N-alkylation of indole via ring-closing metathesis/isomerization/Mannich cascade under ruthenium/chiral phosphoric acid sequential catalysis†
Yan-Chao
Shi
,
Shou-Guo
Wang
,
Qin
Yin
and
Shu-Li
You
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: slyou@sioc.ac.cn; Fax: (+86) 21-54925087
First published on 23rd December 2013
Abstract
A sequential catalysis by combining the Zhan-1B catalyst with chiral phosphoric acid has been utilized for N-alkylation of indole through a ring-closing metathesis/double bond isomerization/Mannich reaction cascade. Enantioenriched γ-lactams were synthesized in up to 92% yield and 95% ee.
Introduction
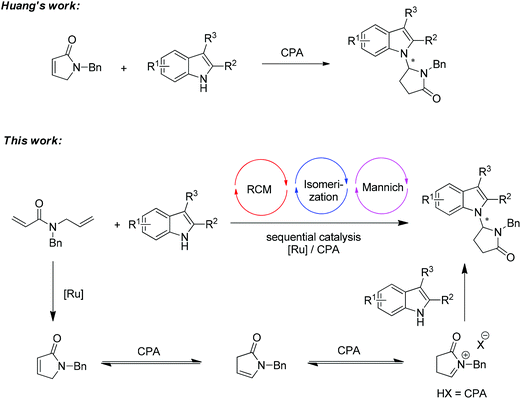
Cascade reactions have broad applications in organic synthesis,1 often reduce time, labor and waste, and enable the construction of complex targets from simple starting materials. It has been proved to be a very efficient strategy to synthesize the indole alkaloids through cascade reactions involving the alkylation of indole.2 Recently, the combination of transition-metal catalyst and chiral phosphoric acid (CPA) has become a subject of intense research for developing new transformations beyond the utilization of the single catalytic system.3,4 Inspired by the pioneering work of Xiao and co-workers on the Ru-catalyzed tandem cross-metathesis/intramolecular Friedel–Crafts alkylation of indoles,5 we recently introduced a sequential catalysis involving a Ru-complex and chiral phosphoric acid.6 Several transformations on the asymmetric alkylation of indole have been realized. However, in general, the asymmetric N-alkylation of indole has been much less explored than the asymmetric Friedel–Crafts alkylation reaction of indole at the C3 position.7,8To be noted, an elegant chiral phosphoric acid catalyzed isomerization of α,β-unsaturated lactam to N-acyl iminium in an enantioselective N-H functionalization of indoles was reported by Huang and co-workers (Scheme 1, top).9 As part of our research program towards the efficient asymmetric transformation of indoles,10 we envisaged that a sequential catalysis combining a Ru-catalyst and chiral phosphoric acid might be able to realize the N-alkylation of indole in a more efficient fashion by employing readily available starting materials (Scheme 1, bottom).
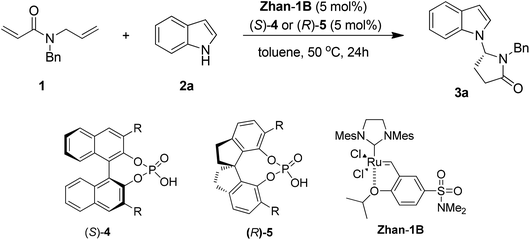
To test our hypothesis, N-allyl-N-benzylacrylamide 1 was treated with 1.2 equivalents of indole (2a) in the presence of 5 mol% chiral phosphoric acid 4a and 5 mol% Zhan-1B in toluene. After stirring at room temperature for 3 days, the reaction gave the desired product 3a in 23% yield and 78% ee (entry 1, Table 1). Prolonging the reaction time to 7 days, the yield of 3a was improved to 52% without decreasing the enantioselectivity (78% ee) (entry 2, Table 1). When the reaction temperature was increased to 50 °C, starting material 1 disappeared after 24 h and the product was obtained in 67% yield and 78% ee (entry 3, Table 1). Under these reaction conditions, more chiral phosphoric acids were tested.
| Entrya | Catalyst | R | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.2 mmol), 2a (0.24 mmol), Zhan-1B (5 mol%), 4 or 5 (5 mol%) in 1.5 mL toluene. b Isolated yield. c Determined by HPLC analysis. d Room temperature for 3 d. e Room temperature for 7 d. | ||||
| 1d | 4a | 2,4,6-(iPr)3-C6H2 | 23 | 78 |
| 2e | 4a | 2,4,6-(iPr)3-C6H2 | 52 | 78 |
| 3 | 4a | 2,4,6-(iPr)3-C6H2 | 67 | 78 |
| 4 | 4b | Ph | 7 | 25 |
| 5 | 4c | 3,5-(CF3)2-C6H3 | 23 | −5 |
| 6 | 4d | 2-Naphthyl | 16 | 21 |
| 7 | 4e | 1-Naphthyl | 8 | 36 |
| 8 | 4f | SiPh3 | 41 | 12 |
| 9 | 4g | 9-Anthryl | 53 | 50 |
| 10 | 4h | 2,6-(iPr)2-4-tBu-C6H2 | 51 | 81 |
| 11 | 5a | 4-Cl-C6H4 | 21 | 29 |
| 12 | 5b | 3,5-(CF3)2-C6H3 | 12 | 33 |
| 13 | 5c | 2,4,6-(iPr)3-C6H2 | 76 | 92 |
First, a series of (S)-BINOL-derived chiral phosphoric acids was tested (entries 1–10, Table 1). The 4-(tert-butyl)-2,6-diisopropylphenyl substituted phosphoric acid afforded an improved enantioselectivity (81% ee, entry 10, Table 1). The sterically congested phosphoric acid catalysts might be crucial for the enantioselective control. Then the (R)-SPINOL-derived phosphoric acids (entries 11–13, Table 1) were screened. To our great delight, the reaction with catalyst 5c bearing 2,4,6-triisopropylphenyl groups led to the best combination of yield and enantioselectivity (76% yield, 92% ee, entry 13, Table 1).
Examination of the reaction temperatures revealed that the enantioselectivity was not influenced at lower temperature (entries 1 and 2, Table 2) but decreased at higher temperatures (entries 1, 3 and 4, Table 2). The yields decreased at 30 °C and 110 °C comparing to those at 50 °C and 80 °C. Investigation of the ruthenium catalysts disclosed that both the Grubbs II and Hoveyda–Grubbs II catalysts gave excellent enantioselectivity. The Hoveyda–Grubbs II catalyst could give a comparable yield (entry 6, Table 2) but the reaction with Grubbs II catalyst resulted in a lower yield (entry 5, Table 2). The reactions in benzene and o-xylene (entries 7 and 8, Table 2) afforded comparable enantioselectivity but decreased yields, while the reaction in DCE led to the drop of both yield and enantioselectivity (entry 9, Table 2). The optimized reaction conditions obtained were as following: 5 mol% Zhan-1B, 5 mol% (R)-5c, 1.2 equivalents of indole in toluene at 50 °C (entry 1, Table 2).
| Entrya | [Ru] catalyst | Solvent | T (%) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.2 mmol), 2a (0.24 mmol), [Ru] (5 mol%), (R)-5c (5 mol%) in 1.5 mL toluene. b Isolated yield. c Determined by HPLC analysis. | |||||
| 1 | Zhan-1B | Toluene | 50 | 76 | 92 |
| 2 | Zhan-1B | Toluene | 30 | 17 | 92 |
| 3 | Zhan-1B | Toluene | 80 | 72 | 85 |
| 4 | Zhan-1B | Toluene | 110 | 29 | 34 |
| 5 | Grubbs II | Toluene | 50 | 19 | 92 |
| 6 | Hoveyda–Grubbs II | Toluene | 50 | 73 | 92 |
| 7 | Zhan-1B | Benzene | 50 | 34 | 90 |
| 8 | Zhan-1B | o-Xylene | 50 | 71 | 91 |
| 9 | Zhan-1B | DCE | 50 | 31 | 83 |
Under the optimized reaction conditions, various indole derivatives were examined to test the generality of the cascade reaction. The results are summarized in Scheme 2. The absolute configuration of product was assigned as R by comparing the sign of the optical rotation with the known compounds reported in the literature.9 In general, all the tested substrates varying substituents on the indole such as 5-Me, 5-OMe, 5-Br, 4-Br, and 6-Cl were tolerated with excellent enantioselectivity (88–94% ee, 3b–3f). The yields are in general moderate to good (52–86% yields) except for 4-Br substituted indole (33% yield). In addition, indole substrates bearing a substituent at the C-3 or C-2 position such as 2-Me, 3-Me, 2,3-(Me)2, and 2,3-(C4H8)- could be well tolerated (67–92% yield, 85–95% ee, 3g–3j).
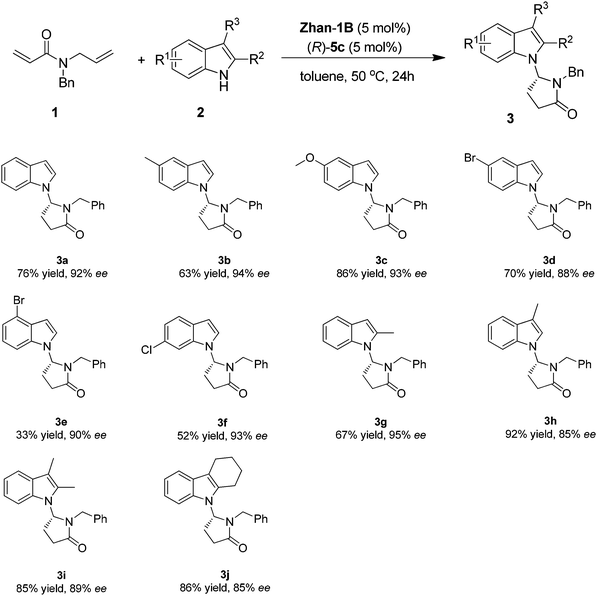 | ||
| Scheme 2 Substrate scope for cascade RCM/isomerization/Mannich reaction. Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), Zhan-1B (5 mol%), (R)-5c (5 mol%) in 1.5 mL toluene. | ||
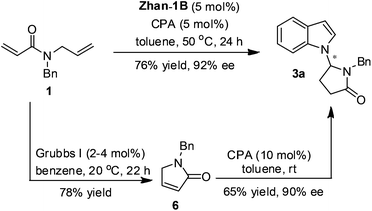
The cascade reaction enabled by the sequential catalysis not only reduced the synthetic steps but also offered a more efficient synthesis overall. For example, with 1 and indole as the substrates, the yield and enantioselectivity of the product obtained from this cascade with those derived from the two step reactions in the literature were compared (Scheme 3). The first RCM step afforded compound 6 in 78% yield in the presence of 5 mol% Grubbs I catalyst in benzene11 and the second asymmetric double bond isomerization and Mannich reactions gave product 3a in 65% yield and 90% ee according to the literature.9 Therefore, the combined yield of the two steps was 51% yield, which is lower than the cascade reaction (76% yield). To be noted, the enantioselectivity of the current study in general is higher comparing with those reported by Huang because two different chiral phosphoric acids were utilized, respectively.
In conclusion, we have developed an efficient N-alkylation of indole through a RCM/isomerization/Mannich reaction cascade. In the presence of a ruthenium complex and chiral phosphoric acid, 5-(1H-indol-1-yl)pyrrolidin-2-one derivatives were synthesized in good yields and excellent enantioselectivity. The sequential catalysis allowing three distinctive steps to be performed in a cascade displayed superior efficiency over the stepwise reactions. Further exploration of efficient reactions based on the Ru complex/chiral phosphoric acid sequential catalysis is undergoing in our lab.
Acknowledgements
We thank the National Basic Research Program of China (973 Program 2010CB833300), the National Natural Science Foundation of China (21025209, 21121062, 21332009), and the Chinese Academy of Sciences for generous financial support.References
- For selected reviews, see: (a) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed. Engl., 1993, 32, 131 CrossRef; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS PubMed; (c) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CAS PubMed; (d) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed; (e) A. M. Walji and D. W. C. MacMillan, Synlett, 2007, 1477 CAS; (f) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed; (g) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS; (h) H. Pellissier, Chem. Rev., 2013, 113, 442 CrossRef CAS PubMed.
- For selected organocatalytic reactions, see: (a) J. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5482 CrossRef CAS PubMed; (b) D. Enders, A. A. Narine, F. Toulgoat and T. Bisschops, Angew. Chem., Int. Ed., 2008, 47, 5661 CrossRef CAS PubMed; (c) C. Gioia, A. Hauville, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2008, 47, 9236 CrossRef CAS PubMed; (d) C. Zheng, Y. Lu, J. Zhang, X. Chen, Z. Chai, W. Ma and G. Zhao, Chem.–Eur. J., 2010, 16, 5853 CrossRef CAS PubMed; (e) D. Enders, C. Wang, M. Mukanova and A. Greb, Chem. Commun., 2010, 46, 2447 RSC; (f) B. Tan, G. Hernández-Torres and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (g) Y.-J. Cao, H.-G. Cheng, L.-Q. Lu, J.-J. Zhang, Y. Cheng, J.-R. Chen and W.-J. Xiao, Adv. Synth. Catal., 2011, 353, 617 CrossRef CAS; (h) X. Wu, X. Dai, H. Fang, L. Nie, J. Chen, W. Cao and G. Zhao, Chem.–Eur. J., 2011, 17, 10510 CrossRef CAS; (i) S. B. Jones, B. Simmons, A. Mastracchio and D. W. C. MacMillan, Nature, 2011, 475, 183 CrossRef CAS PubMed; (j) Z. Zhang and J. C. Antilla, Angew. Chem., Int. Ed., 2012, 51, 11778 CrossRef CAS PubMed; (k) Z. Chen, B. Wang, Z. Wang, G. Zhu and J. Sun, Angew. Chem., Int. Ed., 2013, 52, 2027 CrossRef CAS PubMed ; For selected metal-catalyzed reactions, see: ; (l) B. M. Trost and J. Quancard, J. Am. Chem. Soc., 2006, 128, 6314 CrossRef CAS PubMed; (m) T. Arai and N. Yokoyama, Angew. Chem., Int. Ed., 2008, 47, 4989 CrossRef CAS PubMed; (n) J. Barluenga, E. Tudela, A. Ballesteros and M. Tomás, J. Am. Chem. Soc., 2009, 131, 2096 CrossRef CAS PubMed; (o) Y. Lian and H. M. L. Davies, J. Am. Chem. Soc., 2010, 132, 440 CrossRef CAS PubMed; (p) L. M. Repka, J. Ni and S. E. Reisman, J. Am. Chem. Soc., 2010, 132, 14418 CrossRef CAS PubMed; (q) Y. Duan, M.-W. Chen, Z.-S. Ye, D.-S. Wang, Q.-A. Chen and Y.-G. Zhou, Chem.–Eur. J., 2011, 17, 7193 CrossRef CAS PubMed; (r) G. Cera, M. Chiarucci, A. Mazzanti, M. Mancinelli and M. Bandini, Org. Lett., 2012, 14, 1350 CrossRef CAS PubMed; (s) M. E. Kieffer, L. M. Repka and S. E. Reisman, J. Am. Chem. Soc., 2012, 134, 5131 CrossRef CAS PubMed; (t) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed; (u) H.-G. Cheng, L.-Q. Lu, T. Wang, Q.-Q. Yang, X.-P. Liu, Y. Li, Q.-H. Deng, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2013, 52, 3250 CrossRef CAS PubMed.
- For reviews: (a) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC; (b) M. Rueping, R. M. Koenigs and I. Atodiresei, Chem.–Eur. J., 2010, 16, 9350 CrossRef CAS PubMed; (c) J. Zhou, Chem.–Asian J., 2010, 5, 422 CrossRef CAS PubMed; (d) C. Zhong and X. Shi, Eur. J. Org. Chem., 2010, 2999 CrossRef CAS; (e) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337 RSC; (f) X. Wu, M. Li and L. Gong, Acta Chim. Sin., 2013, 71, 1091 Search PubMed; (g) F. Lv, S. Liu and W. Hu, Asian J. Org. Chem., 2013, 2, 824 CrossRef CAS.
- For selected recent examples: (a) M. Rueping, A. P. Antonchick and C. Brinkmann, Angew. Chem., Int. Ed., 2007, 46, 6903 CrossRef CAS PubMed; (b) S. Mukherjee and B. List, J. Am. Chem. Soc., 2007, 129, 11336 CrossRef CAS PubMed; (c) K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2008, 130, 14452 CrossRef CAS PubMed; (d) W. Hu, X. Xu, J. Zhou, W.-J. Liu, H. Huang, J. Hu, L. Yang and L.-Z. Gong, J. Am. Chem. Soc., 2008, 130, 7782 CrossRef CAS PubMed; (e) Z.-Y. Han, H. Xiao, X.-H. Chen and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 9182 CrossRef CAS PubMed; (f) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796 CrossRef CAS PubMed; (g) M. Terada and Y. Toda, J. Am. Chem. Soc., 2009, 131, 6354 CrossRef CAS PubMed; (h) X.-Y. Liu and C.-M. Che, Org. Lett., 2009, 11, 4204 CrossRef CAS PubMed; (i) C. A. Holloway, M. E. Muratore, R. I. Storer and D. J. Dixon, Org. Lett., 2010, 12, 4720 CrossRef CAS PubMed; (j) J. Jiang, H.-D. Xu, J.-B. Xi, B.-Y. Ren, F.-P. Lv, X. Guo, L.-Q. Jiang, Z.-Y. Zhang and W.-H. Hu, J. Am. Chem. Soc., 2011, 133, 8428 CrossRef CAS PubMed; (k) Q.-A. Chen, D.-S. Wang, Y.-G. Zhou, Y. Duan, H.-J. Fan, Y. Yang and Z. Zhang, J. Am. Chem. Soc., 2011, 133, 6126 CrossRef CAS PubMed; (l) Q.-A. Chen, M.-W. Chen, C.-B. Yu, L. Shi, D.-S. Wang, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2011, 133, 16432 CrossRef CAS PubMed; (m) Z.-Y. Han, D.-F. Chen, Y.-Y. Wang, R. Guo, P.-S. Wang, C. Wang and L.-Z. Gong, J. Am. Chem. Soc., 2012, 134, 6532 CrossRef CAS PubMed; (n) X.-F. Tu and L.-Z. Gong, Angew. Chem., Int. Ed., 2012, 51, 11346 CrossRef CAS PubMed; (o) M. Terada and Y. Toda, Angew. Chem., Int. Ed., 2012, 51, 2093 CrossRef CAS PubMed; (p) H. Qiu, M. Li, L.-Q. Jiang, F.-P. Lv, L. Zan, C.-W. Zhai, M. P. Doyle and W.-H. Hu, Nat. Chem., 2012, 4, 733 CrossRef CAS PubMed; (q) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef CAS PubMed; (r) H. Wu, Y.-P. He and L.-Z. Gong, Org. Lett., 2013, 15, 460 CrossRef CAS PubMed.
- (a) J.-R. Chen, C.-F. Li, X.-L. An, J.-J. Zhang, X.-Y. Zhu and W.-J. Xiao, Angew. Chem., Int. Ed., 2008, 47, 2489 CrossRef CAS PubMed; (b) X.-L. An, J.-R. Chen, C.-F. Li, F.-G. Zhang, Y.-Q. Zou, Y.-C. Guo and W.-J. Xiao, Chem.–Asian J., 2010, 5, 2258 CrossRef CAS PubMed ; Also see: ; (c) E. Ascic, J. F. Jensen and T. E. Nielsen, Angew. Chem., Int. Ed., 2011, 50, 5188 CrossRef CAS PubMed; (d) E. Ascic, S. T. Le Quement, M. Ishoey, M. Daugaard and T. E. Nielsen, ACS Comb. Sci., 2012, 14, 253 CrossRef CAS PubMed; (e) M. T. Petersen and T. E. Nielsen, Org. Lett., 2013, 15, 1986 CrossRef CAS PubMed.
- (a) Q. Cai, Z.-A. Zhao and S.-L. You, Angew. Chem., Int. Ed., 2009, 48, 7428 CrossRef CAS PubMed; (b) Q. Cai, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2010, 49, 8666 CrossRef CAS PubMed; (c) Q. Cai, X.-W. Liang, S.-G. Wang, J.-W. Zhang, X. Zhang and S.-L. You, Org. Lett., 2012, 14, 5022 CrossRef CAS PubMed; (d) Q. Cai, X.-W. Liang, S.-G. Wang and S.-L. You, Org. Biomol. Chem., 2013, 11, 1602 RSC; (e) J.-W. Zhang, Q. Cai, Q. Gu, X.-X. Shi and S.-L. You, Chem. Commun., 2013, 49, 7750 RSC ; Also see a recent example from Yu's group: ; (f) H. Liu, C. Zeng, J. Guo, M. Zhang and S. Yu, RSC Adv., 2013, 3, 1666 RSC.
- For selected asymmetric N-alkylation of indole: (a) B. M. Trost, M. J. Krische, V. Berl and E. M. Grenzer, Org. Lett., 2002, 4, 2005 CrossRef CAS PubMed; (b) M. Bandini, A. Eichholzer, M. Tragni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2008, 47, 3238 CrossRef CAS PubMed; (c) H.-L. Cui, X. Feng, J. Peng, J. Lei, K. Jiang and Y.-C. Chen, Angew. Chem., Int. Ed., 2009, 48, 5737 CrossRef CAS PubMed; (d) L. M. Stanley and J. F. Hartwig, Angew. Chem., Int. Ed., 2009, 48, 7841 CrossRef CAS PubMed; (e) D. Enders, C. Wang and G. Raabe, Synthesis, 2009, 4119 CAS; (f) L. Hong, W. Sun, C. Liu, L. Wang and R. Wang, Chem.–Eur. J., 2010, 16, 440 CrossRef CAS PubMed; (g) D. Enders, A. Greb, K. Deckers, P. Selig and C. Merkens, Chem.–Eur. J., 2012, 18, 10226 CrossRef CAS PubMed; (h) W.-B. Liu, X. Zhang, L.-X. Dai and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 5183 CrossRef CAS PubMed; (i) D. Enders, C. Joie and K. Deckers, Chem.–Eur. J., 2013, 19, 10818 CrossRef CAS PubMed.
- For reviews on asymmetric Friedel–Crafts reaction, see: (a) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS PubMed; (b) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (c) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (d) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (e) V. Terrasson, R. M. Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635 CrossRef CAS; (f) M. Zeng and S.-L. You, Synlett, 2010, 9, 1289 Search PubMed.
- Y. Xie, Y. Zhao, B. Qian, L. Yang, C. Xia and H. Huang, Angew. Chem., Int. Ed., 2011, 50, 5682 CrossRef CAS PubMed.
- For selected examples: (a) Q. Kang, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2007, 129, 1484 CrossRef CAS PubMed; (b) Q.-F. Wu, H. He, W.-B. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418 CrossRef CAS PubMed; (c) Q. Yin and S.-L. You, Chem. Sci., 2011, 2, 1344 RSC; (d) Q. Cai, C. Zheng, J.-W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2011, 50, 8665 CrossRef CAS PubMed; (e) Q.-F. Wu, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 1680 CrossRef CAS PubMed; (f) Q. Cai and S.-L. You, Org. Lett., 2012, 14, 3040 CrossRef CAS PubMed; (g) Q. Cai, C. Liu, X.-W. Liang and S.-L. You, Org. Lett., 2012, 14, 4588 CrossRef CAS PubMed; (h) M. Zeng, W. Zhang and S.-L. You, Chin. J. Chem., 2012, 30, 2615 CrossRef CAS; (i) Q.-L. Xu, L.-X. Dai and S.-L. You, Chem. Sci., 2013, 4, 97 RSC; (j) Q. Yin, S.-G. Wang and S.-L. You, Org. Lett., 2013, 15, 2688 CrossRef CAS PubMed; (k) Q. Yin and S.-L. You, Org. Lett., 2013, 15, 4266 CrossRef CAS PubMed; (l) C.-X. Zhuo, Q.-F. Wu, Q. Zhao, Q.-L. Xu and S.-L. You, J. Am. Chem. Soc., 2013, 135, 8169 CrossRef CAS PubMed.
- G. C. Fu, S. T. Nguyen and R. H. Grubbs, J. Am. Chem. Soc., 1993, 115, 9856 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3qo00008g |
| This journal is © the Partner Organisations 2014 |