Thermo-responsive white-light emission based on tetraphenylethylene- and rhodamine B-containing boronate nanoparticles†
Ayumi
Ozawa
,
Ai
Shimizu
,
Ryuhei
Nishiyabu
and
Yuji
Kubo
*
Department of Applied Chemistry, Graduate School of Urban Environmental Sciences, Tokyo Metropolitan University, 1-1 Minami-ohsawa, Hachioji, Tokyo 192-0397, Japan. E-mail: yujik@tmu.ac.jp; Fax: +81-42-677-3134; Tel: +81-42-677-3134
First published on 3rd November 2014
Abstract
Boronate nanoparticles with built-in tetraphenylethylene (TPE) have been prepared. Their emissive color tuning by altering the amount of rhodamine B grafted onto the surface gave white-light emissive nanoparticles. They showed reversible and thermo-responsive emission in the temperature range of 5–65 °C in water, which enabled them to serve as a bright nanothermometer.
The behavior of chemical and biological systems is affected by temperature. Thus, thermo-responsive materials are among the most fundamental indicators used for a wide range of applications from industry to medical diagnostics.1 In this regard, luminescent materials such as quantum dots2 and lanthanide systems3 have been prepared and elucidated. The fluorescent organic systems, the luminescence depending on temperature, are promising candidates. Indeed, the related systems derived from organic molecules,4 polymers (e.g. poly N-isopropylacrylamide (PNIPAM))5 and triblock copolymers (e.g. poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide))6 have been reported. These systems are capable of monitoring the temperature of solutions by changing their fluorescence intensity due to temperature-dependent photophysical changes in the fluorophores or conformational transitions. Considering thermal imaging of the local environment in fluids,7 it is worthwhile to develop a simple technique with a high color-contrast scheme for the measurement of fluid temperature. With this in mind, white-luminescence nanomaterials are valuable targets, which are made up of components that emit either the three primary colors (red, green, and blue) or two complementary colors to cover the entire visible wavelength region.8 With the help of molecular organization modes such as supramolecular polymers,9 gels,10 self-assembled nanoparticles,11 metal–organic frameworks,12 vesicles,13 micelles,14 organic–inorganic hybrids,15 and supramolecular cages,16 some examples of white-luminescence nanomaterials have been fabricated. However, molecular-based nanothermometers exhibiting white-light emission have not been reported yet. The discovery of a sophisticated interplay between a thermo-responsive luminophore and a complementary emissive color unit to cover the visible region in the solid state would enable us to produce the desired binary system exhibiting white luminescence.
We have recently investigated boronate self-assemblies, which are built by sequential dehydration reactions of diboronic acids with tetraols to form highly dispersible three-dimensional particles through a nucleation process.17 Purposeful surface functionalization of boronate particles allowed us to prepare heterogeneous catalysts18 as well as a recyclable chemosensor for selective monitoring of Cu2+ in water.19 Inspired by these results, we have envisaged that the use of aggregation-induced emission (AIE)-active luminogens, a series of propeller-shaped luminescent molecules,20 as components for boronate assemblies would be a promising way to develop fluorescent particles through restriction of their intramolecular rotation. In the past decade, a number of TPE-derived sensing systems have been proposed by making use of the easy synthetic modification of tetraphenylethylene (TPE). This was possible because of the existence of unique fluorescence switching in response to chemical stimulus-induced conformational transitions as well as changes in the microenvironment.21 In the case of TPE-containing luminescent thermometers, very few examples with PNIPAM have been reported.5b,d This is probably responsible for the limited number of thermo-responsive polymers.
In this study, structurally defined di(boronic acid)-appended tetraphenylethylene (DB-TPE)22 was used to prepare AIE-active blue-emissive boronate nanoparticles (BPs). As described below in detail, tuning of the emission color of these particles has been achieved by varying the amount of rhodamine B grafted onto the surface, which endowed it with white-light emission. It is worth emphasizing that such bright nanoparticles exhibited reversible and thermo-responsive emission in water; this property could be exploited to develop a white-light emissive nanothermometer that can be operated in water.
DB-TPE was straightforwardly synthesized from diphenylmethane, the assignment of the molecule being fully carried out using spectroscopic analysis (see ESI†). It was then allowed to react with pentaerythritol (1) in methanol under ambient conditions; aging the mixture for 2 h produced an opalescent solution (Fig. 1a). After filtration and drying in vacuo, the isolated solid was dispersed in water in which it emits blue light under UV-light irradiation (365 nm) using a handheld UV lamp. The formation of BPs (see FE-SEM, Fig. S1a in the ESI†) was confirmed by means of dynamic light scattering measurements, showing an average diameter of 86 ± 28 nm (Fig. S1b in ESI†). As shown in Fig. 1b, the fluorescence spectrum of BPs has a peak at 479 nm when excited at 365 nm in water. Fig. 1c shows the fluorescence microscopic image of BPs. The emission quantum yield, measured using the method of integrative spheres, was 21%. The component analysis was performed using solid-state 13C-CP-MAS NMR spectroscopy (Fig. S2a in ESI†) where not only signals ranging from 128.95 to 146.77 ppm, arising from the aromatic carbons, but also two signals of methylene carbon at 64.79 ppm and spirocarbon at 36.40 ppm were clearly observed. The use of 11B-DD-MAS NMR allowed us to detect distinct peaks at 9.2 and 15.8 ppm (Fig. S2b in ESI†), which were higher field shifted than those of a typical sp2-hybridized trigonal boron. The line shapes in a quadripolar 11B spectrum have been known to be highly sensitive to the chemical and geometrical bonding environment of boron.23 Thus, the spectrum might be interpreted on the basis of phenyl–boron π-stacking interactions between polymers in the solid state. In addition, the measurement of the infrared (IR) absorption spectrum, using equipment fitted with an attenuated total reflection (ATR) attachment, confirmed the boronate ester bond formation (Fig. S3 in ESI†). The obtained particles were found to be composed of a polymeric dehydrated compound derived from DB-TPE and 1.24 The powder X-ray diffraction (PXRD) spectrum suggests the formation of a structurally ordered aggregate of TPE-containing boronate polymers (Fig. S4 in ESI†).
BPs have a negative zeta potential (−12.14 mV), indicating that the hydroxyl groups at the terminal position of the component polymers are present on the surface.11a We were, therefore, interested in carrying out the grafting reaction using dihydroxyboryl compounds. In order to assess such a hypothesis, we investigated the reaction of BPs with 3,5-di-tert-butylphenylboronic acid 2;25 if 2 could participate in the desired grafting reaction, then the butyl carbon could be monitored by 13C-CP-MAS NMR measurements. NMR measurements of the resultant particles, obtained by aging BPs in a MeOH solution of 2, showed a new signal at 32.55 ppm, attributed to tert-butyl carbon (Fig. S5 in ESI†). These results imply that the surface functionalization is accessible through boronate esterification.
We employed boronic acid-appended rhodamine B (R-dye)19 for dye grafting because the dye emits in a longer wavelength region; this enabled us to tune emission colors from blue to red by changing the amount of the dye grafted onto the surface. As shown in Fig. S6 in the ESI,† the absorption band of the R-dye shows a good overlap with the fluorescence band of BPs ranging from 460 to 600 nm, thus resulting in fluorescence resonance energy transfer (FRET). The grafting of the R-dye onto BPs was carried out by soaking BPs (10.00 mg) in a MeOH solution of the R-dye (20–500 μM) at room temperature for 4 h to yield emissive boronate nanoparticles (R-BPs). All the particles exhibited two peaks at 479 and 590 nm in the fluorescence spectrum when excited at 365 nm in water; these peaks were assignable to the components TPE and rhodamine B, respectively (Fig. 2a). As the amount of the grafted R-dye increased, the emission color clearly changed from blue to red (Fig. 2b). A plausible partial structure where FRET occurs is shown in Fig. 2c. Interestingly, the subsequent trajectory of the Commission Internationale de l’Éclairage (CIE) coordinates in the chromaticity diagram (Fig. 2d) indicates the production of white-light emissive particles R-BP(W), where the CIE chromaticity coordinates were (0.336, 0.354). The fluorescence microscopic image of R-BP(W) is shown in Fig. 2e; the amount of the dye grafted was calculated to be 2.7 × 10−6 mol g−1.26
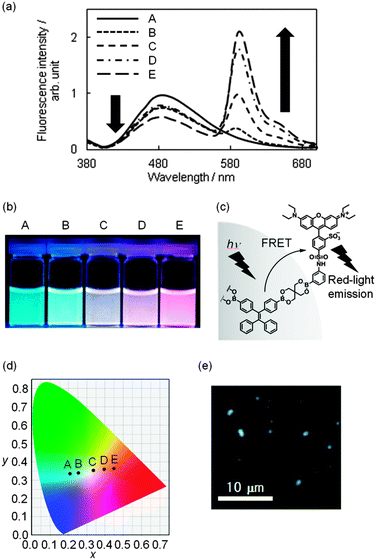 | ||
| Fig. 2 (a) Fluorescence spectra of boronate nanoparticles with binary emissive units (R-BP) in water. λex = 365 nm. The amount of grafted rhodamine B: (A) 0.0 mol g−1; (B) 8.0 × 10−7 mol g−1; (C) 2.7 × 10−6 mol g−1; (D) 3.9 × 10−6 mol g−1; (E) 16 × 10−6 mol g−1. The amount of the grafted dye was assessed by UV/vis absorption spectroscopy. The procedure is described in the ESI.† (b) Photographs of the corresponding boronate nanoparticle dispersion in aqueous media. (c) A plausible partial structure where FRET occurs in R-BP. (d) The composition trajectory to tune colors by changing the amount of rhodamine B shown in the CIE coordinate diagram (λex = 365 nm). (e) The fluorescence microscopic image of white-light emissive particles R-BP(W). | ||
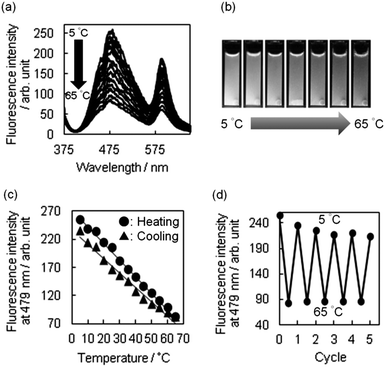
Next, we tried to harness the changes in the white emission as a readily detectable output signal. Therefore, we examined whether R-BP(W) could serve as a bright nanothermometer. Upon heating from 5 to 65 °C, we observed a significant quenching of the white-light emission (Fig. 3a). The change can be seen by the naked eye (Fig. 3b), and the intensities of the fluorescence bands at 479 and 590 nm decreased upon heating. In addition, a representative heating–cooling cycle showed that the particles responded linearly to the range of temperatures studied, with a small hysteresis being observed (Fig. 3c).27 Thus, the temperature sensitivity (S) over the temperature range was defined according to the following equation:1aS = ΔQ/QTΔT × 100% in which QT is the fluorescence intensity at low temperature, ΔQ corresponds to the quenching of fluorescence and is equal to the change in the fluorescence intensity, ΔT is the temperature range, and S is given in % K−1. The value of S for R-BP(W) was determined to be 1.1% K−1, being almost consistent with that of CdTe luminescence thermometry.28
The successful implementation of R-BP(W) for use as a nanothermometer motivated us to examine reversibility of the luminescence spectra between 5 and 65 °C. Fig. 3d shows five heating–cooling cycle experiments for the fluorescence response to temperature variation, indicating a repeatability for such cycles of heating and cooling where the fluorescence intensity at 5 °C reached a similar state but needed 2 additional cycles. Taken together, linear sensitivity and reversible thermo-responsive switching of fluorescence have been successfully achieved by the interplay between TPE immobilized in the particles and rhodamine B grafted onto the surface. Temperature-dependent ring flipping of the unsubstituted phenyl rings in the TPE segments29 plays a significant role in the thermo-responsive function, being followed by the change in emission of rhodamine B through FRET from TPE to the rhodamine B units. In order to strengthen the argument, variable temperature 13C-CP-MAS NMR and DLS measurements of BPs were carried out. In the former case, the shape of signals assignable to methylene carbon and spirocarbon was significantly altered during the increase of temperature from 25 to 65 °C (Fig. S9a in ESI†), possibly due to dynamics in thermo-responsive particles. This speculation has been supported by DLS measurements which allowed us to detect changes in the particle size at different temperatures (Fig. S9b in ESI†). At 5 °C, the averaged diameter of BPs was estimated to be 79 nm, whereas the diameter at 65 °C was 2.8 times larger than that at 5 °C. A synergistic quenching of these emissive units under heating led to a notable change in the emission color, which was detected by the naked eye.
In conclusion, to the best of our knowledge, R-BP(W) represents the first white-light emissive nanothermometer with a temperature sensitivity of 1.1% K−1, which can be operated at physiological temperatures in water. We believe that the proposed boronate-based organization would be a feasible methodology to engineer nanomaterials that can be sensitive to external stimuli.
We thank JASCO for assistance in measuring the emission quantum yield of solid-state BPs. This research was supported by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan (no. 24350075).
Notes and references
- (a) C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799–4829 RSC; (b) X. Wang, O. S. Wolfbeis and R. J. Meier, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- (a) S. Ghosh, W. Hanson, N. Abdollahzadeh and B. Han, Meas. Sci. Technol., 2012, 23, 045704 CrossRef; (b) D. Jaque, B. D. Rosal, E. M. Rodríguez, L. M. Maestro, P. Haro-González and J. G. Solé, Nanomedicine, 2014, 9, 1047–1062 CrossRef CAS PubMed.
- (a) X. Rao, T. Song, J. Gao, Y. Cui, Y. Yang, C. Wu, B. Chen and G. Qian, J. Am. Chem. Soc., 2013, 135, 15559–15564 CrossRef CAS PubMed; (b) R. F. D'Vries, S. álvarez-García, N. Snejko, L. E. Bausá, E. Gutiérrez-Puebla, A. De Andrés and M. A. Monge, J. Mater. Chem. C, 2013, 1, 6316–6324 RSC; (c) Y. Cui, W. Zou, R. Song, J. Yu, W. Zhang, Y. Yang and G. Qian, Chem. Commun., 2014, 50, 719–721 RSC.
- (a) N. Chandrasekharan and L. A. Kelly, J. Am. Chem. Soc., 2001, 123, 9898–9899 CrossRef CAS PubMed; (b) R. E. Brewster, M. J. Kidd and M. D. Schuh, Chem. Commun., 2001, 1134–1135 RSC; (c) Q. Zeng, Z. Li, Y. Dong, C. Di, A. Qin, Y. Hong, L. Ji, Z. Zhu, C. K. W. Jim, G. Yu, Q. Li, Z. Li, Y. Liu, J. Qin and B. Z. Tang, Chem. Commun., 2007, 70–72 RSC; (d) J. Feng, K. Tian, D. Hu, S. Wang, S. Li, Y. Zeng, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2011, 50, 8072–8076 CrossRef CAS PubMed; (e) L. Li, Y. Che, D. E. Gross, H. Huang, J. S. Moore and L. Zang, ACS Macro Lett., 2012, 1, 1335–1338 CrossRef CAS; (f) V. F. Pais, J. M. Lassaletta, R. Fernández, H. S. El-Sheshtawy, A. Ros and U. Pischel, Chem. – Eur. J., 2014, 20, 7638–7645 CrossRef CAS PubMed; (g) S. K. Mellerup and S. Wang, Organometallics, 2014, 33, 5483–5491 CrossRef CAS.
- (a) C. Gota, K. Okabe, T. Funatsu, Y. Harada and S. Uchiyama, J. Am. Chem. Soc., 2009, 131, 2766–2767 CrossRef CAS PubMed; (b) L. Tang, J. K. Jin, A. Qin, W. Z. Yuan, Y. Mao, J. Mei, J. Z. Sun and B. Z. Tang, Chem. Commun., 2009, 4974–4976 RSC; (c) C.-Y. Chen and C.-T. Chen, Chem. Commun., 2011, 47, 994–996 RSC; (d) Y. Jiang, X. Yang, C. Ma, C. Wang, Y. Chen, F. Dong, B. Yang, K. Yu and Q. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 4650–4657 CrossRef CAS PubMed.
- S.-Y. Lee, S. Lee, I.-C. Youn, D. K. Yi, Y. T. Lim, B. H. Chung, J. F. Leary, I. C. Kwon, K. Kim and K. Choi, Chem. – Eur. J., 2009, 15, 6103–6106 CrossRef CAS PubMed.
- D. Ross, M. Gaitan and L. E. Locascio, Anal. Chem., 2001, 73, 4117–4123 CrossRef CAS PubMed.
- (a) H. Wu, L. Ying, W. Yang and Y. Cao, Chem. Soc. Rev., 2009, 38, 3391–3400 RSC; (b) M. C. Gather, A. Köhnen and K. Meerholz, Adv. Mater., 2011, 23, 233–248 CrossRef CAS PubMed; (c) G. M. Farinola and R. Ragni, Chem. Soc. Rev., 2011, 40, 3467–3482 RSC; (d) C. Tang, X.-D. Liu, F. Liu, X.-L. Wang, H. Xu and W. Huang, Macromol. Chem. Phys., 2013, 214, 314–342 CrossRef CAS.
- R. Abbel, C. Grenier, M. J. Pouderoijen, J. W. Stouwdam, P. E. L. G. Leclère, R. P. Sijbesma, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2009, 131, 833–843 CrossRef CAS PubMed.
- (a) R. Abbel, R. van der Weegen, W. Pisula, M. Surin, P. Leclère, R. Lazzaroni, E. W. Meijer and A. P. H. J. Schenning, Chem. – Eur. J., 2009, 15, 9737–9746 CrossRef CAS PubMed; (b) C. Giansante, C. Schäfer, G. Raffy and A. D. Guerzo, J. Phys. Chem. C, 2012, 116, 21706–21716 CrossRef CAS; (c) P. Bairi, B. Roy, P. Chakraborty and A. K. Nandi, ACS Appl. Mater. Interfaces, 2013, 5, 5478–5485 CrossRef CAS PubMed; (d) V. K. Praveen, C. Ranjith and N. Armaroli, Angew. Chem., Int. Ed., 2014, 53, 365–368 CrossRef CAS PubMed.
- (a) C. Vijayakumar, K. Sugiyasu and M. Takeuchi, Chem. Sci., 2011, 2, 291–294 RSC; (b) K.-P. Tseng, F.-C. Fang, J.-J. Shyue, K.-T. Wong, G. Raffy, A. D. Guerzo and D. M. Bassani, Angew. Chem., Int. Ed., 2011, 50, 7032–7036 CrossRef CAS PubMed; (c) R. Abbel, R. van der Weegen, E. W. Meijer and A. P. H. J. Schenning, Chem. Commun., 2009, 1697–1699 RSC; (d) H. Wang, H. Xie, Y. Liang, L. Feng, X. Cheng, H. Lu and S. Feng, J. Mater. Chem. C, 2013, 1, 5367–5372 RSC; (e) S.-C. Chang, S.-J. Chiu, C.-Y. Hsu, Y. Chang and Y.-L. Liu, Polymer, 2012, 53, 4399–4406 CrossRef CAS.
- M.-L. Ma, J.-H. Qin, C. Ji, H. Xu, R. Wang, B.-J. Li, S.-Q. Zang, H.-W. Hou and S. R. Batten, J. Mater. Chem. C, 2014, 2, 1085–1093 RSC.
- X. Zhang, S. Rehm, M. M. Safont-Sempere and F. Würthner, Nat. Chem., 2009, 1, 623–629 CrossRef CAS PubMed.
- X. Zhang, D. Görl and F. Würthner, Chem. Commun., 2013, 49, 8178–8180 RSC.
- (a) F. Gai, T. Zhou, L. Zhang, X. Li, W. Hou, X. Yang, Y. Li, X. Zhao, D. Xu, Y. Liu and Q. Huo, Nanoscale, 2012, 4, 6041–6049 RSC; (b) J. Malinge, C. Allain, A. Brosseau and P. Audebert, Angew. Chem., Int. Ed., 2012, 51, 8534–8537 CrossRef CAS PubMed; (c) Y. Kajiwara, A. Nagai, K. Tanaka and Y. Chujo, J. Mater. Chem. C, 2013, 1, 4437–4444 RSC.
- P. P. Neelakandan, A. Jiménez and J. R. Nitschke, Chem. Sci., 2014, 5, 908–915 RSC.
- R. Nishiyabu, S. Teraoka, Y. Matsushima and Y. Kubo, ChemPlusChem, 2012, 77, 201–209 CrossRef CAS.
- (a) Y. Matsushima, R. Nishiyabu, N. Takanashi, M. Haruta, H. Kimura and Y. Kubo, J. Mater. Chem., 2012, 22, 24124–24131 RSC; (b) S. Fujiwara, N. Takanashi, R. Nishiyabu and Y. Kubo, Green Chem., 2014, 16, 3230–3236 RSC.
- R. Nishiyabu, Y. Sugino and Y. Kubo, Chem. Commun., 2013, 49, 9869–9871 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC; (b) Z. Zhao, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem., 2012, 22, 23726–23740 RSC; (c) R. Hu, N. L. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494–4562 RSC.
- (a) L. Liu, G. Zhang, J. Xiang, D. Zhang and D. Zhu, Org. Lett., 2008, 10, 4581–4584 CrossRef CAS PubMed; (b) C. Park and J.-I. Hong, Tetrahedron Lett., 2010, 51, 1960–1962 CrossRef CAS; (c) Y. Liu, C. Deng, L. Tang, A. Qin, R. Hu, J. Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2011, 133, 660–663 CrossRef CAS PubMed; (d) T. Noguchi, T. Shiraki, A. Dawn, Y. Tsuchiya, L. T. N. Lien, T. Yamamoto and S. Shinkai, Chem. Commun., 2012, 48, 8090–8092 RSC; (e) T. Noguchi, B. Roy, D. Yoshihara, Y. Tsuchiya, T. Yamamoto and S. Shinkai, Chem. – Eur. J., 2014, 20, 381–384 CrossRef CAS PubMed; (f) N.-N. Liu, S. Song, D.-M. Li and Y.-S. Zheng, Chem. Commun., 2012, 48, 4908–4910 RSC; (g) N. B. Shustova, A. F. Cozzolino, S. Reineke, M. Baldo and M. Dincă, J. Am. Chem. Soc., 2013, 135, 13326–13329 CrossRef CAS PubMed; (h) W. Luo, Y. Zhu, J. Zhang, J. He, Z. Chi, P. W. Miller, L. Chen and C.-Y. Su, Chem. Commun., 2014, 50, 11942–11945 RSC.
- Its regioisomers have been synthesized by Tang, et al. and was used as a host for molecular recognition of cyclodextrins; see, Y. Liu, A. Qin, X. Chen, X. Y. Shen, L. Tong, R. Hu, J. Z. Sun and B. Z. Tang, Chem. – Eur. J., 2011, 17, 14736–14740 CrossRef CAS PubMed.
- (a) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed; (b) C. Gervais and F. Babonneau, J. Organomet. Chem., 2002, 657, 75–82 CrossRef CAS.
- Because of low solubility in many solvents, estimation of the number average molecular weight (Mn) and the polydispersity index (PDI) failed.
- A. D. Adams and C. Santini, PCT Pat. Appl., 2007100610A2, 2007 Search PubMed.
- As a control, boronic acid-free rhodamine B was not grafted on the BP surface.
- The stability test has been carried out for the resultant R-BP(W) in water and upon heating. Fig. S7 and S8 show time-dependency on the fluorescence spectra where the spectra in water and upon heating were recorded at several times until 60 min, respectively. As a result, the luminescence properties remained at 90% and 86% of the initial values for 60 min under water and heating conditions, respectively, indicating that R-BP(W) is relatively stable.
- S. Wang, S. Westcott and W. Chen, J. Phys. Chem. B, 2002, 106, 11203–11209 CrossRef CAS.
- N. B. Shustova, T.-C. Ong, A. F. Cozzolino, V. K. Michaelis, R. G. Griffin and M. Dincă, J. Am. Chem. Soc., 2012, 134, 15061–15070 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and data (Fig. S1–S9). See DOI: 10.1039/c4cc07405j |
| This journal is © The Royal Society of Chemistry 2015 |