Open Access Article
Open Access ArticleAcoustic microfluidics for colloidal materials and interface engineering
Xiong
Zhao†
ab,
Zhenzhen
Chen†
ab,
Yinan
Qiu
c and
Nanjing
Hao
 *ab
*ab
aSchool of Chemical Engineering and Technology, Xi’an JiaoTong University, 28 Xianning West Road, Xi’an, Shaanxi 710049, P. R. China. E-mail: nanjing.hao@xjtu.edu.cn
bState Key Laboratory of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, 865 Changning Road, Shanghai 200050, P. R. China
cState Key Laboratory of Technologies in Space Cryogenic Propellants, Beijing, 100028, P. R. China
First published on 19th January 2023
Abstract
The advent of microfluidic and microfabrication technologies provides vast opportunities for the rational design of colloidal materials and interfaces. However, the development of microscale lab-on-a-chip solutions is greatly limited by the lack of flexible control and intensive process kinetics. The integration of acoustics and microfluidics provides tremendous opportunities to manipulate fluids for the spatiotemporal control of engineering applications. In this perspective, we discuss the utility of acoustic microfluidics as an emerging tool for colloidal materials and interface engineering. We outline the underlying principles involved in acoustic microfluidics, assess the achievements and shortcomings of efforts so far in the field, and then highlight the bottlenecks that hinder the practical implementations of acoustic microfluidics from the viewpoints of both academic and industrial applications. We further provide our perspectives toward future research to develop advanced materials and interface systems using acoustic on-chip devices.
1. Introduction
The advent of microfluidics in the 1990s has led to significant achievements and outstanding performance characteristics that range from colloidal materials development and interface engineering applications.1 By virtue of its unique merits in terms of ease-of-use, low-cost, portability, scalability, and automation, microfluidics has demonstrated effectiveness and improvements over conventional benchmark methods.2–7 Although considerable progress has been achieved, the low Reynolds number at the microscale means that microfluidic devices face the critical challenge of process intensification inside the microchannels, which greatly limits their applicability.8–10To overcome this problem, researchers have developed many passive strategies that rely on the optimization of channel geometries and device configurations.11 However, these strategies are not only short of flexible control but lack intensive process kinetics within a short-range proximity.12,13 Alternatively, active strategies that rely on an external energy supply such as acoustics, optics, magnetics, electrics, and heat have emerged. Among them, acoustics-driven microfluidics presents attractive features of precise positioning, high tunability, deep penetration, and good biocompatibility, which provide tremendous opportunities to manipulate fluids for the spatiotemporal control of engineering applications.
This perspective review assesses the promise of acoustic microfluidics for colloidal materials and interface engineering (Fig. 1). We outline the principles underlying acoustic actuation, summarize the successes and failures of past efforts, highlight the current bottlenecks hindering practical implementations, and finally provide ideas to inspire future research in this field.
 | ||
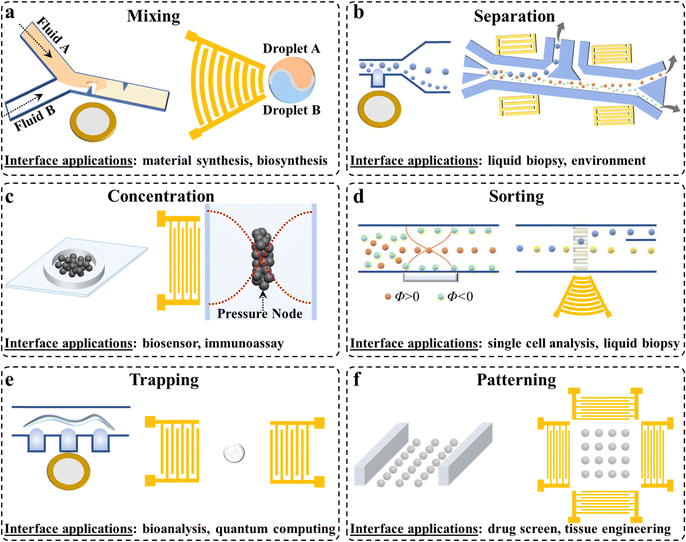
| Fig. 1 Acoustic microfluidics for colloidal materials and interface engineering enabled by the functions of mixing, separation, concentration, sorting, trapping, and patterning. Reproduced with permissions from ref. 24, 32, 38, 48 and 50–57, respectively. | ||
2. Working principles
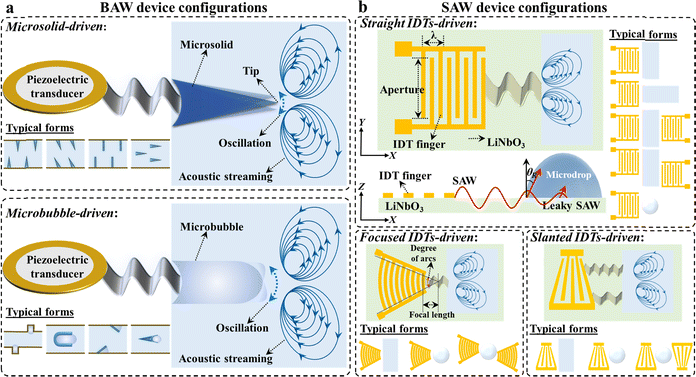
Conventional microfluidic strategies are largely affected by the strong capillary forces and high surface tension in microscale channels, leading to critical challenges for both colloidal materials development and interface engineering applications. The introduction of acoustics enables microfluidic devices to generate acoustic streaming for manipulating liquid precisely and efficiently with controllable reaction and interaction kinetics, providing promising potential for chemical and biomedical engineering.14 According to different driving modes, acoustic microfluidics can be classified into two main groups: bulk acoustic wave (BAW) microfluidics and surface acoustic wave (SAW) microfluidics (Fig. 2).BAW devices function through the propagation of acoustic waves in the direction perpendicular to the surface of the piezoelectric materials (mostly lead zirconate titanate, quartz, and aluminum nitride). BAW microfluidics that usually work under a frequency of several hertz to a few hundred kilohertz can be further divided into microsolid-driven microfluidics and microbubble-driven microfluidics (Fig. 2a). Microsolid-driven microfluidics relies on the oscillation of solid microstructures (mostly triangular objects) inside the microchannels upon acoustic actuation, which can induce a pair of counter-rotating vortices around each solid object.15–17 Such a microstreaming phenomenon enables the facilitation of mass transfer for meeting the specific needs of process intensification at the microscale. The reaction and interaction kinetics regulated by the oscillation amplitude can be adjusted flexibly using the power feature (such as voltage, frequency, and waveform) and microsolid properties (such as the tip angle, number, size, and pattern).17 Similarly, microbubble-driven microfluidics rely on the oscillation of microbubbles that are formed by trapping air pockets in specially designed microchannels with unique cavities or grooves. The strong liquid circulation around microbubbles that is induced by acoustic streaming enables rapid and significant process intensification. It is noted that the resonant frequencies for actuating the microbubbles are directly related to their sizes, providing enough flexibility in adjusting the performance metrics.
SAW devices function through the propagation of acoustic waves in the direction along the surface of the piezoelectric materials (mostly lithium niobate and lithium tantalate) with patterned interdigital transducers (IDTs). Upon actuation of the IDTs, the generated SAW can radiate into the fluid at the Rayleigh angle (θR), giving rise to acoustic streaming. SAW microfluidics, which usually work under a frequency from several hertz to a few hundred megahertz, can be broadly divided further into straight IDTs-driven microfluidics, focused IDTs-driven microfluidics, and slanted IDTs-driven microfluidics (Fig. 2b).18 Straight IDTs-driven microfluidics rely on equal-length straight metal bars (IDT fingers) that are alternately connected on either end. When placing a droplet or microchannel in the wave-propagation path, the SAW energy can induce a strong disturbance in the liquid for process intensification. The reaction and interaction kinetics can be adjusted flexibly via the applied voltage and frequency, size dimensions and location of droplets/microchannels, as well as the configuration of the IDTs. Compared with straight IDTs-driven microfluidics, focused IDTs-driven microfluidics usually rely on curved concentric fingers to generate a stronger SAW energy for intensifying mass transfer. The process kinetics can be controlled well by adjusting a series of design configurations, such as the focal length, degree of arc, finger width, and number of finger pairs. Comparatively, slanted IDTs-driven microfluidics rely on variation of the finger spacing across the transducer width, which enables broad-band frequency excitation for more flexible operations.19,20
3. Colloidal materials development
By virtue of its unique features, such as rapid reaction kinetics, intensive mixing, high integration, and good flexibility, acoustic microfluidics has already demonstrated great potential for the rational design and controllable synthesis of functional micro-/nanomaterials.21 Compared with conventional passive microreactors, acoustics-integrated active microreactors not only enable flexible control but also greatly shorten the mixing distance and simplify the microchannel geometries.22 So far, different kinds of organic materials (such as chitosan,23 PLGA,24 MXene,25,26 MOFs,27 liposomes,24 polyvinyl alcohol,28 and proteins29), inorganic materials (such as Au nanoparticles,23 Ag nanoparticles,30 ZnO nanorods,31,32 and MoS2 quantum dots33), as well as their hybrid materials have been created from BAW and SAW microfluidic reactors (Fig. 3).34 For example, in organic systems, MOFs, including HKUST-1 and Fe-MIL-88B, can be synthesized within 5 min via a SAW platform due to fast convective transport.27 PLGA-PEG nanoparticles of 57.4 nm and liposomes of 73.1 nm have been synthesized using a BAW device.24 Uniform protein crystallization with a 60% size difference drop compared with passive mixing has been realized by the SAW device.29 In inorganic systems, ZnO nanorods of 3 μm length and 200 nm diameter were synthesized inside the glass capillary using a BAW platform.32 Ag nanoparticles with a size of 40–90 nm were synthesized via a SAW device.30 MoS2 quantum dots with a size of 8.25 nm and considerably higher yields compared with conventional methods were synthesized using a SAW device.33 In all, the main effect of acoustic microfluidics, regardless of whether BAW or SAW devices, in the synthesis process is to strongly disturb the flow field and enhance mass transfer. Because of such a process intensification ability, acoustic microfluidics can show a better performance than conventional methods in the synthesis of materials.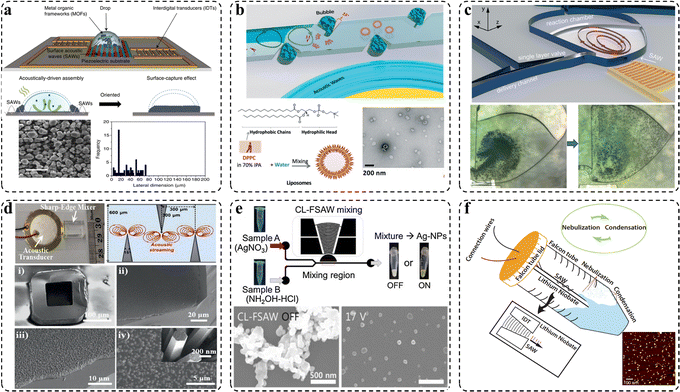 | ||
| Fig. 3 Examples of acoustic microfluidics for colloidal materials development. (a) Straight IDTs-driven synthesis of MOFs. (b) Bubble-driven microreactors for liposome production. (c) Focused SAW microreactor for the synthesis of protein crystals. (d) Sharp-edge acoustic microreactors for the synthesis of a ZnO nanorod array inside a glass capillary. (e) Focused SAW device for the synthesis of Ag nanoparticles. (f) Focused SAW device for the synthesis of MoS2 quantum dots. Reproduced with permissions from ref. 24, 27, 30, 33, 35 and 36, respectively. | ||
In spite of the great achievements that have been made, certain problems remain facing acoustic microfluidics for colloidal materials development. Firstly, compared with conventional microreactors, the flexibility and efficiency of acoustic microreactors have been validated. However, the introduction of acoustics requires the support of affiliated equipment, such as function generators and amplifiers, which will certainly compromise to some extent the portability of microreactor devices. Secondly, the feasibility of acoustic microreactors for the synthesis of organic and inorganic micro-/nanomaterials has been demonstrated. However, systematic investigation of the effect of the reactor operation parameters on the physicochemical properties (such as size, shape, and composition) towards the development of an on-chip material library is still lacking. Thirdly, although numerous studies have identified acoustics-driven intensive mixing inside microchannels and sessile microdrops, the throughput and yield capacity remain a significant challenge for acoustic microreactors. The flow rates in continuous flow microchannels are usually below tens of microliters per minute, while the volumes of the microdrops are generally less than tens of microliters.
4. Interface engineering applications
The introduction of acoustics endows microfluidics with attractive features in terms of precise positioning, flexible control, intensive interactions, and good biocompatibility, which offer great opportunities for advanced interface engineering applications. Compared with conventional microfluidic chips, acoustic microfluidic devices enable the manipulation of particulate objects in a dynamic, predetermined, and controllable manner through acoustic streaming and an acoustic radiation force. So far, a series of BAW- and SAW-based interface engineering solutions have been developed for robust mixing, separation, concentration, sorting, trapping, patterning, etc. (Fig. 4).37The intrinsic advanced functions make acoustic microfluidics possible for benefiting diverse application fields such as biosensing,38,42 nanomedicine,35 drug delivery,39 cell lysis,41 bioliquefaction,43 enzyme bioassays,40 batteries,44 tissue engineering,45 and cell/spheroid/organoid manipulation48,49 (Fig. 5). In spite of its apparent effectiveness, many aspects of acoustic microfluidics in interface engineering applications have still not been explored. Firstly, the engineering applicability of acoustic microfluidics has already been verified with extensive experimental observations. However, considering the complexity of acoustic effects in fluidic environments, the underlying physics and working mechanisms are still not clear enough. Secondly, acoustic microfluidics has successfully demonstrated robust size-based separation and concentration capabilities from nanoscale proteins to microscale cells and milliscale organisms. However, the selective treatment of similar-sized or molecular-sized objects remains an unmet need. Thirdly, due to the actions of acoustic streaming and acoustic radiation forces, acoustic microfluidics can precisely position particulates in a preconceived way at the microscale. Despite the successes achieved at microscopic interfaces, high throughput manipulation remains a challenging issue. Fourthly, high-power acoustic energy is essential for promoting mixing and facilitating the manipulation of particles and droplets at liquid interfaces. However, significant heat may be produced, which not only affects the sound velocity but also the interface actions, especially for applied biological systems.
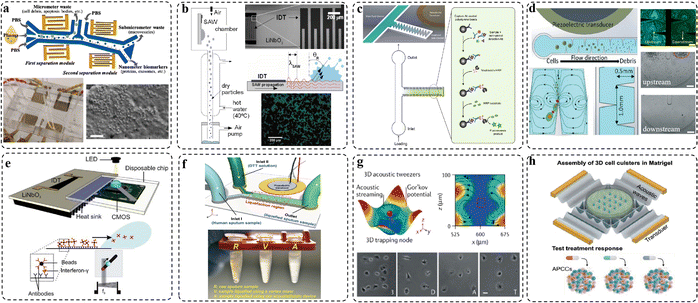 | ||
| Fig. 5 Examples of acoustic microfluidics for interface engineering applications. (a) Straight IDTs-driven separation and analysis of biomarkers. (b) Straight IDTs for protein drug encapsulation and delivery. (c) Sharp-edge acoustic device for enzyme-linked immunosorbent assay. (d) Sharp-edge acoustic device for cell lysis applications. (e) Slanted IDTs as a lens-free detection platform. (f) Sharp-edge-driven acoustic device for sputum liquefaction. (g) Straight IDTs for cell manipulation. (h) Straight IDTs for cell assembly. Reproduced with permissions from ref. 38–43, 48 and 49, respectively. | ||
5. Technological bottlenecks
As a new technology, there is no doubt that acoustic microfluidics has outperformed the conventional materials development and interface application technologies in many aspects. In particular, acoustics not only enables microfluidics to achieve a better performance than existing approaches but also enables process intensification and flexible control to be performed that were not previously available. During the development of applied acoustic microfluidic technology, several technological bottlenecks have gradually been identified, even though they are not yet widely considered by academic and industrial societies.The fabrication of acoustic microfluidic devices relies largely on photolithography in a cleanroom environment and mold replication techniques, which not only require skilled personnel and specialized instruments but also need tedious and lab-intensive protocols (e.g., spin-coating, baking, ultraviolet exposure, rinsing, etc.). These constraints to some extent limit the production efficiency and lead to rising costs. 3D printing represents an emerging and revolutionary technology for novel microfluidics and lab-on-a-chip applications.46 Combined with the portable and versatile 3D printing platform, accelerating the translation of acoustic microfluidics to industrial practice will become possible.
Traditional materials used for BAW and SAW microfluidic devices usually include silicon, glass, lithium niobate, and polydimethylsiloxane. Silicon, glass, and lithium niobate substrates possess high chemical robustness but require complicated and expensive fabrication procedures, while the polydimethylsiloxane polymer cannot endure high temperatures and pressures and may also suffer from severe swelling upon exposure to organic solvents.47 Therefore, it remains imperative to find more robust materials that can meet industrial demands.
The format of acoustic microchips generally consists of a single device coupled with an acoustic signal generation system (including a function generator, amplifier, transducer, etc.). Most of the currently developed acoustic microchips only put the focus on the demonstration of a single chip for colloidal materials and interface engineering. Although acoustic microfluidics has the potential for parallelization and automation of procedures for high-throughput processing, its scalability has rarely been examined. In addition, considering limitations in multiple individual instruments for acoustic control and heat generation during acoustic excitation, integrated open-source systems and advanced heat management solutions should be configured for practical implementation.
Acoustic microfluidics is drawing particular interest due to its desirable mixing, separation, concentration, sorting, trapping, and patterning functions. It is noteworthy that acoustic microfluidic devices have already demonstrated substantial efficacy through intensifying the reaction and interaction kinetics in certain chemical and biomedical engineering fields. However, it is noted that the application range of acoustic microfluidics remains limited and the industrialization process has lagged significantly behind the research output. To broaden and accelerate the global use of acoustic microfluidics, the close collaboration of professionals from different disciplines is pivotal and necessary.
6. Future perspectives and conclusions
The past decade has witnessed significant progress in the research and development of acoustic microfluidics. A number of BAW- and SAW-driven devices have been developed so far for use in the fields of colloidal materials development and interface engineering applications. It can be expected that acoustic microfluidics will merge with a myriad of science/engineering fields that will benefit each other in the future. For example, materials science can grow much faster through acoustic microfluidics as the time for material synthesis can be greatly reduced with the prominent mass transfer; 3D printing provides a straightforward and economic approach to fabricating acoustic microfluidic devices, which will promote scientific research and industrial production; biomaterials derived from acoustic microfluidics offer the fascinating potential for point-of-care biosensing and drug delivery applications. With the fusion of acoustic microfluidics and diversified disciplines, more possibilities will be opened up and a bright future is surely anticipated.Overall, owing to its unique and superior features, especially in terms of flexible control, precise positioning, and deep penetration, acoustics assists microfluidics in being a versatile tool to advance on-chip research and solve unmet practical needs. However, the successful utilization of acoustic microfluidics in practical materials, biology, electronics, and energy fields is still in its infancy and demands continuous effort from both academic and industrial communities. It is anticipated that with emerging technologies in microfabrication, acoustics in combination with microfluidics will provide unprecedented opportunities for revolutionizing conventional practices and expanding the body of knowledge in the field.
Conflicts of interest
The authors have no conflicts of interest to disclose.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 22108212), the open foundations of the State Key Laboratory of Chemical Engineering (Grant No. SKL-ChE-21B06) and the State Key Laboratory of Transducer Technology (Grant No. SKT2101), the Qin Chuang Yuan Talent Program (Grant No. 2021QCYRC4-33), and the “Young Talent Support Plan” of Xi'an Jiaotong University.References
- G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS PubMed.
- K. B. Shanmugasundaram, J. Li, A. I. Sina, A. Wuethrich and M. Trau, Mater. Adv., 2022, 3, 1459–1471 RSC.
- S. Radhakrishnan, M. Mathew and C. S. Rout, Mater. Adv., 2022, 3, 1874–1904 RSC.
- A. Rogolino and G. Savio, Mater. Adv., 2021, 2, 845–855 RSC.
- K. L. Ly, C. B. Raub and X. Luo, Mater. Adv., 2020, 1, 34–44 RSC.
- P. M. Valencia, O. C. Farokhzad, R. Karnik and R. Langer, Nat. Nanotechnol., 2012, 7, 623–629 CrossRef CAS PubMed.
- D. Mark, S. Haeberle, G. Roth, F. von Stetten and R. Zengerle, Chem. Soc. Rev., 2010, 39, 1153 RSC.
- K. S. Elvira, X. Casadevall Solvas, R. C. R. Wootton and A. J. de Mello, Nat. Chem., 2013, 5, 905–915 CrossRef CAS PubMed.
- J. Il Park, A. Saffari, S. Kumar, A. Günther and E. Kumacheva, Annu. Rev. Mater. Res., 2010, 40, 415–443 CrossRef.
- E. Livak-Dahl, I. Sinn and M. Burns, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 325–353 CrossRef CAS PubMed.
- N. Hao, Y. Nie and J. X. J. Zhang, Int. Mater. Rev., 2018, 63, 461–487 CrossRef CAS.
- N. Hao, Y. Nie and J. X. J. Zhang, Biomater. Sci., 2019, 7, 2218–2240 RSC.
- N. Hao, M. Zhang and J. X. J. Zhang, Biomater. Sci., 2020, 8, 1783–1801 RSC.
- W. Connacher, N. Zhang, A. Huang, J. Mei, S. Zhang, T. Gopesh and J. Friend, Lab Chip, 2018, 18, 1952–1996 RSC.
- Z. Chen, P. Liu, X. Zhao, L. Huang, Y. Xiao, Y. Zhang, J. Zhang and N. Hao, Appl. Mater. Today, 2021, 25, 101239 CrossRef.
- C. Zhang, P. Brunet, L. Royon and X. Guo, Chem. Eng. J., 2021, 410, 128252 CrossRef CAS.
- A. A. Doinikov, M. S. Gerlt and J. Dual, Phys. Rev. Lett., 2020, 124, 154501 CrossRef CAS PubMed.
- L. Y. Yeo and J. R. Friend, Annu. Rev. Fluid Mech., 2014, 46, 379–406 CrossRef.
- Y. Q. Fu, J. K. Luo, N. T. Nguyen, A. J. Walton, A. J. Flewitt, X. Zu, Y. Li, G. McHale, A. Matthews, E. Iborra, H. Du and W. I. Milne, Prog. Mater. Sci., 2017, 89, 31–91 CrossRef CAS.
- Y. Gu, C. Chen, Z. Mao, H. Bachman, R. Becker, J. Rufo, Z. Wang, P. Zhang, J. Mai, S. Yang, J. Zhang, S. Zhao, Y. Ouyang, D. T. W. Wong, Y. Sadovsky and T. J. Huang, Sci. Adv., 2021, 7, eabc0467 CrossRef CAS PubMed.
- K. Kulkarni, J. Friend, L. Yeo and P. Perlmutter, Lab Chip, 2009, 9, 754–755 RSC.
- Tandiono, S.-W. Ohl, D. S. W. Ow, E. Klaseboer, V. V. Wong, R. Dumke and C.-D. Ohl, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5996–5998 CrossRef CAS PubMed.
- P. Huang, S. Zhao, H. Bachman, N. Nama, Z. Li, C. Chen, S. Yang, M. Wu, S. P. Zhang and T. J. Huang, Adv. Sci., 2019, 6, 1900913 CrossRef CAS PubMed.
- M. R. Rasouli and M. Tabrizian, Lab Chip, 2019, 19, 3316–3325 RSC.
- H. Alijani, A. R. Rezk, M. M. Khosravi Farsani, H. Ahmed, J. Halim, P. Reineck, B. J. Murdoch, A. El-Ghazaly, J. Rosen and L. Y. Yeo, ACS Nano, 2021, 15, 12099–12108 CrossRef CAS PubMed.
- A. El Ghazaly, H. Ahmed, A. R. Rezk, J. Halim, P. O. Å. Persson, L. Y. Yeo and J. Rosen, ACS Nano, 2021, 15, 4287–4293 CrossRef PubMed.
- H. Ahmed, A. R. Rezk, J. J. Richardson, L. K. Macreadie, R. Babarao, E. L. H. Mayes, L. Lee and L. Y. Yeo, Nat. Commun., 2019, 10, 2282 CrossRef PubMed.
- S. Jin, X. Wei, Z. Yu, J. Ren, Z. Meng and Z. Jiang, ACS Appl. Mater. Interfaces, 2020, 12, 22318–22326 CrossRef CAS PubMed.
- Y. Zhang, C. Devendran, C. Lupton, A. De Marco and A. Neild, Lab Chip, 2019, 19, 262–271 RSC.
- J. Nam, W. S. Jang and C. S. Lim, Sens. Actuators, B, 2018, 258, 991–997 CrossRef CAS.
- N. Hao, P. Liu, H. Bachman, Z. Pei, P. Zhang, J. Rufo, Z. Wang, S. Zhao and T. J. Huang, ACS Nano, 2020, 14, 6150–6163 CrossRef CAS PubMed.
- N. Hao, Z. Pei, P. Liu, H. Bachman, T. D. Naquin, P. Zhang, J. Zhang, L. Shen, S. Yang, K. Yang, S. Zhao and T. J. Huang, Small, 2020, 16, 2005179 CrossRef CAS PubMed.
- S. Marqus, H. Ahmed, M. Ahmed, C. Xu, A. R. Rezk and L. Y. Yeo, ACS Appl. Nano Mater., 2018, 1, 2503–2508 CrossRef CAS.
- Z. Chen, Z. Pei, X. Zhao, J. Zhang, J. Wei and N. Hao, Chem. Eng. J., 2022, 433, 133258 CrossRef CAS.
- Y. Zhang, C. Devendran, C. Lupton, A. De Marco and A. Neild, Lab Chip, 2019, 19, 262–271 RSC.
- N. Hao, Z. Pei, P. Liu, H. Bachman, T. D. Naquin, P. Zhang, J. Zhang, L. Shen, S. Yang, K. Yang, S. Zhao and T. J. Huang, Small, 2020, 16, 2005179 CrossRef CAS PubMed.
- J. Friend and L. Y. Yeo, Rev. Mod. Phys., 2011, 83, 647–704 CrossRef.
- N. Hao, Z. Wang, P. Liu, R. Becker, S. Yang, K. Yang, Z. Pei, P. Zhang, J. Xia, L. Shen, L. Wang, K. A. Welsh-Bohmer, L. H. Sanders, L. P. Lee and T. J. Huang, Biosens. Bioelectron., 2022, 196, 113730 CrossRef CAS PubMed.
- M. Alvarez, L. Y. Yeo, J. R. Friend and M. Jamriska, Biomicrofluidics, 2009, 3, 014102 CrossRef PubMed.
- X. Li, J. Huffman, N. Ranganathan, Z. He and P. Li, Anal. Chim. Acta, 2019, 1079, 129–138 CrossRef CAS PubMed.
- Z. Wang, P. H. Huang, C. Chen, H. Bachman, S. Zhao, S. Yang and T. J. Huang, Lab Chip, 2019, 19, 4021–4032 RSC.
- Y. Bourquin, J. Reboud, R. Wilson, Y. Zhang and J. M. Cooper, Lab Chip, 2011, 11, 2725–2730 RSC.
- P. H. Huang, L. Ren, N. Nama, S. Li, P. Li, X. Yao, R. A. Cuento, C. H. Wei, Y. Chen, Y. Xie, A. A. Nawaz, Y. G. Alevy, M. J. Holtzman, J. P. McCoy, S. J. Levine and T. J. Huang, Lab Chip, 2015, 15, 3125–3131 RSC.
- A. Huang, H. Liu, O. Manor, P. Liu and J. Friend, Adv. Mater., 2020, 32, 1907516 CrossRef CAS PubMed.
- Z. Chen, L. Shen, X. Zhao, H. Chen, Y. Xiao, Y. Zhang, X. Yang, J. Zhang, J. Wei and N. Hao, Appl. Mater. Today, 2022, 26, 101356 CrossRef.
- A. K. Au, W. Huynh, L. F. Horowitz and A. Folch, Angew. Chem., Int. Ed., 2016, 55, 3862–3881 CrossRef CAS PubMed.
- K. Ren, J. Zhou and H. Wu, Acc. Chem. Res., 2013, 46, 2396–2406 CrossRef CAS PubMed.
- F. Guo, Z. Mao, Y. Chen, Z. Xie, J. Lata, P. Li, L. Ren, J. Liu, J. Yang, M. Dao, S. Suresh and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 1522–1527 CrossRef CAS PubMed.
- Z. Ao, Z. Wu, H. Cai, L. Hu, X. Li, C. Kaurich, J. Chang, M. Gu, L. Cheng, X. Lu and F. Guo, Adv. Sci., 2022, 9, 2201478 CrossRef CAS PubMed.
- J. Shi, D. Ahmed, X. Mao, S. S. Lin, A. Lawit and T. J. Huang, Lab Chip, 2009, 9, 2890–2895 RSC.
- P. H. Huang, Y. Xie, D. Ahmed, J. Rufo, N. Nama, Y. Chen, C. Y. Chan and T. J. Huang, Lab Chip, 2013, 13, 3847–3852 RSC.
- P. Li, Z. Mao, Z. P. L. Zhou, Y. Chen, P. H. Huang, C. Truica, J. J. Drabick, W. S. El-Deiry, M. Dao, S. Suresh and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(16), 4970–4975 CrossRef CAS PubMed.
- P. Liu, Z. Tian, K. Yang, T. D. Naquin, N. Hao, H. Huang, J. Chen, Q. Ma, H. Bachman, P. Zhang, X. Xu, J. Hu and T. J. Huang, Sci. Adv., 2022, 8, eabm2592 CrossRef CAS PubMed.
- S. Li, X. Ding, F. Guo, Y. Chen, M. I. Lapsley, S. S. Lin, L. Wang, J. P. McCoy, C. E. Cameron and T. J. Huang, Anal. Chem., 2013, 85(11), 5468–5474 CrossRef CAS PubMed.
- X. Ding, S. S. Lin, M. I. Lapsley, S. Li, X. Guo, C. Y. Chan, I. K. Chiang, L. Wang, J. P. McCoy and T. J. Huang, Lab Chip, 2012, 12, 4228–4231 RSC.
- H. Cai, Z. Wu, Z. Ao, A. Nunez, B. Chen, L. Jiang, M. Bondesson and F. Guo, Biofabrication, 2020, 12, 035025 CrossRef CAS PubMed.
- X. Ding, J. Shi, S. S. Lin, S. Yazdi, B. Kiraly and T. J. Huang, Lab Chip, 2012, 12, 2491–2497 RSC.
Footnote |
| † These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |