Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescent and colorimetric molecular recognition probe for hydrogen bond acceptors†
Sarah J.
Pike
 * and
Christopher A.
Hunter
* and
Christopher A.
Hunter

Department of Chemistry, University of Cambridge, Cambridge, CB2 1EW, UK. E-mail: sp816@cam.ac.uk
First published on 6th November 2017
Abstract
The association constants for formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between a H-bond donor, 1-naphthol, and a diverse range of charged and neutral H-bond acceptors have been measured using UV/vis absorption and fluorescence emission titrations. The performance of 1-naphthol as a dual colorimetric and fluorescent molecular recognition probe for determining the H-bond acceptor (HBA) parameters of charged and neutral solutes has been investigated in three solvents. The data were employed to establish self-consistent H-bond acceptor parameters (β) for benzoate, azide, chloride, thiocyanate anions, a series of phosphine oxides, phosphate ester, sulfoxide and a tertiary amide. The results demonstrate both the transferability of H-bond parameters between different solvents and the utility of the naphthol-based dual molecular recognition probe to exploit orthogonal spectroscopic techniques to determine the HBA properties of neutral and charged solutes. The benzoate anion is the strongest HBA studied with a β parameter of 15.4, and the neutral tertiary amide is the weakest H-bond acceptor investigated with a β parameter of 8.5. The H-bond acceptor strength of the azide anion is higher than that of chloride (12.8 and 12.2 respectively), and the thiocyanate anion has a β value of 10.8 and thus is a significantly weaker H-bond acceptor than both the azide and chloride anions.
1 complexes between a H-bond donor, 1-naphthol, and a diverse range of charged and neutral H-bond acceptors have been measured using UV/vis absorption and fluorescence emission titrations. The performance of 1-naphthol as a dual colorimetric and fluorescent molecular recognition probe for determining the H-bond acceptor (HBA) parameters of charged and neutral solutes has been investigated in three solvents. The data were employed to establish self-consistent H-bond acceptor parameters (β) for benzoate, azide, chloride, thiocyanate anions, a series of phosphine oxides, phosphate ester, sulfoxide and a tertiary amide. The results demonstrate both the transferability of H-bond parameters between different solvents and the utility of the naphthol-based dual molecular recognition probe to exploit orthogonal spectroscopic techniques to determine the HBA properties of neutral and charged solutes. The benzoate anion is the strongest HBA studied with a β parameter of 15.4, and the neutral tertiary amide is the weakest H-bond acceptor investigated with a β parameter of 8.5. The H-bond acceptor strength of the azide anion is higher than that of chloride (12.8 and 12.2 respectively), and the thiocyanate anion has a β value of 10.8 and thus is a significantly weaker H-bond acceptor than both the azide and chloride anions.
Introduction
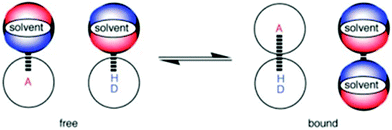
In biological systems, exploitation of the controlled formation of H-bonding interactions to charged or neutral acceptors in molecular recognition motifs plays an essential role in the regulation of structure and function in a wide range of processes.1,2 Molecular recognition events mediated by H-bonding interactions have also been widely employed in supramolecular chemistry3,4 to achieve an operational basis in numerous synthetic systems, finding wide-ranging applications in responsive materials,5 receptors,6 sensing7 and catalysis.8 Given the importance of molecular recognition events involving H-bonding interactions in biological and synthetic systems, the development of H-bond scales that define strength of acceptor and donor species, and thus permit a deeper understanding of the behaviour of solutes in solution, have generated much interest.9–12To develop a quantitative definition of the H-bond properties of solutes in solution, Hunter introduced the electrostatic solvent-competition model to describe the solution-phase equilibrium that exists between H-bonded solutes.13 In this model, the H-bonding interaction formed between two solutes can be interpreted based on pairwise interactions between specific functional group contacts and thus the influence of solvent on the position of equilibrium in the H-bonding interaction can be viewed as a competition between solvent–solute interactions and solvent–solvent interactions (Fig. 1). A variety of UV/vis and NMR spectroscopic molecular recognition probes14–16 have been employed to understand the influence of solvent on solution equilibria but these probes can only be used with a single spectroscopic technique. Dual probes hold distinct advantages over single output systems as they provide orthogonal spectroscopic techniques by which to validate data but dual molecular recognition probes are yet to be reported to study solvation phenomena. Here, we report on the development of a dual molecular recognition probe that employs UV/vis absorption spectroscopy and the complementary spectroscopic technique of fluorescence emission to analyse the influence of solvent on solution phase equilibria.
Using the solvent competition model defined by eqn (1), the Gibbs free energy (ΔG°) of formation of the H-bonded complex between two solutes can be predicted in any solvent environment if the H-bond parameters are known for both the solutes (α and β respectively) and the solvent (αs and βs).
ΔG° (kJ mol−1) = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = −(α − αs)(β − βs) + 6 K = −(α − αs)(β − βs) + 6 | (1) |
Through experimental measurement of the association constants for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H-bonded complexes, eqn (1) can be used to determine the H-bond parameters of solutes and solvents.17–24 For example, eqn (2) may be obtained through rearrangement of eqn (1) and can be employed, with knowledge of α, αs and βs, to determine β values for charged and neutral solutes.
1 H-bonded complexes, eqn (1) can be used to determine the H-bond parameters of solutes and solvents.17–24 For example, eqn (2) may be obtained through rearrangement of eqn (1) and can be employed, with knowledge of α, αs and βs, to determine β values for charged and neutral solutes.
β = βs + (RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K + 6)/(α − αs) K + 6)/(α − αs) | (2) |
Using this method, a diverse range of neutral and charged organic functional groups have been placed on the H-bond acceptor scale. Trialkyl amine oxide and trialkyl phosphine oxides are two of the strongest neutral organic acceptors (β ≈ 11).23,24 Carboxylate anions, benzoate and acetate, have the highest β values (≈15) of the charged acceptors studied.23 The H-bond acceptor properties of the neutral organometallic compound trans-[Ni(F)(2-C5NF4)(PEt3)2] has also been measured (β ≈ 12).24
Whilst the H-bond acceptor properties of a range of charged acceptors have been characterised,23 the thiocyanate and azide anions are of specific interest as they have been shown to have applications in both biological systems and synthetic systems.25 For example, the ability of azide and thiocyanate anions to act as competitive inhibitors of enzymes has been demonstrated26 whilst the effect of thiocyanate anions on protein solubility has been exploited for their use as crystallizing agents in protein crystallography.27 In artificial systems, the formation of H-bonding interactions to azide anions has found applications in crystal engineering28 whilst artificial receptors for both azide and thiocyanate anions have also generated interest.29
Here, we report on the development of a dual molecular recognition probe that employs orthogonal spectroscopic techniques (UV/vis absorption and fluorescence emission) in three solvents to determine the HBA parameters (β) of a range of charged and neutral solutes, including azide and thiocyanate.
Results and discussion
1-Naphthol (1) is a strong H-bond donor (α = 3.9) that has an absorption maximum in the UV/vis region and a fluorescence emission signal both of which undergo significant changes upon formation of a H-bond with a HBA. This permits the measurement of association constants using the two orthogonal spectroscopic techniques and accordingly, the ability of 1 to function as a dual fluorescence and colorimetric molecular recognition probe for HBAs was studied through investigation of the formation of H-bonded complexes of 1 with a series of eleven HBAs (2–12, Schemes 1 and 2) in three different solvents. The four charged acceptors selected as HBAs were benzoate, azide, chloride and thiocyanate anions (2–5). The non-competitive counter-cation, tetrabutylammonium (TBA), was the same in all cases to allow for direct comparison of HBA strength.23,30 The neutral HBAs include a family of phosphine oxides, a phosphate ester, a sulfoxide and a tertiary amide (6–12). The H-bond donor parameter of 1 is comparable to that of phenol,13 at the high end of the α scale. Stable H-bonded complexes are formed by 1 in a range of apolar solvents, even with weaker neutral acceptors, allowing multiple measurements to be obtained for even poor HBAs. Titration experiments were conducted in carbon tetrachloride, chloroform and dichloromethane, so that the transferability of the parameters obtained in different solvent environments could be assessed.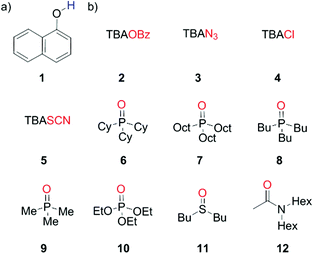 | ||
| Scheme 1 (a) Dual molecular recognition probe, 1-naphthol (1), employed as the H-bond donor (b) charged acceptors (2–5) and neutral acceptors (6–12). | ||
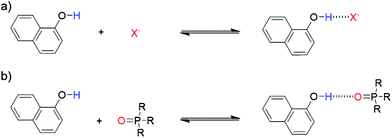 | ||
| Scheme 2 Formation of H-bonded complex between molecular recognition probe 1 and (a) charged or (b) neutral acceptors. | ||
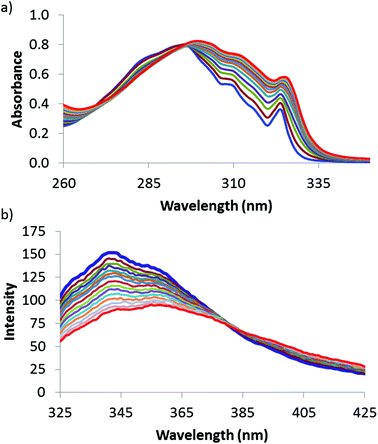
The ability of 1 to function as a dual colorimetric and fluorescent molecular recognition probe was investigated through performing a series of UV/vis absorption and fluorescence emission titration experiments. Representative UV/vis absorption and fluorescence emission spectra are shown in Fig. 2. In the presence of higher concentrations of 2–12, the UV/vis absorption band and fluorescence emission signal of 1 both displayed a marked bathochromic shift (see Fig. 2 and ESI†).18–24,31
By fitting the titration data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm23 or a 1
1 binding isotherm23 or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm that accounts for a second weaker binding interaction,20 a good fit was observed, and consequently, association constants were obtained for the 1·X complexes (where X = 2–12).32,33 The measured association constants are shown in Table 1. There are several instances where acquisition of titration data was not possible either due to overlapping UV/vis signals of the solutes (as for 1·2, 1·3 and 1·5 complexes in carbon tetrachloride and 1·2 and 1·3 complexes in chloroform), or through quenching of the fluorescence signal of 1 (as for 1·2 and 1·3 complexes in dichloromethane and for all the fluorescence titrations undertaken in carbon tetrachloride).34
1 binding isotherm that accounts for a second weaker binding interaction,20 a good fit was observed, and consequently, association constants were obtained for the 1·X complexes (where X = 2–12).32,33 The measured association constants are shown in Table 1. There are several instances where acquisition of titration data was not possible either due to overlapping UV/vis signals of the solutes (as for 1·2, 1·3 and 1·5 complexes in carbon tetrachloride and 1·2 and 1·3 complexes in chloroform), or through quenching of the fluorescence signal of 1 (as for 1·2 and 1·3 complexes in dichloromethane and for all the fluorescence titrations undertaken in carbon tetrachloride).34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with 1 measured by UV/Vis absorption and fluorescence emission titration experiments at 298 Ka
1 complexes with 1 measured by UV/Vis absorption and fluorescence emission titration experiments at 298 Ka
| Acceptor | K/M−1 | |||||
|---|---|---|---|---|---|---|
| UV/vis spectroscopy | Fluorescence spectroscopy | |||||
| CHCl3 | CCl4 | CH2Cl2 | CHCl3 | CH2Cl2 | ||
| a Average of at least two titrations. Errors are quoted at the 95% confidence limit. In all cases greater than 50% saturation of the binding isotherm was achieved. b The absorption of the solute obscured the spectrum. c Quenching of the fluorescence emission of 1 upon addition of increasing amounts of guest. d The association constant was too low to be accurately measured. e Saturation of the binding isotherm was below 50%. | ||||||
| TBAOBz | 2 | —b | —b | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 4400 200 ± 4400 |
2700 ± 700 | —c |
| TBAN3 | 3 | —b | —b | 1300 ± 400 | 440 ± 100 | —c |
| TBACl | 4 | 260 ± 40 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 5000 000 ± 5000 |
810 ± 240 | 270 ± 21 | 700 ± 140 |
| TBASCN | 5 | 110 ± 8 | —b | 200 ± 60 | 120 ± 42 | 210 ± 18 |
| Cy3P(O) | 6 | 136 ± 6 | 5400 ± 200 | 370 ± 14 | 150 ± 60 | 320 ± 48 |
| Oct3P(O) | 7 | 81 ± 16 | 3000 ± 110 | 340 ± 40 | 91 ± 15 | 280 ± 42 |
| Bu3P(O) | 8 | 77 ± 8 | 2500 ± 200 | 260 ± 59 | 74 ± 7 | 200 ± 40 |
| Me3P(O) | 9 | 58 ± 3 | 1900 ± 550 | 180 ± 13 | 52 ± 9 | 140 ± 24 |
| (OEt)3PO | 10 | 29 ± 10 | 340 ± 96 | 47 ± 8 | 21 ± 5 | 52 ± 9 |
| Bu2SO | 11 | —d | 290 ± 51 | —e | —d | 55 ± 14 |
| Acetamide | 12 | —d | 220 ± 76 | —e | —d | 43 ± 9 |
The association constants measured for the 1·X complexes span three orders of magnitude (Table 1). The largest association constants are seen in carbon tetrachloride and the lowest in chloroform whilst the values determined in dichloromethane are intermediate between these two. For example, the association constants measured for the 1·4 complex using UV/vis absorption spectroscopy in carbon tetrachloride is 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, in dichloromethane 810 M−1 and in chloroform 260 M−1. In chloroform, the association constants were too low to be reliably measured for complexes formed with the weaker sulfoxide and tertiary amide HBAs, 11 and 12.
000 M−1, in dichloromethane 810 M−1 and in chloroform 260 M−1. In chloroform, the association constants were too low to be reliably measured for complexes formed with the weaker sulfoxide and tertiary amide HBAs, 11 and 12.
The order of the association constants for different HBAs is consistent in the three solvents:
−OBz > N3− > Cl− > Cy3PO > SCN− ∼ Oct3PO > Bu3PO > Me3PO > (OEt)3PO > Bu2SO > N,N-dihexylacetamide
In general, the stabilities of the H-bonded complexes formed with the anions are stronger than those formed with neutral acceptors which is consistent with the literature.23 Of the charged acceptors, the carboxylate anion forms the most stable complexes with 1 whilst thiocyanate has the lowest association constants. The azide anion forms significantly more stable complexes than the chloride anion. Of the neutral solutes, Cy3PO forms the most stable complexes with 1 in all three solvents whilst the complexes formed with N,N-dihexylacetamide have the lowest association constants. The stability of H-bonded complexes formed with thiocyanate are lower than those obtained for Cy3PO but comparable to those determined for Oct3PO. The association constants measured for H-bonded complexes of the neutral solutes with 1 is highly dependent on the nature of the functional group, following the order; phosphine oxide > phosphate ester > sulfoxide > tertiary amide. This ranking correlates well with literature H-bond acceptor parameters.13 Within the family of phosphine oxide HBAs, 6–9, the stability of the 1·X complex formed depends on the substituents. Cy3PO (6) has slightly larger association constants than analogous complexes formed with the phosphine oxides bearing acyclic aliphatic chains (7–9).
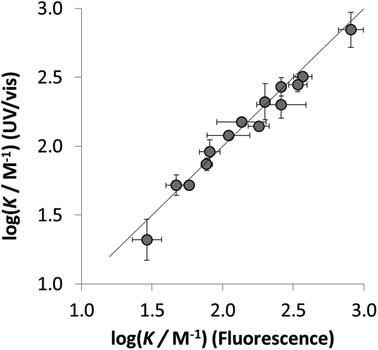
The results obtained using the two orthogonal spectroscopic methods, UV/vis absorption and fluorescence emission, gave comparable association constants for both the neutral and charged 1·X complexes (Fig. 3). The good correlation observed for the association constants measured using the orthogonal spectroscopic techniques in each of solvents indicates consistency in the performance of 1 as a dual molecular recognition probe.
To establish if a set of self-consistent H-bond parameters could be obtained in the three solvents using the two different spectroscopic techniques, the association constants in Table 1 were used in eqn (2) with the solvent H-bond parameters in Table 2 to obtain values of β. Table 3 shows the values of β derived for each of the associations constants in Table 1. Good agreement is observed for the β values for both charged and neutral acceptors utilizing the association constants derived by the two orthogonal spectroscopic techniques and in different solvents. For example, for neutral acceptor, 8 (Oct3PO) displays a β range of 10.7–11.1 for five independently determined values. Consequently, the average HBA parameter can be calculated, as in Table 3, through combining the individual β values and can, therefore, be employed to quantify the HBA strength of charged and neutral solutes in different solvent environments. The excellent correlation observed between the experimentally measured free energies of complexation (ΔG°) and the values calculated using eqn (1) in conjunction with the average β values from Table 3 is shown in Fig. 4.
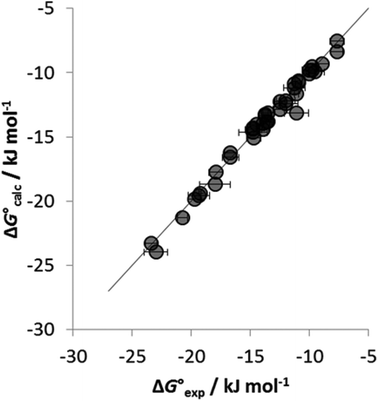 | ||
Fig. 4 Comparison of experimental free energies of complexation  with values calculated using eqn (1) with values calculated using eqn (1) for H-bonded complexes formed with anions and neutral acceptors in carbon tetrachloride, chloroform and dichloromethane using data from both the UV/vis absorption spectroscopy and fluorescence emission spectroscopy titration experiments. The line represents for H-bonded complexes formed with anions and neutral acceptors in carbon tetrachloride, chloroform and dichloromethane using data from both the UV/vis absorption spectroscopy and fluorescence emission spectroscopy titration experiments. The line represents 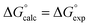 . . | ||
| Acceptor | β | Average β valueb | Literature β value | |||||
|---|---|---|---|---|---|---|---|---|
| UV/vis spectroscopy | Fluorescence spectroscopy | |||||||
| CHCl3 | CCl4 | CH2Cl2 | CHCl3 | CH2Cl2 | ||||
a Errors quoted at twice the standard deviation (2σ) of the individual titrations performed.
b Errors at the 95% confidence limit.
c No experimental data available.
d Based on experimental data obtained for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes measured using UV/vis spectroscopy with three H-bond donors (see ref. 23).
e Based on literature values of βH2 (see ref. 25d and 10). 1 complexes measured using UV/vis spectroscopy with three H-bond donors (see ref. 23).
e Based on literature values of βH2 (see ref. 25d and 10).
|
||||||||
| TBAOBz | 2 | —c | —c | 15.3 ± 0.4 | 15.4 + 0.4 | —c | 15.4 ± 0.1 | 15.1d |
| TBAN3 | 3 | —c | —c | 12.6 ± 0.5 | 12.9 ± 0.3 | —c | 12.8 ± 0.4 | 13.1e |
| TBACl | 4 | 12.2 ± 0.2 | 12.5 ± 0.3 | 12.1 ± 0.3 | 12.3 ± 0.1 | 11.9 ± 0.2 | 12.2 ± 0.4 | 12.1d |
| TBASCN | 5 | 11.1 ± 0.1 | —c | 10.4 ± 0.4 | 11.2 ± 0.5 | 10.5 ± 0.1 | 10.8 ± 0.8 | |
| Cy3P(O) | 6 | 11.3 ± 0.1 | 11.5 ± 0.1 | 11.3 ± 0.1 | 11.5 ± 0.5 | 11.0 ± 0.2 | 11.3 ± 0.4 | |
| Oct3P(O) | 7 | 10.7 ± 0.3 | 11.0 ± 0.2 | 11.0 ± 0.1 | 11.1 ± 0.2 | 10.8 ± 0.1 | 10.9 ± 0.3 | |
| Bu3P(O) | 8 | 10.6 ± 0.2 | 10.7 ± 0.1 | 10.8 ± 0.3 | 10.6 ± 0.1 | 10.5 ± 0.2 | 10.6 ± 0.3 | 10.7d |
| Me3P(O) | 9 | 10.2 ± 0.1 | 10.4 ± 0.3 | 10.3 ± 0.1 | 10.0 ± 0.3 | 10.1 ± 0.3 | 10.2 ± 0.2 | 10.7e |
| (OEt)3PO | 10 | 9.2 ± 0.6 | 8.7 ± 0.2 | 8.8 ± 0.2 | 8.8 ± 0.3 | 9.0 ± 0.2 | 8.9 ± 0.4 | 8.9e |
| Bu2SO | 11 | —c | 8.6 ± 0.2 | —c | —c | 8.9 ± 0.4 | 8.8 ± 0.4 | 8.9e |
| Acetamide | 12 | —c | 8.3 ± 0.3 | —c | —c | 8.6 ± 0.3 | 8.5 ± 0.4 | 8.3e |
The H-bond acceptor properties of the neutral and charged acceptors are depicted in Fig. 5. The β parameters of the anions, BzO− and Cl− are consistent with reported values23 (β = 15.1 and 12.1 respectively) and are, in general, larger than those of neutral acceptors 6–12. N3− has a β value of 12.8 which is close to that of 13.1 calculated from the βH2 value reported by Chabanel et al. in carbon tetrachloride,38 and thus azide is a stronger HBA than Cl−. Chabanel and co-workers qualitatively reported on the weaker HBA ability of thiocyanate compared to azide25d and in this study, we quantify the difference in the HBA strength of the two pseudo-halides, N3− and SCN−, (β values of 12.8 and 10.8 respectively). We have previously shown that the HBA properties of Cl− > NO3− ∼ Br− > I− > ClO4− follow the Hofmeister series,23 which orders anions by their ability to salt out proteins from aqueous solution.39 The β value of SCN− does not fit well within this series as the thiocyanate anion displays HBA properties that are comparable to Br− (β = 10.6) but the Hofmeister series ranks SCN− below I− and ClO4− (β = 8.9 and 8.3 respectively).40 Taylor and Kuntz reported that SCN− displays behavior which does not align with its ranking in the Hofmeister series, when interacting with phenol in apolar organic solvents, exhibiting a HBA strength greater than that of both I− and ClO4−.41 SCN− is a weaker HBA than neutral Cy3PO (β = 11.3).42 Of the studied neutral functional groups, the family of phosphine oxides (6–9) have the highest β values (11.3–10.2) whilst the phosphate ester 10 (8.9) and sulfoxide 11 (8.8) are both stronger H-bond acceptors than tertiary amide, 12 (8.5). Of the four phosphine oxides studied, Cy3PO has the largest β value (11.3) whilst Oct3PO has a β value of 10.8, Bu3PO has a β value of 10.6 and Me3PO the lowest value of 10.2 (Table 3). We have previously reported that trialkyl phosphine oxides have an average β value of 10.7,23 however, there is a degree of variation in the β values of 6–9 (11.3–10.2). The slightly higher β value of Cy3PO compared to the other studied phosphine oxides, 7–9, indicates that the nature of the substituent has an influence on the HBA properties of a functional group. 6 has a HBA strength that is close to that of the strongest neutral acceptor currently placed on the universal scale, trialkyl amine oxide (11.6).24 SCN− is comparable in HBA properties to Oct3PO. The β value of 8.9 for (OEt)3PO matches that determined experimentally by Abraham and co-workers11 whilst the β value of 8.8 obtained for Bu2SO correlates well with reported β value of 8.9.13 In this study we quantify the HBA properties of the sulfoxide demonstrating that they are slightly weaker than that of the phosphonate ester. The tertiary amide is the weakest HBA studied with a β value of 8.5, which correlates well with the reported value of 8.3 calculated from the βH2 value of Abraham.11
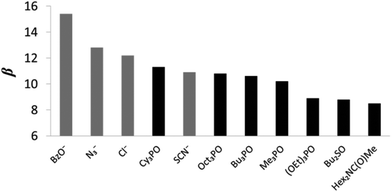 | ||
| Fig. 5 β values for charged and neutral solutes (the anions are shown in grey and the neutral solutes are shown in black). | ||
Conclusions
Fluorescence emission and UV/vis absorption titration experiments have been employed to analyze the formation of H-bonded complexes between eleven H-bond acceptors, of which four were charged and seven were neutral, and the neutral H-bond donor, 1-naphthol, in chloroform, dichloromethane and carbon tetrachloride. The solvent competition model developed by Hunter fully accounts for the spectroscopic data obtained for the H-bonded complexes observed thus permitting the H-bond acceptor parameters (β value) to be determined for the diverse range of anions and the neutral solutes studied. The successful performance of 1-naphthol as a dual molecular recognition probe, with orthogonal spectroscopic readouts, has been demonstrated through the close agreement observed for the data obtained for the H-bonded complexes using both fluorescence emission and UV/vis absorption spectroscopy in different solvents. The transferability of the HBA parameters determined for the neutral and charged species indicates that this data can be readily employed to predict the H-bonding behavior of these anions and neutral solutes in any solvent environment.Benzoate is the strongest H-bond acceptor studied,23 whilst amongst the other charged species investigated, N3− has been shown to be significantly stronger HBA than Cl− whilst SCN− has been identified as a substantially poorer HBA than the halide. The HBA strength of thiocyanate has been shown to be comparable to the neutral solute, Oct3PO. Tertiary amides have been shown to be the weakest HBA investigated whilst the ordering of the studied neutral functional groups follows the ranking; phosphine oxide > phosphate ester > sulfoxide > tertiary amide.
We anticipate that the quantification of the H-bond acceptor parameters of the two pseudo-halides, azide and thiocyanate anions, could facilitate system design in supramolecular architectures which employ these structural motifs. Moreover, the successful performance of 1-naphthol as a dual colorimetric and fluorescent molecular recognition probe represents a new entry into the molecular recognition toolbox providing orthogonal spectroscopic techniques, which are complementary to those that are currently utilized, to study the influence of solvent on solution phase equilibria of H-bond complexes formed between solutes.
General experimental section
All compounds were purchased from Sigma-Aldrich unless otherwise stated. Chloroform was purchased from Acros as 99+% for spectroscopic grade. Tributylphosphine oxide, TBACl, TBASCN, TBAN3, and trioctylphosphine oxide were purchased from Aldrich. TBAOBz and triethyl phosphate were purchased from Fluka. Trimethylphosphine oxide and tricyclohexylphosphine oxide were purchased from Alfa Aesar. All compounds were used as received. The measurements of solids were carried out on a Precisa 125A balance. The following abbreviations are employed: Bz = benzoate, Bu = butyl, Cy = cyclohexyl, Et = ethyl, HBA = H-bond acceptor, HBD = H-bond donor, Hex = hexyl, Me = methyl, Oct = octyl, TBA = tetrabutylammonium.Standard method for UV/vis absorption titrations
Titrations were carried out using a Cary 3 Bio UV/vis spectrophotometer, using standard titration protocols.15 A 10 mL sample of the host, 1-naphthol (1) was prepared at a known concentration (typically between 0.16 mM and 0.20 mM in CHCl3, between 0.14 mM and 0.21 mM in CH2Cl2 and between 0.10 mM and 0.14 mM in CCl4). 2 mL of this solution was removed and added to a quartz cuvette and the UV-Vis spectrum was recorded. The guest (2–12) was then dissolved in 1 mL or 2 mL of the host solution to avoid dilution of the host during the titration and aliquots of this solution were successively added to the cuvette and the UV/vis absorption spectrum was recorded after each addition. The changes in the UV/vis absorption spectra were analysed using a Microsoft Excel spreadsheet to fit the changes in the absorption at fixed wavelengths to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm or a 1
1 binding isotherm or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm accounting for a non-specific interaction optimising the association constant and absorption of the free and bound host using purpose written VBA macros. In all cases, the [guest] was chosen to obtain a binding isotherm of ≥50% saturation.
1 binding isotherm accounting for a non-specific interaction optimising the association constant and absorption of the free and bound host using purpose written VBA macros. In all cases, the [guest] was chosen to obtain a binding isotherm of ≥50% saturation.
H-bond donor 1 displays bathochromic shifting of its characteristic UV/vis absorption band upon complexation with hydrogen bond acceptors 2–12 in the studied solvents.
Standard method for fluorescence titrations
Titrations were carried out using a Cary Eclipse fluorescence spectrophotometer (Agilent). A 10 mL sample of the host, 1-naphthol (1) was prepared at a known concentration (typically between 0.04 mM and 0.09 mM in CHCl3 and between 0.05 mM and 0.06 mM in CH2Cl2). 2 mL of this solution was removed and added to a quartz cuvette and the fluorescence spectrum was recorded. The guest (2–12) was then dissolved in 1 mL or 2 mL of the host solution to avoid dilution of the host during the titration and aliquots of this solution were successively added to the cuvette and fluorescence emission spectrum was recorded after each addition. The changes in the fluorescence emission spectra were analysed using a Microsoft Excel spreadsheet to fit the changes in the absorption at fixed wavelengths to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm or a 1
1 binding isotherm or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm accounting for a non-specific interaction optimising the association constant and absorption of the free and bound host using purpose written VBA macros. In all cases, the [guest] was chosen to obtain a binding isotherm of ≥50% saturation.
1 binding isotherm accounting for a non-specific interaction optimising the association constant and absorption of the free and bound host using purpose written VBA macros. In all cases, the [guest] was chosen to obtain a binding isotherm of ≥50% saturation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the ESPRC. We thank Dr Flore Keymeulen for helpful discussions about the fluorescence emission spectroscopy data.Notes and references
- G. A. Jeffrey and W. Saenger, Hydrogen Bonding in Biological Structures, Springer-Verlag, Berlin, 1991 Search PubMed.
- (a) T. J. Jentsch, Crit. Rev. Biochem. Mol. Biol., 2008, 43, 3 CrossRef CAS PubMed; (b) S. Horowitz and R. C. Trievel, J. Biol. Chem., 2012, 50, 41576–41582 CrossRef PubMed.
- (a) G. Cooke and V. M. Rotello, Chem. Soc. Rev., 2002, 31, 275–286 RSC; (b) J. Cai and J. L. Sessler, Chem. Soc. Rev., 2014, 43, 6198–6213 RSC.
- (a) N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716–11754 CrossRef CAS PubMed; (b) N. Busschaert, C. Caltagirore, W. van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed; (c) A. M. Brouwer, C. Frochot, F. G. Gatti, D. A. Leigh, L. Mottier, F. Paolucci, S. Roffia and G. W. H. Wurpel, Science, 2001, 291, 2124–2128 CrossRef CAS PubMed.
- (a) M. Guo, L. M. Pitet, H. M. Wyss, M. Vos, P. Y. W. Dankers and E. W. Meijer, J. Am. Chem. Soc., 2014, 136, 6969–6977 CrossRef CAS PubMed; (b) J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686–3699 RSC.
- (a) B. W. Tresca, R. J. Hansen, C. V. Chau, B. P. Hay, L. N. Zakharov, M. M. Haley and D. W. Johnson, J. Am. Chem. Soc., 2015, 137, 14959–14967 CrossRef CAS PubMed; (b) S. Goswami, K. Ghosh and M. Halder, Tetrahedron Lett., 1999, 40, 1735–1738 CrossRef CAS; (c) C. R. Bondy and S. J. Loeb, Coord. Chem. Rev., 2003, 240, 77–99 CrossRef CAS; (d) C. Bazzicaupi, A. Benixi, C. Giorgi, V. Lippolis and A. Perra, Inorg. Chem., 2011, 50, 7202–7216 CrossRef PubMed.
- (a) J. W. Grate, Chem. Rev., 2008, 108, 726–745 CrossRef CAS PubMed; (b) R. M. Duke, T. McCabe, W. Schmitt and T. Gunnlaugsson, J. Org. Chem., 2012, 77, 3115–3126 CrossRef CAS PubMed.
- (a) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 571–5743 CrossRef PubMed; (b) Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187–1198 RSC.
- (a) M. H. Abraham and Y. H. Zhao, J. Org. Chem., 2004, 69, 4677–4685 CrossRef CAS PubMed; (b) M. H. Abraham and J. A. Platts, J. Org. Chem., 2001, 66, 3484–3491 CrossRef CAS PubMed; (c) M. H. Abraham, Chem. Soc. Rev., 1993, 22, 73–83 RSC.
- M. H. Abraham, P. L. Grellier, D. V. Prior, J. J. Morris and P. J. Taylor, J. Chem. Soc., Perkin Trans. 2, 1990, 2, 521–529 RSC.
- M. H. Abraham, P. L. Grellier, D. V. Prior, J. J. Morris and P. J. Taylor, J. Chem. Soc., Perkin Trans. 2, 1989, 699–711 RSC.
- M. H. Abraham and W. E. Acree, J. Org. Chem., 2010, 75, 1006–1015 CrossRef CAS PubMed.
- C. A. Hunter, Angew. Chem., Int. Ed., 2004, 43, 5310–5324 CrossRef CAS PubMed.
- R. Cabot and C. A. Hunter, Chem. Soc. Rev., 2012, 41, 3485–3492 RSC.
- V. Amenta, J. L. Cook, C. A. Hunter, C. M. R. Low and J. G. Vinter, J. Phys. Chem. B, 2012, 116, 14433–14400 CrossRef CAS PubMed.
- V. Amenta, J. L. Cook, C. A. Hunter, C. M. R. Low and J. G. Vinter, Org. Biomol. Chem., 2011, 9, 7571–7578 CAS.
- R. Cabot and C. A. Hunter, Org. Biomol. Chem., 2010, 8, 1943–1950 CAS.
- J. L. Cook, C. A. Hunter, C. M. R. Low, A. Perez-Velasco and J. G. Vinter, Angew. Chem., Int. Ed., 2008, 47, 6275–6277 CrossRef CAS PubMed.
- R. Cabot, C. A. Hunter and L. M. Varley, Org. Biomol. Chem., 2010, 8, 1455–1462 CAS.
- J. L. Cook, C. A. Hunter, C. M. R. Low, A. Perez-Velasco and J. G. Vinter, Angew. Chem., Int. Ed., 2007, 46, 3706–3709 CrossRef CAS PubMed.
- V. Amenta, J. L. Cook, C. A. Hunter, C. M. R. Low, H. Sun and J. G. Vinter, J. Am. Chem. Soc., 2013, 135, 12901–12100 CrossRef PubMed.
- N. J. Buurma, J. L. Cook, C. A. Hunter, C. M. R. Low and J. G. Vinter, Chem. Sci., 2010, 1, 242–246 RSC.
- S. J. Pike, J. J. Hutchinson and C. A. Hunter, J. Am. Chem. Soc., 2017, 139, 6700–6706 CrossRef CAS PubMed.
- D. A. Smith, T. Beweries, C. Blasius, N. Jasim, R. Nazir, S. Nazir, C. C. Robertson, A. C. Whitwood, C. A. Hunter, L. Brammer and R. N. Perutz, J. Am. Chem. Soc., 2015, 137, 11820–11831 CrossRef CAS PubMed.
- (a) L. Tchertanov and C. Pascard, Acta Crystallogr., Sect. B: Struct. Sci., 1996, 52, 685–690 CrossRef; (b) J. P. M. Lommerse and J. C. Cole, Acta Crystallogr., Sect. B: Struct. Sci., 1998, 54, 316–322 CrossRef; (c) G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond: In Structural Chemistry and Biology, Oxford University Press, 1999 Search PubMed; (d) P. Goralski, M. Berthelot, J. Rannou, D. Legoff and M. Chabanel, J. Chem. Soc., Perkin Trans. 2, 1994, 2337–2340 RSC.
- L. Tchertanov, Supramol. Chem., 2000, 12, 67–91 CrossRef CAS.
- (a) P. Saludjian, T. Prangé, J. Navaza, R. Ménez, J. P. Guilloteau, M. Riès-Kautt and A. Ducruix, Acta Crystallogr., Sect. B: Struct. Sci., 1992, 48, 520–531 CrossRef; (b) R. Ménez and A. Ducruix, J. Mol. Biol., 1990, 216, 233–234 CrossRef; (c) M. Riès-Kautt and A. Ducruix, J. Biol. Chem., 1989, 264, 745–748 Search PubMed.
- I. S. Bushmarinov, O. G. Nabiev, R. G. Kostyanovsky, M. Y. Antipin and K. A. Lyssenko, CrystEngComm, 2011, 13, 2930–2934 RSC.
- (a) V. Amendola, M. Boiocchi, B. Colasson, L. Fabbrizzi, M. R. Douton and F. Ugozzoli, Angew. Chem., Int. Ed., 2006, 45, 6920–6924 CrossRef CAS PubMed; (b) X. Wang, C. Jia, X. Huang and B. Wu, Inorg. Chem. Commun., 2011, 14, 1508–1510 CrossRef CAS; (c) B. Dietrich, J. Guilhem, J.-M. Lehn, C. Pascard and E. Sonveaux, Helv. Chim. Acta, 1984, 67, 91–104 CrossRef CAS; (d) I. Dilović and K. Užarević, CrystEngComm, 2015, 17, 3153–3161 RSC.
- The nature of the quaternary ammonium counter-cation can influence the strength of the H-bond formed for to H-bond donors in apolar solvents of low dielectric constant: see ref. 23.
- The observed bathochromic shifting of the UV/vis band of 1 in the presence of acceptors 2–12 is due to the formation of a H-bonding interaction which causes a bathochromic shift of the π → π* band of the proton donor. As the molecules in the excited state are more polar, the interaction with the HBA lowers the energy of the excited state more than the ground state leading to a decrease in the energy of the π → π* transition. As energy and wavelength are indirectly proportional this generates a longer wavelength transition and thus leads to red shift of the UV/vis band of 1.
- It is assumed that the binding of the HBD to the thiocyanate anion occurs through the nitrogen atom as has been previously reported: see ref. 29d.
- Through the use of TBASCN and TBAN3 as the guest during the titration experiments, only 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between the guest and host is possible.
1 binding between the guest and host is possible. - P. K. Behem, J. Photochem. Photobiol., A, 1993, 71, 115–118 CrossRef.
- In ref. 20, carbon tetrachloride has been identified to have a αs = 1.4 and βs = 0.6.
- Dichloromethane has previously been identified to have a αs = 1.7 and βs = 1.5 in ref. 19 although it was noted that the experimental data employed to obtain the H-bond parameters of dichloromethane led to a range of possible values; 1.3 < αs < 2.0 and 0.5 < βs < 2.5.
- In ref. 23, the αs of chloroform is given as 2.2 whilst the βs is 1.3.
- In ref. 25d, Chabanel and co-workers obtained association constants for the formation of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H-bonded complexes of TBAN3 and a series of H-bond donors in carbon tetrachloride and from these values calculated the βH2 value of 1.26 for the azide anion. The scales of Abraham and Hunter can be interconverted using the equation β = 10.3(βH2 + 0.06) as in ref. 24 and hence a β value of 13.1 is obtained from the azide anion using the data of Chabanel.
1 H-bonded complexes of TBAN3 and a series of H-bond donors in carbon tetrachloride and from these values calculated the βH2 value of 1.26 for the azide anion. The scales of Abraham and Hunter can be interconverted using the equation β = 10.3(βH2 + 0.06) as in ref. 24 and hence a β value of 13.1 is obtained from the azide anion using the data of Chabanel. - W. Kunz, J. Henle and B. W. Ninham, Curr. Opin. Colloid Interface Sci., 2004, 9, 19–37 CrossRef CAS.
- (a) Y. Zhang and P. S. Cremer, Curr. Opin. Chem. Biol., 2006, 10, 658–663 CrossRef CAS PubMed; (b) Y. Zhang and P. S. Cremer, Annu. Rev. Phys. Chem., 2010, 61, 63–83 CrossRef CAS PubMed.
- R. P. Taylor and I. D. Kuntz Jr., J. Am. Chem. Soc., 1972, 94, 7963–7965 CrossRef CAS.
- The HBA value of the thiocyanate anion is higher than expected and does not follow the Hofmeister series as anticipated from β values obtained for other studied anions; including Cl−, Br−, NO3−, I− and ClO4− see ref. 23. It is unclear as to the reasoning for this high β value of the SCN− although it could, in part, be due to the fact that the anion is linear and thus a different shape to the other anions in the Hofmeister scale that have been studied, which is influencing its ability to interact with the H-bond donor.
Footnote |
| † Electronic supplementary information (ESI) available: Representative UV/vis and fluorescence titration data included. See DOI: 10.1039/c7ob02092a |
| This journal is © The Royal Society of Chemistry 2017 |