Copper-mediated/-catalyzed fluorination reactions: new routes to aryl fluorides
Xin
Mu
and
Guosheng
Liu
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, 345 Lingling Road, Shanghai, China. E-mail: gliu@mail.sioc.ac.cn; Fax: +86-21-64166128; Tel: +86-21-54925346
First published on 6th March 2014
Abstract
Recently, a series of transition metals have been extensively explored for the construction of aryl C–F bonds. The introduction of inexpensive copper reagents instead of Pd and Ag complexes would be promising methods for scalable production of aryl fluorides without concomitant hazardous wastes. This highlight mainly focused on recent advances in copper-mediated/-catalyzed fluorination reactions to construct aryl fluorides.
Widespread application of aryl fluorides in pharmaceuticals, agrichemicals, medical diagnosis (positron emission tomography, PET) and materials science has provoked general interest in the development of methodologies for the construction of aryl C–F bonds.1 Quite recently, the prominence of transition-mediated/-catalyzed fluorination protocols has received much attention. In spite of the tremendous efforts which have been dedicated to the practical procedures, the successful examples were limited to palladium and silver catalytic systems.2 The newly emerged fluorination strategy using copper complexes provides promising alternatives to lower the costs towards potential large scale production. This paper highlights recent progress in this issue.
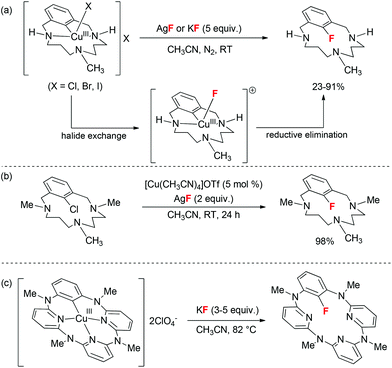
In 2002, Buchwald and co-workers illustrated the first copper-catalyzed halide exchange reactions to synthesize aryl iodides from the corresponding aryl bromides using catalytic amounts of CuI/ligands and NaI as an iodide source.3 However, a comparable mode towards aryl fluorides from aryl bromides and iodides is actually a more challenging issue. In 2006, Grushin reported that stoichiometric amounts of CuF2 can be used to achieve fluorination of phenyl iodide in strong polar solvent (HMPA).4 However, relatively low yield and inseparable dehalogenation byproducts limited its further application. Later on, in 2011, Ribas and co-workers reported well-defined aryl-Cu(III)X (X = Cl, Br, I) complexes to depict the viable involvement of Cu(III) intermediates in these halide exchange processes including the rare aryl C–F bond formation process.5 A sequence involving X-type ligand exchange with the fluoride anion and reductive elimination from the aryl-Cu(III)–F centre was proposed for the scenario (Scheme 1a). Computational studies revealed that aryl C–F reductive elimination is a much more favored process than aryl C–Cl reductive elimination. In addition, the halide exchange on the Cu(III) centre was significantly influenced by the solubility of inorganic fluorides. Finally, the authors also demonstrated the first copper-catalyzed fluorination of aryl chloride within the rigid structure framework (Scheme 1b). Wang and co-workers also independently reported a similar fluorination pattern with a rigid tridentate-nitrogen containing ligand (Scheme 1c).6 These two cases have interpreted the operative aryl C–F bond formation from Cu(III) complexes; however, to advance this protocol towards preparative methods for the aryl fluorides remains a challenging task.
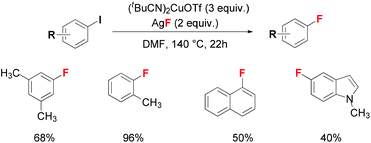
Recently, Hartwig provided a procedure for copper-mediated fluorination of diverse functionalized aryl iodides using AgF as a fluorine source (Scheme 2).7 For less reactive aryl bromides, low conversion and yield was obtained. The author emphasized that an excess amount of copper reagents than AgF is essential for this process due to the side effect of AgF which acted as an oxidant to consume the reactive Cu(I) reagent. Dehalogenation byproducts were also observed during the fluorination reaction. Deuterium-labelling experiments indicated that adventitious water in the reaction system was the hydride source. Furthermore, mechanistic illustration excluded the single-electron-transfer (SET) pathway and an Ar-Cu(III)–F intermediate was proposed for this transformation.
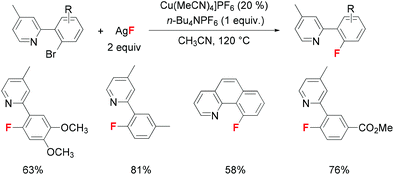
Quite recently, Liu and co-workers reported a novel copper-catalyzed fluorination of allylic bromides and chlorides.8 The success of this halide exchange process relied on pre-installed weak coordinating groups such as carbonyl, ester and phthalimide to facilitate coordination with the copper catalyst, which is helpful to promote the subsequent oxidative addition step. Inspired by this important finding, Liu and co-workers developed a copper-catalyzed fluorination of aryl bromides in the presence of ortho-pyridine coordinating groups (Scheme 3).9 Extensive mechanistic rationale suggested that the pyridyl group will promote oxidative addition toward the aryl C–Br bond and alleviate the oxidation of the copper catalyst by AgF, and this conclusion was further supported by further XANES/EXAFS experiments. In addition, the electron-rich pyridine group significantly promoted the reaction rate than its electron-deficient counterparts which again addressed the vital role of pyridyl directing groups. For the aryl bromide moiety, substrates with electron-rich substituents also exhibited a slight acceleration effect which is consistent with a Cu(I/III) mechanism. Based on the above observations, the authors suggested that this reaction involves a rate-limiting oxidative addition step toward the aryl C–Br bond.
As alternative aryl sources, readily available aryl boron reagents have been successfully applied in copper-mediated fluorination reactions. Recently, Hartwig demonstrated a general procedure to prepare aryl fluorides using electrophilic fluorinating reagents as the fluorine source (Scheme 4a).10 Arylboronate esters provided better yields than other boronic acid derivatives. Inorganic fluoride salts were beneficial to promote the transmetallation step without affecting the base-sensitive fluoro-pyridinium reagents. Both NMR experiments and DFT calculations illustrated two eminent Cu(III)–F structures in THF solutions. Initial oxidation of Cu(I) reagents by fluoro-pyridinium reagents rendered Cu(III)–F species, and the following transmetallation with aryl boron esters and rate-determining reductive elimination produced the aryl fluorides. Meanwhile, Sanford and co-workers described a two-step synthesis of aryl fluorides starting from aryl stannanes and aryl trifluoroborates (Scheme 4b).11 Sequential addition of electrophilic fluorinating reagents and aryl reagents provided much higher yields than the one-pot procedure.
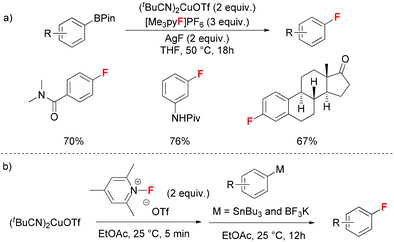 | ||
| Scheme 4 Copper-mediated fluorination of aryl metal reagents with electrophilic fluorinating reagents. | ||
A more desirable method employing inorganic fluoride salts instead of organic electrophilic fluorinating reagents was developed quite recently by Sanford and co-workers (Scheme 5).12 Aryl trifluoroborates could be converted to the corresponding aryl fluorides in the presence of four equivalents of KF and Cu(OTf)2 reagents. The authors emphasized the key oxidation step in which additional Cu(OTf)2 would oxidize the ArCu(II)–F species produced from transmetallation to form the ArCu(III)–F intermediate.
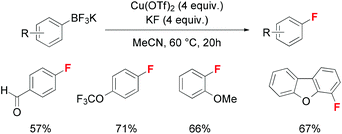 | ||
| Scheme 5 Copper-mediated fluorination of aryl trifluoroborates using KF as nucleophilic fluorinating reagents. | ||
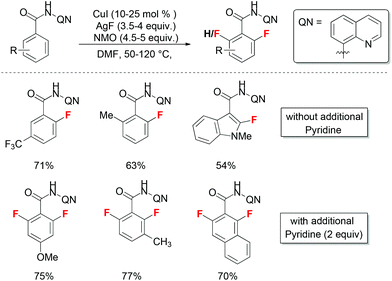
Compared to the above mentioned fluorination of aryl halides and aryl boronates, direct fluorination of the aryl C–H bond would be a more straightforward strategy towards the aryl fluorides. A set of examples have been reported by Daugulis and co-workers using 8-aminoquinoline auxiliary to facilitate copper-catalyzed aryl C–H bond sulfenylation and amination reactions.13 The same directing group was applied in copper catalyzed aryl C–H bond fluorination reactions with AgF as a fluorine source (Scheme 6).14 Two sets of optimal reaction conditions with or without pyridine additive were developed to achieve mono-fluorination or di-fluorination products. This is the only case for copper-catalyzed direct C–H bond fluorination reactions. However, the detailed mechanism is not clear at the moment.
Conclusion and perspective
The copper-mediated/-catalyzed fluorination of aryl reagents has recently received much attention. Although some progress has been achieved, preliminary mechanistic interpretations have been provided to address the formation of aryl C–F bonds. However, this research field is still in its infancy, and the detailed mechanism remains unclear at this stage. In the future, a catalytic version of these reactions would be anticipated based on further detailed analysis of reaction intermediates and elaborate design of new ligands and copper catalysts in order to make this toolbox more applicable.Acknowledgements
We are grateful for the financial support from the National Basic Research Program of China (973-2011CB808700), the National Nature Science Foundation of China (no. 20923005, 21225210, 21202185 and 21121062), the Science and Technology Commission of the Shanghai Municipality (11JC1415000), and the CAS/SAFEA International Partnership Program for Creative Research Teams.Notes and references
- (a) B. E. Smart, Chem. Rev., 1996, 96, 1555 CrossRef CAS PubMed; (b) R. Filler and Y. Koba-yashi, Biomedicinal Aspects of Fluorine Chemistry, Elsevier, Amsterdam, 1982 Search PubMed; (c) Fluorine in Bioorganic Chemistry, ed. J. T. Welch and S. Eswarakrishman, Wiley, New York, 1991 Search PubMed; (d) P. Jeschke, Pest Manag. Sci., 2010, 66, 10 CrossRef CAS PubMed; (e) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (f) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed; (g) P. Jeschke, ChemBioChem, 2004, 5, 570 CrossRef CAS PubMed.
- For recent reviews on transition-mediated fluorination reactions, see: (a) V. V. Grushin, Acc. Chem. Res., 2010, 43, 160 CrossRef CAS PubMed; (b) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed; (c) A. Vigalok, Organometallics, 2011, 30, 4802 CrossRef CAS; (d) C. Hollingworth and V. Gouverneur, Chem. Commun., 2012, 48, 2929 RSC; (e) G. Liu, Org. Biomol. Chem., 2012, 10, 6243 RSC.
- A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2009, 124, 14844 CrossRef PubMed.
- V. V. Grushin and D. E. Hockessin, US, 20060074261, 2006 Search PubMed.
- A. Casitas, M. Canta, M. Sola, M. Costas and X. Ribas, J. Am. Chem. Soc., 2011, 133, 19386 CrossRef CAS PubMed.
- B. Yao, Z.-L. Wang, H. Zhang, D.-X. Wang, L. Zhao and M.-X. Wang, J. Org. Chem., 2012, 77, 3336 CrossRef CAS PubMed.
- P. S. Fier and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 10795 CrossRef CAS PubMed.
- Z. Zhang, F. Wang, X. Mu, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2013, 52, 7549 CrossRef CAS PubMed.
- X. Mu, H. Zhang, P. Chen and G. Liu, Chem. Sci., 2014, 5, 275 RSC.
- P. S. Fier, J. Luo and J. F. Hartwig, J. Am. Chem. Soc., 2013, 135, 2552 CrossRef CAS PubMed.
- Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 4648 CrossRef CAS PubMed.
- Y. Ye, S. D. Schimler, P. S. Hanley and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 16292 CrossRef CAS PubMed.
- (a) L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237 CrossRef CAS PubMed; (b) L. D. Tran, J. Roane and O. Daugulis, Angew. Chem., Int. Ed., 2013, 52, 6043 CrossRef CAS PubMed.
- T. Truong, K. Klimovica and O. Daugulis, J. Am. Chem. Soc., 2013, 135, 9342 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2014 |