Glucose isomerization catalyzed by bone char and the selective production of 5-hydroxymethylfurfural in aqueous media†
Babasaheb M.
Matsagar
 a,
Chi
Van Nguyen
a,
Md. Shahriar A.
Hossain
bc,
Md. Tofazzal
Islam
d,
Yusuke
Yamauchi
a,
Chi
Van Nguyen
a,
Md. Shahriar A.
Hossain
bc,
Md. Tofazzal
Islam
d,
Yusuke
Yamauchi
 befg,
Paresh L.
Dhepe
befg,
Paresh L.
Dhepe
 h and
Kevin C.-W.
Wu
h and
Kevin C.-W.
Wu
 *a
*a
aDepartment of Chemical Engineering, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 10617, Taiwan. E-mail: kevinwu@ntu.edu.tw
bCollege of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
cSchool of Mechanical & Mining Engineering, The University of Queensland, Brisbane, QLD 4072, Australia
dDepartment of Biotechnology, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh
eSchool of Chemical Engineering and Australian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Brisbane, QLD 4072, Australia
fInternational Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
gDepartment of Plant & Environmental New Resources, Kyung Hee University, 1732 Deogyeong-daero, Giheung-gu, Yongin-si, Gyeonggi-do 446-701, South Korea
hCatalysis & Inorganic Chemistry Division, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune 411 008, India
First published on 30th August 2018
Abstract
The selective production of 5-hydroxymethylfurfural (HMF) is important, and it is difficult with glucose substrates in a water solvent. Here we demonstrate a selective method for glucose-to-HMF conversion using the combined catalysis of bone char and 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate acidic ionic liquid catalysts with a high HMF selectivity (54%) in water.
Furans such as 5-hydroxymethylfurfural (HMF) and furfural are of great importance because they are well-known platform molecules and can be further converted into a range of industrially significant fine chemicals.1–5 For example, the hydrogenation of HMF can yield 2,5-dimethylfuran (DMF) which has a potential application as a fuel additive.6 Moreover, HMF can also act as a raw material to synthesize many significant fine chemicals such as a 2,5-furan dicarboxylic acid (FDCA), levulinic acid (LA), γ-valerolactone (GVL), alkyl levulinate, etc.5,7–11
Several reports are available for the dehydration of fructose into HMF with a very high yield.8,12–14 However, the production of HMF from glucose is relatively difficult because of the complex reaction pathways that lead to many undesired side products. The conversion of glucose to HMF generally involves two steps: glucose-to-fructose isomerization which is a reversible reaction and fructose-to-HMF dehydration. The isomerization of glucose into fructose requires a catalyst that exhibits Lewis acidity or basicity15–17 and the dehydration of fructose to HMF is efficiently carried out using a catalyst that has Brønsted acidity.8,18 The direct conversion pathway for the glucose to HMF reaction is less selective compared to the glucose conversion into HMF via the fructose reaction pathway.18–20
For the conversion of fructose and glucose into HMF, a combination of Amberlyst-15 and hydrotalcite as the acidic and basic heterogeneous catalysts was reported in methyl isobutyl ketone (MIBK) and 2-methyltetrahydrofuran (MTHF) solvent systems.21 Recently, ionic liquids (ILs) have been widely used along with high-boiling solvents such as DMSO7,22 and, in these ILs/organic solvent systems, ILs also act as the solvent (e.g. [BMIM][Cl]) as well as the catalyst. They are also used in very large quantities in the presence of homogeneous acidic catalysts (mineral acids), organic acids, Lewis acids, etc.23,24 All of these reported catalytic methods have several drawbacks. For example, the heterogeneous catalysts (Amberlyst-15, H-MOR (Si/Al = 10), HUSY (Si/Al = 15), etc.) used are not recyclable due to their low thermal stability.8,25 The use of high-boiling organic solvents requires energy-intensive steps for separating soluble HMF, has disposal issues and the use of ILs in large quantities is not a cost-effective method.26 The utilization of mineral acid results in reaction system corrosion, and it is difficult to recover and reuse those mineral acid catalysts. Therefore, an efficient and reliable catalytic system for glucose-to-HMF conversion has been highly demanded. Very recently the conversion of glucose into HMF and 5-ethoxymethylfurfural has been reported by combining Lewis (AlCl3·6H2O) and Brønsted acid catalysts (PTSA-POM) in an ethanol/water and GVL/water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixed solvent system that produced a high yield of HMF (up to 60%).16
1 v/v) mixed solvent system that produced a high yield of HMF (up to 60%).16
Although the dehydration of fructose can be catalytically performed efficiently for the synthesis of HMF in high yields,8,26–29 the drawbacks of using fructose as a starting material are its limited supply and higher cost. Hence in the present work, glucose is preferred as the starting sugar for HMF production. We proposed the combination of a Brønsted acidic ionic liquid (BAIL) i.e. 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate with basic bone char for the direct conversion of glucose into HMF (Scheme 1). The BAIL offers excellent chemical and thermal stability,30 and superior activity toward many acid-catalyzed reactions.8,31 Additionally, ionic liquids (ILs) have advantages such as having lower vapor pressure and non-flammability, which make them better solvents and catalysts. Bone char is a natural material obtained from dead animals and exhibits basicity for several catalytic reactions.32 The combination of BAILs and bone char in this study provides a new acid/base catalytic system for glucose-to-HMF conversion with high efficiency. More importantly, we perform this conversion in a water system, which could be beneficial for economic feasibility, technological suitability, sustainability, and environmental superiority. The reaction solvent has a huge impact on the cost of the chemical process and environmental concerns. Hence, the use of a water solvent has been continuously encouraged.
The acidic IL (1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate) synthesis procedure was adapted from a previously described method.1 The acidic IL was synthesized using 1,3-propane sultone and 1-methylimidazole. Equal amounts of 1,3-propane sultone and 1-methylimidazole were refluxed in toluene for 16 h to obtain a white precipitate (zwitterion). Furthermore, the zwitterion was dried at 80 °C for 4 h and then was used for the next step where it reacted with sulfuric acid (equal moles) without any solvent for 12 h at 110 °C to obtain the desired acidic IL. The organic precursors used for the BAIL, such as 1,3-propane sultone and 1-methylimidazole, were completely utilized in the synthesis of the BAIL. Moreover, toluene was used as a solvent for the BAIL synthesis, which could be easily recycled by filtration, because in the first step the solid product yield (zwitterion) was above 96%.
The bone char used in this study was synthesized using cattle bone powder. The synthesis was carried out by the calcination of bone powder at 1200 °C for 2 h under an extremely low oxygen atmosphere.
The prepared acidic IL was characterized using 1H and 13C NMR and the physical and chemical properties of the bone char were characterized with powder X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), nitrogen adsorption/desorption isotherms and CO2-temperature programmed desorption (CO2-TPD). The XRD patterns of the bone char were recorded using a Cu Kα radiation source on a Rigaku-Ultima IV instrument. The morphology of the prepared bone char was observed with SEM (Nova™ NanoSEM 230). N2 adsorption/desorption measurements were conducted using a micromeritics ASAP 2020 for the porous properties including specific surface area and pore size. The basicity of the bone char was examined by CO2-TPD.
The acidic IL used in this study was a viscous liquid at room temperature, and its NMR analysis indicated that it was pure and no additional peaks were seen other than the peaks attributed to the acidic IL (Fig. S1; ESI†). Bone char is a black grainy and porous material consisting mainly of hydroxyapatite. As shown in Fig. S2; ESI†, the XRD patterns of the bone char used in this study exhibited the crystal structure of hydroxyapatite (HAp; Ca5(OH)(PO4)3) because the diffraction peaks of the bone char correspond to the crystal structure of hydroxyapatite samples.33,34 From the EDX spectrum presented in Fig. S3 and Table S1 (ESI†), it was confirmed that the bone char contained 14.1 wt% carbon and had major inorganic elements such as phosphorous (P, 13.6 wt%), calcium (Ca, 23 wt%), and oxygen (O, 46.4 wt%), and minor elements like sodium (Na, 1.4 wt%) and magnesium (Mg, 1.2 wt%). The XPS spectra of the bone char also validated the presence of elements such as Ca, O, C, and P (Fig. S4; ESI†). The CO2-TPD analysis results (Fig. S5; ESI†) indicated that the bone char exhibited medium-strength basic sites (peak at 183 °C) and strong basic sites (peak at 591 °C).35 The specific BET surface area (BET = 98.3 m2 g−1), total pore volume (Vtotal = 0.25 cm2 g−1), and the mean pore diameter (Dp = 10.32 nm) of the bone char were calculated using the BET and N2 adsorption/desorption measurements as shown in Fig. S6, S7 and Table S2 (ESI†). The N2 adsorption/desorption isotherm of the bone char (Fig. S6; ESI†) resembles a typical type IV isotherm (hysteresis loop type H3 above P/Po 0.4 (N2 at −196 °C)) which is common for mesoporous materials.
The glucose isomerization reactions were performed using only bone char as the catalyst in a 25 mL round bottom flask at 90 °C under magnetic stirring in an aqueous medium whereas the conversion of C6 sugars (fructose and glucose) into HMF was carried out in a Parr autoclave (batch reactor) with a heating controller unit. Glucose was added to the reactor along with the catalysts (bone char and the acidic IL) and the solvent (water). Then the reactions were carried out at the desired reaction time and temperature under mechanical stirring (500 rpm).
Glucose-to-fructose isomerization was performed using 10 mL (1 wt%) glucose solution and bone char as the catalyst (0.03 g) in water medium at 90 °C. The result showed 12% fructose yield and 63% HMF selectivity. A further increase in catalyst quantity from 0.03 g to 0.1 g showed a slight improvement in fructose yield from 12% to 15% with a decrease in fructose selectivity from 63% to 47% (ESI; Table S3†). The reactions were also performed at higher temperatures such as 110 °C and 130 °C using 0.05 g of bone char, but the fructose yields were found to be almost similar (13% and 12% (ESI; Table S3†)). Consequently, the non-catalytic glucose isomerization reaction did not show any fructose yield. The results above indicate that bone char can act as a promising catalyst for glucose isomerisation into fructose under lower temperatures.
Subsequently, to understand the effect of solvent systems, various solvents such as water, methanol (MeOH), dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and N,N-dimethylacetamide (DMA) were tested for glucose isomerization using bone char as the catalyst. As shown in Table 1, the highest fructose yield (15%) and selectivity (55%) results were obtained when glucose isomerization was performed in water at 90 °C for 3 h. In contrast, MeOH (10%) DMF (4%) and DMA (3%) showed relatively lower fructose yields. Therefore, for the glucose-to-fructose isomerization reaction, a water solvent system with the bone char catalyst was preferred for optimizing other reaction parameters (Table S3; ESI†). H2O and MeOH have a crucial impact on catalyst activity, probably because of their protic nature which favors proton exchange, unlike DMSO.36
| Entry | Solvent | Conversion (%) | Fructose yield (%) | Fructose selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: glucose 0.1 g, H2O 10 mL, bone char 0.05 g, 90 °C, 3 h. | ||||
| 1 | H2O | 27.2 | 15 | 55 |
| 2 | MeOH | 34.5 | 9.6 | 28 |
| 3 | DMF | 8.2 | 3.3 | 40 |
| 4 | DMSO | 2.6 | 1 | 38 |
| 5 | DMA | 18 | 2.5 | 14 |
Before converting glucose into HMF, initial experiments were performed using fructose as the starting sugar and a 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate ([C3SO3HMIM][HSO4]) BAIL as the catalyst in water medium at 150 °C for 30 min. As summarized in Table 2, a low conversion of fructose (28%) along with a lower yield of HMF (17%) was obtained (entry 1; Table 2). Almost no fructose conversion (4%) and HMF formation were seen for the non-catalytic reaction under similar reaction conditions (entry 3; Table 2). These results indicated that water is not a suitable solvent for fructose-to-HMF dehydration. Consequently, by changing the solvent to a biphasic solvent system (H2O + MIBK) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v, an enhancement in fructose conversion (84%) and HMF yield (73%) was observed (entry 2; Table 2). However, when the same biphasic system was applied with glucose as the starting sugar, only a 15% fructose yield and a 10% HMF yield were observed with around 50% glucose conversion. A further increase in reaction time from 30 min to 4 h showed an increase in HMF yield from 10% to 30% with an increase in glucose conversion from 49% to 89%, respectively (entry 4 and 5; Table 2). We also tested the γ-valerolactone solvent for the glucose dehydration reaction but it was noticed that no HMF yield was obtained for a 3 h reaction performed at 170 °C using BAIL (0.1 g) and bone char (0.1 g) catalysts.
5 v/v, an enhancement in fructose conversion (84%) and HMF yield (73%) was observed (entry 2; Table 2). However, when the same biphasic system was applied with glucose as the starting sugar, only a 15% fructose yield and a 10% HMF yield were observed with around 50% glucose conversion. A further increase in reaction time from 30 min to 4 h showed an increase in HMF yield from 10% to 30% with an increase in glucose conversion from 49% to 89%, respectively (entry 4 and 5; Table 2). We also tested the γ-valerolactone solvent for the glucose dehydration reaction but it was noticed that no HMF yield was obtained for a 3 h reaction performed at 170 °C using BAIL (0.1 g) and bone char (0.1 g) catalysts.
| Entry | C6 sugar | Catalyst | Solvent | Time (h) | Temp. (°C) | Glucose conv. (%) | Fructose yield/conv. (%) | HMF yield (%) |
|---|---|---|---|---|---|---|---|---|
| a Glucose 0.5 g, BAIL 0.025 g, H2O 5 mL, MIBK 25 mL. b Glucose 0.2 g, bone char 0.1 g, BAIL 0.1 g, H2O 20 mL. c Glucose 0.2 g, bone char 0.05 g, BAIL 0.05 g, H2O 20 mL. *Indicates the conversion of fructose. | ||||||||
| 1a | Fructose | BAIL | H2O | 0.5 | 150 | — | 28* | 17 |
| 2a | Fructose | BAIL | H2O + MIBK (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) 5 v/v) |
0.5 | 150 | — | 84* | 73 |
| 3a | Fructose | — | H2O | 0.5 | 150 | — | 4* | — |
| 4a | Glucose | BAIL | H2O + MIBK (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) 5 v/v) |
0.5 | 150 | 49 | 15 | 10 |
| 5a | Glucose | BAIL | H2O + MIBK (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) 5 v/v) |
4 | 150 | 89 | 6 | 30 |
| 6b | Glucose | BAIL + bone char | H2O | 3 | 160 | 16 | 7 | 6 |
| 7b | Glucose | BAIL + bone char | H2O | 3 | 170 | 33 | 9 | 17 |
| 8b | Glucose | Bone char | H2O | 3 | 170 | 78 | 5 | 15 |
| 9b | Glucose | BAIL | H2O | 3 | 170 | 98 | 2 | 19 |
| 10b | Glucose | BAIL + bone char | H2O | 6 | 160 | 25 | 7 | 12 |
| 11b | Glucose | BAIL + bone char | H2O | 24 | 160 | 74 | 3 | 33 |
| 12c | Glucose | BAIL + bone char | H2O | 6 | 170 | 48 | 4.5 | 26 |
| 13c | Glucose | BAIL + bone char | H2O | 9 | 170 | 59 | 5 | 32 |
| 14c | Glucose | BAIL + bone char | H2O | 12 | 170 | 72 | 4 | 39 |
Although an enhanced HMF yield with fructose could be achieved in the biphasic solvent system with the assistance of the BAIL catalyst, the use of an organic solvent would cause environmental pollution. Basically, in the biphasic solvent system, the HMF yield is higher compared to that in water because of the possibility of more side reactions in water. In the present study, our aim was to decrease the formation of side reactions in the water solvent system to improve the selectivity of HMF and make the process environmentally friendly. The results also suggest that the production of HMF from glucose is more difficult compared to the fructose conversion.37 To overcome these problems, we first performed glucose-to-fructose isomerization prior to fructose-to-HMF dehydration in the water system. It is known that glucose-to-fructose isomerization is a reversible reaction, and Lewis acid or Brønsted basic catalysts are beneficial in this reaction.38 On the other hand, the dehydration of fructose into HMF can be efficiently carried out using a Brønsted acid catalyst.39 In the present work, we combined two catalysts: bone char with Lewis and Brønsted basicity and a BAIL that are both helpful for glucose-to-fructose isomerization and fructose-to-HMF dehydration, for producing HMF from glucose in water.
The bone char is in the form of a powder and the BAIL is in the form of a liquid, so it is possible to perform a one-pot glucose-to-fructose-to-HMF reaction. We first studied the effect of reaction temperature, and found that a slight increase in temperature from 160 °C to 170 °C resulted in a double increase in glucose conversion regardless of whether the reaction time was short (3 h, entry 6 and 7 of Table 2) or long (6 h, entry 10 and 12 of Table 2). It is believed that 170 °C is a suitable temperature, and the reactions carried out above 170 °C resulted in the formation of degradation products due to a condensation product of sugars and HMF in the presence of a catalyst.37 The HMF selectivity and yield were influenced by reaction time. Therefore, the reactions were performed for 3 h to 6 h, and 12 h and the results showed that both glucose conversion, (from 33% to 72%) and HMF yield (from 17% to 39%) increased, as shown in entries 7, 12, 13 and 14; Table 2. The optimized reaction showed 72% of glucose conversion, 39% HMF yield (entry 14), and 54% HMF selectivity. The above results indicated that selective glucose-to-fructose-to-HMF conversion is driven by the bone char and BAIL catalysts. The combination of the bone char and BAIL exhibited very high selectivity for HMF (54%), which is the highest value in water systems, even when the reactions were carried out using glucose as the starting sugar.
To demonstrate the stability of bone char under reaction conditions, the fresh bone char and the bone char recovered after the reaction were characterized with XRD. As shown in Fig. 1, the XRD patterns of these two samples are identical, which confirms that the bone char catalyst is stable under reaction conditions. Furthermore, the recovered bone char that was used for the glucose isomerization reaction under optimized reaction conditions showed no drop-in activity (16% fructose yield with 28% glucose conversion). The TGA results also show that the bone char recovered from the reaction mixture is stable under reaction conditions (Fig. S8 and S9 ESI†). Similarly, the BAIL catalyst was also isolated from the reaction mixture and characterized with 1H NMR spectroscopy. The result revealed that the BAIL was also a stable catalyst (Fig. S10 and S11; ESI†). Moreover, the Hammet analysis results showed that the BAIL recovered from the reaction mixture has a similar Hammet acidity (Ho = 2.08; the Ho analysis was performed with 0.075 g BAIL in 50 mL p-nitroaniline indicator solution) to that of the fresh BAIL.
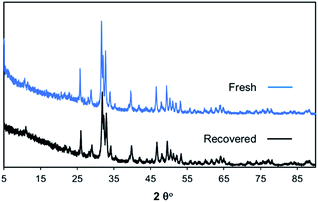 | ||
| Fig. 1 The XRD patterns of the fresh and recovered bone char after the reaction. Reaction conditions: glucose 0.2 g, bone char 0.1 g, BAIL 0.1 g, H2O 20 mL, 170 °C, 3 h. | ||
It has been reported that the use of glucose instead of fructose for the production of HMF always results in more by-products (formic acid, levulinic acid, succinic acid, etc.).40 Additionally, there is an increased chance of side reactions when water is used as a solvent for the dehydration of glucose into HMF. This is because in the presence of only water as the reaction solvent, the sugars can react with the formed HMF in the presence of a catalyst which results in further side products. Our previous study also provided evidence for this phenomenon when using fructose as the starting sugar and a BAIL as the catalyst.8 In this study, the use of only a BAIL catalyst for the glucose dehydration reaction showed the formation of a dark-brown solution due to the formation of by-products such as levulinic acid and formic acid. Additionally, a black solid formation was also seen that could have been humin side-products, as shown in Fig. 2 (right), which drastically decreased the selectivity of HMF (19%). In contrast, the use of combined bone char and acidic IL exhibited a light colored reaction mixture and inhibited the formation of solid side-products in the reaction mixture, as presented in Fig. 2 (left), which resulted in enhanced HMF selectivity.
Although almost similar HMF yields (15% and 19%) are attained using the bone char and BAIL for the 3 h reaction at 170 °C in water, a significant difference was observed in the glucose conversion (Table 1, entries 8 and 9). As it is mentioned in the introduction section, in the presence of a Brønsted acid catalyst (BAIL) the glucose is directly converted into HMF, which results in a higher glucose conversion (98%). On the other hand, when bone char (which has both Lewis and Brønsted basicity) was used for the reaction the glucose is first isomerized into fructose and then converted into HMF. The glucose isomerization reaction is reversible which is responsible for a lower glucose conversion (78%). The bone char has Lewis basicity (the ability to donate lone pairs of electrons, thus capable of coordination to a Lewis acid) because of PO43− and Brønsted basicity (ability to attract an H+ ion from an acid) due to OH−. The acid sites of the BAIL can react with the basic sites of hydroxyapatite part of bone char and show the possibility for neutralization. However, this is a reversible reaction, and the results of the catalyst characterization study confirm that the BAIL and bone char are stable under reaction conditions. The active sites of bone char are not well understood and require a detailed study for a better understanding of the role of the active sites.
As a comparison, reactions were also carried out using only bone char as the catalyst in aqueous media at 170 °C for 3 h. The result showed a low HMF yield (15%) and a higher glucose conversion (78%). This suggests that bone char alone is not a beneficial catalyst for fructose-to-HMF dehydration but it can help with glucose-to-fructose isomerization. Recently, glucose-to-HMF conversion with a high HMF selectivity of 60% was reported using a 1-ethyl-3-methylimidazolium bromide (EMIMBr) IL as the solvent and tin phosphate (SnPO) as a catalyst.41 Glucose conversion into HMF was also reported using acid base bi-functional heteropolyacid-based ionic hybrids in a THF/H2O–NaCl solvent system and 53% HMF selectivity was obtained. In this case, NaCl was used as a co-catalyst.19 In the present study, we achieved an almost similar HMF selectivity (54%) in a water solvent system instead of ionic liquid or THF/H2O–NaCl solvent systems that usually increase the capital cost of the process due to the higher cost of ILs. A BAIL was used in this study as the catalyst (a small amount), instead of a solvent. In addition, the utilization of water as the solvent and bone char as a renewable catalyst makes the current system more economically viable and environmentally friendly.
Conclusions
Selective glucose-to-HMF conversion is demonstrated in aqueous media using a combination of bone char and an acidic IL as the catalysts. The inexpensive basic bone char provides the catalytic ability for glucose-to-fructose isomerization while the acidic ionic liquid catalyst promotes fructose-to-HMF dehydration. The use of a basic bone char and acidic IL catalyst combination shows a synergistic effect on increasing HMF selectivity by reducing the side reactions. The maximum glucose conversion of 72% and HMF yield of 39% were obtained for the first time in water medium using an environmentally benign catalyst. The bone char and BAIL catalysts used in this study are stable under reaction conditions, which indicates that they are feasible for current catalytic biomass conversion and that they are also potentially useful for many other acidic/basic catalytic applications. A detailed characterization of the bone char was performed using XRD, SEM, N2-adsorption/desorption and CO2-TPD and the acidic IL was characterized using NMR (1H and 13C) spectroscopy.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Ministry of Science and Technology (MOST) of Taiwan (104-2628-E-002-008-MY3; 105-2218-E-155-007; 105-2221-E-002-003-MY3; 105-2221-E-002-227-MY3; 105-2622-E-155-003-CC2) and the Aim for Top University Project at National Taiwan University (105R7706) for the funding support. This research is also supported by the Global Connections Fund (Priming grant scheme) of the Australian Academy of Technology and Engineering (ATSE) in 2018, the Australian Research Council (ARC) Future Fellow (grant FT150100479), and JSPS KAKENHI (grants 17H05393 and 17K19044).References
- B. M. Matsagar and P. L. Dhepe, New J. Chem., 2017, 41, 6137–6144 RSC.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- B. M. Matsagar, S. A. Hossain, T. Islam, H. R. Alamri, Z. A. Alothman, Y. Yamauchi, P. L. Dhepe and K. C. W. Wu, Sci. Rep., 2017, 7, 13508 CrossRef PubMed.
- L. Hu, J. Xu, S. Zhou, A. He, X. Tang, L. Lin, J. Xu and Y. Zhao, ACS Catal., 2018, 8, 2959–2980 CrossRef.
- B. Liu and Z. Zhang, ChemSusChem, 2016, 9, 2015–2036 CrossRef PubMed.
- W.-H. Peng, Y.-Y. Lee, C. Wu and K. C. W. Wu, J. Mater. Chem., 2012, 22, 23181–23185 RSC.
- Y. Qu, L. Li, Q. Wei, C. Huang, P. Oleskowicz-Popiel and J. Xu, Sci. Rep., 2016, 6, 26067 CrossRef PubMed.
- B. M. Matsagar, M. K. Munshi, A. A. Kelkar and P. L. Dhepe, Catal. Sci. Technol., 2015, 5, 5086–5090 RSC.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef PubMed.
- C. V. Nguyen, Y.-T. Liao, T.-C. Kang, J. E. Chen, T. Yoshikawa, Y. Nakasaka, T. Masuda and K. C. W. Wu, Green Chem., 2016, 18, 5957–5961 RSC.
- T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass, vol. I, 2004 Search PubMed.
- T. Okano, K. Qiao, Q. Bao, D. Tomida, H. Hagiwara and C. Yokoyama, Appl. Catal., A, 2013, 451, 1–5 CrossRef.
- C. Zhou, J. Zhao, A. E. A. Yagoub, H. Ma, X. Yu, J. Hu, X. Bao and S. Liu, Egypt. J. Pet., 2017, 26, 477–487 CrossRef.
- C. V. Nguyen, D. Lewis, W.-H. Chen, H.-W. Huang, Z. A. Alothman, Y. Yamauchi and K. C. W. Wu, Catal. Today, 2016, 278, 344–349 CrossRef.
- C. Liu, J. M. Carraher, J. L. Swedberg, C. R. Herndon, C. N. Fleitman and J.-P. Tessonnier, ACS Catal., 2014, 4, 4295–4298 CrossRef.
- H. Xin, T. Zhang, W. Li, M. Su, S. Li, Q. Shao and L. Ma, RSC Adv., 2017, 7, 41546–41551 RSC.
- P. Zhou and Z. Zhang, Catal. Sci. Technol., 2016, 6, 3694–3712 RSC.
- Y. J. Pagán-Torres, T. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef.
- P. Zhao, Y. Zhang, Y. Wang, H. Cui, F. Song, X. Sun and L. Zhang, Green Chem., 2018, 20, 1551–1559 RSC.
- C. Yue, G. Li, A. Pidko Evgeny, J. Wiesfeld Jan, M. Rigutto and J. M. Hensen Emiel, ChemSusChem, 2016, 9, 2421–2429 CrossRef PubMed.
- V. V. Ordomsky, V. L. Sushkevich, J. C. Schouten, J. van der Schaaf and T. A. Nijhuis, J. Catal., 2013, 300, 37–46 CrossRef.
- L. Hu, X. Tang, Z. Wu, L. Lin, J. Xu, N. Xu and B. Dai, Chem. Eng. J., 2015, 263, 299–308 CrossRef.
- Y. Li, H. Liu, C. Song, X. Gu, H. Li, W. Zhu, S. Yin and C. Han, Bioresour. Technol., 2013, 133, 347–353 CrossRef PubMed.
- T. Ståhlberg, W. Fu, J. M. Woodley and A. Riisager, ChemSusChem, 2011, 4, 451–458 CrossRef PubMed.
- C. Lansalot-Matras and C. Moreau, Catal. Commun., 2003, 4, 517–520 CrossRef.
- R. Galaverna, M. C. Breitkreitz and J. C. Pastre, ACS Sustainable Chem. Eng., 2018, 6, 4220–4230 CrossRef.
- J. Y. G. Chan and Y. Zhang, ChemSusChem, 2009, 2, 731–734 CrossRef PubMed.
- X. Qi, H. Guo, L. Li and R. L. Smith, ChemSusChem, 2012, 5, 2215–2220 CrossRef PubMed.
- H. Han, H. Zhao, Y. Liu, Z. Li, J. Song, W. Chu and Z. Sun, RSC Adv., 2017, 7, 3790–3795 RSC.
- B. M. Matsagar, S. A. Hossain, T. Islam, Y. Yamauchi and K. C. W. Wu, J. Visualized Exp., 2018, 136 DOI:10.3791/57613.
- B. M. Matsagar and P. L. Dhepe, Catal. Sci. Technol., 2015, 5, 531–539 RSC.
- S. Diallo-Garcia, M. B. Osman, J.-M. Krafft, S. Casale, C. Thomas, J. Kubo and G. Costentin, J. Phys. Chem. C, 2014, 118, 12744–12757 CrossRef.
- N. A. Medellin-Castillo, R. Leyva-Ramos, E. Padilla-Ortega, R. O. Perez, J. V. Flores-Cano and M. S. Berber-Mendoza, J. Ind. Eng. Chem., 2014, 20, 4014–4021 CrossRef.
- S. Patel, J. Han, W. Qiu and W. Gao, J. Environ. Chem. Eng., 2015, 3, 2368–2377 CrossRef.
- E. G. Rodrigues, T. C. Keller, S. Mitchell and J. Perez-Ramirez, Green Chem., 2014, 16, 4870–4874 RSC.
- S. Despax, B. Estrine, N. Hoffmann, J. Le Bras, S. Marinkovic and J. Muzart, Catal. Commun., 2013, 39, 35–38 CrossRef.
- P. Bhaumik and P. L. Dhepe, RSC Adv., 2013, 3, 17156–17165 RSC.
- R. Oozeerally, D. L. Burnett, T. W. Chamberlain, R. I. Walton and V. Degirmenci, Chemcatchem, 2018, 10, 706–709 CrossRef PubMed.
- A. Chinnappan, C. Baskar and H. Kim, RSC Adv., 2016, 6, 63991–64002 RSC.
- S. Jia, Z. Xu and Z. C. Zhang, Chem. Eng. J., 2014, 254, 333–339 CrossRef.
- Q. Hou, M. Zhen, L. Liu, Y. Chen, F. Huang, S. Zhang, W. Li and M. Ju, Appl. Catal., B, 2018, 224, 183–193 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00339d |
| This journal is © The Royal Society of Chemistry 2018 |