Optimized arrangement of non-π-conjugated PO3NH3 units leads to enhanced ultraviolet optical nonlinearity in NaPO3NH3†
Lingli
Wu
abc,
Haotian
Tian
bc,
Chensheng
Lin
 b,
Xin
Zhao
bc,
Huixin
Fan
b,
Pengxiang
Dong
b,
Shunda
Yang
b,
Ning
Ye
d and
Min
Luo
b,
Xin
Zhao
bc,
Huixin
Fan
b,
Pengxiang
Dong
b,
Shunda
Yang
b,
Ning
Ye
d and
Min
Luo
 *ab
*ab
aFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian 350108, China. E-mail: lm8901@fjirsm.ac.cn
bKey Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China
cUniversity of the Chinese Academy of Sciences, Beijing, 100049, China
dTianjin Key Laboratory of Functional Crystal Materials, Institute of Functional Crystal, Tianjin University of Technology, Tianjin, 300384, China
First published on 2nd January 2024
Abstract
The key to achieving nonlinear optical (NLO) crystals with superior optical properties lies in the development of new high-performance functional units and their precise arrangement. In this study, we identify the polar covalent tetrahedron (PO3NH3)−, with its remarkable optical properties, as a novel deep-ultraviolet (DUV) NLO-active unit for the first time. To ensure the (PO3NH3)− units are accurately aligned, we selected NaNH4PO3F·H2O as a template. Subsequently, we successfully synthesized NaPO3NH3, wherein the (PO3NH3)− units were arranged in an orderly manner. This excellent ordered arrangement of (PO3NH3)− units enables NaPO3NH3 to exhibit not only a significant SHG effect (1.2 × KDP), but also the largest birefringence (0.062@546.1 nm) among DUV non-π-conjugated phosphate systems.
Introduction
Deep-ultraviolet (DUV) nonlinear optical (NLO) crystals hold significant potential in extending and regulating laser wavelengths into the DUV region through the frequency-doubling effect. This capability is crucial for applications in semiconductor manufacturing, optical communication, and laser micromachining.1–4 However, achieving a balance between a short cutoff edge (λcut-off), high second harmonic generation (SHG) efficiency, and moderate birefringence (Δn) remains a challenge for DUV crystals.5–7 Previous research has indicated that obtaining suitable birefringence for phase matching in DUV crystals is difficult.8,9 Among the few crystals capable of directly realizing DUV laser output, KBe2BO3F2 (KBBF) stands out.10 Nonetheless, the practical application of KBBF is limited due to its severe layered growth habit and the presence of highly toxic beryllium oxide.Non-π-conjugated systems have garnered significant interest due to their broader optical transmittance window, making phosphate systems a primary research focus. However, phosphate systems are often hindered by weak SHG and small birefringence, which can be ascribed to the low hyperpolarizability and minimal optical anisotropy resulting from the absence of π orbitals.11,12 Numerous studies have been conducted to enhance SHG and birefringence in phosphates. One of the most effective approaches involves the use of NLO-active cations, such as Ti4+,13 Bi3+,14 Sn2+,15 and Pb2+,16 among others.17 Nevertheless, this method invariably induces a red shift of absorption edge, limiting its applicability in the DUV region. To ensure high UV transmission, the selection of cations must be restricted to metal ions with a full shell, including alkali metals, alkaline earth metals, and certain rare earth metal cations.18–20 Therefore, to maintain the wide band gap of phosphates, an alternative strategy to enhance SHG and birefringence is the modification of [PO4]3− group.
Along this path, polar polyphosphates, such as P2O7 dimer,21,22 P3O10 trimer,23,24 and [PO3]∞ chain groups,25,26 have been investigated. However, these crystals exhibit enhancement only in SHG performance. The birefringence of polyphosphates is rarely greater than 0.03@1064 nm, which is attributed to the steric hindrance between groups caused by co-vertex connections, preventing the ordered arrangement of polyphosphate groups. In contrast, isolated tetrahedral groups offer greater flexibility in adjusting their positions to maximize optical anisotropy. Consequently, identifying polar tetrahedral phosphate groups with exceptional properties is crucial. In light of this, Pan and Chen (2018) groups proposed introducing highly electronegative fluorine (F) into [PO4]3− groups to obtain polar (PO3F)2− with excellent DUV optical properties. Subsequent studies discovered crystals such as (NH4)2PO3F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 and [C(NH2)3]2PO3F;28 however, the arrangement of polar tetrahedral groups in these crystals did not favor maximizing enhanced birefringence. In 2019, Chen's group successfully synthesized and grew NaNH4PO3F·H2O,29 which binds fluorophosphate together through hydrogen bonds from NH4+, achieving near-directional arrangement of non-π-conjugated DUV functional units of polar phosphate units for the first time. Ultimately, NaNH4PO3F·H2O exhibited a large SHG and an extremely prominent birefringence of >0.053@589.3 nm while maintaining deep-ultraviolet transmittance. Given that NaNH4PO3F·H2O has been demonstrated to be an outstanding DUV NLO crystal, it potentially serves as a novel parent compound to guide the synthesis and screening of new materials.
27 and [C(NH2)3]2PO3F;28 however, the arrangement of polar tetrahedral groups in these crystals did not favor maximizing enhanced birefringence. In 2019, Chen's group successfully synthesized and grew NaNH4PO3F·H2O,29 which binds fluorophosphate together through hydrogen bonds from NH4+, achieving near-directional arrangement of non-π-conjugated DUV functional units of polar phosphate units for the first time. Ultimately, NaNH4PO3F·H2O exhibited a large SHG and an extremely prominent birefringence of >0.053@589.3 nm while maintaining deep-ultraviolet transmittance. Given that NaNH4PO3F·H2O has been demonstrated to be an outstanding DUV NLO crystal, it potentially serves as a novel parent compound to guide the synthesis and screening of new materials.
In the investigation of the sulfonate system, our research group has discovered that polar tetrahedra, obtained by employing substituents such as amino and methyl groups, exhibit exceptional DUV optical properties.30,31 Consequently, we have identified a series of DUV non-π-conjugated NLO crystals with outstanding characteristics, including M(SO3NH2)2 (M = Ba, Sr),32 sulfamide,33 and KNO3SO3NH3.34 Compared to (SO4)2−, (NH2SO3)− and SO2(NH2)2 demonstrate superior performance in three key aspects: polarizability anisotropy, hyper-polarizability, and the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) gap. Moreover, the incorporation of hydrogen-containing anionic groups facilitates the regulation of their arrangement. For instance, sulfamide employs the hydrogen bond of SO2(NH2)2 to align itself unidirectionally within a layer. Since the geometric structure and optical properties of PO4 closely resemble those of SO4, we anticipate that modifying the phosphate group with amino or methyl groups could yield a novel polar tetrahedron containing hydrogen bonds and possessing exceptional optical properties.
In this study, we employed NaNH4PO3F·H2O as a template. By replacing F− with an NH3 group, we formed the new polar tetrahedron (PO3NH3)−, with the expectation that it would display excellent DUV optical properties and achieve a consistent arrangement through its inherent hydrogen bond. Furthermore, our calculations revealed that the polar (PO3NH3)− unit exhibits improved performance in the aforementioned three key aspects compared to (PO4)3−. Encouraged by these computational findings, we successfully synthesized an amino-phosphate DUV NLO material, NaPO3NH3 (NPNH). Notably, the polar [PO3NH3]− groups in NPNH achieved optimal alignment, which significantly enhanced the (010) in-plane anisotropy and resulted in the largest Δn (0.062) reported to date among DUV non-π-conjugated phosphate systems. Physical measurements demonstrated that NPNH exhibits a substantial second-harmonic generation (SHG) effect (1.2 × KH2PO4 (KDP)) and short cutoff edges (<190 nm).
Experimental section
Reagents
Diphenyl Phosphoramidate (C12H12NO3P, 97%, Adamas), NaOH (≧98%, Titan), Gelatin (Aladdin), Acetic acid glacial (≧99.5%, Titan).Synthesis
Polycrystalline samples of NaPO3NH3 were synthesized according to the method described by E. Hobbs, D. E. C. Corbridge, and B. Raistrick.35 A mixture of Diphenyl Phosphoramidate (8 mmol, 1.994 g), NaOH (32 mmol, 1.280 g), and H2O (10 ml) was heated until the Diphenyl Phosphoramidate was completely hydrolyzed. Then acetic acid glacial was slowly dripped into the solution and the insoluble monosodium salt (NaPO3NH3) was produced according to the following scheme:However, the insoluble nature of NaPO3NH3 prevented the growth of the required crystals from a simple solution. Thus, we adopted the method of crystallizing within the gelatin gels in a U-tube to obtain large crystals for subsequent measurements. For this purpose, the gelatin (1 g) was soaked in 15 ml H2O for 5 minutes and then heated until the gelatin was dissolved. Next, the gelatin solution was poured into a U-tube for 3 hours to solidify. Finally, the solution of (NaO)2PONH2 (10 ml, previously prepared) and acetic acid glacial (10 ml) were filled into the respective arms of the U-tube. After one week, a small number of transparent colorless polycrystalline NaPO3NH3 crystals were obtained. These crystals were stable in the air and hardly water-soluble.
Single-crystal X-ray diffraction
For single-crystal X-ray diffraction measurement, a premium NaPO3NH3 signal crystal was chosen. The graphite-monochromatic Cu-Kα radiation (λ = 1.54184 Å) was used to acquire the diffraction data by using a Rigaku Mercury CCD diffractometer at room temperature. The data was integrated by the Crystal Clear program. The intensities were corrected by Lorentz polarization, air absorption, and absorption attributable to the variation in the path length through the detector faceplate. The crystal structure of NaPO3NH3 was solved through the directed methods and then confirmed by full-matrix least-squares fitting on F2 using SHELXL on the Olex2 package.36,37 The PLATON program was used to check for the structure, and no higher symmetry was found. Crystallographic data and structure refinements of NaPO3NH3 were given in Table S1.† Atomic coordinates, equivalent isotropic displacement parameters, selected bond lengths and angles, and anisotropic displacement parameters for NaPO3NH3 were shown in Tables S2–S4.†Power X-ray diffraction
The power X-ray diffraction (XRD) patterns were collected on the Miniflex-600 powder X-ray diffractometer (Cu Kα radiation with λ = 1.540598 Å) from 10° to 80° at room temperature with a scanning speed of 5° min−1. The experimental powder XRD patterns and the simulated patterns of the single crystal are coincident.Energy-dispersive X-ray spectroscopy analysis
Energy dispersive X-ray spectroscopy (EDS) was tested on the FESEM, SU-8010 scanning electron microscope (SEM) with an X-ray spectroscope. A clean NaPO3NH3 crystal was mounted on an aluminum sample stage with carbon conductive tape.Thermal analysis
The data thermogravimetric analysis (TG) and differential thermal analysis (DTA) were carried out on the Netzsch STA449C simultaneous analyzer. NaPO3NH3 power was packed in an Al2O3 crucible and heated from room temperature to 900 °C at a rate of 10 °C min−1.UV-Vis diffuse reflectance spectroscopy
The UV-Vis diffuse reflection spectrum was measured on PerkinElmer Lamda-950 UV-Vis spectrophotometer scanning with the scope of 190–800 nm to determine the cutoff edge of the NaPO3NH3 crystal. BaSO4 is used as the standard of 100% reflectance.Birefringence measurement
The birefringence measurement was performed on the polarizing microscope (ZEISS Axio Scope. A1) equipped with a Berek compensator. The light source is 546.1 nm. The formula for calculating is expressed as follows: Δn = ΔR/T, while ΔR means the optical path difference, Δn signifies the birefringence, and T represents the thickness of the crystal.Second harmonic generation measurements
The test of polycrystalline powder Second Harmonic Generation (SHG) was executed on a Q-switched Nd: YAG solid-state laser with laser radiation wavelengths of 1064 nm. The sample was divided into 24–45, 45–62, 62–75, 75–109, 109–150, and 150–212 μm, respectively. KH2PO4 (KDP) is used as the standard reference. The samples were pressed between two round glass plates, each sealed with 8 mm diameter and 1 mm thick rubber rings. Then the samples were placed in the antiglare box and irradiated with the pulsed laser from an OPO laser. The signals are detected with a photomultiplier tube connected to a RIGOL DS1052E 50 MHz oscilloscope. The ratio of the SHG intensity outputs for the samples and KDP was calculated.Theoretical calculations
The molecular orbitals and polarizability calculations were carried out using density functional theory (DFT)38 and couple cluster with singles and doubles substitutions (CCSD) implemented in the Gaussian09 package 41. HOMO–LUMO Gap (Eg) of anionic groups were calculated through CCSD/Aug-cc-PVDZ, and the polarizability anisotropy (δ) and hyperpolarizability tensor were calculated through wB97/Aug-cc-PVDZ.Band structure and density of states (DOS) were determined using DFT calculations provided by the CASTEP code in the Material Studio Package. The exchange interaction38 was treated by the generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE).39 The energy cutoff for the plane wave was chosen as 750 eV, and the Monkhorst–Pack k-point sampling of 2 × 2 × 2 in the Brillouin zone of the unit cell was selected for calculation. The valence electrons are Na 2s2 2p6 3s1, P 3s2 3p3, O 2s2 2p4, N 2s2 2p3, and H 1s. The real part of the dielectric function was obtained by the Kramers–Kronig transform, and then the refractive index was determined. The SHG coefficients were obtained by the formula originally proposed by Sipe et al.40 and developed by Cheng et al.41 The SHG density of d33 is calculated out by the band-resolved method.42
Results and discussion
In order to investigate the potential structure-directing optical properties of amino-phosphate, we performed calculations and constructed molecular orbital diagrams for phosphate family groups. As shown in Fig. 1 HOMO, the nonbonding O 2p orbitals occupied the HOMO in three groups, while the F atom and NH3 group were not involved. This indicates that the electrons within the group are not evenly distributed, suggesting that the polarizability anisotropy values of (PO3F)2− and (PO3NH3)− are not lower than those of (PO4)3−. From HOMO to LUMO, the electron distribution in (PO3NH3)− shifts from O to NH3. This extensive electron redistribution correlates with a large electric field, which may enhance the SHG effect. Additionally, we calculated three parameters for (PO4)3−, (PO3F)2− and (PO3NH3)− as references (Table 1). The computed HOMO–LUMO gap of (PO3NH3)− is comparable to that of (PO4)3−, suggesting that amino-phosphate could maintain the advantage of broad UV transmittance characteristic of phosphate. Concurrently, (PO3NH3)− exhibits superior hyperpolarizability, which can be rationalized by the values of the “flexible” chemical bonds within the NLO-active group.43 The flexibility index of P–N in (PO3NH3)− (F = 0.14) is greater than that of P–F in (PO3F)2− (F = 0.09), indicating that the more flexible P–N bonds are beneficial for further enhancing the SHG response. In conclusion, based on anionic group theory, the slightly larger polarizability anisotropy and superior hyperpolarizability could result in exceptional NLO properties.| Units | δ | |β| | E g (eV) |
|---|---|---|---|
| Polarizability anisotropy (δ), hyperpolarizability tensor (β), and HOMO–LUMO gap (Eg). | |||
| (PO 4 ) 3− | 0 | 0 | 7.24 |
| (PO 3 F) 2− | 6.03 | 60.8 | 9.52 |
| (PO 3 NH 3 ) − | 2.16 | 194 | 7.82 |
 | ||
| Fig. 1 Molecular orbitals of the (PO4)3−, (PO3F)2−, and (PO3NH3)− anionic groups. The HOMO and LUMO are shown in the middle and lower panels, respectively. | ||
NaPO3NH3 (NPNH) powder samples were synthesized using the two-step method reported by E. Hobbs et al.35 To obtain large crystals for subsequent measurements, we employed a U-tube gelatin gel crystallization technique. The experimental powder X-ray diffraction (PXRD) pattern in Fig. S1† is consistent with the calculated values, confirming phase purity. Energy-dispersive X-ray spectroscopy (EDS) analysis reveals that the crystal contains Na, P, O, and N elements (Fig. S2†). Thermogravimetric (TG) and differential thermal analysis (DTA) curves for NPNH are presented in Fig. S3,† indicating stability up to 260 °C, which is higher than the thermal stability of NaNH4PO3F·H2O (under 100 °C),29 KNO3SO3NH3 (∼150 °C),34 and KLi(HC3N3O3)·2H2O (125 °C).44 NPNH is insoluble in water and alcohol and remains stable in air at room temperature for at least three months.
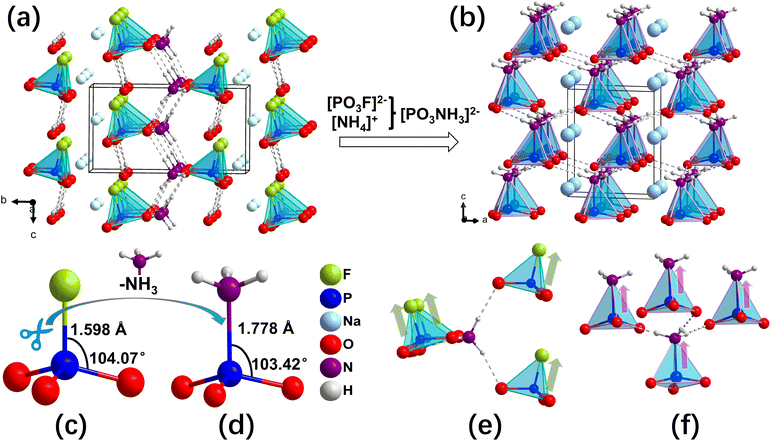
NPNH crystallizes in the hexagonal asymmetric space group P63 (No. 173) with lattice parameters a = 5.7805(1) Å, c = 6.0265(1) Å, which aligns with the findings of E. Hobbs et al. The structure of NPNH is characterized by a single anionic structural motif, (PO3NH3)−. As illustrated in Fig. 2d, each phosphorus atom is tetrahedrally coordinated by three oxygen atoms and an NH3 group, forming a (PO3NH3)− tetrahedron. The P–O distances are approximately 1.509 Å, and the P–N bond length is 1.779 Å. Intriguingly, the (PO3NH3)− groups are interconnected via hydrogen bonds, facilitating the formation of a three-dimensional (3D) structure in NPNH. Sodium cations are embedded within this network, ensuring overall charge neutrality (Fig. 2b). This bonding pattern is reminiscent of the interactions observed between (PO3F)2− groups in NaNH4PO3F·H2O. When contrasted with NaNH4PO3F·H2O, NPNH exhibits a more neatly arrangement of anionic groups (Fig. 2e and f). In NaNH4PO3F·H2O, the (PO3F)2− tetrahedra within each layer are anchored by water molecules and NH4+ groups, forming a robust hydrogen-bonded network that aligns nearly parallel to the c-axis. In contrast, in NPNH, the (PO3F)2− and NH4+ groups are combined into a single (PO3NH3)− group (Fig. 2c and d), and (PO3NH3)− units are able to achieve complete alignment along the c-axis due to their inherent hydrogen bonds (Fig. 2a and b). Consequently, this uniform alignment of polar P–N bonds results in the superposition of dipoles along the c-axis, maximizing macroscopic polarization. This leads to a significant enhancement of the (010) in-plane anisotropy, enabling NPNH to exhibit birefringence along the c-axis. Furthermore, the hydrogen bonds between anions and Na–O ionic bonds give rise to a 3D structure without any layering tendency (Fig. S4†). The lack of crystal water in NPNH potentially enhances its thermal stability.
The Ultraviolet–Visible (UV-Vis) diffuse reflectance spectra of NPNH are presented in Fig. 3a, illustrating an absorption edge below 190 nm, which corresponds to a band gap exceeding 6.5 eV. The electronic and optical properties of NPNH were computed using the first-principles method, indicating a band gap of 5.320 eV (Fig. S5†). The discrepancy between the experimental and computed band gaps can be attributed to the discontinuity of the exchange–correlation energy function.
 | ||
| Fig. 3 (a) The UV/Vis diffuse-reflectance spectroscopy of NaPO3NH3; (b) power SHG measurement at 532 nm; (c and d) the SHG density of d33 in occupied state and unoccupied state. | ||
The SHG response of NPNH was evaluated under fundamental wave laser radiation (λ = 1064 nm), utilizing polycrystalline KDP as a reference. As depicted in Fig. 3b, the SHG response of NPNH is approximately 1.2 times that of KDP within the particle size range of 150–212 μm, exhibiting phase-matchable behavior in accordance with the rule proposed by Kurtz and Perry.45 Given that the space group is P63, two independent SHG tensor components (d31, d33) must be considered. Through the application of the “velocity-gauge” formula developed by Sipe et al.,40,46 the largest tensor component d33 of NPNH is 0.583 pm V−1 at a wavelength of 1064 nm (1.165 eV), while the largest tensor component d14 of KDP is 0.381 pm V−1 at the same wavelength (Fig. S6†). Accordingly, the calculated SHG intensity is approximately 1.5 times that of KDP, aligning closely with the experimental value. Furthermore, the SHG-weighted electron density of d33 was investigated to discern the origin of the SHG responses (refer to Fig. 3c and d). The occupied and unoccupied states suggest that the primary SHG effect stems from the (PO3NH3)− units, which is consistent with the anionic group theory.47
The birefringence measurement was conducted using a ZEISS Axio Scope. A1 polarizing microscope, equipped with a Berek compensator. The resultant values for ΔR and T were 1219.7 nm and 19.64 μm, respectively, as shown in Fig. S7† and Fig. 4a. The calculated birefringence at 546.1 nm, derived using the formula Δn = ΔR/T,48 was 0.062, closely aligning with the theoretical value of 0.063, following the trend of na = nb > nc (Fig. 4b). It is concluded that NPNH exhibits the highest birefringence among DUV phosphates, surpassing that of NaNH4PO3F·H2O (0.053@589.3 nm), as illustrated in Fig. 4e and Table S7.† This significant birefringence can be primarily attributed to two reasons. First, the (PO3NH3)− unit displays a greater polarizability anisotropy, which contributes to enhanced birefringence. Second, these units possess an optimal arrangement, superior to that of NaNH4PO3F·H2O, as the angle between the P–N bond and the crystallophysical axis c is eliminated (Fig. 4c). The computed dipole moment, as shown in Table S6,† vividly reflects this optimized arrangement. The net dipole moment of (PO3NH3)− units is calculated to be 10.02 D in the polar c direction, with 0 D on the a and b directions, indicating that the polarization direction of all (PO3NH3)− units is along the c-axis. This optimal arrangement potentially compensates for the diminished birefringence effect caused by the reduced anisotropy of the (PO3NH3)− unit compared to (PO3F)2−. As shown in Fig. 4d, the uniform arrangement of polar chemical bonds considerably reduces the electron density in the c-axis direction, resulting in a significant decrease in electron density in the c direction compared to the a and b directions. Consequently, the refractive index in the c direction is substantially lower than that in the a and b directions, leading to high birefringence.49 This is further corroborated by the calculated refractive index dispersion curve.
Conclusions
We report the successful synthesis and growth of a novel NLO crystal, NaPO3NH3, utilizing the newly developed polar NLO-active (PO3NH3)− unit. Structural analysis reveals that these polar (PO3NH3)− units achieve complete alignment along the c-axis, resulting in a uniform arrangement of polar P–N bonds. This unique alignment facilitates the superposition of dipoles along the c-axis, thereby maximizing macroscopic polarization. The resultant effect is an enhanced SHG response and a significantly amplified (010) in-plane anisotropy. Furthermore, NaPO3NH3 demonstrates a DUV absorption edge, indicating its potential as a promising DUV NLO crystal. This study not only introduces a novel NLO phosphate anion group but also proposes a new strategy for obtaining NLO crystals with large birefringence.Author contributions
Prof. Min Luo provided research ideas, supervised the entire research, and revised the manuscript. Lingli Wu wrote the initial draft and completed the entire experiment and measurement work. Haotian Tian assisted in the experiments and measurements. Dr Chengshen Lin and Xin Zhao were responsible for theoretical calculations. Dr Huixin Fan, Pengxiang Dong, Dr Shunda Yang, and Prof. Ning Ye offered help with data and experiment analysis. All authors participated in the discussion.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22222510, 21975255, 21921001, and 52302008), the Natural Science Foundation of Fujian Province (2023J02026), the Foundation of Fujian Science & Technology Innovation Laboratory (2021ZR202), and the Self-deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (CXZX-2022-JQ01).References
- N. Savage, Ultraviolet lasers, Nat. Photonics, 2007, 1, 83–85 CrossRef CAS.
- D. Cyranoski, China's crystal cache: a Chinese laboratory is the only source of a valuable crystal. David Cyranoski investigates why it won't share its supplies, Nature, 2009, 457, 953–955 CrossRef CAS PubMed.
- F. Xu, G. Zhang, M. Luo, G. Peng, Y. Chen, T. Yan and N. Ye, A powder method for the high-efficacy evaluation of electro-optic crystals, Natl. Sci. Rev., 2021, 8, nwaa104 CrossRef CAS PubMed.
- K. C. Chen, C. S. Lin, J. D. Chen, G. S. Yang, H. T. Tian, M. Luo, T. Yan, Z. Hu, J. Wang, Y. Wu, N. Ye and G. Peng, Intense d-p Hybridization in Nb3O15 Tripolymer Induced the Largest Second Harmonic Generation Response and Birefringence in Germanates, Angew. Chem., 2023, 135, e202217039 CrossRef.
- P. S. Halasyamani and J. M. Rondinelli, The must-have and nice-to-have experimental and computational requirements for functional frequency doubling deep-UV crystals, Nat. Commun., 2018, 9, 2972 CrossRef PubMed.
- L. Kang, S. Y. Luo, G. Peng, N. Ye, Y. C. Wu, C. T. Chen and Z. S. Lin, First-Principles Design of a Deep-Ultraviolet Nonlinear-Optical Crystal from KBe2BO3F2 to NH4Be2BO3F2, Inorg. Chem., 2015, 54, 10533–10535 CrossRef CAS PubMed.
- L. Xiong, J. Chen, J. Lu, C. Y. Pan and L. M. Wu, Monofluorophosphates: A New Source of Deep-Ultraviolet Nonlinear Optical Materials, Chem. Mater., 2018, 30, 7823–7830 CrossRef CAS.
- C. C. Jin, F. M. Li, X. Q. Li, J. J. Lu, Z. H. Yang, S. L. Pan and M. Mutailipu, Difluoro (oxalato) borates as Short-Wavelength Optical Crystals with Bifunctional [BF2C2O4] Units, Chem. Mater., 2022, 34, 7516 CrossRef CAS.
- H. T. Qiu, F. M. Li, Z. H. Yang, S. L. Pan and M. Mutailipu, Breaking the Inherent Interarrangement of [B3O6] Clusters for Nonlinear Optics with Orbital Hybridization Enhancement, J. Am. Chem. Soc., 2023, 145, 24401–24407 CrossRef CAS PubMed.
- C. T. Chen, G. L. Wang, X. Y. Wang and Z. Y. Xu, Deep-UV nonlinear optical crystal KBe2BO3F2—discovery, growth, optical properties and applications, Appl. Phys. B: Lasers Opt., 2009, 97, 9–25 CrossRef CAS.
- W. Q. Huang, S. G. Zhao and J. H. Luo, Recent Development of Non-π-Conjugated Deep Ultraviolet Nonlinear Optical Materials, Chem. Mater., 2022, 34, 5–28 CrossRef CAS.
- Y. Q. Li, J. H. Luo and S. G. Zhao, Local Polarity-Induced Assembly of Second-Order Nonlinear Optical Materials, Acc. Chem. Res., 2022, 55, 3460–3469 CrossRef CAS PubMed.
- P. S. Halasyamani, Asymmetric Cation Coordination in Oxide Materials: Influence of Lone-Pair Cations on the Intra-octahedral Distortion in d0 Transition Metals, Chem. Mater., 2004, 16, 3586–3592 CrossRef CAS.
- D. Chen, S. Hao, L. Fan, Y. Guo, J. Yao, C. Wolverton, M. G. Kanatzidis, J. Zhao and Q. Liu, Broad Photoluminescence and Second-Harmonic Generation in the Noncentrosymmetric Organic–Inorganic Hybrid Halide (C6H5(CH2)4NH3)4MX7·H2O (M = Bi, In, X = Br or I), Chem. Mater., 2021, 33, 8106–8111 CrossRef CAS.
- J. Y. Guo, A. Tudi, S. J. Han, Z. H. Yang and S. L. Pan, Sn2PO4I: An Excellent Birefringent Material with Giant Optical Anisotropy in Non π-Conjugated Phosphate, Angew. Chem., Int. Ed., 2021, 60, 24901–24904 CrossRef CAS PubMed.
- G. Zou, C. Lin, H. Jo, G. Nam, T.-S. You and K. M. Ok, Pb2BO3Cl: A Tailor–Made Polar Lead Borate Chloride with Very Strong Second Harmonic Generation, Angew. Chem., Int. Ed., 2016, 55, 12078–12082 CrossRef CAS PubMed.
- Q. Wei, C. He, K. Wang, X. F. Duan, X. T. An, J. H. Li and G. M. Wang, Sb6O7(SO4)2: A Promising Ultraviolet Nonlinear Optical Material with an Enhanced Second-Harmonic-Generation Response Activated by SbIII Lone-Pair Stereoactivity, Chem. – Eur. J., 2021, 27, 5880–5884 CrossRef CAS PubMed.
- H. W. Huang, L. J. Liu, S. F. Jin, W. J. Yao, Y. H. Zhang and C. T. Chen, Deep-Ultraviolet Nonlinear Optical Materials: Na2Be4B4O11 and LiNa5Be12B12O33, J. Am. Chem. Soc., 2013, 135, 18319–18322 CrossRef CAS PubMed.
- M. Mutailipu, M. Zhang, H. P. Wu, Z. H. Yang, Y. H. Shen, J. L. Sun and S. L. Pan, Ba3Mg3(BO3)3F3 polymorphs with reversible phase transition and high performances as ultraviolet nonlinear optical materials, Nat. Commun., 2018, 9, 3089 CrossRef PubMed.
- C. Wu, L. Lin, T. Wu, Z. Huang and C. Zhang, Deep-ultraviolet transparent alkali metal–rare earth metal sulfate NaY(SO4)2·H2O as a nonlinear optical crystal: Synthesis and characterization, CrystEngComm, 2021, 23, 2945–2951 RSC.
- S. G. Zhao, X. Y. Yang, Y. Yang, X. J. Kuang, F. Q. Lu, P. Shan, Z. H. Sun, Z. S. Lin, M. C. Hong and J. H. Luo, Non-Centrosymmetric RbNaMgP2O7 with Unprecedented Thermo-Induced Enhancement of Second Harmonic Generation, J. Am. Chem. Soc., 2018, 140, 1592–1595 CrossRef CAS PubMed.
- H. W. Yu, J. S. Young, H. P. Wu, W. G. Zhang, J. M. Rondinelli and P. S. Halasyamani, M4Mg4(P2O7)3 (M = K, Rb): Structural Engineering of Pyrophosphates for Nonlinear Optical Applications, Chem. Mater., 2017, 29, 1845–1855 CrossRef CAS.
- P. Yu, L. M. Wu, L. J. Zhao and L. Chen, Deep-Ultraviolet Nonlinear Optical Crystals: Ba3P3O10X (X = Cl, Br), J. Am. Chem. Soc., 2014, 136, 480–487 CrossRef CAS PubMed.
- S. G. Zhao, P. F. Gong, S. Y. Luo, L. Bai, Z. S. Lin, Y. Y. Tang, Y. L. Zhou, M. C. Hong and J. H. Luo, Tailored Synthesis of a Nonlinear Optical Phosphate with a Short Absorption Edge, Angew. Chem., 2015, 127, 4291–4295 CrossRef.
- S. G. Zhao, P. F. Gong, S. Y. Luo, L. Bai, Z. S. Lin, C. M. Ji, T. L. Chen, M. C. Hong and J. H. Luo, Deep-Ultraviolet Transparent Phosphates RbBa2(PO3)5 and Rb2Ba3(P2O7)2 Show Nonlinear Optical Activity from Condensation of [PO4]3− Units, J. Am. Chem. Soc., 2014, 136, 8560–8563 CrossRef CAS PubMed.
- M. Abudoureheman, S. J. Han, B. H. Lei, Z. H. Yang, X. F. Long and S. L. Pan, KPb2(PO3)5: a novel nonlinear optical lead polyphosphate with a short deep-UV cutoff edge, J. Mater. Chem. C, 2016, 4, 10630–10637 RSC.
- B. B. Zhang, G. P. Han, Y. Wang, X. L. Chen, Z. H. Yang and S. L. Pan, Expanding Frontiers of Ultraviolet Nonlinear Optical Materials with Fluorophosphates, Chem. Mater., 2018, 30, 5397–5403 CrossRef CAS.
- L. Xiong, J. Chen, J. Lu, C. Y. Pan and L. M. Wu, Monofluorophosphates: A new source of deep-ultraviolet nonlinear optical materials, Chem. Mater., 2018, 30, 7823–7830 CrossRef CAS.
- J. Lu, J. N. Yue, L. Xiong, W. K. Zhang, L. Chen and L. M. Wu, Uniform alignment of non-π-conjugated species enhances deep ultraviolet optical nonlinearity, J. Am. Chem. Soc., 2019, 141, 8093–8097 CrossRef CAS PubMed.
- Z. Y. Bai and K. M. Ok, Designing Sulfate Crystals with Strong Optical Anisotropy through π-Conjugated Tailoring, Angew. Chem., Int. Ed., 2023, e202315311 Search PubMed.
- X. F. Wang, X. D. Leng, Y. Kuk, J. Lee, Q. Jing and K. M. Ok, Deep-Ultraviolet Transparent Mixed Metal Sulfamates with Enhanced Nonlinear Optical Properties and Birefringence, Angew. Chem., Int. Ed., 2023, e202315434 Search PubMed.
- X. Hao, M. Luo, C. S. Lin, G. Peng, F. Xu and N. Ye, M(NH2SO3)2 (M=Sr, Ba): Two Deep-Ultraviolet Transparent Sulfamates Exhibiting Strong Second Harmonic Generation Responses and Moderate Birefringence, Angew. Chem., Int. Ed., 2021, 60, 7621–7625 CrossRef CAS PubMed.
- H. T. Tian, N. Ye and M. Luo, Sulfamide: A Promising Deep-Ultraviolet Nonlinear Optical Crystal Assembled from Polar Covalent [SO2(NH2)2] Tetrahedra, Angew. Chem., Int. Ed., 2022, 61, e202200395 CrossRef CAS PubMed.
- H. T. Tian, C. S. Lin, X. Zhao, S. H. Fang, H. Li, C. Wang, N. Ye and M. Luo, Design of a new ultraviolet nonlinear optical material KNO3SO3NH3 exhibiting an unexpected strong second harmonic generation response, Mater. Today Phys., 2022, 28, 100849 CrossRef CAS.
- E. Hobbs, D. E. C. Corbridge and B. Raistrick, The crystal structure of monosodium phosphoramidate, NaHPO3NH2, Acta Crystallogr., 1953, 6, 621–626 CrossRef CAS.
- G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., 2008, A64, 112–122 CrossRef PubMed.
- G. M. Sheldrick and T. R. Schneider, SHELXL: High-resolution refinement, Methods Enzymol., 1997, 277, 319–343 CAS.
- A. M. Rappe, K. M. Rabe, E. Kaxiras and J. D. Joannopoulos, Erratum: Optimized pseudopotentials, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1227–1230 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1997, 78, 1396–1396 CrossRef CAS.
- D. J. Moss, E. Ghahramani, J. E. Sipe and H. M. van Driel, Band-structure calculation of dispersion and anisotropy in χ→(3) for third-harmonic generation in Si, Ge, and GaAs, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1542–1560 CrossRef CAS PubMed.
- W. D. Cheng, C. S. Lin, H. Zhang and G. L. Chai, Theoretical Evaluation on Terahertz Source Generators from Ternary Metal Chalcogenides of PbM6Te10 (M = Ga, In), J. Phys. Chem. C, 2018, 122, 4557–4564 CrossRef CAS.
- M. H. Lee, C. H. Yang and J. H. Jan, Band-resolved analysis of nonlinear optical properties of crystalline and molecular materials, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 235110 CrossRef.
- X. X. Jiang, S. G. Zhao, Z. S. Lin, J. H. Luo, P. D. Bristowe, X. G. Guan and C. C. Tian, The role of dipole moment in determining the nonlinear optical behavior of materials: ab initio studies on quaternary molybdenum tellurite crystals, J. Mater. Chem. C, 2014, 2, 530 RSC.
- D. H. Lin, M. Luo, C. S. Lin, F. Xu and N. Ye, KLi(HC3N3O3)·2H2O: Solvent-drop Grinding Method toward the Hydro-isocyanurate Nonlinear Optical Crystal, J. Am. Chem. Soc., 2019, 141, 3390–3394 CrossRef CAS PubMed.
- S. K. Kurtz and T. T. Perry, A powder technique for the evaluation of nonlinear optical materials, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- W. Cheng, C. Lin, H. Lin and G. Chai, Theoretical Evaluation on Terahertz Source Generators from Ternary Metal Chalcogenides of PbM6Te10 (M = Ga, In), J. Phys. Chem. C, 2018, 122, 4557–4564 CrossRef CAS.
- C. T. Chen, Y. C. Wu and R. K. Li, The anionic group theory of the non-linear optical effect and its applications in the development of new high-quality NLO crystals in the borate series, Int. Rev. Phys. Chem., 1989, 8, 65–91 Search PubMed.
- L. Cao, G. Peng, W. Liao, T. Yan, X. Long and N. Ye, A microcrystal method for the measurement of birefringence, CrystEngComm, 2020, 22, 1956–1961 RSC.
- H. T. Tan, C. S. Lin, Y. Q. Zhou, X. Zhao, H. X. Fan, T. Yan, N. Ye and M. Luo, Design of the Ionic Organic Nonlinear Optical Material NH4[LiC3H(CH3)O4] with Ultrawide Band Gap and Moderate Birefringence, Angew. Chem., Int. Ed., 2023, 62, e202304858 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data and structure date (Tables S1–S5), Dipole moment (Table S6), Comparative table of phosphates (Table S7), and Figures about the measurement results and detail of NaPO3NH3 (Fig. S1–S7). CCDC 2307797 for NaPO3NH3. See DOI: https://doi.org/10.1039/d3qi02465b |
| This journal is © the Partner Organisations 2024 |