Open Access Article
Open Access ArticleDendritic structured palladium complexes: magnetically retrievable, highly efficient heterogeneous nanocatalyst for Suzuki and Heck cross-coupling reactions†
Safoora Sheikh ab,
Mohammad Ali Nasseri
ab,
Mohammad Ali Nasseri *a,
Mohammad Chahkandi
*a,
Mohammad Chahkandi *c,
Oliver Reiser
*c,
Oliver Reiser b and
Ali Allahresani
b and
Ali Allahresani a
a
aDepartment of Chemistry, Faculty of Basic Sciences, University of Birjand, P. O. Box 97175-615, Birjand, Iran. E-mail: manaseri@birjand.ac.ir
bInstitut für Organische Chemie, Universität Regensburg, Universitätsstr. 31, 93053 Regensburg, German
cDepartment of Chemistry, Faculty of Basic Sciences, Hakim Sabzevari University, P. O. Box 96179-76487, Sabzevar, Iran. E-mail: m.chahkandi@hsu.ac.ir
First published on 21st March 2022
Abstract
The recyclable nanomagnetic Pd-complex PAMAM G0-Pd@γ-Fe2O3 is reported for catalytic C–C cross-coupling reactions of challenging substrates. Mainly, a great variety of aryl chlorides can be used as substrates for Suzuki–Miyaura and Mizoroki–Heck reactions under mild reaction conditions (60–90 °C) and low catalyst loading (<1 mol% Pd) in aqueous media. The presence of numerous polar groups in the polymer matrix increases the solubility of the catalyst in water, thus facilitating its operation in aqueous environments. The immobilization of the catalyst on the surface of a magnetic platform allows its effective recovery and reuse without significant loss of catalytic activity for at least six cycles with total leaching of <1% palladium metal, meeting the requirements for acceptable metal residues in the pharmaceutical industry.
1. Introduction
The development of new protocols and methods according to green chemistry standards to meet the growing demands of human society for energy, health, and agricultural purposes remains a pressing challenge for chemistry and the related sciences.1,2 Arguably, catalysis stands at the forefront of such efforts, providing innovation by the development of new chemical transformations. Nevertheless, making catalysis feasible for large-scale applications by improving catalytic activity and selectivity, and especially in the case of transition metal catalysis, obviating metal leaching is equally essential even for well-established transformations.3In this context, palladium-catalyzed cross-couplings have been manifold proven to be of particular significance for synthesizing medicinally relevant compounds even though palladium is one of the scarcest metals in the world.4–6 Efficient recycling of such species is economically desirable; moreover, the considerable toxicity of palladium mandates its removal below 10 ppm in pharmaceuticals.7 Separating homogeneous palladium catalysts from products is challenging; consequently, heterogenization of palladium catalysts is a viable approach to overcome these difficulties. However, typical heterogeneous palladium catalysts such as Pd/C, being most successful for hydrogenations,8 are less efficient in cross-coupling reactions, especially with industrially relevant aryl chlorides.9 Such substrates remain challenging with any catalyst despite some impressive advances in designing molecular palladium complexes with specialized ligands.10–17
Considering large-scale and recycling, many efforts have been made to develop heterogeneous Pd-catalysts,10–14 including molecular palladium–ligand complexes deposited onto various solid supports.15–19 Among them, iron oxide nanoparticles have emerged as an attractive platform for metal catalysts20 and especially for palladium complexes to arrive at eco-friendly and robust heterogeneous catalysts for Suzuki–Miyaura and Heck–Mizoroki C–C cross-coupling reactions.21–24 Such catalysts can be easily manipulated and retrieved by external magnets avoiding cumbersome centrifugation or filtration processes.25–27 Moreover, iron oxide nanoparticles have a high surface-to-volume ratio, which allows generating a large number of active sites that are available to promote catalytic reactions.28,29
Polyamidoamine (PAMAM) dendrimers have a particularly well-defined structure favorable for the encapsulation of metal nanoparticles.30 The nanoparticle size and the metal loading can be controlled by the dendrimer generation, whereas the solubility in common solvents can be adjusted by the nature of the dendritic end group.31 Despite these attractive properties, dendrimers are often difficult to recycle and to reuse; thus, attaching them to magnetic supports holds the promise to combine tunability of the dendritic core to generate a suitable environment of catalytically active moieties with magnetic properties beneficial for recovery and reuse.32
Previously, various homogeneous and heterogeneous systems of palladium nanoparticles encapsulated in modified PAMAM dendrimers of generation G2–G6 have been reported for Suzuki–Miyaura and Mizoroki–Heck cross-coupling reaction.33–36 For example, PAMAM-G3 grafted on functionalized multiwall carbon nanotubes (MWCNTs-NH2) showed good activity for aryl chlorides in Heck reactions employing N-methyl-2-pyrrolidone (NMP) as a solvent,33 and the Fe3O4-G4-PAMAM-Pd(0) could convert aryl chlorides in DMF as a solvent in Suzuki–Miyaura couplings.34 Moving towards environmentally more benign solvent systems, the G6-OH(Pd74Cu73) PAMAM34 dendrimer or Fe3O4@SiO2-PAMAM-Pd36 were competent catalysts for aryl chlorides in Suzuki–Miyaura reactions in a mixture of EtOH/H2O. Similar to the PAMAM structure, polypropylene polybenzyl isocyanate (PPI) dendrimers modified by diphosphines as coordination site for Pd(II) complex have been employed successfully in Suzuki–Miyaura reactions with aryl chlorides in a THF/H2O solvent mixture.37 More Pd-PAMAM-based systems were reported, but these only catalyze the coupling of aryl iodides and bromides effectively and show little activity at best for aryl chlorides.38–42
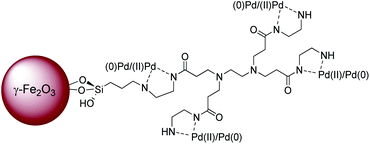
We report here the synthesis of a hybrid material, consisting of a superparamagnetic γ-Fe2O3 core decorated with zero-generation PAMAM dendrimer G0 in which palladium nanoparticles were encapsulated (Fig. 1). This catalytic system shows unusual high activity for Suzuki–Miyaura and Heck coupling reactions, most notably with a wide variety of aryl chlorides as substrates.
2. Experimental section
The preparation of PAMAM G0-Pd@γ-Fe2O3 nanocatalyst is described in detail in the experimental section (ESI, Section 1. Schemes 1–3†). Briefly, at first γ-Fe2O3 nanocore was prepared as the platform for the immobilization, using Fe(II) and Fe(III) chloride salts. Then the iron oxide nanoparticles were functionalized with (3-chloropropyl)triethoxysilane followed by the attachment of the zero-generation of polyamidoamine dendrimer (PAMAM) to the γ-Fe2O3 MNPs through the siloxy linker. Subsequently, PAMAM G0@γ-Fe2O3 was treated with palladium dichloride, which produced the PAMAM G0-Pd@γ-Fe2O3 (ESI, Schemes 1–3†). The physicochemical properties of PAMAM G0-Pd@γ-Fe2O3 were characterized by FT-IR, TEM, XRD, FE-SEM, ICP, TGA/DTG, VSM, MAP, XPS, EDS, and LC-MS analyzes (see the ESI, Fig. S1–S16†).2.1 General procedure for the Suzuki–Miyaura cross-coupling reaction (9a–r)
PAMAM G0-Pd@γ-Fe2O3 (0.47 mol%, 0.007 g) was added to a mixture of K2CO3 (2 mmol), phenylboronic acid (1.2 mmol), and aryl halide (1.0 mmol) in water (2 mL), under stirring and heated at 60 °C (90 °C when we used aryl chlorides). The progress of reaction was monitored by TLC until completion (15 min), then the nanocatalyst was separated by an external magnet. The reaction mixture was extracted with ethyl acetate (3 × 5 mL), and the combined organic layers were dried over anhydrous Na2SO4 and concentrated. The residue was purified on silica to give rise to the pure products 9a–r.2.2 General procedure for the Mizoroki–Heck cross-coupling reaction (12a–r)
PAMAM G0-Pd@γ-Fe2O3 (0.61 mol%, 0.009 g) was added to a mixture of K2CO3 (2 mmol), alkene (1.5 mmol), and aryl halide (1.0 mmol) in water (2 mL), under stirring and heated at 80 °C (90 °C when we used aryl chlorides). The reaction progress was screened by TLC at different time intervals. After the completion of the reaction, the nanocatalyst was separated by an external magnet. The reaction mixture was extracted with ethyl acetate (3 × 5 mL), and the combined organic layers were dried over anhydrous Na2SO4 and concentrated. The residue was purified on silica to give rise to the pure products 12a–r.3. Results and discussion
3.1 Synthesis and characterization of the PAMAM G0-Pd@γ-Fe2O3 MNPs
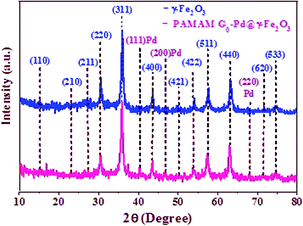
The successful synthesis of PAMAM G0-Pd@γ-Fe2O3 was established by comparing its XRD pattern with those of γ-Fe2O3. The XRD pattern of γ-Fe2O3 (Fig. 2) confirms the cubic magnetite crystal structure according to the peaks at 15, 23.1, 26.9, 30.3, 35.8, 43.6, 50, 54.8, 57.3, 63.2, 71.1, and 74.4° (2θ), corresponding to the (110), (210), (211), (220), (311), (400), (421), (422), (511), (440), (620) and (533) miller indices respectively (JCPDS card no. 39-1346).43,44 The unit cell is assigned as the space group P4132 with the crystallographic parameters a = b = c = 8.3515 Å, and α = β = γ = 90°. The observed diffraction peaks of PAMAM G0-Pd@γ-Fe2O3 at 40.1, 46.7 and 68.1° (2θ) are attributed to the standard crystallographic pattern of Pd NPs corresponding to the (111), (200), and (220) miller indices (JCPDS Card no. 65-2867, Fig. 2).45,46 The decrease of the XRD peak intensity of PAMAM G0-Pd@γ-Fe2O3 can be attributed to the formation of palladium complexes on the surface of γ-Fe2O3 MNPs being stabilized by various functional groups present in the PAMAM dendrimer acting as ligands.The FE–SEM images of γ-Fe2O3 NPs, γ-Fe2O3-CPTES and supported catalyst were shown in Fig. S10.† According to the FE-SEM images, the γ-Fe2O3 and γ-Fe2O3-CPTES NPs have a uniform spherical morphology (Fig. S10A†). Also, the particles of the supported catalyst on the surface of γ-Fe2O3 NPs have a homogeneous spherical morphology and uniform size distribution. This clearly shows the synergistic effect of organic and inorganic diverse ingredients in a homogeneous network of PAMAM G0-Pd@γ-Fe2O3 (6) (Fig. S10B†).
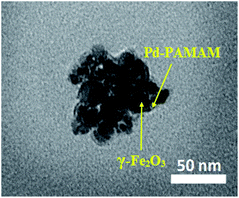
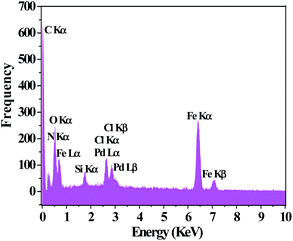
The TEM image of PAMAM G0-Pd@γ-Fe2O3 is in line with the proposed core–shell structure placing palladium nanoparticles at the surface of magnetic Fe2O3 nanoparticles (Fig. 3). Energy-dispersive X-ray (EDX) choosing a point at random on the surface of PAMAM G0-Pd@γ-Fe2O3 NPs (Fig. 4) confirmed the presence of Pd, N, O, Si, Fe, Cl, and C, in 8.10, 7.74, 21.03, 2.45, 43.79, 2.69, and 14.20 wt% respectively.
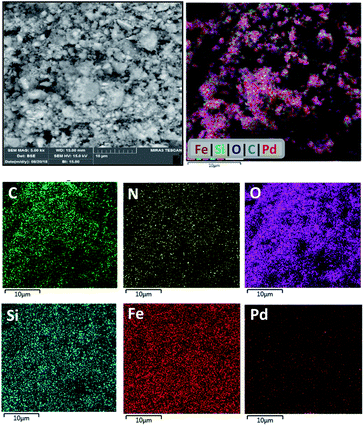
Elemental mapping analysis by energy dispersive spectroscopy (EDS) of PAMAM G0-Pd@γ-Fe2O3 (Fig. 5) indicates that Pd NPs are distributed almost uniformly and without aggregation on the catalyst surface along with the other elements (C, N, O, Si, Fe) present.
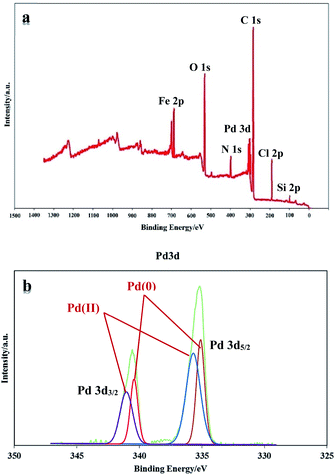
XPS analysis was performed to investigate the chemical state of the surface of PAMAM G0-Pd@γ-Fe2O3 (Fig. 6a). In the XPS elemental survey of PAMAM G0-Pd@γ-Fe2O3, the peaks corresponding to carbon, oxygen, nitrogen, chlorine, silicon, palladium and iron are clearly observed (Fig. 6a). Also, Pd(0)/(II) existence in the catalyst structure were confirmed through XPS analysis through studying binding energy range of 335.15 to 340.97 eV (Fig. 6b). As shown in Fig. 6b, the peaks located at 335.15 (3d5/2) and 340.35 eV (3d3/2) correspond to Pd(II) species, and also the peaks appeared at 336.11 (3d5/2) and 340.97 eV (3d3/2) are attributed Pd(II) (Fig. 6b). According to XPS results, the ratio of Pd(0)/Pd(II) is equal to 0.40 (in atomic number).
3.2 Evaluation of the catalytic activity of PAMAM G0-Pd@γ-Fe2O3 MNPs in cross-coupling reactions
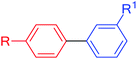
Taking the Suzuki–Miyaura C–C coupling reaction between iodobenzene and phenylboronic acid as the model reaction, PAMAM G0-Pd@γ-Fe2O3 was evaluated varying solvent, temperature, base, and catalyst loading (see ESI for details, Fig. S17†), suggesting that the best results were achieved with polar solvents. Being green and safe, we were pleased to see that the reaction proceeded exceptionally well in water, giving rise to 95% yield with a catalyst loading of 0.47 mol% within 15 minutes of reaction time at 60 °C.With the optimized reaction conditions in hand, we evaluated the substrate scope of PAMAM G0-Pd@γ-Fe2O3 for Suzuki–Miyaura coupling reactions (Table 1). A wide variety of electron-deficient or electron-rich aryl halides or phenylboronic acids were amenable, and functional groups that could be potentially sensitive under the basic, aqueous reaction conditions employed (CHO, CN, NH2) were tolerated well. As expected, the reactivity decreased from aryl iodides via aryl bromides to aryl chlorides, but even for the latter high yields could be obtained by raising the reaction temperature to 90 °C and prolonging the reaction time 100–220 min.
| Prod. | X | R1 | Yield | t (min) | TON | TOF |
|---|---|---|---|---|---|---|
| a Reaction conditions: phenylboronic acid (1.2 mmol), aryl halide (1.00 mmol), K2CO3 (Et3N when we used the aryl chlorides) (2.00 mmol), catalyst (0.007 g, 0.47 mol% of Pd), H2O (2.0 mL), 60 °C (90 °C when we used the aryl chlorides); all yields are isolated. | ||||||
| 9a | I | NH2 | 85 | 120 | 180 | 90 |
| 9a | Cl | NH2 | 65 | 150 | 138 | 39 |
| 9b | I | OMe | 92 | 70 | 195 | 168 |
| 9b | Br | OMe | 90 | 110 | 191 | 104 |
| 9c | I | Me | 85 | 90 | 180 | 120 |
| 9c | Br | Me | 75 | 120 | 159 | 79 |
| 9c | Cl | Me | 65 | 150 | 138 | 55 |
| 9d | I | H | 95 | 15 | 202 | 808 |
| 9d | Br | H | 95 | 20 | 202 | 612 |
| 9d | Cl | H | 92 | 100 | 195 | 117 |
| 9e | I | CHO | 93 | 60 | 197 | 197 |
| 9e | Br | CHO | 85 | 85 | 180 | 128 |
| 9e | Cl | CHO | 75 | 100 | 195 | 96 |
| 9f | Br | COMe | 95 | 90 | 202 | 134 |
| 9g | I | NO2 | 94 | 25 | 200 | 487 |
| 9g | Br | NO2 | 90 | 35 | 191 | 330 |
| 9g | Cl | NO2 | 87 | 55 | 155 | 155 |
| 9h | Br | CN | 80 | 90 | 170 | 113 |
| 9h | Cl | CN | 60 | 180 | 148 | 49 |
Analogously, the catalytic activity of PAMAM G0-Pd@γ-Fe2O3 was evaluated for Mizoroki–Heck C–C coupling reactions. Screening of conditions for the reaction of iodobenzene with styrene (see ESI for details, Fig. S18†) also revealed for this transformation water to be a suitable choice in combination with K2CO3 at a reaction temperature of 80 °C, giving rise to 1,2-diphenylethylene in 95% yield within 60 minutes at a catalyst loading of 0.61 mol%.
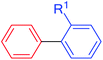
Evaluating the substrate scope under the optimized reaction conditions demonstrated that a great variety of electronically and sterically differentiated aryl halides reacted smoothly in the coupling with styrene or n-butyl acrylate (Table 2). Noteworthy, electron-rich and electron-poor aryl chlorides could be converted, requiring only slightly longer reaction times and higher temperatures (90 °C).
| Prod. | X | R | Yield | t (min) | TON | TOF |
|---|---|---|---|---|---|---|
| a Reaction conditions: styrene or n-butyl acrylate (1.5 mmol), aryl halide (1.00 mmol), K2CO3 (Et3N when we used the aryl chlorides) (2.00 mmol), catalyst (0.009 g, 0.61 mol% of Pd), H2O (2.00 mL), 80 °C (90 °C when we used aryl chlorides); all yields are isolated. | ||||||
| 12a | I | Me | 75 | 180 | 122 | 41 |
| 12a | Br | Me | 65 | 180 | 106 | 35 |
| 12a | Cl | Me | 55 | 220 | 90 | 24 |
| 12b | I | OMe | 90 | 90 | 148 | 98 |
| 12c | I | H | 95 | 60 | 155 | 150 |
| 12c | Br | H | 95 | 80 | 155 | 117 |
| 12c | Cl | H | 90 | 120 | 147 | 73 |
| 12d | I | CN | 85 | 60 | 139 | 139 |
| 12e | I | NO2 | 95 | 45 | 155 | 206 |
| 12e | Br | NO2 | 95 | 50 | 155 | 186 |
| 12e | Cl | NO2 | 90 | 60 | 147 | 147 |
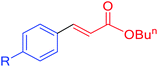 |
||||||
| 12f | I | OH | 90 | 120 | 147 | 73 |
| 12g | I | NH2 | 90 | 120 | 147 | 73 |
| 12h | I | OMe | 92 | 500 | 150 | 181 |
| 12h | Cl | OMe | 65 | 300 | 106 | 21 |
| 12i | I | Me | 80 | 120 | 131 | 65 |
| 12i | Br | Me | 80 | 140 | 131 | 56 |
| 12i | Cl | Me | 70 | 150 | 114 | 45 |
| 12j | I | H | 95 | 50 | 155 | 187 |
| 12j | Br | H | 90 | 75 | 147 | 117 |
| 12j | Cl | H | 80 | 100 | 131 | 78 |
| 12k | I | Cl | 85 | 55 | 139 | 153 |
| 12k | Br | Cl | 70 | 60 | 114 | 114 |
| 12l | I | Br | 75 | 70 | 122 | 105 |
| 12m | I | CO2Et | 95 | 40 | 131 | 65 |
| 12m | Br | CO2Et | 93 | 50 | 152 | 183 |
| 12m | Cl | CO2Et | 90 | 80 | 147 | 110 |
| 12n | I | COMe | 92 | 70 | 150 | 129 |
| 12o | Br | CN | 92 | 50 | 150 | 181 |
| 12o | Cl | CN | 85 | 120 | 139 | 69 |
| 12p | I | NO2 | 95 | 30 | 155 | 77 |
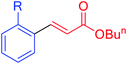 |
||||||
| 12q | Br | Me | 65 | 130 | 106 | 49 |
| 12q | Cl | Me | 50 | 240 | 81 | 20 |
| 12r | I | NO2 | 92 | 70 | 150 | 129 |
Control experiments confirmed for both, the Suzuki–Miyaura as well as the Mizoroki–Heck reactions that no coupling is observed with the parent materials PAMAM G0@γ-Fe2O3 and PAMAMG0, but also that PdCl2 as a stand-alone catalyst does not promote the transformations. The high activity and efficiency of the PAMAM G0-Pd@γ-Fe2O3 catalyst compares well to other catalysts examined for comparison (see Fig. S19†).
3.3 Recyclability study
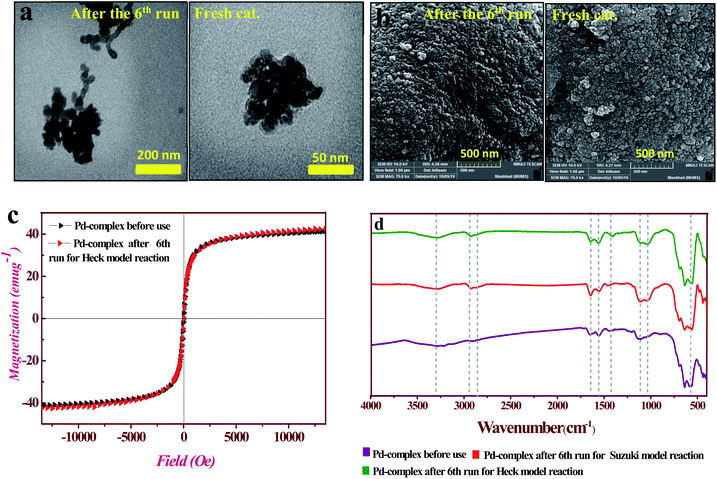
The recyclability, activity, and stability of PAMAM G0-Pd@γ-Fe2O3 catalyst were examined using the hot-filtration method taking the Mizoroki–Heck reaction between iodobenzene and styrene (Table 2, entry 5) as a representative example.After the catalyst was magnetically removed from the reaction medium (28 min, 50% yield of 1,1-diphenylethylene monitored by GC), the reaction, by and large, did not proceed further (53% yield after 90 min, Fig. S20†), indicating that little to no catalytically active species were present outside the support.
ICP analysis of the Mizoroki–Heck model reaction solution was performed to determine the amount of palladium leaching for six consecutive catalytic runs (Fig. S21†).
Base on the results, Pd catalyst, showed high stability, with little leaching of Pd (total less than 1%), while consistent yields (95–93%) of 1,1–diphenylethylene were obtained for each cycle (see ESI for details, Table S1†).
Likewise, recyclability of the PAMAM G0-Pd@γ-Fe2O3 complex was evaluated for the Suzuki–Miyaura reaction between iodobenzene and phenylboronic acid for 6 sequential runs (see ESI for details, Table S1†), giving rise to 93–95% yield in each run. The comparison of TEM and SEM images of fresh and the used catalyst after 6 runs in the Mizoroki–Heck reaction did not show significant changes in size and morphology (Fig. 7a and b). In addition, VSM measurements (Fig. 7c) and FT-IR spectra (Fig. 7e) further confirmed that the catalyst did not change in its structure and magnetic properties. Also, evaluation and comparison leaching, some of hydrophilic/amphiphilic catalysts effective in c–c cross coupling reactions with PAMAM G0-Pd@γ-Fe2O3 catalyst were performed to better understand the activity of the PAMAM G0-Pd@γ-Fe2O3 (see Table 3. ESI† for found more details).
4. Summary and conclusions
In conclusion, we developed a recyclable magnetic nano Pd-complex, formulated as PAMAM G0-Pd@γ-Fe2O3 MNPs, which proved to be an efficient catalyst for the Suzuki–Miyaura and Mizoroki–Heck coupling. Notable features are its high activity in water, which allows the employment of aryl chloride at moderately forcing conditions (90 °C), facile recyclability and reuse, as well as little palladium leaching (<1% in a total of 6 runs).Conflicts of interest
All the authors, declare that they haven't any of conflicts of interest.Acknowledgements
Authors gratefully acknowledge the financial support by the Research Council of University of Birjand, Birjand, Iran and Hakim Sabzevari University, Sabzevar, Iran.References
- R. Karu and S. Gedu, Green Chem., 2018, 20, 369–374 RSC.
- P. Slavík, D. W. Kurka and D. K. Smith, Chem. Sci., 2018, 9, 8673 RSC.
- O. Al-Madanat, Y. AlSalka, M. Curti, R. Dillert and D. W. Bahnemann, ACS Catal., 2020, 10, 7398–7412 CrossRef CAS.
- W. Ji, H.-H. Wu and J. Zhang, ACS Catal., 2020, 10, 1548 CrossRef CAS.
- L. Zhou, S. Li, B. Xu, D. Ji, L. Wu, Y. Liu, Z. M. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2020, 59, 2769 CrossRef CAS PubMed.
- H. Lee, Y. Lee and S. H. Cho, Org. Lett., 2019, 21, 5912 CrossRef CAS PubMed.
- I. V. Gürsel, T. Noël, Q. Wang and V. Hessel, Green Chem., 2015, 17, 2012 RSC.
- L. Stadler, M. Homafar, A. Hartl, S. Najafishirtari, M. Colombo, R. Zboril, P. Martin, M. B. Gawande, J. Zhi and O. Reiser, ACS Sustainable Chem. Eng., 2018, 7, 2388 CrossRef.
- S. Wittmann, J.-P. Majoral, R. N. Grass, W. J. Stark and O. Reiser, Green Process. Synth., 2012, 1, 275 CAS.
- D. J. Snelders, G. van Koten and R. J. Klein Gebbink, J. Am. Chem. Soc., 2009, 131, 11407 CrossRef CAS PubMed.
- Y. Yu, T. Hu, X. Chen, K. Xu, J. Zhang and J. Huang, Chem. Commun., 2011, 47, 3592 RSC.
- F. Wang, J. Mielby, F. H. Richter, G. Wang, G. Prieto, T. Kasama, C. Weidenthaler, H. J. Bongard, S. Kegnæs and A. Fürstner, Angew. Chem., Int. Ed., 2014, 126, 8789 CrossRef.
- J. Zhi, D. Song, Z. Li, X. Lei and A. Hu, Chem. Commun., 2011, 47, 10707 RSC.
- L. Wu, B.-L. Li, Y.-Y. Huang, H.-F. Zhou, Y.-M. He and Q.-H. Fan, Org. Lett., 2006, 8, 3605 CrossRef CAS PubMed.
- A. L. Isfahani, I. Mohammadpoor-Baltork, V. Mirkhani, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and R. Kia, Adv. Synth. Catal., 2013, 355, 957 CrossRef CAS.
- E. Mulahmetovic and G. Hargaden, Rev. J. Chem., 2017, 7, 373 CrossRef CAS.
- Z. B. Shifrina, V. G. Matveeva and L. M. Bronstein, Chem. Rev., 2019, 120, 1350 CrossRef PubMed.
- J. Blümel, Coord. Chem. Rev., 2008, 252, 2410 CrossRef.
- N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217 CrossRef CAS PubMed.
- S. Sobhani and Z. Pakdin-Parizi, Appl. Catal., A, 2014, 479, 112 CrossRef CAS.
- R. N. Baig and R. S. Varma, Green Chem., 2013, 15, 398 RSC.
- R. N. Baig, J. Leazer and R. S. Varma, Clean Technol. Environ. Policy, 2015, 17, 2073 CrossRef.
- M. Nasrollahzadeh, Z. Issaabadi and R. S. Varma, ACS Omega, 2019, 4, 14234 CrossRef CAS PubMed.
- R. Luque, B. Baruwati and R. S. Varma, Green Chem., 2010, 12, 1540 RSC.
- A. K. Rathi, M. B. Gawande, J. Pechousek, J. Tucek, C. Aparicio, M. Petr, O. Tomanec, R. Krikavova, Z. Travnicek and R. S. Varma, Green Chem., 2016, 18, 2363 RSC.
- M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartooni and M. Khalaj, J. Mol. Catal. A: Chem., 2015, 396, 31 CrossRef CAS.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- R. Sedghi, B. Heidari, H. Shahmohamadi, P. Zarshenas and R. S. Varma, Molecules, 2019, 24, 3048 CrossRef CAS PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed.
- R. Ye, A. V. Zhukhovitskiy, C. V. Deraedt, F. D. Toste and G. A. Somorjai, Acc. Chem. Res., 2017, 50, 1894 CrossRef CAS PubMed.
- C. Gao, F. Lyu and Y. Yin, Chem. Rev., 2021, 121(2), 834–881 CrossRef CAS PubMed.
- Y. Zhou, L. Luan, B. Tang, Y. Niu, R. Qu, Y. Liu and W. Xu, Chem. Eng. J., 2020, 398, 125651 CrossRef CAS.
- M. R. Nabid, Y. Bide and S. J. T. Rezaei, Appl. Catal., A, 2011, 406, 124 CrossRef CAS.
- X. Yan, Y. Luo, W. Liu, L. Liang, Y. Gan, Z. Chen, Z. Xu, H. Wan, D. Tang and H. Shi, Phys. Chem. Chem. Phys., 2020, 22, 6222 RSC.
- Y. Zhang, X. Wei and Z. Yao, Chin. J. Chem., 2010, 28, 2274 CrossRef CAS.
- R. Ma, P. Yang and F. Bian, New J. Chem., 2018, 42, 4748 RSC.
- J. Lemo, K. Heuzé and D. Astruc, Org. Lett., 2005, 7, 2253 CrossRef CAS PubMed.
- Y. Yang, S. Ogasawara, G. Li and S. Kato, Polym. J., 2015, 47, 340 CrossRef CAS.
- E. Niknam, A. Moaddeli and A. Khalafi-Nezhad, J. Organomet. Chem., 2020, 923, 121369 CrossRef CAS.
- N. Bahri-Laleh, S. Sadjadi and A. Poater, J. Colloid Interface Sci., 2018, 531, 421 CrossRef CAS PubMed.
- F. Giacalone, V. Campisciano, C. Calabrese, V. La Parola, Z. Syrgiannis, M. Prato and M. Gruttadauria, ACS Nano, 2016, 10, 4627 CrossRef CAS PubMed.
- J. Lemo, K. Heuzé and D. Astruc, Inorg. Chim. Acta, 2006, 359, 4909 CrossRef CAS.
- E. Darezereshki, Mater. Lett., 2010, 64, 1471 CrossRef CAS.
- V. Sreeja and P. Joy, Mater. Res. Bull., 2007, 42, 1570 CrossRef CAS.
- K. Cheng, D. Cao, F. Yang, L. Zhang, Y. Xu and G. Wang, J. Mater. Chem., 2012, 22, 850 RSC.
- J. Cui, N. Zhu, N. Kang, C. Ha, C. Shi and P. Wu, Chem. Eng. J., 2017, 328, 1051 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00487a |
| This journal is © The Royal Society of Chemistry 2022 |