Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity†
Bibiana
Comesaña-Gándara‡
 a,
Jie
Chen‡
a,
C. Grazia
Bezzu
a,
Jie
Chen‡
a,
C. Grazia
Bezzu
 a,
Mariolino
Carta
a,
Mariolino
Carta
 b,
Ian
Rose
a,
Maria-Chiara
Ferrari
c,
Elisa
Esposito
b,
Ian
Rose
a,
Maria-Chiara
Ferrari
c,
Elisa
Esposito
 d,
Alessio
Fuoco
d,
Alessio
Fuoco
 d,
Johannes C.
Jansen
d,
Johannes C.
Jansen
 *d and
Neil B.
McKeown
*d and
Neil B.
McKeown
 *a
*a
aEaStCHEM, School of Chemistry, University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: neil.mckeown@ed.ac.uk
bDepartment of Chemistry, Swansea University, College of Science, Grove Building, Singleton Park, Swansea, SA2 8PP, UK
cInstitute for Materials and Processes, School of Engineering, The University of Edinburgh, Mayfield Road, Edinburgh EH9 3JL, UK
dInstitute on Membrane Technology, ITM-CNR, Via P. Bucci 17/C, 87036 Rende (CS), Italy
First published on 23rd July 2019
Abstract
Membranes composed of Polymers of Intrinsic Microporosity (PIMs) have the potential for energy efficient industrial gas separations. Here we report the synthesis and gas permeability data of a series of ultrapermeable PIMs, of two-dimensional chain conformation and based on benzotriptycene structural units, that demonstrate remarkable ideal selectivity for most gas pairs of importance. In particular, the CO2 ultrapermeability and high selectivity for CO2 over CH4, of key importance for the upgrading of natural gas and biogas, and for CO2 over N2, of importance for cost-effective carbon capture from power plants, exceed the performance of the current state-of-the-art polymers. All of the gas permeability data from this series of benzotriptycene-based PIMs are placed well above the current 2008 Robeson upper bounds for CO2/CH4 and CO2/N2. Indeed, the data for some of these polymers fall into a linear correlation on the benchmark Robeson plots [i.e. log(PCO2/PCH4) versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCO2 and log(PCO2/PN2) versus log
PCO2 and log(PCO2/PN2) versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCO2], which are parallel to, but significantly above, that of the 2008 CO2/CH4 and CO2/N2 upper bounds, allowing their revision. The redefinition of these upper bounds sets new aspirational targets for polymer chemists to aim for and will result in more attractive parametric estimates of energy and cost efficiencies for carbon capture and natural/bio gas upgrading using state-of-the-art CO2 separation membranes.
PCO2], which are parallel to, but significantly above, that of the 2008 CO2/CH4 and CO2/N2 upper bounds, allowing their revision. The redefinition of these upper bounds sets new aspirational targets for polymer chemists to aim for and will result in more attractive parametric estimates of energy and cost efficiencies for carbon capture and natural/bio gas upgrading using state-of-the-art CO2 separation membranes.
Broader contextThe low-cost and energy-effective removal of carbon dioxide (CO2) from natural gas and biogas would help the supply of methane as the cleanest burning and lowest carbon-emitting hydrocarbon fuel. In addition, carbon capture and storage (CCS) from power plant emissions will be required to achieve the goals of the 2015 Paris Agreement, which aspires to maintain global warming to less than 1.5 °C above that of the pre-industrial age by the end of the 21st Century. Indeed, the combined use of biofuels, such as biogas, and CCS technology is regarded as the key negative emissions technology required in order to reach the Agreement's ambitious targets for reduced emissions. Despite the urgent need for CCS, the best technology platform for its delivery is still unclear due to the difficulties in the estimation of costs and the complex evaluation of the advantages and disadvantages associated with each technology. Highly permeable membranes that are selective for CO2 over methane (CO2/CH4) and CO2 over nitrogen (CO2/N2) are of increasing interest for natural gas/biogas upgrading and carbon capture, respectively, due to the inherent efficiency of membrane separations. Here we report the synthesis of a series of ultrapermeable polymers that define the state-of-the-art in the trade-off between permeability and selectivity for all important gas separations and, in particular, for CO2/CH4 and CO2/N2. The data from these polymers were used to redefine the benchmark Robeson upper bounds for these two gas separations at much higher values of selectivity. This enhancement will improve the credibility of polymer membranes for CO2 separations when evaluated against competing processes. Hopefully, this will help to stimulate the fundamental polymer science and applied engineering required to develop membrane systems for these CO2 separations of key importance to energy and the environment. |
Introduction
Membranes based on polymers as the selective layer are used for the energy efficient separation of gas mixtures including those of key relevance to energy and the environment.1–4 The development of new polymers with greater gas permeability and selectivity would further enhance the efficiency of membrane gas separations of current industrial interest,5 including hydrogen recovery during ammonia preparation (H2 from N2), oxygen or nitrogen enrichment of air (O2 from N2)6 and natural gas or biogas upgrading (predominantly CO2 from CH4).7–10 Increasingly, polymer membranes are also being considered as a practical alternative to solvent absorption for large-scale capture of CO2 from power plant flue gas (predominantly CO2 from N2).7,9,11–14 For gas separations on such a massive scale, membranes with very high permeance (i.e. flux) are desirable to minimise energy costs for gas compression and to reduce the active surface area of the membrane, thereby, optimising the overall size and manufacture cost of the membrane system.5,15 However, polymer membrane materials suffer from the well-established trade-off between gas permeability (Px) and selectivity for one gas over another (Px/Py),16,17 so that established ultrapermeable polymers, such as the polyacetylene poly(trimethylsilylpropyne) (PTMSP),18,19 and recently reported examples20 are insufficiently selective for use in gas separations.The general trade-off between polymer permeability and selectivity was first quantified by Robeson in 1991 when he identified upper bounds in plots of log(Px/Py), versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Px for O2/N2, H2/N2, He/N2, H2/CH4, He/CH4, CO2/CH4, and He/H2 gas pairs based on the gas permeability of the best performing polymers at that time.21 Subsequently, for a newly prepared polymer (or a mixed matrix membrane)22,23 the position of its gas permeability data relative to the upper bounds on Robeson plots allows for its potential for gas separations to be estimated. Robeson updated all of the upper bounds in 2008 using initial data for two spirobisindane-based Polymers of Intrinsic Microporosity (PIM-1 and PIM-7; Table S1, ESI†),24 whose rigid and contorted macromolecular structures provided exceptionally high permeability with moderate selectivity.25 In addition, data for these two PIMs were also used to define an upper bound for the CO2/N2 gas pair, which is of key importance to post-combustion carbon capture but had been considered of no practical interest in 1991.24 Since 2008, many PIMs with enhanced rigidity have demonstrated gas permeability data that lie well above some of the 2008 upper bounds.26 These highly shape-persistent PIMs were obtained by replacing the relatively flexible spirobisindane structural unit with spirobifluorene27,28 units or highly rigid bridged bicyclic components such as ethanoanthracene,29–32 triptycene,33–36 methanopentacene37 and Trögers base.29,35 Indeed, in 2015 Pinnau et al.38 proposed that the O2/N2, H2/N2 and H2/CH4 upper bounds should be updated using permeability data from aged films of highly selective triptycene-based PIMs (e.g. PIM-Trip-TB35 and TPIM-133). However, revisions of the upper bound for CO2/N2 and CO2/CH4 were not proposed at that time due to the data for these polymers and other high-performing PIMs being close to the existing 2008 CO2/N2 and CO2/CH4 upper bounds (Table S1, ESI†).
Px for O2/N2, H2/N2, He/N2, H2/CH4, He/CH4, CO2/CH4, and He/H2 gas pairs based on the gas permeability of the best performing polymers at that time.21 Subsequently, for a newly prepared polymer (or a mixed matrix membrane)22,23 the position of its gas permeability data relative to the upper bounds on Robeson plots allows for its potential for gas separations to be estimated. Robeson updated all of the upper bounds in 2008 using initial data for two spirobisindane-based Polymers of Intrinsic Microporosity (PIM-1 and PIM-7; Table S1, ESI†),24 whose rigid and contorted macromolecular structures provided exceptionally high permeability with moderate selectivity.25 In addition, data for these two PIMs were also used to define an upper bound for the CO2/N2 gas pair, which is of key importance to post-combustion carbon capture but had been considered of no practical interest in 1991.24 Since 2008, many PIMs with enhanced rigidity have demonstrated gas permeability data that lie well above some of the 2008 upper bounds.26 These highly shape-persistent PIMs were obtained by replacing the relatively flexible spirobisindane structural unit with spirobifluorene27,28 units or highly rigid bridged bicyclic components such as ethanoanthracene,29–32 triptycene,33–36 methanopentacene37 and Trögers base.29,35 Indeed, in 2015 Pinnau et al.38 proposed that the O2/N2, H2/N2 and H2/CH4 upper bounds should be updated using permeability data from aged films of highly selective triptycene-based PIMs (e.g. PIM-Trip-TB35 and TPIM-133). However, revisions of the upper bound for CO2/N2 and CO2/CH4 were not proposed at that time due to the data for these polymers and other high-performing PIMs being close to the existing 2008 CO2/N2 and CO2/CH4 upper bounds (Table S1, ESI†).
Recently, we introduced a new PIM derived from a benzotriptycene monomer, PIM-TMN-Trip, which proved to be as ultrapermeable to gases as PTMSP due to enhanced intrinsic microporosity arising from its 2D chain structure.39 PIM-TMN-Trip demonstrates higher selectivity than PTMSP due to its greater chain rigidity providing enhanced molecular sieving (i.e. diffusivity selectivity). Furthermore, it was found that the unsubstituted benzotriptycene-based PIM (PIM-BTrip) demonstrates even greater selectivity placing its data above the proposed 2015 O2/N2, H2/N2 and H2/CH4 upper bounds and even above Robeson's 2008 upperbounds for CO2/N2 and CO2/CH4.40,41 Here we report on the synthesis and properties of some new members of the benzotriptycene-based PIM series (Fig. 1), all of which demonstrate high permeability and selectivity. In particular, this polymer series demonstrates permeability data for CO2/N2 and CO2/CH4 that suggest new positions of the Robeson upper bound for these important gas pairs that are of key interest for separations of relevance to energy and the environment.
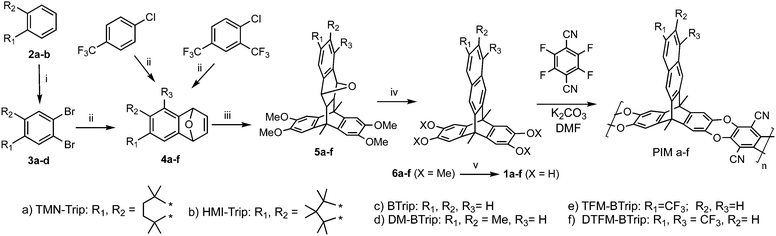 | ||
| Fig. 1 Structure and synthesis of the benzotriptycene PIMs. Reagents and conditions: i. Br2, Fe, DCM, rt, 3 h; ii. n-BuLi, furan, THF, −78 °C, 1.5 h; iii. 9,10-Dimethyl-2,3,6,7-tetramethoxyanthracene, DMF, 250 °C, 7 bar, 2 h, microwave irradiation, iv. TFA or MeSO4H, rt, 24 h.; v. BBr3, DCM. (See ESI† for details). | ||
Results and discussion
Polymer design and synthesis
A further four benzotriptycene PIMs were synthesised along with new batches of PIM-TMN-Trip and PIM-BTrip to allow for direct comparison of their gas permeabilities. The novel polymers include PIM-HMI-Trip, for which the sterically crowded hexamethylindane (HMI)-solubilising group42 would be expected to be more rigid than the tetramethylnaphthalene (TMN) group of PIM-TMN-Trip. Previously for spirobifluorene-based PIMs,43 the introduction of adjacent methyl substituents had been shown to be beneficial to performance, therefore, a PIM based on dimethylbenzotriptycene was prepared (PIM-DM-BTrip). In addition, the potential benefit of introducing one or two trifluoromethyl (TFM) solubilising groups onto the benzotriptycene unit was evaluated by the synthesis of PIM-TFM-BTrip and PIM-DTFM-BTrip, respectively.Each polymer was prepared from its tetrahydroxy benzotriptycene monomer (1a–f) using the well-established benzodioxin-forming polymerisation reaction devised for PIM synthesis (Fig. 1).44 Monomers were prepared by adaptation of the classic benzotriptycene synthesis, involving the Diels–Alder reaction between 2,3,6,7-tetramethoxy-9,10-dimethylanthracene and the appropriate 1,4-dihydro-1,4-epoxynaphthalene39 – with the latter prepared from the Diels–Alder reaction between the appropriate benzyne intermediate and furan.45–47
PIM-TMN-Trip and PIM-HMI-Trip are both soluble in chloroform, facilitating analysis using Gel Permeation Chromatography (GPC) that confirmed that high molecular mass polymer was achieved for both polymers (Table 1). In contrast, PIM-DM-Btrip, PIM-TFM-BTrip and PIM-DTFM-BTrip proved soluble only in quinoline. The success of this high-boiling aromatic solvent for dissolving these otherwise intractable polymers prompted a re-investigation of the solubility of unsubstituted PIM-BTrip, which we had previously described as insoluble.39 Pleasingly, this polymer also proved soluble in quinoline. Although quinoline is not an appropriate solvent for GPC analysis, solutions of PIM-DM-BTrip, PIM-TFM-BTrip, PIM-DTFM-BTrip and PIM-BTrip could be used to cast mechanically flexible and robust films, implying that a reasonably high molecular mass had been achieved during the synthesis. Synthetic and structural characterisation details, including solid state NMR (Fig. S1) are given in the ESI.†
| Polymer | Yield (%) | Solubility | M n (g mol−1) | M w/Mn | η (cm3 g−1) | SABETb (m2 g−1) | V Total (ml g−1) | V M (ml g−1) | CO2 uptakee (mmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Inherent viscosity in quinoline at 25 °C. b BET surface area calculated from N2 adsorption isotherm obtained at 77 K. c Total pore volume estimated from N2 uptake at P/Po = 0.98. d Micropore volume estimated from N2 uptake at P/Po = 0.05. e CO2 adsorption at 1 bar and 273 K. f Relative to polystyrene standards. g Not measured due to insolubility in solvents compatible with GPC analysis. | |||||||||
| PIM-TMN-Trip | 67 | CHCl3 | 52 300f | 3.8 | 74 | 1034 | 0.87 | 0.38 | 3.3 |
| PIM-HMI-Trip | 58 | CHCl3 | 61 300f | 2.4 | 58 | 1033 | 0.71 | 0.38 | 3.0 |
| PIM-BTrip | 78 | Quinoline | —g | —g | 66 | 911 | 0.63 | 0.33 | 3.2 |
| PIM-DM-BTrip | 82 | Quinoline | —g | —g | 72 | 920 | 0.72 | 0.33 | 3.0 |
| PIM-TFM-BTrip | 79 | Quinoline | —g | —g | 37 | 848 | 0.66 | 0.31 | 2.5 |
| PIM-DTFM-BTrip | 84 | Quinoline | —g | —g | 65 | 964 | 1.02 | 0.33 | 2.5 |
Gas adsorption and gas transport properties.
In their powder form, all benzotriptycene-based PIMs adsorb a large amount of nitrogen (N2, 77 K) at low relative pressure. Analysis of the N2 adsorption isotherms (Fig. S1, ESI†) gives apparent Brunauer–Emmett–Teller (BET) surface areas (SABET) within the range of 848–1034 m2 g−1 (Table 1), which are amongst the highest obtained from solution processable polymers.29,39 The shapes of the N2 isotherms are similar for all polymers except for PIM-TMN-Trip and PIM-DTFM-BTrip, for which there is larger uptake at higher pressures associated with a large hysteresis between the adsorption and desorption isotherms. This might be related to the TMN and CF3 substituents protruding out of the 2D plane of the polymer chain and thus interfering with the electrostatic nitrile–nitrile interactions which are likely to dominate polymer cohesion. Adsorption of CO2 at 273 K (Fig. S2, ESI†) shows similar uptakes for the benzotriptycene-PIMs (2.5–3.3 mmol g−1). The uptake for PIM-BTrip is slightly higher at lower pressures, which may be ascribed to a greater concentration of ultramicropores (diameter < 0.7 nm in its pore size distribution (Fig. S3, ESI†)).Solvent cast films (Fig. S4, ESI†) of the benzotriptycene-based PIMs all demonstrate exceptionally high gas permeability (Table 2). However, the evaluation of gas permeability data for a new polymer requires careful consideration of its film history and thickness as these factors influence greatly the observed values.32 Generally, the highest reported values of gas permeability for high free volume polymers such as the PTMSP and PIMs were obtained from films freshly treated with methanol (or ethanol), which removes any residual casting solvent but also induces additional free volume.31,48 The values of gas permeability from freshly methanol treated thick films (135–176 μm) of the benzotriptycene PIMs are some of highest reported for a pure polymer film (e.g., PCO2 = 21–53 × 103 Barrer) and are comparable to those from ethanol treated ultrapermeable polyacetylenes (e.g., PCO2 = 28–47 × 103 Barrer).19,42 For each of the methanol treated films the order of decreasing gas permeability is CO2 > H2 > O2 > He > CH4 > N2 with the exception of those from the less permeable and more size-selective PIM-BTrip for which He permeates faster than O2. The ideal selectivities of all of the methanol treated films are significantly higher than those obtained for the ultrapermeable polyacetylenes and fall in the range of those reported for methanol treated films of less permeable PIMs such as PIM-1 (e.g., PO2/PN2 = 2.6–3.6).48
| PIM-a | lb | P N2 | P O2 | P CO2 | P CH4 | P H2 | P He | P O2/PN2 | P H2/PN2 | P CO2/PN2 | P CO2/PCH4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Number in parentheses is the ageing time in days after methanol treatment. b Thickness did not exhibit significant changes upon ageing. c Data defining the proposed CO2/CH4 upper bound. d Data defining the proposed CO2/N2 upper bound. e Average and standard deviation (in parentheses) of four independent measurements of the same aged sample. f Average and standard deviation (in parentheses) of four independent samples. g Data not included on Robeson plots (Fig. 2). | |||||||||||
| BTrip | 160 | 1190 | 4330 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1690 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
4540 | 3.64 | 10.2 | 18.1 | 12.7 |
| (130)c,d | 160 | 522 | 2570 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
570 | 8440 | 3110 | 4.92 | 16.2 | 25.3 | 23.2 |
| (253)c,d | 160 | 401 | 2170 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
411 | 8930 | 3400 | 5.41 | 22.3 | 26.7 | 26.0 |
| (365)c,d | 160 | 280 | 1580 | 8020 | 282 | 7160 | 2810 | 5.65 | 25.6 | 28.6 | 28.4 |
| (490)c,d | 160 | 195 | 1240 | 6060 | 203 | 6380 | 2650 | 6.34 | 32.6 | 31.0 | 29.9 |
| (633)c,d | 160 | 127 | 935 | 4350 | 130 | 5100 | 2180 | 7.36 | 40.1 | 34.2 | 33.5 |
| (718)e,g | 160 | 112 (±4) | 838 (±48) | 3770 (±166) | 113 (±4) | 4820 (±186) | 2150 (±64) | 7.51 (±0.19) | 43.2 (±0.53) | 33.8 (±0.53) | 33.5 (±0.33) |
| BTripd | 64 | 339 | 1800 | 9200 | 412 | 9430 | 3960 | 5.31 | 27.8 | 27.1 | 22.3 |
| (120) | 64 | 200 | 1160 | 6040 | 237 | 7180 | 3020 | 5.79 | 35.8 | 30.2 | 25.5 |
| (253)d | 64 | 190 | 1143 | 5990 | 225 | 8080 | 3490 | 6.01 | 42.5 | 31.5 | 26.6 |
| (371)c,d | 64 | 154 | 997 | 5150 | 163 | 7730 | 3620 | 6.47 | 50.2 | 33.4 | 31.6 |
| TMN-Trip | 166 | 3540 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
7250 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6490 | 2.94 | 5.31 | 14.9 | 7.28 |
| (120) | 166 | 1970 | 6620 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
3130 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
5600 | 3.36 | 7.77 | 16.9 | 10.6 |
| (253) | 166 | 1470 | 5440 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2030 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5190 | 3.71 | 9.59 | 17.6 | 12.8 |
| (358) | 166 | 1289 | 5082 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 648 648 |
1751 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118 118 |
5290 | 3.94 | 11.0 | 18.4 | 13.5 |
| (426) | 166 | 1100 | 4620 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1440 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5420 | 4.20 | 12.8 | 18.5 | 14.2 |
| HMI-Tripd | 135 | 2560 | 8540 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4870 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5700 | 3.34 | 6.48 | 17.3 | 9.08 |
| (1)f,g | 135 | 2120 (±330) | 7380 (±989) | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (±3680) 000 (±3680) |
3990 (±708) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (±1765) 400 (±1765) |
6500 (±762) | 3.49 (±0.14) | 8.95 (±2.16) | 18.6 (±1.7) | 9.94 (±1.44) |
| (120) | 135 | 1440 | 5180 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2150 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4240 | 3.60 | 8.19 | 18.7 | 12.5 |
| (253) | 135 | 972 | 3930 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1220 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3960 | 4.04 | 11.0 | 19.5 | 15.6 |
| (358) | 135 | 907 | 3760 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 404 404 |
1083 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141 141 |
4245 | 4.15 | 12.3 | 19.2 | 16.1 |
| (426) | 135 | 804 | 3580 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
967 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4150 | 4.45 | 13.7 | 20.4 | 16.9 |
| TFM-BTripc,d | 176 | 1830 | 6210 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2280 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5150 | 3.39 | 7.43 | 18.4 | 14.8 |
| (123)c | 176 | 1090 | 4230 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1250 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
4120 | 3.88 | 9.82 | 20.3 | 17.7 |
| (255)c | 176 | 875 | 3640 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
953 | 9870 | 4050 | 4.15 | 11.3 | 21.0 | 19.3 |
| (367)c | 176 | 791 | 3450 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
873 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
4170 | 4.36 | 12.7 | 21.5 | 19.5 |
| (496) | 176 | 722 | 3260 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
792 | 9760 | 3920 | 4.51 | 13.5 | 21.6 | 19.7 |
| DTFM-BTrip | 112 | 3000 | 7770 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
4340 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
5860 | 2.59 | 4.90 | 14.2 | 9.82 |
| (119) | 112 | 1800 | 5410 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2150 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
4690 | 3.01 | 6.28 | 16.1 | 13.5 |
| (366) | 112 | 1300 | 4460 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1390 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
4590 | 3.41 | 8.23 | 17.5 | 16.4 |
| (490) | 112 | 864 | 3490 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
890 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
4770 | 4.04 | 12.1 | 19.6 | 19.0 |
| (636) | 112 | 741 | 3170 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
728 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4730 | 4.27 | 13.8 | 20.0 | 20.3 |
| DM-BTripd,f | 114 | 1020 (±133) | 3950 (±374) | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (±1071) 000 (±1071) |
1570 (±85) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (±482) 400 (±482) |
4000 (±354) | 3.90 (±0.16) | 11.3 (±1.07) | 21.8 (±2.5) | 14.0 (±1.5) |
| (128)d | 114 | 521 | 2640 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
599 | 9870 | 3650 | 5.07 | 18.9 | 23.4 | 20.4 |
As noted for all PIMs and highly permeable polymers,31,32,49–51 the extremely high values of gas permeability measured initially from the freshly methanol treated films are not maintained on ageing.52 However, the reduction in permeability is accompanied by an increase in ideal selectivity for all gas pairs. In addition, on ageing, He permeability surpasses the value of O2 for all the polymers, indicating enhanced size selectivity. Comparing data from approximately like-for-like samples (i.e. ∼120 day aged and 110–180 μm thick films) the order of decreasing permeability and increasing selectivity for the benzotriptycene PIMs is PIM-TMN-Trip > PIM-DTFM-BTrip > PIM-HMI-Trip > PIM-TFM-Trip > PIM-BTrip ≈ PIM-DM-BTrip. It can be deduced that the bulky TMN and HMI substituents both enhance permeability greatly, with the more rigid HMI substituent providing slightly higher selectivity over TMN. The relatively small –CF3 substituents of PIM-TFM-BTrip and PIM-DTFM-BTrip also enhance permeability relative to unsubstituted PIM-BTrip. Interestingly, the –CF3 substituents appear to slow ageing, with 54% of the value for PO2 of the methanol treated film of PIM-DTFM-BTrip retained after one year, and 56% for PIM-TFM-BTrip, as compared to only 30–36% for films without –CF3 substituents.
Depending on the gas, the standard deviation of the permeability is in the range 4–18% for the freshly MeOH treated PIM-HMI-Trip and PIM-DM-BTrip films, and 3–6% for the aged PIM-BTrip film. These are small compared to the effect of the ageing in this work, and almost negligible when represented on the double-logarithmic Robeson diagrams (Fig. S6, ESI†).
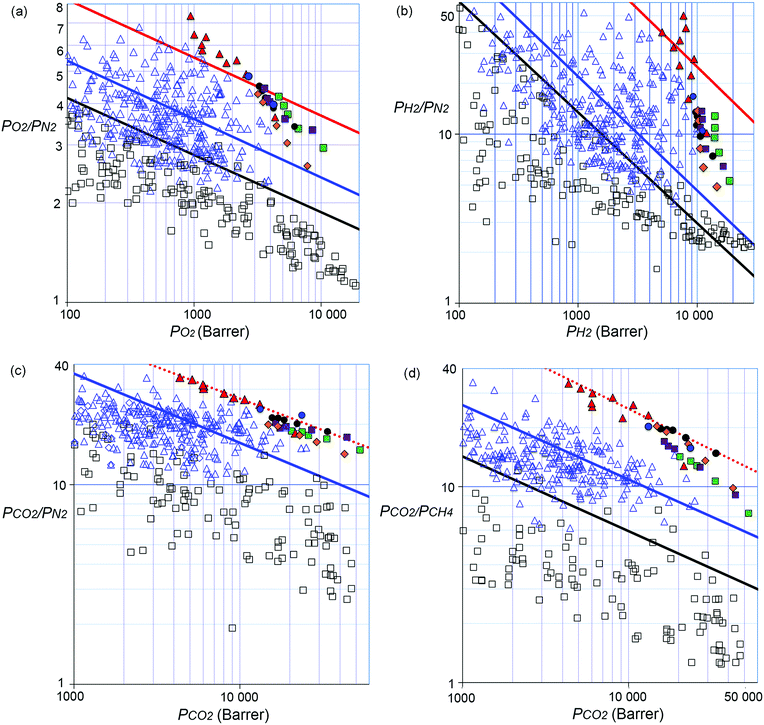
A thinner film of PIM-BTrip (64 μm) demonstrates lower initial permeability after methanol treatment, consistent with the well-established trend that thinner films age more rapidly than thicker films.32,52,53 It is also more size selective than the thicker film of the same polymer with H2 > CO2 > He > O2 > CH4 > N2 the order of decreasing gas permeability. Due to the commonly encountered variability of gas permeability from differing film thicknesses and history, data for a new polymer are best compared to those of existing polymers by using Robeson plots (Fig. 2). As noted, the position of the data from a new polymer relative to the Robeson upper bounds provides a useful indicator of its potential performance as gas separation membranes. All data points for the benzotriptycene polymers lie far above the 2008 upper bounds for O2/N2 (Fig. 2a), H2/N2 (Fig. 2b), H2/CH4, CO2/N2 (Fig. 2c) and CO2/CH4 (Fig. 2d). Data for the ∼1 year aged films for all of the polymers lie close to the proposed 2015 upper bound for O2/N2. In particular, aged PIM-BTrip demonstrates exceptional selectivity for a highly permeable polymer so that its data lie well above the proposed 2015 upper bounds for O2/N2 (Fig. 2a), H2/N2 (Fig. 2b), and H2/CH4. A notable feature of the permeability data from aged samples of the benzotriptycene-PIMs on the O2/N2 and H2/N2 Robeson plots is the near linear correlation at a steeper slope than that of the upper bounds (Fig. S5, ESI†). This reflects the far larger reduction of permeabilities on ageing for gases composed of larger molecules such as N2 and CH4 as compared to those composed of the smaller O2 and H2 molecules.
Gas transport through a polymer is described by the solution-diffusion model54 with Px = Dx × Sx, where Dx is the diffusivity coefficient (Table S2, ESI†) and Sx is the solubility coefficient for gas x (Table S3, ESI†). Therefore, the ideal selectivity (Px/Py) for a polymer comes from a combination of diffusivity selectivity (Dx/Dy) and solubility selectivity (Sx/Sy). The remarkable positions of the data for the benzotriptycene-PIMs on the H2/N2, and O2/N2 Robeson plots are due to very high diffusivity selectivity originating from the size-sieving behaviour of the polymers, which differentiates between gas molecules of differing effective diameters (dx).40 This is best illustrated by the correlation between dx2 and the diffusivity coefficient (Dx),55 which is steepest for PIM-BTrip and less steep for benzotriptycene PIMs that possess a substituent, although the absolute value of the diffusion coefficient is larger (Fig. 3). Ageing decreases the diffusion coefficient for all polymers but steepens the correlation between dx2 and Dx, especially for PIM-BTrip, which is evidence of its further enhanced size selectivity (Fig. S7, ESI†).40 The extraordinary performance of PIM-BTrip can be attributed to its ultramicroporosity, which facilitates the diffusivity of small gas molecules, together with very high chain rigidity,16,54 which hinders the activated transport of larger gas molecules by reducing thermal motions that allow gaps to form between voids. The extreme rigidity of PIM-BTrip accounts for the very high activation energy for the diffusion of larger gases such as N2 and CH4.40 The gas transport properties of PIM-BTrip appears similar to those reported for the two triptycene-derived polymers, PIM-Trip-TB35 and TPIM-1,33 which were used to define the proposed 2015 upper bounds for O2/N2, H2/N2 and H2/CH4.38 It should be noted that the data from PIM-Trip-TB used to define the 2015 upper bounds were taken from a film that was aged for only 100 days after methanol treatment.35 Recent remeasurement of the gas permeability of this film after 1900 days gives data that are also well over the proposed 2015 upper bounds for O2/N2 (i.e. PO2 = 532 Barrer; PO2/PN2 = 8.2) and H2/N2 (i.e. PH2 = 4430 Barrer; PH2/PN2 = 65). Therefore, the design concepts used to obtain the extraordinary size selectivity demonstrated by PIM-BTrip and PIM-Trip-TB are likely to provide PIMs that will provoke future significant revisions of the O2/N2, H2/N2 and H2/CH4 Robeson upper bounds.
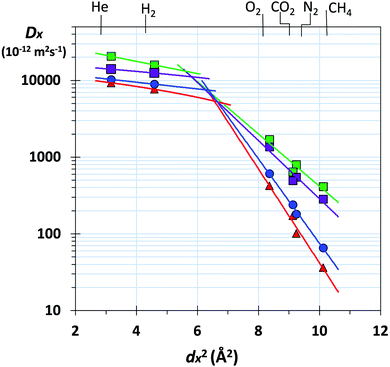 | ||
Fig. 3 Plot of diffusivity coefficient (Dx) versus dx2 (where dx = effective diameter of gas molecule x: He = 1.78; H2 = 2.14; O2 = 2.89; CO2 = 3.02; N2 = 3.04; CH4 = 3.18 Å)55 for freshly methanol treated films of PIM-BTrip ( ), PIM-TMN-Trip ( ), PIM-TMN-Trip ( ), PIM-HMI-Trip ( ), PIM-HMI-Trip ( ), PIM-DM-BTrip ( ), PIM-DM-BTrip ( ). Data for PIM-TFM-BTrip and PIM-DTFM-BTrip are not shown for clarity but are very similar to those for PIM-TMN-Trip and PIM-HMI-Trip, respectively. ). Data for PIM-TFM-BTrip and PIM-DTFM-BTrip are not shown for clarity but are very similar to those for PIM-TMN-Trip and PIM-HMI-Trip, respectively. | ||
Redefining the CO2/N2 and CO2/CH4 upper bounds
Separations involving CO2 are mechanistically more complex than those governed predominately by diffusivity selectivity (e.g. O2/N2 or H2/N2) because SCO2 dominates transport, especially for CO2/N2 due to the similar effective diameters of the two gas molecules. Typically for PIMs, values for SCO2/SN2 lie in the range 15–20 whereas those for DCO2/DN2 lie between 0.9–1.5 and these values are similar for PIMs with both higher and lower PCO2 permeability. In general, solubility selectivity tends to remain fairly constant during ageing, in contrast to the increases observed for ideal selectivity values for transport dominated by diffusivity selectivity.52 Thus, plotting data for previously reported PIMs on the Robeson plot for CO2/N2 shows many data points slightly above the 2008 upper bound at higher permeability (PCO2 > 3000 Barrer) but few at lower values of permeability. Indeed, very few highly permeable polymers possess a CO2/N2 selectivity > 30,56–59 which is the lower limit of interest for a first-pass polymer membrane for post-combustion carbon capture (Table S1, ESI†).12Although all of the data for the benzotriptycene PIMs are above the 2008 upper bound for CO2/N2, the data from PIM-BTrip are particularly promising with both thick and thinner aged films providing PCO2 > 4000 Barrer and PCO2/PN2 > 30. The impressive performance of PIM-BTrip appears to be due to an unusually high DCO2/DN2 of 2.0, whereas that of the substituted members of the series relies on greater SCO2/SN2 resulting from the greater number of CO2 adsorption sites provided by the larger amount of intrinsic microporosity (Table S3, ESI†). The eleven data points on the Robeson plot from four different polymers that fall into a linear correlation parallel to that of the 2008 upper bound allows us to propose a substantially improved new upper bound for CO2/N2 (Fig. 2c and Tables 2 and 3). These data points are distributed over a large PCO2 range of 4400–52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Barrer.
000 Barrer.
| k (Barrer) | n | |
|---|---|---|
| Robeson 2008 upper bounds24 | ||
| CO2/CH4 | 5.369 × 106 | −2.636 |
| CO2/N2 | 30.967 × 106 | −2.888 |
| Proposed upper bounds | ||
| CO2/CH4 | 22.584 × 106 | −2.401 |
| CO2/N2 | 755.58 × 106 | −3.409 |
In addition, the data for all of the benzotriptycene PIMs lie well above the 2008 upper bound for CO2/CH4 at a higher selectivity than those of previously reported polymers. Indeed, only data for the highly rigid “intermolecularly-locked” derivative of PIM-1 (PIM-C1)60 and PIM-SBF-243 come close to those of the benzotriptycene PIMs (Table S1, ESI†). This exceptional performance appears due to a combination of both high diffusivity selectivity, with DCO2/DCH4 in the range 5.7–9.5 for aged films, and good solubility selectivity (SCO2/SCH4 > 3). Ten data points from two different polymers allows us to propose a new upper bound for CO2/CH4 parallel to that of 2008 (Fig. 2d and Tables 2 and 3). The benzotriptycene PIMs that either define or provide data that are very close to this revised upper bound are either unsubstituted (PIM-BTrip) or possess only small substituents (i.e. PIM-DM-BTrip; PIM-TFM-BTrip and PIM-DTFM-BTrip). In contrast, those possessing larger cyclic solubilising groups (i.e. PIM-TMN-Trip and PIM-HMI-Trip) are slightly less selective.
When defining his 2008 CO2/CH4 upper bound, Robeson noted that data for a series of Thermally Rearranged (TR) polymers, reported by Park et al.,15,61 “with exceptional CO2/CH4 separation capabilities”,24 appeared to form an upper bound above that proposed for solution processable polymers. Such insoluble network polymers as the TR polymers often perform above the 2008 upper bounds defined for solution processable polymers due to their rigidity approaching that of carbon molecular sieves (i.e. polymers carbonised at high temperatures). Remarkably, the CO2/CH4 upper bound defined by the solution processable benzotriptycene-based PIMs lies at the same position as that of Robeson's tentatively proposed TR polymer upper bound with a selectivity 2.5 times higher than that for the 2008 upper bound.
Conclusions
The benzotriptycene-based PIMs provide exceptional gas permeability data for most important gas pairs and allow for the redefinition of the CO2/CH4 and CO2/N2 Robeson upper bounds. This is important in order to set aspirational targets for chemists in the design and synthesis of novel polymers. In addition, it will help parametric studies of energy and cost efficiency for carbon capture and natural/bio gas upgrading by providing enhanced but realistic state-of-the-art values for membrane permeability and selectivity. The resulting estimates of energy efficiencies and costs will be more attractive relative to both previous calculations for membrane systems and to competitive CO2 separation processes. The resulting improved credibility of polymer membranes for these crucial separations will stimulate research activity in this technological area of prime importance to energy and the environment.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The research leading to these results has received funding from the EU FP7 Framework Program under grant agreement no. 608490, project M4CO2 and from the EPSRC (UK) grant numbers EP/M01486X/1, EP/R000468/1 and EP/K008102/2.References
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- Y. Yampolskii, Macromolecules, 2012, 45, 3298–3311 CrossRef CAS.
- R. W. Baker and B. T. Low, Macromolecules, 2014, 47, 6999–7013 CrossRef CAS.
- M. Galizia, W. S. Chi, Z. P. Smith, T. C. Merkel, R. W. Baker and B. D. Freeman, Macromolecules, 2017, 50, 7809–7843 CrossRef CAS.
- P. M. Budd and N. B. McKeown, Polym. Chem., 2010, 1, 63–68 RSC.
- R. S. Murali, T. Sankarshana and S. Sridhar, Sep. Purif. Rev., 2013, 42, 130–186 CrossRef CAS.
- S. F. Wang, X. Q. Li, H. Wu, Z. Z. Tian, Q. P. Xin, G. W. He, D. D. Peng, S. L. Chen, Y. Yin, Z. Y. Jiang and M. D. Guiver, Energy Environ. Sci., 2016, 9, 1863–1890 RSC.
- J. K. Adewole and A. L. Ahmad, J. Polym. Res., 2017, 24, 17 CrossRef.
- N. Y. Du, H. B. Park, M. M. Dal-Cin and M. D. Guiver, Energy Environ. Sci., 2012, 5, 7306–7322 RSC.
- E. Esposito, L. Dellamuzia, U. Moretti, A. Fuoco, L. Giorno and J. C. Jansen, Energy Environ. Sci., 2019, 12, 281–289 RSC.
- M. C. Ferrari, D. Bocciardo and S. Brandani, Green Energy Environ., 2016, 1, 211–221 CrossRef.
- T. C. Merkel, H. Q. Lin, X. T. Wei and R. Baker, J. Membr. Sci., 2010, 359, 126–139 CrossRef CAS.
- R. W. Baker, B. Freeman, J. Kniep, X. T. Wei and T. Merkel, Int. J. Greenhouse Gas Control, 2017, 66, 35–47 CrossRef CAS.
- L. S. White, K. D. Amo, T. Wu and T. C. Merkel, J. Membr. Sci., 2017, 542, 217–225 CrossRef CAS.
- S. Kim and Y. M. Lee, Prog. Polym. Sci., 2015, 43, 1–32 CrossRef CAS.
- B. D. Freeman, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, eaab0530 CrossRef PubMed.
- T. Masuda, E. Isobe, T. Higashimura and K. Takada, J. Am. Chem. Soc., 1983, 105, 7473–7474 CrossRef CAS.
- Y. Yampolskii, Polym. Rev., 2017, 57, 200–212 CrossRef CAS.
- Y. He, F. M. Benedetti, S. Lin, C. Liu, Y. Zhao, H.-Z. Ye, T. Van Voorhis, M. G. De Angelis, T. M. Swager and Z. P. Smith, Adv. Mater., 2019, 31, 1807871 CrossRef PubMed.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165–186 CrossRef CAS.
- S. Budhathoki, O. Ajayi, J. A. Steckel and C. E. Wilmer, Energy Environ. Sci., 2019, 12, 1255–1264 RSC.
- C. Y. Chuah, K. Goh, Y. Q. Yang, H. Q. Gong, W. Li, H. E. Karahan, M. D. Guiver, R. Wang and T. H. Bae, Chem. Rev., 2018, 118, 8655–8769 CrossRef CAS PubMed.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- P. M. Budd, K. J. Msayib, C. E. Tattershall, B. S. Ghanem, K. J. Reynolds, N. B. McKeown and D. Fritsch, J. Membr. Sci., 2005, 251, 263–269 CrossRef CAS.
- M. D. Guiver and Y. M. Lee, Science, 2013, 339, 284–285 CrossRef CAS PubMed.
- X. Ma, O. Salinas, E. Litwiller and I. Pinnau, Macromolecules, 2013, 46, 9618–9624 CrossRef CAS.
- X. Ma, B. Ghanem, O. Salines, E. Litwiller and I. Pinnau, ACS Macro Lett., 2015, 4, 231–235 CrossRef CAS.
- M. Carta, R. Malpass-Evans, M. Croad, Y. Rogan, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Science, 2013, 339, 303–307 CrossRef CAS PubMed.
- Y. Rogan, L. Starannikova, V. Ryzhikh, Y. Yampolskii, P. Bernardo, F. Bazzarelli, J. C. Jansen and N. B. McKeown, Polym. Chem., 2013, 4, 3813–3820 RSC.
- E. Tocci, L. De Lorenzo, P. Bernardo, G. Clarizia, F. Bazzarelli, N. B. McKeown, M. Carta, R. Malpass-Evans, K. Friess, K. Pilnacek, M. Lanc, Y. P. Yampolskii, L. Strarannikova, V. Shantarovich, M. Mauri and J. C. Jansen, Macromolecules, 2014, 47, 7900–7916 CrossRef CAS.
- X. H. Ma and I. Pinnau, Macromolecules, 2018, 51, 1069–1076 CrossRef CAS.
- B. S. Ghanem, R. Swaidan, X. H. Ma, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 26, 6696–6700 CrossRef CAS PubMed.
- B. S. Ghanem, R. Swaidan, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 26, 3688–3692 CrossRef CAS PubMed.
- M. Carta, M. Croad, R. Malpass-Evans, J. C. Jansen, P. Bernardo, G. Clarizia, K. Friess, M. Lanc and N. B. McKeown, Adv. Mater., 2014, 26, 3526–3531 CrossRef CAS PubMed.
- I. Rose, M. Carta, R. Malpass-Evans, M.-C. Ferrari, P. Bernardo, G. Clarizia, J. C. Jansen and N. B. McKeown, ACS Macro Lett., 2015, 4, 912–915 CrossRef CAS.
- R. Williams, L. A. Burt, E. Esposito, J. C. Jansen, E. Tocci, C. Rizzuto, M. Lanc, M. Carta and N. B. McKeown, J. Mater. Chem. A, 2018, 6, 5661–5667 RSC.
- R. Swaidan, B. Ghanem and I. Pinnau, ACS Macro Lett., 2015, 4, 947–951 CrossRef CAS.
- I. Rose, C. G. Bezzu, M. Carta, B. Comesana-Gandara, E. Lasseuguette, M. C. Ferrari, P. Bernardo, G. Clarizia, A. Fuoco, J. C. Jansen, K. E. Hart, T. P. Liyana-Arachchi, C. M. Colina and N. B. McKeown, Nat. Mater., 2017, 16, 932–937 CrossRef CAS PubMed.
- A. Fuoco, B. Comesana-Gandara, M. Longo, E. Esposito, M. Monteleone, I. Rose, C. G. Bezzu, M. Carta, N. B. McKeown and J. C. Jansen, ACS Appl. Mater. Interfaces, 2018, 10, 36475–36482 CrossRef CAS PubMed.
- Y. Yin and M. D. Guiver, Nat. Mater., 2017, 16, 880–881 CrossRef CAS PubMed.
- Y. Hu, M. Shiotsuki, F. Sanda, B. D. Freeman and T. Masuda, Macromolecules, 2008, 41, 8525–8532 CrossRef CAS.
- C. G. Bezzu, M. Carta, M. C. Ferrari, J. C. Jansen, M. Monteleone, E. Esposito, A. Fuoco, K. Hart, T. P. Liyana-Arachchi, C. M. Colina and N. B. McKeown, J. Mater. Chem. A, 2018, 6, 10507–10514 RSC.
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230–231 RSC.
- H. Hart, C. Lai, G. C. Nwokogu and S. Shamouilian, Tetrahedron, 1987, 43, 5203–5224 CrossRef CAS.
- F. Bailly, F. Cottet and M. Schlosser, Synthesis, 2005, 791–797, DOI:10.1055/s-2005-861813.
- R. S. Luo, J. H. Liao, L. Xie, W. J. Tang and A. S. C. Chan, Chem. Commun., 2013, 49, 9959–9961 RSC.
- P. M. Budd, N. B. McKeown, B. S. Ghanem, K. J. Msayib, D. Fritsch, L. Starannikova, N. Belov, O. Sanfirova, Y. Yampolskii and V. Shantarovich, J. Membr. Sci., 2008, 325, 851–860 CrossRef CAS.
- P. Bernardo, F. Bazzarelli, F. Tasselli, G. Clarizia, C. R. Mason, L. Maynard-Atem, P. M. Budd, M. Lanc, K. Pilnacek, O. Vopicka, K. Friess, D. Fritsch, Y. P. Yampolskii, V. Shantarovich and J. C. Jansen, Polymer, 2017, 113, 283–294 CrossRef CAS.
- R. Swaidan, B. Ghanem, E. Litwiller and I. Pinnau, Macromolecules, 2015, 48, 6553–6561 CrossRef CAS.
- L. Starannikova, V. Khodzhaeva and Y. Yampolskii, J. Membr. Sci., 2004, 244, 183–191 CrossRef CAS.
- Z. X. Low, P. M. Budd, N. B. McKeown and D. A. Patterson, Chem. Rev., 2018, 118, 5871–5911 CrossRef CAS PubMed.
- K. D. Dorkenoo and P. H. Pfromm, Macromolecules, 2000, 33, 3747–3751 CrossRef CAS.
- L. M. Robeson, B. D. Freeman, D. R. Paul and B. W. Rowe, J. Membr. Sci., 2009, 341, 178–185 CrossRef CAS.
- V. Teplyakov and P. Meares, Gas Sep. Purif., 1990, 4, 66–74 CrossRef CAS.
- R. Swaidan, B. S. Ghanem, E. Litwiller and I. Pinnau, J. Membr. Sci., 2014, 457, 95–102 CrossRef CAS.
- J. Wu, J. T. Liu and T. S. Chung, Adv. Sustainable Syst., 2018, 2, 1800044 CrossRef.
- N. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372–375 CrossRef CAS PubMed.
- C. R. Mason, L. Maynard-Atem, N. M. Al-Harbi, P. M. Budd, P. Bernardo, F. Bazzarelli, G. Clarizia and J. C. Jansen, Macromolecules, 2011, 44, 6471–6479 CrossRef CAS.
- J. Zhang, H. Kang, J. Martin, S. Zhang, S. Thomas, T. C. Merkel and J. Jin, Chem. Commun., 2016, 52, 6553–6556 RSC.
- H. B. Park, C. H. Jung, Y. M. Lee, A. J. Hill, S. J. Pas, S. T. Mudie, E. Van Wagner, B. D. Freeman and D. J. Cookson, Science, 2007, 318, 254–258 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ee01384a |
| ‡ The first two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |