Open Access Article
Open Access ArticleA critical review of single particle inductively coupled plasma mass spectrometry – A step towards an ideal method for nanomaterial characterization†
Darya
Mozhayeva
 a and
Carsten
Engelhard
a and
Carsten
Engelhard
 *ab
*ab
aDepartment of Chemistry and Biology, University of Siegen, Adolf-Reichwein-Str. 2, D-57076 Siegen, Germany. E-mail: engelhard@chemie.uni-siegen.de; Fax: +492717402041
bCenter of Micro- and Nanochemistry and Engineering, University of Siegen, Adolf-Reichwein-Str. 2, D-57076 Siegen, Germany
First published on 25th July 2019
Abstract
Single particle inductively coupled plasma mass spectrometry (spICP-MS or SP-ICP-MS depending on the author) is becoming an important tool for the characterization of nanoparticles (NPs). The method allows determining the size, size distribution, and particle number concentrations of NPs in suspensions after a mere few minutes of measurement. This review is modeled after the concept of “an ideal method for atomic spectroscopy” introduced by Gary M. Hieftje in his publication dedicated to Howard Malmstadt. This review discusses the instrumental developments in spICP-MS of recent years step-by-step, from the sample introduction system to the detector. The authors identify necessary improvements and suggest directions for further developments which have the potential to bring the method closer to “an ideal method for atomic spectroscopy”. The review also discusses the literature on coupling spICP-MS to separation and fractionation techniques including capillary electrophoresis (CE), field flow fractionation (FFF), and differential mobility analysis (DMA). The second part of the review is dedicated to the applications of spICP-MS. Key steps in sample preparation and selected instrumental conditions that were used in the published literature are summarized in a tabular form. Most frequently, spICP-MS is used for silver (Ag), gold (Au), and titanium dioxide (TiO2) nanomaterial analysis. Data acquisition was typically performed with millisecond dwell times in the past while a time resolution of hundreds of microseconds has been used more often in the last five years. The table may serve as a guide to choose an experimental procedure depending on the matrix that is present in the sample under investigation.
1 Introduction
1.1 Nanomaterials
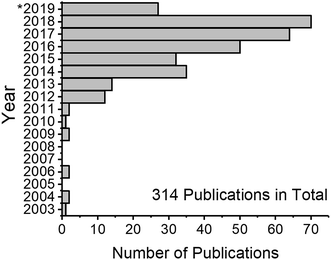
Nanotechnology is a rapidly developing field of science which utilizes materials and their properties at the nanometer (10−9 m) scale. The basic idea of nanotechnology has been formulated already in 1960 by Richard P. Feynman: “What would the properties of materials be if we could really arrange the atoms the way we want them?”2 Indeed, materials at the nanometer scale possess unique properties different from those of chemically identical bulk materials. The fact that matter has distinct size-dependent properties led to the development of colloid chemistry. The first systematic studies in this field were conducted by Michael Faraday when he described the properties of “ruby” gold (Au) suspensions in 1857.3 “The state of division of these particles must be extreme; they have not as yet been seen by any power of the microscope.”3 This statement is proof that the task of analyzing Au nanoparticles (NPs) was a challenge. The variety of states and properties of nanomaterials still presents a challenge for their characterization in analytical chemistry, even after more than 150 years after the term “colloid” was first coined.4 The invention of electron microscopes in the 20th century has allowed scientists to visualize nanomaterials (i.e. particles of any shape with at least one dimension of a size between 1 nm and 100 nm). Although microscopy-based techniques became prominent nanomaterial analysis tools, they have some limitations, namely, difficult sample preparation, limited area of analysis, and measurement of projections (not three-dimensional imaging). Therefore, alternative analysis methods are needed.An alternative method for nanomaterial characterization is single particle inductively coupled plasma mass spectrometry (spICP-MS, also referred to as SP-ICP-MS depending on the author).5 The technique utilizes a standard ICP-MS setup, and makes use of time-resolved detection to probe NPs that are introduced into diluted suspensions (ideally) one by one. Since the first publications, the field has grown rapidly (Fig. 1) and, in the authors' estimation, will continue to grow. There have been several reviews focusing on the topic of spICP-MS,6–9 discussing the principles, potential, limitations, and selected applications. The goal of this review is to critically discuss the latest developments and remaining challenges of spICP-MS and its metrology, to highlight instrumental parameters that are important for NP detection, and to inform the reader about the latest applications of spICP-MS when used with and without particle fractionation methods.
1.2 Principle and early development of spICP-MS
The basic principle of spICP-MS is that NPs can be detected individually if they are introduced sequentially into diluted suspensions and the detector readout frequency is sufficiently high. The constituents of a given single NP generate a discrete pulse of ions at a corresponding mass-to-charge ratio (m/z) on the order of a few hundreds of microseconds above the continuous background.10 The signal abundance is proportional to the mass of an NP after careful calibration of the system. NP size can then be calculated from the NP mass if an element-specific density and particle geometry are assumed. The frequency of the detected signal pulses can be related to the particle number concentration (PNC) in the suspension. Overall, spICP-MS allows obtaining the average size, size distribution, and PNC of NPs after only a few minutes of measurement. Quantification and calibration strategies were summarized and described in detail in other older reviews,6–9 so they will be discussed only shortly below.The history of discrete particle detection has already been described.8 The first utilization of an ICP source for time-resolved particle analysis was published by Kawaguchi et al.11 In their paper, micrometer-sized particles were generated after the desolvation of monodisperse NaCl, Ca(NO3)2, and Cu(NO3)2 droplets. The method was based on optical emission spectrometry (OES) detection and intended for the analysis of particles in air. Time resolved detection of MnCO3 particles in model aerosol samples with ICP-OES was reported by Bochert and Dannecker to obtain a particle size distribution.12 Later, the group of Kawaguchi adapted the technique to ICP-MS (the commercial detector was modified) to achieve 15 times lower mass detection limits (LODs) and detect femtogram amounts of zinc.13 This method utilized monodisperse droplets of Zn(CH3COO)2 and Pb(NO3)2 suspensions that were dried to produce particles, which were then introduced into the ICP-MS. Two years later it was shown that instrumental conditions significantly affect the resulting particle signal.14 For example, the combination of radio frequency (RF) power, sampling position, and carrier gas (also referred to as “nebulizer gas”) flow were shown to influence the signal. For Zn-containing particles, optimal conditions for particle detection were reported to be 1400 W RF power, 14 mm sampling position, and 0.8 L min−1 carrier gas flow; however, no other elements or matrices were tested. At that time, the main future applications for air and aerosol analysis were predicted to be environmental studies (detection of contaminants in air) and control of clean environments (e.g. clean rooms in industrial application).11,13–16 Also, the detection of particles from suspensions with ICP-OES was reported.17,18 For example, Knight et al. studied micrometer-sized particles of refractory oxides and silicates.18 They pointed out that due to incomplete droplet vaporization and particle ionization, the response obtained for 3–7 µm silica particles was not proportional to the mass of the analytes. Furthermore, the mass calibration “still has remained a problem” when the article was published, due to a lack of commercially available particles (detectable by ICP-OES) with narrow size distributions.18
A feasibility study for colloid suspension analysis with spICP-MS was published by Degueldre and Favarger in 2003.5 In the paper, results of spICP-MS with 10 ms dwell time for the analysis of polydisperse 400 nm (median size) TiO2, 150 nm Al2O3, 400 nm FeOOH, and some natural colloids were presented. The choice of isotopes for detection was discussed in detail because of the mass interference experienced by light elements in a single quadrupole ICP-MS (ICP-Q-MS), and 48Ti+, 27Al+, 57Fe+, and 44[SiO]+ were chosen for NP detection in a model water matrix. Similar studies were published by the same authors for 100 nm ZrO2,19 manually milled ThO2 (ref. 20) and UO2,21 and 80 to 250 nm Au particles.22 The studies utilized PNC of 105 to 106 cm−3, and the method was presented as an alternative to microscopy investigations.5,19–22
After the first publications on spICP-MS between 2003 and 2011, the total number of publications first doubled in 2012 (cf.Fig. 1). According to a search in the Web of Science database, interest in this method is steadily growing and over 300 peer-reviewed manuscripts on the topic have been published to date. The next chapter is dedicated to the description of improvements of the spICP-MS method and areas that require further research and where the methodology can be further developed in the opinion of the authors.
2 A step towards an ideal spICP-MS method
The title and idea of the article were inspired by plenary lectures of Gary M. Hieftje23,24 and his publication dedicated to Howard Malmstadt in 2006.1 Howard Malmstadt's research reportedly followed the concept of an “ideal”. He was known to first define the qualities of an ideal “concept, method, device, or system”, and the research itself was then aimed at overcoming the identified weaknesses.1 In the same paper,1 the characteristics of such “an ideal method for atomic spectroscopy” were defined. These characteristics comprise, among others, the LOD of a single atom, no spectral or matrix interference, simultaneous multielement detection, and standardless analysis.1 These ideal characteristics warrant another look here and will be compared to performance characteristics as they are related to spICP-MS and NP analysis to date. The capabilities and advances of the method along with the limitations are critically discussed and future areas of research are identified which would help to bring us closer to what would be an ideal spICP-MS method.2.1 Sample preparation
“An ideal method for atomic spectroscopy” would require no sample preparation, and, ideally, liquid samples could be analyzed with spICP-MS without any sample preparation. In reality, this can be done only for model solutions (and not for unknown samples); however, this still requires an abundance of factors to be considered beforehand, especially when a significant amount of matrix is present, in order not to alter the state of NPs. Nanomaterials possess high surface energy that makes them more reactive compared to bulk materials of the same composition; therefore, the stability of the NP suspensions should always be considered during storage, handling, sample preservation, and sample preparation. Different dispersion media or dilutions, interactions with materials of the sampling or storage containers, storage conditions, and storage time may alter the surface coating or size of the NPs and cause aggregation. Moreover, a certain PNC range is required for analysis to measure the NPs individually. The required PNC range for analysis is discussed here in detail, as it depends on a plethora of factors (nebulization and transport efficiency, type of nebulizer and sample introduction system, elemental composition and size of the NPs etc.). If the samples are too diluted, measurement time can be increased to enhance the number of detected particles. In some cases, matrix interference can be reduced by sample dilution.Nanomaterials often come in complex matrices (e.g. solid matrices and environmental and food samples) and require carefully optimized sample preparation protocols for their successful extraction and spICP-MS analysis. Table 1 presents an overview of all papers which report sample preparation strategies for spICP-MS sorted by the type of matrix (e.g. animal tissue, cell cultures, body fluids, cosmetics etc.) and by the publication year. This table is intended to help the reader to easily grasp the experimental details. In addition, the reader is advised that the main challenges of sample preparation are discussed in some detail in other papers and reviews.25,26
| Year | NP types analyzed | Matrix | Sample preparation | Nebulizer and spray chamber | Plasma parameters | Mass analyzer | Measured isotopes | Dwell time | Size LOD (ESD) | Features | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Note that the entries are grouped by the sample matrix that is the main focus of each study. Papers that report solely on spICP-MS method development are not included. | |||||||||||
| Animal tissue | |||||||||||
| 2012 | <20 nm NM-300K or <15 nm PVP-coated Ag NPs | Rat tissue | Enzymatic digestion with proteinase K | Babington nebulizer | RF power 1400 W | Q | 107Ag+, 109Ag+ | 3 ms | 20 nm | Oral exposure of rats over a period of 28 days | 104 |
| 2013 | 30, 80, and 1500 nm Ag NP powders, 30, 70 nm PVP-coated Ag NPs | Lumbriculus variegatus tissue | Sonication with water and 0.45 µm filtering | n/s | n/s | Q | n/s | n/s | n/s | NPs detected in tissue even after 48 h depuration | 105 |
| 2013 | 100 nm PVP-coated Au NPs, 60 and 100 nm PVP-coated Ag NPs | Spiked ground beef, Daphnia magna, Lumbriculus variegatus tissues, aqueous samples | Alkaline digestion with TMAH | Glass nebulizer, cyclonic spray chamber | n/s | Q | n/s | 10 ms | n/s | 106 | |
| 2013 | Ag nanowires with PVP or aluminum doped SiO2 coatings | Daphnia magna hemolymph, aqueous samples | Dilutions, where necessary | n/s | n/s | Q | 107Ag+, 197Au+ | 10 ms | <30 nm ESD | 107 | |
| 2014 | 60 nm Au NPs | Rat tissue | Alkaline TMAH digestion and enzymatic digestion with proteinase K | MicroFlow PFA nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 0.96–0.99 L min−1 | Q | 197Au+ | 3 ms | Only NPs >44 nm were considered | Intravenous administration of the NPs | 108 |
| 2014 | 60 nm citrate-coated Ag NPs | Spiked chicken meat | Enzymatic digestion with proteinase K | Conical glass concentric nebulizer, quartz impact bead spray chamber | RF power 1400 W, cooling gas 13 L min−1, auxiliary gas 0.7 L min−1, nebulizer gas 1.1 L min−1 | Q | 107Ag+ | 3 ms | n/s | 109 | |
| 2014 | <25 nm anatase TiO2 | Rat spleen | Enzymatic digestion with proteinase K | PFA micronebulizer, heated cyclonic spray chamber, desolvation system | n/s | Q | 49Ti+ | 3 ms | n/s | Oral exposure | 110 |
| 2015 | 50 nm PVP-coated Ag NPs | Earthworm tissue | Enzymatic digestion with proteinase K | Conical glass concentric nebulizer, quartz impact bead spray chamber | RF power 1400 W, cooling gas 13 L min−1, auxiliary gas 0.7 L min−1, nebulizer gas 1.1 L min−1 | Q | 107Ag+ | 3 ms | 30 nm | In vivo exposure in soil | 111 |
| 2015 | 42 nm PVP-coated Ag NPs | Spiked chicken meat | Reference to previous studies | Reference to previous studies | Reference to previous studies | n/s | n/s | n/s | n/s | Two laboratories carried out the analysis with different methods | 112 |
| 2017 | 18–20 nm Ag NPs | Mouse maternal tissues, placentas, foetuses | Alkaline digestion with TMAH | Quartz concentric nebulizer, cyclonic spray chamber | n/s | Q | 107Ag+ | 0.1 ms | 13 nm | Nose-only inhalation of a NP aerosol for pregnant female mice | 113 |
| 2017 | 20 nm PVP-coated Ag NPs | Hen livers and yolks | Enzymatic digestion with proteinase K | n/s | RF power 1000 W, cooling gas 15 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.1 L min−1 | SF mass analyzer was used in low resolution mode | 109Ag+ | 2 ms | 10 nm | Oral administration to hens | 114 |
| 2017 | 30 and 60 nm Au NPs | Caenorhabditis elegans nematode | Alkaline digestion with TMAH | C-type nebulizer, impact bead spray chamber | n/s | Q | 197Au+ | 10 ms | n/s | Sucrose density gradient centrifugation was used to remove non-ingested NPs | 115 |
| 2017 | 40 to 750 nm Pb NPs | Game meat | Enzymatic digestion with proteinase K | Low-flow concentric nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.65 L min−1, nebulizer gas 1.10 L min−1 | Q | 208Pb+ | 5 ms | 40 to 80 nm | Lead from bullets; size LOD was reported to depend on the lead background | 116 |
| 2017 | 26 nm Ag NPs | Chicken meat paste after in vitro model, saliva, gastric, and intestinal digestions | Dilution of digest extracts | MicroMist nebulizer, Scott-type spray chamber | RF power 1500 W, cooling gas 15 L min−1, auxiliary gas 0.73 L min−1, nebulizer gas 0.68 L min−1 (these conditions specified only for total silver analysis) | Q | 107Ag+ | 10 ms | n/s | 117 | |
| 2017 | 15 to 75 nm PVP-coated Ag NPs | Spiked chicken muscle meat | Enzymatic digestion with proteinase K | Varied among the participants | Varied among the participants | Q or n/s | n/s | 3 ms | 15–20 nm | Interlaboratory method performance study with over 10 laboratories | 82 |
| 2017 | 60 and 80 nm Au NPs | LA-SP-ICP-MS imaging of mice heart, lung, spleen, liver, kidney | Intravenous injection | n/s | RF power 1400 W, nebulizer gas 0.7 L min−1 | Q | 197Au+ | 0.1 ms | n/s | 118 | |
| 2018 | CeO2 NPs | Liver and lung tissue of female mice | Enzymatic digestion with proteinase K | Low-flow concentric nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 1.1 L min−1 | Q | 140Ce+ | 3 ms | 18 nm | Intravenous injection of NPs; no NPs in liver were detected after oral exposure | 119 |
| 2018 | 30 and 80 nm PVP-coated Ag and Au NPs | Liver, gill, and intestine tissue of zebrafish (Danio rerio), aqueous samples | Alkaline digestion with TMAH | n/s | RF power 1550 W, nebulizer gas 1.14 L min−1, sampling position 8.0 mm | Q | 107Ag+, 197Au+ | 0.1 ms | n/s | 120 | |
| 2018 | 50 and 100 nm rutile TiO2 NPs | Bivalve mollusk | Ultrasound assisted enzymatic digestion with a pancreatin and lipase mixture | Glass concentric or PFA nebulizer (n/s clearly), cyclonic spray chamber | RF power 1600 W, cooling gas 16 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 0.95 L min−1 | Q | 49Ti+ | 0.1 ms | 24.4–30.4 nm | 121 | |
| 2018 | TiO2 and CeO2 NPs | Spiked zebrafish (Danio rerio) (intestine, liver, gills, and brain) | Enzymatic digestion with proteinase K, H2O2 treatment, SDS stabilization | Conical glass nebulizer, impact bead spray chamber | RF power 1400 W, cooling gas 13 L min−1, auxiliary gas 0.7 L min−1, nebulizer gas 1.1 L min−1 | Q | 48Ti+, 140Ce+ | 3 ms | 100 nm TiO2, 30–40 nm CeO2 | Method for NP extraction from tissue was optimized | 122 |
| Biological applications | |||||||||||
| 2009 | 20, 40, and 80 nm Au NPs | Immunoassay with NPs as tags to antibodies | 2% HNO3 to release the tags, dilution | Glass concentric nebulizer, impact bead spray chamber | RF power 1400 W, cooling gas 13 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 0.85 L min−1 | Q | 197Au+ | 10 ms | 15 nm | 123 | |
| 2014 | 25 nm Au, 25 nm Ag, and 20 nm Pt citrate-coated NPs | Multiplex DNA assay with NP tags | Melting wash, dilution | n/s | RF power 1200 W, cooling gas 13 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 0.82 L min−1 | Q | 107Ag+, 197Au+, 195Pt+ | 0.5 ms | 124 | ||
| 2015 | 80 nm citrate-coated Au NPs | Primary human umbilical vein endothelial cells | Alkaline digestion with TMAH | MicroFlow PFA nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 0.96–0.99 L min−1 | Q | 197Au+ | 10 ms | n/s | 125 | |
| 2016 | 7 and 20 nm TiO2 NPs; 50 and 75 nm citrate-coated Ag NPs | Mouse neuroblastoma cells | Lysis in Triton X-100 | n/s | RF power 1400 W, additional gas flow (Ar) 0.95 L min−1 | Q | 107Ag+, 48Ti+ | 3 ms | 22 nm Ag, 69 nm TiO2 | 126 | |
| 2016 | 10, 30, and 70 nm PEG- BPEI-, and citrate-coated Ag NPs | OECD 201 culture medium with Pseudokirchiniella subcapitata | Filtration (0.45 µm pore size), dilution | n/s | n/s | TQ | 107Ag+ | 3 ms | n/s | 127 | |
| 2018 | 18 nm Al NPs, 20 nm Al2O3 NPs, 25 nm TiO2 NPs | Model cell culture medium | n/s | PFA nebulizer, cyclonic spray chamber | Cooling gas 13 L min−1, auxiliary gas 0.7 L min−1, nebulizer gas 0.89 L min−1 | Q | n/s | 3 ms | 54 nm Al, 50 nm Al2O3, 60–100 nm TiO2 | Several methods for NP characterization are described | 128 |
| 2018 | NPs formed from CrIII salts | Medium for algal ecotoxicity testing | Dilution | MicroMist nebulizer | RF power 1500 W, cooling gas 13.5 L min−1, auxiliary gas 0.77 L min−1, nebulizer gas 0.87 L min−1 | Q | 52Cr+ | 0.05 ms | 90 nm Cr(OH)3 | 129 | |
| 2019 | 30 nm citrate-coated Au NPs | Oligonucleotide-functionalized Au NPs after sandwich hybridization reaction-capture | See article for detailed procedure | Glass concentric nebulizer, impact bead spray chamber | RF power 1750 W, cooling gas 17 L min−1, auxiliary gas 1 L min−1, nebulizer gas 1.05 L min−1 | Q | 197Au+ | 3 ms | n/s | 130 | |
| Body fluids and tissue | |||||||||||
| 2012 | 60 nm citrate-coated Ag NPs | Model saliva, gastric, duodenal, and bile juices with and without proteins | Dilution | Babington type nebulizer, impact bead spray chamber | RF power 1400 W, cooling gas 13 L min−1, auxiliary gas 0.7 L min−1, nebulizer gas 1.1 L min−1 | Q | 107Ag+ | n/s | n/s | 131 | |
| 2015 | 44.5 ± 9.2 nm citrate-coated Au NPs | Spiked whole blood of rats | Dilution | MicroMist glass nebulizer | Nebulizer gas 1.05 L min−1 | Q | 197Au+ | 10 ms | n/s | 132 | |
| 2017 | Respirable crystalline silica | Exhaled breath condensate | n/s | n/s | Q with KED (He) | 28Si+ | 3 ms | 300 nm | 133 | ||
| 2017 | 10, 30, 50, 60, 80, and 100 nm citrate- or carboxylic acid-coated Ag and Au NPs | Spiked human whole blood | Dilution with Triton X, TMAH, and water | Concentric glass nebulizer, conical spray chamber | Nebulizer gas 1.06 L min−1 | Q | 107Ag+, 197Au+ | 0.05 ms | 30 nm | 134 | |
| 2018 | 40 nm PEG-coated Ag NPs, broadly distributed PEG-, sodium carboxylate-coated Ag NPs | Spiked human placental tissue | Alkaline digestion with TMAH, enzymatic digestion with proteinase K | MicroMist nebulizer, Scott spray chamber | RF power 1550 W, cooling gas 15 L min−1, nebulizer gas 1.03 L min−1 | TQ | 107Ag+ | 3 ms | 25 nm | 135 | |
| 2018 | Broadly distributed PEG-, sodium carboxylate-coated Ag NPs | Human ex vivo placenta perfusion model | Enzymatic digestion with proteinase K | MicroMist nebulizer, Scott spray chamber for TQ; MicroFlow PFA nebulizer, cyclonic spray chamber for Q | RF power 1550 W, cooling gas 15 L min−1, nebulizer gas 1.03–1.05 L min−1 | Q, TQ | 107Ag+ | 3 ms | 25 nm | 136 | |
| 2019 | 20 nm citrate-coated Au NPs | Water, RPMI 1640 culture medium, cell and exosome lysates | Dilution, sonication | PFA-ST nebulizer | RF power 1500 W | Q | 197Au+ | 5 ms | 10 nm | 137 | |
| Carbon nanotubes (CNTs) | |||||||||||
| 2013 | Intercalated Co and Y NPs | Single walled CNT dispersions | Dilution of dispersed CNTs | n/s | n/s | Q | 89Y+, 59Co+ | 10 ms | n/s | Detection of trace catalytic metals intercalated in the CNTs | 138 |
| 2016 | Intercalated Y | Single walled CNT dispersions, Daphnia magna in nanopure water after CNT exposure | Dilution and sonication for Daphnia magna samples | n/s | RF power 1600 W, cooling gas 16 L min−1, auxiliary gas 1.02 L min−1, nebulizer gas 0.85–1 L min−1 | Q | 89Y+ | 0.1, 10 ms | n/s | 139 | |
| 2017 | Intercalated Y | Single walled CNTs, release supernatants containing degradation products | Surfactant addition, sonication, dilution | n/s | n/s | Q | 89Y+ | 0.1 ms | n/s | CNT fragments were released due to photodegradation of CNTs and polycaprolactone nanocomposite | 140 |
| Cosmetics | |||||||||||
| 2015 | 32 to 40 nm TiO2 NPs | Sunscreens | Dispersion in Triton X-100, dilution | Cyclonic spray chamber, Meinhard nebulizer | RF power 1600 W, nebulizer gas 1.06–1.08 L min−1 | Q | 48Ti+ | 0.1 ms | 27–29 nm | 141 | |
| 2017 | 30 to 120 nm TiO2 NPs | Cosmetics and personal care products | Defatting with hexane, suspension in water, dilution or suspension in SDS, dilution | Cyclonic spray chamber, Meinhard nebulizer | RF power 1450 W | Q | 48Ti+, 197Au+ | 0.1 ms | 35 nm TiO2 | No Au NPs were found in the tested samples | 142 |
| 2018 | ≤107 nm TiO2 and ≤98 nm ZnO NPs | Cream and spray sunscreens | Dispersion in Triton X-100, dilution | PFA nebulizer | n/s | Q with KED (He) | 48Ti+, 64Zn+ | 5 ms | 25 nm TiO2, 50 nm ZnO | 143 | |
| 2018 | TiO2 NPs | Sunscreen, coating of chocolate candies | Defatting with hexane, filtration, dilution for sunscreen; extraction with water, sonication, filtration, dilution for coating | Meinhard nebulizer, cyclonic spray chamber | n/s | Q | 48Ti+ | 0.1 ms | 32 nm | 144 | |
| 2018 | TiO2 and ZnO NPs | Sunscreen powder | Dispersion in Triton X-100, dilution | n/s | n/s | n/s | n/s | n/s | n/s | 145 | |
| 2018 | Al2O3, TiO2, SiO2 NPs | AF4 fractions after toothpaste fractionation | Dilution, see the article for detailed sample preparation procedure | Low-flow concentric nebulizer, cyclonic spray chamber | RF power 1549 W, cooling gas 14 L min−1, auxiliary gas 0.79 L min−1, nebulizer gas 1.04 L min−1 | Q | 27Al+, 47Ti+ | 10 ms | 55–65 nm Al2O3 and TiO2 NPs | 146 | |
| Model environmental aqueous samples | |||||||||||
| 2013 | Nanoparticulate Zn, Mo, and Ag in leachates | Model freshwater, seawater, acidic rainwater | Leaching | n/s | RF power 1550 W, cooling gas 15 L min−1, auxiliary gas 0.35 L min−1, nebulizer gas 0.79 L min−1 | Q with KED (He) | 66Zn+, 98Mo+, 107Ag+ | 30 ms | n/s | Leaching of CIGS and OPV cells into model water was studied | 147 |
| 2014 | 50 nm PVP-coated Ag NPs | Spiked littoral mesocosms on a lake | Spiking, dilution | n/s | n/s | Q | 107Ag+ | 10 ms | 30 nm | 148 | |
| 2014 | 60 and 100 nm citrate-, tannic acid-, and PVP-coated Ag NPs | Spiked deionized, tap, surface, and EPA moderately hard reconstituted laboratory water | Spiking | n/s | n/s | Q | 107Ag+ | 10 ms | 25–30 nm | NP dissolution kinetic study | 149 |
| 2014 | 50 nm citrate-coated and 80 nm PVP-coated Ag NPs | Spiked purified water, waste water influent and effluent, river water | Filtration (0.45 µm pore size), spiking | Glass conical nebulizer, conical spray chamber with impact bead | RF power 1450 W, cooling gas 15 L min−1, nebulizer gas 0.85 and 0.93 L min−1 | Q | 107Ag+, 109Ag+ | 5 ms | 40 nm | Internal calibration with isotope dilution (109Ag enriched silver standard) was used, both silver isotopes were monitored in one run with 1.9 ms settling time | 66 |
| 2016 | 80–200 nm ZnO NPs, 30–50 nm CeO2 NPs, Zn- and Ce-containing NPs | Spiked river water after real and model drinking water treatment, river water | Spiking, water treatment, dilution or no treatment | Meinhard concentric nebulizer, cyclonic spray chamber | RF power 1600 W, cooling gas 18 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.02–1.06 L min−1 | Q | 67Zn+, 140Ce+ | 0.1 ms | 35–40 nm ZnO, 18–20 nm CeO2 | 150 | |
| 2016 | Ti-containing NPs; 100 and 160 nm TiO2 NPs; 40, 70, and 100 nm citrate-coated Ag NPs; 50, 80, and 100 nm citrate-coated Au NPs | Spiked river water after real and model drinking water treatment, river water | Spiking, water treatment, dilution or no treatment | Meinhard concentric nebulizer, cyclonic spray chamber | RF power 1600 W, cooling gas 18 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.02–1.06 L min−1 | Q | 47Ti+, 197Au+, 107Ag+ | 0.1 ms | 65–70 nm TiO2, 21–23 nm Ag, 27–30 nm Au | 151 | |
| 2016 | 80 nm citrate- and PVP-coated Ag NPs | Spiked waste water effluent and mixed liquor | Filtration (0.45 µm pore size), spiking | Concentric glass nebulizer, cyclonic spray chamber | RF power 1600 W | Q | 107Ag+ | 0.1 ms, 10 ms | 10 nm for double deionized water | 152 | |
| 2016 | 20 and 50 nm citrate-, PVP-, and lipoic acid-coated Ag NPs | Spiked lake water | Filtration (0.22 µm pore size), spiking | n/s | RF power 1550 W, cooling gas 15 L min−1 | Q | 107Ag+, 109Ag+ | 5 ms | 24 nm | 153 | |
| 2016 | 30, 60, 80, and 100 nm tannic acid-coated Au NPs | Spiked river and waste water | Filtration (0.45 µm pore size), spiking | MicroMist nebulizer | RF power 1550 W, auxiliary gas 0.1 L min−1, nebulizer gas 1.05 L min−1 | Q | 197Au+ | 3 ms | 19 nm for ultrapure water, 31 nm for 0.1 µg L−1 Au3+ | 154 | |
| 2016 | 75 nm PVP-coated Ag NPs | Reaction in aerated, sulfide-containing water and EPA moderately hard reconstituted water standard | Spiking, dilution | Microflow concentric PFA nebulizer, impact bead spray chamber | n/s | Q | 107Ag+ | 10 ms | 15 nm | 155 | |
| 2016 | 40 and 80 nm citrate-coated Ag NPs | Spiked waste water effluent and influent, river water | Filtration (0.45 µm pore size), spiking, HDC | Concentric nebulizer | n/s | Q | 107Ag+ | 0.1 ms | 24 nm | 102 | |
| 2016 | 50 and 80 nm citrate- or PVP-coated Ag NPs | Spiked MilliQ water, chloride containing MilliQ water, MilliQ water at pH 5, 7, and 7.6 | Spiking | Conical nebulizer, impact bead spray chamber | RF power 1450 W, cooling gas 15 L min−1, nebulizer gas 0.85 L min−1 | Q | 107Ag+ | 5 ms | 40 nm without Ag+ | Ozonation was used for selected samples | 156 |
| 2017 | 50 nm citrate- and tannic acid-coated Ag NPs | Spiked waste water effluent, MilliQ water, modified TAP medium | Spiking, dilution, IEC | n/s | n/s | Q | n/s | 0.5 ms | 17 nm | 157 | |
| 2017 | 10, 20, 30, 40, 50, 60, 70, 80, and 100 nm PVP-coated Ag NPs | Spiked lake and tap water, liquid consumer products, migration solutions from plasters | Spiking, dilution | Cyclonic spray chamber, Meinhard concentric nebulizer | n/s | Q | 107Ag+ | 0.05 ms | 12–15 nm | 158 | |
| 2017 | 20, 40, 80, 100, and 200 nm PVP-coated and commercial Ag NPs | Spiked waste water effluent, environmental water | Spiking, centrifugation | n/s | n/s | Q | n/s | 10 ms | n/s | 159 | |
| 2017 | 10–25 nm TiO2 and 10–30 nm ZnO NPs | Spiked river water | Spiking, dilution | Reference to previous studies | Reference to previous studies | Q | 47Ti+, 66Zn+ | 0.1 ms | 64 nm TiO2, 43 nm ZnO | 160 | |
| 2017 | 30–50 nm PVP-coated Ag NPs | Spiked MilliQ and lake water with gum arabic | Sonication, dilution | n/s | n/s | Q | 107Ag+ | 5 ms | 40 nm | 161 | |
| 2017 | 60 nm Ag–Ag core–shell NPs (30 nm core, 15 nm shell) | Spiked EPA moderately hard water with or without fulvic acid | Spiking | Cyclonic spray chamber, Meinhard concentric nebulizer | RF power 1600 W | Q | 107Ag+, 197Au+ | 0.1 ms | 15.5 nm Ag | 162 | |
| 2017 | 40, 80, and 100 nm citrate- or PVP-coated Ag NPs | Spiked wastewater biosolids (raw or supernatant) | Filtration (0.45 µm pore size), spiking | Cyclonic spray chamber, type C0.5 concentric glass nebulizer | n/s | Q | 107Ag+ | 0.5 ms | n/s | 163 | |
| 2017 | 40 and 60 nm BPEI- and PVP-coated Ag NPs | Spiked microcosm tanks with seawater | Spiking | Flow injection, pneumatic nebulizer | Reference to previous studies | Q | 107Ag+ | 10 ms | n/s | 164 | |
| 2017 | 40 nm citrate-coated Ag NPs | Spiked WWTP mesocosm | Filtration (0.1 mm pore size), spiking | Cyclonic spray chamber, Burgener Mira Mist nebulizer | RF power 1205 W, cooling gas 15.01 L min−1, auxiliary gas 0.75 L min−1, nebulizer gas 0.520 L min−1 | SF | n/s | 0.1 ms | From 5.4 nm to 30–40 nm | 62 | |
| 2018 | Aged 34 nm citrate-coated Ag NPs, Ag-containing aggregates | Remobilization medium (remobilization from a model sediment) | Dilution, filtration (1 µm pore size) or no filtration | n/s | n/s | Q | 107Ag+ | 5 ms | 30 nm | 165 | |
| 2018 | 40, 70, and 100 nm citrate-coated Ag; 50, 80, and 100 nm citrate-coated Au; 100 nm TiO2; 30–50 nm CeO2; 80–200 nm ZnO NPs | Spiked river and lake water with alum, ferric oxides, or ferric sulfate | Dilution | Reference to previous studies | Reference to previous studies | Q | 47Ti+ | 0.1 ms | 25 nm Ag, 30 nm Au, 70 nm TiO2, 23 nm CeO2, 44 nm ZnO | 166 | |
| 2018 | 25 nm PVP-coated Ag NPs, 5 nm TiO2 NPs | Effluent of a lab-scale WWTP | Sonication, dilution | n/s | n/s | Q | 107Ag+ | 3 ms | n/s | 167 | |
| 2018 | 30, 50, 80, and 100 nm citrate-coated and 60 and 100 nm PVP-coated Ag NPs | Spiked tap, river water, waste water influent | Spiking, Ag+ was adsorbed by magnetic reduced graphene oxide | n/s | RF power 1550 W, cooling gas 15 L min−1 | Q | 107Ag+ | 3 ms | 20 nm | 168 | |
| 2018 | 30–50 nm PVP-coated Ag NPs | Spiked surface water of a boreal oligotrophic lake | Lake spiking | Reference to previous studies | Reference to previous studies | SF | 107Ag+ | 0.05 ms | 12 nm | 169 | |
| 2018 | 30–50 nm PVP-coated Ag NPs | Spiked surface water of a boreal oligotrophic lake | Lake spiking | Glass conical nebulizer | RF power 1450 W, cooling gas 15 L min−1 | Q | 107Ag+ | 5 ms | 45 ± 5 nm | 170 | |
| 2018 | 50 nm zero-valent iron NPs, Cd2+ sorbed to the NPs | Spiked Milli-Q water, synthetic and effluent waste water | Spiking, shaking | Scott spray chamber | RF power 1550 W, cooling gas 15.0 L min−1, auxiliary gas 0.90 L min−1, nebulizer gas 1.09 L min−1, sampling position 8 mm | TQ | 56Fe+, 111Cd+ | 3 ms | 36 nm | H2 was used as a reaction gas | 171 |
| 2019 | 20,40, and 60 nm citrate-coated Ag NPs | Spiked Milli-Q water, spiked wastewater | CPE | PFA microflow nebulizer, cyclonic spray chamber | RF power 1600 W, cooling gas 18.0 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 0.83 L min−1 | Q | 107Ag+ | 0.1 ms | >20 nm | Optimization of CPE was performed | 172 |
| Environmental aqueous samples | |||||||||||
| 2014 | TiO2 NPs released from sunscreen products | Suspended particulate matter of old Danube Lake, Vienna, Austria | Dilution | n/s | n/s | Q | 47Ti+ | 10 ms | 130 nm | Interference of 48Ti+ with 48Ca+ | 173 |
| 2015 | 50 nm ZnO NPs and Zn-containing NPs | Spiked and unspiked surface water, effluent waste water (Des Prairies River, Montreal WWTP, Canada) | Spiking, dilution or IEC (Chelex 100) | Type C0.5 concentric glass nebulizer, cyclonic spray chamber | n/s | Q | n/s | 0.5 ms | 32 nm for Milli-Q water, 70 nm for river water, 126 nm for waste water | 174 | |
| 2016 | Ag-containing NPs | WWTP and surface water from the River Isar, Germany; pre-alpine lake water, Germany | CPE, dilution | n/s | n/s | Q | 107Ag+ | 3 ms | 14 nm | 175 | |
| 2016 | Citrate-coated Ag NPs, tannic acid-coated Au NPs, Ag- and Au-containing NPs | Spiked filtered natural and waste water, unspiked natural water (Guiyu and Xiangjiang Rivers and Chendian Lake, China) and waste water (HuNan University, China) | Filtration with a nylon membrane (0.45 and 0.22 µm pore size) before spiking; dilution for unspiked samples | MicroMist nebulizer | RF power 1550 W, auxiliary gas 0.1 L min−1, nebulizer gas 1.05 L min−1, sampling position 8 mm | Q | 107Ag+, 197Au+ | 3 ms | 20 nm Ag, 19 nm Au | 103 | |
| 2017 | 71 and 145 nm TiO2 NPs | Influent sewage, aeration tank contents of a WWTP (Hyderabad, India) | Microwave digestion (HNO3 and H2O2), filtration with a cellulose acetate membrane (0.22 µm pore size), sonication | n/s | RF power 1550 W, nebulizer gas 1.05 L min−1 | n/s | 47Ti+ | 3 and 10 ms | n/s | 176 | |
| 2017 | Ti-containing NPs | Surface water of clear Creek in Golden, Colorado, USA | n/s | n/s | n/s | Q, SF | 49Ti+ at quadrupole, 48Ti+ at SF | 3 ms | 79 nm TiO2 for Q, 42 nm TiO2 for SF | SF measurements were presented as a proof of concept | 177 |
| 2018 | Ti-containing natural NPs and engineered TiO2 NPs | Water samples from old Danube Lake, Vienna, Austria | Sonication, centrifugation for TQ; sonication, dialysis for TOF | n/s | n/s | TQ, TOF | 63TiNH+ for TQ, MA for TOF | 4 ms for TQ, 3 ms for TOF | 81 nm TiO2 for TQ | NH3 and He were used as reaction/collision gases | 73 |
| 2018 | Ag-containing NPs | Water from Vltava, Prague, Czech Republic | 1% (w/w) gelatin for stabilization | PTFE concentric nebulizer, cyclonic spray chamber | RF power 1100 W, cooling gas 11 L min−1, auxiliary gas 1.0 L min−1, nebulizer gas 0.85 L min−1 | Q | 107Ag+ | 0.1 ms | 15 nm | 178 | |
| 2018 | Ag-containing NPs | Bottom sediments and labile sediments from Lake Ontario, Canada, freeze-dried samples | Sonication with water, centrifugation, filtration (0.45 µm pore size) | Reference to previous studies | Reference to previous studies | SF mass analyzer was used in low resolution mode | 107Ag+ | 0.05 ms | 16 nm | 179 | |
| 2018 | Ag, CeO2, and TiO2 NPs | Surface water of the Meuse and Ijssel Rivers, The Netherlands | Sonication | MicroFlow PFA nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 1.1 L min−1 | Q | 107Ag+, 140Ce+, 48Ti+, 139La+ | 3 ms | 14 nm Ag, 10 nm CeO2, 100 nm TiO2 | MA was used with 100 µs dwell time to detect 140Ce+ and 139La+ | 180 |
| 2018 | Ti-containing NPs | Water from the Salt River, pools, Arizona, USA | Filtration (0.7 µm pore size) | n/s | n/s | Q | 49Ti+ | 10 ms | 148 ± 3 nm for river water, 173 ± 15 nm for pool water | 181 | |
| 2018 | Ag-containing NPs | Water from Lake Königssee and Lake Waginger see, Bavaria, Germany | CPE, dilution | n/s | n/s | Q | 107Ag+ | 0.1 ms | 10 nm | 182 | |
| 2018 | Pb-, Fe-, Sn-, Cu-, Ag-, and Ti-containing particles | Tap water from Phoenix, Arizona, USA | n/s | n/s | Q with KED for 56Fe+ | 208Pb+, 56Fe+, 118Sn+, 107Ag+, 65Cu+, 49Ti+ | 10 ms | 11.3 nm Pb, 55 nm Fe, 26 nm Sn, 40 nm Cu, 75 nm Ti, 13 nm Ag | No Ti- and Ag-containing NPs were discovered | 183 | |
| 2019 | Engineered TiO2 NPs | Sanitary sewage spills | Tetrasodium pyrophosphate treatment, stirring, sonication, centrifugation, dilution | n/s | n/s | TOF | 48Ti+, MA | 33 kHz | 40 nm TiO2 | Split-particle events were summed up | 184 |
| Model food samples | |||||||||||
| 2014 | 20, 40, and 100 nm PVP-coated Ag NPs | Food simulants (distilled water and 10% ethanol) | Dilution | Varied among the participants | Varied among the participants | n/s | n/s | 3 ms | Varied among the participants | Interlaboratory method performance study with over 23 laboratories | 83 |
| 2014 | 30 and 60 nm citrate-coated Au NPs, 60 nm citrate-coated Ag NPs | Spiked Milli-Q water, chicken digest (enzymatic digestion) | Dilution | Varied among the participants | Varied among the participants | n/s | 107Ag+, 197Au+ | 3 ms | Varied among the participants | Interlaboratory method performance study with over 9 laboratories, 3 of which used SP-ICP-MS | 185 |
| 2016 | 60 nm PVP-coated Ag NPs | Food simulants (water, 10% ethanol, and 3% acetic acid) | Dilution | MicroMist nebulizer | RF power 1550 W, cooling gas 15 L min−1 | Q | 107Ag+ | 3 ms | n/s | 186 | |
| 2016 | 10, 30, 50, 60, and 100 nm Ag NPs, 10, 20, 30, 50, 60, 70, and 80 nm Au NPs | Spiked water, orange juice, apple juice | Dilution | n/s | n/s | Q | 107Ag+, 197Au+ | 0.05 ms | 31–34 nm | The coatings were not specified for each size of the NPs. Citrate-, PVP-coated Ag NPs, citrate-, carboxylic acid-coated, and PBS-buffered Au NPs were used | 187 |
| 2018 | 40 nm PEG-, citrate-coated Ag NPs | Food simulants (10%, 20%, and 50% ethanol; 3% acetic acid; olive oil), low fat cow milk, 2% NaCl | Dilution, Triton X-100 was used to create an olive oil emulsion in water | Low-flow concentric nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.80 L min−1, nebulizer gas 0.96 L min−1 | Q | 107Ag+ | 3 ms | 10–20 nm | 188 | |
| Food | |||||||||||
| 2014 | TiO2 NPs | Food grade TiO2 (E171), food and personal care products | Food grade TiO2 suspension in BSA, heating with H2O2 and resuspension in BSA for other products | Conical glass concentric nebulizer | RF power 1400 W | Q | 48Ti+ | 3 ms | 50 nm | 189 | |
| 2015 | Ag NPs | Decoration of pastry (“pearls”) | Dissolution in water, dilution | MicroMist nebulizer | n/s | Q | 107Ag+ | 3 ms | 13 nm | 190 | |
| 2018 | TiO2 NPs | Candy products | Sonication in water, dilution | MicroMist nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 1.03 L min−1 | TQ | 48Ti+ with O2 gas had the highest sensitivity | 10 ms | 26 nm | Optimization of TQ detection was performed with different gases | 57 |
| 2018 | TiO2, Cu, and Ag NPs | Drinks and food | Sample preparation varied depending on the product | Meinhard nebulizer, cyclonic spray chamber | n/s | Q | 107Ag+, 63Cu+, 48Ti+ | 0.1 ms | 32 nm TiO2, 30 nm Ag | 191 | |
| 2018 | Al-containing NPs | Chinese noodles | Enzymatic digestion with α-amylase | Low–low concentric nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 13.9 L min−1, auxiliary gas 0.79 L min−1, nebulizer gas 1.07 L min−1 | Q | 27Al+ | 3 ms | 54–83 nm Al2O3 | 192 | |
| 2019 | TiO2 NPs | Surimi (crab sticks) | Enzymatic digestion with pancreatin and lipase, dilution with 1% glycerol, sonication | n/s | RF power 1600 W, cooling gas 16 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 0.95 L min−1 | Q | 49Ti+ | 0.1 ms | 31.3–37.1 nm | 193 | |
| Plant exposure | |||||||||||
| 2015 | 40 nm PVP-coated; 10, 12, 15, 20, 30, 40, 50, 80, and 100 nm citrate-coated Au NPs | Tomato plants | Enzymatic digestion with Macerozyme R-10, dilution | Meinhard nebulizer, cyclonic spray chamber | RF power 1600 W, cooling gas 18 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.08 L min−1 | Q | 197Au+ | 0.1 ms | 20 nm | 194 | |
| 2016 | 10 nm citrate-coated Ag NPs | Arabidopsis thaliana plants’ roots and shoots | Enzymatic digestion with Macerozyme R-10, dilution | n/s | n/s | Q | 107Ag+ | 0.05 ms | 10 nm | 195 | |
| 2016 | 30–50 nm and 50–100 nm CeO2 NPs | Shoots of cucumber (C. sativus), tomato (S. lycopersicum L.), soybean (Glycine max), pumpkin (Cucurbita pepo) | Enzymatic digestion with Macerozyme R-10 | Meinhard nebulizer, cyclonic spray chamber | RF power 1600 W, cooling gas 18 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.06 L min−1 | Q | 140Ce+ | 0.1 ms | 23–25 nm | 196 | |
| 2016 | 70 nm citrate-coated Pt NPs | Lepidium sativum, Sinapis alba plants | Enzymatic digestion with Macerozyme R-10, filtration (0.45 µm pore size), dilution | Meinhard glass microconcentric nebulizer, cyclonic spray chamber | RF power 1450 W, cooling gas 15.0 L min−1, auxiliary gas 1.0 L min−1, nebulizer gas 0.98 L min−1 | Q | 195Pt+ | 0.1 ms | n/s | 197 | |
| 2017 | Cu-containing NPs from fungicide residues (copper oxychloride) | Vine leaves | Rainfall washoff, through fall sampling, filtration (0.45 µm pore size), dilution | n/s | n/s | Q | 63Cu+ | 0.1 ms | 8–60 nm Cu | 198 | |
| 2017 | 17 nm PVP-coated Ag NPs | Soybean, rice (root and foliar exposures) | Enzymatic digestion with Macerozyme R-10, dilution | Meinhard nebulizer, cyclonic spray chamber | n/s | Q | 107Ag+ | 0.05 ms | 14 nm | 199 | |
| 2017 | PVP-coated Ag2S | Dicotyledonous cucumber (Cucumis sativus), monocotyladonous wheat (Triticum aestivum L.) | Enzymatic digestion with Macerozyme R-10, dilution | n/s | n/s | TQ | 107Ag+ | 3 ms | 20–25 nm | 200 | |
| 2018 | 20–100 nm CuO NPs | Leaves of vegetables, kale (Brassica oleracea, var. Acephala Lacinato), lettuce (Lactuca sativa var. green leaf cultivar), collard green (Brassica oleracea, var. Acephala) | Exposure to NPs, rinsing with ultrapure water or enzymatic digestion with Macerozyme R-10, dilution | Glass concentric nebulizer | RF power 1550 W, nebulizer gas 0.67 L min−1, sampling position 8.0 mm | Q | 63Cu+ | 0.1 ms | n/s | 201 | |
| 2018 | Pd NPs | Sinapis alba leaves, stems, roots | Enzymatic digestion with Macerozyme R-10, filtration (0.45 µm pore size), dilution | Meinhard glass microconcentric nebulizer, cyclonic spray chamber | RF power 1450 W, cooling gas 15.0 L min−1, auxiliary gas 0.9 L min−1, nebulizer gas 1.10 L min−1 | Q | 105Pd+ | 0.1 ms | 25–30 nm | 202 | |
| 2018 | “Green synthesis” of Ag NPs | Leaf sap extract from Aloe arborescens | AgNO3 addition to the leaf sap extract induces the formation of Ag NPs under sunlight, centrifugation, dilution | Concentric quartz nebulizer, baffle-type cyclonic spray chamber | RF power 1500 W, cooling gas 17 L min−1, auxiliary gas 1.4 L min−1, nebulizer gas 0.8 L min−1 | Q | 107Ag+ | 5 ms | n/s | 203 | |
| 2018 | Isotopically labelled Ag, Cu, ZnO NPs | Arabidopsis thaliana shoot and roots | Macerozyme R-10, filtration (0.22 µm pore size), dilution | MicroMist nebulizer | Cooling gas 15 L min−1, auxiliary gas 1.0 L min−1, nebulizer gas 1.05 L min−1 | Q | 107Ag+, 65Cu+, 70Zn+ | 3 ms | n/s | Cu and ZnO NPs were not detected in shoots or roots because of the high background | 204 |
| 2019 | “Green synthesis” of Ag NPs | Cucumber leaf extract | AgNO3 addition to the leaf extract, pH 10.0, 4 h at 80 °C | n/s | RF power 1550 W, nebulizer gas 0.67 L min−1, sampling position 8.0 mm | Q | 107Ag+ | 0.1 ms | n/s | 205 | |
| 2019 | 80–200 nm ZnO NPs | Lettuce Lactuca sativa L. growth medium, roots and leaves | Dilution, Macerozyme R-10 for roots and leaves | n/s | n/s | Q | 66Zn+ | 0.1 ms | n/s | 206 | |
| Model soil samples | |||||||||||
| 2014 | 25 nm PVA-coated Ag NPs, 30 nm ZnO NPs, 42 nm TiO2 NPs, 35 nm CeO2 NPs | Soil spiked with biosolids that were enriched with NPs | Spiking, water extraction, centrifugation, filtration (0.45 µm pore size) | n/s | n/s | Q | n/s | 10 ms | 18 nm Ag, 70–80 nm TiO2, 10 nm CeO2 | 207 | |
| 2015 | CuO NPs | Spiked topsoil colloid extracts | Extraction, dilution, spiking | MicroMist nebulizer | RF power 1550 W, cooling gas 15 L min−1, auxiliary gas 0.15 L min−1, nebulizer gas 0.98 L min−1 | Q with KED (He) | 63Cu+ | 5 ms or 0.1 ms | 15 ± 10 nm | 208 | |
| 2017 | 10, 30, 60 nm citrate-coated Au NPs, 30 nm BPEI- and PVP-coated Au NPs | Spiked soil colloidal extracts | Water extraction, centrifugation, filtration (0.45 µm pore size), spiking, CPE | Concentric MicroMist nebulizer, Scott spray chamber | n/s | Q | 197Au+ | 10 ms | n/s | 209 | |
| 2017 | 40 nm PVP-coated Ag NPs | Natural sandy loam soil spiked with biosolids that were enriched with NPs | Spiking, TSPP extraction, gravimetric sedimentation, dilution | Low pressure PFA nebulizer | RF power 1600 W, nebulizer gas 1.04 L min−1 | Q | 107Ag+ | 0.05 ms | 20 nm | 210 | |
| 2017 | 40 nm PVP-coated Ag NPs | Nanopure water with NaNO3 and KNO3, filtered sandy loam soil extracts with NaNO3 and KNO3 | KNO3 extraction, centrifugation, filtration (0.45 µm and 0.22 µm pore size), spiking, dilution | Low pressure PFA nebulizer, cyclonic spray chamber | RF power 1600 W, nebulizer gas 1.04 L min−1 | Q | 107Ag+ | 0.05 ms | 19 nm | 211 | |
| 2017 | 25 and 40 nm PVP-coated Ag NPs | Spiked sandy loam soil and biosolid extracts | Spiking, TSPP extraction, sonication, filtration (0.45 µm and 0.22 µm pore size), dilution | Low pressure PFA nebulizer, cyclonic spray chamber | RF power 1600 W, nebulizer gas 1.04 L min−1 | Q | 107Ag+ | 0.05 ms | 19 nm | Optimization of the NP extraction conditions from soil, only the most efficient conditions were mentioned | 212 |
| 2018 | 30 nm citrate-, BPEI-, PVP-, PEG-, NOM-coated Au NPs | Standard soil water extracts, estuarine sediment in moderately hard water | Spiking, moderately hard water extraction, centrifugation, dilution | Concentric nebulizer, Scott spray chamber | n/s | Q | 197Au+ | 10 ms | n/s | 213 | |
| 2018 | 40 and 100 nm citrate-coated; 75 and 100 nm PVP- and PEG-coated Ag NPs | Consumer product (band aid) water extracts, spiked soil water extracts | Spiking, Milli-Q water extraction, filtration (0.45 µm pore size) | n/s | RF power 1500 W, cooling gas 15 L min−1 | Q | 107Ag+ | 10 ms | n/s | 214 | |
| Solid environmental samples | |||||||||||
| 2016 | As-containing NPs | Leachate from mine wastes | Leaching with 1 mM KCl, centrifugation | Glass concentric slurry nebulizer, cyclonic spray chamber | RF power 1200 W, cooling gas 15 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.0 L min−1 | Q | 75As+ | 5 ms | 117 nm FeIIIAsVO4·2H2O | Settling time of 3 ms | 215 |
| 2017 | Zn-, Fe-, and Ti- containing NPs | Sewage sludge | Acetic acid extraction, dilution | n/s | n/s | Q with KED for 56Fe+ | 47Ti+, 66Zn+, 56Fe+ | 0.1 ms | 15–20 nm Ti, 15–16 nm Zn, 12–17 nm Fe | 216 | |
| 2017 | Ce-containing natural NPs and <50 nm CeO2 NPs | Spiked topsoil samples | Spiking, shaking, wet-sieving (32 µm pore size), freeze-drying, aqueous colloid extraction, dilution | Pneumatic nebulizer, cyclonic spray chamber for TOF, Miramist nebulizer for Q | RF power 1400 W, cooling gas 16 L min−1, auxiliary gas 1.1 L min−1, and nebulizer gas 1.2 L min−1 for TOF; RF power 1550 W, cooling gas 15 L min−1, auxiliary gas 0.4 L min−1, and nebulizer gas 0.8 L min−1 for Q | TOF at 33 kHz complete mass spectrum in 300 µs, Q | MA for TOF; 140Ce+ and 139La+ for Q | n/a for TOF, 5 ms for Q | 0.10–0.17 fg Ce, 0.13 fg La for TOF; 0.13–0.57 fg Ce, 0.21–0.34 fg La for Q | Natural and engineered NPs were identified with multielement fingerprinting | 72 |
| 2018 | Pt NPs | Road dust leachate, catalyst material | Ultrasonic extraction with stormwater runoff, filtration (0.45 µm pore size) | Quartz nebulizer | n/s | Q | 195Pt+ | 5 ms | 7.4 nm | 217 | |
| 2019 | Th- and U-containing NPs | Leachates of tailings of a niobium mine | Leaching with different solutions of 2–10 pH; Ca, Mg, and Na at 0–13 mmol L−1; fulvic acid at 0–20 ng L−1; centrifugation | n/s | RF power 1300 W, cooling gas 13 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 0.7–1.0 L min−1 | SF | 232Th+, 238U+ | 0.05 ms | 3 nm U, Th | 218 | |
| Model water samples | |||||||||||
| 2013 | 1–10 nm sodium polyacrylate-coated; 20, 40, and 80 Ag NPs | NP water suspensions | Dilution | n/s | n/s | Q | n/s | 3 ms | 20 nm | 219 | |
| 2015 | 100 nm citrate-coated Ag NPs; 60, 100 nm Au NPs, Au/Ag 48 nm core/15 nm shell | Spiked water with laundry detergents | Spiking, filtration or no filtration, dilution | Type-C MiraMist nebulizer, cyclonic spray chamber | n/s | Q | n/s | 3 ms | 30 nm Ag | 220 | |
| 2015 | 252 nm DNA/SiO2 NPs, 350 nm SiO2 NPs | Spiked ultrapure and drinking water | Dilution | Quartz MicroMist nebulizer, cyclonic spray chamber | RF power 950 W, cooling gas 16 L min−1, auxiliary gas 0.6 L min−1, nebulizer gas 1.2 L min−1 | SF | 28Si+ | 5 ms | n/s | 221 | |
| 2017 | 60, 100 nm citrate- and PVP-coated Ag NPs and their aggregates | NaNO3 or NaNO3 and Ca(NO3)2 | Dilution or dialysis | MicroMist nebulizer | RF power 1400 W, cooling gas 18.0 L min−1, auxiliary gas 1.30 L min−1, nebulizer gas 1.44 L min−1 | Q | n/s | 10 ms | n/s | 222 | |
| Other applications | |||||||||||
| 2016 | Cu2O NPs | Antifouling paint | Dilution, sonication | n/s | n/s | Q | n/s | 0.1 ms | n/s | 223 | |
| 2016 | Ag NPs | Release from plastic food containers into food simulants (Milli-Q water, 10% ethanol, 3% acetic acid) | Incubation | n/s | Sampling position 7 mm | n/s | n/s | 3 ms | n/s | 224 | |
| 2016 | Ag NPs | Release from plastic food containers and baby feeding bottles into food simulants (Milli-Q water, 10% and 90% ethanol, 3% acetic acid) | Incubation, sonication, evaporation of ethanol and reconstitution in Milli-Q water | MicroMist nebulizer, Scott spray chamber | RF power 1550 W, cooling gas 15 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1 L min−1 | Q | 107Ag+ | 10 ms | n/s | 225 | |
| 2016 | Ag NPs | Release from nanosilver conductive ink, ink itself | Ink dilution | n/s | n/s | Q | n/s | 0.1 ms | n/s | 226 | |
| 2017 | Ag NPs | Glass slides coated with Ag NPs, structured SiO2-based nanocomposites with a single layer of Ag NPs | Ultrapure water extraction and dilution for glass slides; MOPS extraction, algae treatment, centrifugation, dilution for nanocomposites | Glass concentric slurry nebulizer, cyclonic spray chamber | RF power 1200 W, cooling gas 15 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.0 L min−1 | Q | 107Ag+ and 109Ag+ | 5 ms | 24 to 40 nm | 227 | |
| 2017 | Ag NPs | Toothbrushes | Release of NPs to tap water | n/s | Sampling position 7 mm | n/s | n/s | 3 ms | 35 nm | 228 | |
| 2017 | TiO2 NPs | Textiles (table placemats, wet wipes, microfiber cloths, and baby bodysuits) | Release of NPs into deionized water, sonication, shaking, dilution and addition of Triton-X | n/s | n/s | n/s | 48Ti+, 44Ca+ | 0.1 ms | 27–33 nm | 229 | |
| 2017 | Iron based Fe2O3 nanopigment | Nanopigments in a polymer matrix: release from cryo-milled debris into Milli-Q water, moderately hard water, water with a humic acid | Rotation end over end | MicroMist nebulizer | RF power 1550 W, cooling gas 15 L min−1, auxiliary gas 0.19 L min−1, nebulizer gas 0.98 L min−1 | Q with KED (H2) | 56Fe+ | 5 ms | n/s | 230 | |
| 2017 | Ag NPs | Release from antibacterial leather and leatherette into Milli-Q water | Milli-Q water extraction | n/s | n/s | Q | 107Ag+ | 0.05 ms | n/s | 231 | |
| 2017 | TiO2, Al2O3, Cu-phthalocyanine, and CuO NPs | Tattoo inks | Dilution | PFA-ST nebulizer | RF power 1400 W, cooling gas 15 L min−1, auxiliary gas 1.2 L min−1, nebulizer gas 1.05 L min−1 | Q with KED (He) | 27Al+, 63Cu+, 47Ti+ | 5 ms | n/s | 232 | |
| 2017 | Mo- and Fe-containing NPs | Asphaltene solutions | Dilution with o-xylene, sonication | Concentric glass nebulizer | RF power 1600 W, nebulizer gas 0.35 L min−1, option gas 0.35 L min−1 (Ar, 80%; O2, 20%), sampling position 10 mm | TQ | 51V+, 56Fe+, 60Ni+, 95Mo+ | 0.1 ms | n/s | 233 | |
| 2017 | Pt/SiO2 nanocomposite with ultra-small Pt NPs | Pt/SiO2 nanocomposite | Dilution | MicroMist pneumatic nebulizer, Scott-type spray chamber | RF power 1500 W, nebulizer gas 1.05 L min−1, sampling position 8.0 mm | Q with KED (He) | 195Pt+ | 10 ms | 17.2 nm Pt | 234 | |
| 2018 | Al-, Si-, and Ti-containing NPs | Release from ceramic cookware during simulated linear abrasion | Wash with Liquinox, release into 3% acetic acid, dilution | MicroMist nebulizer | n/s | TQ | 27Al+, 28Si+, 48Ti+ | 3 ms | n/s | H2 was used as a reaction gas | 235 |
| 2018 | Ag NPs | Consumer sprays | Dilution | n/s | n/s | Q | n/s | 10 ms | 17.3–35.3 nm | 236 | |
| 2018 | Sb-, Pb-, and Ba-containing NPs | Gunshot residue wash from shooters' hands | Wash with ultrapure water with 0.2% formaldehyde or hand swabbing with cotton swabs and sonication | n/s | n/s | Q | 121Sb+, 137Ba+, 208Pb+, 121Sb+ and 137Ba+, 121Sb+ and 208Pb+, 137Ba+ and 208Pb+, 206Pb+ and 208Pb+, 207Pb+ and 208Pb+, 206Pb+ and 207Pb+ | 29 µs (Sb, Ba), 30 µs (Pb) | n/s | Single and dual element modes were used. Monitoring of two isotopes with 145 µs settling time, 150 µs settling time for lead isotopes | 237 |
| 2018 | As-containing NPs | Cigarette smoke | Smoke collection with electrostatic trapping, wash with methanol, dilution with deionized water | Dual-port spray chamber | n/s | Q | 75As+ | 0.1 ms | n/s | No As-containing NPs were found | 238 |
| 2018 | Biogenic Se NPs; 50 and 100 nm Se NPs | Se-rich yeast | Enzymatic digestion with Driselase, protease | Concentric nebulizer, cyclonic spray chamber | RF power 1550 W, cooling gas 15 L min−1, auxiliary gas 0.9 L min−1, nebulizer gas 1.10 L min−1 | Q with KED (H2) | 78Se+, 80Se+ | 5 ms, 0.1 ms | 18 nm | 239 | |
| 2018 | Cu NPs | Cuprum metallicum, Gelsemium sempervirens homeopathy medicines | n/s | n/s | n/s | Q | n/s | n/s | 45 nm Cu, 52 nm Cu2O | 240 | |
| 2019 | Niobium and titanium carbonitride particles | Microalloyed steel | Etching in H2SO4 and Disperbyk-2012, centrifugation to remove dissolved iron, dilution | Pneumatic nebulizer, cyclonic spray chamber | n/s | TOF at 555 Hz | Ma, 48Ti+, 93Nb+ | n/a | 27.5 nm NbCN, 50.5 nm TiNbCN | 241 | |
It is fundamentally important when using complex matrices to consider that the state of NPs may change due to filtering (NPs may interact with filter membranes), species interconversion (NPs may partially dissolve and form ionic species or ionic species can be reduced to corresponding metals), extraction and digestion procedures, or storage. At the current state of knowledge and as it is used today, spICP-MS is considered to be very suitable for the analysis of liquid samples without any sample preparation but only in the case of a rather simple matrix. In all other cases, a careful sample preparation method development is required for the analysis of complex, in particular, solid matrices to ensure that NPs do not change in their size, form, or aggregation state.
2.2 Sample introduction
In an ideal world, a sample introduction system would exist for spICP-MS that features a 100% transport efficiency and a high tolerance to all kinds of different matrices. Today, commercially available nebulizers do not achieve a 100% particle transport efficiency, which necessitates the precise determination of the nebulizer transport efficiency for system calibration. Pneumatic nebulizers achieve only approximately 0.5–2% transport efficiency with a 1 mL min−1 sample uptake rate.27 The aerosol transport through a spray chamber is aimed to eliminate larger droplets, which helps to reduce the solvent load and to improve analyte signal stability, but at the same time a considerable amount of the analytes is also lost. An alternative to high-flow pneumatic nebulizers (e.g. 1 mL min−1 sample uptake rate) are micronebulizers with considerably lower sample flow rates. With micronebulizers (e.g. at a 10 µL min−1 sample uptake rate) the transport efficiency can be improved to 60 or up to 80%.27 Micronebulizers utilize low-volume spray chambers (e.g. 15 cm3) and help to improve the transport efficiency. For example, a transport efficiency of approximately 93% was reportedly achieved for 70 nm Pt NPs with a large-bore concentric nebulizer and a small-volume on-axis cylinder chamber.28 A loss of 7% was discussed to be likely due to adsorption to nebulizer and spray chamber walls, NP surface charges, and assumptions made during PNC determination.28 In general, the higher the sample flow of a nebulizer, the lower its transport efficiency typically is. However, the matrix tolerance decreases from higher to lower sample uptake rates. Micronebulizers can be more difficult to operate and maintain due to the dimensions of the inlet capillary (e.g. 0.15 mm),28 which might get obstructed, and sample interchange can also be tedious. When compared to standard pneumatic nebulizers, however, micronebulizers are considered to be advantageous in the field of spICP-MS for low-volume samples and simple matrices, when they are used to interface with separation devices, or to achieve lower PNC LODs.Another approach to achieve high transport efficiency for NPs is through a microdroplet generator (MDG), in which monodisperse droplets are generated by a piezoelectrically actuated quartz capillary.29 The droplets generated at a controlled volume and speed are transported into the ICP, and a transport efficiency of over 95% can be achieved.30 The advantage of the MDG introduction is that calibration may be performed with dissolved metal standards if reference materials of the NPs are not available.30,31 Also, a combination of a pneumatic nebulizer and an MDG was recently reported as a means to exchange different sample matrixes faster and to calibrate the NP signal using traceable elemental standards without the need to use NP reference materials.31,32 In this setup, the MDG was used for system calibration, and the pneumatic nebulizer was used for sample introduction.
A comparison of pneumatic nebulizers and MDG-based sample introduction systems was performed in order to highlight the advantages and disadvantages of the techniques for NP quantification.33,34 It was found that losses are still possible at the sample introduction stage affecting both NPs and dissolved species. Future improvements of sample introduction systems are still needed to ensure high NP and dissolved ion transport efficiency, robust operation, automated sample introduction, and a high tolerance toward different matrices.
One approach to the introduction of solid samples into the ICP-MS is laser ablation (LA). Recent research has demonstrated a possible coupling of LA to spICP-MS.35 Instrumental parameters were optimized, and imaging of a sunflower plant root (cross section), which was previously exposed to Au NPs (60 nm citrate-coated, PNC: 1.83 × 109 NP mL−1), has been performed. With 307![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points obtained per line scan, the obtained results show that Au NPs retained their original size and were concentrated on the surface of the root and rhizodermis (Fig. 2). It is recommended by the authors of the study “that the laser fluence is kept below 1 J cm−2 to avoid NP degradation”.35
000 data points obtained per line scan, the obtained results show that Au NPs retained their original size and were concentrated on the surface of the root and rhizodermis (Fig. 2). It is recommended by the authors of the study “that the laser fluence is kept below 1 J cm−2 to avoid NP degradation”.35
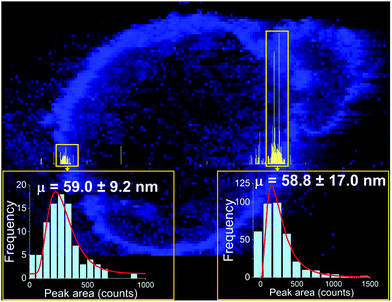 | ||
| Fig. 2 Image (in blue) showing the distribution of gold in a root cross section from a sunflower plant exposed to gold NPs with a mean size of 60 nm, overlaid with a high-resolution time-resolved signal of a single LA-spICP-MS line scan (in yellow). The pixel size in the image is 5 × 5 µm2, and the line-scan signal was recorded every 100 µs. Reprinted with permission from Metarapi et al.35 Copyright 2019 American Chemical Society. (https://pubs.acs.org/doi/10.1021/acs.analchem.9b00853, further permissions related to the material excerpted should be directed to the American Chemical Society). | ||
2.3 NPs in the ICP source
When NPs enter the ICP, they would ideally get fully vaporized, atomized, and ionized, regardless of their elemental composition, size, and matrix they are in. However, it is important to consider differences in the physicochemical properties of the elements (and other species such as their oxides) that the particles are made of including boiling points and ionization potentials. These differences are likely to result in different optimal experimental conditions for the best spICP-MS performance. In fact, the fundamental aspects of micrometer-sized particles were studied by LA-ICP-MS and it was found that the particle size can significantly affect the vaporization, atomization, and ionization efficiency.36,37 While our understanding of the behavior of micrometer-sized particles in the ICP has improved in recent years, the number of fundamental studies on the effects of nanometer-sized particles in spICP-MS is still very limited. For example, Ho et al. focused on the determination of the maximum signal intensity as a function of the ion sampling position (frequently referred to as “sampling depth”) for different elements in aqueous solution and a selection of Au and ZrO2 NPs.38 It was shown that different elements have a different signal maximum in their sampling position profiles depending on the combination of element ionization potentials and boiling points of the corresponding oxides. 150 nm and 250 nm Au and 80 nm ZrO2 NPs were investigated in the same study, and they were found to have different complete ionization positions (±0.5 mm) in the ICP compared to dissolved metal analysis (Fig. 3).38 Consequently, when calibration with dissolved metals is performed in spICP-MS, it is important to determine the position of the maximum signal in sampling position profiles for method optimization and minimization of systematic errors.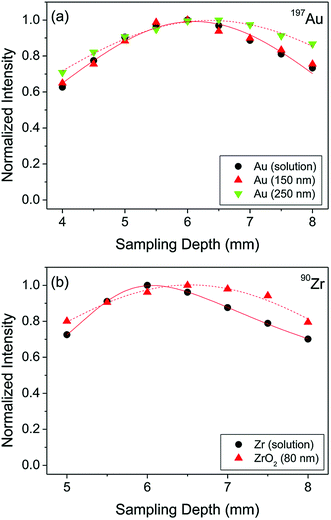 | ||
| Fig. 3 Sampling depth profiles of (a) Au and (b) Zr in the form of aqueous solution with a concentration of 10 µg L−1 and discrete NPs. Reproduced from Ho et al.38 with permission from the Royal Society of Chemistry. | ||
Incomplete ionization may occur due to a relatively larger mass of individual NPs, and, in turn, would lead to a limited upper size dynamic range for NP analysis. Additionally, matrix ions that reach the plasma together with the NPs may affect the ionization of the NPs. For example, Niemax et al. utilized an MDG to study atomization processes in the plasma.39 They reported a local plasma cooling effect during atomization which is dependent on the analyte mass. Another finding was that the matrix elements in the droplets affect the droplet atomization. Later they confirmed experimentally that the position of atomization and ionization of analytes in the ICP strongly depends on the injector gas flow, the size of the introduced droplet, and also on the mass of the analyte (e.g. particles).40 The presence of a matrix (SiO2 particles in a Ca2+ matrix) affects both particle and matrix component atomization. For example, there was a delay in complete atomization of two 1.55 µm SiO2 particles compared to one 0.83 µm SiO2 particle that translates into “a spatial shift of about 8 mm in the ICP.”40 It has also been shown that the position of atomization and ionization is important for ion sampling. If the ions are sampled too early, when atomization and ionization are still not complete, then the detected signal per particle decreases. If the sampling is performed too late, then after the particles are ionized, diffusion occurs, and the signal per particle may also decrease.27
Ho et al. performed a simulation study focusing on incomplete particle vaporization.41 It was shown that ion sampling requires knowledge of the point of complete particle ionization. For example, they reported that the mass calibration leveled off at higher mass values (above 34 fg) at the 8 mm sampling position and concluded that Au particles larger than 150 nm may experience incomplete ionization; further experiments to confirm this hypothesis were not conducted in the study. A sampling position upstream in the plasma (closer to the coil) resulted in an even narrower linear dynamic range (LDR) for Au NP detection (e.g. 6 mm in the simulations results in incomplete vaporization of Au NPs above 60 nm). Additionally, smaller NPs are subjected to diffusion to a greater extent, causing analyte losses for smaller particles that already completely vaporize early in the ICP. Therefore, it was pointed out that it is important to match the NP masses used for calibration with the analyzed particles. A literature search41 was done to determine the detected signal of the particles at which the size calibration is no longer linear (100 nm for Ag NPs34 and 150 nm for Au NPs41); however, to what extent incomplete particle ionization and the limited LDR of the detector influence the obtained values was not studied. Borovinskaya et al. demonstrated that droplets that are off the central axis of the plasma experience a temporal shift in their ICP-MS signals due to diffusion in the plasma.42 A computational study confirmed the advantages of introducing the samples on-axis to achieve higher transport efficiencies of the ions into the MS.43 Chan and Hieftje demonstrated that injection of droplets (deionized water) into the ICP causes a noticeable influence on it; the plasma is locally cooled (the cooling lasts for more than 2 ms after the droplet leaves the load-coil) and is then reheated to a temperature above equilibrium (this effect lasts up to 4 ms after the droplet leaves the load coil); therefore, these effects last longer than the residence time of droplets in the plasma.44 Here, the OH molecular band and Ar I and H I emission lines were measured with a monochromatic imaging spectrometer every 100 µs.
The studies presented in the paragraph above demonstrate that it is indeed important to optimize the plasma conditions for a precise and sensitive NP detection. For example, the injector gas flow (only Ar and not He was considered in this review), plasma power, sampling position, and injector diameter should be optimized based on the analytes and matrix used. Other studies were done to find an optimal sampling position. They studied the effect of the ICP-MS sampling position on the signal intensity of Ag and Au NPs.45 It was shown that it is necessary to optimize the sampling position because it can decrease the size LODs by 25–30% for the studied NPs compared to the standard instrument tuning procedure. For example a sampling position of 4 mm was found to be optimal for Ag and Au NPs to obtain the highest signal intensity, and the signal of dissolved silver and gold standards followed the same trend.45 It is important to note that the optimal sampling position would be different for different instruments, and the elements of different mass ranges, and the formation rate of doubly charged ions and oxides should be accounted for some elements. Chun et al. used a double-viewing-position single particle ICP-OES approach to study and select an appropriate sampling position.46 The approach can be used to elucidate a potentially incomplete ionization of particles, and, therefore, provides information for spICP-MS that sampling from these positions would not be suitable.
spICP-MS is highly dependent on the plasma conditions, and more studies are required in this respect to develop robust protocols to establish optimal plasma conditions for different NPs and different matrices. The plasma conditions that were used in spICP-MS application papers are summarized in Table 1 and discussed in the corresponding chapter. Apart from the choice of the nebulizer, torch injectors of a smaller diameter (1 or 1.5 mm inner diameter) may help to guide NPs on a central axis movement towards the sampler tip. The combination of three parameters, namely injector gas flow, plasma power, and ion sampling efficiency (depending on sampling position), significantly affects NP ionization and, in turn, the recorded signals, and should be optimized prior to analyses. The aim is to achieve the conditions under which the ionization is complete for the required NP size range in a specific matrix, and to sample the ions into the MS from the point of complete ionization to limit ion cloud diffusion in the plasma and a loss of ions per particle.
2.4 Ion transport
All analyte ions produced in the ICP would ideally be transferred completely into the mass spectrometer. However, the step of ion extraction is associated with losses. Ion extraction from the atmospheric-pressure ICP is typically performed by using a two-stage (sampler and skimmer cones) and sometimes a three-stage aperture interface. Downstream of the skimmer orifice, positively charged analyte ions are separated from other plasma species using ion guide devices. While optimal ion lens voltages may differ from element to element, typically a standard tuning protocol is established with a multielement solution to determine only one “ideal” set of voltages for the whole mass range. The maximum sensitivity for a particular ion may be achieved by fine tuning. Additionally, space charge effects, namely ion losses due to charge repulsion and defocusing of the ion beam downstream of the skimmer and the ion optics, may introduce mass-dependent artifacts in nanoparticle analysis similarly to what is known for standard elemental analysis. Niu and Houk47 described fundamental aspects of ion extraction in ICP-MS, and highlighted that the understanding of the processes occurring during the transport of the ions to the mass analyzer would help to reduce ion losses at this stage. Typically, low-mass isotopes have lower ion kinetic energies compared to high-mass isotopes; therefore, low-mass isotopes get forced out to the edges of the ion beam by high-mass isotopes and a relative loss of sensitivity for low-mass isotopes is observed.48 To the best of our knowledge, papers on space-charge effect investigations specifically for NP analysis have not been published yet. Clearly, such ion sampling and transport effects as are mentioned above will affect ions from NPs in a similar fashion, and, in turn, lead to possible partial losses of the number of ions per NP that were generated in the plasma, partial losses of the background ions, losses due to the extraction of positively charged ions, space charge effects etc. All of these losses will likely decrease the overall instrument sensitivity and contribute to an increase in the size LOD for NPs in spICP-MS. However, the order-of-magnitude compared to other fundamental aspects in spICP-MS is not clear to date and more fundamental research is required.2.5 Mass analyzers
An ideal mass analyzer for NPs would be able to have a high mass resolution to provide isotopic information along with simultaneous rapid multielement detection of short (few hundreds of microseconds)10 NP signals. The mass analyzers that are available today are suitable for different types of applications and still have some room for improvement. ICP-Q-MS is widely used because of its comparatively low cost and capability for fast NP detection. However, ICP-Q-MS instruments are limited in terms of multielement detection and resolution (one m/z unit at a time). Switching between different m/z ratios requires some settling time (on the order of 100 µs)49 for the new set of conditions to be stable (ion travel time through the mass analyzer etc.). If one decides to perform isotope-hopping over the course of a fast transient NP signal, the settling time leads to a limited signal coverage, which also significantly limits the number of counts detected per NP. A proof-of-concept for a two-element detection was recently demonstrated, where Au/Ag core/shell NPs were detected with 100 µs dwell time and 100 µs settling time.49 Interference is another limitation of ICP-Q-MS due to its comparatively low mass resolution. A large number of elements suffer from interference in ICP-Q-MS,50 especially in the presence of a matrix. If the interfering species is present only as the background and not in the form of NPs, then NPs could still be detected to a certain extent as signal pulses above the continuous background. However, as the variation of the background signal rises with increasing signal level,51,52 the NP size LOD rapidly increases (from 18 nm to 32 nm for Ag NPs, when 0.3 µg L−1 Ag+ was added, and 5 ms dwell time).53 One approach to remove interference may be the use of a collision-reaction cell with kinetic energy discrimination. The collision-reaction cell was purposely used to reduce the sensitivity of the instrument to be able to detect Au NPs up to 200–250 nm in diameter.54,55 After passing through the mass analyzer, the ion detection itself is performed usually by using a discrete dynode electron multiplier. The crucial parameter to set here is the detector dwell time, which will be discussed in the next chapter. In spite of all limitations discussed above, ICP-Q-MS is still the most widely used instrument (compared to other mass analyzers) for NP detection in terms of the number of publications.The utilization of triple quadrupole (TQ or QQQ) technology allows overcoming matrix interference not only in solution analysis but also in particle analysis. For example, the use of CH3F or H2 for reactions/collisions in ICP-QQQ-MS allowed quantifying SiO2 NPs (high natural background of N2) in the range from 80 to 400 nm using on-mass detection with H2 (28Si+) and mass-shift detection with CH3F (28Si19F+) and significantly improved the size LODs (Fig. 4).56 TiO2 NPs can be quantified with the use of NH3 as the reaction gas in candy products57 and water matrices with a high Ca content58 (48Ca+ interferes with the most abundant 48Ti+ isotope, and the mass-shift detection of [48Ti(14N1H3)3(14N1H)]+ has been performed). In contrast to ICP-Q-MS and ICP-QQQ-MS, sector field (SF)-ICP-MS and multicollector instruments feature a higher mass resolution and sensitivity compared to ICP-Q/QQQ-MS and can also be used for NP detection.30,58–64 For example, a high mass resolution makes it possible to distinguish 48Ti+ (m/z = 47.948) and 48Ca+ (m/z = 47.953) during the analysis of TiO2 NPs in calcium rich matrices.58 The feasibility of spICP-MS for isotope analysis in erbium oxide particles was demonstrated with multi-collector (MC)-ICP-MS.65 Isotope dilution analysis was introduced for Ag NP analysis and quantification with ICP-Q-MS.66,67 Here, spiked samples with isotopically enriched 109Ag+ solution were introduced for quantification.
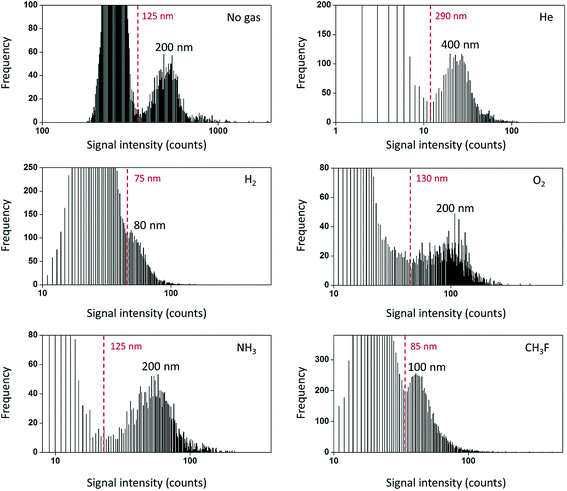 | ||
| Fig. 4 Frequency distribution for the lowest NP sizes detectable using different reaction gases in ICP-TQ-MS for SiO2 particle analysis. Practical LODssize are indicated in red in each figure. Frequency refers to the number of events of each type (background or NPs) detected. Reproduced from Bolea-Fernandez et al.56 with permission from the Royal Society of Chemistry. | ||
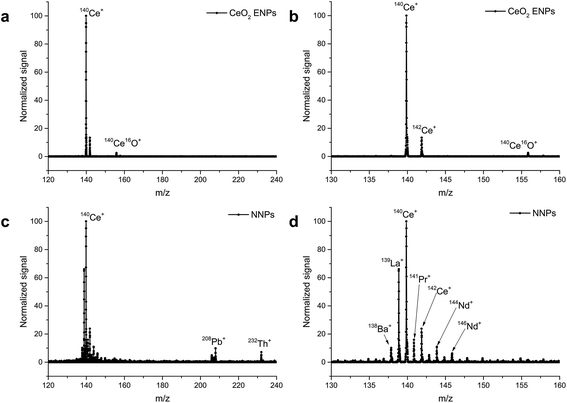
A limitation of scanning-type mass analyzers is the fact that only one isotope (m/z) can be examined at once. Quasi-simultaneous multielement analysis can be performed with time-of-flight ICP-MS (ICP-TOF-MS).68 While ICP-TOF-MS instruments were offered by manufactures in the past but did not seem to find their way into the routine elemental analysis market, the recent interest in nanoparticle analysis led researchers to revisit this type of mass analyzer. A prototype instrument was developed by the Günther group at ETH Zurich which features a 30 kHz spectral acquisition rate. Particle size LODs of 46 nm, 32 nm, and 22 nm for Ag, Au, and U NPs respectively were reported (at that time higher than that with ICP-Q-MS34,69,70). In a follow-up study, ICP-TOF-MS was used to perform the analysis of e.g. Au/Ag core/shell NPs. It was successfully shown that this core/shell material could be identified even in the presence of Ag NPs in the same sample. Improved size-related LODs of 19 nm and 27 nm for Au and Ag NPs respectively were reported (values determined with Poisson statistics).71 The benefit of all-isotope-information in a sampled ion cloud was recently exploited to distinguish natural from engineered CeO2 NPs72 (Fig. 5) and TiO2 NPs.73 The commercial ICP-TOF-MS is reported to achieve 29 nm, 14 nm, and 7 nm LODs for Ti, Mo, and Au containing NPs respectively.74 It was used, for example, for Bi containing NPs and NPs of steel to obtain the elemental composition of these industrial materials.74
 | ||
| Fig. 5 ICP-TOF-MS mass spectra of CeO2 engineered NPs and natural Ce-containing NPs. Averaged mass spectra for 20 discrete single nanoparticle events from both a suspension of CeO2 engineered NPs (a and b (zoomed on Ce)) and a pristine soil sample with natural Ce-containing NPs (c and d (zoomed on Ce and neighboring isotopes)). The engineered NP sample is characterized solely by the Ce ion signal, while the geogenic Ce-containing NNP sample shows, in addition to the Ce signal, detectable levels of La, Ba, Pr, Nd, and Th within single-particle events. Reproduced from Praetorius et al.72 with permission from the Royal Society of Chemistry. | ||
2.6 Detector dwell time
Ideally, spICP-MS requires fast time-resolved detection to get accurate information (number of counts) for each detected NP over the whole required duration of the measurement. In this paper, we focus on secondary electron multiplier (SEM) detectors as they are most frequently used for ICP-Q-MS. Usually, ion detection occurs sequentially within defined time intervals called dwell times. In spICP-MS, dwell times in the millisecond time range are still the most frequently used (Table 1, also determined by the available settings of the instruments). As was demonstrated earlier, for example in a study on the effect of a CE buffer matrix on the particle ion cloud duration in CE-spICP-MS,10 NPs typically result in ion cloud event durations on the order of a few hundreds of microseconds. One fundamental limitation of millisecond dwell times is that only one data point is used to describe a shorter transient. Additionally, a dead time between the individual dwell times5 may interrupt the time-resolved measurements and lead to count losses in pulse-counting mode of the SEM. The occurrence of a NP between two adjacent dwell time intervals may cause one NP to be detected as two smaller ones (split-particle events). Similarly, towards higher particle numbers in a suspension, two or more particles may fall into one dwell time (particle coincidence), which results in a skewed PNC. Therefore, the users of millisecond dwell times in spICP-MS should always consider a suitable PNC range for their measurements and be aware of the limitations of the method when the data are used to draw conclusions e.g. from particle stability and toxicology studies.One possible approach to overcome the measurement artifacts is to use integration times that are significantly shorter than the duration of NP ion clouds (on the microsecond time scale). This way allows for obtaining time-resolved profiles of NP ion clouds with an adequate number of data points per transient. The main challenge then arises in the data acquisition, storage, and processing of µs time-resolved data. For example, if the dwell time would be 10 µs, then each 1 s 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points are obtained. Therefore, a special data processing for visualization and quantification is required that is different from standard ICP-MS data acquisition (DAQ) and software, respectively. In addition, the accurate extraction of NP ion clouds and their unambiguous identification above possible background counts are critical in µs-spICP-MS. To the best of our knowledge, the first system for time-resolved particle analysis with ICP-MS was presented by Nomizu et al. in 2002.16 The detection was performed with 20 µs time resolution for 1 min in the pulse-counting mode; however, it is stated that the measurement time was limited by the computer hard disc space. Later, ICP-MS became commercially available which allows data acquisition with 100, 50 and 10 µs dwell times. For example, several authors utilized a dwell time as low as 10 µs and highlighted the advantages and disadvantages compared to millisecond time.49,75,76 In the study by Montaño et al., NP signal extraction from the background was carried out by applying a three time standard deviation (SD) of the background criterion.49 One limitation of commercially available ICP-MS instrumentation is the fact that the total measurement time with high time resolution is currently limited to minutes. An ideal spICP-MS instrument would be able to operate continuously with microsecond time resolution (hours rather than minutes), without significant dead time, and be able to process the data online. As a contribution from our group to help to get closer to such an ideal system, we presented a DAQ system developed in-house for spICP-MS with 5 µs time resolution and truly continuous data acquisition (Fig. 6).77 The system allows performing acquisition for any measurement duration (only limited by the hard disk space). It was used for continuous measurements for up to 60 min with the coupling of a separation technique.10 The obtained data were processed with in-house written software and particle events were extracted on a particle-by particle level by setting defined count thresholds.77
000 data points are obtained. Therefore, a special data processing for visualization and quantification is required that is different from standard ICP-MS data acquisition (DAQ) and software, respectively. In addition, the accurate extraction of NP ion clouds and their unambiguous identification above possible background counts are critical in µs-spICP-MS. To the best of our knowledge, the first system for time-resolved particle analysis with ICP-MS was presented by Nomizu et al. in 2002.16 The detection was performed with 20 µs time resolution for 1 min in the pulse-counting mode; however, it is stated that the measurement time was limited by the computer hard disc space. Later, ICP-MS became commercially available which allows data acquisition with 100, 50 and 10 µs dwell times. For example, several authors utilized a dwell time as low as 10 µs and highlighted the advantages and disadvantages compared to millisecond time.49,75,76 In the study by Montaño et al., NP signal extraction from the background was carried out by applying a three time standard deviation (SD) of the background criterion.49 One limitation of commercially available ICP-MS instrumentation is the fact that the total measurement time with high time resolution is currently limited to minutes. An ideal spICP-MS instrument would be able to operate continuously with microsecond time resolution (hours rather than minutes), without significant dead time, and be able to process the data online. As a contribution from our group to help to get closer to such an ideal system, we presented a DAQ system developed in-house for spICP-MS with 5 µs time resolution and truly continuous data acquisition (Fig. 6).77 The system allows performing acquisition for any measurement duration (only limited by the hard disk space). It was used for continuous measurements for up to 60 min with the coupling of a separation technique.10 The obtained data were processed with in-house written software and particle events were extracted on a particle-by particle level by setting defined count thresholds.77
 | ||
| Fig. 6 Representative ICP-MS signal (monitoring m/z197Au+) due to 30 nm Au NPs (CNP = 2.5 × 105 NP mL−1) acquired simultaneously for 2 s with (a) 10 ms dwell time (vendor software), and (b) 5 µs dwell time (home-built data acquisition system). First zoom level shows several particle events in (c) and (d) for 500 ms (of the highlighted section in a and b). Second zoom level (e) shows the temporal profile of a single particle's ion cloud identified with the home-built data acquisition system in (d). Reproduced from Strenge and Engelhard77 with permission from the Royal Society of Chemistry. | ||
SF-ICP-MS has also been used with microsecond time resolution (as short as 10 µs).59,60,63,64 NP identification in the raw data was carried out by determining the peak maxima above a certain threshold.59,64 Tuoriniemi et al. introduced a peak recognition algorithm into an SF-ICP-MS using a 100 µs dwell time based on cluster detection.60 Another mass analyzer that can be used for fast detection of NPs is an ICP-TOF-MS that can be operated with a speed of up to 30 kHz.68
While the majority of spICP-MS studies investigate spherically shaped nanomaterials (or assume a spherical shape), first attempts have been undertaken to distinguish NPs with different shapes and high aspect ratios. For example, microsecond time resolution helped to distinguish spherical NPs from nanorods and to perform dimensional characterization of the NPs based on their ion cloud signal duration.78 The composition of NPs of gold and silver alloys has also been assessed using profiles of the ion clouds.79 The detection of silica colloids, which otherwise would require the use of a collision gas to remove polyatomic interference (from nitrogen dimer ions), has been simplified with microsecond time resolution detection.80
As reported above, the advent of microsecond time resolution helped to significantly improve the performance of spICP-MS compared to millisecond time resolved data. The number of data points per ion cloud event is improved, the background is divided between adjacent dwells,76,77 and, thus, the detection of NPs is possible in a wider range of PNCs and in the presence of a higher background and dissolved ion concentrations. However, it should also be noted that the data obtained with microsecond time resolution represent in most of the cases only several counts per dwell time (with 5–10 µs dwell times) and that the normal distribution statistics may not apply to these data anymore. In fact, we suggest that Poisson statistics should be considered in order to differentiate NPs from the background.81
2.7 Quantification considerations
The principles of quantification with spICP-MS were described in previous reviews in detail.6,8,9 Briefly speaking, quantification can be performed using NP standards of the same elemental composition or dissolved standard solutions of the element after taking into account the nebulization efficiency in order to obtain particle size and size distributions with a pneumatic nebulizer. The PNC determination requires a NP standard with the known PNC of the same element, or of a different element, if the same transport and nebulization efficiencies are assumed. The main limitation today is the fact that only a limited variety of the NP standards of different compositions and certified PNCs exist,9 and difficulties in determination of the nebulization efficiency can occur.82 Interlaboratory studies have shown that the determination of median particle diameter (2–5% repeatability SD and 15–25% reproducibility SD) is much more repeatable and reproducible compared to the determination of PNC (7–18% repeatability SD and 70–90% reproducibility SD). The lack of stability of the NPs in initial suspensions and different matrices depending on the handling and storage conditions may have a significant contribution to this fact.82,83 Recently, a metrological study assessing the determination and validation of Au NP size and size distribution was performed.84 High-resolution scanning electron microscopy (HR-SEM) was used as one of the methods to validate the results obtained with spICP-MS. The two methods show a good agreement with a relative precision of 0.5%. It was emphasized that the NP size characterization provided by their suppliers is not sufficient, and that more characterization is needed if the NPs are intended to be used in research. Alternative methods including the use of a MDG,30,32,33 isotope dilution,66,67 and flow injection85,86 are promising new quantification approaches. However, more studies on the metrology of these methods are required to ensure accurate NP analysis.The counting stage of the electron multiplier is typically used up to 2 × 106 cps (ref. 27) because there is a detector dead time (on the order of 50 ns)77 between the acquisitions caused by physical and detector construction limitations. Because NPs result in short but intense ion signals, some of the counts per NP are lost due to the dead time (e.g. 6.2% for 40 nm and 24.4% for 60 nm Au NPs).77 This phenomenon leads to a limited LDR for NP size detection. Liu et al. extended the LDR for Au NPs from 10 nm to 70 nm in “highest sensitivity mode” to 200 nm in “less sensitive modes”.54 The approaches that can be used to extend the LDR are based on decreasing the temporal ion signal abundance by the use of low extraction voltage54 or collision-reaction cells.54,55 The effect of the plasma conditions on the LDR for Au NPs was investigated by Lee and Chan and 250 nm Au NPs were reportedly outside of the LDR.87
The size LODs depend on the sensitivity of the instrument, and an ideal LOD of one atom cannot be achieved nowadays with the current ICP-MS systems. The main reasons are a low nebulization efficiency, low ionization efficiencies of some elements in the argon plasma, and ion transfer into and inside the mass spectrometer. Lee et al. calculated the size LODs for 40 elements for an ICP-Q-MS.70 So far, most of the elements still have LODs well above 10 nm (ref. 6 and 70) and spICP-MS instruments are yet to be developed that can cover the complete nanoscale from 1 nm to 100 nm routinely.
PNC and size LODs are both based on a statistical evaluation of the data; therefore, data processing plays an important role in spICP-MS. For millisecond time resolution, the size LOD is usually determined as 3 × SDBG (SD of the background) or 5 × SDBG above the background.69,88 Real world samples may have higher size LODs due to a continuous background. If the blank is well known and no NP events are detected, then the PNC LOD was proposed to be three detected NP events by Laborda et al.88 based on the Currie Poisson–Normal approximation ( for a “well-known” blank). This PNC LOD may need recalculation if some NP events are detected even in blanks. The data obtained with microsecond time resolution usually require even more data processing, because each NP is represented by several data points. Until now there is still no established approach to extract NPs from the raw data, and each developed system utilizes its own algorithm (discussed in the previous chapter). Therefore, there is still a need to develop statistical approaches based on counting statistics for the quantitative extraction of NPs from time-resolved data.
for a “well-known” blank). This PNC LOD may need recalculation if some NP events are detected even in blanks. The data obtained with microsecond time resolution usually require even more data processing, because each NP is represented by several data points. Until now there is still no established approach to extract NPs from the raw data, and each developed system utilizes its own algorithm (discussed in the previous chapter). Therefore, there is still a need to develop statistical approaches based on counting statistics for the quantitative extraction of NPs from time-resolved data.
Another issue in NP quantification is the differentiation of NPs from the background. The continuous background in ICP-MS may be a result of dissolved ions, natural background, or interference. Bi et al. proposed an approach to differentiate NPs from the background with the use of K-means clustering to improve the differentiation of the NPs from the BG compared to the “traditional standard deviation approach”.89 Cornelis and Hassellöv developed an approach for data deconvolution taking into the account the noise components of ICP-MS to differentiate the NPs that are not fully resolved from the background.90 An approach that utilizes modelling of the background based on the noise components with Monte Carlo simulation was developed for the data obtained with ICP-TOF-MS with 200 Hz resolution.91 The method allows distinguishing small NPs from the background, and the decision criteria for NP detection were revisited. Alternatively, dissolved ions can be removed with ion exchange resins92 or the samples can be analyzed after dilutions.93 Microsecond time resolution helps to distinguish NPs from a continuous background (up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cps) and quantify both the dissolved ions and NPs.81
000 cps) and quantify both the dissolved ions and NPs.81
2.8 Coupling of spICP-MS to separation techniques
A promising approach to obtain more information about mixtures of NPs is the online coupling of spICP-MS to a separation technique. As spICP-MS is used for NP size and size distribution determination, different separation techniques allow obtaining complimentary information. However, the main challenge is that spICP-MS requires the discrete detection of individual NPs while separation techniques will result in a local preconcentration of analytes of a certain type (in a peak), which then elute/migrate together from the column/capillary. Additionally, separation techniques usually require a separation medium (mainly organic compounds) that is introduced into the ICP-MS and may cause matrix effects. Therefore, the combined use of a separation/fractionation technique and spICP-MS requires a careful method development to ensure that:• The NPs are separated based on their properties but not focused in time to the extent that the detection of single NPs is significantly hindered.
• The organic buffer does not interfere with the NP detection (instrumental parameter optimization).
•A suitable dwell time is chosen.
•The NPs do not undergo size transformations during the separation.
• The best size and PNC LODs are achieved.
An overview of the separation techniques that were coupled online to spICP-MS is presented in Table 2, and the main features are highlighted. The first online coupling of spICP-MS to hydrodynamic chromatography (HDC) was presented by Pergantis et al. in 2012, where Au NPs were separated by their size.94 In 2016, spICP-MS was coupled online to asymmetric field flow fractionation (AF4) to fractionate the NPs by their size and also core–shell NPs (Ag core with a SiO2 shell) from mono-component NPs (Ag NPs) (Fig. 7).95 Electrospray-differential mobility analysis (ES-DMA) was also coupled online to spICP-MS.96 This method allows distinguishing different sizes of NPs, assessing their aggregation,96 and distinguishing nanorods from spherical NPs.97 The coupling of capillary electrophoresis (CE) to spICP-MS98 allows separation of the NPs not only by their size, but also in some cases by their different coatings (Fig. 8).99 According to Table 2, most of the separation methods utilize surfactants, most commonly sodium dodecyl sulfate (SDS), to enhance the separation of NPs from each other. The coupling of separation techniques online to spICP-MS has the potential to answer non-trivial questions in NP mixtures analysis, where spICP-MS alone does not provide sufficient information.
| Technique coupled online | Analytes | Nebulizer and spray chamber | Plasma parameters | Dwell time | Separation features | Ref. |
|---|---|---|---|---|---|---|
| CE | 10, 20, 30, 40, and 60 nm citrate-coated Au NPs | Microflow nebulizer with a low volume spray chamber | RF power 1500 W, cooling gas 15 L min−1, auxiliary gas 1 L min−1, nebulizer gas 0.8 L min−1, sampling position 7 mm | 2 ms | 70 mM SDS and 10 mM CAPS in pH 10 buffer | 98 |
| CE | 20, 40, and 60 nm citrate-coated Ag NPs | Microflow nebulizer with a low volume spray chamber | RF power 1450 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 0.8 L min−1, sampling position 3.5 mm | 5 µs | 60 mM SDS and 10 mM CAPS in pH 10 buffer, online preconcentration | 10 |
| CE | 20, 40, and 60 nm citrate-coated; 20, 40, and 60 nm PVP-coated; 40 and 60 nm PEG-coated; 40 nm BPEI-coated Ag NPs | Microflow nebulizer with a low volume spray chamber | RF power 1450 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 0.8 L min−1, sampling position 3.5 mm | 5 µs | 60 mM SDS and 10 mM CAPS in pH 10 buffer, online preconcentration, separation of NPs with different coatings | 99 |
| ES-DMA | 30, 40, 60, 80, and 100 nm Au NPs | n/s | n/s | 10 ms | Ammonium acetate was used for electrospray, aggregate detection | 96 |
| ES-DMA | CTAB- and citrate-coated Au nanorods (diameters 11.8 to 38.2 nm and aspect ratios 1.8 to 6.9) | n/s | n/s | 10 ms | Quantification of the length and diameter of nanorods | 97 |
| FFF | 40, 60, 80, and 100 nm citrate-coated Ag NPs, 60 nm citrate coated Au NPs, 51 nm Ag core and 21.6 nm SiO2 shell citrate-coated NPs | Concentric nebulizer with a cyclonic spray chamber | Nebulizer gas 0.88–0.96 L min−1 | 5 ms | 10 kDa regenerated cellulose membrane, 0.02% FL-70 carrier, separation of Au/SiO2 core/shell NPs from Au NPs | 95 |
| FFF | AgPURE® (<20 nm polyoxyethylene fatty acid ester-coated) in food simulants (water, 10% ethanol, and 3% acetic acid) extracted from model films | Concentric nebulizer with a cyclonic spray chamber | RF power 1550 W, cooling gas 14 L min−1, auxiliary gas 0.8 L min−1, nebulizer gas 1 L min−1 | 5 ms | 10 kDa regenerated cellulose membrane, ultrapure water as the mobile phase | 100 |
| HDC | 30, 60, 80, and 100 nm citrate-coated Au NPs | V-groove nebulizer with a double pass Scott spray chamber | n/s | 10 ms | 10 mM SDS in pH 11 eluent | 94 |
| HDC | 10, 30, 50, 100, and 150, 250 nm citrate-coated Au NPs | PTFE spray chamber | RF power 1400 W, auxiliary gas 0.82 L min−1, nebulizer gas 0.78 L min−1, sampling position 40 mm | 5 ms | 2 mM Na2PO4, 60 mM formaldehyde, 1.8 mM SDS, 3.2 mM Brij L23, and 3.2 mM Triton X-100 in pH 7.5–8 eluent | 101 |
| HDC | 40 and 80 nm Ag NPs spiked in Milli Q water, WWTP influents and effluents | n/s | n/s | 100 µs | 1 mM NaNO3, 0.0013% w/w SDS, and 0.0013% w/w Triton X-100 in pH 7.5 eluent | 102 |
 | ||
Fig. 7 (a) Size distribution of a mixture containing 40 (1 ng L−1), 60 (2 ng L−1), and 80 nm (6 ng L−1) Ag NPs and Ag–SiO2 NPs (1 ng Ag per L) obtained using spICP-MS. (b) Contour plot result of an AF4-spICP-MS analysis on a suspension containing 40, 60, and 80 nm Ag NPs (678 ng L−1, 1.39 µg L−1, and 3.73 µg L−1, respectively) and Ag–SiO2 NPs (624 ng Ag per L). In (a) and (b), the Ag mass concentration ratio of 40, 60, and 80 nm AgNPs, and Ag–SiO2 NPs was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Reprinted with permission from Huynh et al.95 Copyright 2016 American Chemical Society. 1. Reprinted with permission from Huynh et al.95 Copyright 2016 American Chemical Society. | ||
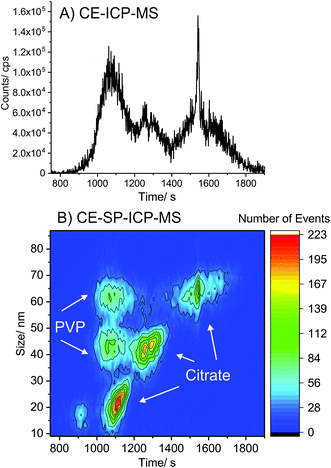 | ||
| Fig. 8 Comparison of a standard CE-ICP-MS plot (A) and first CE-spICP-MS two-dimensional color map (B) acquired from a complex five-component mixture of different nanomaterials (5 µg L−1 citrate-coated 20 nm sized, 35 µg L−1 each citrate and PVP-coated 40 nm sized, 100 µg L−1 PVP-coated 60 nm sized, and 200 µg L−1 citrate-coated 60 nm sized Ag NPs). The analysis was conducted by monitoring at 107Ag+ with 5 µs dwell time, using 110 s injection and REPSM at 20 kV. Reprinted with permission from Mozhayeva et al.99 Copyright 2017 American Chemical Society. | ||
3 Applications of spICP-MS
There has been a significant increase in the number of published studies that utilize spICP-MS in recent years (Fig. 1) and the majority of these publications are dedicated to applications thereof. Table 1 summarizes the papers that include applications of spICP-MS for the analysis of different samples and different matrices (note that fundamental studies on spICP-MS are not included). The studies included in Table 1 are grouped by the analysis matrix and then sorted by the year of publication. Table 1 is a summary of the articles with a short description of the sample preparation and selected instrumental parameters. The reader is advised to check the original publications for more details.It became apparent when compiling this table that many publications do not include all experimental conditions that the authors of this review consider important for spICP-MS. As discussed above, the combination of RF power, sampling position, and carrier gas flow is crucial for the best spICP-MS performance. The parameters dwell time and measured isotopes are very important as well. Most of the articles state the dwell time that was used for the measurements, with microsecond time resolution (most frequently 0.1 ms dwell time) becoming more widely used in recent years. The majority of the articles do not include the sampling position or injector diameter in the experimental descriptions. Some articles cite their previous studies and do not cite the exact conditions that were used for the study.
The majority of the spICP-MS application papers (Table 1) utilize a method for direct analysis of aqueous media (exposure media, model and real environmental water samples, etc.) with or without dilution. Dilution is an effective tool to reduce the matrix load. A filtration step is introduced frequently to avoid clogging of the nebulizer; however, this step may lead to partial losses of NPs due to interactions with the filter membrane materials, even if the NPs are smaller than the membrane pores.103 Therefore, more research is required to determine suitable filter materials for NPs with different coatings to reduce these interactions or identify membranes that show a somewhat reproducible adsorption behavior. When enzymatic or alkaline digestions are used for more complex matrices (tissue, plants, etc.), care must be taken to ensure that the NPs keep their initial state after these procedures. The ultimate goal of any sample preparation step must be a high particle recovery rate and little to no species transformation.
4 Conclusion
The past two decades have witnessed the commercial realization of new and powerful ICP-MS instrumentation and methods, including instruments with faster data acquisition, enhanced detection power, alternative mass analyzers, off-the-shelf interfaces to couple liquid chromatography, CE, etc. to ICP-MS, and novel separation and fractionation methods. While these instruments were successfully used for nanomaterial characterization and the number of published studies of spICP-MS is steadily increasing, there are some remaining challenges that need to be addressed to ultimately reach the top of the nanoparticle peak.Total consumption microflow nebulizers or droplet generators are attractive due to a high particle transport efficiency. However, microflow nebulizers sometimes suffer from clogging (in the presence of agglomerates or organic matter) and commercially available droplet generators reportedly suffer from a limited day-to-day reproducibility and cannot be coupled to autosamplers in the state in which they are available today. Future research in the area of sample introduction for both stand-alone spICP-MS and when interfaced with separation methods (e.g. CE-spICP-MS) is encouraged to address these and other challenges with the ultimate goal of a high-throughput and robust sample introduction system for single particle (and single-cell) ICP-MS. While sample introduction is a potential source of error, sample preparation is often overlooked but may play an even bigger role, especially when particle number concentrations are to be determined. Here, more fundamental studies on potential analyte losses and species transformation (oxidation, release of ions, change of size, and agglomeration) during sampling, storage, and sample preparation are required. For example, a common sample preparation step is filtration to remove unwanted organic matter and larger particle fractions. However, particle losses might occur depending on the particle size and surface coating interaction with the filter material and are often overlooked when particle number concentrations are reported. Similarly to conventional analytical methods, the analyte (particle) recovery should become a parameter that is always reported in future spICP-MS studies.
Based on the publications discussed in this review and from our own findings, we would like to stress that a careful optimization of the plasma conditions and dwell time is required to achieve better NP size detection limits and accurate particle size and number information respectively. In addition, instrumental developments to improve the ion sampling/transfer efficiency in ICP-MS would help to further decrease the size detection limits for single particles and also to gain access to information on NPs of mixed elemental composition and core/shell materials.
While quadrupole-based ICP-MS systems were widely used in past spICP-MS studies, we assume that mass analyzers that provide fast time-resolved and multielement detection such as ICP-TOF-MS will play an important role in this field in the future. However, even the best instrument is worthless if it cannot be calibrated properly, and there is still the lack of appropriate reference materials for calibration. In the future, the field would benefit from more well-characterized and certified nanomaterials to ensure accurate and precise quantification.
It can be concluded that spICP-MS is a very useful method for NP analysis today but there is still room for fundamental studies, instrumental improvements, and methodological advances to come closer to what would be an ideal method for nanomaterial characterization.
Conflicts of interest
There are no conflicts to declare. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. The authors declare no competing financial interest.Note added after first publication
This article replaces the version published on 5th August 2019, which contained errors in Table 1.List of abbreviations
| AF4 | asymmetrical flow field-flow fractionation |
| BPEI | branched polyethyleneimine |
| BSA | bovine serum albumin |
| CAPS | 3-cyclohexylamoniuopropanesulfonic acid |
| CE | capillary electrophoresis |
| CIGS | copper indium gallium selenide cells |
| CNT | carbon nanotube |
| CPE | cloud point extraction |
| CTAB | cetyltrimethylammonium bromide |
| DAQ | data acquisition |
| DMEM | Dulbecco's modified eagle medium |
| EPA | Environmental Protection Agency |
| ESD | equivalent spherical diameter |
| ES-DMA | electrospray-differential mobility analysis |
| FFF | field flow fractionation |
| HDC | hydrodynamic chromatography |
| HR-SEM | high-resolution scanning electron microscopy |
| ICP-Q-MS | single quadrupole ICP-MS |
| IEC | ion-exchange column |
| KED | kinetic energy discrimination |
| LA | laser ablation |
| LDR | linear dynamic range |
| LOD | detection limit |
| m/z | mass-to-charge ratio |
| MA | multielement analysis |
| MC | multi-collector |
| MDG | microdroplet generator |
| MOPS | 3-morpholinopropane-1-sulfonic acid |
| n/a | not applicable |
| n/s | not specified |
| NOM | natural organic matter |
| NP | nanoparticle |
| OECD | The Organization for Economic Co-operation and Development |
| OES | optical emission spectrometry |
| OPV | organic photovoltaic cells |
| PBS | phosphate buffered saline |
| PEG | polyethylene glycol |
| PFA | perfluoroalkoxy alkane |
| PNC | particle number concentration |
| PTFE | polytetrafluoroethylene |
| PVA | polyvinyl alcohol |
| PVP | polyvinylpyrrolidone |
| Q | quadrupole |
| QQQ | triple quadrupole |
| RF | radio frequency |
| SD | standard deviation |
| SDS | sodium dodecyl sulfate |
| SEM | secondary electron multiplier |
| SF | sector field |
| spICP-MS | single particle inductively coupled plasma mass spectrometry |
| TAP | tris-acetate-phosphate |
| TMAH | tetramethylammonium hydroxide |
| TOF | time-of-flight |
| TQ | triple quadrupole |
| TSPP | tetrasodium pyrophosphate |
| WWTP | waste water treatment plant |
Acknowledgements
We acknowledge the House of Young Talents of the University of Siegen for providing funding for Darya Mozhayeva.References
- G. M. Hieftje, Howard Malmstadt – Toward the ideal, Spectrochim. Acta, Part B, 2006, 61, 597–598 CrossRef.
- R. P. Feynman, There's plenty of room at the bottom, Eng. Sci., 1960, 23, 22–36 Search PubMed.
- M. Faraday, Experimental relations of gold (and other metals) to light, Philos. Trans. R. Soc. London, 1857, 147, 145–181 CrossRef.
- F. R. S. Thomas Graham, Liquid diffusion applied to analysis, Philos. Trans. R. Soc. London, 1861, 151, 183–224 CrossRef.
- C. Degueldre and P. Y. Favarger, Colloid analysis by single particle inductively coupled plasma-mass spectroscopy: a feasibility study, Colloids Surf., A, 2003, 217, 137–142 CrossRef CAS.
- F. Laborda, E. Bolea and J. Jiménez-Lamana, Single particle inductively coupled plasma mass spectrometry: a powerful tool for nanoanalysis, Anal. Chem., 2014, 86, 2270–2278 CrossRef CAS PubMed.
- F. Laborda, E. Bolea and J. Jiménez-Lamana, Single particle inductively coupled plasma mass spectrometry for the analysis of inorganic engineered nanoparticles in environmental samples, Trends Environ. Anal. Chem., 2016, 9, 15–23 CrossRef CAS.
- M. D. Montaño, J. W. Olesik, A. G. Barber, K. Challis and J. F. Ranville, Single particle ICP-MS: advances toward routine analysis of nanomaterials, Anal. Bioanal. Chem., 2016, 408, 5053–5074 CrossRef.
- B. Meermann and V. Nischwitz, ICP-MS for the analysis at the nanoscale – a tutorial review, J. Anal. At. Spectrom., 2018, 33, 1432–1468 RSC.
- D. Mozhayeva, I. Strenge and C. Engelhard, Implementation of online preconcentration and microsecond time resolution to capillary electrophoresis single particle inductively coupled plasma mass spectrometry (CE-SP-ICP-MS) and its application in silver nanoparticle analysis, Anal. Chem., 2017, 89, 7152–7159 CrossRef CAS.
- H. Kawaguchi, N. Fukasawa and A. Mizuike, Investigation of airborne particles by inductively coupled plasma emission-spectrometry calibrated with monodisperse aerosols, Spectrochim. Acta, Part B, 1986, 41, 1277–1286 CrossRef.
- U. K. Bochert and W. Dannecker, On-line aerosol analysis by atomic emission spectroscopy, J. Aerosol Sci., 1989, 20, 1525–1528 CrossRef CAS.
- T. Nomizu, S. Kaneco, T. Tanaka, T. Yamamoto and H. Kawaguchi, Determination of femto-gram amounts of zinc and lead in individual airborne particles by inductively coupled plasma mass spectrometry with direct air-sample introduction, Anal. Sci., 1993, 9, 843–846 CrossRef CAS.
- S. Kaneco, T. Nomizu, T. Tanaka, N. Mizutani and H. Kawaguchi, Optimization of operating conditions in individual airborne particle analysis by inductively coupled plasma mass spectrometry, Anal. Sci., 1995, 11, 835–840 CrossRef CAS.
- T. Nomizu, H. Nakashima, Y. Hotta, T. Tanaka and H. Kawaguchi, Simultaneous measurement of the elemental content and size of airborne particles by inductively coupled plasma emission spectrometry combined with the laser light-scattering method, Anal. Sci., 1992, 8, 527–531 CrossRef CAS.
- T. Nomizu, H. Hayashi, N. Hoshino, T. Tanaka, H. Kawaguchi, K. Kitagawa and S. Kaneco, Determination of zinc in individual airborne particles by inductively coupled plasma mass spectrometry with digital signal processing, J. Anal. At. Spectrom., 2002, 17, 592–595 RSC.
- M. Thompson, C. D. Flint, S. Chenery and K. Knight, Time resolved system for the analysis of particles in the inductively coupled plasma - preliminary studies, J. Anal. At. Spectrom., 1992, 7, 1099–1102 RSC.
- K. Knight, S. Chenery, S. W. Zochowski, M. Thompson and C. D. Flint, Time-resolved signals from particles injected into the inductively coupled plasma, J. Anal. At. Spectrom., 1996, 11, 53–56 RSC.
- C. Degueldre, P. Y. Favarger and C. Bitea, Zirconia colloid analysis by single particle inductively coupled plasma–mass spectrometry, Anal. Chim. Acta, 2004, 518, 137–142 CrossRef CAS.
- C. Degueldre and P. Y. Favarger, Thorium colloid analysis by single particle inductively coupled plasma-mass spectrometry, Talanta, 2004, 62, 1051–1054 CrossRef CAS.
- C. Degueldre, P. Y. Favarger, R. Rosse and S. Wold, Uranium colloid analysis by single particle inductively coupled plasma-mass spectrometry, Talanta, 2006, 68, 623–628 CrossRef CAS.
- C. Degueldre, P. Y. Favarger and S. Wold, Gold colloid analysis by inductively coupled plasma-mass spectrometry in a single particle mode, Anal. Chim. Acta, 2006, 555, 263–268 CrossRef CAS.
- G. M. Hieftje, presented in part at the European Winter Conference on Plasma Spectrochemistry, Sankt Anton am Arlberg, Austria, 2017 Search PubMed.
- G. M. Hieftje, presented in part at the Winter Conference on Plasma Spectrochemistry, Amelia Island, FL, U.S.A., 2018 Search PubMed.
- F. Laborda, E. Bolea, G. Cepriá, M. T. Gómez, M. S. Jiménez, J. Pérez-Arantegui and J. R. Castillo, Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples, Anal. Chim. Acta, 2016, 904, 10–32 CrossRef CAS.
- I. De la Calle, M. Menta and F. Séby, Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: a review, Spectrochim. Acta, Part B, 2016, 125, 66–96 CrossRef CAS.
- J. W. Olesik and P. J. Gray, Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle, J. Anal. At. Spectrom., 2012, 27, 1143–1155 RSC.
- S. I. Miyashita, H. Mitsuhashi, S. I. Fujii, A. Takatsu, K. Inagaki and T. Fujimoto, High transport efficiency of nanoparticles through a total-consumption sample introduction system and its beneficial application for particle size evaluation in single-particle ICP-MS, Anal. Bioanal. Chem., 2017, 409, 1531–1545 CrossRef CAS.
- S. Gschwind, L. Flamigni, J. Koch, O. Borovinskaya, S. Groh, K. Niemax and D. Günther, Capabilities of inductively coupled plasma mass spectrometry for the detection of nanoparticles carried by monodisperse microdroplets, J. Anal. At. Spectrom., 2011, 26, 1166–1174 RSC.
- S. Gschwind, H. Hagendorfer, D. A. Frick and D. Günther, Mass quantification of nanoparticles by single droplet calibration using inductively coupled plasma mass spectrometry, Anal. Chem., 2013, 85, 5875–5883 CrossRef CAS.
- L. Hendriks, B. Ramkorun-Schmidt, A. Gundlach-Graham, J. Koch, R. N. Grass, N. Jakubowski and D. Günther, Single-particle ICP-MS with online microdroplet calibration: toward matrix independent nanoparticle sizing, J. Anal. At. Spectrom., 2019, 34, 716–728 RSC.
- B. Ramkorun-Schmidt, S. A. Pergantis, D. Esteban-Fernández, N. Jakubowski and D. Günther, Investigation of a combined microdroplet generator and pneumatic nebulization system for quantitative determination of metal-containing nanoparticles using ICPMS, Anal. Chem., 2015, 87, 8687–86894 CrossRef CAS.
- S. Gschwind, M. d. L. A. Montes and D. Günther, Comparison of sp-ICP-MS and MDG-ICP-MS for the determination of particle number concentration, Anal. Bioanal. Chem., 2015, 407, 4035–4044 CrossRef CAS PubMed.
- B. Franze, I. Strenge and C. Engelhard, Single particle inductively coupled plasma mass spectrometry: evaluation of three different pneumatic and piezo-based sample introduction systems for the characterization of silver nanoparticles, J. Anal. At. Spectrom., 2012, 27, 1074–1083 RSC.
- D. Metarapi, M. Sala, K. Vogel-Mikus, V. S. Selih and J. T. van Elteren, Nanoparticle analysis in biomaterials using laser ablation-single particle-inductively coupled plasma mass spectrometry, Anal. Chem., 2019, 91, 6200–6205 CrossRef CAS PubMed.
- M. Guillong and D. Günther, Effect of particle size distribution on ICP-induced elemental fractionation in laser ablation-inductively coupled plasma-mass spectrometry, J. Anal. At. Spectrom., 2002, 17, 831–837 RSC.
- M. Guillong, H.-R. Kuhn and D. Günther, Application of a particle separation device to reduce inductively coupled plasma-enhanced elemental fractionation in laser ablation-inductively coupled plasma-mass spectrometry, Spectrochim. Acta, Part B, 2003, 58, 211–220 CrossRef.
- K. S. Ho, W. W. Lee and W. T. Chan, Effects of ionization potential of an element and boiling point of the corresponding oxide on the sensitivity of ICP-MS, J. Anal. At. Spectrom., 2015, 30, 2066–2073 RSC.
- S. Groh, C. C. Garcia, A. Murtazin, V. Horvatic and K. Niemax, Local effects of atomizing analyte droplets on the plasma parameters of the inductively coupled plasma, Spectrochim. Acta, Part B, 2009, 64, 247–254 CrossRef.
- A. Murtazin, S. Groh and K. Niemax, Investigation of sample introduction- and plasma-related matrix effects in inductively coupled plasma spectrometry applying single analyte droplet and particle injection, Spectrochim. Acta, Part B, 2012, 67, 3–16 CrossRef CAS.
- K. S. Ho, K. O. Lui, K. H. Lee and W. T. Chan, Considerations of particle vaporization and analyte diffusion in single-particle inductively coupled plasma-mass spectrometry, Spectrochim. Acta, Part B, 2013, 89, 30–39 CrossRef CAS.
- O. Borovinskaya, M. Aghaei, L. Flamigni, B. Hattendorf, M. Tanner, A. Bogaerts and D. Günther, Diffusion- and velocity-driven spatial separation of analytes from single droplets entering an ICP off-axis, J. Anal. At. Spectrom., 2014, 29, 262–271 RSC.
- M. Aghaei and A. Bogaerts, Particle transport through an inductively coupled plasma torch: elemental droplet evaporation, J. Anal. At. Spectrom., 2016, 31, 631–641 RSC.
- G. C. Y. Chan and G. M. Hieftje, Local cooling, plasma reheating and thermal pinching induced by single aerosol droplets injected into an inductively coupled plasma, Spectrochim. Acta, Part B, 2016, 121, 55–66 CrossRef CAS.
- I. Kálomista, A. Kéri and G. Galbács, Optimization of plasma sampling depth and aerosol gas flow rates for single particle inductively coupled plasma mass spectrometry analysis, Talanta, 2017, 172, 147–154 CrossRef.
- K. H. Chun, H. Zhang and W. T. Chan, Double-viewing-position single-particle inductively coupled plasma-atomic emission spectrometry for the selection of ICP sampling position in SP-ICP measurements, Anal. Sci., 2018, 34, 711–717 CrossRef CAS.
- H. Niu and R. S. Houk, Fundamental aspects of ion extraction in inductively coupled plasma mass spectrometry, Spectrochim. Acta, Part B, 1996, 51, 779–815 CrossRef.
- S. J. Hill, A. Fisher and M. Liezers, in Inductively Coupled Plasma Mass Spectrometry Handbook, ed. S. M. Nelms, Blackwell Publishing Ltd, Oxford, UK, 2005, pp. 1–25 Search PubMed.
- M. D. Montaño, H. R. Badiei, S. Bazargan and J. F. Ranville, Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times, Environ. Sci.: Nano, 2014, 1, 338–346 RSC.
- T. W. May and R. H. Wiedmeyer, A table of polyatomic interferences in ICP-MS, Atom. Spectrosc., 1998, 19, 150–155 CAS.
- F. Laborda, J. Medrano and J. R. Castillo, Quality of quantitative and semiquantitative results in inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom., 2001, 16, 732–738 RSC.
- A. T. Ince, J. G. Williams and A. L. Gray, Noise in inductively-coupled plasma-mass spectrometry - some preliminary measurements, J. Anal. At. Spectrom., 1993, 8, 899–903 RSC.
- F. Laborda, J. Jiménez-Lamana, E. Bolea and J. R. Castillo, Selective identification, characterization and determination of dissolved silver(I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom., 2011, 26, 1362–1371 RSC.
- J. Liu, K. E. Murphy, R. I. MacCuspie and M. R. Winchester, Capabilities of single particle inductively coupled plasma mass spectrometry for the size measurement of nanoparticles: a case study on gold nanoparticles, Anal. Chem., 2014, 86, 3405–3414 CrossRef CAS PubMed.
- L. A. Rush, M. C. Endres, M. Liezers, J. D. Ward, G. C. Eiden and A. M. Duffin, Collisional dampening for improved quantification in single particle inductively coupled plasma mass spectrometry, Talanta, 2018, 189, 268–273 CrossRef CAS PubMed.
- E. Bolea-Fernandez, D. Leite, A. Rua-Ibarz, L. Balcaen, M. Aramendia, M. Resano and F. Vanhaecke, Characterization of SiO2 nanoparticles by single particle-inductively coupled plasma-tandem mass spectrometry (SP-ICP-MS/MS), J. Anal. At. Spectrom., 2017, 32, 2140–2152 RSC.
- S. Candás-Zapico, D. J. Kutscher, M. Montes-Bayón and J. Bettmer, Single particle analysis of TiO2 in candy products using triple quadrupole ICP-MS, Talanta, 2018, 180, 309–315 CrossRef PubMed.
- M. Tharaud, A. P. Gondikas, M. F. Benedetti, F. von der Kammer, T. Hofmann and G. Cornelis, TiO2 nanomaterial detection in calcium rich matrices by spICPMS. A matter of resolution and treatment, J. Anal. At. Spectrom., 2017, 32, 1400–1411 RSC.
- P. Shaw and A. Donard, Nano-particle analysis using dwell times between 10 µs and 70 µs with an upper counting limit of greater than 3 × 107 cps and a gold nanoparticle detection limit of less than 10 nm diameter, J. Anal. At. Spectrom., 2016, 31, 1234–1242 RSC.
- J. Tuoriniemi, G. Cornelis and M. Hassellöv, A new peak recognition algorithm for detection of ultra-small nano-particles by single particle ICP-MS using rapid time resolved data acquisition on a sector-field mass spectrometer, J. Anal. At. Spectrom., 2015, 30, 1723–1729 RSC.
- J. Tuoriniemi, G. Cornelis and M. Hassellöv, Improving the accuracy of single particle ICPMS for measurement of size distributions and number concentrations of nanoparticles by determining analyte partitioning during nebulisation, J. Anal. At. Spectrom., 2014, 29, 743–752 RSC.
- J. Tuoriniemi, M. D. Jurgens, M. Hassellöv and G. Cornelis, Size dependence of silver nanoparticle removal in a wastewater treatment plant mesocosm measured by FAST single particle ICP-MS, Environ. Sci.: Nano, 2017, 4, 1189–1197 RSC.
- A. J. Managh, D. N. Douglas, K. M. Cowen, H. J. Reid and B. L. Sharp, Acquisition of fast transient signals in ICP-MS with enhanced time resolution, J. Anal. At. Spectrom., 2016, 31, 1688–1692 RSC.
- K. Newman, C. Metcalfe, J. Martin, H. Hintelmann, P. Shaw and A. Donard, Improved single particle ICP-MS characterization of silver nanoparticles at environmentally relevant concentrations, J. Anal. At. Spectrom., 2016, 31, 2069–2077 RSC.
- S. Yongyang, W. Wei, L. Zhiming, D. Hu, Z. Guoqing, X. Jiang and R. Xiangjun, Direct detection and isotope analysis of individual particles in suspension by single particle mode MC-ICP-MS for nuclear safety, J. Anal. At. Spectrom., 2015, 30, 1184–1190 RSC.
- L. Telgmann, C. D. Metcalfe and H. Hintelmann, Rapid size characterization of silver nanoparticles by single particle ICP-MS and isotope dilution, J. Anal. At. Spectrom., 2014, 29, 1265–1272 RSC.
- C. A. Sötebier, D. J. Kutscher, L. Rottmann, N. Jakubowski, U. Panne and J. Bettmer, Combination of single particle ICP-QMS and isotope dilution analysis for the determination of size, particle number and number size distribution of silver nanoparticles, J. Anal. At. Spectrom., 2016, 31, 2045–2052 RSC.
- O. Borovinskaya, B. Hattendorf, M. Tanner, S. Gschwind and D. Günther, A prototype of a new inductively coupled plasma time-of-flight mass spectrometer providing temporally resolved, multi-element detection of short signals generated by single particles and droplets, J. Anal. At. Spectrom., 2013, 28, 226–233 RSC.
- J. Tuoriniemi, G. Cornelis and M. Hassellöv, Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles, Anal. Chem., 2012, 84, 3965–3972 CrossRef CAS.
- S. Lee, X. Bi, R. B. Reed, J. F. Ranville, P. Herckes and P. Westerhoff, Nanoparticle size detection limits by single particle ICP-MS for 40 elements, Environ. Sci. Technol., 2014, 48, 10291–10300 CrossRef CAS.
- O. Borovinskaya, S. Gschwind, B. Hattendorf, M. Tanner and D. Günther, Simultaneous mass quantification of nanoparticles of different composition in a mixture by microdroplet generator-ICPTOFMS, Anal. Chem., 2014, 86, 8142–8148 CrossRef CAS.
- A. Praetorius, A. Gundlach-Graham, E. Goldberg, W. Fabienke, J. Navratilova, A. Gondikas, R. Kaegi, D. Günther, T. Hofmann and F. von der Kammer, Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils, Environ. Sci.: Nano, 2017, 4, 307–314 RSC.
- A. Gondikas, F. von der Kammer, R. Kaegi, O. Borovinskaya, E. Neubauer, J. Navratilova, A. Praetorius, G. Cornelis and T. Hofmann, Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters, Environ. Sci.: Nano, 2018, 5, 313–326 RSC.
- S. Naasz, S. Weigel, O. Borovinskaya, A. Serva, C. Cascio, A. K. Undas, F. C. Simeone, H. J. P. Marvin and R. J. B. Peters, Multi-element analysis of single nanoparticles by ICP-MS using quadrupole and time-of-flight technologies, J. Anal. At. Spectrom., 2018, 33, 835–845 RSC.
- A. Hineman and C. Stephan, Effect of dwell time on single particle inductively coupled plasma mass spectrometry data acquisition quality, J. Anal. At. Spectrom., 2014, 29, 1252–1257 RSC.
- I. Abad-Álvaro, E. Pena-Vázquez, E. Bolea, P. Bermejo-Barrera, J. R. Castillo and F. Laborda, Evaluation of number concentration quantification by single-particle inductively coupled plasma mass spectrometry: microsecond vs. millisecond dwell times, Anal. Bioanal. Chem., 2016, 408, 5089–5097 CrossRef PubMed.
- I. Strenge and C. Engelhard, Capabilities of fast data acquisition with microsecond time resolution in inductively coupled plasma mass spectrometry and identification of signal artifacts from millisecond dwell times during detection of single gold nanoparticles, J. Anal. At. Spectrom., 2016, 31, 135–144 RSC.
- I. Kálomista, A. Kéri, D. Ungor, E. Csapó, I. Dékány, T. Prohaska and G. Galbács, Dimensional characterization of gold nanorods by combining millisecond and microsecond temporal resolution single particle ICP-MS measurements, J. Anal. At. Spectrom., 2017, 32, 2455–2462 RSC.
- A. Kéri, I. Kálomista, D. Ungor, A. Bélteki, E. Csapó, I. Dékány, T. Prohaska and G. Galbács, Determination of the structure and composition of Au-Ag bimetallic spherical nanoparticles using single particle ICP-MS measurements performed with normal and high temporal resolution, Talanta, 2018, 179, 193–199 CrossRef PubMed.
- M. D. Montaño, B. J. Majestic, A. K. Jamting, P. Westerhoff and J. F. Ranville, Methods for the detection and characterization of silica colloids by microsecond spICP-MS, Anal. Chem., 2016, 88, 4733–4741 CrossRef PubMed.
- D. Mozhayeva and C. Engelhard, A quantitative nanoparticle extraction method for microsecond time resolved single-particle ICP-MS data in the presence of a high background, J. Anal. At. Spectrom., 2019 10.1039/c9ja00042a.
- S. Weigel, R. Peters, K. Loeschner, R. Grombe and T. P. J. Linsinger, Results of an interlaboratory method performance study for the size determination and quantification of silver nanoparticles in chicken meat by single-particle inductively coupled plasma mass spectrometry (sp-ICP-MS), Anal. Bioanal. Chem., 2017, 409, 4839–4848 CrossRef CAS.
- T. P. Linsinger, R. Peters and S. Weigel, International interlaboratory study for sizing and quantification of Ag nanoparticles in food simulants by single-particle ICPMS, Anal. Bioanal. Chem., 2014, 406, 3835–3843 CrossRef CAS PubMed.
- A. R. Montoro Bustos, K. P. Purushotham, A. Possolo, N. Farkas, A. E. Vladar, K. E. Murphy and M. R. Winchester, Validation of single particle ICP-MS for routine measurements of nanoparticle size and number size distribution, Anal. Chem., 2018, 90, 14376–14386 CrossRef CAS PubMed.
- R. P. Lamsal, G. Jerkiewicz and D. Beauchemin, Flow injection single particle inductively coupled plasma mass spectrometry: an original simple approach for the characterization of metal-based nanoparticles, Anal. Chem., 2016, 88, 10552–10558 CrossRef CAS.
- C. Toncelli, K. Mylona, M. Tsapakis and S. A. Pergantis, Flow injection with on-line dilution and single particle inductively coupled plasma – mass spectrometry for monitoring silver nanoparticles in seawater and in marine microorganisms, J. Anal. At. Spectrom., 2016, 31, 1430–1439 RSC.
- W. W. Lee and W. T. Chan, Calibration of single-particle inductively coupled plasma-mass spectrometry (SP-ICP-MS), J. Anal. At. Spectrom., 2015, 30, 1245–1254 RSC.
- F. Laborda, J. Jiménez-Lamana, E. Bolea and J. R. Castillo, Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS, J. Anal. At. Spectrom., 2013, 28, 1220–1232 RSC.
- X. Y. Bi, S. Lee, J. F. Ranville, P. Sattigeri, A. Spanias, P. Herckes and P. Westerhoff, Quantitative resolution of nanoparticle sizes using single particle inductively coupled plasma mass spectrometry with the K-means clustering algorithm, J. Anal. At. Spectrom., 2014, 29, 1630–1639 RSC.
- G. Cornelis and M. Hassellöv, A signal deconvolution method to discriminate smaller nanoparticles in single particle ICP-MS, J. Anal. At. Spectrom., 2014, 29, 134–144 RSC.
- A. Gundlach-Graham, L. Hendriks, K. Mehrabi and D. Günther, Monte Carlo simulation of low-count signals in time-of-flight mass spectrometry and its application to single-particle detection, Anal. Chem., 2018, 90, 11847–11855 CrossRef CAS.
- M. Hadioui, C. Peyrot and K. J. Wilkinson, Improvements to single particle ICPMS by the online coupling of ion exchange resins, Anal. Chem., 2014, 86, 4668–4674 CrossRef CAS PubMed.
- D. M. Schwertfeger, J. R. Velicogna, A. H. Jesmer, R. P. Scroggins and J. I. Princz, Single particle-inductively coupled plasma mass spectroscopy analysis of metallic nanoparticles in environmental samples with large dissolved analyte fractions, Anal. Chem., 2016, 88, 9908–9914 CrossRef CAS PubMed.
- S. A. Pergantis, T. L. Jones-Lepp and E. M. Heithmar, Hydrodynamic chromatography online with single particle-inductively coupled plasma mass spectrometry for ultratrace detection of metal-containing nanoparticles, Anal. Chem., 2012, 84, 6454–6462 CrossRef CAS.
- K. A. Huynh, E. Siska, E. Heithmar, S. Tadjiki and S. A. Pergantis, Detection and quantification of silver nanoparticles at environmentally relevant concentrations using asymmetric flow field-flow fractionation online with single particle inductively coupled plasma mass spectrometry, Anal. Chem., 2016, 88, 4909–4916 CrossRef CAS.
- J. Tan, J. Liu, M. Li, H. El Hadri, V. A. Hackley and M. R. Zachariah, Electrospray-differential mobility hyphenated with single particle inductively coupled plasma mass spectrometry for characterization of nanoparticles and their aggregates, Anal. Chem., 2016, 88, 8548–8555 CrossRef CAS PubMed.
- J. Tan, Y. Yang, H. El Hadri, M. Li, V. A. Hackley and M. R. Zachariah, Fast quantification of nanorod geometry by DMA-spICP-MS, Analyst, 2019, 144, 2275–2283 RSC.
- B. Franze, I. Strenge and C. Engelhard, Separation and detection of gold nanoparticles with capillary electrophoresis and ICP-MS in single particle mode (CE-SP-ICP-MS), J. Anal. At. Spectrom., 2017, 32, 1481–1489 RSC.
- D. Mozhayeva and C. Engelhard, Separation of silver nanoparticles with different coatings by capillary electrophoresis coupled to ICP-MS in single particle mode, Anal. Chem., 2017, 89, 9767–9774 CrossRef CAS.
- B. Hetzer, A. Burcza, V. Graf, E. Walz and R. Greiner, Online-coupling of AF4 and single particle-ICP-MS as an analytical approach for the selective detection of nanosilver release from model food packaging films into food simulants, Food Control, 2017, 80, 113–124 CrossRef CAS.
- D. Rakcheev, A. Philippe and G. E. Schaumann, Hydrodynamic chromatography coupled with single particle-inductively coupled plasma mass spectrometry for investigating nanoparticles agglomerates, Anal. Chem., 2013, 85, 10643–10647 CrossRef CAS PubMed.
- K. Proulx, M. Hadioui and K. J. Wilkinson, Separation, detection and characterization of nanomaterials in municipal wastewaters using hydrodynamic chromatography coupled to ICPMS and single particle ICPMS, Anal. Bioanal. Chem., 2016, 408, 5147–5155 CrossRef CAS PubMed.
- Y. Yang, C. L. Long, H. P. Li, Q. Wang and Z. G. Yang, Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry, Sci. Total Environ., 2016, 563–564, 996–1007 CrossRef CAS PubMed.
- M. van der Zande, R. J. Vandebriel, E. Van Doren, E. Kramer, Z. Herrera Rivera, C. S. Serrano-Rojero, E. R. Gremmer, J. Mast, R. J. Peters, P. C. Hollman, P. J. Hendriksen, H. J. Marvin, A. A. Peijnenburg and H. Bouwmeester, Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure, ACS Nano, 2012, 6, 7427–7442 CrossRef CAS PubMed.
- J. G. Coleman, A. J. Kennedy, A. J. Bednar, J. F. Ranville, J. G. Laird, A. R. Harmon, C. A. Hayes, E. P. Gray, C. P. Higgins, G. Lotufo and J. A. Steevens, Comparing the effects of nanosilver size and coating variations on bioavailability, internalization, and elimination, using Lumbriculus variegatus, Environ. Toxicol. Chem., 2013, 32, 2069–2077 CrossRef CAS PubMed.
- E. P. Gray, J. G. Coleman, A. J. Bednar, A. J. Kennedy, J. F. Ranville and C. P. Higgins, Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry, Environ. Sci. Technol., 2013, 47, 14315–14323 CrossRef CAS PubMed.
- L. D. Scanlan, R. B. Reed, A. V. Loguinov, P. Antczak, A. Tagmount, S. Aloni, D. T. Nowinski, P. Luong, C. Tran, N. Karunaratne, D. Pham, X. X. Lin, F. Falciani, C. P. Higgins, J. F. Ranville, C. D. Vulpe and B. Gilbert, Silver nanowire exposure results in internalization and toxicity to Daphnia magna, ACS Nano, 2013, 7, 10681–10694 CrossRef CAS PubMed.
- K. Loeschner, M. S. Brabrand, J. J. Sloth and E. H. Larsen, Use of alkaline or enzymatic sample pretreatment prior to characterization of gold nanoparticles in animal tissue by single-particle ICPMS, Anal. Bioanal. Chem., 2014, 406, 3845–3851 CrossRef CAS.
- R. J. Peters, Z. H. Rivera, G. van Bemmel, H. J. Marvin, S. Weigel and H. Bouwmeester, Development and validation of single particle ICP-MS for sizing and quantitative determination of nano-silver in chicken meat, Anal. Bioanal. Chem., 2014, 406, 3875–3885 CAS.
- R. Tassinari, F. Cubadda, G. Moracci, F. Aureli, M. D'Amato, M. Valeri, B. De Berardis, A. Raggi, A. Mantovani, D. Passeri, M. Rossi and F. Maranghi, Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen, Nanotoxicology, 2014, 8, 654–662 CrossRef CAS PubMed.
- S. Makama, R. Peters, A. Undas and N. W. van den Brink, A novel method for the quantification, characterisation and speciation of silver nanoparticles in earthworms exposed in soil, Environ. Chem., 2015, 12, 643–651 CrossRef CAS.
- R. Grombe, G. Allmaier, J. Charoud-Got, A. Dudkiewicz, H. Emteborg, T. Hofmann, E. H. Larsen, A. Lehner, M. Llinàs, K. Loeschner, K. Mølhave, R. J. Peters, J. Seghers, C. Solans, F. von der Kammer, S. Wagner, S. Weigel and T. P. J. Linsinger, Feasibility of the development of reference materials for the detection of Ag nanoparticles in food: neat dispersions and spiked chicken meat, Accredit. Qual. Assur., 2015, 20, 3–16 CrossRef CAS.
- L. Campagnolo, M. Massimiani, L. Vecchione, D. Piccirilli, N. Toschi, A. Magrini, E. Bonanno, M. Scimeca, L. Castagnozzi, G. Buonanno, L. Stabile, F. Cubadda, F. Aureli, P. H. Fokkens, W. G. Kreyling, F. R. Cassee and A. Pietroiusti, Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus, Nanotoxicology, 2017, 11, 687–698 CrossRef CAS.
- F. Gallocchio, G. Biancotto, V. Cibin, C. Losasso, S. Belluco, R. Peters, G. van Bemmel, C. Cascio, S. Weigel, P. Tromp, F. Gobbo, S. Catania and A. Ricci, Transfer study of silver nanoparticles in poultry production, J. Agric. Food Chem., 2017, 65, 3767–3774 CrossRef CAS PubMed.
- M. E. Johnson, S. K. Hanna, A. R. Montoro Bustos, C. M. Sims, L. C. Elliott, A. Lingayat, A. C. Johnston, B. Nikoobakht, J. T. Elliott, R. D. Holbrook, K. C. Scott, K. E. Murphy, E. J. Petersen, L. L. Yu and B. C. Nelson, Separation, sizing, and quantitation of engineered nanoparticles in an organism model using inductively coupled plasma mass spectrometry and image analysis, ACS Nano, 2017, 11, 526–540 CrossRef CAS PubMed.
- B. Kollander, F. Widemo, E. Agren, E. H. Larsen and K. Loeschner, Detection of lead nanoparticles in game meat by single particle ICP-MS following use of lead-containing bullets, Anal. Bioanal. Chem., 2017, 409, 1877–1885 CrossRef CAS PubMed.
- K. Ramos, L. Ramos and M. M. Gómez-Gómez, Simultaneous characterisation of silver nanoparticles and determination of dissolved silver in chicken meat subjected to in vitro human gastrointestinal digestion using single particle inductively coupled plasma mass spectrometry, Food Chem., 2017, 221, 822–828 CrossRef CAS PubMed.
- Q. Li, Z. Wang, J. Mo, G. Zhang, Y. Chen and C. Huang, Imaging gold nanoparticles in mouse liver by laser ablation inductively coupled plasma mass spectrometry, Sci. Rep., 2017, 7, 2965 CrossRef.
- J. Modrzynska, T. Berthing, G. Ravn-Haren, K. Kling, A. Mortensen, R. R. Rasmussen, E. H. Larsen, A. T. Saber, U. Vogel and K. Loeschner, In vivo-induced size transformation of cerium oxide nanoparticles in both lung and liver does not affect long-term hepatic accumulation following pulmonary exposure, PLoS One, 2018, 13, e0202477 CrossRef PubMed.
- H. K. Sung, E. Jo, E. Kim, S. K. Yoo, J. W. Lee, P. J. Kim, Y. Kim and I. C. Eom, Analysis of gold and silver nanoparticles internalized by zebrafish (Danio rerio) using single particle-inductively coupled plasma-mass spectrometry, Chemosphere, 2018, 209, 815–822 CrossRef CAS.
- M. V. Taboada-López, S. Iglesias-López, P. Herbello-Hermelo, P. Bermejo-Barrera and A. Moreda-Piñeiro, Ultrasound assisted enzymatic hydrolysis for isolating titanium dioxide nanoparticles from bivalve mollusk before sp-ICP-MS, Anal. Chim. Acta, 2018, 1018, 16–25 CrossRef PubMed.
- F. Abdolahpur Monikh, L. Chupani, E. Zuskova, R. Peters, M. Vancová, M. G. Vijver, P. Porcal and W. J. Peijnenburg, Method for extraction and quantification of metal-based nanoparticles in biological media: number-based biodistribution and bioconcentration, Environ. Sci. Technol., 2019, 53, 946–953 CrossRef CAS PubMed.
- S. Hu, R. Liu, S. Zhang, Z. Huang, Z. Xing and X. Zhang, A new strategy for highly sensitive immunoassay based on single-particle mode detection by inductively coupled plasma mass spectrometry, J. Am. Soc. Mass Spectrom., 2009, 20, 1096–1103 CrossRef CAS PubMed.
- S. Zhang, G. Han, Z. Xing, S. Zhang and X. Zhang, Multiplex DNA assay based on nanoparticle probes by single particle inductively coupled plasma mass spectrometry, Anal. Chem., 2014, 86, 3541–3547 CrossRef CAS.
- H. Klingberg, L. B. Oddershede, K. Loeschner, E. H. Larsen, S. Loft and P. Møller, Uptake of gold nanoparticles in primary human endothelial cells, Toxicol. Res., 2015, 4, 655–666 RSC.
- I. L. Hsiao, F. S. Bierkandt, P. Reichardt, A. Luch, Y. J. Huang, N. Jakubowski, J. Tentschert and A. Haase, Quantification and visualization of cellular uptake of TiO2 and Ag nanoparticles: comparison of different ICP-MS techniques, J. Nanobiotechnol., 2016, 14, 50 CrossRef.
- A. Malysheva, N. Voelcker, P. E. Holm and E. Lombi, Unraveling the complex behavior of AgNPs driving NP-cell interactions and toxicity to algal cells, Environ. Sci. Technol., 2016, 50, 12455–12463 CrossRef CAS.
- B. Krause, T. Meyer, H. Sieg, C. Kästner, P. Reichardt, J. Tentschert, H. Jungnickel, I. Estrela-Lopis, A. Burel, S. Chevance, F. Gauffre, P. Jalili, J. Meijer, L. Böhmert, A. Braeuning, A. F. Thünemann, F. Emmerling, V. Fessard, P. Laux, A. Lampen and A. Luch, Characterization of aluminum, aluminum oxide and titanium dioxide nanomaterials using a combination of methods for particle surface and size analysis, RSC Adv., 2018, 8, 14377–14388 RSC.
- I. Aharchaou, J. S. Py, S. Cambier, J. L. Loizeau, G. Cornelis, P. Rousselle, E. Battaglia and D. A. L. Vignati, Chromium hazard and risk assessment: New insights from a detailed speciation study in a standard test medium, Environ. Toxicol. Chem., 2018, 37, 983–992 CrossRef CAS PubMed.
- X. Xu, J. Chen, B. Li, L. Tang and J. Jiang, Single particle ICP-MS-based absolute and relative quantification of E. coli O157 16S rRNA using sandwich hybridization capture, Analyst, 2019, 144, 1725–1730 RSC.
- A. P. Walczak, R. Fokkink, R. Peters, P. Tromp, Z. E. Herrera Rivera, I. M. Rietjens, P. J. Hendriksen and H. Bouwmeester, Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model, Nanotoxicology, 2013, 7, 1198–1210 CrossRef CAS.
- S. V. Jenkins, H. Qu, T. Mudalige, T. M. Ingle, R. Wang, F. Wang, P. C. Howard, J. Chen and Y. Zhang, Rapid determination of plasmonic nanoparticle agglomeration status in blood, Biomaterials, 2015, 51, 226–237 CrossRef CAS PubMed.
- E. Leese, J. F. Staff, V. A. Carolan and J. Morton, Exhaled breath condensate: a novel matrix for biological monitoring to assess occupational exposure to respirable crystalline silica, Ann. Work Exposures Health, 2017, 61, 902–906 CrossRef CAS PubMed.
- M. Witzler, F. Küllmer and K. Günther, Validating a single-particle ICP-MS method to measure nanoparticles in human whole blood for nanotoxicology, Anal. Lett., 2017, 51, 587–599 CrossRef.
- J. Vidmar, T. Buerki-Thurnherr and K. Loeschner, Comparison of the suitability of alkaline or enzymatic sample pre-treatment for characterization of silver nanoparticles in human tissue by single particle ICP-MS, J. Anal. At. Spectrom., 2018, 33, 752–761 RSC.
- J. Vidmar, K. Loeschner, M. Correia, E. H. Larsen, P. Manser, A. Wichser, K. Boodhia, Z. S. Al-Ahmady, J. Ruiz, D. Astruc and T. Buerki-Thurnherr, Translocation of silver nanoparticles in the ex vivo human placenta perfusion model characterized by single particle ICP-MS, Nanoscale, 2018, 10, 11980–11991 RSC.
- M. Logozzi, D. Mizzoni, B. Bocca, R. Di Raimo, F. Petrucci, S. Caimi, A. Alimonti, M. Falchi, F. Cappello, C. Campanella, C. C. Bavisotto, S. David, F. Bucchieri, D. F. Angelini, L. Battistini and S. Fais, Human primary macrophages scavenge AuNPs and eliminate it through exosomes. A natural shuttling for nanomaterials, Eur. J. Pharm. Biopharm., 2019, 137, 23–36 CrossRef CAS.
- R. B. Reed, D. G. Goodwin, K. L. Marsh, S. S. Capracotta, C. P. Higgins, D. H. Fairbrother and J. F. Ranville, Detection of single walled carbon nanotubes by monitoring embedded metals, Environ. Sci.: Processes Impacts, 2013, 15, 204–213 RSC.
- J. J. Wang, R. S. Lankone, R. B. Reed, D. H. Fairbrother and J. F. Ranville, Analysis of single-walled carbon nanotubes using spICP-MS with microsecond dwell time, NanoImpact, 2016, 1, 65–72 CrossRef.
- R. S. Lankone, J. Wang, J. F. Ranville and D. H. Fairbrother, Photodegradation of polymer-CNT nanocomposites: effect of CNT loading and CNT release characteristics, Environ. Sci.: Nano, 2017, 4, 967–982 RSC.
- Y. Dan, H. Shi, C. Stephan and X. Liang, Rapid analysis of titanium dioxide nanoparticles in sunscreens using single particle inductively coupled plasma–mass spectrometry, Microchem. J., 2015, 122, 119–126 CrossRef CAS.
- I. de la Calle, M. Menta, M. Klein and F. Seby, Screening of TiO2 and Au nanoparticles in cosmetics and determination of elemental impurities by multiple techniques (DLS, SP-ICP-MS, ICP-MS and ICP-OES), Talanta, 2017, 171, 291–306 CrossRef CAS.
- B. Bocca, S. Caimi, O. Senofonte, A. Alimonti and F. Petrucci, ICP-MS based methods to characterize nanoparticles of TiO2 and ZnO in sunscreens with focus on regulatory and safety issues, Sci. Total Environ., 2018, 630, 922–930 CrossRef CAS PubMed.
- I. de la Calle, M. Menta, M. Klein, B. Maxit and F. Séby, Towards routine analysis of TiO2 (nano-)particle size in consumer products: Evaluation of potential techniques, Spectrochim. Acta, Part B, 2018, 147, 28–42 CrossRef CAS.
- P. J. Lu, S. W. Fang, W. L. Cheng, S. C. Huang, M. C. Huang and H. F. Cheng, Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods, J. Food Drug Anal., 2018, 26, 1192–1200 CrossRef CAS PubMed.
- M. Correia, T. Uusimäki, A. Philippe and K. Loeschner, Challenges in determining the size distribution of nanoparticles in consumer products by asymmetric flow field-flow fractionation coupled to inductively coupled plasma-mass spectrometry: the example of Al2O3, TiO2, and SiO2 nanoparticles in toothpaste, Separations, 2018, 5, 56 CrossRef CAS.
- Y. S. Zimmermann, A. Schaffer, P. F. Corvini and M. Lenz, Thin-film photovoltaic cells: long-term metal(loid) leaching at their end-of-life, Environ. Sci. Technol., 2013, 47, 13151–13159 CrossRef CAS PubMed.
- L. M. Furtado, M. E. Hoque, D. F. Mitrano, J. F. Ranville, B. Cheever, P. C. Frost, M. A. Xenopoulos, H. Hintelmann and C. D. Metcalfe, The persistence and transformation of silver nanoparticles in littoral lake mesocosms monitored using various analytical techniques, Environ. Chem., 2014, 11, 419–430 CrossRef CAS.
- D. M. Mitrano, J. F. Ranville, A. Bednar, K. Kazor, A. S. Hering and C. P. Higgins, Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS), Environ. Sci.: Nano, 2014, 1, 248–259 RSC.
- A. R. Donovan, C. D. Adams, Y. Ma, C. Stephan, T. Eichholz and H. Shi, Detection of zinc oxide and cerium dioxide nanoparticles during drinking water treatment by rapid single particle ICP-MS methods, Anal. Bioanal. Chem., 2016, 408, 5137–5145 CrossRef CAS PubMed.
- A. R. Donovan, C. D. Adams, Y. Ma, C. Stephan, T. Eichholz and H. Shi, Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment, Chemosphere, 2016, 144, 148–153 CrossRef CAS PubMed.
- M. Azodi, Y. Sultan and S. Ghoshal, Dissolution behavior of silver nanoparticles and formation of secondary silver nanoparticles in municipal wastewater by single-particle ICP-MS, Environ. Sci. Technol., 2016, 50, 13318–13327 CrossRef CAS PubMed.
- J. Jiménez-Lamana and V. I. Slaveykova, Silver nanoparticle behaviour in lake water depends on their surface coating, Sci. Total Environ., 2016, 573, 946–953 CrossRef.
- C.-l. Long, Z.-g. Yang, Y. Yang, H.-p. Li and Q. Wang, Determination of gold nanoparticles in natural water using single particle-ICP-MS, J. Cent. South Univ., 2016, 23, 1611–1617 CrossRef CAS.
- J. M. Pettibone and J. Liu, In situ methods for monitoring silver nanoparticle sulfidation in simulated waters, Environ. Sci. Technol., 2016, 50, 11145–11153 CrossRef CAS.
- L. Telgmann, M. T. Nguyen, L. Shen, V. Yargeau, H. Hintelmann and C. D. Metcalfe, Single particle ICP-MS as a tool for determining the stability of silver nanoparticles in aquatic matrixes under various environmental conditions, including treatment by ozonation, Anal. Bioanal. Chem., 2016, 408, 5169–5177 CrossRef CAS.
- A. Azimzada, N. Tufenkji and K. J. Wilkinson, Transformations of silver nanoparticles in wastewater effluents: links to Ag bioavailability, Environ. Sci.: Nano, 2017, 4, 1339–1349 RSC.
- R. Aznar, F. Barahona, O. Geiss, J. Ponti, T. Jose Luis and J. Barrero-Moreno, Quantification and size characterisation of silver nanoparticles in environmental aqueous samples and consumer products by single particle-ICPMS, Talanta, 2017, 175, 200–208 CrossRef CAS PubMed.
- Y. J. Chang, Y. H. Shih, C. H. Su and H. C. Ho, Comparison of three analytical methods to measure the size of silver nanoparticles in real environmental water and wastewater samples, J. Hazard. Mater., 2017, 322, 95–104 CrossRef CAS.
- N. Londono, A. R. Donovan, H. Shi, M. Geisler and Y. Liang, Impact of TiO2 and ZnO nanoparticles on an aquatic microbial community: effect at environmentally relevant concentrations, Nanotoxicology, 2017, 11, 1140–1156 CrossRef CAS PubMed.
- J. D. Martin, L. Telgmann and C. D. Metcalfe, A method for preparing silver nanoparticle suspensions in bulk for ecotoxicity testing and ecological risk assessment, Bull. Environ. Contam. Toxicol., 2017, 98, 589–594 CrossRef CAS PubMed.
- R. C. Merrifield, C. Stephan and J. Lead, Determining the concentration dependent transformations of Ag nanoparticles in complex media: using SP-ICP-MS and Au@Ag core-shell nanoparticles as tracers, Environ. Sci. Technol., 2017, 51, 3206–3213 CrossRef CAS.
- T. Théoret and K. J. Wilkinson, Evaluation of enhanced darkfield microscopy and hyperspectral analysis to analyse the fate of silver nanoparticles in wastewaters, Anal. Methods, 2017, 9, 3920–3928 RSC.
- C. Toncelli, K. Mylona, I. Kalantzi, A. Tsiola, P. Pitta, M. Tsapakis and S. A. Pergantis, Silver nanoparticles in seawater: A dynamic mass balance at part per trillion silver concentrations, Sci. Total Environ., 2017, 601–602, 15–21 CrossRef CAS.
- L. Degenkolb, G. Metreveli, A. Philippe, A. Brandt, K. Leopold, L. Zehlike, H. J. Vogel, G. E. Schaumann, T. Baumann, M. Kaupenjohann, F. Lang, S. Kumahor and S. Klitzke, Retention and remobilization mechanisms of environmentally aged silver nanoparticles in an artificial riverbank filtration system, Sci. Total Environ., 2018, 645, 192–204 CrossRef CAS PubMed.
- A. R. Donovan, C. D. Adams, Y. Ma, C. Stephan, T. Eichholz and H. Shi, Fate of nanoparticles during alum and ferric coagulation monitored using single particle ICP-MS, Chemosphere, 2018, 195, 531–541 CrossRef CAS.
- A. Georgantzopoulou, P. Almeida Carvalho, C. Vogelsang, M. Tilahun, K. Ndungu, A. M. Booth, K. V. Thomas and A. Macken, Ecotoxicological effects of transformed silver and titanium dioxide nanoparticles in the effluent from a lab-scale wastewater treatment system, Environ. Sci. Technol., 2018, 52, 9431–9441 CrossRef CAS.
- L. Luo, Y. Yang, H. Li, R. Ding, Q. Wang and Z. Yang, Size characterization of silver nanoparticles after separation from silver ions in environmental water using magnetic reduced graphene oxide, Sci. Total Environ., 2018, 612, 1215–1222 CrossRef CAS PubMed.
- J. D. Martin, P. C. Frost, H. Hintelmann, K. Newman, M. J. Paterson, L. Hayhurst, M. D. Rennie, M. A. Xenopoulos, V. Yargeau and C. D. Metcalfe, Accumulation of silver in yellow perch (Perca flavescens) and northern pike (Esox lucius) From a lake dosed with nanosilver, Environ. Sci. Technol., 2018, 52, 11114–11122 CrossRef CAS PubMed.
- D. C. Rearick, L. Telgmann, H. Hintelmann, P. C. Frost and M. A. Xenopoulos, Spatial and temporal trends in the fate of silver nanoparticles in a whole-lake addition study, PLoS One, 2018, 13, e0201412 CrossRef PubMed.
- J. Vidmar, P. Oprčkal, R. Milačič, A. Mladenovič and J. Ščančar, Investigation of the behaviour of zero-valent iron nanoparticles and their interactions with Cd(2+) in wastewater by single particle ICP-MS, Sci. Total Environ., 2018, 634, 1259–1268 CrossRef CAS PubMed.
- J. J. López-Mayán, B. Cerneira-Temperán, E. Peña-Vázquez, M. C. Barciela-Alonso, M. R. Domínguez-González and P. Bermejo-Barrera, Evaluation of a cloud point extraction method for the preconcentration and quantification of silver nanoparticles in water samples by ETAAS, Int. J. Environ. Anal. Chem., 2019, 98, 1434–1447 CrossRef.
- A. P. Gondikas, F. von der Kammer, R. B. Reed, S. Wagner, J. F. Ranville and T. Hofmann, Release of TiO2 nanoparticles from sunscreens into surface waters: a one-year survey at the old Danube recreational Lake, Environ. Sci. Technol., 2014, 48, 5415–5422 CrossRef CAS PubMed.
- M. Hadioui, V. Merdzan and K. J. Wilkinson, Detection and characterization of ZnO nanoparticles in surface and waste waters using single particle ICPMS, Environ. Sci. Technol., 2015, 49, 6141–6148 CrossRef CAS PubMed.
- L. Li, M. Stoiber, A. Wimmer, Z. Xu, C. Lindenblatt, B. Helmreich and M. Schuster, To what extent can full-scale wastewater treatment plant effluent influence the occurrence of silver-based nanoparticles in surface waters?, Environ. Sci. Technol., 2016, 50, 6327–6333 CrossRef CAS PubMed.
- S. P. Bitragunta, S. G. Palani, A. Gopala, S. K. Sarkar and V. R. Kandukuri, Detection of TiO2 nanoparticles in municipal sewage treatment plant and their characterization using single particle ICP-MS, Bull. Environ. Contam. Toxicol., 2017, 98, 595–600 CrossRef CAS PubMed.
- R. B. Reed, D. P. Martin, A. J. Bednar, M. D. Montaño, P. Westerhoff and J. F. Ranville, Multi-day diurnal measurements of Ti-containing nanoparticle and organic sunscreen chemical release during recreational use of a natural surface water, Environ. Sci.: Nano, 2017, 4, 69–77 RSC.
- M. Loula, A. Kaňa, R. Koplík, J. Hanuš, M. Vosmanská and O. Mestek, Analysis of silver nanoparticles using single-particle inductively coupled plasma – mass spectrometry (ICP-MS): parameters affecting the quality of results, Anal. Lett., 2018, 52, 288–307 CrossRef.
- C. D. Metcalfe, T. Sultana, J. Martin, K. Newman, P. Helm, S. Kleywegt, L. Shen and V. Yargeau, Silver near municipal wastewater discharges into western Lake Ontario, Canada, Environ. Monit. Assess., 2018, 190, 555 CrossRef PubMed.
- R. J. B. Peters, G. van Bemmel, N. B. L. Milani, G. C. T. den Hertog, A. K. Undas, M. van der Lee and H. Bouwmeester, Detection of nanoparticles in Dutch surface waters, Sci. Total Environ., 2018, 621, 210–218 CrossRef CAS PubMed.
- A. K. Venkatesan, R. B. Reed, S. Lee, X. Bi, D. Hanigan, Y. Yang, J. F. Ranville, P. Herckes and P. Westerhoff, Detection and sizing of Ti-containing particles in recreational waters using single particle ICP-MS, Bull. Environ. Contam. Toxicol., 2018, 100, 120–126 CrossRef CAS PubMed.
- A. Wimmer, A. Kalinnik and M. Schuster, New insights into the formation of silver-based nanoparticles under natural and semi-natural conditions, Water Res., 2018, 141, 227–234 CrossRef CAS PubMed.
- A. K. Venkatesan, B. T. Rodríguez, A. R. Marcotte, X. Bi, J. Schoepf, J. F. Ranville, P. Herckes and P. Westerhoff, Using single-particle ICP-MS for monitoring metal-containing particles in tap water, Environ. Sci.: Water Res. Technol., 2018, 4, 1923–1932 RSC.
- F. Loosli, J. Wang, S. Rothenberg, M. Bizimis, C. Winkler, O. Borovinskaya, L. Flamigni and M. Baalousha, Sewage spills are a major source of titanium dioxide engineered (nano)-particle release into the environment, Environ. Sci.: Nano, 2019, 6, 763–777 RSC.
- R. J. B. Peters, Z. H. Rivera, H. Bouwmeester, S. Weigel and H. J. P. Marvin, Advanced analytical techniques for the measurement of nanomaterials in complex samples: a comparison, Qual. Assur. Saf. Crops, 2014, 6, 281–290 CrossRef CAS.
- S. Addo Ntim, T. A. Thomas and G. O. Noonan, Influence of aqueous food simulants on potential nanoparticle detection in migration studies involving nanoenabled food-contact substances, Food Addit. Contam., Part A, 2016, 33, 905–912 CrossRef CAS.
- M. Witzler, F. Kullmer, A. Hirtz and K. Günther, Validation of gold and silver nanoparticle analysis in fruit juices by single-particle ICP-MS without sample pretreatment, J. Agric. Food Chem., 2016, 64, 4165–4170 CrossRef CAS PubMed.
- M. Jokar, M. Correia and K. Loeschner, Behavior of silver nanoparticles and ions in food simulants and low fat cow milk under migration conditions, Food Control, 2018, 89, 77–85 CrossRef CAS.
- R. J. Peters, G. van Bemmel, Z. Herrera-Rivera, H. P. Helsper, H. J. Marvin, S. Weigel, P. C. Tromp, A. G. Oomen, A. G. Rietveld and H. Bouwmeester, Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles, J. Agric. Food Chem., 2014, 62, 6285–6293 CrossRef CAS PubMed.
- E. Verleysen, E. Van Doren, N. Waegeneers, P. J. De Temmerman, M. Abi Daoud Francisco and J. Mast, TEM and SP-ICP-MS analysis of the release of silver nanoparticles from decoration of pastry, J. Agric. Food Chem., 2015, 63, 3570–3578 CrossRef CAS PubMed.
- I. de la Calle, M. Menta, M. Klein and F. Seby, Study of the presence of micro- and nanoparticles in drinks and foods by multiple analytical techniques, Food Chem., 2018, 266, 133–145 CrossRef CAS PubMed.
- K. Loeschner, M. Correia, C. Lopez Chaves, I. Rokkjaer and J. J. Sloth, Detection and characterisation of aluminium-containing nanoparticles in Chinese noodles by single particle ICP-MS, Food Addit. Contam., Part A, 2018, 35, 86–93 CrossRef CAS.
- M. V. Taboada-López, P. Herbello-Hermelo, R. Domínguez-González, P. Bermejo-Barrera and A. Moreda-Piñeiro, Enzymatic hydrolysis as a sample pre-treatment for titanium dioxide nanoparticles assessment in surimi (crab sticks) by single particle ICP-MS, Talanta, 2019, 195, 23–32 CrossRef.
- Y. Dan, W. Zhang, R. Xue, X. Ma, C. Stephan and H. Shi, Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma-mass spectrometry analysis, Environ. Sci. Technol., 2015, 49, 3007–3014 CrossRef CAS.
- D. Bao, Z. G. Oh and Z. Chen, Characterization of silver nanoparticles internalized by arabidopsis plants using single particle ICP-MS analysis, Front. Plant Sci., 2016, 7, 32 Search PubMed.
- Y. Dan, X. Ma, W. Zhang, K. Liu, C. Stephan and H. Shi, Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO2 nanoparticles, Anal. Bioanal. Chem., 2016, 408, 5157–5167 CrossRef CAS.
- J. Jiménez-Lamana, J. Wojcieszek, M. Jakubiak, M. Asztemborska and J. Szpunar, Single particle ICP-MS characterization of platinum nanoparticles uptake and bioaccumulation by Lepidium sativum and Sinapis alba plants, J. Anal. At. Spectrom., 2016, 31, 2321–2329 RSC.
- I. D. la Calle, P. Pérez-Rodríguez, D. Soto-Gómez and J. E. López-Periago, Detection and characterization of Cu-bearing particles in throughfall samples from vine leaves by DLS, AF4-MALLS (-ICP-MS) and SP-ICP-MS, Microchem. J., 2017, 133, 293–301 CrossRef.
- C. C. Li, F. Dang, M. Li, M. Zhu, H. Zhong, H. Hintelmann and D. M. Zhou, Effects of exposure pathways on the accumulation and phytotoxicity of silver nanoparticles in soybean and rice, Nanotoxicology, 2017, 11, 699–709 CrossRef CAS PubMed.
- P. Wang, E. Lombi, S. Sun, K. G. Scheckel, A. Malysheva, B. A. McKenna, N. W. Menzies, F.-J. Zhao and P. M. Kopittke, Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants, Environ. Sci.: Nano, 2017, 4, 448–460 RSC.
- A. A. Keller, Y. Huang and J. Nelson, Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS, J. Nanoparticle Res., 2018, 20 CrossRef CAS.
- K. Kinska, J. Jiménez-Lamana, J. Kowalska, B. Krasnodebska-Ostrega and J. Szpunar, Study of the uptake and bioaccumulation of palladium nanoparticles by Sinapis alba using single particle ICP-MS, Sci. Total Environ., 2018, 615, 1078–1085 CrossRef CAS PubMed.
- S. S. D. Kumar, N. N. Houreld, E. M. Kroukamp and H. Abrahamse, Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria, J. Photochem. Photobiol. B, 2018, 178, 259–269 CrossRef CAS PubMed.
- J. Nath, I. Dror, P. Landa, T. Vanek, I. Kaplan-Ashiri and B. Berkowitz, Synthesis and characterization of isotopically-labeled silver, copper and zinc oxide nanoparticles for tracing studies in plants, Environ. Pollut., 2018, 242, 1827–1837 CrossRef CAS PubMed.
- H. Zhang, Y. Huang, J. Gu, A. Keller, Y. Qin, Y. Bian, K. Tang, X. Qu, R. Ji and L. Zhao, Single particle ICP-MS and GC-MS provide a new insight into the formation mechanisms during the green synthesis of AgNPs, New J. Chem., 2019, 43, 3946–3955 RSC.
- J. Wojcieszek, J. Jiménez-Lamana, K. Bierla, M. Asztemborska, L. Ruzik, M. Jarosz and J. Szpunar, Elucidation of the fate of zinc in model plants using single particle ICP-MS and ESI tandem MS, J. Anal. At. Spectrom., 2019, 34, 683–693 RSC.
- Y. Yang, X. Bi, P. Westerhoff, K. Hristovski and J. E. McLain, Engineered nanomaterials impact biological carbon conversion in soils, Environ. Eng. Sci., 2014, 31, 381–392 CrossRef CAS.
- J. Navratilova, A. Praetorius, A. Gondikas, W. Fabienke, F. von der Kammer and T. Hofmann, Detection of engineered copper nanoparticles in soil using single particle ICP-MS, Int. J. Environ. Res. Public Health, 2015, 12, 15756–15768 CrossRef CAS PubMed.
- H. E. Hadri and V. A. Hackley, Investigation of cloud point extraction for the analysis of metallic nanoparticles in a soil matrix, Environ. Sci.: Nano, 2017, 4, 105–116 RSC.
- A. H. Jesmer, J. R. Velicogna, D. M. Schwertfeger, R. P. Scroggins and J. I. Princz, The toxicity of silver to soil organisms exposed to silver nanoparticles and silver nitrate in biosolids-amended field soil, Environ. Toxicol. Chem., 2017, 36, 2756–2765 CrossRef CAS PubMed.
- D. Schwertfeger, J. Velicogna, A. Jesmer, H. McShane, R. Scroggins and J. Princz, Ion exchange technique (IET) to characterise Ag+ exposure in soil extracts contaminated with engineered silver nanoparticles, Environ. Chem., 2017, 14, 123–133 CrossRef CAS.
- D. M. Schwertfeger, J. R. Velicogna, A. H. Jesmer, S. Saatcioglu, H. McShane, R. P. Scroggins and J. I. Princz, Extracting metallic nanoparticles from soils for quantitative analysis: method development using engineered silver nanoparticles and SP-ICP-MS, Anal. Chem., 2017, 89, 2505–2513 CrossRef CAS PubMed.
- H. El Hadri, S. M. Louie and V. A. Hackley, Assessing the interactions of metal nanoparticles in soil and sediment matrices – a quantitative analytical multi-technique approach, Environ. Sci.: Nano, 2018, 5, 203–214 RSC.
- L. Torrent, M. Iglesias, M. Hidalgo and E. Marguí, Determination of silver nanoparticles in complex aqueous matrices by total reflection X-ray fluorescence spectrometry combined with cloud point extraction, J. Anal. At. Spectrom., 2018, 33, 383–394 RSC.
- M. A. Gomez-Gonzalez, E. Bolea, P. A. O'Day, J. Garcia-Guinea, F. Garrido and F. Laborda, Combining single-particle inductively coupled plasma mass spectrometry and X-ray absorption spectroscopy to evaluate the release of colloidal arsenic from environmental samples, Anal. Bioanal. Chem., 2016, 408, 5125–5135 CrossRef CAS.
- F. Tou, Y. Yang, J. Feng, Z. Niu, H. Pan, Y. Qin, X. Guo, X. Meng, M. Liu and M. F. Hochella, Environmental risk implications of metals in sludges from waste water treatment plants: the discovery of vast stores of metal-containing nanoparticles, Environ. Sci. Technol., 2017, 51, 4831–4840 CrossRef CAS.
- K. Folens, T. Van Acker, E. Bolea-Fernandez, G. Cornelis, F. Vanhaecke, G. Du Laing and S. Rauch, Identification of platinum nanoparticles in road dust leachate by single particle inductively coupled plasma-mass spectrometry, Sci. Total Environ., 2018, 615, 849–856 CrossRef CAS PubMed.
- Z. Li, M. Hadioui and K. J. Wilkinson, Conditions affecting the release of thorium and uranium from the tailings of a niobium mine, Environ. Pollut., 2019, 247, 206–215 CrossRef CAS PubMed.
- M. Hadioui, S. Leclerc and K. J. Wilkinson, Multimethod quantification of Ag+ release from nanosilver, Talanta, 2013, 105, 15–19 CrossRef CAS PubMed.
- D. M. Mitrano, Y. Arroyo Rojas Dasilva and B. Nowack, Effect of variations of washing solution chemistry on nanomaterial physicochemical changes in the laundry cycle, Environ. Sci. Technol., 2015, 49, 9665–9673 CrossRef CAS.
- D. Paunescu, C. A. Mora, L. Querci, R. Heckel, M. Puddu, B. Hattendorf, D. Günther and R. N. Grass, Detecting and number counting of single engineered nanoparticles by digital particle polymerase chain reaction, ACS Nano, 2015, 9, 9564–9572 CrossRef CAS.
- H. A. Kim, B. T. Lee, S. Y. Na, K. W. Kim, J. F. Ranville, S. O. Kim, E. Jo and I. C. Eom, Characterization of silver nanoparticle aggregates using single particle-inductively coupled plasma-mass spectrometry (spICP-MS), Chemosphere, 2017, 171, 468–475 CrossRef CAS.
- A. S. Adeleye, E. A. Oranu, M. Tao and A. A. Keller, Release and detection of nanosized copper from a commercial antifouling paint, Water Res., 2016, 102, 374–382 CrossRef CAS.
- A. Mackevica, M. E. Olsson and S. F. Hansen, Silver nanoparticle release from commercially available plastic food containers into food simulants, J. Nanoparticle Res., 2016, 18, 5 CrossRef.
- K. Ramos, M. M. Gómez-Gómez, C. Cámara and L. Ramos, Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICPMS, Talanta, 2016, 151, 83–90 CrossRef CAS PubMed.
- D. P. Martin, N. L. Melby, S. M. Jordan, A. J. Bednar, A. J. Kennedy, M. E. Negrete, M. A. Chappell and A. R. Poda, Nanosilver conductive ink: A case study for evaluating the potential risk of nanotechnology under hypothetical use scenarios, Chemosphere, 2016, 162, 222–227 CrossRef CAS PubMed.
- I. Abad-Alvaro, E. Bolea, F. Laborda and J. R. Castillo, An ICP-MS-based platform for release studies on silver-based nanomaterials, J. Anal. At. Spectrom., 2017, 32, 1101–1108 RSC.
- A. Mackevica, M. E. Olsson and S. F. Hansen, The release of silver nanoparticles from commercial toothbrushes, J. Hazard. Mater., 2017, 322, 270–275 CrossRef CAS PubMed.
- A. Mackevica, M. E. Olsson and S. F. Hansen, Quantitative characterization of TiO2 nanoparticle release from textiles by conventional and single particle ICP-MS, J. Nanoparticle Res., 2017, 20 Search PubMed.
- N. Neubauer, L. Scifo, J. Navratilova, A. Gondikas, A. Mackevica, D. Borschneck, P. Chaurand, V. Vidal, J. Rose, F. von der Kammer and W. Wohlleben, Nanoscale coloristic pigments: upper limits on releases from pigmented plastic during environmental aging, in food contact, and by leaching, Environ. Sci. Technol., 2017, 51, 11669–11680 CrossRef CAS PubMed.
- M. C. Sportelli, R. A. Picca, F. Paladini, A. Mangone, L. C. Giannossa, C. D. Franco, A. L. Gallo, A. Valentini, A. Sannino, M. Pollini and N. Cioffi, Spectroscopic characterization and nanosafety of Ag-modified antibacterial leather and leatherette, Nanomaterials, 2017, 7, E203 CrossRef PubMed.
- B. Bocca, E. Sabbioni, I. Mičetić, A. Alimonti and F. Petrucci, Size and metal composition characterization of nano- and microparticles in tattoo inks by a combination of analytical techniques, J. Anal. At. Spectrom., 2017, 32, 616–628 RSC.
- J. Nelson, M. Yamanaka, F. Lopez-Linares, L. Poirier and E. Rogel, Characterization of dissolved metals and metallic nanoparticles in asphaltene solutions by single-particle inductively coupled plasma mass spectrometry, Energy Fuels, 2017, 31, 11971–11976 CrossRef CAS.
- A. Sápi, A. Kéri, I. Kálomista, D. G. Dobó, Á. S. Ákos Szamosvölgyi, K. L. Juhász, Á. K. Ákos Kukovecz, Z. Kónya and G. Galbács, Determination of the platinum concentration of a Pt/silica nanocomposite decorated with ultra small Pt nanoparticles using single particle inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom., 2017, 32, 996–1003 RSC.
- S. Addo Ntim, S. Norris, K. Scott, T. A. Thomas and G. O. Noonan, Consumer use effects on nanoparticle release from commercially available ceramic cookware, Food Control, 2018, 87, 31–39 CrossRef CAS.
- J. Therkorn, L. Calderon, B. Cartledge, N. Thomas, B. Majestic and G. Mainelis, Inactivation of pure bacterial biofilms by impaction of aerosolized consumer products containing nanoparticulate metals, Environ. Sci.: Nano, 2018, 5, 544–555 RSC.
- R. D. Heringer and J. F. Ranville, Gunshot residue (GSR) analysis by single particle inductively coupled plasma mass spectrometry (spICP-MS), Forensic Sci. Int., 2018, 288, e20–e25 CrossRef CAS PubMed.
- J.-R. Huang, P. Li, J.-H. Wen, X. Hu, Y.-J. Chen, D.-H. Yin and H.-Z. Lian, Determination of arsenic species in mainstream cigarette smoke based on inductively coupled plasma mass spectrometry, Spectrosc. Lett., 2018, 51, 252–256 CrossRef CAS.
- J. Jiménez-Lamana, I. Abad-Álvaro, K. Bierla, F. Laborda, J. Szpunar and R. Lobinski, Detection and characterization of biogenic selenium nanoparticles in selenium-rich yeast by single particle ICPMS, J. Anal. At. Spectrom., 2018, 33, 452–460 RSC.
- M. Van Wassenhoven, M. Goyens, E. Capieaux, P. Devos and P. Dorfman, Nanoparticle characterisation of traditional homeopathically manufactured Cuprum metallicum and Gelsemium sempervirens medicines and controls, Homeopathy, 2018, 107, 244–263 CrossRef PubMed.
- A. Hegetschweiler, O. Borovinskaya, T. Staudt and T. Kraus, Single particle mass spectrometry of titanium and niobium carbonitride precipitates in steels, Anal. Chem., 2018, 91, 943–950 CrossRef PubMed.
Footnote |
| † Dedicated to Professor Gary M. Hieftje on the occasion of his retirement from Indiana University. |
| This journal is © The Royal Society of Chemistry 2020 |