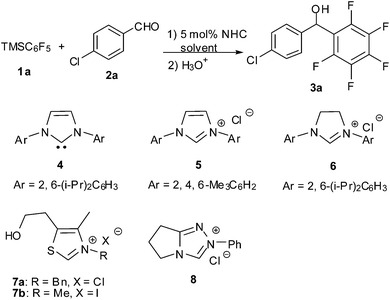
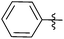
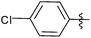
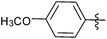
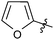
N-heterocyclic carbene-catalysed pentafluorophenylation of aldehydes†
Guang-Fen Duab,
Fen Xingb,
Cheng-Zhi Gu*b,
Bin Dai*b and
Lin Heb
aSchool of Chemical Engineering and Technology, Tianjin University. Tianjin 300072, China
bSchool of Chemistry and Chemical Engineering, Shihezi University, Xinjiang Uygur Autonomous Region, 832000, China. E-mail: gcz_tea@shzu.edu.cn; db_tea@shzu.edu.cn; Fax: +86-993-2057270
First published on 13th April 2015
Abstract
N-heterocyclic carbenes have been utilized as highly efficient organocatalysts to catalyse multifluorophenylation of aldehydes with pentafluorophenyltrimethylsilane or bis(trimethylsilyl)tetrafluorobenzene to afford the corresponding fluorinated adducts in 49–99% yields.
The last decade has witnessed an explosive growth in N-heterocyclic carbenes (NHCs) catalysis.1 As an important type of organocatalyst, NHCs have been utilized widely in organic synthesis. Aside from the classical benzoin reaction2 and Stetter reaction,3 a large number of NHC catalysed transformations, such as the homoenolate reaction of enals,4 redox reaction of functional aldehydes,5 cycloaddition of ketenes,6 transesterification,7 Michael addition8 and other reactions9 have been studied. To date, three different activation modes based on the corresponding ambiphilicity, nucleophilicity and basicity of NHCs have been established.1d More interestingly, NHCs exhibit extremely high reactivity toward the activation of silicon-based nucleophiles.10 For example, Song and co-workers reported that only 0.01 mol% of NHC was enough to efficiently catalyse the cyanosilylation reaction of TMSCN and aldehydes.11 Based on this nucleophilic activation strategy, several NHC catalysed reactions such as trifluoromethylation reaction,12a Mukaiyama aldol reaction,12b–d ring opening reaction,12e silyl-Reformasky reaction,12f,g group-transfer polymerization13 and ring-opening polymerization14 have been developed recently. Despite remarkable progress made in this research field, NHC catalysed activation of Ar–Si bonds remains elusive.
Organofluorine compounds are widely applied in pharmaceutical and agricultural chemistry as well as material science.15 Owing to the special electronic properties of fluorine, the incorporation of fluorinated moieties can modify the biological and physiological properties of a known molecule significantly, which has become a routine strategy in new drug discovery. Therefore, the development of efficient methods for the introduction of fluorinated groups into organic molecules has attracted considerable research interest.
Among different fluorinated moieties, pentafluorophenyl group is an important subset, and compounds containing this moiety are widely utilized in pharmaceuticals, functional material and other fields.16 The nucleophilic addition of pentafluorophenyl lithium or the Grignard reagent to carbonyl compounds is the most straightforward approach17 for the construction of these vital fluorinated molecules. However, many sensitive functional groups can't be well tolerated and the reaction suffers from harsh reaction conditions. Therefore, the development of mild and highly efficient pentafluorophenylation reaction is highly desirable.
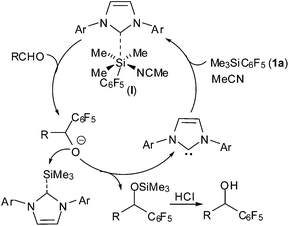
The commercially available pentafluorophenyltrimethylsilane 1a can serve as precursor of pentafluorophenyl carbanion to react with carbonyl compounds. TASF and the toxic KCN can catalyse the addition reactions,18 but with very limited substrate scope. Recently, Lam and co-workers reported19 that transition metal catalyst Cu(OAc)2·dppe can catalyse pentafluorophenylation of aldehydes and active ketones. However, some heteroaromatic aldehydes are not suitable for the reaction. We have developed NHCs-catalysed vinylogous Mukaiyama aldol reaction, phospha-aldol reaction and silyl-Reformasky reaction via nucleophilic activation of silylated nucleophiles.12 We envisioned that NHCs can activate Ar–Si bonds to catalyse pentafluorophenylation reaction of aldehydes. Herein, we would like to disclose this result.
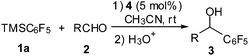
Our studies commenced with the reaction of pentafluorophenyltrimethylsilane 1a and 4-chlorobenzaldehyde 2a. To our delight, under the catalysis of 5 mol% NHC 4 (1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, IPr),20 the reaction proceeded smoothly in THF to produce the desired product 3a in 66% yield (Table 1, entry 1). Encouraged by this result, other common NHCs were next examined. NHCs generated in situ from imidazolium, imidazolinium and different bases can catalyse the reaction efficiently (Table 1, entries 2–5). Whereas NHCs derived from thiazolium and triazolium showed very low efficiency (Table 1, entries 6–8). A brief screening of reaction media indicated that the polar solvents of acetonitrile and formdimethylamide (DMF) can give high yields (Table 1, entries 9–12). Reduction of catalyst loading to 1 mol% led to decrease of reaction rate, but with high yield maintained (Table 1, entry 13).
| Entry | Conditions | Time (h) | Yieldb (%) |
|---|---|---|---|
| a 1a (1.5 equiv.), 2a (1.0 equiv.).b Isolated yield.c Using 1 mol% NHC 4. | |||
| 1 | 4, THF | 2 | 66% |
| 2 | 5, tBuOK, THF | 2 | 64% |
| 3 | 6, tBuOK, THF | 3 | 47% |
| 4 | 5, DBU, THF | 2 | 63% |
| 5 | 5, Cs2CO3, THF | 2 | 55% |
| 6 | 7a, DBU, THF | 6 | 18% |
| 7 | 7b, DBU, THF | 6 | 14% |
| 8 | 8, DBU, THF | 6 | <10% |
| 9 | 4, PhCH3 | 2 | 67% |
| 10 | 4, CH2Cl2 | 2 | 45% |
| 11 | 4, CH3CN | 2 | 88% |
| 12 | 4, DMF | 2 | 84% |
| 13c | 4, CH3CN | 12 | 85% |
With the optimal reaction conditions in hand, the generality of the reaction was next investigated and the results are summarized in Table 2. Both aromatic and aliphatic aldehydes can undergo the addition smoothly to produce the corresponding products. Aromatic aldehydes with electron-withdrawing (entries 1–5), -neutral (entries 6–9), and donating groups (entries 9 and 10) can participate in the reaction smoothly to afford the corresponding products in excellent yields. Meanwhile, different positions of the substituents showed no obvious impact on the reaction yields (entries 11–17). Interestingly, heteroaromatic aldehydes and cinnamaldehyde were proved to be good candidates for the addition, releasing the desired adducts in excellent yields (entries 18–20). The experiment results indicate that aliphatic aldehydes underwent smooth reaction to produce the corresponding products in moderate to high yields (entries 21–23). It is noteworthy that these pentafluorophenylation reactions of aldehydes can be conveniently conducted on gram-scale without sacrificing reaction yield (entry 24).
| Entry | R | Time (h) | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 5 mol% of NHC 4, 1.5 equiv. of 1a, 0.3 mol L−1 of 2, room temperature for 1–3 h.b Isolated yield.c 1a (18 mmol), 2a (15 mmol), using 1 mol% of NHC 4, anhydrous acetonitrile 10.0 mL, room temperature for 12 h. | ||||
| 1 | 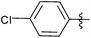 |
2 | 3a | 88 |
| 2 | 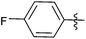 |
1 | 3b | 96 |
| 3 | 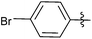 |
2 | 3c | 93 |
| 4 | 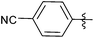 |
1 | 3d | 92 |
| 5 | 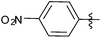 |
1 | 3e | 94 |
| 6 | 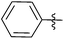 |
2 | 3f | 99 |
| 7 | 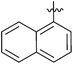 |
2 | 3g | 94 |
| 8 | 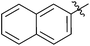 |
2 | 3h | 91 |
| 9 | 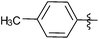 |
2 | 3i | 88 |
| 10 | 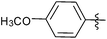 |
4 | 3j | 95 |
| 11 | 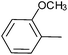 |
3 | 3k | 81 |
| 12 | 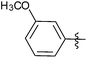 |
1 | 3l | 91 |
| 13 | 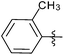 |
2 | 3m | 93 |
| 14 | 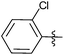 |
1 | 3n | 92 |
| 15 | 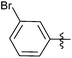 |
1 | 3o | 98 |
| 16 | 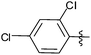 |
1 | 3p | 99 |
| 17 | 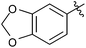 |
3 | 3p | 93 |
| 18 | 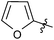 |
2 | 3q | 92 |
| 19 | 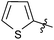 |
2 | 3r | 90 |
| 20 | 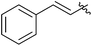 |
2 | 3s | 92 |
| 21 | 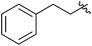 |
2 | 3t | 49 |
| 22 | 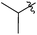 |
2 | 3u | 69 |
| 23 | 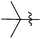 |
2 | 3v | 86 |
| 24c | 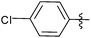 |
12 | 3a | 92 |
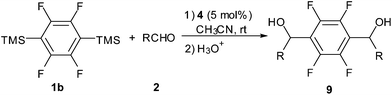
We also found that NHCs can facilitate tetrafluorophenylation of aldehydes efficiently (Table 3). Under the optimized reaction conditions, 1,4-bis(trimethylsilyl)tetrafluorobenzene 1b served as a synthon of tetrafluorobenzene dianion to undergo dual addition with two equiv. of aldehydes to produce tetrafluorophenylation adducts 9 in moderated to good yields.
Based on the pioneering work of NHCs-catalysed nucleophilic addition of silylated reagents with carbonyl compounds,11,12 a plausible mechanism is speculated and illustrated in Scheme 1. NHC attacks the trimethylsilyl group of 1a to form a reactive hexavalent silicon species21 (I), which might initiate the following addition to aldehyde and produce the desired product after acidic work up.
Conclusions
In summary, NHC-catalysed pentafluorophenylation and dual tetrafluorophenylation of aldehydes have been developed. The extremely mild reaction conditions, simple procedure and high yields provide a novel and efficient organocatalytic protocol for the incorporation of multifluorophenyl groups into aldehydes. Further exploration of NHCs-catalysed introduction of other fluorinated moieties are on-going in our laboratory.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21262027) and the Natural Science Foundation of Shihezi university (no. 2011ZRKETD-04, 2012ZRKXJQ06).Notes and references
- (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (b) A. T. Biju, N. Kuhl and F. Glorius, Acc. Chem. Res., 2011, 44, 1182 CrossRef CAS PubMed; (c) J. Mahatthananchai and J. W. Bode, Chem. Sci., 2012, 3, 192–197 RSC; (d) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314 CrossRef CAS PubMed; (e) N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef PubMed.
- (a) D. Enders and U. Kallfass, Angew. Chem., Int. Ed., 2002, 41, 1743 CrossRef CAS; (b) Y. Hachisu, J. W. Bode and K. Suzuki, J. Am. Chem. Soc., 2003, 135, 8432 CrossRef PubMed; (c) D. Enders, O. Niemeier and T. Balensiefer, Angew. Chem., Int. Ed., 2006, 45, 1463 CrossRef CAS PubMed; (d) H. Takikawa, Y. Hachisu, J. W. Bode and K. Suzuki, Angew. Chem., Int. Ed., 2006, 45, 3492 CrossRef CAS PubMed; (e) L. H. Sun, Z. Q. Liang, W. Q. Jia and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 5803–5806 CrossRef CAS PubMed; (f) K. J. Wu, G. Q. Li, Y. Li, L. X. Dai and S. L. You, Chem. Commun., 2011, 47, 493–495 RSC; (g) G. Q. Li, L. X. Dai and S. L. You, Chem. Commun., 2007, 852–854 RSC.
- (a) H. Stetter and M. Schreckenberg, Angew. Chem., Int. Ed., 1973, 12, 81 CrossRef PubMed; (b) M. S. Kerr, J. Read de Alaniz and T. Rovis, J. Am. Chem. Soc., 2002, 124, 10298 CrossRef CAS PubMed; (c) M. S. Kerr and T. Rovis, J. Am. Chem. Soc., 2004, 126, 8876 CrossRef CAS PubMed; (d) J. Read de Alaniz and T. Rovis, J. Am. Chem. Soc., 2005, 127, 6284 CrossRef CAS PubMed; (e) A. E. Mattson, A. M. Zuhl, T. E. Reynolds and K. A. Scheidt, J. Am. Chem. Soc., 2006, 128, 4932 CrossRef CAS PubMed; (f) Q. Liu, S. Perreault and T. Rovis, J. Am. Chem. Soc., 2008, 130, 14066–14067 CrossRef CAS PubMed; (g) D. Enders, J. W. Han and A. Henseler, Chem. Commun., 2008, 3989–3991 RSC; (h) D. A. Dirocco, K. M. Oberg, D. M. Dalton and T. Rovis, J. Am. Chem. Soc., 2009, 131, 10872 CrossRef CAS PubMed; (i) K. Hirano, A. T. Biju, I. Piel and F. Glorius, J. Am. Chem. Soc., 2009, 131, 14190–14191 CrossRef CAS PubMed; (j) D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2011, 133, 10402 CrossRef CAS PubMed.
- For an excellent review, see: (a) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. Paul, A. Jose and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336 RSC . For selected examples, see:; (b) C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205 CrossRef CAS PubMed; (c) S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370 CrossRef CAS PubMed; (d) V. Nair, S. Vellalath, M. Poonoth and E. Suresh, J. Am. Chem. Soc., 2006, 128, 8736–8737 CrossRef CAS PubMed; (e) J. Izquierdo and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634 CrossRef CAS PubMed; (f) X. Chen, X. Fang and Y. R. Chi, Chem. Sci., 2013, 4, 2613–2618 RSC; (g) S. Bera, R. C. Samanta, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 9622–9626 CrossRef CAS PubMed; (h) H. Lv, W.-Q. Jia, L. H. Sun and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 8607–8610 CrossRef CAS PubMed; (i) N. A. White and T. Rovis, J. Am. Chem. Soc., 2014, 136, 14674–14677 CrossRef CAS PubMed; (j) C. Guo, B. Sahoo, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2014, 136(50), 17402–17405 CrossRef CAS PubMed.
- For an excellent review, see: (a) H. U. Vora, P. Wheeler and T. Rovis, Adv. Synth. Catal., 2012, 354, 1617 CrossRef CAS PubMed ; for selected examples, see:; (b) N. T. Reynolds, J. R. D. Alaniz and T. Rovis, J. Am. Chem. Soc., 2004, 126, 9518 CrossRef CAS PubMed; (c) K. Y.-K. Chow and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 8126 CrossRef CAS PubMed; (d) H. U. Vora and T. Rovis, J. Am. Chem. Soc., 2007, 129, 13796 CrossRef CAS PubMed; (e) J. W. Bode and S. S. Sohn, J. Am. Chem. Soc., 2007, 129, 13798 CrossRef CAS PubMed; (f) G.-Q. Li, Y. Li, L.-X. Dai and S.-L. You, Org. Lett., 2007, 9, 3519 CrossRef CAS PubMed; (g) J. Mo, X. Chen and Y. R. Chi, J. Am. Chem. Soc., 2012, 134, 8810 CrossRef CAS PubMed; (h) Q. Ni, H. Zhang, A. Grossmann, C. C. J. Loh, C. Merkens and D. Enders, Angew. Chem., Int. Ed., 2013, 52, 13562–13566 CrossRef CAS PubMed; (i) J. Izquierdo and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634 CrossRef CAS PubMed; (j) L. Candish, A. Levens and D. W. Lupton, Chem. Sci., 2015, 6, 2366–2370 RSC.
- (a) For an excellent review, see: X.-Y. Chen and S. Ye, Synlett, 2013, 24, 1614–1622 CrossRef CAS PubMedfor selected examples, see: (b) Y. R. Zhang, L. He, X. Wu, P. L. Shao and S. Ye, Org. Lett., 2008, 10, 277 CrossRef CAS PubMed; (c) N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith, Org. Biomol. Chem., 2008, 6, 1108 RSC; (d) X.-L. Huang, L. He, P.-L. Shao and S. Ye, Angew. Chem., Int. Ed., 2009, 48, 192 CrossRef CAS PubMed; (e) T. Y. Jian, L. He, C. Tang and S. Ye, Angew. Chem., Int. Ed., 2011, 50, 9104 CrossRef CAS PubMed; (f) P.-L. Shao, X.-Y. Chen and S. Ye, Angew. Chem., Int. Ed., 2010, 49, 8412 CrossRef CAS PubMed; (g) T. Y. Jian, X. Y. Chen, L. H. Sun and Y. Song, Chem. Commun., 2013, 11, 158–163 CAS; (h) J. Douglas, J. E. Taylor, G. Churchill, A. M. Z. Slawin and A. D. Smith, J. Org. Chem., 2013, 78, 3925 CrossRef CAS PubMed; (i) H.-M. Zhang, Z.-H. Gao and S. Ye, Org. Lett., 2014, 16, 3079–3081 CrossRef CAS PubMed.
- (a) G. A. Grasa, R. M. Kissling and S. P. Nolan, Org. Lett., 2002, 4, 3583 CrossRef CAS PubMed; (b) G. W. Nyce, J. A. Lamboy, E. F. Connor, R. M. Waymouth and J. L. Hedrick, Org. Lett., 2002, 4, 3587 CrossRef CAS PubMed; (c) G. A. Grasa, T. Güveli, R. Singh and S. P. Nolan, J. Org. Chem., 2003, 68, 2812 CrossRef CAS PubMed; (d) R. Singh, R. M. Kissling, M.-A. Letellier and S. P. Nolan, J. Org. Chem., 2004, 69, 209 CrossRef CAS PubMed; (e) T. Kano, K. Sasaki and K. Maruoka, Org. Lett., 2005, 7, 1347 CrossRef CAS PubMed.
- (a) T. Boddaert, Y. Coquerel and J. Rodriguez, Adv. Synth. Catal., 2009, 351, 1744–1748 CrossRef CAS PubMed; (b) T. Boddaert, Y. Coquerel and J. Rodriguez, Chem.–Eur. J., 2011, 17, 2266–2271 CrossRef CAS PubMed; (c) E. M. Phillips, M. Riedrich and K. A. Scheidt, J. Am. Chem. Soc., 2010, 132, 13179 CrossRef CAS PubMed; (d) Q. Kang and Y. Zhang, Org. Biomol. Chem., 2011, 9, 6715 RSC; (e) M. Hans, L. Delaude, J. Rodriguez and Y. Coquerel, J. Org. Chem., 2014, 79, 2758–2764 CrossRef CAS PubMed; (f) Y. Z. Li, Y. Wang, G. F. Du, H. Y. Zhang, H. L. Yang and L. He, Asian J. Org. Chem., 2015, 4, 327–332 CrossRef CAS PubMed.
- For selected examples, see: (a) L. He, H. Guo, Y. Z. Li, G. F. Du and B. Dai, Chem. Commun., 2014, 50, 3719 RSC; (b) Z. Fu, J. Xu, T. Zhu, W. W. Y. Leong and Y. R. Chi, Nat. Chem., 2013, 5, 835 CrossRef CAS PubMed; (c) P. Chauhan and D. Enders, Angew. Chem., Int. Ed., 2014, 53, 1485 CrossRef CAS PubMed; (d) X. Bugaut, F. Liu and F. Glorius, J. Am. Chem. Soc., 2011, 133, 8130–8133 CrossRef CAS PubMed; (e) M. T. Hovey, C. T. Check, A. F. Sipher and K. A. Scheidt, Angew. Chem., Int. Ed., 2014, 9603–9607 CrossRef CAS PubMed; (f) X. Chen, Z. Gao, C. Song, C. Zhang, Z. Wang and S. Ye, Angew. Chem., Int. Ed., 2014, 53, 11611 CrossRef CAS PubMed; (g) F. Li, Z. Wu and J. Wang, Angew. Chem., Int. Ed., 2015, 54, 656 CAS; (h) X. Dong, W. Yang, W. Hu and J. Sun, Angew. Chem., Int. Ed., 2015, 54, 660 CAS; (i) Z. Wu, F. Li and J. Wang, Angew. Chem., Int. Ed., 2015, 54, 1629 CrossRef CAS PubMed.
- For reviews see: (a) M. J. Fuchter, Chem.–Eur. J., 2010, 16, 12286 CrossRef CAS PubMed; (b) L. He, H. Guo, Y. Wang, G.-F. Du and B. Dai, Tetrahedron Lett., 2015, 56, 972 CrossRef CAS PubMed.
- For NHCs-catalysed cyanation reactions, see: (a) Y. Fukuda, Y. Maeda, S. Ishii, K. Kondo and T. Aoyama, Synthesis, 2006, 2006, 589 CrossRef PubMed; (b) J. J. Song, F. Gallou, J. T. Reeves, Z. Tan, N. K. Yee and C. H. Senanayake, J. Org. Chem., 2006, 71, 1273 CrossRef CAS PubMed; (c) Y. Suzuki, M. Bakar, K. Muramatsu and M. Sato, Tetrahedron, 2006, 62, 4227 CrossRef CAS PubMed; (d) M. Tan, Y. Zhang and J. Y. Ying, Adv. Synth. Catal., 2009, 351, 1390 CrossRef CAS PubMed.
- (a) J. J. Song, Z. Tan, J. T. Reeves, F. Gallou, N. K. Yee and C. H. Senanayake, Org. Lett., 2005, 7, 2193 CrossRef CAS PubMed; (b) J. J. Song, Z. Tan, J. T. Reeves, N. K. Yee and C. H. Senanayake, Org. Lett., 2007, 9, 1013 CrossRef CAS PubMed; (c) G. F. Du, L. He, C. Z. Gu and B. Dai, Synlett, 2010, 16, 2513 Search PubMed; (d) Z. H. Cai, G. F. Du, L. He, C. Z. Gu and B. Dai, Synthesis, 2011, 2011, 2073 CrossRef PubMed; (e) J. Wu, X. Sun, S. Ye and W. Sun, Tetrahedron Lett., 2006, 47, 4813 CrossRef CAS PubMed; (f) X. L. Zou, G. F. Du, W. F. Sun, L. He, X. W. Ma, C. Z. Gu and B. Dai, Tetrahedron, 2013, 69, 607 CrossRef CAS PubMed; (g) Y. C. Fan, G. F. Du, W. F. Sun, W. Kang and L. He, Tetrahedron Lett., 2012, 53, 2231 CrossRef CAS PubMed.
- (a) J. Raynaud, A. Ciolino, A. Baceiredo, M. Destarac, F. Bonnette, T. Kato, Y. Gnanou and D. Taton, Angew. Chem., Int. Ed., 2008, 47, 5390 CrossRef CAS PubMed; (b) J. Raynaud, N. Liu, Y. Gnanou and D. Taton, Macromolecules, 2010, 43, 8853 CrossRef CAS; (c) J. Raynaud, N. Liu, M. Fèvre, Y. Gnanou and D. Taton, Polym. Chem., 2011, 2, 1706 RSC.
- (a) B. G. Lohmeijer, G. Dubois, F. Leibfarth, R. C. Pratt, F. Nederberg, A. Nelson, R. M. Waymouth, C. Wade and J. L. Hedrick, Org. Lett., 2006, 8, 4683 CrossRef CAS PubMed; (b) H. A. Brown, Y. A. Chang and R. M. Waymouth, J. Am. Chem. Soc., 2013, 135, 18738 CrossRef CAS PubMed.
- (a) J. Wang, M. Sánchez-Roselló, J. Luis Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (b) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2nd edn, 2013 Search PubMed; (c) I. Ojima, J. Org. Chem., 2013, 78, 6358 CrossRef CAS PubMed; (d) K. Uneyama, J. Fluorine Chem., 2008, 129, 550–576 CrossRef CAS PubMed; (e) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed; (f) Fluorinated Materials for Energy Conversion, ed. T. Nakajima and H. Groult, Elsevier, 2005 Search PubMed; (g) M. Pagliaro and R. Ciriminna, J. Mater. Chem., 2005, 15, 4981–4991 RSC.
- (a) A. Zahn, C. Brotschi and C. J. Leumann, Chem. Eur. J., 2005, 11, 2125 CrossRef CAS PubMed; (b) J. S. Reddy, V. G. Anand and J. Am, Chem. Soc., 2008, 130, 3718–3719 CrossRef CAS PubMed; (c) V. A. Montes, G. Li, R. Pohl, J. Shinar and P. Anzenbacher, Adv. Mater., 2004, 16, 2001 CrossRef CAS PubMed; (d) Y. Sakamoto, T. Suzuki, A. Miura, H. Fujikawa, S. Tokito and Y. Taga, J. Am. Chem. Soc., 2000, 122, 1832 CrossRef CAS; (e) M. Matsui, K. Shirai, N. Tanaka, K. Funabiki, H. Muramatsu, K. Shibata, Y. Abe and Y. Ohgomori, J. Mater. Chem., 1999, 9, 2755 RSC; (f) M. L. Clarke, J. Organomet. Chem., 2003, 665, 65 CrossRef CAS.
- (a) M. E. Jung, Y. H. Jung and Y. Miyazawa, Tetrahedron Lett., 1990, 31, 6983 CrossRef CAS; (b) H. K. Gupta, M. Stradiotto, D. W. Hughes and M. J. McGlinchey, J. Organomet. Chem., 2000, 65, 3652 CrossRef CAS PubMed; (c) S. Elshani, H. Du, K. E. Laintz, N. R. Natale, C. M. Wai, N. S. A. Elkarim and R. A. Bartsch, Tetrahedron, 2000, 56, 4651 CrossRef CAS.
- (a) M. Fujita, M. Obayashi and T. Hiyama, Tetrahedron, 1988, 44, 4135 CrossRef CAS; (b) B. A. Gostevskii, O. A. Kruglaya, A. I. Albanov and N. S. Vyazankin, J. Organomet. Chem., 1980, 187, 157 CrossRef CAS; (c) A. F. Webb, D. S. Sethi and H. Gilman, J. Organomet. Chem., 1970, 21, P61 CrossRef CAS.
- S. Brogan, N. B. Carter and H. W. Lam, Synlett, 2010, 4, 615–617 Search PubMed.
- A. J. Arduengo III, R. Krafczyk and R. Schmutzler, Tetrahedron, 1999, 55, 14523 CrossRef.
- For recent study of penta- and hexacoordinate silicon–NHC complexes see: R. S. Ghadwal, S. S. Sen, H. W. Roesky, G. Tavcar, S. Merkel and D. Stalke, Organometallics, 2009, 28, 6374 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c5ra05487g |
| This journal is © The Royal Society of Chemistry 2015 |