Z)-3-hexen-1-ol (9) with two other green leaf volatiles, the esters hexyl acetate and (Z)-3-hexenyl acetate, was strongly attractive in Y-tube choice tests to the Vespula pollinators of the nectar rewarding Epipactis helleborine.50 It is thought that the orchid emits these green leaf volatiles, usually associated with wounding by herbivorous insects, as a deceptive signal to mimic the presence of herbivorous prey species and attract its predatory wasp pollinator.
In the mushroom-scented Dracula lafleurii, the mushroom alcohol, 1-octen-3-ol (10), is emitted by the attractive labellum. This compound is predicted to be a key attractant of the small fly pollinators – although single compound bioassays have yet to be conducted.51
In some food rewarding and food deceptive systems, the short chain 1-heptanol (11), 1-octanol, and 1-decanol were EAD-active,52,53 while the longer chain 1-hexadecanol, 1-octadecanol (7), and their unsaturated analogs were EAD-active components of attractive orchid extracts in two sexually deceptive Ophrys species.34 However, the precise roles of these primary alcohols in pollination remain to be more fully tested.
2.3 Aldehydes
Across the plant kingdom, aliphatic aldehydes are ubiquitous floral and vegetative volatiles. It is not surprising that these compounds have been reported across a wide range of orchid species (for example ref. 54–59), yet a decisive role for aldehydes in orchid pollination has rarely been confirmed. Lahondère et al.53 showed that the short chain aldehydes heptanal (12), octanal, and nonanal (13) were emitted by Platanthera obtusata and that 13 was critical to the attraction of its mosquito pollinators.53 Mosquito pollination is unusual in this orchid genus, with other species predominantly pollinated by moths, where terpenes often play a key role in pollinator attraction (see Section 3 for further details).Aldehydes (C7–C26) have been found to be present in many species of sexually deceptive Ophrys species, and EAD-active in several.28,34–37,60–62 Cuervo et al.63 found 20 EAD-active compounds in common between orchids and associated female Eucera kullenbergi bees, including seven aldehydes (C7–C16), as well as alcohols, fatty acids, and hydrocarbons.63 Synthetic blends of all EAD-active compounds in females and orchids, as well as polar compounds only, aldehydes only, and alkanes only, elicited more sexual attraction than the control in field bioassays, but were not as attractive as female bee and orchid extracts. The presence of aldehydes, in both females and orchids, their EAD-activity, and some sexual attraction indicate a possible role as sex pheromone components, although their importance in the overall blend remains unclear.
Sexually deceptive Ophrys sphegodes exhibit within-inflorescence variation in the relative amount of aldehydes and esters produced, with the uppermost flowers producing less of these compounds than the second uppermost flower and consequently attract fewer Andrena nigroanea pollinators.39 Ayasse et al.,39 however, showed that this reduction in visitation could be avoided by applying aldehydes and esters to the uppermost flowers, suggesting that bees may learn the scent of individual flowers in part due to aldehydes and esters.
In Telipogon peruvianus, tetradecanal (14) is present in both orchids and female Eudejeania sp. aff. browni tachinid flies, and is the only non-hydrocarbon EAD-active to males.45 The blend of hydrocarbons and tetradecanal is sexually attractive in field bioassays, although the role of tetradecanal alone has not been reported.
2.4 Carboxylic acids
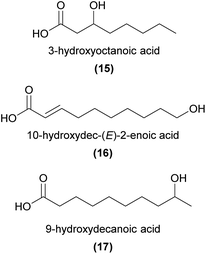
Carboxylic acids, with chain lengths between C6 to C18, are relatively common floral volatiles,9 and have frequently been found in orchids.57,64–66 Despite their prevalence, carboxylic acids have rarely been shown to function in orchid pollinator attraction, with two notable exceptions.In a fascinating pollination system, Cymbidium floribundum attracts swarms of the Japanese honeybee, Apis cerana japonica, by emitting bee aggregation pheromones.6 Sugahara et al.6 identified 3-hydroxyoctanoic acid (15) and 10-hydroxydec-(E)-2-enoic acid (16) as attractive components from the flowers. They also noted that 15 and similar unsaturated acids are known mandibular gland components of A. cerana,67 and that 16 is a major component of royal jelly in A. mellifera.68 Interestingly, neither compound was attractive alone, but instead a specific blend of both compounds was required to attract the bees.
Ophrys speculum is a sexually deceptive, scoliid wasp-pollinated species in an otherwise mostly bee-pollinated genus. Eight EAD-active compounds are shared between the flowers and associated female wasps.31,60 These comprised saturated (ω-1)-hydroxy acids (e.g.17) and (ω-1)-oxo acids, aldehydes and ethyl esters. In combination, at blend ratios comparable to those found in the orchid and wasp, these compounds elicited high rates of attempted copulation in field bioassays.60 Notably, the enantiomeric composition of 7-hydroxyoctanoic acid and 9-hydroxydecanoic acid (17) was similar in orchids and wasps (R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 6
S = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and this enantiomeric ratio was essential for strong sexual attraction in the field bioassays.
4), and this enantiomeric ratio was essential for strong sexual attraction in the field bioassays.
2.5 Esters
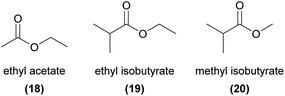
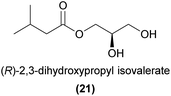
Esters are reported as floral volatiles across a range of orchids with different pollination strategies, although few studies have conclusively linked these molecules to orchid pollinator attraction. One important exception is the non-photosynthetic Gastrodia similis, which attracts its specific drosophilid fly pollinator, Scaptodrosophila bangi, by emitting ethyl acetate (18), ethyl isobutyrate (19), and methyl isobutyrate (20).69 The flies were strongly attracted to a synthetic mix of the three esters, and each compound on its own was also partially attractive.Another interesting case of specific chemical attraction of pollinators by an ester is found in the Japanese orchid Luisia teres, which sexually attracts male Protaetia pryeri pryeri beetles.70,71 Wakamura et al.71 showed that male beetles were exclusively attracted to (R)-2,3-dihydroxypropyl isovalerate (21), but not to the (S)-enantiomer or the racemate. The (R)-enantiomer was also found in virgin female beetles but did not occur in males, strongly suggesting that this compound is related to sex signalling in this species.
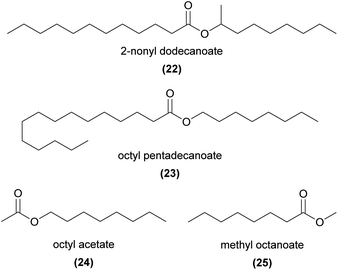
Several esters with relatively long parent alcohol and acid chains are EAD-active in sexually deceptive Ophrys. Examples include 2-nonyl dodecanoate (22) and dodecyl tetradecanoate in O. sphegodes,28,39 octyl and nonyl pentadecanoate (23) in O. aymoninii,33 and 2-nonyl hexadecanoate reported in O. iricolor,62O. lupercalis,36 and O. fabrella.37
Shorter ethyl- and methyl esters and acetates are reported in several food rewarding and food deceptive species, but their role in pollination also remains unresolved. Examples include C10, C12, and C14 EAD-active alkyl and alkenyl acetates in the food rewarding Gymnadenia conopsea,72 a series of C6 to C16 alkyl acetates (e.g.24) in G. conopsea73 and the food deceptive Cypripedium calceolus,52 methyl octanoate (25) and methyl decanoate in the food deceptive Orchis mascula,74 a series of C10 to C16 methyl and ethyl esters in the food rewarding Cremastra appendiculata,75 and methyl hexadecanoate in the rewarding Platanthera bifolia.76,77
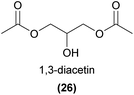
Oil rewarding plants partake in highly specialized interactions with ‘oil bees’, by secreting oils comprised of various acylglycerols and free fatty acids as a reward for pollination. The bees collect and use these oils for larval provisioning, brood cell water-proofing, or food for adults.78 Due to the low volatility of floral oils, we do not focus on these here, however a related volatile acetylated glycerol has been identified as a common compound associated with oil rewarding plants that may function as a signal of oil reward. 1,3-Diacetin (26) and both enantiomers of 1,2-diacetin have been detected from many oil rewarding plants, including several orchid species.79,80 Interestingly, these compounds were shown to be electrophysiologically and behaviourally active in oil bees, but not detectable to co-occurring non-oil bee species, suggesting that these compounds might represent a ‘private communication channel’ between oil flowers and oil bees.79
2.6 Lactones
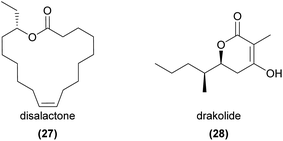
In a remarkable demonstration of the potential of chemical ecology, Cohen et al.81 were able to successfully identify the sexual attractant from a single flower of the exceedingly rare South African orchid Disa forficaria. Using GC-EAD and HRMS, the authors identified a single active compound, (16S,9Z)-16-ethyl hexadec-9-enolide (termed disalactone, 27), that was highly attractive and elicited prolonged copulatory behaviour from male Chorothyse hessei longhorn beetle pollinators in field bioassays. Further bioassays with individual isomers showed that the (16R,9Z)-stereoisomer was only weakly attractive, and (rac,9E)-16-ethyl hexadec-9-enolide and (rac,8Z)-16-ethyl hexadec-8-enolide were also weakly attractive.81 While this is the only macrolide presently known to play a role in pollination, several macrolides are known insect pheromones,82 suggesting similar compounds may be more broadly exploited. We further note that (ω-1)-hydroxy acids (e.g.17) such as those identified by Ayasse et al.60 may readily cyclise to form macrolides.In the rare Australian orchid Drakaea micrantha, a blend of three compounds, likely from two separate biosynthetic pathways, elicit strong sexual behaviour from male Zeleboria sp. thynnine wasp pollinators. Similar to other Drakaea spp., two hydroxymethylpyrazines are part of the blend, however the pyrazines are barely attractive on their own and a β-hydroxylactone (4-hydroxy-3-methyl-6S-(pentan-2S-yl)-5,6-dihydro-2H-pyran-2-one, termed drakolide (28)), is required to elicit strong sexual behaviour.83 Further investigations showed that both the naturally occurring stereoisomer of 28 and a blend of four stereoisomers prepared from racemic reagents elicited strong sexual behaviour from male wasps in field bioassays when presented in combination with the two hydroxymethylpyrazines.84 Field bioassays with six structural analogues of drakolide featuring substituents at positions 3 and 6 revealed reduced sexual behaviour and varying levels of attractiveness.84
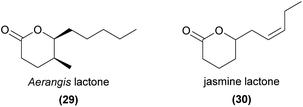
Several other lactones, such as the ‘Aerangis lactone’ ((5S,6S)-6-pentyl-5-methyltetrahydropyran-2-one, 29)10,85 and the jasmine lactone ((Z)-6-(pent-2-en-1-yl)tetrahydro-2H-pyran-2-one, 30),86 have been identified as orchid volatiles. Noted for their pleasant scents to humans, these may be important for pollination, but their biological activities have not yet been reported.
2.7 Tetrahydrofuran derivatives
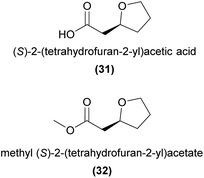
Using bioassay-guided fractionation in combination with field bioassays, Bohman et al.87 demonstrated that (S)-2-(tetrahydrofuran-2-yl)acetic acid (31) produced by the sexually deceptive Australian orchid Cryptostylis ovata, is attractive to male Lissopimpla excelsa wasp pollinators.87 The ester derivatives methyl (S)-2-(tetrahydrofuran-2-yl)acetate (32) and ethyl (S)-2-(tetrahydrofuran-2-yl)acetate, present in small amounts in the orchid, were similarly attractive to male wasps. However, few landings and no attempted copulations were observed in response to either orchid solvent extracts, 31, the ester derivatives, or a combination of all three, indicating that additional compounds or other non-chemical cues are required to induce the strong copulatory behaviour observed at the flower.2.8 Chiloglottones
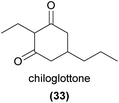
In a first step towards understanding the chemical basis of sexual pheromone mimicry in the many sexually deceptive orchids of Australia, Schiestl et al.88 demonstrated that the sole EAD-active compound in the male thynnine wasp pollinated Chiloglottis trapeziformis orchid was 2-ethyl-5-propylcyclohexan-1,3-dione, termed chiloglottone (33),88 which was also confirmed to be the female sex pheromone of the pollinator species. In field bioassays, synthetic 33 was equally as attractive as orchid extracts, whole flowers, and female wasps. Subsequent investigations revealed the presence of five additional related compounds in other species of Chiloglottis, with compounds differing in the alkyl or alkenyl chains at positions 2 and 5.89,90 Each of eleven Chiloglottis species investigated was found to produce one or two chiloglottones, with some sharing of chiloglottones among allopatric taxa and occasional sharing of chiloglottones among sympatric taxa.90,91 Chiloglottones have also been detected in the allied Arthrochilus and Paracaleana, and are EAD-active in the latter genus, although their role in pollination is yet to be confirmed.903 Isoprenoids
Isoprenoids are a common group of hydrocarbon compounds based on condensations of five-carbon (isoprene) building blocks derived from dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP).92 Isoprenoids represent the largest class of natural products93 and can be classified by the number of isoprene units as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), etc. Terpenoids are terpenes that have been modified in some way, usually through oxidation. They constitute a wide and structurally diverse group of specialised metabolites produced by plants and have many roles in defence and pollinator attraction.15 Below we include terpenoids within the respective group of terpenes.3.1 Monoterpenes
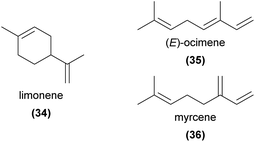
Monoterpenes are among the most common of all floral volatiles, being found across the plant kingdom and commonly reported in orchid floral volatile profiles. Here we focus on compounds shown via bioassays to be directly involved in orchid pollination, but we note that given their prevalence, monoterpenes likely play roles in pollination of many other orchid species.Of particular note, while limonene (34), (E)-ocimene (35), and myrcene (36) are the three most commonly occurring floral volatiles across studied plant families9 and are also common in orchid flowers, they have rarely been confirmed to be involved in pollination. One exception is in the perfume rewarding pollination systems, where monoterpenes frequently appear in detailed lists of compounds that are attractive to perfume-collecting male euglossine bee pollinators.94,95 We discuss some of these compounds here, but refer interested readers to these reviews and references therein for a comprehensive account of the compounds potentially96 or known to be94,95 involved.
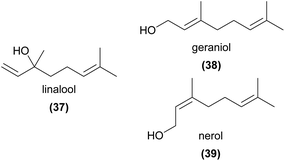
Linalool (37) is also one of very few monoterpenoids to be implicated in pollination by sexual deception. Borg-Karlson et al.101 first showed that the (S)-enantiomer was attractive to male Colletes cunnicularius bees, the pollinator of Ophrys exaltata, whereas the racemate was less attractive. Mant et al.29 also found racemic linalool rarely induced attempted copulation. However, in blends with 12 EAD-active hydrocarbons (odd chain lengths of C21–C31 alkanes, alkenes, and one alkadiene), contacts increased, indicating linalool acts as a long-range attractant, while the hydrocarbons are essential to induce sexual behaviour.
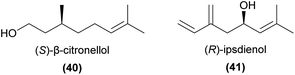
To our knowledge, the only other monoterpenoid shown to play a key role in sexual deception is (S)-β-citronellol (40).102 In Caladenia plicata, this compound in an optimal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blend with 2-hydroxy-6-methylacetophenone, secures pollination by male Zeleboria sp. thynnine wasps. Field bioassays revealed neither compound was active on its own,102 while further field tests confirmed replacements with (R)-β-citronellol and alternative regioisomers of the acetophenone derivative were barely attractive.103
4 blend with 2-hydroxy-6-methylacetophenone, secures pollination by male Zeleboria sp. thynnine wasps. Field bioassays revealed neither compound was active on its own,102 while further field tests confirmed replacements with (R)-β-citronellol and alternative regioisomers of the acetophenone derivative were barely attractive.103
Ipsdienol (41) has also been found from several perfume rewarding orchids, and is known to play a key role in specific attraction of perfume-collecting bees.99,104 Schorkopf et al.105 further showed that the (R)-enantiomer was attractive, but the (S)-enantiomer was not.
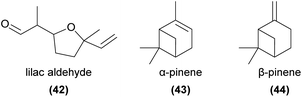
Nearly two decades later, another detailed and innovative study focused on the pollination chemistry of Platanthera species. Lahondère et al.53 showed that in P. obtusata, the unusual attraction of mosquitoes was achieved primarily via the enhanced emission of nonanal (13) relative to lilac aldehydes (42), and that related species not pollinated by mosquitoes had much lower levels of 13 and much greater amounts of 42 and linalool (37), as well as several other EAD-active monoterpenes. The same study confirmed through behavioural and physiological experiments that increasing the amount of 42 (a mixture of three isomers) in a synthetic blend approximating the orchid scents reduced attractiveness to mosquitoes.
In tandem, these studies of Platanthera species highlight the complexity of plant–pollinator interactions mediated by chemistry, wherein one compound can be attractive to some pollinator species while deterring others. It remains unclear which of the eight possible lilac aldehyde stereoisomers were important to attraction or deterrence in each study, which may add further complexity given that pollinator antennae may respond differently to each isomer.106
Both α- and β-pinene (43, 44) are key components of the oviposition site mimicry employed by Epipactis veratrifolia, which mimics the volatile compounds of aphids to attract several species of hoverflies as pollinators.4 These hoverflies seek to lay eggs near aphid colonies, as their larvae feed on aphids. Stökl et al.4 found that the floral volatiles of E. veratrifolia were very similar to those emitted as alarm pheromones from the aphid Megoura viciae, comprising primarily 43, 44, myrcene (36), and β-phellandrene, inducing the pollinator to lay eggs near the orchid. EAD experiments showed that each of these compounds were physiologically active in Episyrphus balteatus antennae, and further showed that when these compounds were added to bean plants, E. balteatus females laid significantly more eggs on the dosed plants compared to controls. A later study confirmed that a synthetic mixture of 43 and 44 alone was sufficient to attract hoverflies in field bioassays.107 The monoterpene 44 also attracted the hoverfly pollinators of Cypripedium subtropicum in field trapping experiments.108
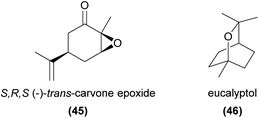
Carvone epoxide (45) stands out as one of the more unusual monoterpenoids to be experimentally confirmed as a key to the attraction of the perfume-collecting euglossine bee pollinators of various orchids.109,110 Recently, Brandt et al.111 investigated the relative attractiveness and prevalence of all four isomers of carvone epoxide, across five Catasetum species pollinated specifically by Eulama bee species. Only the (S,R,S)-(−)-trans-isomer was produced in these species and this isomer elicited both the strongest bee antennal response and the strongest attraction in field bioassays when compared to the other isomers.
Eucalyptol (1,8-cineole, 46) has been known to be attractive to euglossine bees for over 50 years.2 It has also been confirmed as an EAD-active component of attractive synthetic blends in several food rewarding and food deceptive orchids,53,74,112 and thus may warrant further attention in field bioassays.
3.2 Sesquiterpenes
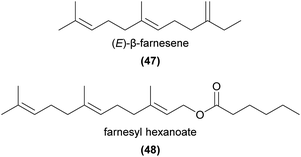
Sesquiterpenes are composed of three isoprene units, and can be further modified to produce a great diversity of floral volatile compounds. However, fewer sesquiterpenes have been shown to be involved in orchid pollination than monoterpenes, and their roles are often less well defined.Farnesyl hexanoate (48) is one of few terpenoids known to play a role in sexual deception, where it acts as a repellent rather than an attractant. Schiestl and Ayasse115 showed that Ophrys sphegodes flowers exhibited a large increase in 48 after pollination. Furthermore, addition of 48 to unpollinated flowers substantially reduced their attractiveness to the male Andrena nigroaenea pollinator,115 in line with previous observations that production of this compound by mated female bees lowers their attractiveness to males.116
3.3 Other terpenoids
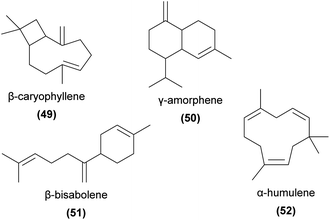
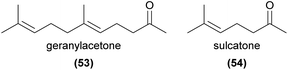
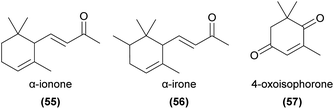
Geranylacetone (53) has been found to be a major floral volatile component of the aphid mimicking Cypripedium subtropicum and is weakly attractive to its hoverfly pollinators in field trapping experiments.108 This compound has also been identified from several other orchids.80,120 6-Methyl-5-hepten-2-one (sulcatone, 54) has also been found in many orchid species.57,59,66,120–125 It is EAD-active to the pollinators of Gymnadenia conopsea and Orchis mascula,73,74 but appears not to have been tested in field bioassays.Apocarotenoids are degradation products of C40-carotenoids, usually formed through oxidative cleavage of pigment compounds.126 The products often play important signalling roles (for example, the phytohormones abscisic acid and strigolactones), and several are known as floral volatiles. Relevant examples of volatile apocarotenoids are ionones (e.g.55) and their derivatives, which are known to be responsible for the smell of violets127 and are common in some orchid species.10 α-Ionone, β-ionone and α-irone (56) are known to be attractive to perfume-collecting bees,94,128 and β-ionone elicits strong antennal responses from some perfume-collecting bees.98 4-Oxoisophorone (57) has been found to be EAD-active in pollinators of Cypripedium calceolus52 and has been detected in several other orchid species,122,129–132 as have several ionone isomers,59,64,129,133–140 though their functions in pollination are generally unknown.
4 Benzenoids
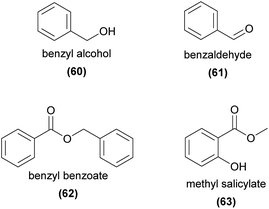
Benzenoids are widespread plant volatile compounds and are usually biosynthesized from the aromatic amino acid phenylalanine, which itself is derived from the shikimate pathway.16 They can be classified based on the length of the main side chain, (C6–C0–4, for side chains comprising 0–4 carbons) with modifications such as methylation, hydroxylation, and acetylation leading to considerable structural diversity.13Several C6–C1 benzenoids, such as benzyl alcohol (60), benzaldehyde (62), and benzyl benzoate (63) are amongst the most common of all floral volatiles, being found in more than half of the angiosperm families studied.9 Unsurprisingly, these compounds and many other benzenoids are well-represented across the orchids, and some have been shown to be relevant to pollination.
4.1 C6–C0
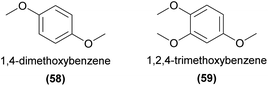
Some relatively common C6–C0 compounds found in orchid flowers are the dimethoxybenzenes (e.g.58). While each of the isomers are known as orchid floral volatiles, to our knowledge only the ortho and para compounds have been confirmed as pollinator attractants in field bioassays. For example, 1,4-dimethoxybenzene (58) has been confirmed to be attractive in field bioassays to various perfume-collecting bee species and is reported to be an abundant floral volatile in members of the orchid genera Embreea, Gongora, and Mormodes.94 More recently, this isomer has also been shown to elicit strong antennal responses from many other species of perfume-collecting bees across several genera.98 The related 1,2,4-trimethoxybenzene (59) has also been shown to elicit antennal responses in several perfume-collecting bee species.98,113Outside of perfume rewarding systems, Salzmann et al.141 found that the volatile profile of the bumblebee-pollinated Anacamptis coriophora was dominated by benzenoids, most notably 58 and 4-methoxybenzaldehyde. These two compounds were the only compounds emitted by the orchid found to elicit a response from Bombus terrestris queen antennae.
4.2 C6–C1
Benzyl alcohol (60) is emitted from many orchid species, and has been shown to be attractive to various perfume-collecting bee species.94,100 It has also been shown to be EAD-active to pollinators of perfume rewarding,98 food rewarding,73,77,142 food deceptive,52 and sexually deceptive63 orchids, as well as to pollinators of the honeybee alarm pheromone mimicking Dendrobium sinense.5Similarly, the common benzenoids benzaldehyde (61),50,53,73,77,142,143 benzyl acetate,5,52,73,76,98,99,142,143 benzyl benzoate (62),77,99,143 and methyl salicylate (63)77,98,99,117 have been found to be EAD-active in several orchid pollinators.
4.3 C6–C2
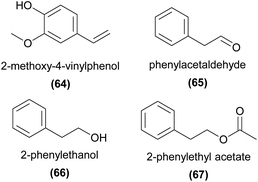
In an interesting study, Nunes et al.144 found that Dichaea pendula almost exclusively emitted 2-methoxy-4-vinylphenol (64). They further showed that this compound was not only attractive to several perfume-collecting bee pollinator species, but that it was even more strongly attractive to florivorous weevils, potentially representing a major evolutionary trade-off between beneficial and detrimental ecological interactions.Phenylacetaldehyde (65) has been demonstrated to be attractive to moth pollinators of Gymnadenia odoratissima as a trap bait in field tests143 and has been shown to be EAD-active in several other lepidopteran orchid pollinators.73,142
Other common C6–C2 compounds have also been shown to be attractive to perfume-collecting bees in the field,94 while 2-phenylethanol (66)52,73,142 and 2-phenylethyl acetate (67)52,73,142 are EAD-active in moth and bee pollinators. More recently, Chapurlat et al.73 also demonstrated that phenylacetaldehyde (65), 66, 60, 67, and the phenylpropanoids methylisoeugenol and eugenol, are EAD-active components of the floral scent of Gymnadenia conopsea to two moth pollinators, Deilephila porcellus and Aglais urticae.
4.4 C6–C3
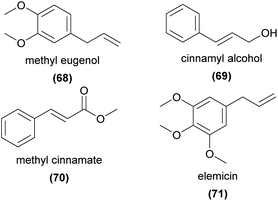
Methyl eugenol (68) is strongly attractive to the fruit fly pollinators of several Bulbophyllum orchid species.145 Isomers of 68 and eugenol have also been shown to be EAD-active in moth pollinators of various food rewarding orchids,72,73,143 beetle pollinators of Satyrium microrrhynchum,117 and bee pollinators of various perfume rewarding orchid species.94,98,99,128Cinnamyl alcohol (69) and its methyl ester, methyl cinnamate (70), are relatively common orchid volatiles. Both compounds are known as attractants of perfume-collecting bees and found in perfume rewarding flowers.94,128 The alcohol 69 is weakly attractive to the moth pollinators of Platanthera bifolia,77 and is EAD-active in the moth pollinators of Gymnadenia conopsea72 where emission rates are correlated with pollinator activity.146 The ester 70 is also EAD-active in the bumblebee pollinators of Orchis mascula.74 Elemicin (71) is known to attract the fruit fly pollinators of Bulbophyllum orchids147 as well as perfume-collecting bees,94 and is EAD-active in moth72,73 and beetle117 pollinators.
4.5 C6–C4
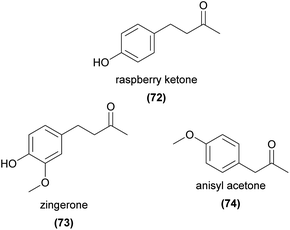
Several phenylbutanoid compounds have been shown to be key attractants in the well-studied ‘fruit fly orchids’. In a compelling early study, Nishida et al.148 showed that male Zeugodacus cucurbitae flies were attracted to and fed from a single spot on a developed TLC plate separating the crude extract of Dendrobium superbum petals. They further showed that the attractive compound, ‘raspberry ketone’ (4-(4-hydroxyphenyl)-2-butanone, 72), was sequestered in the rectal glands of the male flies, and was only present in rectal gland extracts of males that fed on the flower. Raspberry ketone (72) was later found in other Bulbophyllum species,149–152 and a methoxylated derivative, ‘zingerone’ (4-(4-hydroxy-3-methoxyphenyl)-2-butanone, 73), has been identified as a fruit fly attractant from several Bulbophyllum species as well.151–154 Other studies have found these and related phenylbutanoids such as anisyl acetone (74), zingerol, rhododendrol, and 4-(4-methoxyphenyl)-2-butanol as significant components of the floral scent of Bulbophyllum species,149,152 all of which were also attractive to some extent to male Zeugodacus cucurbitae flies in laboratory bioassays.1495 Other compounds
5.1 Nitrogenous compounds
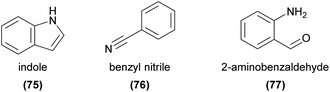
The nitrogen atom in organic compounds usually originates from an amino acid.16 In many alkaloids, building blocks from the acetate, shikimate, and terpene pathways are often also incorporated leading to a diverse group of natural products.Indole (75) is a particularly common nitrogenous plant volatile, being derived from the same pathway as the amino acid tryptophan.15 While known as an attractant and modifier of perfume rewarding orchid fragrances,94,10075 has not been directly shown to be responsible for pollinator attraction in any other orchid species. However, it has been found to be EAD-active in several orchid pollinators,52,73,99 and is present widely in orchids with a variety of pollination strategies.54,55,131,135,155,156 It has been implicated in oviposition site mimicry3 and has been detected in a few orchids with this strategy.56,157 However, a direct role in orchid pollinator attraction has not yet been established.
Other nitrogenous compounds commonly found in orchids include benzyl nitrile (76),55,66,131,136 2-aminobenzaldehyde (77),73 and several aldoximes,55,132,136,155,158,159 but to our knowledge their roles in orchid pollination have not been established. However, these and many other nitrogenous compounds are known to be EAD- and behaviourally active in various other, non-orchid pollination systems (e.g. ref. 160–162), which indicates likely roles in orchid pollination as well.
Pyrazines are volatile heterocyclic nitrogenous compounds predicted to be derived from oxidative dimerisation of α-aminocarbonyls derived from amino acids. They are widely distributed in plants, insects, fungi and bacteria, but to date, their biosynthesis has only be established in bacteria.163
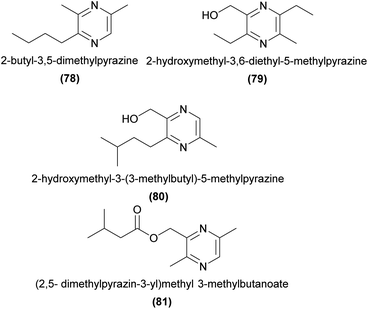
The sexually deceptive orchid genus Drakaea attracts male thynnine wasp pollinators primarily using various tetrasubstituted pyrazines. In Drakaea glyptodon, three alkyl pyrazines and one hydroxymethylpyrazine are EAD-active to its male Zaspilothynnus trilobatus thynnine wasp pollinator. Only two of these compounds, 2-butyl-3,5-dimethylpyrazine (78) and 2-hydroxymethyl-3,6-diethyl-5-methylpyrazine (79), in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, are required to elicit rates of sexual behaviour in field bioassays comparable to the flower.164 The related species D. micrantha elicits sexual behaviour from its male Zeleboria sp. thynnine wasp pollinator with a combination of drakolide (28) and two hydroxymethylpyrazines.83
1 ratio, are required to elicit rates of sexual behaviour in field bioassays comparable to the flower.164 The related species D. micrantha elicits sexual behaviour from its male Zeleboria sp. thynnine wasp pollinator with a combination of drakolide (28) and two hydroxymethylpyrazines.83
In a D. livida ecotype pollinated by male Zaspilothynnus nigripes thynnine wasps, 2-hydroxymethyl-3-(3-methylbutyl)-5-methylpyrazine (80) is EAD-active and present in both orchids and female wasps.165 In another D. livida ecotype pollinated by male Catocheilus sp. thynnine wasps, four tetrasubstituted pyrazines and (2,5-dimethylpyrazin-3-yl)methyl 3-methylbutanoate (81) have been shown to be EAD-active.166
5.2 Sulfurous compounds
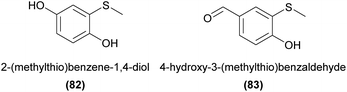
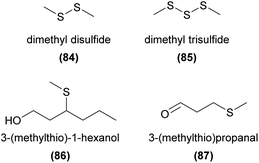
Natural compounds containing sulfur are generally derived from cysteine or methionine amino acid building blocks with cysteine being the major sulfur donor for thiosulfinates and glucosinolates. A large portion of volatile sulfurous compounds are the result of enzymatic decomposition of these latter biosynthetic products.167 (Methylthio)phenols have been identified from Caladenia and Drakaea orchids and found to act as pollinator attractants in two sexually deceptive Caladenia spider orchids. A set of four (methylthio)phenols were found to be used by C. crebra to sexually deceive its thynnine wasp pollinator Campylothynnus flavopictus. Three of the four semiochemicals had not previously been found as natural products.168 Two of these compounds (82 and 83) have also been confirmed to attract another Campylothynnus sp. pollinator to Caladenia attingens.169 More recently, all three previously unknown (methylthio)phenols have also been identified in one of the ecotypes of Drakaea livida, although no function has yet been reported.170Dimethyl disulfide (84) and dimethyl trisulfide (85) are strongly correlated with oviposition site mimicry, and are frequently emitted from flowers utilizing this pollination strategy.3 Surprisingly, to our knowledge these compounds have only twice been reported in orchids, without any confirmation of function.10,157 Several other sulfurous compounds have also been identified from orchids, again without any confirmed function, such as 3-(methylthio)-1-hexanol (86)134 and 3-(methylthio)propanal (87).139
6 Methods
Deciphering the chemical basis of pollinator attraction is no easy task. The floral volatile signals involved are often a complex blend with many of the components stored and emitted in sub-microgram amounts against a background of tens to hundreds of other compounds. For headspace sampling, detection may be hampered by slow release rates, while solvent extractions may fail to find compounds that are emitted as soon as they are produced. Even in highly specialised pollination systems, where just one or a few active compounds are involved in pollinator attraction, pinpointing candidate compounds can be challenging. Furthermore, in such cases the compounds involved are often unusual, making them challenging to identify without detailed structural elucidation.Here, we highlight studies that illustrate some of the key methods that have facilitated new chemical discoveries and broadened the field of pollination chemistry. We commence with an overview of methods for isolation, before covering identification and data analysis. We conclude this section by covering some of the synthetic methods that have made it possible to confirm the identity and activity of unusual specific compounds in selected studies.
6.1 Isolation
To date, most studies of wild orchids have not attempted isolation of pure compounds directly, due to limited availability of flowers, and the generally low abundance of semiochemicals. Much more commonly, gas chromatography with mass spectrometry (GC-MS) is used to separate and analyse the compounds, with gas chromatography coupled with electroantennographic detection (GC-EAD) frequently used in parallel to narrow down the candidate compounds.Sampling of volatiles has generally been done with solvent extraction or headspace collection methods. Solvent extractions involve direct immersion of floral tissue in solvents of various polarities, and are particularly useful for analysing less volatile compounds, stored compounds, and to quickly answer questions about tissue specificity of volatiles. Headspace sampling captures volatile compounds from the air surrounding flowers by adsorption or absorption onto one of a variety of stationary phases, and is particularly useful for assessing the composition of volatiles emitted by flowers and that might realistically be encountered by pollinators in the environment. Headspace methods utilise either static sampling (e.g. Solid Phase Microextraction, SPME), or dynamic sampling, where volatiles are actively pumped onto adsorbents, and subsequently eluted with solvents or desorbed in a heated GC inlet.
The effectiveness of these techniques depends on the volatility, solubility, and polarity of each compound, which is often unknown at the outset. There are often large qualitative and quantitative differences between headspace analysis and solvent extraction. Generally, headspace analysis is most effective when relatively large amounts of volatiles are released from the plants, while solvent extraction is superior for plants that have large storage of semiochemicals and low release rates. For example, Gervasi et al.171 isolated a range of electrophysiologically active long-chain hydrocarbons by dichloromethane extraction of Ophrys insectifera, while Bohman et al.38 employed SPME to extract shorter C15- and C17-hydrocarbons from the same species that were also found to be EAD-active to the pollinator Argogorytes mystaceus. Similarly, studies utilising both headspace and solvent extractions often find limited overlap between the results of each method.47,172 On the other hand, SPME has been unsuccessful for isolation of bioactive compounds from Australian Drakaea flowers despite multiple attempts (B. Bohman, pers. comm.), possibly due to low release rates of semiochemicals, while solvent extraction with dichloromethane has yielded the compounds of interest for several species.83,164,166 Another option to sample less volatile floral compounds with a non-destructive method is to sample floral tissue surfaces by rubbing with SPME fibres.40,173
Adsorption of pheromone components from glass surfaces followed by desorption by a solvent such as diethyl ether is another effective way of isolating semiochemicals emitted at low rates and quantities. Wakamura et al.71 used this method to isolate sufficient amounts of 2,3-dihydroxypropyl isovalerate (21) to obtain 2D-NMR data using a cryoprobe following column chromatography, and identified this ester as the sex pheromone of the sexually deceived scarab beetle Protaetia pryeri pryeri.71 In short, the beetles were placed in a stainless steel net cage inside a closed glass beaker not allowing the beetles to touch the glass. Later, the cage was removed and the beaker rinsed with diethyl ether. Some studies of orchids pollinated by oil collecting bees have also used traditional natural product isolation procedures with column chromatography and NMR spectroscopy to identify less volatile compounds such as triterpenes and glycosides, although whether these compounds are involved in pollination remains to be confirmed.174
For sexually deceptive orchids in particular, there are excellent examples of detailed chemical studies involving preparative chromatography methods.175 One example is the study by Cuervo et al.63 that fractionated extracts of the European Ophrys leochroma, pollinated by Eucera bees. These authors found that polar fractions eluted from a silica column with chloroform, dichloromethane, and diethyl ether, were more attractive to the bee pollinators than non-polar fractions of hexane elutions. This finding, together with the work of Ayasse et al.60 on wasp-pollinated Ophrys, show that both these orchids lure their sexually deceived pollinators with polar compounds. In contrast, the vast majority of studies of Ophrys have only used non-polar solvents such as hexanes, with hydrocarbons predominantly identified as pollinator attractants.7
Three recent Australian studies report using semi-preparative gas chromatography to isolate bioactive floral compounds for NMR analysis (using a microprobe) and bioassays. These studies involve three diverse pollinator taxa; fungus gnats,41 ichneumonid wasps,87 and thynnine wasps.83 Hayashi et al.41 collected fractions from Pterostylis orbiculata for laboratory choice-bioassays with Mycomya fungus gnats, in order to successfully isolate the unsaturated hydrocarbons (6Z,9Z)-6,9-tricosadiene and (6Z,9Z)-1,6,9-tricosatriene (6) present in the flowers that elicited attraction. Bohman et al.87 used SPME and semi-preparative gas chromatography concurrently to determine the set of long-range tetrahydrofuranyl acid derivatives 31 and 32 as attractants for the ichneumonid wasp Lissopimpla excelsa that pollinates Cryptostylis ovata. Thynnine wasp attractants have also been isolated with semi-preparative GC methods. Here, the hydroxymethylpyrazine 79 was initially identified as a candidate from Drakaea micrantha to attract its Zeleboria sp. pollinator, while the second component of the blend was unknown. By testing a combination of 79 and different GC-fractions of the orchid extract until a single fraction containing one main compound was confirmed as attractive to the thynnine pollinator, the first drakolide (28) was isolated.83
6.2 Identification and data analysis
In most cases, especially for less specific pollination systems, candidate compounds are tentatively identified by GC-MS data comparisons with increasingly comprehensive databases. Commercially available reference libraries now provide EI-MS and retention index data for several hundred thousand compounds, and synthetic standards can often be readily obtained for confirmation. Nonetheless, it can still be difficult to distinguish between compounds with very similar properties based only on mass spectrometric fragmentation and retention time data. Furthermore, recently discovered compounds, including known pollinator attractants, will not be present in the commercial databases. Thus, their identification is often only possible by careful interpretation of GC-MS data after detection with electroantennography or isolation with preparative chromatography. For example, Bohman et al.164–166 tentatively identified novel hydroxymethylpyrazines from Drakaea livida and D. glyptodon, attracting thynnine wasp pollinators, by extrapolating mass fragmentations from other known pyrazines, in combination with GC retention data. These identifications were then confirmed by comparison with synthesised compounds. Similarly, Cohen et al.81 identified a novel macrolide, 27, that attracts the cerambycid beetle pollinator of the exceptionally rare South African orchid Disa forficaria with GC-MS alone, and confirmed this identification by synthesis.Additional techniques such as GC-FTIR89,176 are useful to differentiate between isomers while purification and NMR6,41,147 are helpful in elucidating new natural products when a sufficient amount of floral material is available – although this can be particularly challenging when the orchid species is rare. For example, the amount of sample required for a 1H NMR is at least a thousand-fold higher than for a routine GC-MS analysis, and more comprehensive 2D-experiments require even higher amounts of isolated compound.
Differentiating between many possible structural isomers of certain compounds often requires additional methods to unambiguously assign a structure. Derivatisation with dimethyl disulphide (DMDS) is frequently used to determine alkene double bond positions, and while the studies that developed these techniques for volatile and semi-volatile compounds (ref. 177 and 178) are regularly cited, often very little experimental detail about the analysis is described. It should be noted that in the original papers, each candidate compound was synthesised and derivatised to enable direct comparison with derivatised biological extracts. The adduct formation upon treatment of alkenes is far from straightforward, particularly for complex extracts and for compounds containing more than one double bond179 or other functional groups, such as alcohols or carbonyls.180 Consequently, it would be advisable to provide more detailed experimental data when these experiments are carried out, such as clearly stating whether synthetic compounds have been derivatised and co-injected,181 and whether other isomers were ruled out. Including mass spectra and retention indices of adducts on which the identification is based would also provide the reader greater confidence in the identifications, as is common practice for identification of natural products by GC-MS. If semi-preparative GC is available, the analysis can be simplified by fractionating before treating each fraction with DMDS.38
More generally, it is also important to be aware that obtaining synthetic standards and confirming retention times and mass spectra may not be sufficient for unambiguous identification if other structurally similar compounds cannot be ruled out. For example, in the identification of drakolide (28),83 several structural analogues were synthesised and exhibited virtually identical GC-MS retention and mass spectral data. Furthermore, two of these isomers (differing in branching within a sidechain) required comparison across four GC-columns before they could be separated and the natural product confirmed. (Ref. 83 and B. Bohman pers. comm). Unfortunately, not even biological activity is always proof of a correctly identified natural product, as structural analogues may also show very similar activity.84,182 Overall, multiple GC-columns of differing stationary phase and carefully optimised methods should be employed in order to adequately compare retention data and mass spectra between natural products and synthetic standards.
Chiral semiochemicals also require additional identification efforts. Wakamura et al.71 used HPLC with a chiral-phase column to separate the two enantiomers of 2,3-dihydroxypropyl isovalerate, while chiral monoterpenes, pyrazines and drakolides have been easily separated by chiral-phase GC.4,83,165 In order to determine the absolute configuration, chromatographic separation of stereoisomers (usually using a chiral stationary phase) in combination with methods such as X-ray crystallography or measurement of the optical rotation or electronic circular dichroism (ECD) of synthetic standards can be applied. Then, the peak of the natural product can be aligned chromatographically with the corresponding peak of the fully characterised synthetic standard. This methodology was used in the confirmation of the absolute configuration of the sexually attractive drakolide from Drakaea micrantha.83
Chemical analysis may also involve comparative analysis of the recorded data. (Semi)automated analysis of GC-MS data with peak-deconvolution and alignment software, statistical treatment, and graphical visualisation of data have undergone rapid development over the last decade, largely due to the expansion of the field of metabolomics.183,184 These developments have facilitated comparative studies of comprehensive floral volatilomes, which complement structural elucidation of new specific semiochemicals to enable new insights into the roles of chemical communication. Such “chemical phenotyping” studies have, for example, revealed relationships between chemical composition and the phylogeny in taxa of perfume rewarding orchids,59,125,135 disentangled visual and olfactory signals in mushroom-mimicking Dracula orchids,51 showed correlations between floral cuticular hydrocarbon compositions and fly pollinators in Neotinea,47 and distinguished chemotypes of sexually deceptive Drakaea orchids.170 Here, it is important to note that to identify any specific compounds revealed by such analyses as potentially biologically relevant, comparison of retention data and mass spectral fragmentations with those of fully characterised reference compounds is critical.
6.3 Synthesis
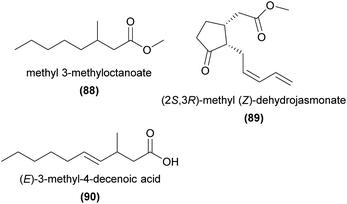
Many of the unusual compounds found in orchids, particularly from the highly specialised sexually deceptive orchid taxa, have required the development of new routes of synthesis.Excellent examples of earlier work in preparing orchid natural products by enantioselective methods include the suite of papers by Kitahara and coworkers,185–188 who synthesised candidate stereoisomers of a range of natural products from orchids, and confirmed the absolute configuration with chiral-phase GC. Both enantiomers of ethyl 3-methyloctanoate (88) were prepared in nine steps from methyl 3-hydroxy-2-methylpropanoate. Comparing both enantiomers by chiral-phase GC revealed that the natural product in the African Aerangis spp. orchid studied had S-configuration.186 Methyl cis(Z)-dihydrojasmonate (89) was determined to have (2S,3R)-configuration in the Asian Cymbidium goeringii after synthesis of all four stereoisomers from an in-house available intermediate in eight steps.187 Similarly, (E)-3-methyl-4-decenoic acid (90) and derivatives were prepared to determine the configuration of a range of European and Indo-Australian orchids185 and both enantiomers of cis-3-methyl-4-decanolide were synthesised in eight steps from (1S,5R)-2-oxabicyclo[3.3.0]-oct-6-en-3-one (29).188
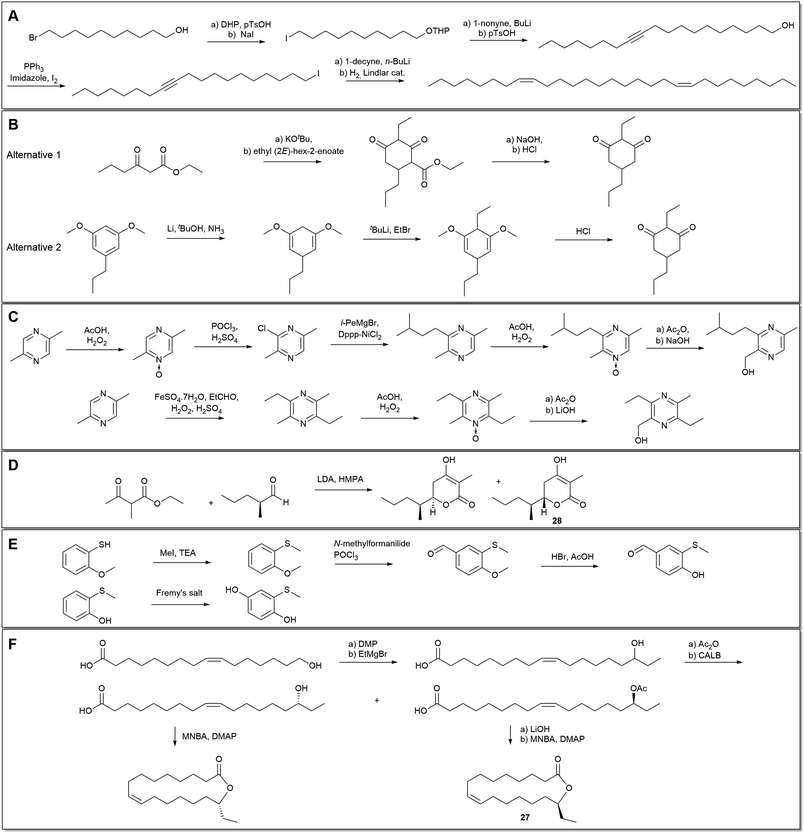
In the case of the many European Ophrys studies, few have reported the detailed synthesis of alkenes. The more unusual (8Z,20Z)-nonacosadiene, found in O. exaltata, was however prepared in five steps from the commercially available 10-bromo-1-decanol, via Lindlar hydrogenation of the corresponding alkyne (Scheme 1A). Chromatography on silica provided the (Z)-alkene in 94% purity.29 Another unusual unsaturated hydrocarbon, (6Z,9Z)-1,6,9-tricosatriene (6), found in Australian Pterostylis orbiculata, was identified, synthesised in five steps from propargyl alcohol, and confirmed to be a sexual attractant to the male fungus gnat pollinator.41
A nice example where orchid chemistry has motivated the modification of existing methods is provided by Delle-Vedove et al.,158 who developed a new stereoselective method for synthesis of (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT). An aqueous Wittig–Horner–Emmons reaction in combination with a Cu-catalysed decarboxylation to complement the classic Wittig-approach yielded a 38/62 (Z/E) ratio of the product (as determined by NMR) allowing the determination of the configuration of the natural product.158
Chiloglottones, which were the first orchid pollinator attractants to be identified in Australia,88 have been synthesised by multiple methods. Initially, a condensation approach between ethyl 3-oxohexanoate and ethyl (2E)-hex-2-enoate was taken to prepare chiloglottone (33).89 This protocol was later replaced by a more general route from 1,3-dimethoxybenzoic acid, via resorcinol derivatives, allowing a greater flexibility of substituents (Scheme 1B).189
Several semiochemicals from Drakaea orchids have also been synthesised via alternative routes, with the new refinements improving flexibility. For hydroxymethylpyrazines, two synthetic methods have been reported. In the earlier work, Kumada–Corriu cross-couplings were combined with Boekelheide hydroxylations to prepare for example 2-hydroxymethyl-3-(3-methylbutyl)-5-methylpyrazine (80) from 2,5-dimethylpyrazine in six steps.165 Later, a more convenient four-step method was used based on Minisci-type chemistry (Scheme 1C).164,190 Drakolide (28), was prepared from (S)-2-methylpentanal and ethyl-2-methyl-3-oxobutanoate (Scheme 1D), with the resulting diastereoisomers separated with HPLC and enantiomeric purity enhanced with chiral-phase HPLC. The absolute configuration of 28 was determined with X-ray crystallography of the synthetic stereoisomer and confirmed to match the natural product by chiral-phase HPLC and GC.83 Another recent example from Australia is the synthesis of three (methylthio)phenols, used as wasp pollinator attractants by spider orchids such as Caladenia crebra. 4-Hydroxy-3-(methylthio)benzaldehyde (83) was prepared from 2-methoxythiophenol through an S-methylation, Vilsmeier–Haack formylation, and O-demethylation. The corresponding alcohol (82) was formed through a sodium borohydride reduction. 2-(Methylthio)benzene-1,4-diol was prepared by oxidising the commercially available 2-methylthiophenol using Fremy's salt (Scheme 1E).168
Most recently, Katte et al.149 have provided good examples of orchid semiochemical synthesis by preparing ‘syringerone’ (4-(4-hydroxy-3,5-dimethoxyphenyl)-2-butanone), ‘raspberry ketone’ (72) and its acetate from Bulbophyllum fruit fly orchids of Asia. In South Africa, Cohen et al.81 synthesised the macrolide 27 found in Disa forficaria in five steps from (9Z)-16-hydroxy-octadec-9-enoic acid (Scheme 1F).
7 Final remarks
7.1 The importance of behavioural bioassays
There are no shortcuts in establishing the biological role of particular compounds in specific orchid–pollinator interactions. Instead, behavioural bioassays are required to determine which compounds are relevant, and to what extent.Often there are tens to hundreds of compounds present in floral extracts, and so for simplicity it may be tempting to draw conclusions about the function of chemical compounds in one system based on their function in another. Specifically, when compounds are known to be key to pollinator attraction in one orchid, finding them in another might suggest a similar role. In some cases this may indeed be a valid inference, but there are also many examples in which attractive compounds in one system are seemingly unimportant or play contrasting roles in another. As a case in point, two studies on Platanthera species show that shared compounds do not always have the same function for pollinator attraction even in related orchids. Plepys et al.77 showed that lilac aldehydes (42) were key floral attractants to the hawkmoth pollinators of P. bifolia, while Lahondère et al.53 showed that lilac aldehydes (42) reduced attraction of the mosquito pollinators of P. obtusata. These studies highlight the need for behavioural investigations of pollination chemistry to determine function, and both provide good examples of behavioural and physiological investigations to understand the chemical basis of pollinator attraction.
Measuring electroantennographic physiological activity (EAG or GC-EAD) has proven a powerful tool for pinpointing only the compounds perceptible to pollinators, but does not in itself provide information about the function of those compounds. For example, in the hornet pollinators of Dendrobium sinense, benzyl acetate and benzyl alcohol (60) are the most strongly EAD-active compounds from the orchid volatile profile, but are not required to attract pollinators.5 Similarly, Huber et al.143 found that benzaldehyde (61), phenylacetaldehyde (65), 2-phenylethyl acetate (67), benzyl acetate, eugenol, and 1-phenyl-2,3-butanedione were emitted from Gymnadenia odoratissima and were EAD-active to their pyralid moth pollinators, but only phenylacetaldehyde (65) was attractive in the field.
It is worth noting that EAD-active compounds that do not appear to be involved in pollinator attraction may still warrant closer research attention, since they may play roles beyond pollinator attraction. For example, the alkanes and alkenes may be particularly interesting in this context. Given their prevalence in orchid extracts and their very wide use as insect signalling compounds, it is plausible that some chemical information could be encoded by these compounds. For example, honey bees and bumblebees are known to be able to detect hydrocarbon ‘footprints’ from conspecifics present on flowers and avoid visiting these flowers, thereby saving time that might be wasted visiting a flower that had recently had nectar or pollen resources collected.191,192 While there is currently no evidence that hydrocarbons play this role in orchids, it serves as a reminder that there may be many subtle cues perceptible to pollinators that are as yet unknown.
7.2 Considering absolute configuration
The absolute configuration of semiochemicals, including orchid pollinator attractants, is often fundamental for biological activity. As a result, it is often important to analyse samples enantioselectively and to test stereoisomers separately in bioassays in order to accurately determine any biological activity. For example, Schorkopf et al.105 assessed pollinator discrimination between enantiomers of ipsdienol, a monoterpene alcohol known from several perfume rewarding species.104,113 Male Euglossa cyanura bees were attracted to the (R)-enantiomer (41) and the racemate, but showed virtually no attraction to the (S)-enantiomer. They further showed that male E. cyanura antennae were consistently more responsive to the (R)-enantiomer, potentially contributing to the difference in behavioural response.Similar observations, but with the (S)-enantiomer and racemate rather than the (R)-enantiomer and racemate being active have been reported in an analysis of the field activity of β-citronellol (40) to the thynnine wasp pollinator of Caladenia plicata.103 In other cases, while one enantiomer is attractive, the presence of the other reduces or completely prevents attraction to the racemate. For example, Williams and Whitten94 described baiting with both enantiomers of α-pinene, and found that while the (−)-enantiomer (1S,5S) was attractive to Eulama nigrita, the (+)-enantiomer (1R,5R) was not. Furthermore, the racemate was also unattractive, suggesting that the (+)-enantiomer was either actively repellent or masked the effect of the (−)-enantiomer to this species. More recently, Wakamura et al.71 showed that male Protaetia beetles were exclusively attracted to the (R)-enantiomer of 2,3-dihydroxypropyl isovalerate (21), but were not attracted to the (S)-enantiomer or to the racemate, again suggesting that the presence of the (S)-enantiomer prevents attraction to the (R)-enantiomer. These findings highlight the critical importance of not ruling out the attractiveness of compounds based on bioassays using mixtures of stereoisomers. Instead, pure stereoisomers are required for unambiguous evaluation of biological activity.
7.3 Lessons from sexual deception
The chemistry of pollination by sexual deception has been frequently highlighted throughout this review. Indeed, there has been remarkable progress on this topic in the 20+ years since Schiestl et al.27 first confirmed the chemical sexual mimicry of female Andrena bees by Ophrys orchids, and this research continues to grow. Since 2016, the chemistry of sexual deception has been confirmed in diverse Australian genera,41,102,168 and for the first time in Africa,81 Asia,71 and South America.45 These recent chemical discoveries include the first examples from sexually deceptive systems pollinated by Diptera41,45 and Coleoptera.71,81 Notably these ‘solved’ cases represent a small fraction of the hundreds of known cases of sexual deception, meaning that there are many more discoveries to be made.The ongoing progress in this area is likely because sexually deceptive systems are particularly amenable to investigations of pollination chemistry. They often have somewhat simpler floral volatile profiles than more generalist systems, and in some studies the dominant volatiles are the active ones (e.g. ref. 41 and 102). The strong sexual responses of males to sex pheromones also make them particularly amenable to experimental bioassays in the laboratory and field, as there are typically many rapid responses to the ‘correct’ compound or mixture of compounds. Finally, investigations of sexually deceptive orchids often integrate multiple lines of evidence and methods to establish active chemical compounds. For example, the ability to reduce the number of candidate compounds by comparing chemical extracts of active and non-active floral tissues with extracts of the female of the pollinator being mimicked has proven powerful. The addition of GC-EAD and/or semi preparative techniques, particularly when combined with iterative field and/or lab bioassays further helps to pinpoint the compounds involved.175
While sexual deception is undoubtedly among the most highly specialised of all plant pollination strategies, the dependence of an orchid species on just a few pollinator species is common.193 It follows that the strategies used to disentangle the chemistry of sexual deception can be applied to many hundreds or even thousands of other orchid species. Other types of mimicry-based pollination strategies are particularly ripe for investigation. Indeed, this review has highlighted several cases where the chemistry of unusual plant–pollinator interactions has been solved by integrating information from the orchid, the pollinator, and the putative model (e.g. mimicry of honeybee alarm pheromones,5 mimicry of green leaf volatiles50etc.).
In less specific pollination systems, where a wider variety of species are attracted to general scents indicative of a food reward, the particular compounds responsible for attraction can be more challenging to reliably elucidate than in more specialised cases. Here, attraction is often due to a large ‘bouquet’ of compounds, each contributing partially to pollinator attraction. For this reason, studies attempting to disentangle the effects of individual compounds in food rewarding and food deceptive systems are comparatively rare (but see ref. 53, 77 and 143). We predict that a chemical phenotyping approach, where taxonomic units are compared, and diagnostic compounds are subsequently identified, could be the first steps towards a more comprehensive understanding of the role of chemistry in diverse pollination systems. Examples of this approach have already been successfully applied in perfume rewarding and sexually deceptive pollination systems.47,59,135,170 Chemical phenotyping may also provide important clues about the evolution of pollination strategy shifts and the evolution of novel semiochemicals. Thus, we recommend this approach as a potentially fruitful area for further research.
8 Conclusion
This review has demonstrated that orchids are a rich source of interesting and unusual natural products. Yet, despite many decades of interdisciplinary studies, we have most likely only identified a very small fraction of the compounds pivotal for pollination of orchids. Given the huge number and great diversity of orchid species, the opportunities for new discoveries in this space are near endless.9 Conflicts of interest
There are no conflicts to declare.10 Acknowledgements
This work was supported by the Australian Research Council DP210100471 (R. P. and G. R. F.) and an Australian Government Research Training Program Scholarship to J. P.11 References
- R. Govaerts, P. Bernet, K. Kratochvil, G. Gerlach, G. Carr, P. Alrich, A. M. Pridgeon, J. Pfahl, M. A. Campacci, D. Holland Baptista, H. Tigges, J. Shaw, P. Cribb, A. George, K. Kreuz and J. Wood, World Checklist of Orchidaceae. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet, (accessed August 28, 2022) Search PubMed.
- C. H. Dodson, R. L. Dressler, H. G. Hills, R. M. Adams and N. H. Williams, Science, 1969, 164, 1243–1249 CrossRef CAS PubMed.
- A. Jürgens, S.-L. Wee, A. Shuttleworth and S. D. Johnson, Ecol. Lett., 2013, 16, 1157–1167 CrossRef PubMed.
- J. Stökl, J. Brodmann, A. Dafni, M. Ayasse and B. S. Hansson, Proc. R. Soc. B, 2011, 278, 1216–1222 CrossRef PubMed.
- J. Brodmann, R. Twele, W. Francke, L. Yi-bo, S. Xi-qiang and M. Ayasse, Curr. Biol., 2009, 19, 1368–1372 CrossRef CAS PubMed.
- M. Sugahara, K. Izutsu, Y. Nishimura and F. Sakamoto, Zool. Sci., 2013, 30, 99–104 CrossRef CAS PubMed.
- B. Bohman, G. R. Flematti, R. A. Barrow, E. Pichersky and R. Peakall, Curr. Opin. Plant Biol., 2016, 32, 37–46 CrossRef CAS PubMed.
- F. P. Schiestl, Naturwissenschaften, 2005, 92, 255–264 CrossRef CAS PubMed.
- J. T. Knudsen, R. Eriksson, J. Gershenzon and B. Ståhl, Bot. Rev., 2006, 72, 1–120 CrossRef.
- R. Kaiser, The Scent of Orchids. Olfactory and chemical investigations, Elsevier, Amsterdam, 1993 Search PubMed.
- R. Kaiser, Meaningful Scents Around the World: Olfactory, Chemical, Biological, and Cultural Considerations, Wiley, 2006 Search PubMed.
- J. T. Knudsen and J. Gershenzon, in Biology of Plant Volatiles, ed. E. Pichersky and N. Dudareva, CRC Press, 2020, pp. 57–79 Search PubMed.
- J. K. Muhlemann, A. Klempien and N. Dudareva, Plant, Cell Environ., 2014, 37, 1936–1949 CrossRef PubMed.
- F. P. Schiestl and F. Marion-Poll, in Analysis of Taste and Aroma, ed. J. F. Jackson and H. F. Linskens, Springer, Berlin, Heidelberg, 2002, pp. 173–198 Search PubMed.
- E. Pichersky, J. P. Noel and N. Dudareva, Science, 2006, 311, 808–811 CrossRef CAS PubMed.
- P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, John Wiley & Sons, 2009 Search PubMed.
- X. Yang, I. A. Guschina, S. Hurst, S. Wood, M. Langford, T. Hawkes and J. L. Harwood, Pest Manage. Sci., 2010, 66, 794–800 CrossRef CAS PubMed.
- J. W. Campbell and J. E. Cronan, Annu. Rev. Microbiol., 2001, 55, 305–332 CrossRef CAS PubMed.
- A. Bernard, F. Domergue, S. Pascal, R. Jetter, C. Renne, J.-D. Faure, R. P. Haslam, J. A. Napier, R. Lessire and J. Joubès, Plant Cell, 2012, 24, 3106–3118 CrossRef CAS PubMed.
- Y. Qiu, C. Tittiger, C. Wicker-Thomas, G. Le Goff, S. Young, E. Wajnberg, T. Fricaux, N. Taquet, G. J. Blomquist and R. Feyereisen, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 14858–14863 CrossRef CAS PubMed.
- P. M. Schlüter, S. Xu, V. Gagliardini, E. Whittle, J. Shanklin, U. Grossniklaus and F. P. Schiestl, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5696–5701 CrossRef PubMed.
- S. B. Lee and M. C. Suh, Plant Cell Rep., 2015, 34, 557–572 CrossRef CAS PubMed.
- L. Samuels, L. Kunst and R. Jetter, Annu. Rev. Plant Biol., 2008, 59, 683–707 CrossRef CAS PubMed.
- G. J. Blomquist, C. Tittiger and R. Jurenka, in Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate, ed. H. Wilkes, Springer International Publishing, Cham, 2018, pp. 1–32 Search PubMed.
- H. Holze, L. Schrader and J. Buellesbach, Heredity, 2021, 126, 219–234 CrossRef PubMed.
- H. Chung and S. B. Carroll, BioEssays, 2015, 37, 822–830 CrossRef CAS PubMed.
- F. P. Schiestl, M. Ayasse, H. F. Paulus, C. Löfstedt, B. S. Hansson, F. Ibarra and W. Francke, Nature, 1999, 399, 421 CrossRef CAS.
- F. P. Schiestl, M. Ayasse, H. F. Paulus, C. Löfstedt, B. S. Hansson, F. Ibarra and W. Francke, J. Comp. Physiol. A, 2000, 186, 567–574 CrossRef CAS PubMed.
- J. Mant, C. Brändli, N. J. Vereecken, C. M. Schulz, W. Francke and F. P. Schiestl, J. Chem. Ecol., 2005, 31, 1765–1787 CrossRef CAS PubMed.
- M. Ayasse, in Biology of Floral Scent, ed. N. Dudareva and E. Pichersky, CRC Press, 2006, pp. 219–241 Search PubMed.
- M. Ayasse, J. Stökl and W. Francke, Phytochemistry, 2011, 72, 1667–1677 CrossRef CAS PubMed.
- R. Peakall, D. C. Wong, B. Bohman, G. R. Flematti and E. Pichersky, in Biology of Plant Volatiles, ed. E. Pichersky and N. Dudareva, CRC Press, 2020, pp. 271–295 Search PubMed.
- D. D. L. Gervasi, M.-A. Selosse, M. Sauve, W. Francke, N. J. Vereecken, S. Cozzolino and F. P. Schiestl, Ecol. Evol., 2017, 7, 6023–6034 CrossRef PubMed.
- J. Gögler, J. Stökl, A. Sramkova, R. Twele, W. Francke, S. Cozzolino, P. Cortis, A. Scrugli and M. Ayasse, Evolution, 2009, 63, 2222–2234 CrossRef PubMed.
- J. Stökl, H. Paulus, A. Dafni, C. Schulz, W. Francke and M. Ayasse, Plant Syst. Evol., 2005, 254, 105–120 CrossRef.
- J. Stökl, P. M. Schlüter, T. F. Stuessy, H. F. Paulus, G. Assum and M. Ayasse, Am. J. Bot., 2008, 95, 472–481 CrossRef PubMed.
- J. Stökl, P. M. Schlüter, T. F. Stuessy, H. F. Paulus, R. Fraberger, D. Erdmann, C. Schulz, W. Francke, G. Assum and M. Ayasse, Biol. J. Linn. Soc., 2009, 98, 439–451 CrossRef.
- B. Bohman, A. M. Weinstein, R. Mozuraitis, G. R. Flematti and A.-K. Borg-Karlson, Int. J. Mol. Sci., 2020, 21, 620 CrossRef CAS PubMed.
- M. Ayasse, F. P. Schiestl, H. F. Paulus, C. Löfstedt, B. Hansson, F. Ibarra and W. Francke, Evolution, 2000, 54, 1995–2006 CAS.
- N. Joffard, V. Arnal, B. Buatois, B. Schatz and C. Montgelard, Plant Biol., 2020, 22, 881–889 CrossRef CAS PubMed.
- T. Hayashi, B. Bohman, A. Scaffidi, R. Peakall and G. R. Flematti, Curr. Biol., 2021, 31, 1954–1961 CrossRef CAS PubMed.
- C. Löfstedt, N. Wahlberg and J. G. Millar, in Pheromone Communication in Moths, ed. J. Allison and R. T. Cardè, University of California Press, 2016, pp. 43–78 Search PubMed.
- A. El-Sayed, The pherobase: database of insect pheromones and semiochemicals, https://www.pherobase.com, (accessed August 29, 2022) Search PubMed.
- C. Martel, L. Cairampoma, F. W. Stauffer and M. Ayasse, PLoS One, 2016, 11, e0165896 CrossRef PubMed.
- C. Martel, W. Francke and M. Ayasse, New Phytol., 2019, 223, 1989–2001 CrossRef CAS PubMed.
- A. Flach, A. J. Marsaioli, R. B. Singer, M. D. C. E. Amaral, C. Menezes, W. E. Kerr, L. G. Batista-Pereira and A. G. Corrêa, J. Chem. Ecol., 2006, 32, 59–70 CrossRef CAS PubMed.
- C. Martel, D. Rakosy, P. E. Romero, J. Jersáková and M. Ayasse, J. Systemat. Evol., 2021 DOI:10.1111/jse.12812.
- F. P. Schiestl and S. Cozzolino, BMC Evol. Biol., 2008, 8, 27 CrossRef PubMed.
- N. J. Vereecken, C. A. Wilson, S. Hötling, S. Schulz, S. A. Banketov and P. Mardulyn, Proc. R. Soc. B, 2012, 279, 4786–4794 CrossRef PubMed.
- J. Brodmann, R. Twele, W. Francke, G. Hölzler, Q.-H. Zhang and M. Ayasse, Curr. Biol., 2008, 18, 740–744 CrossRef CAS PubMed.
- T. Policha, A. Davis, M. Barnadas, B. T. M. Dentinger, R. A. Raguso and B. A. Roy, New Phytol., 2016, 210, 1058–1071 CrossRef CAS PubMed.
- H. Braunschmid, B. Mükisch, T. Rupp, I. Schäffler, P. Zito, D. Birtele and S. Dötterl, Arthropod-Plant Interact., 2017, 11, 363–379 CrossRef.
- C. Lahondère, C. Vinauger, R. P. Okubo, G. H. Wolff, J. K. Chan, O. S. Akbari and J. A. Riffell, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 708–716 CrossRef PubMed.
- J. M. R. B. V. Aguiar, G. D. S. Ferreira, P. A. Sanches, J. M. S. Bento and M. Sazima, Phytochemistry, 2021, 182, 112591 CrossRef CAS PubMed.
- M. G. Balducci, T. Van Der Niet and S. D. Johnson, Ann. Bot., 2020, 126, 1155–1164 CrossRef PubMed.
- L. Humeau, C. Micheneau, H. Jacquemyn, A. Gauvin-Bialecki, J. Fournel and T. Pailler, J. Trop. Ecol., 2011, 27, 591–599 CrossRef.
- N. Joffard, I. Le Roncé, A. Langlois, J. Renoult, B. Buatois, L. Dormont and B. Schatz, J. Evol. Biol., 2020, 33, 1028–1038 CrossRef CAS PubMed.
- F. S. Robustelli della Cuna, J. Calevo, M. Bazzicalupo, C. Sottani, E. Grignani and S. Preda, Plants, 2021, 10, 1718 CrossRef CAS PubMed.
- D. W. Roubik and J. T. Knudsen, Flora, 2017, 232, 117–127 CrossRef.
- M. Ayasse, F. P. Schiestl, H. F. Paulus, F. Ibarra and W. Francke, Proc. R. Soc. B, 2003, 270, 517–522 CrossRef CAS PubMed.
- J. Mant, R. Peakall and F. P. Schiestl, Evolution, 2005, 59, 1449–1463 Search PubMed.
- J. Stökl, R. Twele, D. H. Erdmann, W. Francke and M. Ayasse, Chemoecology, 2007, 17, 231–233 CrossRef.
- M. Cuervo, D. Rakosy, C. Martel, S. Schulz and M. Ayasse, J. Chem. Ecol., 2017, 43, 469–479 CrossRef CAS PubMed.
- J. Calevo, M. Bazzicalupo, M. Adamo, F. S. Robustelli della Cuna, S. Voyron, M. Girlanda, K. J. Duffy, A. Giovannini and L. Cornara, Diversity, 2021, 13, 550 CrossRef CAS.
- A. Manzo, S. Panseri, I. Vagge and A. Giorgi, Molecules, 2014, 19, 7913–7936 CrossRef PubMed.
- C. I. Peter and S. D. Johnson, Ann. Bot., 2014, 113, 277–288 CrossRef PubMed.
- C. I. Keeling, G. W. Otis, S. Hadisoesilo and K. N. Slessor, Apidologie, 2001, 32, 243–252 CrossRef CAS.
- G. Lercker, P. Capella, L. S. Conte, F. Ruini and G. Giordani, Lipids, 1981, 16, 912–919 CrossRef CAS.
- F. Martos, M.-L. Cariou, T. Pailler, J. Fournel, B. Bytebier and S. D. Johnson, New Phytol., 2015, 207, 225–234 CrossRef CAS PubMed.
- N. Arakaki, K. Yasuda, S. Kanayama, S. Jitsuno, M. Oike and S. Wakamura, Appl. Entomol. Zool., 2016, 51, 241–246 CrossRef.
- S. Wakamura, N. Arakaki, D. Moriyama, S. Kanayama, M. Oike, A. Kimura, S. Wajima, H. Ono and H. Yasui, Chemoecology, 2020, 30, 49–57 CrossRef CAS.
- J. Jersáková, S. Castro, N. Sonk, K. Milchreit, I. Schödelbauerová, T. Tolasch and S. Dötterl, Evol. Ecol., 2010, 24, 1199–1218 CrossRef.
- E. Chapurlat, J. Ågren, J. Anderson, M. Friberg and N. Sletvold, New Phytol., 2019, 222, 2009–2022 CrossRef PubMed.
- L. Dormont, T. Fort, J.-M. Bessière, M. Proffit, E. Garcia Hidalgo, B. Buatois and B. Schatz, Acta Oecol., 2020, 107, 103600 CrossRef.
- R. Kubo and M. Ono, Can. Entomol., 2017, 149, 372–376 CrossRef.
- D. Plepys, F. Ibarra, W. Francke and C. Löfstedt, Oikos, 2002, 99, 75–82 CrossRef CAS.
- D. Plepys, F. Ibarra and C. Löfstedt, Oikos, 2002, 99, 69–74 CrossRef CAS.
- S. L. Buchmann, Annu. Rev. Ecol. Syst., 1987, 18, 343–369 CrossRef.
- I. Schäffler, K. E. Steiner, M. Haid, S. S. van Berkel, G. Gerlach, S. D. Johnson, L. Wessjohann and S. Dötterl, Sci. Rep., 2015, 5, 12779 CrossRef PubMed.
- M. Castañeda-Zárate, S. D. Johnson and T. van der Niet, Curr. Biol., 2021, 31, 238–246 CrossRef PubMed.
- C. Cohen, W. R. Liltved, J. F. Colville, A. Shuttleworth, J. Weissflog, A. Svatoš, B. Bytebier and S. D. Johnson, Curr. Biol., 2021, 31, 1962–1969 CrossRef CAS PubMed.
- S. Schulz and S. Hötling, Nat. Prod. Rep., 2015, 32, 1042–1066 RSC.
- B. Bohman, M. M. Y. Tan, R. D. Phillips, A. Scaffidi, A. N. Sobolev, S. A. Moggach, G. R. Flematti and R. Peakall, Angew. Chem., Int. Ed., 2020, 59, 1124–1128 CrossRef CAS PubMed.
- B. Bohman, M. M. Y. Tan, G. R. Flematti and R. Peakall, J. Chem. Ecol., 2022, 48, 323–336 CrossRef CAS PubMed.
- D. Bartschat, D. Lehmann, A. Dietrich, A. Mosandl and R. Kaiser, Phytochem. Anal., 1995, 6, 130–134 CrossRef CAS.
- R. Luyt and S. D. Johnson, Plant Syst. Evol., 2001, 228, 49–62 CrossRef.
- B. Bohman, A. M. Weinstein, R. D. Phillips, R. Peakall and G. R. Flematti, J. Nat. Prod., 2019, 82, 1107–1113 CrossRef CAS PubMed.
- F. P. Schiestl, R. Peakall, J. G. Mant, F. Ibarra, C. Schulz, S. Franke and W. Francke, Science, 2003, 302, 437–438 CrossRef CAS PubMed.
- S. Franke, F. Ibarra, C. M. Schulz, R. Twele, J. Poldy, R. A. Barrow, R. Peakall, F. P. Schiestl and W. Francke, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8877–8882 CrossRef CAS PubMed.
- R. Peakall, D. Ebert, J. Poldy, R. A. Barrow, W. Francke, C. C. Bower and F. P. Schiestl, New Phytol., 2010, 188, 437–450 CrossRef CAS PubMed.
- F. P. Schiestl and R. Peakall, Funct. Ecol., 2005, 19, 674–680 CrossRef.
- Z. Yin and J. S. Dickschat, Nat. Prod. Rep., 2022 10.1039/D2NP00020B.
- J. Gershenzon and N. Dudareva, Nat. Chem. Biol., 2007, 3, 408–414 CrossRef CAS PubMed.
- N. H. Williams and W. M. Whitten, Biol. Bull., 1983, 164, 355–395 CrossRef CAS.
- S. Ramírez, R. L. Dressle and M. Ospina, Biota Colomb., 2002, 3, 7–118 Search PubMed.
- G. Gerlach and R. Schill, Bot. Acta, 1991, 104, 385–391 CrossRef.
- N. S. L. Albuquerque, P. Milet-Pinheiro, D. D. Cruz, D. M. A. F. Navarro and I. C. Machado, Plant Biol., 2021, 23, 719–727 CrossRef CAS PubMed.
- K. Brandt, S. Dötterl, S. R. Ramírez, F. Etl, I. C. Machado, D. M. do A. F. Navarro, D. Dobler, O. Reiser, M. Ayasse and P. Milet-Pinheiro, Front. Ecol. Evol., 2021, 9, 727471 Search PubMed.
- P. Milet-Pinheiro, D. M. Do Amaral Ferraz Navarro, S. Dötterl, A. T. Carvalho, C. E. Pinto, M. Ayasse and C. Schlindwein, Phytochemistry, 2015, 116, 149–161 CrossRef CAS PubMed.
- E. R. Pansarin and M. C. E. Amaral, Flora, 2009, 204, 238–249 CrossRef.
- A.-K. Borg-Karlson, J. Tengö, I. Valterová, C. R. Unelius, T. Taghizadeh, T. Tolasch and W. Francke, J. Chem. Ecol., 2003, 29, 1–14 CrossRef CAS PubMed.
- H. Xu, B. Bohman, D. C. J. Wong, C. Rodriguez-Delgado, A. Scaffidi, G. R. Flematti, R. D. Phillips, E. Pichersky and R. Peakall, Curr. Biol., 2017, 27, 1867–1877 CrossRef CAS PubMed.
- B. Bohman, A. Karton, G. R. Flematti, A. Scaffidi and R. Peakall, J. Chem. Ecol., 2018, 44, 436–443 CrossRef CAS PubMed.
- W. M. Whitten, H. G. Hills and N. H. Williams, Phytochemistry, 1988, 27, 2759–2760 CrossRef CAS.
- D. L. P. Schorkopf, L. Mitko and T. Eltz, J. Chem. Ecol., 2011, 37, 953 CrossRef CAS PubMed.
- S. Dötterl, D. Burkhardt, B. Weißbecker, A. Jürgens, S. Schütz and A. Mosandl, J. Chromatogr. A, 2006, 1113, 231–238 CrossRef PubMed.
- X.-H. Jin, Z.-X. Ren, S.-Z. Xu, H. Wang, D.-Z. Li and Z.-Y. Li, BMC Plant Biol., 2014, 14, 63 CrossRef PubMed.
- H. Jiang, J.-J. Kong, H.-C. Chen, Z.-Y. Xiang, W.-P. Zhang, Z.-D. Han, P.-C. Liao and Y.-I. Lee, New Phytol., 2020, 227, 1213–1221 CrossRef CAS PubMed.
- N. Lindquist, M. A. Battiste, W. Mark Whitten, N. H. Williams and L. Strekowski, Phytochemistry, 1985, 24, 863–865 CrossRef CAS.
- P. Milet-Pinheiro and G. Gerlach, Perspect. Plant Ecol. Evol. Systemat., 2017, 27, 23–34 CrossRef.
- K. Brandt, S. Dötterl, R. Fuchs, D. M. A. F. Navarro, I. C. S. Machado, D. Dobler, O. Reiser, M. Ayasse and P. Milet-Pinheiro, J. Chem. Ecol., 2019, 45, 464–473 CrossRef CAS PubMed.
- F. P. Schiestl and F. Glaser, Alpine Bot., 2012, 122, 1–9 CrossRef.
- P. Milet-Pinheiro, D. M. do A. F. Navarro, S. Dötterl, A. T. Carvalho, C. E. Pinto, M. Ayasse and C. Schlindwein, Phytochemistry, 2015, 116, 149–161 CrossRef CAS PubMed.
- A.-K. Borg-Karlson, Phytochemistry, 1990, 29, 1359–1387 CrossRef CAS.
- F. P. Schiestl and M. Ayasse, Oecologia, 2001, 126, 531–534 CrossRef PubMed.
- F. P. Schiestl and M. Ayasse, Behav. Ecol. Sociobiol., 2000, 48, 303–307 CrossRef.
- S. D. Johnson, A. Ellis and S. Dötterl, Am. J. Bot., 2007, 94, 47–55 CrossRef CAS PubMed.
- H. Bouwmeester, R. C. Schuurink, P. M. Bleeker and F. Schiestl, Plant J., 2019, 100, 892–907 CrossRef CAS PubMed.
- M. Huang, A. M. Sanchez-Moreiras, C. Abel, R. Sohrabi, S. Lee, J. Gershenzon and D. Tholl, New Phytol., 2012, 193, 997–1008 CrossRef CAS PubMed.
- T. van der Niet, A. Jürgens and S. D. Johnson, Plant Biol., 2015, 17, 226–237 CrossRef CAS PubMed.
- M. G. Balducci, D. J. Martins and S. D. Johnson, Plant Syst. Evol., 2019, 305, 765–775 CrossRef.
- H. Braunschmid and S. Dötterl, Front. Plant Sci., 2020, 11, 584081 CrossRef PubMed.
- K. Gross, M. Sun and F. P. Schiestl, PLoS One, 2016, 11, e0147975 CrossRef PubMed.
- C. Micheneau, J. Fournel, B. H. Warren, S. Hugel, A. Gauvin-Bialecki, T. Pailler, D. Strasberg and M. W. Chase, Ann. Bot., 2010, 105, 355–364 CrossRef PubMed.
- K. E. Steiner, R. Kaiser and S. Dötterl, Am. J. Bot., 2011, 98, 1663–1679 CrossRef CAS PubMed.
- A. Felemban, J. Braguy, M. D. Zurbriggen and S. Al-Babili, Front. Plant Sci., 2019, 10, 1168 Search PubMed.
- L. Aloum, E. Alefishat, A. Adem and G. Petroianu, Molecules, 2020, 25, 5822 CrossRef CAS PubMed.
- N. H. Williams and C. H. Dodson, Evolution, 1972, 26, 84–95 CrossRef PubMed.
- H. Braunschmid, R. Guilhot and S. Dötterl, Diversity, 2021, 13, 1–15 Search PubMed.
- L. Dormont, R. Delle-Vedove, J.-M. Bessière and B. Schatz, Phytochemistry, 2014, 100, 51–59 CrossRef CAS PubMed.
- J. Jersáková, J. Spaethe, M. Streinzer, J. Neumayer, H. Paulus, S. Dötterl and S. D. Johnson, Bot. J. Linn. Soc., 2016, 180, 269–294 CrossRef.
- T. Van der Niet, A. Jürgens and S. D. Johnson, S. Afr. J. Bot., 2010, 76, 726–738 CrossRef CAS.
- K. Brandt, I. C. Machado, D. M. do Amaral Ferraz Navarro, S. Dötterl, M. Ayasse and P. Milet-Pinheiro, AoB Plants, 2020, 12, plaa030 CrossRef.
- A. Flach, R. C. Dondon, R. B. Singer, S. Koehler, M. D. C. E. Amaral and A. J. Marsaioli, J. Chem. Ecol., 2004, 30, 1045–1056 CrossRef CAS PubMed.
- M. C. Hetherington-Rauth and S. R. Ramírez, Ann. Bot., 2016, 118, 135–148 CrossRef CAS PubMed.
- S. D. Johnson, Plant Syst. Evol., 2005, 251, 153–160 CrossRef.
- G. C. Kite and G. A. Salazar, Rev. Mex. Biodivers., 2008, 79, 153–157 Search PubMed.
- J. Li, G.-F. Zhu and Z.-H. Wang, J. Essent. Oil-Bear. Plants, 2017, 20, 385–394 CrossRef CAS.
- A. Tava, R. Cecotti and M. Confalonieri, J. Essent. Oil Res., 2012, 24, 39–44 CrossRef CAS.
- T. van der Niet, R. J. Cozien, B. Bytebier and S. D. Johnson, Plant Syst. Evol., 2017, 303, 387–401 CrossRef.
- C. C. Salzmann, S. Cozzolino and F. P. Schiestl, Plant Biol., 2007, 9, 720–729 CrossRef CAS PubMed.
- S. D. Johnson, M. G. Balducci and A. Shuttleworth, Biol. J. Linn. Soc., 2020, 129, 213–226 Search PubMed.
- F. K. Huber, R. Kaiser, W. Sauter and F. P. Schiestl, Oecologia, 2005, 142, 564–575 CrossRef PubMed.
- C. E. P. Nunes, M. F. G. V. Peñaflor, J. M. S. Bento, M. J. Salvador and M. Sazima, Oecologia, 2016, 182, 933–946 CrossRef PubMed.
- K. H. Tan and R. Nishida, J. Insect Sci., 2012, 12, 56 CrossRef PubMed.
- R. Steen, H. R. Norli and G. Thöming, Arthropod-Plant Interact., 2019, 13, 581–592 CrossRef.
- R. Nishida, K.-H. Tan, S.-L. Wee, A. K.-W. Hee and Y.-C. Toong, Biochem. Syst. Ecol., 2004, 32, 245–252 CrossRef CAS.
- R. Nishida, O. Iwahashi and K. H. Tan, J. Chem. Ecol., 1993, 19, 713–722 CrossRef CAS PubMed.
- T. Katte, K. H. Tan, Z.-H. Su, H. Ono and R. Nishida, Appl. Entomol. Zool., 2020, 55, 55–64 CrossRef CAS.
- T. Keng-Hong and R. Nishida, J. Chem. Ecol., 2005, 31, 497–507 CrossRef PubMed.
- M. Nakahira, H. Ono, S. L. Wee, K. H. Tan and R. Nishida, Biochem. Syst. Ecol., 2018, 81, 86–95 CrossRef CAS.
- K.-H. Tan, J. J. Vermeulen, T. Katte, H. Ono and R. Nishida, Arthropod-Plant Interact., 2021, 15, 447–455 CrossRef CAS.
- K. H. Tan and R. Nishida, Biochem. Syst. Ecol., 2007, 35, 334–341 CrossRef CAS.
- K.-H. Tan and R. Nishida, J. Chem. Ecol., 2000, 26, 533–546 CrossRef CAS.
- S. D. Johnson and N. Hobbhahn, S. Afr. J. Bot., 2010, 76, 739–748 CrossRef CAS.
- P. Milet-Pinheiro, J. B. F. Silva, D. M. A. F. Navarro, I. C. S. Machado and G. Gerlach, Plant Species Biol., 2018, 33, 158–163 CrossRef.
- T. Van Der Niet, D. M. Hansen and S. D. Johnson, Ann. Bot., 2011, 107, 981–992 CrossRef CAS PubMed.
- R. Delle-Vedove, N. Juillet, J.-M. Bessire, C. Grison, N. Barthes, T. Pailler, L. Dormont and B. Schatz, Phytochemistry, 2011, 72, 735–742 CrossRef CAS PubMed.
- L. J. Nielsen and B. L. Møller, Phytochemistry, 2015, 120, 3–18 CrossRef CAS PubMed.
- A. Jürgens, U. Glück, G. Aas and S. Dötterl, Bot. J. Linn. Soc., 2014, 175, 624–640 CrossRef.
- F. Mas, A. Harper, R. Horner, T. Welsh, P. Jaksons and D. M. Suckling, J. Sci. Food Agric., 2018, 98, 4445–4453 CrossRef CAS PubMed.
- S. Dötterl, A. Jürgens, K. Seifert, T. Laube, B. Weißbecker and S. Schütz, New Phytol., 2006, 169, 707–718 CrossRef PubMed.
- F. B. Mortzfeld, C. Hashem, K. Vranková, M. Winkler and F. Rudroff, Biotechnol. J., 2020, 15, 2000064 CrossRef CAS.
- B. Bohman, R. D. Phillips, M. H. M. Menz, B. W. Berntsson, G. R. Flematti, R. A. Barrow, K. W. Dixon and R. Peakall, New Phytol., 2014, 203, 939–952 CrossRef CAS PubMed.
- B. Bohman, L. Jeffares, G. Flematti, R. D. Phillips, K. W. Dixon, R. Peakall and R. A. Barrow, Org. Lett., 2012, 14, 2576–2578 CrossRef CAS PubMed.
- B. Bohman, L. Jeffares, G. Flematti, L. T. Byrne, B. W. Skelton, R. D. Phillips, K. W. Dixon, R. Peakall and R. A. Barrow, J. Nat. Prod., 2012, 75, 1589–1594 CrossRef CAS PubMed.
- M. Iranshahi, J. Essent. Oil Res., 2012, 24, 393–434 CrossRef CAS.
- B. Bohman, R. D. Phillips, G. R. Flematti, R. A. Barrow and R. Peakall, Angew. Chem., Int. Ed., 2017, 56, 8455–8458 CrossRef CAS PubMed.
- B. Bohman, R. D. Phillips, G. R. Flematti and R. Peakall, Fitoterapia, 2018, 126, 78–82 CrossRef CAS PubMed.
- A. M. Weinstein, B. Bohman, G. R. Flematti and R. D. Phillips, Plants, 2022, 11, 260 CrossRef CAS PubMed.
- D. D. Gervasi and F. P. Schiestl, Nat. Commun., 2017, 8, 1–8 CrossRef PubMed.
- P. Zito, S. Rosselli, M. Bruno, A. Maggio and M. Sajeva, Plant Signaling Behav., 2019, 14, 1552056 CrossRef PubMed.
- N. Joffard, B. Buatois and B. Schatz, Sci. Nat., 2016, 103, 77 CrossRef PubMed.
- N. P. Ferreira, L. U. R. Chiavelli, C. R. Savaris, S. M. Oliveira, D. L. Lucca, M. A. Milaneze-Gutierre, R. T. Faria and A. M. Pomini, Biochem. Syst. Ecol., 2019, 86, 103918 CrossRef CAS.
- B. Bohman, A.-K. Borg-Karlson and R. Peakall, in Biology of Plant Volatiles, ed. E. Pichersky and N. Dudareva, CRC Press, 2020, pp. 39–56 Search PubMed.
- L. Jirovetz, J. E. Gonzalez, G. Silvera, A. Nikiforov and A. Woidich, J. Essent. Oil Res., 1992, 4, 435–438 CrossRef CAS.
- H. R. Buser, H. Arn, P. Guerin and S. Rauscher, Anal. Chem., 1983, 55, 818–822 CrossRef CAS.
- E. Dunkelblum, S. H. Tan and P. J. Silk, J. Chem. Ecol., 1985, 11, 265–277 CrossRef CAS PubMed.
- T. W. Mitchell, H. Pham, M. C. Thomas and S. J. Blanksby, J. Chromatogr. B, 2009, 877, 2722–2735 CrossRef CAS PubMed.
- L. Xu, H. Guan, L. Liu, S. Mao, J. Feng, Z. Su and L. Liu, J. Chromatogr. A, 2022, 1672, 463009 CrossRef CAS PubMed.
- N. Shimizu, N. Mori and Y. Kuwahara, Biosci., Biotechnol., Biochem., 1999, 63, 1478–1480 CrossRef CAS PubMed.
- B. Bohman, A. Karton, R. C. M. Dixon, R. A. Barrow and R. Peakall, J. Chem. Ecol., 2016, 42, 17–23 CrossRef CAS PubMed.
- P. A. Aronov and B. D. Hammock, Mass Spectrom. Rev., 2007, 26, 51–78 CrossRef PubMed.
- R. Spicer, R. M. Salek, P. Moreno, D. Cañueto and C. Steinbeck, Metabolomics, 2017, 13, 106 CrossRef PubMed.
- S. H. Kang and T. Kitahara, Nat. Prod. Lett., 1996, 9, 13–20 CrossRef CAS.
- T. Kitahara, K. S. Hyun and M. Santelli, Nat. Prod. Lett., 1994, 5, 157–164 CrossRef CAS.
- T. Kitahara, M. Inoue, S. Tamogami and R. Kaiser, Tetrahedron, 1996, 52, 1487–1492 CrossRef CAS.
- Y. Masuzawa, S. Tamogami and T. Kitahara, Nat. Prod. Lett., 1999, 13, 239–246 CrossRef CAS.
- J. Poldy, R. Peakall and R. A. Barrow, Org. Biomol. Chem., 2009, 7, 4296–4300 RSC.
- B. Bohman, B. Berntsson, R. C. M. Dixon, C. D. Stewart and R. A. Barrow, Org. Lett., 2014, 16, 2787–2789 CrossRef CAS PubMed.
- T. Eltz, J. Chem. Ecol., 2006, 32, 907–915 CrossRef CAS PubMed.
- D. Goulson, J. W. Chapman and W. O. H. Hughes, J. Insect Behav., 2001, 14, 669–678 CrossRef.
- R. D. Phillips, N. Reiter and R. Peakall, Ann. Bot., 2020, 126, 345–362 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |