‘All three-in-one’: ferromagnetic interactions, single-molecule magnetism and magnetocaloric properties in a new family of [Cu4Ln] (LnIII = Gd, Tb, Dy) clusters†
Paul
Richardson
a,
Dimitris I.
Alexandropoulos
a,
Luís
Cunha-Silva
c,
Giulia
Lorusso
d,
Marco
Evangelisti
d,
Jinkui
Tang
*b and
Theocharis C.
Stamatatos
*a
aDepartment of Chemistry, Brock University, St. Catharines, Ontario, L2S3A1, Canada. E-mail: tstamatatos@brocku.ca; Tel: (+1)-905-688-5550 Ext. 3400
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
cREQUIMTE/LAQV & Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto, 4169-007 Porto, Portugal
dInstituto de Ciencia de Materiales de Aragón (ICMA) and Departamento de Física de la Materia Condensada, CSIC-Universidad de Zaragoza, 50009 Zaragoza, Spain
First published on 9th September 2015
Abstract
A new family of isomorphous [Cu4Ln] clusters (LnIII = Gd, Tb, Dy) with a ‘propeller’-like topology was obtained from the use of naphthalene-2,3-diol in CuII/LnIII chemistry; all complexes are ferromagnetically-coupled and display either magnetocaloric properties or SMM behavior depending on the central Ln ion.
The field of inorganic and modern coordination chemistry has been undoubtedly flourished over the last three decades, or so, from the synthesis of new polynuclear metal complexes with aesthetically appealing structures,1 exciting supramolecular architectures,2 and interesting magnetic properties such as ferromagnetism,3 single-molecule magnetism (SMM),4 molecular magnetic refrigeration,5 and multifunctional materials.6 In an ideal case, ferromagnetic interactions result from the accidental orthogonality of the interacting magnetic orbitals of two or more paramagnetic metal ions, especially if these orbitals are the eg ones.7 The magnetic exchange interactions are propagated by the donor atoms (i.e., O atoms) of bridging ligands; it has been established that M–O–M (M = metal) angles close to 90° facilitate the orthogonality of the magnetic orbitals.8
Further, when the individual metal ions possess an appreciable number of unpaired electrons, ferromagnetism leads to high-spin molecules. High-spin molecules consisting of isotropic metal ions have shown a remarkable ability to act as magnetic refrigerants.9 Magnetic refrigeration is based on the magnetocaloric effect (MCE), i.e., the change of magnetic entropy (ΔSm) and adiabatic temperature (ΔTad) following a change of the applied magnetic field (ΔB), and can be used for cooling purposes via adiabatic demagnetization.10 In contrast, when a high-spin molecule is made of anisotropic metal ions, SMM properties may emerge. SMMs are molecular species that exhibit an energy barrier, U, to the relaxation of magnetization.11 Experimentally, an SMM expresses superparamagnet-like properties, showing frequency-dependent out-of-phase alternating-current (ac) magnetic susceptibility signals and hysteresis loops, the diagnostic property of a magnet.
Apparently, a combination of metal ions which fulfills all the above objectives is that of CuII with primarily GdIII (8S7/2, S = 7/2, L = 0, g = 2), and subsequently all paramagnetic and anisotropic LnIII ions (i.e., TbIII and DyIII). In addition, the choice of the organic bridging ligand has been always a challenging task. Such a ligand needs to be able to aggregate the metal ions into a polymetallic motif and simultaneously block the extensive polymerization by providing molecular systems with the necessary thermodynamic stability through, for instance, the chelate effect. To that end, we have decided to employ naphthalene-2,3-diol (ndH2) chelate12 in CuII/4f-metal cluster chemistry as a means of obtaining heterometallic compounds with new structural motifs and interesting magnetic properties. We herein report a new family of isomorphous pentanuclear (NHEt3)5[Cu4Ln(nd)8] (Ln = Gd (1), Tb (2), Dy (3)) clusters resulted from the general reaction of Cu(ClO4)2·6H2O, Ln(ClO4)3·xH2O, ndH2 and NEt3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 ratio in MeOH.† The obtained compounds exhibit a ‘propeller’-like topology, they are all ferromagnetically-coupled, and they show either MCE or SMM properties depending on the nature of the LnIII ion. The chemical and structural identities of the complexes were confirmed by single-crystal X-ray crystallography (complete data sets for 1 and 2), elemental analyses (C, H, N), and IR spectral comparison.†
14 ratio in MeOH.† The obtained compounds exhibit a ‘propeller’-like topology, they are all ferromagnetically-coupled, and they show either MCE or SMM properties depending on the nature of the LnIII ion. The chemical and structural identities of the complexes were confirmed by single-crystal X-ray crystallography (complete data sets for 1 and 2), elemental analyses (C, H, N), and IR spectral comparison.†
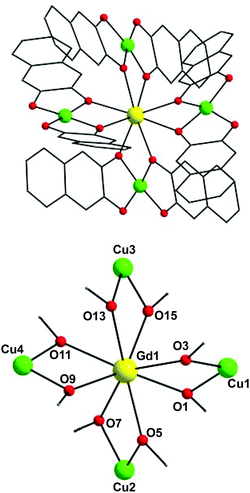
In view of the structural similarities of 1–3, only the structure of (NHEt3)5[Cu4Gd(nd)8] (1) will be described as a representative example. The molecular structure of the anion of 1 (Fig. 1, top) consists of a central GdIII ion surrounded by four CuII atoms in a ‘propeller’-like conformation. Eight doubly-deprotonated nd2− ligands serve to bridge the GdIII atom with the outer CuII atoms using one of the two alkoxido arms; these ligands are thus η1:η2:μ. The non-bridging O atoms of nd2− are strongly hydrogen-bonded to the Et3NH+ countecations, thus enhancing the overall stability and crystallinity of 1. Peripheral ligation about the [Cu4Gd(μ-OR)8]3+ core (Fig. 1, bottom) is provided by the chelating part of the eight naphthalene-2,3-diol groups. The four CuII atoms occupy the vertices of a distorted, non-planar Cu4 rectangle (Fig. S1†) while the central GdIII ion is displaced 0.14 Å away from the Cu4 best-mean-plane. The CuII atoms are not directly linked to each other but only through the GdIII mediator; hence, there are no significant interactions between the 3d-metal ions. The CuII–O–GdIII angles span the range 97.1(2)–102.7(2)°.
All CuII ions are four-coordinate with distorted square planar geometries: the cis- and trans-angles lie in the 86.3–99.7° and 164.9–174.5° ranges, deviating only slightly from the 90° and 180°, respectively, of an ideal square plane. The tetrahedrality13 calculated for Cu1, Cu2, Cu3, and Cu4 in 1 gives average dihedral angles of ∼18, ∼8, ∼11, and ∼10°, respectively, supporting the distorted square planar geometry for the basal Cu(1,2,3,4)O4 planes. The central GdIII is eight-coordinate with a triangular dodecahedral coordination geometry (CShM value = 0.76, program SHAPE;14 see Fig. S2 and Table S2†). TbIII ion of complex 2 is also eight-coordinate with triangular dodecahedral coordination geometry (CShM value = 0.81). Finally, the reported compounds join only a handful of previously reported {Cu4Ln} clusters15 albeit they are the first ‘propeller’-like clusters exhibiting SMM behaviour and MCE properties (vide infra).
Solid-state direct-current (dc) magnetic susceptibility (χM) data on dried and analytically-pure samples of 1–3 were collected in the 2.0–300 K range in an applied field of 0.1 T, and are plotted as χMT vs. T in Fig. 2. The experimental χMT values at 300 K for all complexes are in excellent agreement with the theoretical ones expected for four CuII (S = 1/2, g = 2.2) and one GdIII (9.69 cm3 K mol−1) or TbIII (13.64 cm3 K mol−1) or DyIII (15.99 cm3 K mol−1) non-interacting ions. The magnetic behaviour of 1–3 indicates ferromagnetic exchange interactions between the CuII and LnIII centres, with the χMT products steadily increasing with decreasing temperature to reach the values of 17.91 (1), 18.72 (2), and 21.67 (3) cm3 K mol−1 at T = 2, 3, and 2.5 K, respectively. The χMT value of 1 at 2 K is in excellent agreement with the value of 17.88 cm3 K mol−1, expected for an S = 11/2 system with g = 2. For the anisotropic analogues 2 and 3, the χMT products slightly decrease below ∼3 K, indicating the presence of magnetic anisotropy and/or depopulation of the excited MJ states. For the isotropic analogue 1, we used the PHI program16 and the spin-Hamiltonian H = −J(ŜCu1·ŜGd1 + ŜCu2·ŜGd1 + ŜCu3·ŜGd1 + ŜCu4·ŜGd1) to fit the susceptibility and magnetization data. An excellent fit of the experimental data (solid blue line in Fig. 2) gave as best-fit parameters: J = +1.31(4) cm−1 and g = 2.00(4), thus confirming the intramolecular ferromagnetic interactions between the metal centres and the stabilization of an S = 11/2 spin ground state for 1. Note that the introduction of an additional J-coupling constant, to consider any possible Cu⋯Cu magnetic interactions, in the above spin Hamiltonian did not improve the fit and gave almost identical JCu⋯Gd (+1.36 cm−1) and JCu⋯Cu ∼ 0 cm−1 values. The obtained J value of 1 is of the same order of magnitude, although somewhat smaller than those observed in other Cu4Gd clusters in which the CuII ions are magnetically coupled to each other (JCu⋯Cu ≠ 0).15 The weak ferromagnetic interactions in 1 are likely resulted from the orthogonality of the d and f-metal orbitals.8,15,17
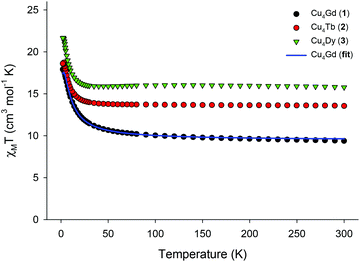 | ||
| Fig. 2 χ M T vs. T plots for 1–3 in an applied field of 0.1 T. Solid blue line is the result of the fit for the Cu4Gd compound, as described in the text. | ||
Magnetization (M) vs. field (H) studies for 1–3 (Fig. S3†) show fast saturated variations, further confirming the presence of predominant ferromagnetic interactions in all compounds. The magnetization of 1 at 2 K saturates fast and in small fields to a value of ∼11NμB consistent with an S = 11/2 spin ground state. To evaluate the accuracy of the susceptibility data, the magnetization data of 1 were also fitted using the above spin-Hamiltonian. Best-fit parameters are: J = +1.34 cm−1 and g = 2.02 (Fig. S3†). The M vs. H plots for 2 and 3 between 1.9 and 5 K justify the presence of magnetic anisotropy, since the data are not superimposed on a single master curve.
Given the recent interest in Cu/Gd-metal clusters as low-temperature magnetic coolers,18 and the large magnetization value of 1 as a result of the ferromagnetic interactions between the metal centres, we decided to pursue magnetocaloric studies. In Fig. 3 we report the magnetic entropy change, −ΔSm, as derived from heat capacity (Fig. S4,† top) and magnetization data. Values of ΔSm can be obtained from the T and field dependencies of the entropy (Fig. S4,† bottom). The maximum −ΔSm for 1 was achieved with ΔB = 7 T at T = 3 K; however, the obtained value of ∼10 J kg−1 K−1 is much lower than the maximum entropy value per mole (4.85R ∼ 18.43 J kg−1 K−1) for 4 CuII and 1 GdIII fully decoupled ions. This indicates that the magnetic coupling between the five metal centres is accountable for the obtained −ΔSm value. Indeed, for an S = 11/2 spin system the maximum −ΔSm expected is given by R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2ST + 1) = R
ln(2ST + 1) = R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 = 2.5R ∼ 9.5 J kg−1 K−1; the latter value is in excellent agreement with the experimental −ΔSm for 1, highlighting the effect of the magnitude of J on the MCE.
12 = 2.5R ∼ 9.5 J kg−1 K−1; the latter value is in excellent agreement with the experimental −ΔSm for 1, highlighting the effect of the magnitude of J on the MCE.
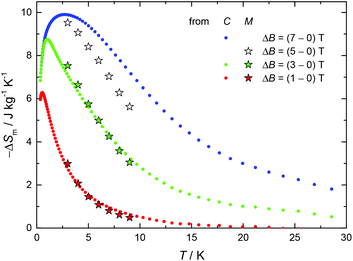 | ||
| Fig. 3 Magnetic entropy change for Cu4Gd (1), as obtained from heat capacity (C) and magnetization (M) experiments for the indicated applied field changes. | ||
Ac magnetic susceptibility studies have been also carried out in order to investigate the magnetization dynamics of 2 (Fig. S5†) and 3 in the absence of an external dc magnetic field. Only the Cu4Dy analogue showed frequency-dependent in-phase ( ) and out-of-phase (
) and out-of-phase ( ) tails of signals at temperatures below ∼5 K (Fig. 4), characteristic of the slow magnetization relaxation of a fast-relaxing SMM with a relatively small energy barrier for the magnetization reversal. Such behavior most likely arises from predominant single-ion effects of the DyIII centre within 3; note that DyIII is a Kramers ion, and irrespective of the ligand field it is expected to possess a bistable ground state.19 On the other hand, TbIII is a non-Kramers ion and so its complexes will have a bistable ground state only if it has an axially-symmetric ligand field.19 Efforts to shift the
) tails of signals at temperatures below ∼5 K (Fig. 4), characteristic of the slow magnetization relaxation of a fast-relaxing SMM with a relatively small energy barrier for the magnetization reversal. Such behavior most likely arises from predominant single-ion effects of the DyIII centre within 3; note that DyIII is a Kramers ion, and irrespective of the ligand field it is expected to possess a bistable ground state.19 On the other hand, TbIII is a non-Kramers ion and so its complexes will have a bistable ground state only if it has an axially-symmetric ligand field.19 Efforts to shift the  tails of signals of 3 to higher temperatures and surpass the fast tunnelling of both 2 and 3 by applying an external dc field are currently in progress.
tails of signals of 3 to higher temperatures and surpass the fast tunnelling of both 2 and 3 by applying an external dc field are currently in progress.
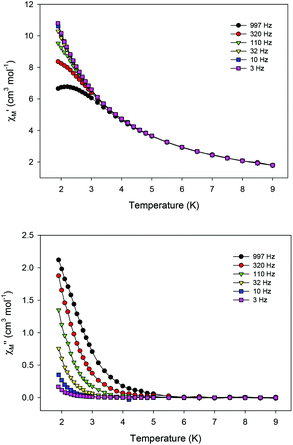 | ||
| Fig. 4 Temperature dependence of the in-phase (top) and out-of-phase (bottom) ac susceptibility signals of 3 in a 3 G field oscillating at the indicated frequencies. | ||
Conclusions
In conclusion, we have shown that the choice of a capable organic chelating/bridging ligand in Cu/Ln chemistry can provide the means of obtaining new cluster compounds with interesting topologies and diverse magnetic properties, such as high-spin ferromagnetic complexes, SMMs and molecular magnetic refrigerants for low-temperature cooling applications. Work in progress includes the synthesis and complete characterization of the remaining members of this family of Cu4Ln clusters, the elucidation of their photophysical properties, and the extension of this research to other polyaromatic diols and 3d/4f-metal combinations.Acknowledgements
This work was supported by Brock University, NSERC-DG and ERA (to Th. C. S), the Ontario Trillium Foundation (graduate scholarship to D. I. A), Fundação para a Ciência e a Tecnologia (FCT, Portugal) for financial support to REQUIMTE/LAQV (UID/QUI/50006/2013), the MINECO (MAT2012-38318-C03, to M. E), and the National Natural Science Foundation of China (grants 21371166, 21331003 and 21221061 to J. T).Notes and references
- (a) A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117 CrossRef CAS PubMed; (b) G. F. S. Whitehead, F. Moro, G. A. Timco, W. Wernsdorfer, S. J. Teat and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2013, 125, 10116 CrossRef PubMed; (c) M. Manoli, R. Inglis, M. J. Manos, V. Nastopoulos, W. Wernsdorfer, E. K. Brechin and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2011, 50, 4441 CrossRef CAS PubMed; (d) M. Murugesu, R. Clérac, C. E. Anson and A. K. Powell, Inorg. Chem., 2004, 43, 7269 CrossRef CAS PubMed; (e) Y. Bi, X.-T. Wang, W. Liao, X. Wang, X. Wang, H. Zhang and S. Gao, J. Am. Chem. Soc., 2009, 131, 11650 CrossRef CAS PubMed; (f) P. Alborés and E. Rentschler, Angew. Chem., Int. Ed., 2009, 48, 9366 CrossRef PubMed; (g) S. K. Langley, R. A. Scott, N. F. Chilton, B. Moubaraki and K. S. Murray, Chem. Commun., 2011, 47, 6281 RSC; (h) E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, J. Am. Chem. Soc., 2010, 132, 16146 CrossRef CAS PubMed.
- (a) J. Fielden and L. Cronin, Encyclopaedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, 2005. DOI:10.1081/E-ESMC-120024346; (b) R. E. P. Winpenny, Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Amsterdam, 2003, ch. 7.3, pp. 125–175 Search PubMed.
- (a) A. M. Ako, I. J. Hewitt, V. Mereacre, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 4926 CrossRef CAS PubMed; (b) E. E. Moushi, Th. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Inorg. Chem., 2009, 48, 5049 CrossRef CAS PubMed; (c) M. Murugesu, M. Habrych, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2004, 126, 4766 CrossRef CAS PubMed; (d) Th. C. Stamatatos, K. A. Abboud, W. Wernsdorfer and G. Christou, Angew. Chem., Int. Ed., 2006, 45, 4134 CrossRef CAS PubMed.
- (a) G. Aromí and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef; (b) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed; (c) J. D. Rinehart and J. R. Long, J. Am. Chem. Soc., 2009, 131, 12558 CrossRef CAS PubMed.
- (a) M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC; (b) J.-L. Liu, Y.-C. Chen, F.-S. Guo and M.-L. Tong, Coord. Chem. Rev., 2014, 281, 26 CrossRef CAS PubMed.
- D. I. Alexandropoulos, A. M. Mowson, M. Pilkington, V. Bekiari, G. Christou and Th. C. Stamatatos, Dalton Trans., 2014, 43, 1965 RSC.
- (a) K. K. Nanda, R. Das, L. K. Thompson, K. Venkatsubramanian and K. Nag, Inorg. Chem., 1994, 33, 1188 CrossRef CAS; (b) S. Banerjee, M. G. B. Drew, C. Z. Lu, J. Tercero, C. Diaz and A. Ghosh, Eur. J. Inorg. Chem., 2005, 2376 CrossRef CAS PubMed; (c) L. Rodríguez, E. Labisbal, A. Sousa-Pedrares, J. A. García-Vázquez, J. Romero, M. L. Durán, J. A. Real and A. Sousa, Inorg. Chem., 2006, 45, 7903 CrossRef PubMed; (d) J. M. Clemente-Juan, B. Chansou, B. Donnadieu and J. P. Tuchagues, Inorg. Chem., 2000, 39, 5515 CrossRef CAS; (e) S. Das, S. Hossain, A. Dey, S. Biswas, E. Pardo, F. Lloret and V. Chandrasekhar, Eur. J. Inorg. Chem., 2014, 3393 CrossRef CAS PubMed.
- O. Kahn, Molecular Magnetism, VCH Publishers, New York, 1993 Search PubMed.
- M. Evangelisti, in Molecular Magnets, NanoScience and Technology, ed. J. Bartolomé, F. Luis and J. F. Fernández, Springer-Verlag, Berlin, Heidelberg, 2014, pp. 365–387 Search PubMed.
- M. Evangelisti, F. Luis, L. J. de Jongh and M. Affronte, J. Mater. Chem., 2006, 16, 2534 RSC.
- D. Gatteschi, R. Sessoli and J. Villain, in Molecular Nanomagnets, Oxford University Press, Oxford, 2006 Search PubMed.
- D. I. Alexandropoulos, A. Fournet, L. Cunha-Silva, A. M. Mowson, V. Bekiari, G. Christou and Th. C. Stamatatos, Inorg. Chem., 2014, 53, 5420 CrossRef CAS PubMed.
- E. S. Koumousi, M. Zampakou, C. P. Raptopoulou, V. Psycharis, C. M. Beavers, S. J. Teat, G. Psomas and Th. C. Stamatatos, Inorg. Chem., 2012, 51, 7699 CrossRef CAS PubMed.
- S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell and D. Avnir, Coord. Chem. Rev., 2005, 249, 1693 CrossRef CAS PubMed.
- (a) Y.-Q. Sun, M. Liang, W. Dong, G.-M. Yang, D.-Z. Liao, Z.-H. Jiang, S.-P. Yan and P. Cheng, Eur. J. Inorg. Chem., 2004, 1514 CrossRef CAS PubMed; (b) L. Zhang, S.-B. Wang, G.-M. Yang, J. Tang, D.-Z. Liao, Z.-H. Jiang, S.-P. Yan and P. Cheng, Inorg. Chem., 2003, 42, 1462 CrossRef CAS PubMed; (c) J. L. Sanz, R. Ruiz, A. Gleizes, F. Lloret, J. Faus, M. Julve, J. J. Borras-Almenar and Y. Journaux, Inorg. Chem., 1996, 35, 7384 CrossRef CAS PubMed; (d) Q. Zhu, S. Xiang, T. Sheng, D. Yuan, C. Shen, C. Tan, S. Hu and X. Wu, Chem. Commun., 2012, 48, 10736 RSC.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS PubMed.
- M. Andruh, I. Ramade, E. Codjovi, O. Guillou, O. Kahn and J.-C. Trombe, J. Am. Chem. Soc., 1993, 115, 1822 CrossRef CAS.
- (a) T. N. Hooper, J. Schnack, S. Piligkos, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2012, 51, 4633 CrossRef CAS PubMed; (b) S. K. Langley, N. F. Chilton, B. Moubaraki, T. Hooper, E. K. Brechin, M. Evangelisti and K. S. Murray, Chem. Sci., 2011, 2, 1166 RSC; (c) J.-D. Leng, J.-L. Liu and M.-L. Tong, Chem. Commun., 2012, 48, 5286 RSC.
- (a) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed; (b) J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC; (c) R. J. Blagg, L. Ungur, F. Tuna, J. Speak, P. Comar, D. N. Collison, W. Wernsdorfer, E. J. L. McInnes, L. Chibotaru and R. E. P. Winpenny, Nat. Chem., 2013, 5, 673 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full synthetic and crystallographic discussion, structural figures, and magnetic data. CCDC 1418227 and 1418228. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qi00146c |
| This journal is © the Partner Organisations 2015 |