Open Access Article
Open Access ArticleControlling the speciation and reactivity of carbon-supported gold nanostructures for catalysed acetylene hydrochlorination†
Selina K.
Kaiser‡
a,
Ronghe
Lin‡
 a,
Sharon
Mitchell
a,
Sharon
Mitchell
 a,
Edvin
Fako
b,
Frank
Krumeich
a,
Roland
Hauert
c,
Olga V.
Safonova
d,
Vita A.
Kondratenko
e,
Evgenii V.
Kondratenko
a,
Edvin
Fako
b,
Frank
Krumeich
a,
Roland
Hauert
c,
Olga V.
Safonova
d,
Vita A.
Kondratenko
e,
Evgenii V.
Kondratenko
 e,
Sean M.
Collins
e,
Sean M.
Collins
 f,
Paul A.
Midgley
f,
Núria
López
f,
Paul A.
Midgley
f,
Núria
López
 b and
Javier
Pérez-Ramírez
b and
Javier
Pérez-Ramírez
 *a
*a
aInstitute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich, Vladimir-Prelog-Weg 1, 8093 Zurich, Switzerland. E-mail: jpr@chem.ethz.ch
bInstitute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
cSwiss Federal Laboratories for Materials Science and Technology, EMPA, Überlandstrasse 129, 8600 Dübendorf, Switzerland
dPaul Scherrer Institut, 5232 Villigen PSI, Switzerland
eLeibniz-Institut für Katalyse e. V., Albert-Einstein-Straße 29a, 18059 Rostock, Germany
fDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge CB3 0FS, UK
First published on 22nd November 2018
Abstract
Carbon-supported gold catalysts have the potential to replace the toxic mercuric chloride-based system applied industrially for acetylene hydrochlorination, a key technology for the manufacture of polyvinyl chloride. However, the design of an optimal catalyst is essentially hindered by the difficulties in assessing the nature of the active site. Herein, we present a platform of carbon supported gold nanostructures at a fixed metal loading, ranging from single atoms of tunable oxidation state and coordination to metallic nanoparticles, by varying the structure of functionalised carbons and use of thermal activation. While on activated carbon particle aggregation occurs progressively above 473 K, on nitrogen-doped carbon gold single atoms exhibit outstanding stability up to temperatures of 1073 K and under reaction conditions. By combining steady-state experiments, density functional theory, and transient mechanistic studies, we assess the relation between the metal speciation, electronic properties, and catalytic activity. The results indicate that the activity of gold-based catalysts correlates with the population of Au(I)Cl single atoms and the reaction follows a Langmuir–Hinshelwood mechanism. Strong interaction with HCl and thermodynamically favoured acetylene activation were identified as the key features of the Au(I)Cl sites that endow their superior catalytic performance in comparison to N-stabilised Au(III) counterparts and gold nanoparticles. Finally, we show that the carrier (activated carbon versus nitrogen-doped carbon) does not affect the catalytic response, but determines the deactivation mechanism (gold particle aggregation and pore blockage, respectively), which opens up different options for the development of stable, high-performance hydrochlorination catalysts.
Introduction
The growing demand for plastics coupled with the volatility of oil and natural gas prices and concerns over supply security have revived interest in coal as a low-cost and long-term feedstock for the chemical industry.1 In particular, this development gives a new impulse to acetylene hydrochlorination, a key technology for the production of polyvinyl chloride (PVC). Already today, China, the global player in the PVC market, exploits its vast coal reserves to produce more than 70% of this commodity (13 Mton per year) from acetylene.2–4 Unfortunately, the present process still relies on a highly toxic and volatile mercuric chloride catalyst supported on activated carbon (10–15 wt% HgCl2/AC), the use of which will be banned from 2022.5 Among possible alternatives, carbon-supported gold catalysts stand out as the most promising candidates to ultimately replace the mercuric chloride-based system and enable a sustainable expansion of the technology.3 However, wide industrial implementation is still hindered by the low durability of the gold catalysts under reaction conditions (T = 403–453 K, P = 1.5 bar, HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H2 = 1.1
C2H2 = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Specifically, the deactivation has been ascribed to the reduction of the Au(I)/Au(III) species to Au(0), while also sintering of gold clusters into particles and the formation of carbonaceous deposits have been reported.6–9 To guide the rational design of a stable gold catalyst, it is crucial to gain a deeper understanding on the nature of the active site. While to date, various gold species have been identified to be potentially active, including particles,6,10,11 clusters,12,13 and recently also single atoms,14 there remains considerable controversy on their relative activity and the prevailing reaction and deactivation mechanisms. The foremost barriers for a systematic assessment of the influence of gold dispersion and speciation as central catalytic descriptors are: (i) the co-existence of single atoms, clusters and particles in carbon supported gold-based catalysts and (ii) their tendency to sinter under reaction conditions.6–9 The development of suitable synthetic approaches to overcome these issues is restricted by the generally limited understanding on the interaction of single atoms with carbon-based carriers.15 The intrinsic stability of single-atom catalysts is determined by the ability of the host to provide suitable anchoring sites (e.g., N or O) for metal coordination.16 For example, gold single atoms hosted on zeolites or metal oxides exhibit remarkable stability, which can be attributed to the presence of oxygen linkages.17–19 However, under the harsh reaction conditions of acetylene hydrochlorination metal oxide-supported systems (e.g., Al2O3 or TiO2) quickly deactivate due to bulk chlorination or coking. In fact, only amorphous carbon materials (e.g., activated carbon, carbon black) are practically applicable supports for gold-based catalysts in acetylene hydrochlorination.3,7 In this respect, doping of amorphous carbon materials with p-block elements could provide the necessary sites to entrap gold single atoms and improve their thermal stability and durability under reaction conditions.15,20 In particular, nitrogen-doped carbon (NC) has demonstrated unique suitability to entrap atomically dispersed transition metals (e.g., Pd, Pt, Fe, Co).21–23 Interestingly, also gold nanoparticles showed improved catalytic performance on N-doped carbon in comparison to activated carbon in acetylene hydrochlorination.24,25
1). Specifically, the deactivation has been ascribed to the reduction of the Au(I)/Au(III) species to Au(0), while also sintering of gold clusters into particles and the formation of carbonaceous deposits have been reported.6–9 To guide the rational design of a stable gold catalyst, it is crucial to gain a deeper understanding on the nature of the active site. While to date, various gold species have been identified to be potentially active, including particles,6,10,11 clusters,12,13 and recently also single atoms,14 there remains considerable controversy on their relative activity and the prevailing reaction and deactivation mechanisms. The foremost barriers for a systematic assessment of the influence of gold dispersion and speciation as central catalytic descriptors are: (i) the co-existence of single atoms, clusters and particles in carbon supported gold-based catalysts and (ii) their tendency to sinter under reaction conditions.6–9 The development of suitable synthetic approaches to overcome these issues is restricted by the generally limited understanding on the interaction of single atoms with carbon-based carriers.15 The intrinsic stability of single-atom catalysts is determined by the ability of the host to provide suitable anchoring sites (e.g., N or O) for metal coordination.16 For example, gold single atoms hosted on zeolites or metal oxides exhibit remarkable stability, which can be attributed to the presence of oxygen linkages.17–19 However, under the harsh reaction conditions of acetylene hydrochlorination metal oxide-supported systems (e.g., Al2O3 or TiO2) quickly deactivate due to bulk chlorination or coking. In fact, only amorphous carbon materials (e.g., activated carbon, carbon black) are practically applicable supports for gold-based catalysts in acetylene hydrochlorination.3,7 In this respect, doping of amorphous carbon materials with p-block elements could provide the necessary sites to entrap gold single atoms and improve their thermal stability and durability under reaction conditions.15,20 In particular, nitrogen-doped carbon (NC) has demonstrated unique suitability to entrap atomically dispersed transition metals (e.g., Pd, Pt, Fe, Co).21–23 Interestingly, also gold nanoparticles showed improved catalytic performance on N-doped carbon in comparison to activated carbon in acetylene hydrochlorination.24,25
Herein, we show that gold single atoms hosted on N-doped carbon, derived by the controlled pyrolysis of polyaniline, exhibit remarkable stability in a wide temperature range of 413–1073 K and in acetylene hydrochlorination. This contrasts with the progressive aggregation of gold observed on the Au/AC reference systems above 473 K and under reaction conditions. The exclusive presence of gold single atoms on NC was confirmed by aberration-corrected scanning transmission electron microscopy (STEM) and extended X-ray absorption fine structure spectroscopy (EXAFS). Changes in the metal-carrier interaction upon thermal activation further enable a gradual tuning of the gold oxidation state as confirmed by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near edge spectroscopy (XANES), and rationalised by density functional theory (DFT). With a platform of AC- and NC-supported gold catalysts of varying speciation, from single atom to nanoparticle, we systematically assess the relation between the metal speciation, electronic properties, and catalytic activity in acetylene hydrochlorination. Finally, we combine DFT and transient mechanistic studies from temporal analysis of products (TAP) to rationalise the structure-performance relationships of different gold sites.
Results and discussion
Evolution of the gold speciation during thermal activation
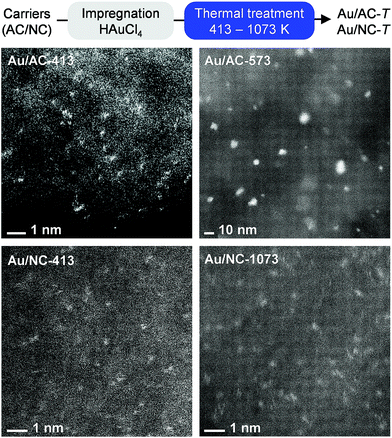 | ||
| Fig. 1 Synthesis of gold single-atom catalysts hosted on activated carbon (AC) and N-doped carbon (NC), accompanied by STEM (top) and aberration-corrected STEM images (bottom) of selected samples of both series. The critical departing temperature of gold single atoms on AC is ca. 473 K, albeit even at 673 K single atoms are still present (Fig. S4†). On NC, gold single atoms are stable up to 1073 K. | ||
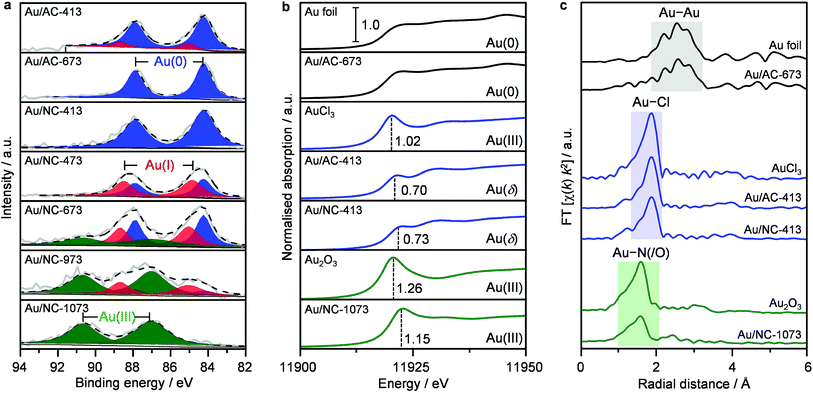
From the normalised Au L3-edge XANES it is possible to derive the gold oxidation states by comparing the white line intensity and other spectral features with appropriate standards (e.g., Au foil, AuCl3). In accordance to expectations, the white line intensities of the low-temperature samples Au/AC-413 and Au/NC-413 are 0.70 and 0.73, respectively. These values are lower than the reference AuCl3 intensity of 1.02 (Fig. 2b), but close to the reported value of 0.6 for AuCl,14 thus indicating the predominant presence of AuCl. With linear combination fitting, the Au(I)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au(III) ratio was estimated to be 2.6 and 4.0, respectively, for Au/NC-413 and Au/AC-413, which agrees well with previous literature reports.14 The extended X-ray absorption fine structure (EXAFS) analysis of Au/NC-413 and Au/AC-413, indicates 2.0 and 2.5 Cl neighbours, respectively, at distances similar to those in AuCl2 (RAu–Cl = 2.272(5) Å and RAu–Cl = 2.269(3) Å, respectively) while no Au–Au contribution was found (Fig. 2c and Table S3†). In accordance with the XPS results, Au/NC-1073 has a much higher white line intensity of 1.15, suggesting the presence of Au(III) species. Interestingly, EXAFS FT analysis of Au/NC-1073 does not indicate the presence of Au–Cl entities, but rather Au–O or Au–N species. A more specific assignment is not possible with the XAS technique, as oxygen and nitrogen scattering signals cannot be distinguished. Fitting of the first coordination shell revealed 4.0 N/O neighbours around Au at a distance of RAu–N/O = 1.96(5) Å. Importantly to notice, no Au–Au characteristic peaks were detected for the whole Au/NC-T series, highlighting the exclusive presence of gold single atoms on the NC carrier. On the contrary, for Au/AC-673, a significant Au–Au contribution (RAu–Au = 2.99(1) Å) predominates in the EXAFS spectrum, while no white line feature at 11
Au(III) ratio was estimated to be 2.6 and 4.0, respectively, for Au/NC-413 and Au/AC-413, which agrees well with previous literature reports.14 The extended X-ray absorption fine structure (EXAFS) analysis of Au/NC-413 and Au/AC-413, indicates 2.0 and 2.5 Cl neighbours, respectively, at distances similar to those in AuCl2 (RAu–Cl = 2.272(5) Å and RAu–Cl = 2.269(3) Å, respectively) while no Au–Au contribution was found (Fig. 2c and Table S3†). In accordance with the XPS results, Au/NC-1073 has a much higher white line intensity of 1.15, suggesting the presence of Au(III) species. Interestingly, EXAFS FT analysis of Au/NC-1073 does not indicate the presence of Au–Cl entities, but rather Au–O or Au–N species. A more specific assignment is not possible with the XAS technique, as oxygen and nitrogen scattering signals cannot be distinguished. Fitting of the first coordination shell revealed 4.0 N/O neighbours around Au at a distance of RAu–N/O = 1.96(5) Å. Importantly to notice, no Au–Au characteristic peaks were detected for the whole Au/NC-T series, highlighting the exclusive presence of gold single atoms on the NC carrier. On the contrary, for Au/AC-673, a significant Au–Au contribution (RAu–Au = 2.99(1) Å) predominates in the EXAFS spectrum, while no white line feature at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 eV is observed in XANES, corroborating with the metallic nature of gold in this catalyst according to the microscopic observations. The integrated approach of combining XPS and XAS techniques enables clear distinction of the nature of different gold species on the carbon hosts.
919 eV is observed in XANES, corroborating with the metallic nature of gold in this catalyst according to the microscopic observations. The integrated approach of combining XPS and XAS techniques enables clear distinction of the nature of different gold species on the carbon hosts.
Role of the gold anchoring site
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
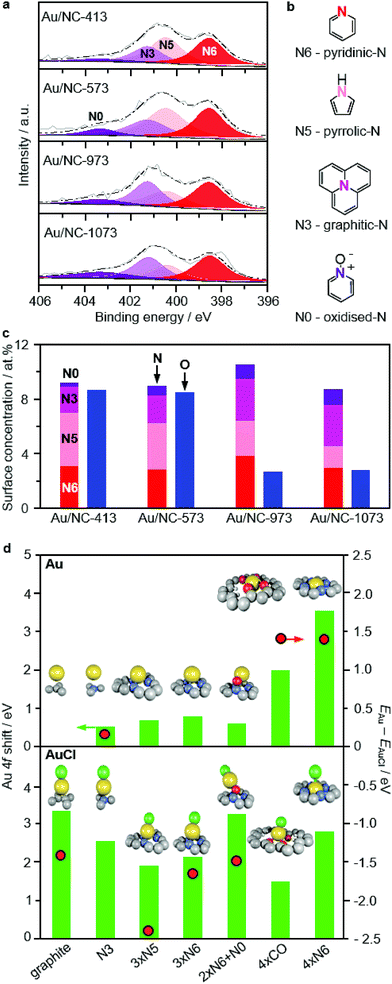
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratios of ca. 37. This ratio first decreases to 25 at 673 K, and further drops to <2 at above 973 K accompanied with the disappearance of the C–Cl peaks for the two high-temperature catalysts (Fig. S10†). A very similar trend is found for the oxygen content of the Au/NC-T series, as for T = 413–573, the total content is ca. 9 at% and for T = 973–1073 it drops below 3 at% (Fig. 3c). On the contrary, the N content (ca. 10 at%) is remarkably constant over the whole investigated temperature range. Overall, these insights from XPS substantiate the above conclusions from EXAFS and XANES and further help to rationalise the evolution of gold species with rising catalyst treatment temperatures. Since nitrogen functionalities have clearly superior thermal stability to oxygen functionalities, we can speculate that the gold species in Au/NC-1073 rather comprises nitrogen than oxygen binding sites. On the basis of the N 1s fitting results it is further possible to foreshadow a leading role in stabilising gold single atoms to pyridinic-N, based on their superb stability and high surface concentration in the Au/NC-1073 sample.
Au ratios of ca. 37. This ratio first decreases to 25 at 673 K, and further drops to <2 at above 973 K accompanied with the disappearance of the C–Cl peaks for the two high-temperature catalysts (Fig. S10†). A very similar trend is found for the oxygen content of the Au/NC-T series, as for T = 413–573, the total content is ca. 9 at% and for T = 973–1073 it drops below 3 at% (Fig. 3c). On the contrary, the N content (ca. 10 at%) is remarkably constant over the whole investigated temperature range. Overall, these insights from XPS substantiate the above conclusions from EXAFS and XANES and further help to rationalise the evolution of gold species with rising catalyst treatment temperatures. Since nitrogen functionalities have clearly superior thermal stability to oxygen functionalities, we can speculate that the gold species in Au/NC-1073 rather comprises nitrogen than oxygen binding sites. On the basis of the N 1s fitting results it is further possible to foreshadow a leading role in stabilising gold single atoms to pyridinic-N, based on their superb stability and high surface concentration in the Au/NC-1073 sample.
| Sample | Au speciationa | Aub at% | Nb at% | Ob at% | Clb at% | V total cm3 g−1 | V micro cm3 g−1 | S BET m2 g−1 |
|---|---|---|---|---|---|---|---|---|
| a SA: single atom, NP: nanoparticle. b XPS. c Volume of Ar at p/p0 = 0.98. d t-Plot method. e BET method. | ||||||||
| NC | — | 0.00 | 9.3 | 6.4 | 0.05 | 0.42 | 0.21 | 517 |
| AC | — | 0.00 | 0.3 | 4.4 | 0.00 | 0.84 | 0.69 | 1795 |
| Au/NC-413 | SA | 0.05 | 9.2 | 8.7 | 1.8 | 0.32 | 0.16 | 376 |
| Au/NC-473 | SA | 0.04 | 10.4 | 8.6 | 1.5 | 0.20 | 0.14 | 356 |
| Au/NC-673 | SA | 0.02 | 9.8 | 7.6 | 0.8 | 0.33 | 0.20 | 551 |
| Au/NC-973 | SA | 0.05 | 10.6 | 2.7 | 0.1 | 0.17 | 0.16 | 389 |
| Au/NC-1073 | SA | 0.02 | 8.7 | 2.8 | 0.02 | 0.39 | 0.22 | 496 |
| Au/AC-413 | SA | 0.04 | 0.2 | 9.5 | 1.1 | 0.69 | 0.56 | 1278 |
| Au/AC-473 | SA | 0.08 | 0.5 | 7.8 | 0.7 | 0.60 | 0.54 | 1150 |
| Au/AC-673 | SA + NP | 0.04 | 0.3 | 7.7 | 0.6 | 0.84 | 0.67 | 1773 |
| Au/NC-473-12 h | SA | 0.03 | 9.1 | 8.1 | 1.3 | 0.07 | 0.02 | 90 |
| Au/AC-473-12 h | SA + NP | 0.04 | 0.6 | 6.6 | 0.9 | 0.58 | 0.50 | 1100 |
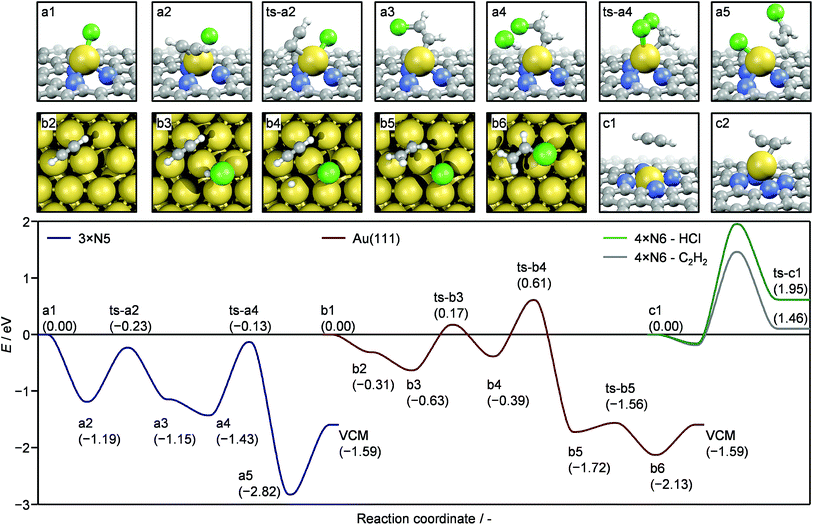
The remarkable ability of the NC carrier to stabilise gold atoms was rationalised by DFT. A set of 21 graphite defects (Fig. S11†) was used to represent the AC and NC support materials. The models were built in the basal plane according to stoichiometry: regular, N-containing: N-pyridinic (N6), N-pyrrolic (N5); O-containing: epoxide, keto, hydroxyl; plus combinations of those and the corresponding step models. These structures were evaluated for their ability to bind Au and AuCl. The differences in their formation energies, calculated as ΔE = EAu − EAuCl, (Fig. 3d; Table S4†) indicate that in general AuCl species are thermodynamically favoured and thus more stable than their Au counterparts. In accordance with the XAS analysis, simulation of Au 4f XPS shift for these AuCl species suggests they are significantly oxidised Au(δ) (I < δ < III), but are likely observed as Au(0) by XPS due to photoreduction effects. Similar results are obtained for the AuCl2 and AuCl3 species (Table S5†). Exceptions to the general trend occur only when the cavity has a suitable size and geometry to provide a square planar coordination pattern (four pyridinic-4xN6 or four keto environments 4xCO) for the gold atom (Fig. S12†). On the basis of the results presented above, AuCl species are dominantly present in the low-temperature Au/NC-T catalysts and are responsible for the XPS signal. With increasing catalyst treatment temperature, the Cl content is lowered and the mobility of the gold atoms increases (Fig. S13†). This leads to a larger population of configurations observed as more oxidised gold species since these are deeper wells in the potential energy surface of the doped carbons (Table S4†). A further increase in temperature enhances the diffusion of gold species as they are able to escape local diffusion minima and enter the 4xN6 cavities, adopting a four-fold N-coordinated planar geometry where they are trapped. With an Au 4f XPS shift of 3.67 eV, this structure represents the global diffusion minimum (adsorption E = −3.61 eV with respect to gas-phase Au) and is observed in Au/NC-1073 by XPS and XANES as Au(III) that is more resistant to X-ray reduction (Fig. S7†).
Catalytic performance in acetylene hydrochlorination
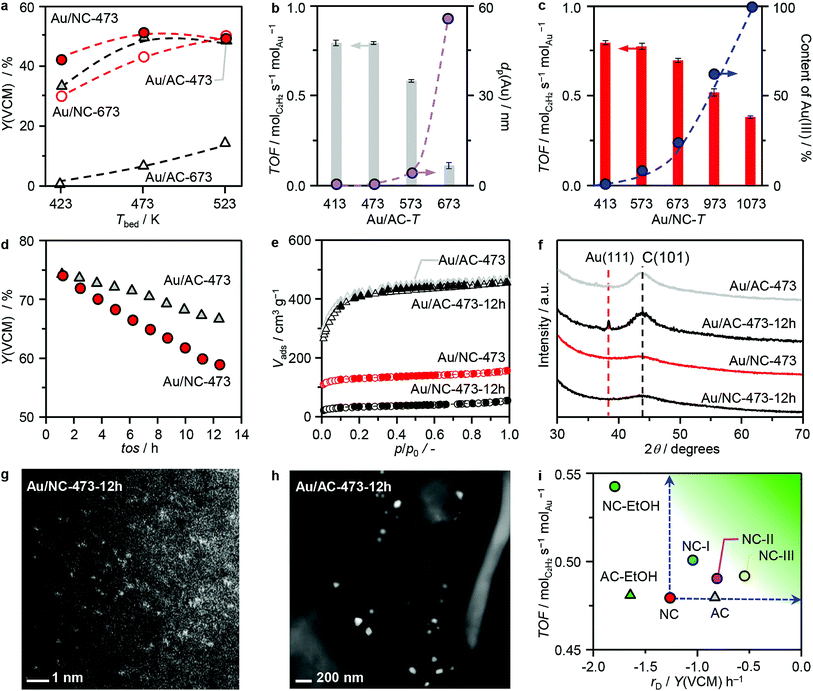 | ||
Fig. 4 (a–d) Catalytic performance of AC- and NC-supported gold catalysts in acetylene hydrochlorination, expressed as the yield of vinyl chloride monomer Y(VCM) or the turnover frequency (TOF) at (a) different reaction temperatures and as a function of (b) the mean particle size of gold (dp) for Au/AC and (c) the percentage of Au(III) for Au/NC, as well as (d) during a short-term stability test. (e) Argon sorption isotherms, (f) XRD, and (g and h) HAADF-STEM images of the catalysts after 12 h time on stream (tos). (i) Comparison of TOFs and deactivation rate, rD, of the catalysts developed in this work with Au/AC-473 and Au/NC-473 as reference systems (Fig. S15a†). Reaction conditions: Tbed = 473 K, Wcat = 0.1 g (a–c) and 0.25 g (d and i), HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H2 C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 44 Ar = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, FT = 15 cm3 min−1, P = 1 bar. 16, FT = 15 cm3 min−1, P = 1 bar. | ||
Towards the wide implementation of an economically viable and green technology based on gold catalysts, another challenge to face is the elimination of highly corrosive and hazardous aqua regia from the catalyst synthesis protocols. Several studies have demonstrated the central role of this reagent in oxidising and dispersing the active gold species on the carbon support, being the key for high catalytic activity.4,26,33 In fact, no alternative solvents could yield atomic dispersion of gold on carbon supports, without nanoparticle formation. As rationalised in the previous section, the stabilisation effect of gold single atoms on NC originates from the nitrogen dopant and consequently there should be no need to use a strong oxidising acid. In this respect, we applied ethanol, as an environmental benign solvent, in the impregnation step and obtained another catalyst of fully atomically dispersed gold species on N-doped carbon (Au/NC–EtOH) as evident from HAADF-STEM and XRD analysis (Fig. S15b and c†). On the contrary, following the same approach with activated carbon yielded the sample Au/AC–EtOH, which showed Au(111) diffraction peaks in XRD, indicating a contribution of gold nanoparticles. Remarkably, in terms of initial catalytic activity Au/NC–EtOH outperformed all other catalysts developed in this study, demonstrating the potential to replace aqua regia in the synthesis protocol and underlining the central role of the nitrogen dopant rather than the impregnation solvent to disperse and stabilise gold single atoms. However, despite the high initial activity, Au/NC–EtOH deactivates rather quickly, comparable to Au/AC–EtOH. While the latter suffers from severe particle aggregation, as evident from a clear increase in the gold diffraction peaks observed with XRD, Au/NC–EtOH mainly deactivates due to pore blockage and/or coking (Table S6†), solidifying the importance of the carrier properties and the need for careful engineering of the support.
Mechanistic studies
Computational and kinetic studies enable the detailed understanding of catalytic action, guiding the design towards stable high-performance single atom catalysts.34,35 To date, acetylene hydrochlorination is widely believed to follow an Eley–Rideal mechanism, in which HCl reacts from the gas phase with the initially formed C2H2–Au(III) complex.36–38 Based on DFT calculations it was shown that C2H2 is the preferred ligand for the Au(III) site and it was further concluded that the addition of HCl to the C2H2–metal complex is the rate-determining step.39,40 Recently, an alternative mechanism has been proposed that proceeds via the oxidative addition of HCl to Au(I)Cl, followed by the addition of C2H2 to the formed Au(III) complex and finally the reductive elimination of C2H3Cl.14 Except for the dynamic transformation between Au(I) and Au(III) species, observed by in situ EXAFS,14 there is limited experimental evidence to support either of the two proposed mechanisms. To gain further experimental insights into the mechanism of acetylene hydrochlorination over Au single-atom catalysts, we conducted kinetic studies on Au/AC-473 and Au/NC-473 (Fig. S16†). In good agreement with the similar initial catalytic activity, the kinetic parameters were found to be quite comparable as well, which suggests that the two catalysts promote the same reaction mechanism. Specifically, apparent activation energies of 28.0 and 30.4 kJ mol−1 were derived for Au/NC-473 and Au/AC-473, respectively. The partial reaction orders of C2H2 and HCl were determined to be 1.05 and 0.72 for Au/NC-473 and 1.02 and 0.76 for Au/AC-473, which indicates a dependence of the reaction rate on the surface coverage of both reactants. In this respect, a detailed analysis of the interactions of HCl, C2H2, and C2H3Cl with distinct gold sites could provide further insights.| Catalyst | HCl | C2H2 | C2H3Cl | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k ads,eff, s−1 | k des, s−1 | K , — | k ads,eff, s−1 | k des, s−1 | K , — | k ads,eff, s−1 | k des s−1 | K , — | |
a Complete consumption of HCl due to irreversible adsorption.
b C2H2 simply diffused through the reactor.
c The ratio of kads,eff![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kdes. kdes.
|
|||||||||
| NC | —a | —a | —a | 1.2 × 103 | 1.4 × 102 | 8.6 | 1.2 × 103 | 14 | 90.6 |
| Au/NC-473 | 8 × 103 | 2 × 10−3 | 4 × 106 | 9.3 × 102 | 1.3 × 102 | 7.2 | 1.2 × 103 | 17 | 70.9 |
| Au/NC-1073 | —a | —a | —a | 4.7 × 102 | 87 | 5.4 | 1.2 × 103 | 14 | 84.6 |
| Au/NC-473-12 h | 5 × 103 | 2 × 10−2 | 3 × 105 | —b | —b | —b | 6.7 × 102 | 19 | 34.9 |
| AC | —a | —a | —a | 5.5 × 103 | 4.5 × 102 | 12.3 | 8.1 × 104 | 13 | 6.2 × 103 |
| Au/AC-473 | 3 × 103 | 1 × 10−6 | 3 × 109 | 5.0 × 102 | 1.6 × 102 | 3.1 | 7.1 × 106 | 1.2 × 105 | 58.0 |
| Au/AC-673 | 5 × 102 | 4 × 102 | 1.3 | 4.1 × 102 | 1.2 × 102 | 3.4 | 6.7 × 106 | 7.7 × 104 | 87.0 |
| Au/AC-473-12 h | 1 × 102 | 0.5 | 2 × 102 | 1.8 × 102 | 1.0 × 102 | 1.8 | 2.3 × 103 | 44 | 53.0 |
Conclusions
In this study we presented a strategy to gradually tune the electronic properties and the stability of individual gold atoms by varying the structure of functionalised carbons and use of thermal activation. Owing to the presence of suitable metal anchoring sites in polyaniline-derived N-doped carbon, gold remains atomically dispersed up to 1073 K, but experiences a gradual increase in the metal oxidation state with rising activation temperature, as is evident from aberration-corrected STEM, XANES, and EXAFS analysis. In the absence of nitrogen sites, progressive agglomeration of gold atoms occurs already above 473 K. This distinct evolution of gold atoms as a function of the carbon carrier enabled the development of a unique platform of gold nanostructures suitable to assess the relation between the metal speciation, electronic properties, and catalytic performance in acetylene hydrochlorination. On the basis of steady-state catalytic tests, computational and transient mechanistic studies, we showed that the activity of gold-based catalysts correlates with the population of Au(I)Cl single atoms and the reaction follows a Langmuir–Hinshelwood mechanism. Accordingly, strong interaction with HCl and the thermodynamically favourable activation of acetylene are the key features of the Au(I)Cl sites that endow their superior catalytic performance. In line with the substantially lower catalytic activity, gold nanoparticles and N-coordinated Au(III) single atom ensembles were found to exhibit higher energy barriers for the activation of both reactants. Remarkably, the choice of carbon host does not affect the catalytic response, but determines the deactivation mechanism (pore blockage for Au/NC and gold particle aggregation for Au/AC), suggesting different strategies for catalyst optimisation. Going beyond acetylene hydrochlorination, the herein developed approach of controlling the reactivity of individual atoms provides exiting perspectives for the design of metal species for targeted applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by ETH Reserach Grant ETH-40 17, the Swiss National Science Foundation (project no. 200021-169679), and Spanish MINECO (CTQ2015-68770-R). E. F. thanks MINECO La Caixa Severo Ochoa for a predoctoral grant through Severo Ochoa Excellence Accreditation 2014 2018 (SEV 2013 0319). BSC-RES for providing generous computational resources. V. A. K and E. V. K acknowledge the State of Mecklenburg-Vorpommern for financial support. S. M. C. acknowledges support from the Henslow Research Fellowship and Girton College, Cambridge. P. A. M. acknowledges the EPSRC for funding under grant number EP/R008779/1. The Scientific Centre for Optical and Electron Microscopy (ScopeM) at ETH Zurich is acknowledged for the use of their facilities.References
- I. T. Trotus, T. Zimmermann and F. Schüth, Chem. Rev., 2014, 114, 1761 CrossRef CAS PubMed.
- R. Lin, A. P. Amrute and J. Pérez-Ramírez, Chem. Rev., 2017, 117, 4182 CrossRef CAS PubMed.
- G. Malta, S. J. Freakley, S. A. Kondrat and G. J. Hutchings, Chem. Commun., 2017, 53, 11733 RSC.
- P. Johnston, N. Carthey and G. J. Hutchings, J. Am. Chem. Soc., 2015, 137, 14548 CrossRef CAS PubMed.
- United Nations Environment Programme, Minamata Convention on Mercury, www.mercuryconvention.org/, accessed July 2018.
- M. Conte, C. J. Davies, D. J. Morgan, T. E. Davies, A. F. Carley, P. Johnston and G. J. Hutchings, Catal. Sci. Technol., 2013, 3, 128 RSC.
- B. Nkosi, J. Catal., 1991, 128, 366 CrossRef CAS.
- B. Dai, Q. Wang, F. Yu and M. Zhu, Sci. Rep., 2015, 5, 10553 CrossRef PubMed.
- K. C. O'Connell, J. R. Monnier and J. R. Regalbuto, Appl. Catal., B, 2018, 225, 264 CrossRef.
- X. H. Tian, G. T. Hong, B. B. Jiang, F. P. Lu, Z. W. Liao, J. D. Wang and Y. R. Yang, RSC Adv., 2015, 5, 46366 RSC.
- J. Oliver-Meseguer, A. Doménech-Carbó, M. Boronat, A. Leyva-Pérez and A. Corma, Angew. Chem., Int. Ed., 2017, 56, 6435 CrossRef CAS PubMed.
- F. Zhao, Y. Wang and L. Kang, Can. J. Chem., 2016, 94, 842 CrossRef CAS.
- Y. Wang, M. Zhu, L. Kang and B. Dai, RSC Adv., 2014, 4, 38466 RSC.
- G. Malta, S. A. Kondrat, S. J. Freakley, C. J. Davies, L. Lu, S. Dawson, A. Thetford, E. K. Gibson, D. J. Morgan, W. Jones, P. P. Wells, P. Johnston, C. R. Catlow, C. J. Kiely and G. J. Hutchings, Science, 2017, 355, 1399 CrossRef CAS PubMed.
- H. Li, H.-X. Zhang, X.-L. Yan, B.-S. Xu and J.-J. Guo, New Carbon Mater., 2018, 33, 1 CrossRef.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem., 2018, 2, 65 CrossRef CAS.
- M. Yang, S. Li, Y. Wang, J. A. Herron, Y. Xu, L. F. Allard, S. Lee, J. Huang, M. Mavrikakis and M. Flytzani-Stephanopoulos, Science, 2014, 346, 1498 CrossRef CAS PubMed.
- M. Yang and M. Flytzani-Stephanopoulos, Catal. Today, 2017, 298, 216 CrossRef CAS.
- B. Qiao, J.-X. Liang, A. Wang, C.-Q. Xu, J. Li, T. Zhang and J. J. Liu, Nano Res., 2015, 8, 2913 CrossRef CAS.
- D. Cazorla-Amorós, Frontiers in Materials, 2014, 1, 1 Search PubMed.
- Y. J. Chen, S. F. Ji, Y. G. Wang, J. C. Dong, W. X. Chen, Z. Li, R. A. Shen, L. R. Zheng, Z. B. Zhuang, D. S. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2017, 56, 6937 CrossRef CAS PubMed.
- Y. Han, Y.-G. Wang, W. Chen, R. Xu, L. Zheng, J. Zhang, J. Luo, R.-A. Shen, Y. Zhu, W.-C. Cheong, C. Chen, Q. Peng, D. Wang and Y. Li, J. Am. Chem. Soc., 2017, 139, 17269 CrossRef CAS PubMed.
- D. A. Bulushev, M. Zacharska, E. V. Shlyakhova, A. L. Chuvilin, Y. N. Guo, S. Beloshapkin, A. V. Okotrub and L. G. Bulusheva, ACS Catal., 2016, 6, 681 CrossRef CAS.
- J. Zhao, J. Xu, J. Xu, T. Zhang, X. Di, J. Ni and X. Li, Chem. Eng. J., 2015, 262, 1152 CrossRef CAS.
- X. Li, M. Zhu and B. Dai, Appl. Catal., B, 2013, 142, 234 CrossRef.
- M. Conte, C. J. Davies, D. J. Morgan, T. E. Davies, D. J. Elias, A. F. Carley, P. Johnston and G. J. Hutchings, J. Catal., 2013, 297, 128 CrossRef CAS.
- T. Furnival, R. K. Leary, E. C. Tyo, S. Vajda, Q. M. Ramasse, J. M. Thomas, P. D. Bristowe and P. A. Midgley, Chem. Phys. Lett., 2017, 683, 370 CrossRef CAS.
- Y. Y. Fong, B. R. Visser, J. R. Gascooke, B. C. Cowie, L. Thomsen, G. F. Metha, M. A. Buntine and H. H. Harris, Langmuir, 2011, 27, 8099 CrossRef CAS PubMed.
- F. Karadas, G. Ertas, E. Ozkaraoglu and S. Suzer, Langmuir, 2005, 21, 437 CrossRef CAS PubMed.
- X. Liu, M. Conte, D. Elias, L. Lu, D. J. Morgan, S. J. Freakley, P. Johnston, C. J. Kiely and G. J. Hutchings, Catal. Sci. Technol., 2016, 6, 5144 RSC.
- G. Hong, X. Tian, B. Jiang, Z. Liao, J. Wang, Y. Yang and J. Zheng, RSC Adv., 2016, 6, 3806 RSC.
- R. Lin, S. K. Kaiser, R. Hauert and J. Pérez-Ramírez, ACS Catal., 2018, 8, 1114 CrossRef CAS.
- P. T. Bishop, N. A. Carthey and P. Johnston, U.S. Pat. 9,409, vol. 161, 2016.
- T. Z. H. Gani and H. J. Kulik, ACS Catal., 2018, 8, 975 CrossRef CAS.
- J. C. Liu, Y. G. Wang and J. Li, J. Am. Chem. Soc., 2017, 139, 6190 CrossRef CAS PubMed.
- S. Wang, B. Shen and Q. Song, Catal. Lett., 2009, 134, 102 CrossRef.
- J. Zhong, Y. Xu and Z. Liu, Green Chem., 2018, 20, 2412 RSC.
- M. Conte, A. Carley, C. Heirene, D. Willock, P. Johnston, A. Herzing, C. Kiely and G. Hutchings, J. Catal., 2007, 250, 231 CrossRef CAS.
- J. Ma, S. Wang and B. Shen, React. Kinet., Mech. Catal., 2013, 110, 177 CrossRef CAS.
- T. V. Krasnyakova, I. V. Zhikharev, R. S. Mitchenko, V. I. Burkhovetski, A. M. Korduban, T. V. Kryshchuk and S. A. Mitchenko, J. Catal., 2012, 288, 33 CrossRef CAS.
- J. Pérez-Ramírez and E. Kondratenko, Catal. Today, 2007, 121, 160 CrossRef.
- K. Morgan, N. Maguire, R. Fushimi, J. T. Gleaves, A. Goguet, M. P. Harold, E. V. Kondratenko, U. Menon, Y. Schuurman and G. S. Yablonsky, Catal. Sci. Technol., 2017, 7, 2416 RSC.
- J. T. Gleaves, G. S. Yablonskii, P. Phanawadee and Y. Schuurman, Appl. Catal., A, 1997, 160, 55 CrossRef CAS.
- M. Álvarez-Moreno, C. de Graaf, N. López, F. Maseras, J. M. Poblet and C. Bo, J. Chem. Inf. Model., 2015, 55, 95 CrossRef PubMed.
- E. Fako, ioChem-BD Collection, DOI:10.19061/iochem-bd-1-74.
- M. García-Mota, N. Cabello, F. Maseras, A. M. Echavarren, J. Pérez-Ramírez and N. López, ChemPhysChem, 2008, 9, 1624 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, additional computational details, TAP experiments, microscopy images and videos, and catalytic data. See DOI: 10.1039/c8sc03186j |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2019 |