Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic environmental interactions shaped by vegetative plant volatiles
Rocío
Escobar-Bravo†
 a,
Po-An
Lin†
a,
Po-An
Lin†
 b,
Jamie M.
Waterman†
b,
Jamie M.
Waterman†
 *a and
Matthias
Erb
*a and
Matthias
Erb
 *a
*a
aInstitute of Plant Sciences, University of Bern, Bern, Switzerland. E-mail: jamie.waterman@unibe.ch; matthias.erb@unibe.ch
bDepartment of Entomology, National Taiwan University, Taipei, Taiwan
First published on 2nd February 2023
Abstract
Covering: up to November 2022
Plants shape terrestrial ecosystems through physical and chemical interactions. Plant-derived volatile organic compounds in particular influence the behavior and performance of other organisms. In this review, we discuss how vegetative plant volatiles derived from leaves, stems and roots are produced and released into the environment, how their production and release is modified by abiotic and biotic factors, and how they influence other organisms. Vegetative plant volatiles are derived from different biosynthesis and degradation pathways and are released via distinct routes. Both biosynthesis and release are regulated by other organisms as well as abiotic factors. In turn, vegetative plant volatiles modify the physiology and the behavior of a wide range of organisms, from microbes to mammals. Several concepts and frameworks can help to explain and predict the evolution and ecology of vegetative plant volatile emission patterns of specific pathways: multifunctionality of specialized metabolites, chemical communication displays and the information arms race, and volatile physiochemistry. We discuss how these frameworks can be leveraged to understand the evolution and expression patterns of vegetative plant volatiles. The multifaceted roles of vegetative plant volatiles provide fertile grounds to understand ecosystem dynamics and harness their power for sustainable agriculture.
1. Introduction
Plants shape terrestrial ecosystems by producing and releasing organic chemicals. Chemicals with low boiling points and high vapor pressure can be released as volatiles1 and mediate interactions with the environment at a distance.2,3 Floral volatiles are often constitutively released and can serve as attractants of pollinators and defenses against florivores4 but can also mediate interactions with other organisms.5,6 Vegetative plant volatiles are often highly inducible by biotic and abiotic stressors and play important roles in plant interactions with other organisms and the environment.2,4,7–9Work over the past years has uncovered how vegetative plant volatiles are synthesized and released, how they are modified by their environment, and how they modulate interactions between plants and other organisms.10–15 An increasing number of studies have begun to investigate the dynamic patterns that emerge from the multilayered interactions between plants and the biotic and abiotic environment.16–18 Understanding the role of vegetative plant volatiles in agroecosystems has become ever more pressing due to climate change, as vegetative volatiles may be used to promote plant resilience in dynamic environments.19,20 A robust understanding of the physiological and ecological mechanisms governing volatile-mediated interactions is essential to harness their potential in this context.
Recent reviews have discussed biological functions of vegetative plant volatiles,21 the molecular mechanisms of volatile production and emission,22 their ecological functions and evolution,3 and their physical and chemical behavior in the atmosphere.23 Here, we aim to complement this work by reviewing and integrating the most recent findings on the regulation, release, transfer, and biological impacts of vegetative plant volatiles. By highlighting how the environment regulates these volatiles and how they regulate the environment in turn, we aim at gaining a deeper understanding on the dynamics that underpin volatile-mediated ecosystem processes.
2. Biosynthesis and release of vegetative plant volatiles
2.1 Biosynthesis of vegetative plant volatiles
Plants emit diverse blends of vegetative volatiles. These blends include terpenoids, phenylpropanoids/benzenoids, fatty acid derivatives, nitrogen and Sulphur-containing compounds (Fig. 1), all of which are produced via distinct biochemical pathways.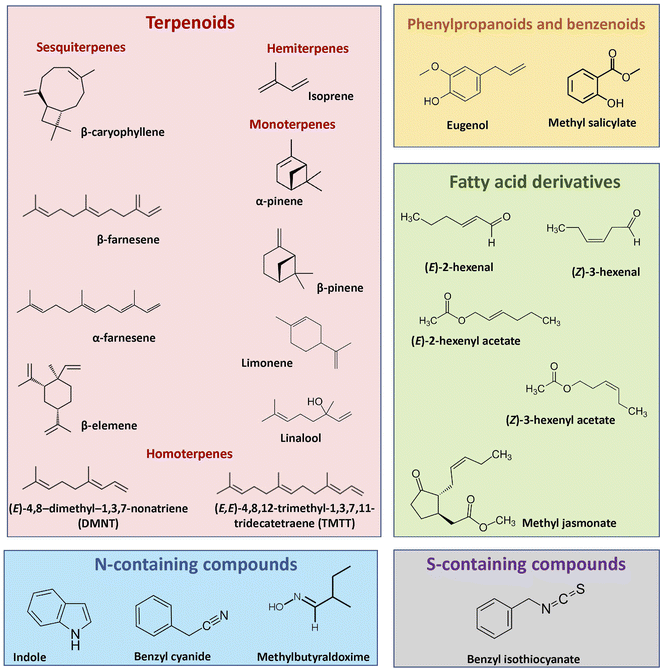 | ||
| Fig. 1 Examples of structures of vegetative plant volatiles that play important roles in plant interactions with other organisms and the environment. | ||
Terpenoids comprise over 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 known structures.24 They are derived from two five-carbon precursors, the isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP), via two independent pathways: the mevalonic acid (MVA) and methylerythritol phosphate (MEP). The MVA pathway gives rise to volatile sesquiterpenes, while the MEP pathway provides precursors to volatile hemiterpenes, monoterpenes and diterpenes. In both pathways, IPP and DMAPP are condensed head-to-tail by short-chain prenyltransferases to form the prenyl diphosphate precursors of various chain lengths, including geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15), and geranylgeranyl diphosphate (GGPP, C20). These precursors are used as substrates by a family of terpene synthases (TPSs) to generate C5-hemiterpenes, C10-monoterpenoids, C15-sesquiterpenoids, or C20-diterpenoids.25,26 Many TPSs can synthesize multiple products from a single prenyl diphosphate substrate or accept more than one substrate, thus contributing to terpenoid diversity.10 TPS products can be modified further via hydroxylation, dehydrogenation, acylation, or other reactions.11,27 For instance, oxidative degradation of the TPS2 products (E,E)-geranyllinalool and (E)-nerolidol by two specific P450 monooxygenases results in the biosynthesis of the acyclic homoterpenes (E)-3,8-dimethyl-1,4,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT).11
000 known structures.24 They are derived from two five-carbon precursors, the isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP), via two independent pathways: the mevalonic acid (MVA) and methylerythritol phosphate (MEP). The MVA pathway gives rise to volatile sesquiterpenes, while the MEP pathway provides precursors to volatile hemiterpenes, monoterpenes and diterpenes. In both pathways, IPP and DMAPP are condensed head-to-tail by short-chain prenyltransferases to form the prenyl diphosphate precursors of various chain lengths, including geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15), and geranylgeranyl diphosphate (GGPP, C20). These precursors are used as substrates by a family of terpene synthases (TPSs) to generate C5-hemiterpenes, C10-monoterpenoids, C15-sesquiterpenoids, or C20-diterpenoids.25,26 Many TPSs can synthesize multiple products from a single prenyl diphosphate substrate or accept more than one substrate, thus contributing to terpenoid diversity.10 TPS products can be modified further via hydroxylation, dehydrogenation, acylation, or other reactions.11,27 For instance, oxidative degradation of the TPS2 products (E,E)-geranyllinalool and (E)-nerolidol by two specific P450 monooxygenases results in the biosynthesis of the acyclic homoterpenes (E)-3,8-dimethyl-1,4,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT).11
Phenylpropanoid and benzenoid compounds are derived from the shikimate pathway. They are defined by their mostly planar and cyclic ring structures with conjugated double bonds and can be classified based on the length of the side chain as phenylpropenes (C6–C3) and benzenoid (C6–C1) compounds.28 The first committed step in their biosynthesis is catalyzed by the L-phenylalanine ammonia lyase (PAL) enzyme, which deaminates the aromatic amino acid phenyl alanine to trans-cinnamic acid (CA). CA is transformed via several enzymatic reactions into para-coumaroyl CoA, the general precursor for a wide range of products including anthocyanins, flavonoids, lignin and phenylpropenes. Phenyl propenes are, together with terpenes, major constituents of essential oils, such as methylchavicol or eugenol, which are stored in the trichome glands of basil (Ocimum basilicum) leaves.29,30 Formation of benzenoids from CA results from the shortening of the propyl side chain by two carbons via β-oxidative or non-β-oxidative pathways.28
Plants also release several nitrogen-containing volatiles that are derived from amino acid biosynthesis. Indole, a precursor of tryptophan biosynthesis, is produced and emitted by many plant species upon herbivory.31–33 Indole synthesis and emission have been best characterized in maize (Zea mays), where 1-(2-carboxyphenylamino)-l-deoxyribulose-5-phosphate is first converted into indole-3-glycerolphosphate by specific indole-3-glycerolphosphate synthases, and then into volatile indole by the action of an indole-3-glycerolphosphate lyase.34 Aldoximes and nitriles occur as intermediates in the biosynthesis of several nitrogenous defense compounds such as cyanogenic glycosides, hydroxynitrile glycosides and camalexin. The biosynthesis of these compounds has been well characterized in Western balsam poplar (Populus trichocarpa). Volatile aldoximes such as 2- and 3-methylbutyraldoxime, and nitriles such as benzyl cyanide, which are induced upon herbivory, are derived from amino acids by the action of two cytochrome P450 (CYP) monooxigenases of the CYP79 family.35 Nitriles can be produced by two additional P450 enzymes belonging to the CYP71 family, which catalyze the conversion of aldoximes to nitriles.36
Volatile fatty acid derivatives include green leaf volatiles (GLVs), consisting of C6 compounds including alcohols, aldehydes, esters, as well as the phytohormone methyl jasmonate (MeJA). GLVs and jasmonates are produced via the lipoxygenase (LOX) pathway, which begins when C18-polyunsaturated fatty acids, linoleic and linolenic acids, are cleaved from cell membranes by lipases and dioxygenated by either 9- or 13-LOXs to form 9- and 13-hydroperoxides, respectively. These hydroperoxides act as substrates in the hydroperoxide lyases (HPLs) and allene oxide synthases (AOSs) pathways to produce GLVs and jasmonic acid (JA), respectively. For GLV biosynthesis, 13-hydroperoxy octadecatrienoic acid is cleaved by HPL to form (Z)-3-hexenal, which is spontaneously or enzymatically converted into other C6 compounds, including (E)-2-hexenal, (Z)-3-hexenol and (Z)-3-hexenyl acetate.37 Biosynthesis of MeJA is synthesized in the peroxisome from a 13-hydroperoxide intermediate via sequential reduction and β-oxidation steps, which results in the formation of JA, and it is followed by the methylation of JA by a JA carboxyl methyltransferase.38
Volatiles can also result from the breakdown of plant secondary metabolites. Isothiocyanates, for instance, are sulfur-containing volatiles that are characteristic of the Brassicaceae and produced by the myrosinase-mediated hydrolysis of glucosinolates.39 Glucosinolates are a structurally diverse group of β-thioglucoside-N-hydroxysulfates classified according to their precursor amino acid as aliphatic (from alanine, valine, leucine, isoleucine, or methionine), aromatic (from phenylalanine or tyrosine), or indolic glucosinolate (from tryptophan). Their biosynthesis starts with the modification of the precursor amino acid to form the glucosinolate core. In some instances, side chain elongation or secondary modifications can occur prior to and after core biosynthesis.40 Plants producing these compounds also synthesize myrosinases, which are specific β-thioglucosidases that are kept separately from their substrates. When plant tissues are disrupted, the myrosinases hydrolyse the glucose residue of glucosinolates releasing an unstable intermediate, which rearranges to form non-volatile and volatile isothiocyanates and nitriles.40
In summary, vegetative plant volatiles are produced through several different pathways and mechanisms. While some volatiles are synthesized from simpler precursors, others are breakdown products of more complex compounds. These diverse origins contribute to the temporal, spatial and environmental complexity of plant volatile blends, and provide many independent attack points for ecological and evolutionary processes to shape these blends.
2.2 Mechanisms of vegetative plant volatile release
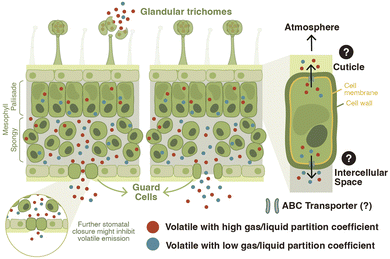
The mere production of a volatile organic compound is not sufficient for its release into the environment. Vegetative plant volatiles produced in the epidermal cells of roots25 or in the mesophyll cells of green-leaf tissues have to cross different cellular boundaries such as the cell membrane and wall, the intercellular spaces, and the stomata or cuticle before being released into the environment (Fig. 2).41–43 Depending on their physicochemical characteristics, volatile compounds can accumulate in the lipid or aqueous phase.44 The emission of volatiles driven solely by diffusion can lead to toxic volatile accumulation in membranes because of preferential partitioning of some of these compounds into lipid bilayers.45 To enhance export and avoid toxicity, volatile phenylpropanoid and benzenoid compounds are transported across the plasma membrane by an adenosine triphosphate–binding cassette (ABC) transporter in Petunia hybrida flowers.12 Recent discoveries have furthermore shown that a pleiotropic drug resistance transporter 3 (AaPDR3) from the ABC transporter superfamily is involved in the transport of the sesquiterpene β-caryophyllene in annual mugwort (Artemisia annua) trichomes.46 Thus, volatile transport can be regulated via membrane transporters.Following their transfer across membranes, leaf volatiles have three routes to reach the atmosphere: diffusion across the cuticle, release via stomata, or direct release from wounded cells. In Petunia hybrida flowers, the cuticle acts as a sink/concentrator for volatiles, and a reduction in cuticle thickness alters volatile emission, internal pools and biosynthesis.47 In the leaves, significant correlations have been observed between stomatal aperture and volatile emission.42,43,48 When stomata are closed, sesquiterpenes for instance can accumulate in the leaf, suggesting that their release is constrained by stomatal aperture.42,43 Detailed kinetic analyses in Pinus pinea revealed a partial disconnect between the release of the monoterpene β-ocimene and stomatal conductance.49 This phenomenon may be explained by the higher gas/liquid phase partition coefficient of this volatile, also called Henry's law constant (H). Volatiles with a high H accumulate at higher intercellular partial pressure, resulting in less sensitivity to stomatal closure49 (Fig. 2).
Some plant species have specialized secretory structures in their aerial tissues such as resin ducts, idioblasts, and/or glandular trichomes where they produce and store volatiles. Glandular trichomes are hair-like epidermal structures that can produce and accumulate essential oils.30 In the tomato clade of the Solanum genus, for instance, plants produce leaf glandular trichomes that synthesize and store volatile terpenoids.50,51 Emission of type-VI trichome-derived terpenoids is very low in intact tomato leaves. Upon trichome disruption, however, these compounds are strongly emitted, suggesting this as the main mechanism for volatile release52 (Fig. 2). In tomato, after biosynthesis, volatile terpenoids are directed into an intercellular storage cavity outside of the gland cells.53 It has been suggested that the low emission from intact trichomes might be explained by the cell wall material surrounding this storage cavity, which could prevent the escape of the volatiles and re-entry into the secretory cells.54
In summary, plants can release volatiles via several different routes, including active transport and passive diffusion (Fig. 2). By consequence, plants can potentially regulate volatile release via biosynthesis, transporter activity, stomatal opening, and cuticular composition. The latter processes are intimately linked to other biological functions. Transporters, for instance, are likely to transport other small molecules, stomata are essential for gas exchange, and the cuticle provides and important physical barrier against abiotic and biotic stresses. The regulation of volatile release through processes such as stomatal opening and cuticular structure is thus associated with functional trade-offs that may complicate associated evolutionary trajectories. Therefore, the regulation of volatile release for compounds that can be produced de novo via the production of specific enzymes may be accomplished more effectively by regulating their biosynthesis. Storage and wound release are also an efficient way of tailoring volatile release to environmental stress.
3. Environmental regulation of vegetative plant volatile biosynthesis and release
3.1 Regulation by biotic stressors
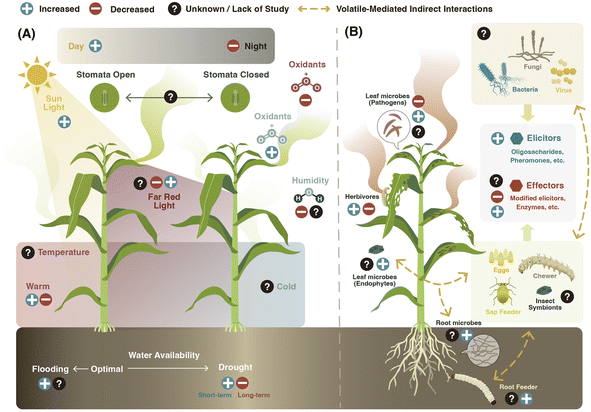
Volatile composition and emission by plants vary depending on the identity of the attacker. For example, feeding by thrips (cell content-feeder), spider mites (cell content-feeder), or aphids (phloem-feeder) in cucumber (Cucumis sativus) leaves induces the expression of different terpene synthases, which translates into different volatile emission patterns.58 In addition, attacks by chewing herbivores tend to trigger higher emission of volatiles than by piercing-sucking insects, suggesting that different types and levels of damage induce distinct volatile responses in plants.8,59 Simultaneous or sequential attack by multiple herbivores can result in distinct volatile emissions as well.60,61 Aboveground attack by the fall armyworm (Spodoptera frugiperda), for instance, induces volatile emission in the roots of maize (Zea mays) plants that repels Western corn rootworm (Diabrotica virgifera virgifera) larvae. Yet, when the Western corn rootworm feeds on the plant first, the induction of the repellent volatiles is suppressed.61
The modulation of vegetative plant volatile emission by herbivores is mediated by the recognition of damage-associated molecular patterns as well as herbivore-associated molecular patterns present in the oral secretions, saliva, ovipositional fluids, digestive waste products, and pheromones of the herbivore.62 Depending on their ability to induce or suppress plant defense responses, these are called elicitors or effectors.63 Herbivore-derived elicitors like β-glucosidases,64N-acyl-glutamines such as volicitin,65 and cyclic disulfide-bridged inceptin-related peptides66 found in the oral secretions of lepidopteran larvae, as well as monounsaturated disulfooxy fatty acids (caeliferins) present in the oral secretions of non-lepidopteran insects,67 have all been shown to induce volatile emissions in plants. Recently, four oligosaccharides detected in the oral secretions of the cotton leafworm (Spodoptera littoralis) larvae induced volatile emissions in Tansy (Tanacetum vulgare) plants.68 Herbivore-derived proteins from eggs69,70 and frass71 modulate volatile emissions as well. For instance, an annexin-like protein “diprionin” isolated from egg-associated secretions in sawfly (Diprion pini) was recently found to trigger (E)-β-farnesene emission in Scots pine (Pinus sylvestris).70 Interestingly, volatiles released by insects, such as sexual and aggregation pheromones, can also modulate volatile emissions by enhancing herbivory-induced plant defense responses72 and volatile release.73
Herbivore-derived effectors can suppress volatile emissions.63 Silkworms (Bombix mori),74 the fall armyworm (S. frugiperda),57 velvet bean caterpillar (Anticarsia gemmatalis),75 tomato fruitworm (Helicoverpa zea), and tobacco hornworm (Manduca sexta)76 have been all reported to suppress induced volatile emissions in plants. In B. mori larval oral secretions, a fatty acid hydroperoxide dehydratase (BmFHD) suppresses GLVs production.74 Similar enzymes are present in the oral secretions of other lepidopteran species.77 The truncated form of inceptin (Vu-In−A) produced by the legume-specialist velvet bean caterpillar antagonizes inceptin-induced responses, including the production of the homoterpene DMNT in cowpea (Vigna unguiculata).75 Recently, in H. zea, the salivary enzyme glucose oxidase has been shown to inhibit volatile emission in different host plants.43 This inhibition was linked to the suppression of stomatal aperture.78 Besides the induction or suppression of volatile emissions, some herbivores can modulate the chemical profile of the released plant volatile compounds. In M. sexta, the larval oral secretions are enriched in an (3Z):(2E)-hexenal isomerase that modulates the release of plant GLVs by re-arranging (Z)-3-hexenal to (E)-2-hexenal.79
In summary, the release of plant volatiles following herbivore attack is strongly influenced by the action of elicitors and effectors (Fig. 3B). Elicitors act by activating the biosynthesis of plant volatiles, but to what extent these compounds also regulate transport and release processes is not well established. Herbivore effectors are known to target both the biosynthesis and volatile release processes. Understanding the interplay between elicitors and effectors in regulating volatile release is an important research frontier.
Pathogenic bacteria can modulate volatile release in plants. A virulent strain of Pseudomonas syringae, for instance, increases the emission of TMTT, β-ionone, and α-farnesene in Arabidopsis.81 In tobacco (Nicotiana tabacum), both avirulent and virulent P. syringae strains induce the emission of MeSA, monoterpenes, and sesquiterpenes in infected plants.82 Gram-negative bacterial pathogens such as P. syringae have a type III secretion system (a molecular syringe) that injects effectors into the host cell to suppress the plant immune system and promote bacterial infection. Tobacco plants treated with P. syringae mutants deficient in this secretion system emit less volatiles upon infection.82 More recently, it has been shown that plant recognition of one of these injected P. syringae effectors, AvrRpm1, triggers the emission of vegetative volatiles in Arabidopsis.83
Vegetative plant volatile emissions can be also regulated by fungal infections.84 Infection with fungal pathogens elicits greater GLVs emissions than wounding alone or insect herbivore attack.8 Although the mechanisms of this phenomenon are still unknown, it has been suggested that enhanced GLVs production might be controlled by the action of fungal effectors. In nature, however, pathogen infections can co-occur with herbivore attacks resulting in distinct volatile profiles.85 These patterns may be explained by hormonal cross-talk.85–87 For instance, the rust fungus (Melampsora laricipopulina) reduces the biosynthesis and emission of herbivore-induced plant volatiles in black poplar (Poplus nigra) by activating the SA pathway and suppressing JA associated defenses.87
Plant viruses regulate vegetative plant volatile emissions as well.88 For instance, the Tomato spotted wilt virus (TSWV), mainly transmitted by Western flower thrips (Frankliniella occidentalis), reduces the expression of terpene synthases and emission of monoterpenes in thrips-infested pepper (Capsicum annuum) plants. This suppression is mediated by a non-structural protein of TSWV that directly interacts with the MYC2 transcription factor, a JA signaling regulator.89 MYC2 is also targeted by the viral genetic factor βC1 of Tomato yellow leaf curl China virus, which suppresses plant terpene biosynthesis in Arabidopsis and Nicotiana benthamiana plants.90 In a recent study, infection with the Cucumber mosaic virus was found to reduce the production of the monoterpenes 2-carene and β-phellandrene through the action of a viral 2b RNA silencing suppressor protein in tomato (Solanum lycopersicum), implicating microRNAs in the regulation of volatile emissions as well.91
Apart from pathogens, microbes that live in symbiosis with plants and herbivores can also influence constitutive and stress-induced vegetative plant volatiles. Root colonization by nitrogen-fixing rhizobia, for instance, increases indole and MeSA emission while suppressing β-caryophyllene production in leaves of JA-induced lima bean plants (Phaseolus Lunatus).92 Soil inoculation with the rhizobacteria Pseudomonas fluorescens WCS417r and P. putida SJ04 enhances aboveground emissions of menthone, menthol, and pulegone terpenoid compounds in peppermint (Mentha × piperita) plants.93 In addition, colonization with rhizobacteria alters the expression of genes responsible for the synthesis of sesquiterpenes and indole in maize roots.94 Similar systemic effects have been also observed for aboveground colonizing microbes. The endophytic fungus Neotyphodium uncinatum, which colonizes the aerial parts of the grass hybrid Festuca pratensis × Lolium perenne, can reduce the emission of root volatiles.95 Effects of beneficial microbes might be mediated by the increased N availability and other resources needed for volatile biosynthesis,96 but also by the modulation of defense signaling.97 In the case of insect symbionts, the presence of the endosymbiont Hamiltonella defensa in pea aphid (Acyrthosiphon pisum) has been shown to compromise plant indirect defenses (i.e., parasitoid attraction) by altering volatile emission upon aphid feeding.98
In summary, plant pathogenic and beneficial microbes can induce and suppress plant vegetative volatiles, locally or systematically, via microbial elicitors and effectors that target plant defense signaling. Recent studies have shown that microbes present in herbivores and parasites of herbivores can also affect the volatile emissions of their host plant, thus adding an additional layer to the biological regulation of plant volatile release.99
3.2 Regulation by abiotic stressors
Short periods of high temperature can affect plant volatile emissions. In the Mediterranean shrub (Halimium halimifolium), a 10 days heat wave resulted in an increase in volatile emission during the first two days.103 A 13C-labeling approach revealed that plants allocated more carbon into de novo biosynthesis of plant volatiles. Carbon investment for de novo volatile biosynthesis was also enhanced in Salix and Betula spp upon warming, suggesting the role of these volatiles in stress protection.104 Overall, plant volatile emissions increase at higher temperatures despite a negative carbon balance under heat stress. The effect of temperature on plant volatile emission is likely associated with the level of temperature changes and the physiological tolerance of each plant species.
Short-term heat stress can modulate vegetative plant volatile emissions via multiple pathways. Heat stress triggers emission of oxygenated volatiles, such as acetaldehyde. The enhanced acetaldehyde emission can be explained by induced ROS accumulation and fatty acid peroxidation.105,106 Heat stress also enhances the emission of methanol in some plant species.103 Methanol can originate from the action of methyltransferase proteins and protein repair reactions, both induced under heat stress.107 Emission of terpenoids can be also modulated by the direct heat effects on the volatility of these compounds, and indirectly through the induction of de novo biosynthesis.44 Nevetheless, work is needed to understand how temperature influences the biosynthesis, transport, and release of vegetative plant volatiles.
In addition to soil water availability, air humidity can also influence plant physiological processes itself. For example, high humidity usually leads to larger stomata and pores, as well as impaired stomatal function.110 This can affect photosynthetic processes and volatile biosynthesis,111 and also volatile release rate through changes in stomatal aperture.42 Low air humidity effects on stomatal closure might lead to lower ozone uptake by plants and, therefore, higher ozone concentration in the atmosphere.44,112,113 Increased ozone levels can, in turn, alter plant volatile production and release (see Section 3.2.4).
As drought is often associated with heat stress,114 many studies have investigated how the combination of these two stressors influence plant volatile emissions.2,19 In piñon pine (Pinus edulis), elevated temperature alone increased monoterpene emissions, but the opposite was observed in combination with severe water deficit conditions, when the carbon assimilation rates drop to zero.115 In the tropical rainforest tree, Couepia longipendula, high leaf temperatures and water deficit resulted in a strong decrease in photosynthesis, transpiration, and emissions of volatile terpenoids with a simultaneous stimulation of GLVs.116 Lower terpenoid emissions under severe abiotic stress conditions are likely caused by lower photosynthetic activity, higher membrane peroxidation, lower net carbon assimilation, excessive accumulation of reactive oxygen species, and higher lipid peroxidation.116
Drought can also modulate herbivore- and microbial-induced plant volatile emissions by itself.117 In tea (Camellia sinensis var. sinensis) plants, for instance, water deficit enhances MeJA-induced volatile emissions.118 In tomato, water deficit can increase the emission of herbivore-induced vegetative plant volatiles that function as repellents to insects.119 By contrast, water deficit reduces the emission of MeSA upon infection with the bacterial pathogen Candidatus Liberibacter in citrus plants.120 In general, drought can be expected to suppress the emission of leaf volatiles with a low Henry's law constant (H) by increasing stomatal resistance (see Section 2.2).
Compared to water deficit, less is known about the effect of water saturation and flooding on vegetative plant volatile emission. Flooding significantly changes the quality of the volatile blend in maize plants and reduces total volatile emission in certain maize genotypes but not others. However, when flooded maize plants are damaged by S. frugiperda caterpillars, they produce a significantly higher amount of volatiles.121
Changes in light spectral composition can also modulate vegetative plant volatile emissions. In dense canopies, the absorption of red (R) and reflection of far-red (FR) light by photosynthetic tissues causes a decrease in R to FR ratios. Plants respond to this light shift by investing in growth to outcompete their neighbors124 and suppressing defenses.125 Both constitutive and inducible volatile emission are decreased in Arabidopsis thaliana upon low R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FR ratio treatment.126 In tomato, the JA-induced production of volatile compounds is also modulated by low R
FR ratio treatment.126 In tomato, the JA-induced production of volatile compounds is also modulated by low R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FR ratios or genetic inactivation of the FR-associated photoreceptor, resulting in specific volatile blends.127 In addition, decreased proportions of ultraviolet radiation A, blue and green-yellow light, together with increases in R and FR, can decrease the concentrations and emissions of the phenylpropanoid eugenol and the monoterpenes linalool and 1,8-cineole in basil (Ocimum basilicum).128 Enhanced blue light treatment, however, can decrease the emission rates of monoterpenes in Norway spruce (Picea abies).129 FR supplementation can enhance the emission of maize (Z. mays) volatiles in response to herbivory or volatile cues emitted by neighboring plants.130 These effects may be in part explained by the positive impact of supplemental FR on stomatal conductance and photosynthetic rate.130 Similar effects have also been found in goldenrod (Solidago altissima), where FR enhanced plant volatile emissions in response to volatile cues from non-damaged neighbors.131
FR ratios or genetic inactivation of the FR-associated photoreceptor, resulting in specific volatile blends.127 In addition, decreased proportions of ultraviolet radiation A, blue and green-yellow light, together with increases in R and FR, can decrease the concentrations and emissions of the phenylpropanoid eugenol and the monoterpenes linalool and 1,8-cineole in basil (Ocimum basilicum).128 Enhanced blue light treatment, however, can decrease the emission rates of monoterpenes in Norway spruce (Picea abies).129 FR supplementation can enhance the emission of maize (Z. mays) volatiles in response to herbivory or volatile cues emitted by neighboring plants.130 These effects may be in part explained by the positive impact of supplemental FR on stomatal conductance and photosynthetic rate.130 Similar effects have also been found in goldenrod (Solidago altissima), where FR enhanced plant volatile emissions in response to volatile cues from non-damaged neighbors.131
The impact of other abiotic factors, such as minerals, heavy metals, low temperature, rain, and wind on plant volatile emission is less clear. Salt stress increases the emission of sesquiterpenes and certain monoterpenes in tropical daisies (Egletes viscosa).139 In addition, plants can respond to cold stress by emitting more volatile terpenoids and GLVs.140
In summary, abiotic factors strongly modulate vegetative plant volatile emissions. Given the diverse origins and release pathways, it is not surprising that the changes in plant metabolism and physiology triggered by variations in the abiotic environment leads to distinct volatile release patterns. Further work on the link between the perception of abiotic stress and the regulation of plant volatile release will be an important next step to understand the adaptive context of these responses.
4. Transfer, degradation, and uptake of vegetative plant volatiles after release
As vegetative plant volatiles are released into the atmosphere, these compounds must travel through the atmosphere to their targets, such as neighboring plants or sensory organs of other organisms, to realize their biological function.141,142 Several environmental factors can modulate this transfer.4.1 Oxidants
Plant volatiles can react with oxidants (e.g., OH, NO3, O3) in the atmosphere (See ref. 148 for detailed graphics). For example, isoprene has been shown to be reactive with OH13 and terpenoids with O3.143,144 These oxidative processes are responsible for the majority (68–69%) of biogenic secondary aerosols,145 which are important for absorbing radiation from the sun, the formation of cloud droplets, precipitation patterns and humidity in general.146,147 Vegetative plant volatiles can also contribute to the generation of atmospheric oxidants. The oxidation of plant volatiles can generate peroxyl radicals that react with NO to form NO2, which is then photolyzed to generate O3.148 It is estimated that forest-emitted isoprene contributes to the generation of 15–18% of the tropospheric ozone.149Atmospheric oxidants are also involved in reducing the availability of plant volatiles.150 Ozone fumigation reduces the atmospheric concentration of mono- and sesquiterpenes released by oilseed rape (Brassica napus). The reduction in sesquiterpene concentration was likely linked to ozonolysis at the leaf surface, whereas reduction in monoterpene concentration was linked to reactions with ozonolysis-derived OH radicals in the air.151 It was also shown that increased ambient ozone reduces the adsorption of the monoterpene myrcene in white cabbage (Brassica oleracea convar. capitata var. alba).152 The reactivity of vegetative volatiles in the atmosphere is compound-specific, with reaction lifetimes that range from days to seconds.150
4.2 Air humidity
Air humidity can influence the availability of volatiles in the atmosphere by regulating their breakdown processes and the formation of secondary aerosols.153,154 In addition, a negative relationship between ozone levels and humidity is constantly observed in air quality data. High vapor pressure deficit (VPD) has a strong correlation with higher ozone levels.155–157 This correlation is partly caused by the lower dry deposition of ozone by plant stomata due to stomatal closure under high VPD.158 Since the late 1990s, there has been an increase in VPD consistently associated with the decrease in primary production, which can offset the positive effect of elevated CO2 on plant growth.159 This high VPD can affect the concentrations of ozone in the atmosphere and, therefore, also the chemical interactions between plant vegetative volatiles and ozone (see Section 4.1). The dynamics of these interactions, however, are still unknown and need further research especially in the context of plant ecological interactions.In addition to atmospheric humidity, the humidity in the soil can influence the diffusion and degradation of belowground vegetative plant volatiles.160 In maize, for instance, the diffusion of β-caryophyllene produced by roots was found to be much faster in drier soils.161
4.3 Other organisms
Vegetative volatiles can be absorbed or degraded by other organisms after their release.162 In tomato, (Z)-3-hexenol from damaged neighboring plants can be taken up and converted into (Z)-3-hexenylvicianoside, which negatively influences herbivores.163 Uptake of plant volatiles by plants can also be observed at larger scales.164 In a winter wheat (Triticum aestivum) field, volatile emissions were observed to differ significantly across the growing season.165 Different types of vegetative volatiles exhibited specific bi-directional exchanges between the field and atmosphere, involving plants, soil, or both.165 Similar to the role of plants as volatile sinks, soil microbes can rapidly mineralize volatiles, a process that is highly dependent on the microbial community and specific volatile compounds.166Vegetative volatiles can stick to the surface of other plants. This absorption can lead to reductions in atmospheric volatiles and re-emission of these volatiles into the atmosphere.167–169 Wild rosemary (Rhododendron tomentosum) is a strong volatile releaser that produces many insect-repelling volatiles. The volatiles released by R. tomentosum have been found to be adsorbed and re-release by neighboring birch and can lead to enhanced resistance of birch against green leaf weevils (Polydrusus flavipes).169 In summary, biotic interactions influence the availability of atmospheric vegetative volatiles. However, detailed knowledge on how this exchange of volatile between the biosphere and atmosphere is still lacking.
5. The role of vegetative plant volatiles in biotic interactions
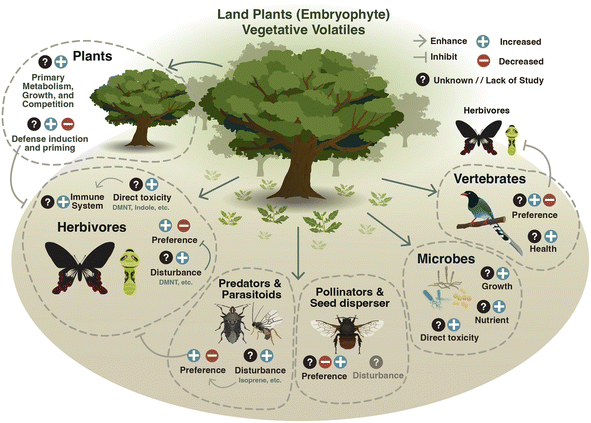
Once released, vegetative volatiles can influence the behavior and physiology of a wide range of organisms (Fig. 4). These effects have important consequences for ecosystem functioning and organismal behaviour.5.1 Effects on plants
Vegetative plant volatiles reprogram plant defenses.14,170 Induced volatiles, for instance, act as stress cues that can be perceived by non-attacked tissues and neighboring plants and trigger defense responses.170 GLVs are released rapidly by plants upon wounding.8,171,172 GLVs such as hexenal, hexenol and hexenyl acetate can prime defenses in neighboring plants, whereby there is a potentiated defence capacity without induction until plants are subsequently challenged with a stressor.8,171,173 GLVs can also directly induce plant defense responses. In maize, exposure to (Z)-3-hexenol for 20–60 min induces defense gene expression to a much greater degree than other stress-induced volatiles (MeJA and MeSA).174 The sensitivity of plants to these early damage-induced volatile signals is likely to help them fend off subsequent attacks.173 Hexenal can be taken up by plants and enzymatically reduced into hexenol and subsequently into hexenyl acetate.172,175 Further, these GLVs are converted into defences (e.g., GLV-glycosides) that can help plants combat environmental stressors.176Indole is a nitrogen-containing volatile that is also emitted relatively early after attack.32 Indole can prime defense responses to herbivory or pathogen infection.31,32,173,177 In rice (Oryza sativa) and tea (Camelia sinensis) indole-mediated priming was shown to be dependent on mitogen-activated protein kinase and downstream WRKY transcription factors, suggesting that this priming occurs in a JA-dependent fashion.31,178 In maize, pre-treatment with indole primed up-regulation of anti-necrotrophic fungal defense genes and significantly reduced disease severity upon exposure to Fusarium graminearum.177 Indole also exhibits synergistic priming effects together with GLVs,173 demonstrating that different volatiles can act in concert to reprogram plant defenses.179
Other volatiles have also been shown to reprogram plant defenses21 and have been implicated in genotype-specific responses.14,180,181 Nonanal and β-ionone trigger systemic acquired resistance against the hemi-biotrophic pathogen Blumeria graminis in barley (Hordeum vulgare).182 Further, a blend of α- and β-pinene induced the production of reactive oxygen species and SA defences in Arabidopsis.83 The homoterpene DMNT induced the activity of Sporamin protease inhibitors, and when Spodoptera larvae were fed on DMNT-exposed plants, their performance was significantly reduced compared to unexposed plants.183 Exposure to plant volatiles can also alter plant primary metabolism. A recent study found that Scots pine seedlings exposed to vegetative volatiles released from large pine weevil (Hylobius abietis)-damaged plants display increased stomatal conductance and photosynthesis, enabling plants to accumulate a larger pool of C-based metabolites.184 Volatile information transfer between plants can also have negative consequences for receiver plants. For example, the blend of induced volatiles emitted from tomato plants following whitefly (Bemisia tabaci) infestation were shown to suppress anti-whitefly defences in receiver plants, rendering them more susceptible to whiteflies.185
In addition to inducible volatiles, constitutive volatile emission can also play a role in information transfer between plants. Volatiles constitutively emitted by spotted knapweed (Centaurea stoebe) roots were shown to increase carbohydrate and protein levels within the roots of common dandelion (Taraxacum officinale), which in turn made T. officinale plants more susceptible to cockchafer beetle (Melolontha melolontha) larvae.186 When potato (Solanum tuberosum) plants were exposed to vegetative volatiles constitutively emitted from onion (Allium cepa) plants, they emitted higher quantities of (E)-nerolidol and TMTT.187 Sesquiterpenes from the spotted knapweed such as β-caryophyllene, can promote growth and germination in sympatric neighboring plants, but the mechanisms of this interaction remain unclear.188
Plants are often grown in close proximity to other plants24 and are thus exposed to complex mixtures of constitutive and induced vegetative plant volatiles.189,190 The abundance and persistence of specific vegetative volatiles within plant canopies is dependent on the diversity and density of plants in a given ecosystem.191 However, we currently know little about the impact of volatile transfer across plant communities. Exposing healthy, uninfected Arabidopsis thaliana plants to monoterpenes emitted from plants infested with Pseudomonas syringae and then subsequently exposing the volatiles from a healthy plant to a third healthy plant enhances the resistance of the third plant to subsequent P. syringae infection.192 Thus, volatile defense cues may be transmitted across plant populations. Volatile blends of odor plumes become less similar to the original composition with increasing distance from the odor source, which may modulate population and community level effects.193 In beech (Fagus), volatile defense induction was strongest at distances <5–7 m, suggesting strong spatial dependency.142
5.2 Effects on invertebrates
Volatiles can also act as repellents against herbivores in a temporally dependent fashion. In tobacco, herbivore-induced volatiles emitted at night repel nocturnal ovipositing by the tobacco budworm (Heliothis virescens) moths.202 Furthermore, some volatiles can act directly as toxins against herbivores. DMNT can damage the peritrophic matrix in the diamondback moth (Plutella xylostella) midguts making them more susceptible to microbial infection and, ultimately, leading to strong reductions in herbivore performance and survivability.203 Indole has also been shown to reduce the survival of the beet armyworm (Spodoptera exigua) when infused into artificial diet.204 Exposure to natural blends of tomato volatiles is associated with increased expression of cytochrome p450 enzymes and increased larval growth on artificial diet containing trypsin inhibitors, suggesting that plant volatiles may boost herbivore detoxification processes.205
Volatile concentration plays a crucial role in determining their effects on herbivores. For example, attraction to indole of S. littoralis caterpillars is strongest at lower concentrations whereas attraction to hexenol is strongest at higher concentrations.195 Indole can also repel S. littoralis, an effect that is lost when the caterpillars are in the proximity of parasitoids.18 The performance of the Western corn root worm was shown to be dependent on the density of conspecific larvae also feeding on maize roots, further, larvae used (E)-β-caryophyllene emission levels associated with the suitable density of conspecifics to locate host plants.206 Additionally, in cabbage, only certain herbivore species resulted in dose-dependent volatile emission. When fed upon by cabbage white (Pieris rapae) plants emitted volatiles in a larval-density-dependent fashion with commensurate parasitoid attraction. However, when fed upon by the P. xylostella, volatile emission was constant across varying larval densities.207
5.3 Effects on vertebrates, including humans
Vegetative plant volatiles can influence the behavior and performance of larger vertebrates. The great tit (Parus major) can distinguish between herbivore-infested and herbivore-free apple trees using volatile cues.215 Terpenes such as α-farnesene and DMNT have been implicated as potential cues as both are significantly induced in herbivore-infested plants, which are more attractive to insect-eating birds.215–217Many reptiles are also olfactory foragers. The active foraging lizard (Sceloporous virgatus) responds to the GLV (E)-2-hexenal primarily by inducing tongue-flicking, which is a known chemosensory behavior.218 Apart from this example, little is known about the impact of plant volatiles on reptiles.218,219
Several herbivorous mammals are known to use vegetative plant volatiles as foraging cues. Goats respond negatively to terpenes emitted by galls formed during gregarious aphid (Salvum wertheimae) infestation of wild pistachio (Pistacia atlantica) trees.220 Elephants use the monoterpenes β-ocimene and linalool, the sesquiterpenes β-caryophyllene and 1-methylpyrrole, as well as the tryptophan-derived indole for food choice.221
Vegetative plant volatiles can also directly impact human behaviour. Certain volatile compounds in edible foods such as coriander (Coriandrum sativum) have been linked with positive emotions, increased salivation, and enhanced theta band activity in the cerebral cortex.222 Additionally, several studies have highlighted the positive impacts of plant-dominated areas on mental health.223,224 Forest bathing, an activity whereby a person spends a prolonged period in a forest, is associated with the amelioration of a number of physical ailments and has been partially linked to volatile cues.225In vitro, 1,8-cineole and β-caryophyllene have been shown to reduce neuro-inflammation (associated with disorders such as Alzheimer's disease).226,227 Phellandrene, a terpene emitted by a wide range in plants has also been associated with anti-inflammatory response in human cell cultures.228 There is evidence that inhaled terpenes such as limonene and pinene can end up in the human bloodstream, although considering they are expelled in unchanged form, they do not seem to be metabolized.225,229
5.4. Effects on microorganisms
Vegetative plant volatiles influence interactions of plants with multitude of microorganisms, such as viruses, bacteria, archaea, protozoa, fungi, algae, and nematodes.230–232 Plant-derived terpenoids, benzenoids, and aldehydes can all exert antibacterial or antifungal effects. For instance, the monoterpenes limonene and β-pinene, and several aldehydes, which are major constituents of the volatile bouquet emitted by pine trees, display antimicrobial activities against airborne bacteria.233 Similarly, GLVs such as (E)-2- and (Z)-3-hexenal show antimicrobial effects against plant pathogenic bacteria in vitro.234 Yet, exceptions to this pattern have been reported for the sesquiterpene (E)-β-caryophyllene, which can directly stimulate the growth of the hemi-biotrophic fungus Colletotrichum graminicola.235 In rice, the induced emission of the monoterpene (S)-limonene upon infection with the pathogenic fungus Magnaporthe oryzae can inhibit the germination of M. oryzae spores.236Besides antimicrobial activities, vegetative plant volatiles can influence the establishment of microbial communities by acting as attractants, nutrients, or substrates to produce bioactive compounds.237 Linalool, 2-phenylethanol, and nonanal emitted by strawberry (Fragaria ananassa) plants can be chemically transformed by leaf epiphytic bacteria and enhance their competitive performance in the presence of the pathogenic fungi Botrytis cinerea.238 Root-derived volatiles emitted by tomato plants can attract certain soil bacteria.239 These authors also observed that some bacteria might be more strongly attracted by plant volatiles under soil nutrient limitation, implicating root volatiles as info-chemicals that provide information about a nearby nutrient-rich environment. Whether active recruitment of microorganisms by vegetative plant volatiles affects plant performance or resilience against biotic and abiotic stressors, however, is not well explored.
In addition to direct effects, microbial communities can indirectly be affected by the interplay of vegetative plant volatiles with other plants or higher trophic organisms. For instance, pathogen-induced emission of aboveground vegetative volatiles in infected plants can alter the responses of non-infected neighbors to the same microbial pathogen either by increasing or reducing susceptibility.240 More recently, vegetative volatiles emitted aboveground by tomato plants inoculated with beneficial root-associated bacteria were shown to modulate the rhizosphere microbiota of surrounding conspecific plants via volatile-induced changes in root exudates.241 The ecological and physiological implications for both the emitter and receiver plants, as well as their associated soil microbiota, were not further investigated. Still, this novel phenomenon adds further support for the potential use of above- and/or below-ground volatiles to fine-tune the rhizosphere microbiota in agricultural systems.
6. Synthesis: biological frameworks to explain patterns of vegetative plant volatile release
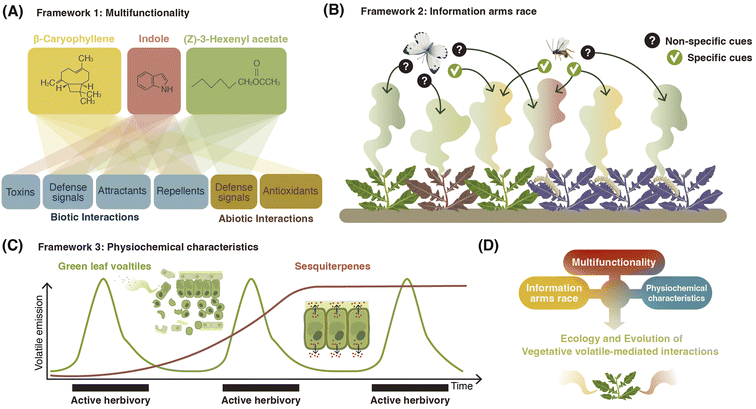
Vegetative plant volatiles are produced via a multitude of pathways and follow different routes to end up in the environment. The biosynthesis and release of volatiles is modulated by both biotic and abiotic factors. Together, this results in highly dynamic and diverse volatile release patterns. Once the volatiles have left the plant, they interact with organisms of all known kingdoms, both at behavioral and physiological levels. These interactions again shape the genetics of volatile release and production over evolutionary time. Given this substantial physiological and ecological complexity, one has to wonder whether there are any specific biological frameworks beyond the most fundamental physical, chemical, and biological principles that explain vegetative volatile release patterns. Over the last years, a number of frameworks have started emerging that may help to explain volatile release patterns (Fig. 5).6.1. Multifunctionality
There is now ample evidence that the same volatile can have multiple functions for plants242 (Fig. 5). GLVs, for instance, act as within-plant defense signals, antimicrobials, and attractants of natural enemies.8 Indole primes plant defenses, it acts as a repellent at high doses, and it can directly intoxicate herbivores.18,32,196,204 The homoterpene DMNT can act as an herbivore toxin when ingested, and it also interferes with the location of the herbivore's host plant when perceived by olfactory receptors.201,203 β-caryophyllene has been implicated in biotic interactions as a volatile cue both above- and below-ground, where the impacts on plants range from beneficial to detrimental. For example, β-caryophyllene can attract herbivores' natural enemies243 or help Diabrotica virgifera virgifera larvae to locate their host plant roots.206 β-caryophyllene has also been implicated as a critical defense against the fungal pathogen Pseudomonas syringae.244 The regulation of the production and release of these compounds likely mirrors their multiple roles and benefits for plants. During insect herbivory, biosynthetic similarity between compounds is associated with higher covariance between compounds, suggesting that there may be constraints at the biosynthetic level for covariation patterns between different volatile groups.245 For example, multiple compounds can be produced from a single enzyme/substrate pair. Further, conditions such as catalytic metal ion availability and pH can influence the relative abundances of terpene products in vitro.246 It is thus possible that, under variable conditions within plant tissues, the relative abundance of products of the same substrate/enzyme might vary. Developing a deeper understanding of the multifunctionality of volatiles can thus help to uncover ecological and evolutionary rationales for their biosynthesis and emission.6.2. Chemical communication displays and the information arms race
Vegetative plant volatiles can provide information to beneficial organisms such as herbivore natural enemies, but also to antagonists such as herbivores. These conflicting functions and effects can be described as an information arms race, whereby the plant tries to hide from herbivores while being visible to herbivore natural enemies.247 In this framework, the ability of plants to produce volatiles that are protective but not necessarily unique (i.e., other nearby plants are also producing them) is most effective for minimizing fitness costs associated with volatile emission.247 Volatile emission can be considered as a chemical communication display that serves as information (Fig. 5B). In plants, covariation between volatiles is relatively low, particularly following herbivory, suggesting that single or few compounds are likely responsible for mediating a particular information transfer between a plant and another organism.245 If herbivores rely on fixed ratios of compounds, then the further reduction of covariance among herbivory-induced plant volatiles may interfere with the host location of subsequent herbivores.248 Further exploration regarding how multifunctionality, not only of the volatile pool but also of individual compounds, influences the ecological interactions in complex ecosystems is essential to identify how volatiles ultimately impact plant fitness and evolutionary trajectories.6.3. Physiochemistry
Depending on their physiochemical characteristics, vegetative plant volatiles show distinct spatiotemporal release and transfer patterns. Some are breakdown products and thus released rapidly from wounded tissues, while others are synthesized via enzymatic cascades and thus produced more slowly8,32 (Fig. 5C). Some are stable and diffuse across large distances, and others are unstable and rapidly degraded.249 Thus, vegetative plant volatiles have distinct physiochemical fingerprints, which constrain their adaptive plasticity and ecological effects. GLVs, for instance, are breakdown products rapidly released following stress, mostly from wound sites.250 They thus provide a timely, but unspecific cue of active damage, and may be used by natural enemies accordingly.251 Similarly, the above properties predispose GLVs to act as damage-associated molecular patterns in plant defense regulation.252 Volatiles such as terpenes, on the other hand, diffuse slowly and take longer periods to be produced because of dedicated biosynthetic machinery. Accordingly, they can act as highly specific cues for certain herbivores and other plants, as well as constitutive defenses or phytoanticipins. Hence, terpenoids might be less suited as rapid, localized defense signals (Fig. 5C). There are, however, exceptions to this; terpenes stored in trichomes for instance can be released rapidly upon damage (see Section 2.2).In summary, we propose that the release patterns of a given class of volatiles can be explained, at least in part, by their multifunctionality, the information arms race, and their physiochemical properties. A better understanding of the physical properties and ecology of individual volatile classes is indispensable to leverage the power of these frameworks (Fig. 5D).
7. Open questions and future research on vegetative plant volatiles
Although the field is rapidly gaining a deep understanding of the physiology and ecology of vegetative plant volatiles, several important open questions remain.7.1 Mechanisms of transport and release
While the biosynthesis of vegetative plant volatiles is understood in substantial detail, the mechanisms governing transport and release are not well known yet (Fig. 2). Most molecular work so far has focused on floral volatiles,253 and it remains to be seen whether vegetative volatile release is governed by the same mechanisms. A particular challenge will be to understand the relative importance of transport and/or diffusion across cell membranes, cuticular barriers, stomata, trichomes, and wounds. Given the vast diversity of physiochemical characteristics across vegetative plant volatiles, it is likely that different volatiles will have different preferred release pathways mediated by the physiological characteristics of plant tissues.41,44 Understanding the major transport routes of individual volatiles will be imperative to investigate their regulation, ecology, and evolution in an environmental context.7.2 Interactions of multiple stresses on volatile production and release
Plant volatile responses to individual biotic and abiotic factors are well described, and we have also gained more detailed understanding on how different abiotic factors interact in this context.254 However, much remains to be learned regarding the impact of multivariate environments on plant volatile production and release. Interactions between multiple abiotic factors at different levels of stress severity, for instance, are not well known. Severe stress might trigger completely different responses in comparison to mild stress. Climate change has led to an increase in multi-stress scenarios worldwide,114 which will most likely affect plant volatile emissions and plant interactions as well. Investigating how plant volatiles profiles respond to these dynamic abiotic pressures will help to predict plant performance in the context of herbivory and beyond.7.3 Transmission dynamics in the field
A number of methods has been developed to detect, identify, and quantify plant volatiles.255 The pros and cons of various plant volatile measurement techniques are reviewed in detail by Tholl et al.256 Most of these methods rely on collection approaches with low temporal resolution and/or involve highly restricted headspace environments such as glass bottles.257,258 For example, volatiles are often collected and concentrated over time (often several hours) and collections are then analyzed using techniques such as gas chromatography coupled with mass-spectrometry (GC-MS).256 As samples are pooled, the precise time point in which these volatiles are emitted is difficult to determine. Even if samples are collected for shorter periods of time, materials and lengthy analytical program run times can cause temporal experimental bottlenecks. Techniques such as proton-transfer reaction time-of-flight mass spectrometry (PTR-ToF-MS) do not require a time-consuming separation phase and allows for direct injection of airborne volatiles into the mass spectrometer. Still, PTR-ToF-MS cannot differentiate chemical structures with identical molecular weights, e.g., different monoterpenes.It is clear that there is high spatial variability in vegetative plant volatile emissions, whether it be differing plant tissues and plant species or between plants under variable environmental pressures.259–261 Capturing this variation in more detail will be important in the future. Recently, technological advancements have allowed for real-time measurements of volatiles across multiple plants, however this has yet to be implemented in a field setting.130 By consequence, we lack a detailed understanding of spatial and temporal volatile dynamics at the plant and interplant scale within plant canopies.256 Developing our understanding of how vegetation creates a “volatile landscape” in terrestrial ecosystems is important to understand how ecosystem-scale volatile dynamics mediate ecosystem functioning. The development of adequate devices/technologies will play an essential role in the study of plant volatiles at the canopy level.
7.4 Mechanisms of uptake, perception, and physiological responses
Plant volatiles are perceived and, in some cases, taken up by plants and other organisms.163,179,262–265 The mechanisms underlying these processes, however, are not well understood. Volatile uptake by plants, for instance, is thought to occur through the stomata or via diffusion through the leaf cuticle,253,266 but neither of these mechanisms have been explored in detail. To date, a receptor-based mechanism in plants has only been reported for the volatile phytohormone ethylene.267 Volatile perception has been explored at the molecular level for a number of insects, and different mechanisms are being validated for plants.164,268,269 Outside of perception, volatiles' targets that may trigger physiological responses in insects and mammals are unknown and deserve further study to harness the potential of plant volatiles as pest control agents and drugs.7.5 Identifying major evolutionary forces driving vegetative plant volatile patterns
Given the many functions and effects of vegetative plant volatiles, understanding the major forces that govern their evolution remains challenging. Volatiles have been implicated as driving forces in shaping resistance to herbivory in receiver plants, sometimes in a taxa specific manner. There is also genotype-specific information transfer between plants, where signals from kin have been shown to result in the highest level of resistance to herbivory in receiver plants.14,270 The existence of elicitors and effectors produced by both arthropods and microbes modulating volatile emissions has reinforced the idea that vegetative plant volatiles are important actors in the ecology of plant biotic interactions and might have shaped the evolution of these traits.43,271 In nature, however, plants are exposed to all these components either simultaneously or sequentially through their lifetime. Thus, it is likely that volatile-related traits have evolved under the influence of a dynamic environment. Understanding how vegetative plant volatiles respond to multiple biotic factors is, therefore, essential to unravel their ecological functions.8. Author contributions
All authors conceived the idea for this review. REB, PAL and JMW contributed equally to the writing of the first draft (order of authorship determined by alphabetical order of last name). ME wrote additional sections and made critical revisions to subsequent drafts. All authors made substantial contributions to the final draft.9. Conflicts of interest
The authors have no conflicts of interest to declare.10. Acknowledgement
This work is supported by the Swiss National Science foundation, grant numbers 200355 (to ME) and 210651 (to JMW), the State Secretariat for Education, Research and Innovation of Switzerland, project CANWAS (to ME), the Velux Foundation, grant number 1231 (to ME and REB) and the University of Bern.11. References
- J. Takabayashi and K. Shiojiri, Multifunctionality of herbivory-induced plant volatiles in chemical communication in tritrophic interactions, Curr. Opin. Insect Sci., 2019, 32, 110–117 CrossRef PubMed.
- F. Loreto and J. P. Schnitzler, Abiotic stresses and induced BVOCs, Trends Plant Sci., 2010, 15, 154–166 CrossRef CAS PubMed.
- S. Zhou and G. Jander, Molecular ecology of plant volatiles in interactions with insect herbivores, J. Exp. Bot., 2021, 73, 449–462 CrossRef PubMed.
- R. A. Raguso, Wake Up and Smell the Roses: The Ecology and Evolution of Floral Scent, Annu. Rev. Ecol. Evol. Syst., 2008, 39, 549–569 CrossRef.
- D. Kessler, C. Diezel, D. G. Clark, T. A. Colquhoun and I. T. Baldwin, Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends, Ecol. Lett., 2013, 16, 299–306 CrossRef PubMed.
- C. M. Caruso and A. L. Parachnowitsch, Do Plants Eavesdrop on Floral Scent Signals?, Trends Plant Sci., 2016, 21, 9–15 CrossRef CAS PubMed.
- T. C. J. Turlings and M. Erb, Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential, Annu. Rev. Entomol., 2018, 63, 433–452 CrossRef CAS PubMed.
- M. Ameye, S. Allmann, J. Verwaeren, G. Smagghe, G. Haesaert, R. C. Schuurink and K. Audenaert, Green leaf volatile production by plants: a meta-analysis, New Phytol., 2018, 220, 666–683 CrossRef CAS PubMed.
- B. M. Delory, P. Delaplace, M.-L. Fauconnier and P. Du Jardin, Root-emitted volatile organic compounds: can they mediate belowground plant-plant interactions?, Plant Soil, 2016, 402, 1–26 CrossRef CAS.
- N. Dudareva, A. Klempien, J. K. Muhlemann and I. Kaplan, Biosynthesis, function and metabolic engineering of plant volatile organic compounds, New Phytol., 2013, 198, 16–32 CrossRef CAS PubMed.
- A. Richter, C. Schaff, Z. Zhang, A. E. Lipka, F. Tian, T. G. Köllner, C. Schnee, S. Preiß, S. Irmisch, G. Jander, W. Boland, J. Gershenzon, E. S. Buckler and J. Degenhardt, Characterization of Biosynthetic Pathways for the Production of the Volatile Homoterpenes DMNT and TMTT in Zea mays, Plant Cell, 2016, 28, 2651–2665 CrossRef CAS PubMed.
- F. Adebesin, J. R. Widhalm, B. Boachon, F. Lefèvre, B. Pierman, J. H. Lynch, I. Alam, B. Junqueira, R. Benke, S. Ray, J. A. Porter, M. Yanagisawa, H. Y. Wetzstein, J. A. Morgan, M. Boutry, R. C. Schuurink and N. Dudareva, Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter, Science, 2017, 356, 1386–1388 CrossRef CAS PubMed.
- A. C. Nölscher, A. M. Yañez-Serrano, S. Wolff, A. C. de Araujo, J. V. Lavrič, J. Kesselmeier and J. Williams, Unexpected seasonality in quantity and composition of Amazon rainforest air reactivity, Nat. Commun., 2016, 7, 10383 CrossRef PubMed.
- A. Kalske, K. Shiojiri, A. Uesugi, Y. Sakata, K. Morrell and A. Kessler, Insect Herbivory Selects for Volatile-Mediated Plant-Plant Communication, Curr. Biol., 2019, 29, 3128–3133 CrossRef CAS PubMed.
- T. C. Turlings and J. H. Tumlinson, Systemic release of chemical signals by herbivore-injured corn, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 8399–8402 CrossRef CAS PubMed.
- R. P. Ghimire, T. Silfver, K. Myller, E. Oksanen, J. K. Holopainen and J. Mikola, BVOC Emissions From a Subarctic Ecosystem, as Controlled by Insect Herbivore Pressure and Temperature, Ecosystems, 2021, 25, 872–891 CrossRef.
- T. Li, T. Holst, A. Michelsen and R. Rinnan, Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra, Nat. Plants, 2019, 5, 568–574 CrossRef PubMed.
- M. Ye, N. Veyrat, H. Xu, L. Hu, T. C. J. Turlings and M. Erb, An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars, Sci. Adv., 2018, 4, eaar4767 CrossRef PubMed.
- J. Peñuelas and M. Staudt, BVOCs and global change, Trends Plant Sci., 2010, 15, 133–144 CrossRef PubMed.
- E. K. Meineke, C. C. Davis and T. J. Davies, Phenological sensitivity to temperature mediates herbivory, Global Change Biol., 2021, 27, 2315–2327 CrossRef CAS PubMed.
- M. Rosenkranz, Y. Chen, P. Zhu and A. C. Vlot, Volatile terpenes - mediators of plant-to-plant communication, Plant J., 2021, 108, 617–631 CrossRef CAS PubMed.
- L. Hu, K. Zhang, Z. Wu, J. Xu and M. Erb, Plant volatiles as regulators of plant defense and herbivore immunity: molecular mechanisms and unanswered questions, Curr. Opin. Insect Sci., 2021, 44, 82–88 CrossRef PubMed.
- A. M. Yáñez-Serrano, E. Bourtsoukidis, E. G. Alves, M. Bauwens, T. Stavrakou, J. Llusià, I. Filella, A. Guenther, J. Williams, P. Artaxo, K. Sindelarova, J. Doubalova, J. Kesselmeier and J. Peñuelas, Amazonian biogenic volatile organic compounds under global change, Global Change Biol., 2020, 26, 4722–4751 CrossRef PubMed.
- F. Zhou and E. Pichersky, More is better: the diversity of terpene metabolism in plants, Curr. Opin. Plant Biol., 2020, 55, 1–10 CrossRef CAS PubMed.
- F. Chen, D.-K. Ro, J. Petri, J. Gershenzon, J. Bohlmann, E. Pichersky and D. Tholl, Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole, Plant Physiol., 2004, 135, 1956–1966 CrossRef CAS PubMed.
- Y. Liu, S.-H. Luo, J. Hua, D.-S. Li, Y. Ling, Q. Luo and S.-H. Li, Characterization of defensive cadinenes and a novel sesquiterpene synthase responsible for their biosynthesis from the invasive Eupatorium adenophorum, New Phytol., 2021, 229, 1740–1754 CrossRef CAS.
- D. Liu, X. Huang, W. Jing, X. An, Q. Zhang, H. Zhang, J. Zhou, Y. Zhang and Y. Guo, Identification and functional analysis of two P450 enzymes of Gossypium hirsutum involved in DMNT and TMTT biosynthesis, Plant Biotechnol. J., 2018, 16, 581–590 CrossRef CAS PubMed.
- T. Vogt, Phenylpropanoid biosynthesis, Mol. Plant, 2010, 3, 2–20 CrossRef CAS.
- D. R. Gang, J. Wang, N. Dudareva, K. H. Nam, J. E. Simon, E. Lewinsohn and E. Pichersky, An investigation of the storage and biosynthesis of phenylpropenes in sweet basil, Plant Physiol., 2001, 125, 539–555 CrossRef CAS PubMed.
- J. J. Glas, B. C. J. Schimmel, J. M. Alba, R. Escobar-Bravo, R. C. Schuurink and M. R. Kant, Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores, Int. J. Mol. Sci., 2012, 13, 17077–17103 CrossRef CAS PubMed.
- M. Ye, M. Liu, M. Erb, G. Glauser, J. Zhang, X. Li and X. Sun, Indole primes defence signalling and increases herbivore resistance in tea plants, Plant, Cell Environ., 2021, 44, 1165–1177 CrossRef CAS PubMed.
- M. Erb, N. Veyrat, C. A. M. Robert, H. Xu, M. Frey, J. Ton and T. C. J. Turlings, Indole is an essential herbivore-induced volatile priming signal in maize, Nat. Commun., 2015, 6, 6273 CrossRef CAS PubMed.
- X. Zhuang, A. Fiesselmann, N. Zhao, H. Chen, M. Frey and F. Chen, Biosynthesis and emission of insect herbivory-induced volatile indole in rice, Phytochemistry, 2012, 73, 15–22 CrossRef CAS PubMed.
- A. Richter, A. F. Powell, M. Mirzaei, L. J. Wang, N. Movahed, J. K. Miller, M. A. Piñeros and G. Jander, Indole-3-glycerolphosphate synthase, a branchpoint for the biosynthesis of tryptophan, indole, and benzoxazinoids in maize, Plant J., 2021, 106, 245–257 CrossRef CAS PubMed.
- S. Irmisch, A. C. McCormick, G. A. Boeckler, A. Schmidt, M. Reichelt, B. Schneider, K. Block, J.-P. Schnitzler, J. Gershenzon, S. B. Unsicker and T. G. Köllner, Two herbivore-induced cytochrome P450 enzymes CYP79D6 and CYP79D7 catalyze the formation of volatile aldoximes involved in poplar defense, Plant Cell, 2013, 25, 4737–4754 CrossRef CAS PubMed.
- S. Irmisch, A. Clavijo McCormick, J. Günther, A. Schmidt, G. A. Boeckler, J. Gershenzon, S. B. Unsicker and T. G. Köllner, Herbivore-induced poplar cytochrome P450 enzymes of the CYP71 family convert aldoximes to nitriles which repel a generalist caterpillar, Plant J., 2014, 80, 1095–1107 CrossRef CAS.
- J. C. D'Auria, E. Pichersky, A. Schaub, A. Hansel and J. Gershenzon, Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana, Plant J., 2007, 49, 194–207 CrossRef PubMed.
- J.-J. Cheong and Y. D. Choi, Methyl jasmonate as a vital substance in plants, Trends Genet., 2003, 19, 409–413 CrossRef CAS.
- B. A. Halkier and J. Gershenzon, Biology and biochemistry of glucosinolates, Annu. Rev. Plant Biol., 2006, 57, 303–333 CrossRef CAS PubMed.
- I. Blažević, S. Montaut, F. Burčul, C. E. Olsen, M. Burow, P. Rollin and N. Agerbirk, Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants, Phytochemistry, 2020, 169, 112100 CrossRef PubMed.
- Ü. Niinemets, Controls on the emission of plant volatiles through stomata: A sensitivity analysis, J. Geophys. Res., 2003, 108, 4211 CrossRef.
- I. Seidl-Adams, A. Richter, K. B. Boomer, N. Yoshinaga, J. Degenhardt and J. H. Tumlinson, Emission of herbivore elicitor-induced sesquiterpenes is regulated by stomatal aperture in maize (Zea mays) seedlings, Plant, Cell Environ., 2015, 38, 23–34 CrossRef CAS PubMed.
- P.-A. Lin, Y. Chen, D. Chaverra-Rodriguez, C. C. Heu, N. B. Zainuddin, J. S. Sidhu, M. Peiffer, C.-W. Tan, A. Helms, D. Kim, J. Ali, J. L. Rasgon, J. Lynch, C. T. Anderson and G. W. Felton, Silencing the alarm: an insect salivary enzyme closes plant stomata and inhibits volatile release, New Phytol., 2021, 230, 793–803 CrossRef CAS.
- U. Niinemets, F. Loreto and M. Reichstein, Physiological and physicochemical controls on foliar volatile organic compound emissions, Trends Plant Sci., 2004, 9, 180–186 CrossRef CAS.
- J. R. Widhalm, R. Jaini, J. A. Morgan and N. Dudareva, Rethinking how volatiles are released from plant cells, Trends Plant Sci., 2015, 20, 545–550 CrossRef CAS PubMed.
- X. Fu, P. Shi, Q. He, Q. Shen, Y. Tang, Q. Pan, Y. Ma, T. Yan, M. Chen, X. Hao, P. Liu, L. Li, Y. Wang, X. Sun and K. Tang, AaPDR3, a PDR Transporter 3, Is Involved in Sesquiterpene β-Caryophyllene Transport in Artemisia annua, Front. Plant Sci., 2017, 8, 723 CrossRef PubMed.
- P. Liao, S. Ray, B. Boachon, J. H. Lynch, A. Deshpande, S. McAdam, J. A. Morgan and N. Dudareva, Cuticle thickness affects dynamics of volatile emission from petunia flowers, Nat. Chem. Biol., 2021, 17, 138–145 CrossRef CAS PubMed.
- A. Block, M. M. Vaughan, S. A. Christensen, H. T. Alborn and J. H. Tumlinson, Elevated carbon dioxide reduces emission of herbivore-induced volatiles in Zea mays, Plant, Cell Environ., 2017, 40, 1725–1734 CrossRef CAS PubMed.
- U. Niinemets, M. Reichstein, M. Staudt, G. Seufert and J. D. Tenhunen, Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea, Plant Physiol., 2002, 130, 1371–1385 CrossRef CAS PubMed.
- S. Zabel, W. Brandt, A. Porzel, B. Athmer, S. Bennewitz, P. Schäfer, R. Kortbeek, P. Bleeker and A. Tissier, A single cytochrome P450 oxidase from Solanum habrochaites sequentially oxidizes 7-epi-zingiberene to derivatives toxic to whiteflies and various microorganisms, Plant J., 2021, 105, 1309–1325 CrossRef CAS PubMed.
- R. W. J. Kortbeek, M. D. Galland, A. Muras, F. M. van der Kloet, B. André, M. Heilijgers, S. A. F. T. van Hijum, M. A. Haring, R. C. Schuurink and P. M. Bleeker, Natural variation in wild tomato trichomes; selecting metabolites that contribute to insect resistance using a random forest approach, BMC Plant Biol., 2021, 21, 315 CrossRef CAS PubMed.
- J.-H. Kang, G. Liu, F. Shi, A. D. Jones, R. M. Beaudry and G. A. Howe, The Tomato odorless-2 Mutant Is Defective in Trichome-Based Production of Diverse Specialized Metabolites and Broad-Spectrum Resistance to Insect Herbivores, Plant Physiol., 2010, 154, 262–272 CrossRef CAS PubMed.
- N. Bergau, S. Bennewitz, F. Syrowatka, G. Hause and A. Tissier, The development of type VI glandular trichomes in the cultivated tomato Solanum lycopersicum and a related wild species S. habrochaites, BMC Plant Biol., 2015, 15, 289 CrossRef PubMed.
- A. Tissier, J. A. Morgan and N. Dudareva, Plant Volatiles: Going 'In' but not 'Out' of Trichome Cavities, Trends Plant Sci., 2017, 22, 930–938 CrossRef CAS PubMed.
- M. Erb, S. Meldau and G. A. Howe, Role of phytohormones in insect-specific plant reactions, Trends Plant Sci., 2012, 17, 250–259 CrossRef CAS PubMed.
- T. Lin, K. Vrieling, D. Laplanche, P. G. L. Klinkhamer, Y. Lou, L. Bekooy, T. Degen, C. Bustos-Segura, T. C. J. Turlings and G. A. Desurmont, Evolutionary changes in an invasive plant support the defensive role of plant volatiles, Curr. Biol., 2021, 31, 3450–3456 CrossRef CAS PubMed.
- E. S. de Lange, D. Laplanche, H. Guo, W. Xu, M. Vlimant, M. Erb, J. Ton and T. C. J. Turlings, Spodoptera frugiperda Caterpillars Suppress Herbivore-Induced Volatile Emissions in Maize, J. Chem. Ecol., 2020, 46, 344–360 CrossRef CAS PubMed.
- J. He, F. Verstappen, A. Jiao, M. Dicke, H. J. Bouwmeester and I. F. Kappers, Terpene synthases in cucumber (Cucumis sativus) and their contribution to herbivore-induced volatile terpenoid emission, New Phytol., 2022, 233, 862–877 CrossRef CAS PubMed.
- E. Rowen and I. Kaplan, Eco-evolutionary factors drive induced plant volatiles: a meta-analysis, New Phytol., 2016, 210, 284–294 CrossRef CAS PubMed.
- D. M. Magalhães, M. Borges, R. A. Laumann and M. C. Blassioli Moraes, Influence of multiple- and single-species infestations on herbivore-induced cotton volatiles and Anthonomus grandis behaviour, J. Pest Sci., 2018, 91, 1019–1032 CrossRef.
- W. Huang, C. A. M. Robert, M. R. Hervé, L. Hu, Z. Bont and M. Erb, A mechanism for sequence specificity in plant-mediated interactions between herbivores, New Phytol., 2017, 214, 169–179 CrossRef CAS PubMed.
- G. Malik, R. Chaturvedi and S. Hooda, in Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology, ed. I. K. Singh and A. Singh, Springer Singapore, Singapore, 2021, pp. 1–29 Search PubMed.
- A. C. Jones, G. W. Felton and J. H. Tumlinson, The dual function of elicitors and effectors from insects: reviewing the 'arms race' against plant defenses, Plant Mol. Biol., 2022, 109, 427–445 CrossRef CAS PubMed.
- L. Mattiacci, M. Dicke and M. A. Posthumus, Beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 2036–2040 CrossRef CAS PubMed.
- T. C. J. Turlings, H. T. Alborn, J. H. Loughrin and J. H. Tumlinson, Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: isolation and bioactivity, J. Chem. Ecol., 2000, 26, 189–202 CrossRef CAS.
- E. A. Schmelz, M. J. Carroll, S. LeClere, S. M. Phipps, J. Meredith, P. S. Chourey, H. T. Alborn and P. E. A. Teal, Fragments of ATP synthase mediate plant perception of insect attack, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8894–8899 CrossRef CAS PubMed.
- H. T. Alborn, T. V. Hansen, T. H. Jones, D. C. Bennett, J. H. Tumlinson, E. A. Schmelz and P. E. A. Teal, Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12976–12981 CrossRef CAS PubMed.
- L. Mack, P. Gros, J. Burkhardt and K. Seifert, Elicitors of tansy volatiles from cotton leafworm larval oral secretion, Phytochemistry, 2013, 96, 158–169 CrossRef CAS PubMed.
- G. Salerno, F. Frati, E. Conti, E. Peri, S. Colazza and A. Cusumano, Mating Status of an Herbivorous Stink Bug Female Affects the Emission of Oviposition-Induced Plant Volatiles Exploited by an Egg Parasitoid, Front. Physiol., 2019, 10, 398 CrossRef PubMed.
- J. Hundacker, N. Bittner, C. Weise, G. Bröhan, M. Varama and M. Hilker, Pine defense against eggs of an herbivorous sawfly is elicited by an annexin-like protein present in egg-associated secretion, Plant, Cell Environ., 2022, 45, 1033–1048 CrossRef CAS PubMed.
- S. Ray, A. M. Helms, N. L. Matulis, E. Davidson-Lowe, W. Grisales and J. G. Ali, Asymmetry in Herbivore Effector Responses: Caterpillar Frass Effectors Reduce Performance of a Subsequent Herbivore, J. Chem. Ecol., 2020, 46, 76–83 CrossRef CAS PubMed.
- A. M. Helms, C. M. de Moraes, A. Tröger, H. T. Alborn, W. Francke, J. F. Tooker and M. C. Mescher, Identification of an insect-produced olfactory cue that primes plant defenses, Nat. Commun., 2017, 8, 337 CrossRef PubMed.
- D. M. Magalhães, I. T. F. A. Da Silva, M. Borges, R. A. Laumann and M. C. Blassioli-Moraes, Anthonomus grandis aggregation pheromone induces cotton indirect defence and attracts the parasitic wasp Bracon vulgaris, J. Exp. Biol., 2019, 70, 1891–1901 Search PubMed.
- H. Takai, R. Ozawa, J. Takabayashi, S. Fujii, K. Arai, R. T. Ichiki, T. Koeduka, H. Dohra, T. Ohnishi, S. Taketazu, J. Kobayashi, Y. Kainoh, S. Nakamura, T. Fujii, Y. Ishikawa, T. Kiuchi, S. Katsuma, M. Uefune, T. Shimada and K. Matsui, Silkworms suppress the release of green leaf volatiles by mulberry leaves with an enzyme from their spinnerets, Sci. Rep., 2018, 8, 11942 CrossRef PubMed.
- E. A. Schmelz, A. Huffaker, M. J. Carroll, H. T. Alborn, J. G. Ali and P. E. A. Teal, An amino acid substitution inhibits specialist herbivore production of an antagonist effector and recovers insect-induced plant defenses, Plant Physiol., 2012, 160, 1468–1478 CrossRef CAS PubMed.
- S. Allmann and I. T. Baldwin, Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles, Science, 2010, 329, 1075–1078 CrossRef CAS PubMed.
- A. C. Jones, T. M. Cofer, J. Engelberth and J. H. Tumlinson, Herbivorous Caterpillars and the Green Leaf Volatile (GLV) Quandary, J. Chem. Ecol., 2022, 48, 337–345 CrossRef CAS PubMed.
- P.-A. Lin, Y. Chen, G. Ponce, F. E. Acevedo, J. P. Lynch, C. T. Anderson, J. G. Ali and G. W. Felton, Stomata-mediated interactions between plants, herbivores, and the environment, Trends Plant Sci., 2022, 27, 287–300 CrossRef CAS PubMed.
- Y.-H. Lin, J. Silven, N. Wybouw, R. Fandino, H. Dekker, H. Vogel, Y.-L. Wu, C. de Koster, E. Große-Wilde, M. Haring, R. Schuurink and S. Allmann, A salivary GMC oxidoreductase of Manduca sexta re-arranges the green leaf volatile profile of its host plant, 2022 Search PubMed.
- R. Sharifi, S. M. Lee and C. M. Ryu, Microbe-induced plant volatiles, New Phytol., 2018, 220, 684–691 CrossRef PubMed.
- E. Attaran, M. Rostás and J. Zeier, Pseudomonas syringae elicits emission of the terpenoid (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene in Arabidopsis leaves via jasmonate signaling and expression of the terpene synthase TPS4, Mol. Plant-Microbe Interact., 2008, 21, 1482–1497 CrossRef CAS PubMed.
- J. Huang, Y. J. Cardoza, E. A. Schmelz, R. Raina, J. Engelberth and J. H. Tumlinson, Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae, Planta, 2003, 217, 767–775 CrossRef CAS PubMed.
- M. Riedlmeier, A. Ghirardo, M. Wenig, C. Knappe, K. Koch, E. Georgii, S. Dey, J. E. Parker, J.-P. Schnitzler and A. C. Vlot, Monoterpenes Support Systemic Acquired Resistance within and between Plants, Plant Cell, 2017, 29, 1440–1459 CrossRef CAS PubMed.
- H. D. Castelyn, J. J. Appelgryn, M. S. Mafa, Z. A. Pretorius and B. Visser, Volatiles emitted by leaf rust infected wheat induce a defence response in exposed uninfected wheat seedlings, Australas. Plant Pathol., 2015, 44, 245–254 CrossRef CAS.
- G. A. Desurmont, H. Xu and T. C. J. Turlings, Powdery mildew suppresses herbivore-induced plant volatiles and interferes with parasitoid attraction in Brassica rapa, Trends Ecol. Evol., 2016, 39, 1920–1927 CAS.
- C. Ponzio, R. Gols, C. M. J. Pieterse and M. Dicke, Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens, Funct. Ecol., 2013, 27, 587–598 CrossRef.
- F. Eberl, A. Hammerbacher, J. Gershenzon and S. B. Unsicker, Leaf rust infection reduces herbivore-induced volatile emission in black poplar and attracts a generalist herbivore, New Phytol., 2018, 220, 760–772 CrossRef CAS PubMed.
- R.-L. Wang, K. Zhu-Salzman, M. E. A. Elzaki, Q.-Q. Huang, S. Chen, Z.-H. Ma, S.-W. Liu and J.-E. Zhang, Mikania Micrantha Wilt Virus Alters Insect Vector’s Host Preference to Enhance Its Own Spread, Viruses, 2019, 11, 336 CrossRef CAS PubMed.
- X. Wu, S. Xu, P. Zhao, X. Zhang, X. Yao, Y. Sun, R. Fang and J. Ye, The Orthotospovirus nonstructural protein NSs suppresses plant MYC-regulated jasmonate signaling leading to enhanced vector attraction and performance, PLoS Pathog., 2019, 15, e1007897 CrossRef CAS PubMed.
- R. Li, B. T. Weldegergis, J. Li, C. Jung, J. Qu, Y. Sun, H. Qian, C. Tee, J. J. A. van Loon, M. Dicke, N.-H. Chua, S.-S. Liu and J. Ye, Virulence Factors of Geminivirus Interact with MYC2 to Subvert Plant Resistance and Promote Vector Performance, Plant Cell, 2014, 26, 4991–5008 CrossRef CAS PubMed.
- S. C. Groen, S. Jiang, A. M. Murphy, N. J. Cunniffe, J. H. Westwood, M. P. Davey, T. J. A. Bruce, J. C. Caulfield, O. J. Furzer, A. Reed, S. I. Robinson, E. Miller, C. N. Davis, J. A. Pickett, H. M. Whitney, B. J. Glover and J. P. Carr, Virus Infection of Plants Alters Pollinator Preference: A Payback for Susceptible Hosts?, PLoS Pathog., 2016, 12, e1005790 CrossRef PubMed.
- D. J. Ballhorn, S. Kautz and M. Schädler, Induced plant defense via volatile production is dependent on rhizobial symbiosis, Oecologia, 2013, 172, 833–846 CrossRef PubMed.
- L. R. Del Cappellari, J. Chiappero, M. V. Santoro, W. Giordano and E. Banchio, Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria, Sci. Hortic, 2017, 220, 193–198 CrossRef.
- C. Planchamp, G. Glauser and B. Mauch-Mani, Root inoculation with Pseudomonas putida KT2440 induces transcriptional and metabolic changes and systemic resistance in maize plants, Front. Plant Sci., 2015, 5 Search PubMed.
- M. Rostás, M. G. Cripps and P. Silcock, Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect, Oecologia, 2015, 177, 487–497 CrossRef PubMed.
- E. A. Schmelz, H. T. Alborn, J. Engelberth and J. H. Tumlinson, Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize, Plant Physiol., 2003, 133, 295–306 CrossRef CAS PubMed.
- S. Kloppholz, H. Kuhn and N. Requena, A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy, Curr. Biol., 2011, 21, 1204–1209 CrossRef CAS PubMed.
- E. Frago, M. Mala, B. T. Weldegergis, C. Yang, A. McLean, H. C. J. Godfray, R. Gols and M. Dicke, Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles, Nat. Commun., 2017, 8, 1860 CrossRef PubMed.
- F. Zhu, A. Cusumano, J. Bloem, B. T. Weldegergis, A. Villela, N. E. Fatouros, J. J. A. van Loon, M. Dicke, J. A. Harvey, H. Vogel and E. H. Poelman, Symbiotic polydnavirus and venom reveal parasitoid to its hyperparasitoids, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5205–5210 CrossRef CAS PubMed.
- P. Faubert, P. Tiiva, Å. Rinnan, A. Michelsen, J. K. Holopainen and R. Rinnan, Doubled volatile organic compound emissions from subarctic tundra under simulated climate warming, New Phytol., 2010, 187, 199–208 CrossRef CAS PubMed.
- C. A. Deutsch, J. J. Tewksbury, M. Tigchelaar, D. S. Battisti, S. C. Merrill, R. B. Huey and R. L. Naylor, Increase in crop losses to insect pests in a warming climate, Science, 2018, 361, 916–919 CrossRef CAS PubMed.
- F. Ndah, H. Valolahti, M. Schollert, A. Michelsen, R. Rinnan and M. Kivimäenpää, Influence of increased nutrient availability on biogenic volatile organic compound (BVOC) emissions and leaf anatomy of subarctic dwarf shrubs under climate warming and increased cloudiness, Ann. Bot., 2022, 129, 443–455 CrossRef CAS PubMed.
- C. Werner, L. Fasbender, K. M. Romek, A. M. Yáñez-Serrano and J. Kreuzwieser, Heat Waves Change Plant Carbon Allocation Among Primary and Secondary Metabolism Altering CO2 Assimilation, Respiration, and VOC Emissions, Front. Plant Sci., 2020, 11 DOI:10.3389/fpls.2020.01242.
- A. Ghirardo, F. Lindstein, K. Koch, F. Buegger, M. Schloter, A. Albert, A. Michelsen, J. B. Winkler, J.-P. Schnitzler and R. Rinnan, Origin of volatile organic compound emissions from subarctic tundra under global warming, Global Change Biol., 2020, 26, 1908–1925 CrossRef PubMed.
- K. Jardine, T. Karl, M. Lerdau, P. Harley, A. Guenther and J. E. Mak, Carbon isotope analysis of acetaldehyde emitted from leaves following mechanical stress and anoxia, Plant Biol., 2009, 11, 591–597 CrossRef CAS PubMed.
- K. Behnke, A. Ghirardo, D. Janz, B. Kanawati, J. Esperschütz, I. Zimmer, P. Schmitt-Kopplin, Ü. Niinemets, A. Polle, J. P. Schnitzler and M. Rosenkranz, Isoprene function in two contrasting poplars under salt and sunflecks, Tree Physiol., 2013, 33, 562–578 CrossRef CAS PubMed.
- J. Kreuzwieser, J.-P. Schnitzler and R. Steinbrecher, Biosynthesis of Organic Compounds Emitted by Plants, Plant Biol., 1999, 1, 149–159 CrossRef CAS.
- E. Perreca, J. Rohwer, D. González-Cabanelas, F. Loreto, A. Schmidt, J. Gershenzon and L. P. Wright, Effect of Drought on the Methylerythritol 4-Phosphate (MEP) Pathway in the Isoprene Emitting Conifer Picea glauca, Front. Plant Sci., 2020, 11 DOI:10.3389/fpls.2020.546295.
- J. Kreuzwieser, M. Meischner, M. Grün, A. M. Yáñez-Serrano, L. Fasbender and C. Werner, Drought affects carbon partitioning into volatile organic compound (VOC) biosynthesis in Scots pine needles, New Phytol., 2021, 232, 1930–1943 CrossRef CAS PubMed.
- D. Fanourakis, S. Aliniaeifard, A. Sellin, H. Giday, O. Körner, A. Rezaei Nejad, C. Delis, D. Bouranis, G. Koubouris, E. Kambourakis, N. Nikoloudakis and G. Tsaniklidis, Stomatal behavior following mid- or long-term exposure to high relative air humidity: A review, Plant Physiol. Biochem., 2020, 153, 92–105 CrossRef CAS PubMed.
- G. Arimura, S. Köpke, M. Kunert, V. Volpe, A. David, P. Brand, P. Dabrowska, M. E. Maffei and W. Boland, Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission, Plant Physiol., 2008, 146, 965–973 CrossRef CAS PubMed.
- G. Gerosa, A. Finco, S. Mereu, M. Vitale, F. Manes and A. B. Denti, Comparison of seasonal variations of ozone exposure and fluxes in a Mediterranean Holm oak forest between the exceptionally dry 2003 and the following year, Environ. Pollut., 2009, 157, 1737–1744 CrossRef CAS PubMed.
- G. W. Schade and A. H. Goldstein, Plant physiological influences on the fluxes of oxygenated volatile organic compounds from ponderosa pine trees, J. Geophys. Res.: Atmos., 2002, 107, ACH 2-1–ACH 2-8 CrossRef.
- IPCC, Climate Change 2021: The Physical Science Basis, IPCC, Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 2021 Search PubMed.
- A. M. Trowbridge, P. C. Stoy, H. D. Adams, D. J. Law, D. D. Breshears, D. Helmig and R. K. Monson, Drought supersedes warming in determining volatile and tissue defenses of piñon pine (Pinus edulis), Environ. Res. Lett., 2019, 14, 65006 CrossRef CAS.
- K. Jardine, J. Chambers, J. Holm, A. Jardine, C. Fontes, R. Zorzanelli, K. Meyers, V. de Souza, S. Garcia, B. Gimenez, L. Piva, N. Higuchi, P. Artaxo, S. Martin and A. Manzi, Green Leaf Volatile Emissions during High Temperature and Drought Stress in a Central Amazon Rainforest, Plants, 2015, 4, 678–690 CrossRef CAS PubMed.
- S. I. Zandalinas and R. Mittler, Plant responses to multifactorial stress combination, New Phytol., 2022, 234, 1161–1167 CrossRef PubMed.
- E. R. Scott, X. Li, N. Kfoury, J. Morimoto, W.-Y. Han, S. Ahmed, S. B. Cash, T. S. Griffin, J. R. Stepp, A. Robbat and C. M. Orians, Interactive effects of drought severity and simulated herbivory on tea (Camellia sinensis) volatile and non-volatile metabolites, Environ. Exp. Bot., 2018, 157, 283–292 CrossRef.
- P.-A. Lin, S. Paudel, N. Bin Zainuddin, C.-W. Tan, A. Helms, J. G. Ali and G. W. Felton, Low water availability enhances volatile-mediated direct defences but disturbs indirect defences against herbivores, J. Ecol., 2022, 110, 2759–2771 CrossRef.
- X. Martini and L. L. Stelinski, Drought stress affects response of phytopathogen vectors and their parasitoids to infection- and damage-induced plant volatile cues, Ecol. Entomol., 2017, 42, 721–730 CrossRef.
- E. N. Ngumbi and C. M. Ugarte, Flooding and Herbivory Interact to Alter Volatile Organic Compound Emissions in Two Maize Hybrids, J. Chem. Ecol., 2021, 47, 707–718 CrossRef CAS PubMed.
- J. He, R. Halitschke, M. C. Schuman and I. T. Baldwin, Light dominates the diurnal emissions of herbivore-induced volatiles in wild tobacco, BMC Plant Biol., 2021, 21, 401 CrossRef CAS PubMed.
- R. Escobar-Bravo, J. Ruijgrok, H. K. Kim, K. Grosser, N. M. van Dam, P. G. L. Klinkhamer and K. A. Leiss, Light Intensity-Mediated Induction of Trichome-Associated Allelochemicals Increases Resistance Against Thrips in Tomato, Plant Cell Physiol., 2018, 59, 2462–2475 CAS.
- G. L. Fernández-Milmanda and C. L. Ballaré, Shade Avoidance: Expanding the Color and Hormone Palette, Trends Plant Sci., 2021, 26, 509–523 CrossRef PubMed.
- M. Leone, M. M. Keller, I. Cerrudo and C. L. Ballaré, To grow or defend? Low red : far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability, New Phytol., 2014, 204, 355–367 CrossRef CAS PubMed.
- W. Kegge, B. T. Weldegergis, R. Soler, M. V.-V. Eijk, M. Dicke, L. A. C. J. Voesenek and R. Pierik, Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana, New Phytol., 2013, 200, 861–874 CrossRef CAS PubMed.
- L. E. Cortés, B. T. Weldegergis, H. E. Boccalandro, M. Dicke and C. L. Ballaré, Trading direct for indirect defense? Phytochrome B inactivation in tomato attenuates direct anti-herbivore defenses whilst enhancing volatile-mediated attraction of predators, New Phytol., 2016, 212, 1057–1071 CrossRef PubMed.
- M. Kivimäenpä, A. Mofikoya, A. M. Abd El-Raheem, J. Riikonen, R. Julkunen-Tiitto and J. K. Holopainen, Alteration in Light Spectra Causes Opposite Responses in Volatile Phenylpropanoids and Terpenoids Compared with Phenolic Acids in Sweet Basil (Ocimum basilicum) Leaves, J. Agric. Food Chem., 2022, 70, 12287–12296 CrossRef PubMed.
- M. Kivimäenpää, V. Virjamo, R. P. Ghimire, J. K. Holopainen, R. Julkunen-Tiitto, F. Martz, K. Nissinen and J. Riikonen, Changes in light spectra modify secondary compound concentrations and BVOC emissions of Norway spruce seedlings, Can. J. For. Res., 2021, 51, 1218–1229 CrossRef.
- R. Escobar-Bravo, B. C. Schimmel, Y. Zhang, C. A. Robert, G. Glauser, C. L. Ballaré and M. Erb, Maize integrates light and volatile cues from neighboring plants into unique defense responses, 2022, DOI:10.1101/2022.09.12.507519.
- A. Chautá and A. Kessler, Metabolic Integration of Spectral and Chemical Cues Mediating Plant Responses to Competitors and Herbivores, Plants, 2022, 11, 2768 CrossRef PubMed.
- D. F. Karnosky, J. M. Skelly, K. E. Percy and A. H. Chappelka, Perspectives regarding 50years of research on effects of tropospheric ozone air pollution on US forests, Environ. Pollut., 2007, 147, 489–506 CrossRef CAS PubMed.
- A. Kanagendran, L. Pazouki, S. Li, B. Liu, A. Kännaste and Ü. Niinemets, Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’, J. Exp. Biol., 2018, 69, 681–697 CAS.
- E. Khaling, T. Agyei, S. Jokinen, J. K. Holopainen and J. D. Blande, The phytotoxic air-pollutant O3 enhances the emission of herbivore-induced volatile organic compounds (VOCs) and affects the susceptibility of black mustard plants to pest attack, Environ. Pollut., 2020, 265, 115030 CrossRef CAS PubMed.
- A. Brosset, A. Saunier, A. O. Mofikoya, M. Kivimäenpää and J. D. Blande, The Effects of Ozone on Herbivore-Induced Volatile Emissions of Cultivated and Wild Brassica Rapa, Atmosphere, 2020, 11, 1213 CrossRef.
- A. Kanagendran, L. Pazouki and Ü. Niinemets, Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake, Environ. Exp. Bot., 2018, 145, 21–38 CrossRef CAS PubMed.
- Z. Feng, X. Yuan, S. Fares, F. Loreto, P. Li, Y. Hoshika and E. Paoletti, Isoprene is more affected by climate drivers than monoterpenes: A meta-analytic review on plant isoprenoid emissions, Plant, Cell Environ., 2019, 42, 1939–1949 CrossRef CAS PubMed.
- H. Yu and J. D. Blande, Diurnal variation in BVOC emission and CO2 gas exchange from above- and belowground parts of two coniferous species and their responses to elevated O3, Environ. Pollut., 2021, 278, 116830 CrossRef CAS PubMed.
- V. C. V. Batista, I. M. C. Pereira, S. d. O. Paula-Marinho, K. M. Canuto, R. Pereira de Cássia Alves, T. H. S. Rodrigues, D. d. M. Daloso, E. Gomes-Filho and H. H. de Carvalho, Salicylic acid modulates primary and volatile metabolites to alleviate salt stress-induced photosynthesis impairment on medicinal plant Egletes viscosa, Environ. Exp. Bot., 2019, 167, 103870 CrossRef CAS.
- L. Copolovici, A. Kännaste, L. Pazouki and Ü. Niinemets, Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments, J. Plant Physiol., 2012, 169, 664–672 CrossRef CAS PubMed.
- M. Renou and S. Anton, Insect olfactory communication in a complex and changing world, Curr. Opin. Insect Sci., 2020, 42, 1–7 CrossRef PubMed.
- T. Hagiwara, M. I. Ishihara, J. Takabayashi, T. Hiura and K. Shiojiri, Effective distance of volatile cues for plant–plant communication in beech, Ecol. Evol., 2021, 11, 12445–12452 CrossRef PubMed.
- A. M. Yáñez-Serrano, A. C. Nölscher, E. Bourtsoukidis, E. Gomes Alves, L. Ganzeveld, B. Bonn, S. Wolff, M. Sa, M. Yamasoe, J. Williams, M. O. Andreae and J. Kesselmeier, Monoterpene chemical speciation in a tropical rainforest:\breakvariation with season, height, and time of day\breakat the Amazon Tall Tower Observatory (ATTO), Atmos. Chem. Phys., 2018, 18, 3403–3418 CrossRef.
- L. D. Yee, G. Isaacman-Vanwertz, R. A. Wernis, M. Meng, V. Rivera, N. M. Kreisberg, S. V. Hering, M. S. Bering, M. Glasius, M. A. Upshur, A. Gray Bé, R. J. Thomson, F. M. Geiger, J. H. Offenberg, M. Lewandowski, I. Kourtchev, M. Kalberer, S. de Sá, S. T. Martin, M. L. Alexander, B. B. Palm, W. Hu, P. Campuzano-Jost, D. A. Day, J. L. Jimenez, Y. Liu, K. A. McKinney, P. Artaxo, J. Viegas, A. Manzi, M. B. Oliveira, R. de Souza, L. A. T. Machado, K. Longo and A. H. Goldstein, Observations of sesquiterpenes and their oxidation products in central Amazonia during the wet and dry seasons, Atmos. Chem. Phys., 2018, 18, 10433–10457 CrossRef CAS PubMed.
- M. Shrivastava, M. O. Andreae, P. Artaxo, H. M. J. Barbosa, L. K. Berg, J. Brito, J. Ching, R. C. Easter, J. Fan, J. D. Fast, Z. Feng, J. D. Fuentes, M. Glasius, A. H. Goldstein, E. G. Alves, H. Gomes, D. Gu, A. Guenther, S. H. Jathar, S. Kim, Y. Liu, S. Lou, S. T. Martin, V. F. McNeill, A. Medeiros, S. S. de Sá, J. E. Shilling, S. R. Springston, R. A. F. Souza, J. A. Thornton, G. Isaacman-Vanwertz, L. D. Yee, R. Ynoue, R. A. Zaveri, A. Zelenyuk and C. Zhao, Urban pollution greatly enhances formation of natural aerosols over the Amazon rainforest, Nat. Commun., 2019, 10, 1046 CrossRef PubMed.
- T. C. Bond and R. W. Bergstrom, Light Absorption by Carbonaceous Particles: An Investigative Review, Aerosol Sci. Technol., 2006, 40, 27–67 CrossRef CAS.
- U. Pöschl, S. T. Martin, B. Sinha, Q. Chen, S. S. Gunthe, J. A. Huffman, S. Borrmann, D. K. Farmer, R. M. Garland, G. Helas, J. L. Jimenez, S. M. King, A. Manzi, E. Mikhailov, T. Pauliquevis, M. D. Petters, A. J. Prenni, P. Roldin, D. Rose, J. Schneider, H. Su, S. R. Zorn, P. Artaxo and M. O. Andreae, Rainforest Aerosols as Biogenic Nuclei of Clouds and Precipitation in the Amazon, Science, 2010, 329, 1513–1516 CrossRef PubMed.
- J. M. Wedow, E. A. Ainsworth and S. Li, Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response, Trends Biochem. Sci., 2021, 46, 992–1002 CrossRef CAS PubMed.
- R. K. Monson and E. A. Holland, Biospheric Trace Gas Fluxes and Their Control Over Tropospheric Chemistry, Annu. Rev. Ecol. Evol. Syst., 2001, 32, 547–576 CrossRef.
- R. Atkinson and J. Arey, Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review, Atmos. Environ., 2003, 37, 197–219 CrossRef.
- W. J. F. Acton, W. Jud, A. Ghirardo, G. Wohlfahrt, C. N. Hewitt, J. E. Taylor and A. Hansel, The effect of ozone fumigation on the biogenic volatile organic compounds (BVOCs) emitted from Brassica napus above- and below-ground, PLoS One, 2018, 13, e0208825 CrossRef CAS PubMed.
- A. O. Mofikoya, T. H. Kim, A. M. Abd El-Raheem, J. D. Blande, M. Kivimäenpää and J. K. Holopainen, Passive Adsorption of Volatile Monoterpene in Pest Control: Aided by Proximity and Disrupted by Ozone, J. Agric. Food Chem., 2017, 65, 9579–9586 CrossRef CAS PubMed.
- H. Zhang, Y.-H. Lin, Z. Zhang, X. Zhang, S. L. Shaw, E. M. Knipping, R. J. Weber, A. Gold, R. M. Kamens and J. D. Surratt, Secondary organic aerosol formation from methacrolein photooxidation: roles of NOx level, relative humidity and aerosol acidity, Environ. Chem., 2012, 9, 247–262 CrossRef CAS.
- A. Vallat, H. Gu and S. Dorn, How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ, Phytochemistry, 2005, 66, 1540–1550 CrossRef CAS PubMed.
- T. Agyei, S. Juráň, K. K. Ofori-Amanfo, L. Šigut, O. Urban and M. V. Marek, The impact of drought on total ozone flux in a mountain Norway spruce forest, J. For. Sci., 2020, 66, 280 CrossRef CAS.
- L. Huang, E. C. McDonald-Buller, G. McGaughey, Y. Kimura and D. T. Allen, The impact of drought on ozone dry deposition over eastern Texas, Atmos. Environ., 2016, 127, 176–186 CrossRef CAS.
- M. Ma, Y. Gao, Y. Wang, S. Zhang, L. R. Leung, C. Liu, S. Wang, B. Zhao, X. Chang, H. Su, T. Zhang, L. Sheng, X. Yao and H. Gao, Substantial ozone enhancement over the North China Plain from increased biogenic emissions due to heat waves and land cover in summer 2017, Atmos. Chem. Phys., 2019, 19, 12195–12207 CrossRef CAS.
- S. C. Kavassalis and J. G. Murphy, Understanding ozone-meteorology correlations: A role for dry deposition, Geophys. Res. Lett., 2017, 44, 2922–2931 CrossRef.
- W. Yuan, Y. Zheng, S. Piao, P. Ciais, D. Lombardozzi, Y. Wang, Y. Ryu, G. Chen, W. Dong, Z. Hu, A. K. Jain, C. Jiang, E. Kato, S. Li, S. Lienert, S. Liu, J. E. M. S. Nabel, Z. Qin, T. Quine, S. Sitch, W. K. Smith, F. Wang, C. Wu, Z. Xiao and S. Yang, Increased atmospheric vapor pressure deficit reduces global vegetation growth, Sci. Adv., 2019, 5, eaax1396 CrossRef CAS PubMed.
- S. Som, D. S. Willett and H. T. Alborn, Dynamics of belowground volatile diffusion and degradation, Rhizosphere, 2017, 4, 70–74 CrossRef.
- I. Hiltpold and T. C. J. Turlings, Belowground Chemical Signaling in Maize: When Simplicity Rhymes with Efficiency, J. Chem. Ecol., 2008, 34, 628–635 CrossRef CAS PubMed.
- Ü. Niinemets, S. Fares, P. Harley and K. J. Jardine, Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition, Plant, Cell Environ., 2014, 37, 1790–1809 CrossRef PubMed.
- K. Sugimoto, K. Matsui, Y. Iijima, A. Yoshihiko, M. Shoko, R. Ozawa, U. Masayoshi, R. Sasaki, M. d. Alamgir Kabir, A. Shota, N. Tatsunori, G. Ivan, A. Koh, S. Daisuke and T. Junji, Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7144–7149 CrossRef CAS PubMed.
- F. Loreto and S. D'Auria, How do plants sense volatiles sent by other plants?, Trends Plant Sci., 2022, 27, 29–38 CrossRef CAS PubMed.
- A. Bachy, M. Aubinet, C. Amelynck, N. Schoon, B. Bodson, P. Delaplace, A. de Ligne, A. Digrado, P. Du Jardin, M. L. Fauconnier, A. Mozaffar, J. F. Müller and B. Heinesch, Dynamics and mechanisms of volatile organic compound exchanges in a winter wheat field, Atmos. Environ., 2020, 221, 117105 CrossRef CAS.
- C. N. Albers, M. Kramshøj and R. Rinnan, Rapid mineralization of biogenic volatile organic compounds in temperate and Arctic soils, Biogeosciences, 2018, 15, 3591–3601 CrossRef CAS.
- T. Li and J. D. Blande, Associational susceptibility in broccoli: mediated by plant volatiles, impeded by ozone, Global Change Biol., 2015, 21, 1993–2004 CrossRef PubMed.
- A. O. Mofikoya, M. Kivimäenpää, J. D. Blande and J. K. Holopainen, Ozone disrupts adsorption of Rhododendron tomentosum volatiles to neighbouring plant surfaces, but does not disturb herbivore repellency, Environ. Pollut., 2018, 240, 775–780 CrossRef CAS PubMed.
- S. J. Himanen, J. D. Blande, T. Klemola, J. Pulkkinen, J. Heijari and J. K. Holopainen, Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants – a mechanism for associational herbivore resistance?, New Phytol., 2010, 186, 722–732 CrossRef CAS PubMed.
- R. Karban, Plant Communication, Annu. Rev. Ecol. Evol. Syst., 2021, 52, 1–24 CrossRef.
- S. Tian, R. Guo, X. Zou, X. Zhang, X. Yu, Y. Zhan, D. Ci, M. Wang, Y. Wang and T. Si, Priming With the Green Leaf Volatile (Z)-3-Hexeny-1-yl Acetate Enhances Salinity Stress Tolerance in Peanut (Arachis hypogaea L.) Seedlings, Front. Plant Sci., 2019, 10 Search PubMed.
- K. Matsui, K. Sugimoto, J. Mano, R. Ozawa and J. Takabayashi, Differential Metabolisms of Green Leaf Volatiles in Injured and Intact Parts of a Wounded Leaf Meet Distinct Ecophysiological Requirements, PLoS One, 2012, 7, e36433 CrossRef CAS PubMed.
- L. Hu, M. Ye and M. Erb, Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance, Plant, Cell Environ., 2019, 42, 959–971 CrossRef CAS PubMed.
- J. Engelberth, C. F. Contreras, C. Dalvi, T. Li and M. Engelberth, Early Transcriptome Analyses of Z-3-Hexenol-Treated Zea mays Revealed Distinct Transcriptional Networks and Anti-Herbivore Defense Potential of Green Leaf Volatiles, PLoS One, 2013, 8, e77465 CrossRef CAS PubMed.
- M. A. Farag, M. Fokar, H. Abd, H. Zhang, R. D. Allen and P. W. Paré, (Z)-3-Hexenol induces defense genes and downstream metabolites in maize, Planta, 2005, 220, 900–909 CrossRef CAS PubMed.
- K. Sugimoto, Y. Iijima, J. Takabayashi and K. Matsui, Processing of Airborne Green Leaf Volatiles for Their Glycosylation in the Exposed Plants, Front. Plant Sci., 2021, 12 Search PubMed.
- Q. Shen, L. Liu, L. Wang and Q. Wang, Indole primes plant defense against necrotrophic fungal pathogen infection, PLoS One, 2018, 13, e0207607 CrossRef PubMed.
- M. Ye, G. Glauser, Y. Lou, M. Erb and L. Hu, Molecular Dissection of Early Defense Signaling Underlying Volatile-Mediated Defense Regulation and Herbivore Resistance in Rice, Plant Cell, 2019, 31, 687–698 CrossRef CAS PubMed.
- M. Erb, Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling, Curr. Opin. Plant Biol., 2018, 44, 117–121 CrossRef CAS PubMed.
- R. Karban, W. C. Wetzel, K. Shiojiri, S. Ishizaki, S. R. Ramirez and J. D. Blande, Deciphering the language of plant communication: volatile chemotypes of sagebrush, New Phytol., 2014, 204, 380–385 CrossRef PubMed.
- R. Karban, K. Shiojiri, S. Ishizaki, W. C. Wetzel and R. Y. Evans, Kin recognition affects plant communication and defence, Proc. R. Soc. B, 2013, 280, 20123062 CrossRef PubMed.
- A. Brambilla, A. Sommer, A. Ghirardo, M. Wenig, C. Knappe, B. Weber, M. Amesmaier, M. Lenk, J.-P. Schnitzler and A. C. Vlot, Immunity-associated volatile emissions of β-ionone and nonanal propagate defence responses in neighbouring barley (Hordeum vulgare) plants, J. Exp. Bot., 2021, 73, 615–630 CrossRef PubMed.
- A. K. Meents, S.-P. Chen, M. Reichelt, H.-H. Lu, S. Bartram, K.-W. Yeh and A. Mithöfer, Volatile DMNT systemically induces jasmonate-independent direct anti-herbivore defense in leaves of sweet potato (Ipomoea batatas) plants, Sci. Rep., 2019, 9, 17431 CrossRef PubMed.
- H. Yu, M. Kivimäenpää and J. D. Blande, Volatile-mediated between-plant communication in Scots pine and the effects of elevated ozone, Proc. R. Soc. B, 2022, 289, 20220963 CrossRef PubMed.
- P.-J. Zhang, J.-N. Wei, C. Zhao, Y.-F. Zhang, C.-Y. Li, S.-S. Liu, M. Dicke, X.-P. Yu and T. C. J. Turlings, Airborne host-plant manipulation by whiteflies via an inducible blend of plant volatiles, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 7387–7396 CrossRef CAS PubMed.
- W. Huang, V. Gfeller and M. Erb, Root volatiles in plant-plant interactions II: Root volatiles alter root chemistry and plant-herbivore interactions of neighbouring plants, Plant, Cell Environ., 2019, 42, 1964–1973 CrossRef CAS PubMed.
- V. Ninkovic, I. Dahlin, A. Vucetic, O. Petrovic-Obradovic, R. Glinwood and B. Webster, Volatile Exchange between Undamaged Plants – a New Mechanism Affecting Insect Orientation in Intercropping, PLoS One, 2013, 8, e69431 CrossRef CAS PubMed.
- V. Gfeller, M. Huber, C. Förster, W. Huang, T. G. Köllner and M. Erb, Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours, Plant, Cell Environ., 2019, 42, 1950–1963 CrossRef CAS PubMed.
- A. Kessler, R. Halitschke, C. Diezel and I. T. Baldwin, Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata, Oecologia, 2006, 148, 280–292 CrossRef PubMed.
- V. Ninkovic, D. Markovic and I. Dahlin, Decoding neighbour volatiles in preparation for future competition and implications for tritrophic interactions, Perspect. Plant Ecol. Evol. Syst., 2016, 23, 11–17 CrossRef.
- R. N. Kigathi, W. W. Weisser, M. REICHELT, J. Gershenzon and S. B. Unsicker, Plant volatile emission depends on the species composition of the neighboring plant community, BMC Plant Biol., 2019, 19, 58 CrossRef PubMed.
- M. Wenig, A. Ghirardo, J. H. Sales, E. S. Pabst, H. H. Breitenbach, F. Antritter, B. Weber, B. Lange, M. Lenk, R. K. Cameron, J.-P. Schnitzler and A. C. Vlot, Systemic acquired resistance networks amplify airborne defense cues, Nat. Commun., 2019, 10, 3813 CrossRef PubMed.
- X. Cai, Y. Guo, L. Bian, Z. Luo, Z. Li, C. Xiu, N. Fu and Z. Chen, Variation in the ratio of compounds in a plant volatile blend during transmission by wind, Sci. Rep., 2022, 12, 6176 CrossRef CAS PubMed.
- A. M. El-Sayed, A. L. Knight, J. A. Byers, G. J. R. Judd and D. M. Suckling, Caterpillar-induced plant volatiles attract conspecific adults in nature, Sci. Rep., 2016, 6, 37555 CrossRef CAS PubMed.
- A. de Fouchier, X. Sun, G. Caballero-Vidal, S. Travaillard, E. Jacquin-Joly and N. Montagné, Behavioral Effect of Plant Volatiles Binding to Spodoptera littoralis Larval Odorant Receptors, Front. Behav. Neurosci., 2018, 12 DOI:10.3389/fnbeh.2018.00264.
- N. Veyrat, C. A. M. Robert, T. C. J. Turlings and M. Erb, Herbivore intoxication as a potential primary function of an inducible volatile plant signal, J. Ecol., 2016, 104, 591–600 CrossRef CAS.
- C. C. Arce, V. Theepan, B. C. Schimmel, G. Jaffuel, M. Erb and R. A. Machado, Plant-associated CO2 mediates long-distance host location and foraging behaviour of a root herbivore, eLife, 2021, 10, e65575 CrossRef CAS PubMed.
- R. A. R. Machado, V. Theepan, C. A. M. Robert, T. Züst, L. Hu, Q. Su, B. C. J. Schimmel and M. Erb, The plant metabolome guides fitness-relevant foraging decisions of a specialist herbivore, PLoS Biol., 2021, 19, e3001114 CrossRef CAS PubMed.
- C. A. M. Robert, M. Erb, M. Duployer, C. Zwahlen, G. R. Doyen and T. C. J. Turlings, Herbivore-induced plant volatiles mediate host selection by a root herbivore, New Phytol., 2012, 194, 1061–1069 CrossRef CAS PubMed.
- P. W. Paré and J. H. Tumlinson, Plant Volatiles as a Defense against Insect Herbivores, Plant Physiol., 1999, 121, 325–332 CrossRef.
- E. Hatano, A. M. Saveer, F. Borrero-Echeverry, M. Strauch, A. Zakir, M. Bengtsson, R. Ignell, P. Anderson, P. G. Becher, P. Witzgall and T. Dekker, A herbivore-induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signalling pathways, BMC Biol., 2015, 13 DOI:10.1186/s12915-015-0188-3.
- C. M. de Moraes, M. C. Mescher and J. H. Tumlinson, Caterpillar-induced nocturnal plant volatiles repel conspecific females, Nature, 2001, 410, 577–580 CrossRef CAS PubMed.
- C. Chen, H. Chen, S. Huang, T. Jiang, C. Wang, Z. Tao, C. He, Q. Tang and P. Li, Volatile DMNT directly protects plants against Plutella xylostella by disrupting the peritrophic matrix barrier in insect midgut, eLife, 2021, 10, e63938 CrossRef CAS PubMed.
- A. K. Maurya, R. C. Patel and C. J. Frost, Acute toxicity of the plant volatile indole depends on herbivore specialization, J. Pest Sci., 2020, 93, 1107–1117 CrossRef.
- Z. Sun, Y. Lin, R. Wang, Q. Li, Q. Shi, S. R. Baerson, L. Chen, R. Zeng and Y. Song, Olfactory perception of herbivore-induced plant volatiles elicits counter-defences in larvae of the tobacco cutworm, Funct. Ecol., 2021, 35, 384–397 CrossRef CAS.
- C. A. M. Robert, M. Erb, B. E. Hibbard, B. Wade French, C. Zwahlen and T. C. J. Turlings, A specialist root herbivore reduces plant resistance and uses an induced plant volatile to aggregate in a density-dependent manner, Funct. Ecol., 2012, 26, 1429–1440 CrossRef.
- K. Shiojiri, R. Ozawa, S. Kugimiya, M. Uefune, M. van Wijk, M. W. Sabelis and J. Takabayashi, Herbivore-Specific, Density-Dependent Induction of Plant Volatiles: Honest or “Cry Wolf” Signals?, PLoS One, 2010, 5, e12161 CrossRef PubMed.
- J. G. de Boer, M. A. Posthumus and M. Dicke, Identification of Volatiles That Are Used in Discrimination Between Plants Infested with Prey or Nonprey Herbivores by a Predatory Mite, J. Chem. Ecol., 2004, 30, 2215–2230 CrossRef CAS PubMed.
- R. Soler, J. A. HARVEY and T. Bezemer, Foraging efficiency of a parasitoid of a leaf herbivore is influenced by root herbivory on neighbouring plants, Funct. Ecol., 2007, 21, 969–974 CrossRef.
- E. H. Poelman, M. Bruinsma, F. Zhu, B. T. Weldegergis, A. E. Boursault, Y. Jongema, J. J. A. van Loon, L. E. M. Vet, J. A. Harvey and M. Dicke, Hyperparasitoids Use Herbivore-Induced Plant Volatiles to Locate Their Parasitoid Host, PLoS Biol., 2012, 10, e1001435 CrossRef CAS PubMed.
- M. Loivamaki, R. Mumm, M. Dicke and J. P. Schnitzler, Isoprene interferes with the attraction of bodyguards by herbaceous plants, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17430–17435 CrossRef PubMed.
- D. J. Jacobsen and R. A. Raguso, Leaf Induction Impacts Behavior and Performance of a Pollinating Herbivore, Front. Plant Sci., 2021, 12 Search PubMed.
- R.-M. Qin, P. Wen, R. T. Corlett, Y. Zhang, G. Wang and J. Chen, Plant-defense mimicry facilitates rapid dispersal of short-lived seeds by hornets, Curr. Biol., 2022, 32, 3429–3435 CrossRef CAS PubMed.
- A. Kessler, R. Halitschke and K. Poveda, Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant–pollinator interactions, Ecology, 2011, 92, 1769–1780 CrossRef PubMed.
- L. Amo, J. J. Jansen, N. M. van Dam, M. Dicke and M. E. Visser, Birds exploit herbivore-induced plant volatiles to locate herbivorous prey, Ecol. Lett., 2013, 16, 1348–1355 CrossRef PubMed.
- A. Mrazova, K. Sam and L. Amo, What do we know about birds' use of plant volatile cues in tritrophic interactions?, Curr. Opin. Insect Sci., 2019, 32, 131–136 CrossRef PubMed.
- T.-M. Koski, T. Laaksonen, E. Mäntylä, S. Ruuskanen, T. Li, P. S. Girón-Calva, L. Huttunen, J. D. Blande, J. K. Holopainen and T. Klemola, Do Insectivorous Birds use Volatile Organic Compounds from Plants as Olfactory Foraging Cues? Three Experimental Tests, Ethology, 2015, 121, 1131–1144 CrossRef.
- J. K. Goldberg, G. Pintel, S. L. Weiss and E. P. Martins, Predatory lizards perceive plant-derived volatile odorants, Ecol. Evol., 2019, 9, 4733–4738 CrossRef PubMed.
- A. Pérez-Cembranos, V. Pérez-Mellado and W. E. Cooper, Balearic lizards use chemical cues from a complex deceptive mimicry to capture attracted pollinators, Ethology, 2018, 124, 260–268 CrossRef.
- M. Rostás, D. Maag, M. Ikegami and M. Inbar, Gall volatiles defend aphids against a browsing mammal, BMC Evol. Biol., 2013, 13, 193 CrossRef PubMed.
- C. McArthur, P. B. Finnerty, M. H. Schmitt, A. Shuttleworth and A. M. Shrader, Plant volatiles are a salient cue for foraging mammals: elephants target preferred plants despite background plant odour, Anim. Behav., 2019, 155, 199–216 CrossRef.
- W. Zhang, Z. Li, L. Wang, H. Liu and H. Liu, Effect of Coriander Plants on Human Emotions, Brain Electrophysiology, and Salivary Secretion, Biology, 2021, 10, 1283 CrossRef CAS PubMed.
- E. P. Barboza, M. Cirach, S. Khomenko, T. Iungman, N. Mueller, J. Barrera-Gómez, D. Rojas-Rueda, M. Kondo and M. Nieuwenhuijsen, Green space and mortality in European cities: a health impact assessment study, Lancet Planet. Health, 2021, 5, e718–e730 CrossRef PubMed.
- B.-Y. Yang, T. Zhao, L.-X. Hu, M. H. Browning, J. Heinrich, S. C. Dharmage, B. Jalaludin, L. D. Knibbs, X.-X. Liu, Y.-N. Luo, P. James, S. Li, W.-Z. Huang, G. Chen, X.-W. Zeng, L.-W. Hu, Y. Yu and G.-H. Dong, Greenspace and human health: An umbrella review, Innov., 2021, 2, 100164 Search PubMed.
- M. Antonelli, D. Donelli, G. Barbieri, M. Valussi, V. Maggini and F. Firenzuoli, Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review, Int. J. Environ. Res. Public Health, 2020, 17, 6506 CrossRef PubMed.
- A. Khan, K. Vaibhav, H. Javed, R. Tabassum, M. E. Ahmed, M. M. Khan, M. B. Khan, P. Shrivastava, F. Islam, M. S. Siddiqui, M. M. Safhi and F. Islam, 1,8-Cineole (Eucalyptol) Mitigates Inflammation in Amyloid Beta Toxicated PC12 Cells: Relevance to Alzheimer's Disease, Neurochem. Res., 2014, 39, 344–352 CrossRef CAS PubMed.
- Y. Hu, Z. Zeng, B. Wang and S. Guo, Trans-caryophyllene inhibits amyloid β(Aβ) oligomer-induced neuroinflammation in BV-2 microglial cells, Int. Immunopharmacol., 2017, 51, 91–98 CrossRef CAS PubMed.
- T. Kim, B. Song, K. S. Cho and I.-S. Lee, Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases, Int. J. Mol. Sci., 2020, 21, 2187 CrossRef CAS PubMed.
- K. Sumitomo, H. Akutsu, S. Fukuyama, A. Minoshima, S. Kukita, Y. Yamamura, Y. Sato, T. Hayasaka, S. Osanai, H. Funakoshi, N. Hasebe and M. Nakamura, Conifer-Derived Monoterpenes and Forest Walking, Mass Spectrom., 2015, 4, A0042 CrossRef PubMed.
- G. Farré-Armengol, I. Filella, J. Llusia and J. Peñuelas, Bidirectional Interaction between Phyllospheric Microbiotas and Plant Volatile Emissions, Trends Plant Sci., 2016, 21, 854–860 CrossRef PubMed.
- A. Hammerbacher, T. A. Coutinho and J. Gershenzon, Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles, Plant, Cell Environ., 2019, 42, 2827–2843 CrossRef CAS PubMed.
- B. K. Ehlers, M. P. Berg, M. Staudt, M. Holmstrup, M. Glasius, J. Ellers, S. Tomiolo, R. B. Madsen, S. Slotsbo and J. Penuelas, Plant Secondary Compounds in Soil and Their Role in Belowground Species Interactions, Trends Ecol. Evol., 2020, 35, 716–730 CrossRef PubMed.
- Y. GAO, Y.-J. JIN, H.-D. LI and H.-J. CHEN, Volatile Organic Compounds and Their Roles in Bacteriostasis in Five Conifer Species, J. Integr. Plant Biol., 2005, 47, 499–507 CrossRef CAS.
- I. Prost, S. Dhondt, G. Rothe, J. Vicente, M. J. Rodriguez, N. Kift, F. Carbonne, G. Griffiths, M.-T. Esquerré-Tugayé, S. Rosahl, C. Castresana, M. Hamberg and J. Fournier, Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens, Plant Physiol., 2005, 139, 1902–1913 CrossRef CAS PubMed.
- C. A. Fantaye, D. Köpke, J. Gershenzon and J. Degenhardt, Restoring (E)-β-Caryophyllene Production in a Non-producing Maize Line Compromises its Resistance against the Fungus Colletotrichum graminicola, J. Chem. Ecol., 2015, 41, 213–223 CrossRef CAS PubMed.
- X. Chen, H. Chen, J. S. Yuan, T. G. Köllner, Y. Chen, Y. Guo, X. Zhuang, X. Chen, Y.-J. Zhang, J. Fu, A. Nebenführ, Z. Guo and F. Chen, The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae, Plant Biotechnol. J., 2018, 16, 1778–1787 CrossRef CAS PubMed.
- N. M. van Dam, A. Weinhold and P. Garbeva, in Deciphering Chemical Language of Plant Communication, Springer, Cham, 2016, pp. 175–210 Search PubMed.
- D. Abanda-Nkpwatt, U. Krimm, H. A. Coiner, L. Schreiber and W. Schwab, Plant volatiles can minimize the growth suppression of epiphytic bacteria by the phytopathogenic fungus Botrytis cinerea in co-culture experiments, Environ. Exp. Bot., 2006, 56, 108–119 CrossRef CAS.
- K. Schulz-Bohm, S. Gerards, M. Hundscheid, J. Melenhorst, W. de Boer and P. Garbeva, Calling from distance: attraction of soil bacteria by plant root volatiles, ISME J., 2018, 12, 1252–1262 CrossRef CAS PubMed.
- H.-S. Yi, M. Heil, R. M. Adame-Alvarez, D. J. Ballhorn and C.-M. Ryu, Airborne induction and priming of plant defenses against a bacterial pathogen, Plant Physiol., 2009, 151, 2152–2161 CrossRef CAS PubMed.
- H. G. Kong, G. C. Song, H.-J. Sim and C.-M. Ryu, Achieving similar root microbiota composition in neighbouring plants through airborne signalling, ISME J., 2021, 15, 397–408 CrossRef CAS PubMed.
- M. Erb and D. J. Kliebenstein, Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy, Plant Physiol., 2020, 184, 39–52 CrossRef CAS PubMed.
- I. Hiltpold, M. Baroni, S. Toepfer, U. Kuhlmann and T. C. J. Turlings, Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest, J. Exp. Biol., 2010, 213, 2417–2423 CrossRef CAS PubMed.
- M. Huang, A. M. Sanchez-Moreiras, C. Abel, R. Sohrabi, S. Lee, J. Gershenzon and D. Tholl, The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen, New Phytol., 2012, 193, 997–1008 CrossRef CAS PubMed.
- R. R. Junker, J. Kuppler, L. Amo, J. D. Blande, R. M. Borges, N. M. van Dam, M. Dicke, S. Dötterl, B. K. Ehlers, F. Etl, J. Gershenzon, R. Glinwood, R. Gols, A. T. Groot, M. Heil, M. Hoffmeister, J. K. Holopainen, S. Jarau, L. John, A. Kessler, J. T. Knudsen, C. Kost, A.-A. C. Larue-Kontic, S. D. Leonhardt, D. Lucas-Barbosa, C. J. Majetic, F. Menzel, A. L. Parachnowitsch, R. S. Pasquet, E. H. Poelman, R. A. Raguso, J. Ruther, F. P. Schiestl, T. Schmitt, D. Tholl, S. B. Unsicker, N. Verhulst, M. E. Visser, B. T. Weldegergis and T. G. Köllner, Covariation and phenotypic integration in chemical communication displays: biosynthetic constraints and eco-evolutionary implications, New Phytol., 2018, 220, 739–749 CrossRef PubMed.
- A. Vattekkatte, S. Garms, W. Brandt and W. Boland, Enhanced structural diversity in terpenoid biosynthesis: enzymes, substrates and cofactors, Org. Biomol. Chem., 2018, 16, 348–362 RSC.
- P. Zu, K. Boege, E. Del-Val, M. C. Schuman, P. C. Stevenson, A. Zaldivar-Riverón and S. Saavedra, Information arms race explains plant-herbivore chemical communication in ecological communities, Science, 2020, 368, 1377–1381 CrossRef CAS PubMed.
- T. J. Bruce and J. A. Pickett, Perception of plant volatile blends by herbivorous insects – Finding the right mix, Phytochemistry, 2011, 72, 1605–1611 CrossRef CAS PubMed.
- J. K. Holopainen and J. D. Blande, Where do herbivore-induced plant volatiles go?, Front. Plant Sci., 2013, 4, 185 Search PubMed.
- K. Matsui, S. Kurishita, A. Hisamitsu and T. Kajiwara, A lipid-hydrolysing activity involved in hexenal formation, Biochem. Soc. Trans., 2000, 28, 857–860 CrossRef CAS PubMed.
- K. Shiojiri, K. Kishimoto, R. Ozawa, S. Kugimiya, S. Urashimo, G. Arimura, J. Horiuchi, T. Nishioka, K. Matsui and J. Takabayashi, Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 16672–16676 CrossRef CAS PubMed.
- D. Duran-Flores and M. Heil, Sources of specificity in plant damaged-self recognition, Curr. Opin. Plant Biol., 2016, 32, 77–87 CrossRef CAS PubMed.
- B. Boachon, J. H. Lynch, S. Ray, J. Yuan, K. M. P. Caldo, R. R. Junker, S. A. Kessler, J. A. Morgan and N. Dudareva, Natural fumigation as a mechanism for volatile transport between flower organs, Nat. Chem. Biol., 2019, 15, 583–588 CrossRef CAS PubMed.
- J. K. Holopainen and J. Gershenzon, Multiple stress factors and the emission of plant VOCs, Trends Plant Sci., 2010, 15, 176–184 CrossRef CAS PubMed.
- D. Materić, D. Bruhn, C. Turner, G. Morgan, N. Mason and V. Gauci, Methods in plant foliar volatile organic compounds research, Appl. Plant Sci., 2015, 3 DOI:10.3732/apps.1500044.
- D. Tholl, O. Hossain, A. Weinhold, U. S. R. Röse and Q. Wei, Trends and applications in plant volatile sampling and analysis, Plant J., 2021, 106, 314–325 CrossRef CAS PubMed.
- H. T. Alborn, A Technique for Thermal Desorption Analyses Suitable for Thermally-Labile, Volatile Compounds, J. Chem. Ecol., 2018, 44, 103–110 CrossRef CAS PubMed.
- M. Kallenbach, Y. Oh, E. J. Eilers, D. VEIT, I. T. Baldwin and M. C. Schuman, A robust, simple, high-throughput technique for time-resolved plant volatile analysis in field experiments, Plant J., 2014, 78, 1060–1072 CrossRef CAS PubMed.
- D. B. Silva, B. T. Weldegergis, J. J. van Loon and V. H. P. Bueno, Qualitative and Quantitative Differences in Herbivore-Induced Plant Volatile Blends from Tomato Plants Infested by Either Tuta absoluta or Bemisia tabaci, J. Chem. Ecol., 2017, 43, 53–65 CrossRef CAS PubMed.
- Z. Badra, S. Larsson Herrera, L. Cappellin, F. Biasioli, T. Dekker, S. Angeli and M. Tasin, Species-Specific Induction of Plant Volatiles by Two Aphid Species in Apple: Real Time Measurement of Plant Emission and Attraction of Lacewings in the Wind Tunnel, J. Chem. Ecol., 2021, 47, 653–663 CrossRef CAS PubMed.
- G. Vivaldo, E. Masi, C. Taiti, G. Caldarelli and S. Mancuso, The network of plants volatile organic compounds, Sci. Rep., 2017, 7, 11050 CrossRef PubMed.
- K. G. S. Dani and F. Loreto, Plant volatiles as regulators of hormone homeostasis, New Phytol., 2022, 234, 804–812 CrossRef CAS PubMed.
- B. Wang, J. Chu, T. Yu, X. Qian, X. Sun, J. Yuan, G. Xiong, G. Wang, Y. Wang and J. Li, Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4821–4826 CrossRef CAS PubMed.
- M. Zhao, N. Zhang, T. Gao, J. Jin, T. Jing, J. Wang, Y. Wu, X. Wan, W. Schwab and C. Song, Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants, New Phytol., 2020, 226, 362–372 CrossRef CAS PubMed.
- T. Jing, N. Zhang, T. Gao, M. Zhao, J. Jin, Y. Chen, M. Xu, X. Wan, W. Schwab and C. Song, Glucosylation of (Z)-3-hexenol informs intraspecies interactions in plants: A case study in Camellia sinensis, Plant, Cell Environ., 2019, 42, 1352–1367 CrossRef CAS PubMed.
- K. Matsui, A portion of plant airborne communication is endorsed by uptake and metabolism of volatile organic compounds, Curr. Opin. Plant Biol., 2016, 32, 24–30 CrossRef CAS PubMed.
- D. R. Gallie, Ethylene receptors in plants – why so much complexity?, F1000 Med. Rep., 2015, 7, 39 Search PubMed.
- J. Del Mármol, M. A. Yedlin and V. Ruta, The structural basis of odorant recognition in insect olfactory receptors, Nature, 2021, 597, 126–131 CrossRef PubMed.
- S.-H. Son, J.-E. Kim, G. Park, Y.-J. Ko, B. H. Sung, J. Seo, S. S. Oh and J. Y. Lee, Metabolite trafficking enables membrane-impermeable-terpene secretion by yeast, Nat. Commun., 2022, 13, 2605 CrossRef CAS PubMed.
- K. Shiojiri, S. Ishizaki and Y. Ando, Plant–plant communication and community of herbivores on tall goldenrod, Ecol. Evol., 2021, 11, 7439–7447 CrossRef PubMed.
- G. Bonaventure, A. VanDoorn and I. T. Baldwin, Herbivore-associated elicitors: FAC signaling and metabolism, Trends Plant Sci., 2011, 16, 294–299 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to the review. |
| This journal is © The Royal Society of Chemistry 2023 |