Open Access Article
Open Access ArticleSimplifying and expanding the scope of boron imidazolate framework (BIF) synthesis using mechanochemistry†
Cameron B.
Lennox
 ab,
Jean-Louis
Do
ab,
Jean-Louis
Do
 ab,
Joshua G.
Crew
ac,
Mihails
Arhangelskis
ab,
Joshua G.
Crew
ac,
Mihails
Arhangelskis
 ad,
Hatem M.
Titi
ad,
Hatem M.
Titi
 ab,
Ashlee J.
Howarth
ab,
Ashlee J.
Howarth
 bef,
Omar K.
Farha
bef,
Omar K.
Farha
 f and
Tomislav
Friščić
f and
Tomislav
Friščić
 *ab
*ab
aDepartment of Chemistry, McGill University, 801 Sherbrooke St. W, H3A 0B8 Montreal, Canada. E-mail: tomislav.friscic@mcgill.ca
bFRQNT Quebec Centre for Advanced Materials (QCAM/CQMF), Montreal, Canada
cSchool of Chemistry, Cardiff University, Main Building. Park Place, Cardiff CF10 3AT, UK
dFaculty of Chemistry, University of Warsaw, 1 Pasteura St, 02-093 Warsaw, Poland
eDepartment of Biochemistry and Chemistry, Concordia University, 7141 Sherbrooke St. W, H4B 1R6 Montreal, Canada
fInternational Institute for Nanotechnology, Department of Chemistry, Northwestern University, 2145 Sheridan Road, 60208 Evanston, Il, USA
First published on 14th September 2021
Abstract
Mechanochemistry enables rapid access to boron imidazolate frameworks (BIFs), including ultralight materials based on Li and Cu(I) nodes, as well as new, previously unexplored systems based on Ag(I) nodes. Compared to solution methods, mechanochemistry is faster, provides materials with improved porosity, and replaces harsh reactants (e.g. n-butylithium) with simpler and safer oxides, carbonates or hydroxides. Periodic density-functional theory (DFT) calculations on polymorphic pairs of BIFs based on Li+, Cu+ and Ag+ nodes reveals that heavy-atom nodes increase the stability of the open SOD-framework relative to the non-porous dia-polymorph.
Mechanochemistry1–7 has emerged as a versatile methodology for the synthesis and discovery of advanced materials, including nanoparticle systems8–10 and metal–organic frameworks (MOFs),11–15 giving rise to materials that are challenging to obtain using conventional solution-based techniques.16–18 Mechanochemical techniques such as ball milling, twin screw extrusion19 and acoustic mixing20,21 have simplified and advanced the synthesis of a wide range of MOFs, permitting the use of simple starting materials such as metal oxides, hydroxides or carbonates,22,23 at room temperature and without bulk solvents, yielding products of comparable stability and, after activation, higher surface areas than solution-generated counterparts.24–29 The efficiency of mechanochemistry in MOF synthesis was recently highlighted by accessing zeolitic imidazolate frameworks (ZIFs)30,31 that were theoretically predicted, but not accessible under conventional solution-based conditions.17
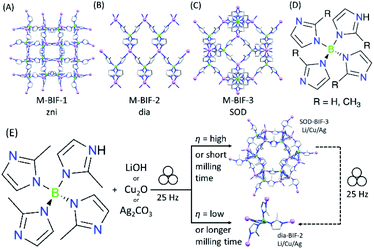
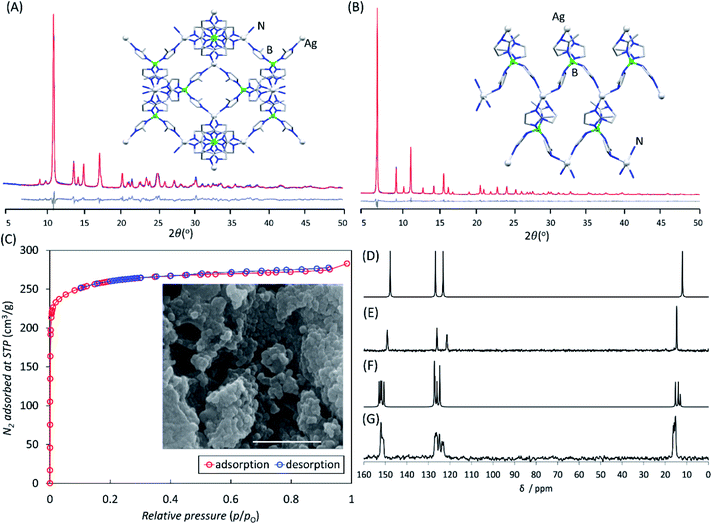
The advantages of mechanochemistry in MOF chemistry led us to address the possibility of synthesizing boron imidazolate frameworks (BIFs),32–34 an intriguing but poorly developed class of microporous materials analogous to ZIFs, comprising equimolar combinations of tetrahedrally coordinated boron(III) and monovalent Li+ or Cu+ cations as nodes (Fig. 1A–C). Although BIFs offer an attractive opportunity to access microporous MOFs with lower molecular weights, particularly in the case of “ultralight” systems based on Li+ and B(III) centers, this family of materials has remained largely unexplored – potentially due to the need for harsh synthetic conditions, including the use of n-butyllithium in a solvothermal environment.32–34
We now show how switching to the mechanochemical environment enables lithium- and copper(I)-based BIFs to be prepared rapidly (i.e., within 60–90 minutes), without elevated temperatures or bulk solvents, and from readily accessible solid reactants, such as hydroxides and oxides (Fig. 1D and E). While the mechanochemically-prepared BIFs exhibit significantly higher surface areas than the solvothermally-prepared counterparts, mechanochemistry allows for expanding this class of materials towards previously not reported Ag+ nodes. The introduction of BIFs isostructural with those based on Li+ or Cu+ but comprising of Ag+ ions, enables a periodic density-functional theory (DFT) evaluation of their stability. This reveals that switching to heavier elements as tetrahedral nodes improves the stability of sodalite topology (SOD) open BIFs with respect to close-packed diamondoid (dia) topology polymorphs.
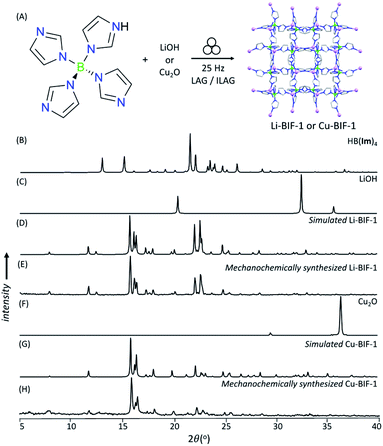
As a first attempt at mechanochemically synthesis of BIFs, we targeted the synthesis of previously reported zni-topology LiB(Im)4 and CuB(Im)4 frameworks (Li-BIF-1 and Cu-BIF-1, respectively, Fig. 1A) using a salt exchange reaction between LiCl or CuCl with commercially available sodium tetrakis(imidazolyl)borate (Na[B(Im)4]) (Fig. 2A). Milling of LiCl and Na[B(Im)4] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for up to 60 minutes led to the appearance of Bragg reflections consistent with the target Li-BIF-1 (CSD MOXJEP) and the anticipated NaCl byproduct. The reaction was, however, incomplete, as seen by X-ray reflections of Na[B(Im)4] starting material. In order to improve reactant conversion, we explored liquid-assisted grinding (LAG), i.e. milling in the presence of a small amount of a liquid phase (measured by the liquid-to-solid ratio η35 in the range of ca. 0–2 μL mg−1). Using LAG conditions with acetonitrile (MeCN, 120 μL, η = 0.5 μL mg−1) led to the complete disappearance of reactant X-ray reflections, concomitant with the formation of Li-BIF-1 alongside NaCl within 60 minutes.
1 stoichiometric ratio for up to 60 minutes led to the appearance of Bragg reflections consistent with the target Li-BIF-1 (CSD MOXJEP) and the anticipated NaCl byproduct. The reaction was, however, incomplete, as seen by X-ray reflections of Na[B(Im)4] starting material. In order to improve reactant conversion, we explored liquid-assisted grinding (LAG), i.e. milling in the presence of a small amount of a liquid phase (measured by the liquid-to-solid ratio η35 in the range of ca. 0–2 μL mg−1). Using LAG conditions with acetonitrile (MeCN, 120 μL, η = 0.5 μL mg−1) led to the complete disappearance of reactant X-ray reflections, concomitant with the formation of Li-BIF-1 alongside NaCl within 60 minutes.
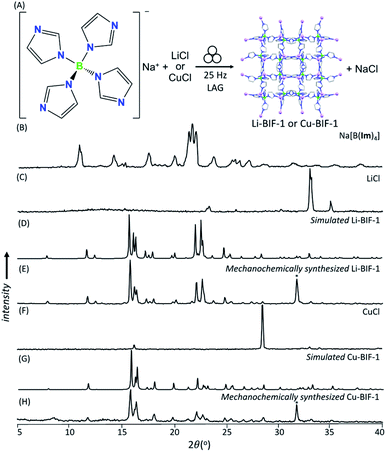 | ||
| Fig. 2 (A) Reaction scheme for the mechanochemical synthesis of Li-BIF-1 by a salt metathesis strategy. Selected PXRD patterns for: (B) Na[B(Im)4] (C) LiCl, (D) simulated Li-BIF-1 (CSD MOXJPEP) and (E) synthesized BIF-1-Li by LAG for 60 minutes with MeCN (η = 0.5 μL mg−1), (F) CuCl, (G) simulated Cu-BIF-1 (CSD MOXJIT), and (H) synthesized BIF-1-Cu by LAG for 60 minutes with MeOH (η = 0.50 μL mg−1). Asterisks denote NaCl, a byproduct of the metathesis reaction. (Fig. 2B–E, also see ESI†). The copper-based zni-CuB(Im)4 (Cu-BIF-1) was readily obtained from CuCl within 60 minutes using similar LAG conditions. We also explored LAG with methanol (MeOH), revealing that the exchange reaction to form NaCl took place with both LiCl and CuCl starting materials. With LiCl, however, the PXRD pattern of the product could not be matched to known phases involving Li+ and B(Im)4− (see ESI†). With CuCl as a reactant, LAG with MeOH (η = 0.5 μL mg−1) cleanly produced Cu-BIF-1 alongside NaCl (see ESI†). | ||
Next, we explored an alternative synthesis approach, analogous to that previously used to form ZIFs and other MOFs: an acid–base reaction between a metal oxide or hydroxide and the acid form of the linker: tetrakis(imidazolato)boric acid, HB(Im)4 (Fig. 3A).36–40 Neat milling LiOH with one equivalent of HB(Im)4 in a stainless steel milling assembly led to the partial formation of Li-BIF-1, as evidenced by PXRD analysis (see ESI†). Complete conversion of reactants into Li-BIF-1 was achieved in 60 minutes by LAG with MeCN (η = 0.25 μL mg−1), as indicated by PXRD analysis (Fig. 3B–E), Fourier transform infrared attenuated total reflectance spectroscopy (FTIR-ATR), thermogravimetric analysis (TGA) in air, and analysis of metal content by inductively-coupled plasma mass spectrometry (ICP-MS) (see ESI†).
Neat milling of HB(Im)4 with Cu2O under similar conditions gave a largely non-crystalline material, as evidenced by PXRD (see ESI†). Switching to the ion- and liquid-assisted grinding (ILAG) methodology, in which the reactivity of a metal oxide is enhanced by a small amount of a weakly acidic ammonium salt, and which was introduced to prepare zinc and cadmium ZIFs from respective oxides,37–40 enabled the synthesis of Cu-BIF-1 from Cu2O. Specifically, PXRD analysis revealed complete disappearance of the oxide in samples obtained by ILAG with either MeOH or MeCN (η = 0.5 μL mg−1) in the presence of NH4NO3 additive (5% by weight, see ESI†). Notably, achieving complete disappearance of Cu2O reactant signals also required switching from stainless steel to a zirconia-based milling assembly, presumably due to more efficient energy delivery.41 After washing with MeOH, the material was characterized by FTIR-ATR, TGA in air, and analysis of metal content by ICP-MS (see ESI†).
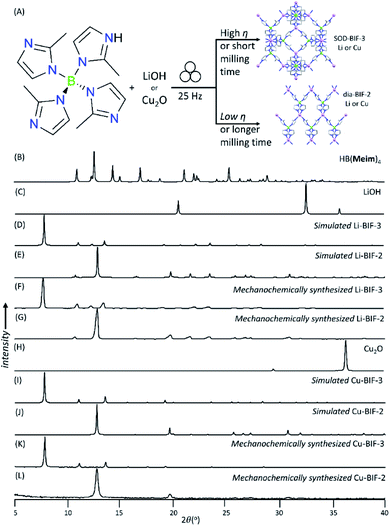
Whereas both the metathesis and acid–base approaches can be used to mechanochemically generate Li- and Cu-BIF-1, the latter approach has a clear advantage of circumventing the formation of the NaCl byproduct. Consequently, in order to further the development of mechanochemical routes to other BIFs, we focused on the acid–base strategy. As next targets, we turned to MOFs based on tetrakis(2-methylimidazole)boric acid H[B(Meim)4],36 previously reported32 to adopt either a non-porous diamondoid (dia) topology (BIF-2) or a microporous sodalite (SOD) topology (BIF-3) with either Li+ or Cu+ as nodes (Fig. 4). Attempts to selectively synthesize either Li-BIF-2 or Li-BIF-3 by neat milling or LAG (using MeOH or MeCN as liquid additives) with LiOH and a stoichiometric amount of HB(Meim)4 were not successful. Exploration of different milling times and η-values produced only mixtures of residual reactants with Li-BIF-2, Li-BIF-3, and/or not yet identified phases (see ESI†). Consequently, we explored milling in the presence of 2-aminobutanol (amb), which is a ubiquitous component of solvent systems used in the solvothermal syntheses of BIFs.32,33 Gratifyingly, using a mixture of amb and MeCN in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio by volume as the milling liquid led to an effective strategy for the selective synthesis of both the dia-topology Li-BIF-2 (CSD code MOXKUG), and the SOD-topology Li-BIF-3 (CSD code MUCLOM).‡ The selective formation of phase-pure samples of Li-BIF-2 and Li-BIF-3 was confirmed by PXRD analysis, which revealed an excellent match to diffractograms simulated based on the previously reported structures (Fig. 4B–G). Systematic exploration of reaction conditions, including time (between 15 and 60 minutes) and η value (between 0.25 and 1 μL mg−1) revealed that the open framework Li-BIF-3 is readily obtained at η either 0.75 or 1 μL mg−1 after milling for 45 minutes or longer (Fig. 4B–G, also see ESI†).§ Lower η-values of 0.25 and 0.5 μL mg−1 preferred the formation of the dia-topology Li-BIF-2, which was obtained as a phase-pure material upon 60 minutes milling at η = 0.5 μL mg−1, following the initial appearance of a yet unidentified intermediate. The preferred formation of Li-BIF-2 at lower η-values is consistent with our previous observations that lower amounts of liquid promote mechanochemical formation of denser MOF polymorphs.37
3 ratio by volume as the milling liquid led to an effective strategy for the selective synthesis of both the dia-topology Li-BIF-2 (CSD code MOXKUG), and the SOD-topology Li-BIF-3 (CSD code MUCLOM).‡ The selective formation of phase-pure samples of Li-BIF-2 and Li-BIF-3 was confirmed by PXRD analysis, which revealed an excellent match to diffractograms simulated based on the previously reported structures (Fig. 4B–G). Systematic exploration of reaction conditions, including time (between 15 and 60 minutes) and η value (between 0.25 and 1 μL mg−1) revealed that the open framework Li-BIF-3 is readily obtained at η either 0.75 or 1 μL mg−1 after milling for 45 minutes or longer (Fig. 4B–G, also see ESI†).§ Lower η-values of 0.25 and 0.5 μL mg−1 preferred the formation of the dia-topology Li-BIF-2, which was obtained as a phase-pure material upon 60 minutes milling at η = 0.5 μL mg−1, following the initial appearance of a yet unidentified intermediate. The preferred formation of Li-BIF-2 at lower η-values is consistent with our previous observations that lower amounts of liquid promote mechanochemical formation of denser MOF polymorphs.37
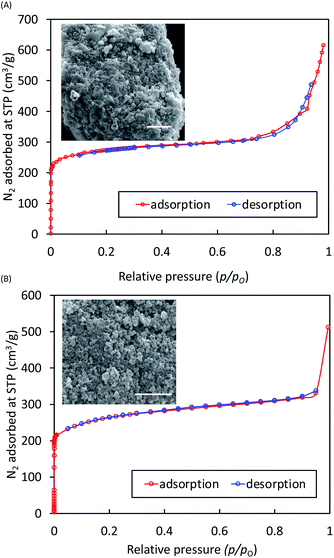
Samples of both Li-BIF-2 and Li-BIF-3 after washing with MeCN were further characterized by FTIR-ATR, TGA in air, and analysis of metal content by ICP-MS (see ESI†). Nitrogen sorption measurement on the mechanochemically obtained Li-BIF-3, after washing with MeCN and evacuation at 85 °C, revealed a highly microporous material with a Brunauer–Emmett–Teller (BET) surface area of 1010 m2 g−1 (Table 1 and Fig. 5A), which is close to the value expected from the crystal structure of the material (1200 m2 g−1, Table 1).32 For direct comparison with previous work,32 we also calculated the Langmuir surface area, revealing an almost 40% increase (1060 m2 g−1) compared to samples made solvothermally (762.5 m2 g−1) (Table 1). Analysis of Li-BIF-3 by scanning electron microscopy (SEM) revealed particles with sizes of hundreds of nanometres, forming larger aggregates several micrometres across (Fig. 5A, inset).
| Material | Surface area (m2 g−1) | Particle sizeb (nm) | Electronic energy per formula unit (eV) | ΔE (kJ mol−1) | |||
|---|---|---|---|---|---|---|---|
| Mechanochemical, BET | Mechanochemical, Langmuir | Prior work, Langmuir![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
Theoreticala | ||||
| a Calculated using MOF Explorer (see ESI). b Determined from SEM measurements, where n corresponds to number of particles observed. | |||||||
| dia-Li-BIF-2 | — | — | — | — | — | −2679.174 | 14.25 |
| SOD-Li-BIF-3 | 1010 | 1060 | 762.5 | 1200 | 217 (n = 24) | −2679.026 | |
| dia-Cu-BIF-2 | — | — | — | — | — | −3417.091 | 9.67 |
| SOD-Cu-BIF-3 | 935 | 1196 | 182.3 | 1100 | 611 (n = 500) | −3416.991 | |
| dia-Ag-BIF-2 | — | — | — | — | — | −4738.959 | 8.66 |
| SOD-Ag-BIF-3 | 1020 | 1205 | — | 1170 | 500 (n = 25) | −4738.869 | |
The analogous copper(I)-based BIF-2 and BIF-3 frameworks were readily accessible by ILAG, by controlling the volume of the liquid additive and milling time (Fig. 4H–L, also see ESI†). Similarly to our previous studies of ZIFs,17,24,37,39 increased milling times preferred the formation of the close-packed polymorph, dia-topology Cu-BIF-2. While the PXRD pattern of the reaction mixture after 60 minutes ILAG with MeOH (η = 0.5 μL mg−1) and NH4NO3 (5% wt/wt) indicated the presence of the SOD-topology Cu-BIF-3, longer milling led to the appearance of the dia-phase (see ESI†). The materials were identified through comparison of experimental PXRD patterns to those simulated from published structures (CSD codes MUCLIG and MOXJOZ for Cu-BIF-2 and Cu-BIF-3, respectively).32 Quantitative synthesis of Cu-BIF-2 from Cu2O was readily accomplished by ILAG for 90 minutes (Fig. 4H–L). Following washing and drying, the products were characterized by PXRD, FTIR-ATR, TGA in air and ICP-MS elemental analysis of metal content.
In order to achieve the synthesis of phase-pure microporous Cu-BIF-3, reaction conditions were modified by increasing η to 1 μL mg−1. This modification enabled the reproducible and quantitative synthesis of Cu-BIF-3 in 60 minutes milling (Fig. 4H–L), confirmed by PXRD, FTIR-ATR, TGA and elemental analysis of metal content (see ESI†). Analyses by SEM and nitrogen sorption were performed on the mechanochemical product after washing and drying in vacuo at 85 °C, revealing that the sample consists of sub-micron particles and exhibits a high BET surface area of 935 m2 g−1, which is close to the theoretically expected value of 1100 m2 g−1 (Table 1 and Fig. 5B). To enable direct comparison with previously reported work,32 we also calculated the Langmuir surface area, revealing a 7-fold increase (1196 m2 g−1) compared to samples made solvothermally (182.3 m2 g−1) (Table 1).
To verify whether the improvement in surface area was attributable to the mechanochemical synthesis or the different activation procedure, a sample of Cu-BIF-3 was synthesized according to the published solvothermal methodology and activated in the same way as the mechanochemically prepared material. The resulting Cu-BIF-3 exhibited a BET surface area of 616 m2 g−1 (see ESI†), i.e. 34% lower compared to the mechanochemically synthesized sample, illustrating a clear benefit of mechanochemistry in providing a simpler, more efficient synthesis, as well as materials of improved porosity.32,42
The mechanochemical approaches to Li- and Cu-based BIFs are surprisingly simple compared to previously reported solvothermal methods,32,33,42 not only avoiding bulk solvents and high temperatures (85 °C for Li-based, 120 °C for Cu-based BIFs), but also enabling the use of simple, easily handled solids LiOH and Cu2O as starting materials compared to, for example, n-BuLi.42 Notably, while the reported solvothermal synthesis of these materials also requires the use of amb for the preparation of both Li- and Cu-BIFs, the use mechanochemical conditions enabled amb-free synthesis of copper-based BIFs. Such simplifications of the synthetic procedure encouraged us to explore the possibility to extend this family of materials towards previously not reported silver(I) derivatives.
As a starting material for the synthesis of Ag(I)-based BIFs we focused on Ag2CO3, generated in situ from readily accessible AgNO3 and K2CO3. One-pot milling reaction of HB(Meim)4, AgNO3, and K2CO3 in the respective stoichiometric ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2, using MeCN as the milling additive (η = 0.25 μL mg−1) readily produced the targeted AgB(Meim)4 material along with the side product KNO3 (Fig. 5, also see ESI†). Specifically, analysis of the reaction mixtures by PXRD revealed that, similar to the lithium and copper(I) analogues,32 the silver-based BIF appears in two polymorphs which could be selectively synthesized by varying the milling time. The BIF products were readily separated from the KNO3 by-product after sequential washing with cold MeOH and acetone, and their respective structures were further validated by structure determination from PXRD data measured on washed and dried materials.
1/2, using MeCN as the milling additive (η = 0.25 μL mg−1) readily produced the targeted AgB(Meim)4 material along with the side product KNO3 (Fig. 5, also see ESI†). Specifically, analysis of the reaction mixtures by PXRD revealed that, similar to the lithium and copper(I) analogues,32 the silver-based BIF appears in two polymorphs which could be selectively synthesized by varying the milling time. The BIF products were readily separated from the KNO3 by-product after sequential washing with cold MeOH and acetone, and their respective structures were further validated by structure determination from PXRD data measured on washed and dried materials.
Specifically, milling for 30 minutes led to the formation of a material (Ag-BIF-3) which, based on PXRD analysis, was isostructural to the SOD-topology Li-BIF-3 and Cu-BIF-3. Consequently, the crystal structure of Ag-BIF-3 (Fig. 5A) was determined through Rietveld refinement of a structural model based on the Cu-BIF-3 structure, in which the copper(I) sites have been replaced by Ag(I), giving rise to a cubic unit cell (space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3n as in the analogous Cu-BIF-3 and Li-BIF-3 structures) with a = 16.6659(3) Å. Composition of Ag-BIF-3 was verified by TGA/DSC and elemental analysis of metal content (see ESI†). The microporous nature of the material was confirmed by N2 sorption analysis, which revealed a high BET surface area of 1020 m2 g−1. Sample analysis by SEM revealed dense aggregates of particles, with sizes below 100 nm (Fig. 5). The 13C cross-polarisation magic angle spinning (CP-MAS) solid-state nuclear magnetic resonance (ssNMR) spectrum of Ag-BIF-3 was consistent with the crystal structure, revealing three signals in the imidazolate region 100–160 ppm and the –CH3 group signal at ∼16 ppm (Fig. 6).
3n as in the analogous Cu-BIF-3 and Li-BIF-3 structures) with a = 16.6659(3) Å. Composition of Ag-BIF-3 was verified by TGA/DSC and elemental analysis of metal content (see ESI†). The microporous nature of the material was confirmed by N2 sorption analysis, which revealed a high BET surface area of 1020 m2 g−1. Sample analysis by SEM revealed dense aggregates of particles, with sizes below 100 nm (Fig. 5). The 13C cross-polarisation magic angle spinning (CP-MAS) solid-state nuclear magnetic resonance (ssNMR) spectrum of Ag-BIF-3 was consistent with the crystal structure, revealing three signals in the imidazolate region 100–160 ppm and the –CH3 group signal at ∼16 ppm (Fig. 6).
Milling for 60 minutes under otherwise identical conditions led to a material whose PXRD pattern was very similar, but not identical, to that of dia-topology Li-BIF-2 and Cu-BIF-2 materials, with additional Bragg reflections indicating possible lower symmetry. The structure of this material (Fig. 6B) was determined by simulated annealing structure solution from PXRD data, revealing a monoclinic (space group P21) unit cell with a = 7.5198(4) Å, b = 16.3763(9) Å, c = 7.5876(4) Å and β = 90.136(6)o. In contrast to structures of Li-BIF-2 and Cu-BIF-2, which all exhibited one symmetrically independent Meim− ligand in a tetragonal I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) space group, the structure of Ag-BIF-3 displays each tetrahedral node surrounded by four symmetrically non-equivalent imidazolate ligands. This much higher multiplicity is clearly reflected by the ssNMR spectrum of the material, validating the structure (Fig. 6). The composition of the material was similarly confirmed by TGA and by elemental analysis of the metal content (see ESI†). For both Ag-BIF-2 and Ag-BIF-3 the measured 13C ssNMR chemical shifts were consistent with those calculated from the herein determined crystal structures (Fig. 6D–G). Notably, while materials based on silver(I) ions are often expected to be light sensitive, the herein reported Ag-BIF-2 and Ag-BIF-3 both appeared unchanged following six months exposure storage in a transparent vial on the bench.
space group, the structure of Ag-BIF-3 displays each tetrahedral node surrounded by four symmetrically non-equivalent imidazolate ligands. This much higher multiplicity is clearly reflected by the ssNMR spectrum of the material, validating the structure (Fig. 6). The composition of the material was similarly confirmed by TGA and by elemental analysis of the metal content (see ESI†). For both Ag-BIF-2 and Ag-BIF-3 the measured 13C ssNMR chemical shifts were consistent with those calculated from the herein determined crystal structures (Fig. 6D–G). Notably, while materials based on silver(I) ions are often expected to be light sensitive, the herein reported Ag-BIF-2 and Ag-BIF-3 both appeared unchanged following six months exposure storage in a transparent vial on the bench.
The crystal structures of Li-, Cu- and Ag-based BIFs provide a unique opportunity to evaluate the effect of changes in the metal node on the relative stability of BIF polymorphs with SOD- and dia-topology across three metals.43–45 The calculations were done using CASTEP plane-wave density-functional theory (DFT)46 code. The previously published crystal structures of Li- and Cu-BIFs with Meim− linkers, as well as the structures of Ag-BIFs herein determined, were geometry-optimized using the PBE47 functional combined with many-body dispersion (MBD*)48–50 correction scheme. The PBE + MBD* approach has previously shown excellent agreement with experimental calorimetric measurements of ZIF polymorphs,24 therefore we expected the same approach to perform reliably for the structures of BIFs. In addition to calculating the relative energies of SOD- and dia-polymorphs, we have performed Gauge Including Projector Augmented Waves (GIPAW)51 simulation of the solid-state NMR spectra of Ag-BIFs to compare the simulated spectra with their experimental counterparts, confirming the low symmetry Ag-BIF-2 structure derived from PXRD data (Fig. 6D–G).
Comparison of calculated energies reveals that increasing the atomic number of the metal node results in increased stabilization of the SOD-topology open framework with respect to the close-packed dia-polymorph. The energy differences (ΔE) between SOD- and dia-topology polymorphs for each pair of Li-, Cu-, and Ag-based frameworks are shown in Table 1. Specifically, whereas Li-BIF-3 structure is calculated to be ca. 14 kJ mol−1 less stable compared to its dia-counterpart, the corresponding differences for Cu-BIF-3 and Ag-BIF-3 are calculated to be considerably smaller, at 9.7 kJ mol−1 and 8.7 kJ mol−1, respectively. The calculated energies are in a similar range to the calculated (15.2 kJ mol−1) and measured (10.6 kJ mol−1) ones for the analogous zinc-based ZIFs, indicating that switching between transition metal and main group-based nodes, or switching between using only a divalent node and a combination of monovalent and trivalent nodes, does not significantly influence the difference in stability of SOD- and dia-topology frameworks. The improvement in the relative stability of open BIF structure upon switching from Li+ to Cu+ and Ag+ nodes is notable, considering the recent interest in the development of MOFs based on heavy-atom nodes.52–54
The simulated ssNMR spectra of Ag-BIF-2 and Ag-BIF-3 showed excellent agreement with the experiment (Fig. 6) in terms of overall chemical shift and the number of distinct NMR signals arising from the crystallographic symmetry. The spectrum of the SOD polymorph is consistent with a single symmetrically unique Meim− linker, while the signal splitting found in the spectrum of the dia-polymorph corresponds to four distinct 2-methylimidazolate units. The NMR simulation fully supports the structural models derived from PXRD data, with calculated chemical shifts underlining the accuracy of the herein used theoretical approach.
Conclusions
In summary, we have shown that the application of mechanochemical methodologies can greatly simplify the synthesis of zeolitic boron imidazolate frameworks, providing a rapid, room-temperature approach to this class of materials. Contrary to previously reported materials obtained by solvothermal synthesis, the open framework SOD-topology BIFs made mechanochemically show higher surface areas, that are very close to the theoretically calculated ones. The use of mechanochemistry also expanded the scope of the zeolitic BIF class of materials, by enabling the synthesis of previously not reported silver-based systems.¶ Theoretical investigation of isostructural BIFs based on Li+, Cu+ and Ag+ reveals that the use of increasingly heavier tetrahedral metal nodes leads to the stabilization of open-framework SOD-topology structure with respect to the corresponding close-packed dia polymorph. Overall, the presented results and synthetic methodologies should provide simpler, faster access to an intriguing, but so far poorly developed, class of ultralight zeolitic MOFs.Data availability
Details of experimental and theoretical procedures, and selected PXRD, TGA, FTIR-ATR, ICP-MS, and nitrogen sorption data are provided as part of the ESI.† Crystallographic data for compounds BIF-2-Ag, BIF-3-Ag and [Hamb+][B(Meim)4−]·2H2O have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition codes 2081806, 2081807, and 2084944, respectively.Author contributions
All authors have contributed to the writing of this manuscript. Development of mechanochemical synthesis procedures and characterization of new materials was performed by C. B. L., J. G. C., J.-L. D. The materials characterization was done by H. M. T., A. J. H., and theoretical studies by M. A. The research was organized and coordinated by T. F.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge support of NSERC Discovery Grant (RGPIN-2017-06467), Discovery Accelerator Award (RGPAS 507837-17), Strategic Grant (STPGP 521582-18), and PGS-D Scholarship (to C. B. L.). MA thanks the National Science Center of Poland (NCN) for the support via SONATA grant (2018/31/D/ST5/03619). We also thank PL-Grid for access to the Prometheus supercomputer. Dr Robin S. Stein is acknowledged for help in acquiring NMR data. Anna Jung of McGill Department of Earth and Planetary Sciences for ICP-MS measurements, and Dr David Liu of the Facility for Electron Microscopy Research (FEMR), McGill University, for SEM imaging.Notes and references
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 13, 1018–1029 CrossRef PubMed.
- N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC.
- E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC.
- A. Porcheddu, E. Colacino, L. De Luca and F. Delogu, ACS Catal., 2020, 10, 8344–8394 CrossRef CAS.
- J. M. Andersen and J. Mack, Chem. Sci., 2017, 8, 5447–5453 RSC.
- C. Bolm and J. G. Hernández, Angew. Chem., Int. Ed., 2019, 58, 3285–3299 CrossRef CAS PubMed.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- B. G. Fiss, N.-N. Vu, G. Douglas, T.-O. Do, T. Friščić and A. Moores, ACS Sustain. Chem. Eng., 2020, 8, 12014–12024 CrossRef CAS.
- D. Prochowicz, M. Saski, P. Yadav, M. Grätzel and J. Lewiński, Acc. Chem. Res., 2019, 52, 3233–3243 CrossRef CAS PubMed.
- D. DeSantis, J. A. Mason, B. D. James, C. Houchins, J. R. Long and M. Veenstra, Energy Fuels, 2017, 31, 2024–2032 CrossRef CAS.
- T. Stolar and K. Užarević, CrystEngComm, 2020, 22, 4511–4525 RSC.
- R. Riccò, O. Linder-Patton, K. Sumida, M. J. Styles, K. Liang, H. Amenitsch, C. J. Doonan and P. Falcaro, Chem. Mater., 2018, 30, 5630–5638 CrossRef.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and L. S. James, Chem. Sci., 2015, 6, 1645–1649 RSC.
- K. Užarević, T. C. Wang, S. Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Chem. Commun., 2016, 52, 2133–2136 RSC.
- A. D. Katsenis, A. Puškarić, V. Štrukil, C. Mottillo, P. A. Julien, K. Užarević, M. H. Pham, T. O. Do, S. A. J. Kimber, P. Lazić, O. Magdysyuk, R. E. Dinnebier, I. Halasz and T. Friščić, Nat. Commun., 2015, 6, 6662 CrossRef CAS PubMed.
- M. Arhangelskis, A. D. Katsenis, N. Novendra, Z. Akimbekov, D. Gandrath, J. M. Marrett, G. Ayoub, A. J. Morris, O. K. Farha, T. Friščić and A. Navrotsky, Chem. Mater., 2019, 31, 3777–3783 CrossRef CAS.
- W. Xu, H. Chen, K. Jie, Z. Yang, T. Li and S. Dai, Angew. Chem., Int. Ed., 2019, 58, 5018–5022 CrossRef CAS PubMed.
- D. E. Crawford and J. Casaban, Adv. Mater., 2016, 28, 5747–5754 CrossRef CAS PubMed.
- A. A. L. Michalchuk, K. S. Hope, S. R. Kennedy, M. V. Blanco, E. V. Boldyreva and C. R. Pulham, Chem. Commun., 2018, 54, 4033–4036 RSC.
- H. M. Titi, J.-L. Do, A. J. Howarth, K. Nagapudi and T. Friščić, Chem. Sci., 2020, 11, 7578–7584 RSC.
- P. A. Julien, C. Mottillo and T. Friščić, Green Chem., 2017, 19, 2729–2747 RSC.
- T. Friščić and L. Fábián, CrystEngComm, 2009, 11, 743–745 RSC.
- Z. Akimbekov, A. D. Katsenis, G. P. Nagabhushana, G. Ayoub, M. Arhangelskis, A. J. Morris, T. Friščić and A. Navrotsky, J. Am. Chem. Soc., 2017, 139, 7952–7957 CrossRef CAS PubMed.
- N. Novendra, J. M. Marrett, A. D. Katsenis, H. M. Titi, M. Arhangelskis, T. Friščić and A. Navrotsky, J. Am. Chem. Soc., 2020, 142, 21720–21729 CrossRef CAS PubMed.
- M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS.
- K. Užarević, T. C. Wang, S.-Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Chem. Commun., 2016, 52, 2133–2136 RSC.
- B. Karadeniz, A. J. Howarth, T. Stolar, T. Islamoglu, I. Dejanovic, M. Tireli, M. C. Wasson, S.-Y. Moon, O. K. Farha, T. Friščić and K. Užarević, ACS Sustainable Chem. Eng., 2018, 6, 15841–15849 CrossRef CAS.
- P. A. Julien, K. Užarević, A. D. Katsenis, S. A. J. Kimber, T. Wang, O. K. Farha, Y. Zhang, J. Casaban, L. S. Germann, M. Etter, R. E. Dinnebier, S. L. James, I. Halasz and T. Friščić, J. Am. Chem. Soc., 2016, 138, 2929–2932 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côte, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U.S.A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed.
- J. Zhang, T. Wu, C. Zhou, S. Chen, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2009, 48, 2542–2545 CrossRef CAS PubMed.
- T. Wu, J. Zhang, C. Zhou, L. Wang, X. Bu and P. Feng, J. Am. Chem. Soc., 2009, 131, 6111–6113 CrossRef CAS PubMed.
- V. I. Isaeva, K. E. Papathanasiou and L. M. Kustov, Crystals, 2020, 10, 617 CrossRef CAS.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418–426 RSC.
- P. J. Bailey, D. Lorono-Gonzales, C. McCormack, F. Millican, S. Parsons, R. Pfeifer, P. P. Pinho, F. Rudolphi and A. Sanchez Perucha, Chem. Eur. J., 2006, 12, 5293–5300 CrossRef CAS PubMed.
- T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimäki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef PubMed.
- T. Friščić, D. G. Reid, I. Halasz, R. S. Stein, R. E. Dinnebier and M. J. Duer, Angew. Chem., Int. Ed., 2010, 49, 712–715 CrossRef PubMed.
- P. J. Beldon, L. F. Fabain, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef CAS PubMed.
- C. A. O'Keeffe, C. Mottillo, J. Vainauskas, L. Fábián, T. Friščić and R. W. Schurko, Chem. Mater., 2020, 32, 4273–4281 CrossRef.
- L. S. Germann, M. Arhangelskis, M. Etter, R. E. Dinnebier and T. Friščić, Chem. Sci., 2020, 11, 10092–10100 RSC.
- The previously reported synthesis of Li-BIF-3 involves the use of HB(Meim)4 with n-BuLi, in a mixture of amb and acetonitrile at a temperature of 85 °C, for 5 days. The reported synthesis of Cu-BIF-3 in solution is achieved from HB(Meim)4, CuI, and amb in acetonitrile solution after 5 days at 120 °C (ref. 32).
- R. Galvelis, B. Slater, A. K. Cheetham and C. Mellot-Draznieks, CrystEngComm, 2012, 14, 374–378 RSC.
- C. Mellot-Draznieks and B. Kerkeni, Mol. Simul., 2014, 40, 25–32 CrossRef CAS.
- I. A. Baburin and S. Leoni, J. Mater. Chem., 2012, 22, 10152–10154 RSC.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, Z. Krist., 2009, 220, 567–570 Search PubMed.
- A. Vela, J. C. Pacheco-Kato, J. L. Gázquez, J. M. del Campo and S. B. Trickey, J. Chem. Phys., 2012, 136, 144115 CrossRef PubMed.
- A. Tkatchenko, R. A. DiStasio, R. Car and M. Scheffler, Phys. Rev. Lett., 2012, 108, 236402 CrossRef PubMed.
- A. Ambrosetti, A. M. Reilly, R. A. DiStasio and A. Tkatchenko, J. Chem. Phys., 2014, 140, 18A508 CrossRef PubMed.
- A. M. Reilly and A. Tkatchenko, Chem. Sci., 2015, 6, 3289–3301 RSC.
- J. R. Yates, C. J. Pickard and F. Mauri, Phys. Rev. B, 2007, 76, 024401 CrossRef.
- K. P. Carter, J. A. Ridenour, M. Kalaj and C. L. Cahill, Chem. Eur. J., 2019, 25, 7114–7118 CrossRef CAS PubMed.
- S. E. Gilson, P. Li, J. E. S. Szymanowski, J. White, D. Ray, L. Gagliardi, O. K. Farha and P. C. Burns, J. Am. Chem. Soc., 2019, 141, 11842–11846 CrossRef CAS PubMed.
- I. R. Speight, I. Huskic, M. Arhangelskis, H. M. Titi, R. S. Stein, T. P. Hanusa and T. Friščić, Chem. Eur. J., 2020, 26, 1811–1818 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and theoretical procedures, and selected PXRD, TGA, FTIR-ATR, ICP-MS, nitrogen sorption data. Crystallographic data for BIF-2-Ag, BIF-3-Ag and [Hamb+][B(Meim)4−]·2H2O. CCDC 2081806, 2081807 and 2084944. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc03665c |
‡ The use of amb in the synthesis of BIFs has not been explained.32–34 Based on the herein reported mechanochemical studies, we believe amb provides a means to increase the reactivity of the HB(Meim)4 ligand, potentially through salt formation, and also hinders the re-arrangement of open-structure Li-BIF-3 into Li-BIF-2. For example, 30 minutes milling of LiOH and HB(Meim)4 in presence of MeCN at η = 1 μL mg−1 leads to a mixture of Li-BIF-2, Li-BIF-3 and HB(Meim)4, which is evident by PXRD analysis. Milling in presence of a mixture of MeCN and amb (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume, η = 1 μL mg−1), however, leads to complete disappearance of PXRD signals of HB(Meim)4, and Li-BIF-3 as the only product. 1 by volume, η = 1 μL mg−1), however, leads to complete disappearance of PXRD signals of HB(Meim)4, and Li-BIF-3 as the only product. |
| § In some cases during screening reaction conditions, the PXRD pattern of the product exhibited Bragg reflections that could not be assigned to neither Li-BIF-2, LiBIF-3, or any of the reactants. These were subsequently found to result from the parasitic formation of the hydrated salt [Hamb+][B(Meim)4−]·2H2O, whose structure was determined from separately prepared single crystals (see ESI,† CCDC deposition code 2084944). The formation of [Hamb+][B(Meim)4−]·2H2O, however, can be avoided by drying both MeCN and amb over molecular sieves before use. |
| ¶ Preliminary attempts to synthesize the Ag-BIFs from acetonitrile solution, using the methodology that was reported for Cu-BIF-3, gave a yet unidentified material. Replacing the solvent with MeOH gave a mixture of phases containing Ag-BIF-2, along with significant amount of metallic silver, evident by the black color of the material. |
| This journal is © The Royal Society of Chemistry 2021 |