1,4-Azaborine based unfused non-fullerene acceptors for organic solar cells†
Shihao
Chen‡
a,
Minghao
Dong‡
a,
Yuanqing
Bai
a,
Yuting
Chen
a,
Yuang
Fu
b,
Lin
Shao
a,
Xinhui
Lu
b,
Chunchen
Liu
 *a,
Kai
Zhang
*a,
Kai
Zhang
 a,
Hongbin
Wu
a,
Hongbin
Wu
 a and
Fei
Huang
a and
Fei
Huang
 *a
*a
aState Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, School of Materials Science and Engineering, South China University of Technology, Guangzhou, 510640, P. R. China. E-mail: mscliu@scut.edu.cn; msfhuang@scut.edu.cn
bDepartment of Physics, The Chinese University of Hong Kong, New Territories, Hong Kong 999077, China
First published on 16th January 2023
Abstract
1,4-Azaborine, containing both boron and nitrogen in an aromatic hydrocarbon, displays unique electronic properties compared with its all-carbon analogue and shows great potential as a multiresonant thermally activated delayed fluorescence material. However, conjugated molecules featuring 1,4-azaborine for organic solar cells (OSCs) have not yet been explored. In this study, two novel acceptor–donor–acceptor (A–D–A) conjugated molecules, ABBT-BO and ABBT-DT, using dithieno-1,4-azaborine as the core D unit have been designed and synthesized. Both molecules exhibited excellent solubility, strong and broad absorption and appropriate energy levels, enabling them to be promising candidates for non-fullerene acceptors (NFAs). It was found that the shorter alkyl chain of ABBT-BO could endow the device with superior morphology, resulting in more sufficient exciton dissociation, improved carrier transport and suppressed charge recombination. Impressively, PM6:ABBT-BO based OSCs achieved a power conversion efficiency (PCE) of 10.07% with an open-circuit voltage (VOC) of 0.900 V, a short-circuit current density (JSC) of 16.41 mA cm−2, and a fill factor (FF) of 68.16%, which is the highest efficiency reported for NFAs featuring BN-heteroarenes. This work demonstrates the significant potential of 1,4-BN-heteroarenes for constructing novel NFAs toward high-performance OSCs.
Introduction
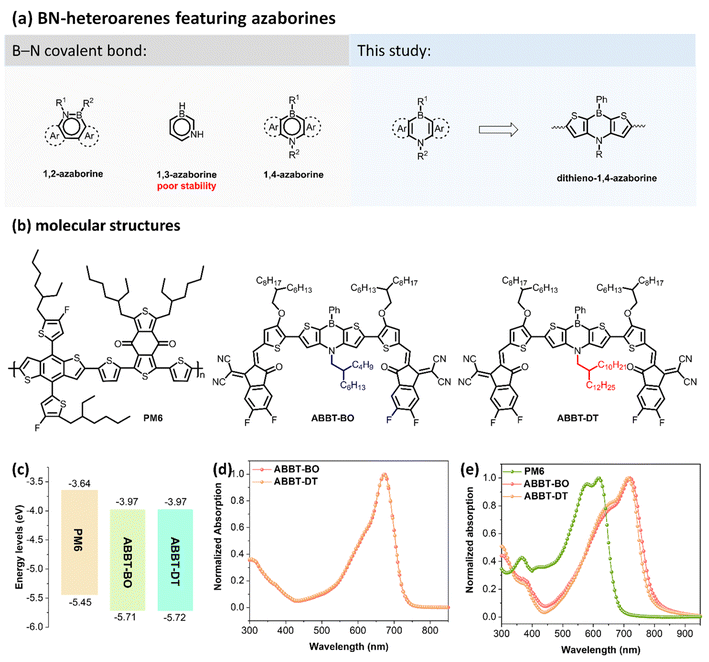
Organic solar cells (OSCs), as a promising renewable energy technology, have attracted great attention due to their distinct advantages of light weight, flexibility, semitransparency and solution processability.1–6 In the past few decades, great efforts have been devoted to exploring various photoactive materials to promote the power conversion efficiency (PCE) of OSCs.7–13 In the past few years, the PCE of OSCs has soared to 19% mainly owing to the discovery of small molecule non-fullerene acceptors (NFAs).14,15 In contrast to the fullerene acceptors, NFAs possessed strong near-infrared (NIR) absorption and easy structure-modification and readily tuned electronic energy levels.16,17 Nowadays, a lot of attention has been paid to the structure optimization of typical NFAs of ITIC and Y6 derivatives, whereas NFAs based on novel conjugated backbones lack exploration.18 Therefore, it is of significance to design and synthesize appealing heteroarenes for constructing novel NFAs toward high-performance OSCs.19–22BN-heteroarenes, which employ both boron and nitrogen in aromatic hydrocarbons, display unique electronic properties compared with their all-carbon analogues and have shown great potential for applications in organic electronics.23 Boron and nitrogen atoms can form a B←N coordinate bond or a B–N covalent bond (Fig. 1a).24 BN-heteroarenes embedded with a B←N coordinate bond exhibit an electron-deficient nature because of the positive charge of the nitrogen atom, which thus has been widely utilized for constructing various polymer and small molecule acceptors in OSCs.25–27 Meanwhile, BN-heteroarenes featuring a B–N covalent bond are also competitive candidates for constructing conjugated materials for organic electronics.28 Azaborines is the basic B–N covalent heteroarene, where two carbon atoms are respectively replaced with boron and nitrogen atoms. According to the relative positions of B and N atoms, azaborines are classified as three isomers: 1,2-azaborine, 1,3-azaborine and 1,4-azaborine (Fig. 1a). Due to its worst thermodynamic stability, conjugated polymers/molecules containing 1,3-azaborine have not yet been explored.29 Very recently, Duan and co-workers successfully employed 1,2-azaborine to construct polymer donor and small molecule NFAs for OSCs, and considerable device performances were achieved.30,31
Compared with its 1,2- and 1,3-isomers, 1,4-azaborine exhibits moderate thermodynamic stability32 and some unique electronic properties due to the different π-electron distributions and dipole moments.29 Since the first 1,4-azaborine-containing anthracene was reported in 1961,33 numerous 1,4-BN-heteroarenes have been designed and synthesized. In particular, π-extended conjugated molecules featuring 1,4-azaborine have attracted great interest due to their potential in multi-resonant thermally activated delayed fluorescence (MR-TADF) materials.34–36 However, to the best of our knowledge, conjugated molecules featuring 1,4-azaborine for OSCs are still unexplored. Therefore, it is necessary to design and synthesize 1,4-BN-heteroarene based donor/acceptors for OSCs and investigate the impact of 1,4-azaborine on material properties as well as device performance.
Herein, dithieno-1,4-azaborine was used, for the first time, as the core unit to construct two novel A–D–A-type small molecule NFAs, ABBT-BO and ABBT-DT (Fig. 1a and b). The molecular skeleton exhibits good planarity because of the nearly planar structure of 1,4-azaborine and intramolecular S⋯O noncovalent interactions. Both molecules exhibit good solubility, strong NIR absorption, and appropriate energy levels, making them competitive candidates for NFAs. As a result, OSCs with ABBT-BO/DT as acceptors have been successfully fabricated. It was found that the length of branched alkyl chains in the nitrogen atom of 1,4-azaborine will make a distinct difference to the exciton dissociation, carrier transport and charge recombination in devices. Eventually, the optimal PM6:ABBT-BO device achieved a PCE of over 10% due to its superior morphology, higher exciton dissociation, increased carrier lifetime and suppressed charge recombination. To the best of our knowledge, it is the highest performance for OSCs based on small molecule NFAs featuring BN-heteroarenes. As the first attempt to design and synthesize 1,4-BN-heteroarene based NFAs, this study may provide some inspiration for developing more BN-containing NFAs for high-performance OSCs.
Results and discussion
Materials synthesis and characterization
The synthetic routes of ABBT-BO and ABBT-DT are shown in Fig. S1 and S2.† Intermediates 2 with a butyl-octyl chain and 6 with a decyltetradecyl chain were both obtained in a one-pot palladium-catalysed Buchwald–Hartwig cross-coupling reaction with sequential formation of two C–N bonds. The incorporation of a long alkyl chain on the nitrogen atom was essential to ensure sufficient solubility for the fabrication of devices using the spin-coating method. The key intermediates 3 and 7 were then generated by a selective Friedel–Crafts-type C–H borylation from commercially available reagent PhBCl2.37 After converting compound 3 and 7 to their respective bis-tributyltin derivatives, 4 and 8, target NFAs (ABBT-BO and ABBT-DT) were then obtained via a Stille coupling reaction followed by a Knoevenagel condensation reaction. Notably, the incorporation of alkoxy groups was in favor of the coplanarity of the target molecules for the conformational “locks” via S⋯O interactions38,39 as well as upshifting of HOMO energy levels for a red-shifted absorption.40 The molecular structures were thoroughly confirmed by 1H/13C nuclear magnetic resonance (NMR) and matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) analyses (Fig. S11–S22†). Both NFAs exhibited sufficient solubility in common organic solvents, such as chloroform, chlorobenzene and toluene, enabling the solution processability for neat and blend films.Photophysical properties
The highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) energy levels were measured to be −3.97/−5.71 eV for ABBT-BO and 3.97/−5.72 eV for ABBT-DT by cyclic voltammetry (CV) tests (Fig. 1c and S3†). A negligible difference was observed for the electronic energy levels of these two molecules. Absorption spectra of ABBT-BO and ABBT-DT in chloroform solution and neat films are shown in Fig. 1d and e, and the corresponding data are outlined in Table 1.| Acceptors | λ max | λ onset [film] | E optg [eV] | E HOMO [eV] | E LUMO [eV] | E g [eV] | ε [M−1 cm−1] | |
|---|---|---|---|---|---|---|---|---|
| Solution | Film | |||||||
| ABBT-BO | 673 | 719 | 792 | 1.57 | −5.71 | −3.97 | 1.74 | 1.44 × 105 |
| ABBT-DT | 673 | 713 | 778 | 1.59 | −5.72 | −3.97 | 1.75 | 1.45 × 105 |
The length of the branched alkyl chain in 1,4-azaborine made negligible difference to the solution absorption for ABBT-BO and ABBT-DT, both of which exhibited an identical peak located at 673 nm (Fig. 1d). Notably, both ABBT-BO and ABBT-DT exhibited a maximum absorption coefficient of over 1.40 × 105 M−1 cm−1, (Fig. S8†) which was comparable to that of the star acceptor Y6.41 In thin films, the absorption spectra of both molecules exhibited a red-shift over 40 nm relative to those in solution, resulting in a strong and broad absorption in the region of 500–800 nm (Fig. 1e). The ABBT-BO film exhibited an absorption peak at 792 nm, slightly red-shifted relative to that at 778 nm of ABBT-DT. Accordingly, optical bandgaps of 1.57 and 1.59 eV were deduced for ABBT-BO and ABBT-DT, respectively. The complementary absorption with PM6 and appropriate energy levels make these two 1,4-azaborine-containing molecules competitive candidates for NFAs in OSCs.
Calculated geometry and electronic properties
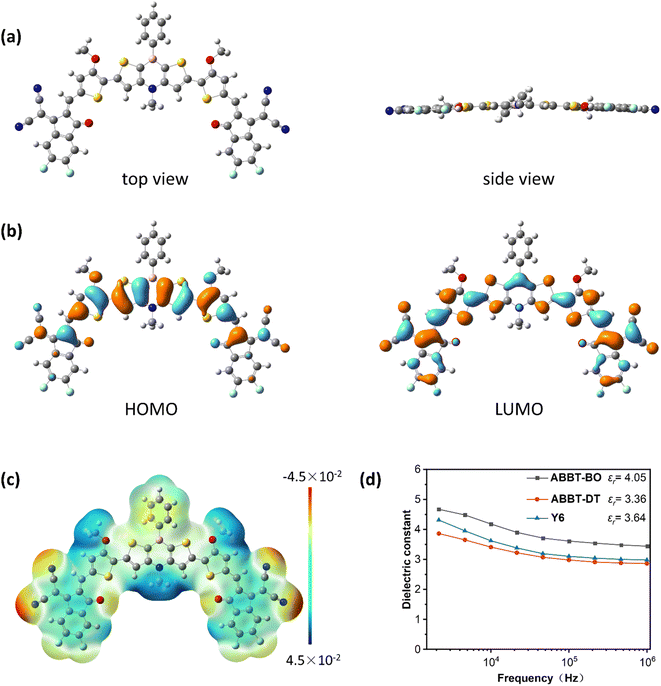
Density-functional theory (DFT) calculations were conducted to investigate the molecular geometry and energy levels at the B3LYP/6-311+g(d,p) level using the Gaussian 16 package. To simplify the calculations, all alkoxy and alkyl chains were respectively replaced by methoxyl and methyl groups. As shown in Fig. 2a, the conjugated skeleton exhibited good coplanarity due to the nearly planar 1,4-azaborine and S⋯O noncovalent interactions, which might be beneficial for the intermolecular packing and thus, improving the charge mobility.42–46 As shown in Fig. 2b, the HOMO energy level was mainly delocalized on the core dithieno-1,4-azaborine and alkoxyl thiophene moieties, whereas the LUMO energy level was distributed along the whole molecule, indicating efficient p–π* conjugation between boron atoms with adjacent thiophenes.47 The calculated HOMO/LUMO was −5.99/−3.94 eV. The discrepancy between DFT calculation results and CV measurements may be derived from the intermolecular packing of ABBT-BO/ABBT-DT that was generally ignored in the calculations.48The electrostatic potential (ESP) is a physical property that can reflect the static charge distribution of a molecule, and it has been usually investigated to speculate the molecular packing behaviour49 and the exciton dissociation progress in OSCs.50 As shown in Fig. 2c and S4,† negative ESP distributions for boron and its adjacent carbon atoms and positive distributions for nitrogen and its adjacent carbon atoms were observed, indicating the obvious dipole moment between boron and nitrogen atoms, which was similar to the ESP distribution in the core of Y-series NFAs. The negative ESP distribution at the B-side region was supposed to enhance the electron-accepting ability. Eventually, due to the incorporation of electron-deficient B atoms, these molecules displayed similar ESP distributions with Y-series NFAs51,52 along the conjugated backbone, which was speculated to facilitate the intermolecular packing53,54 and exciton dissociation.
Dielectric constant measurement
Organic semiconductors typically have low dielectric constants (εr < 3), resulting in high exciton binding energy of hundreds of meVs. A higher εr is persistently pursued for organic semiconductors to promote exciton dissociation and charge transport accompanied by decreased geminate and nongeminate recombinations.55 Considering the unique dipole moment and electronic properties of a 1,4-azaborine unit, the εr values of ABBT-BO, ABBT-DT and Y6 were measured using the parallel-plate capacitance measurement with impedance spectroscopy. The εr values were obtained from the equation: C = ε0εrA/d, where C is the capacitance, ε0 is the permittivity of free space, A is the device area, and d is the thickness of the test film. As shown in Fig. 2d, the εr values were plotted as a function of the frequency. Y6 showed an εr of 3.64, consistent with the reported studies.56 A slightly lower εr of 3.36 was observed for ABBT-DT; however, ABBT-BO exhibited an εr value of 4.05, even higher than that of Y6, which was speculated to facilitate the exciton dissociation in organic electronics.57,58 The lower dielectric constant of ABBT-DT may be ascribed to the longer alkyl chain that reduces the number of polarizable molecular units in a given volume.59Photovoltaic performance
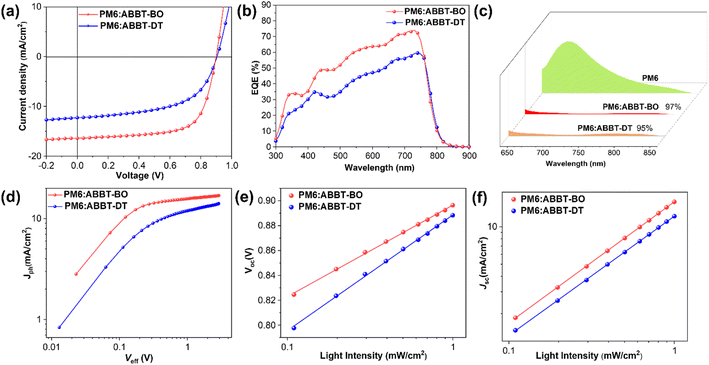
Photovoltaic devices were fabricated with a conventional configuration of ITO/PEDOT:PSS/PM6:ABBT-BO or ABBT-DT/PNDIT-F3N60/Ag. PM6 was selected as the polymer donor due to its matched energy levels and complementary absorption with ABBT-BO/DT. The current density–voltage (J–V) curves of the devices are shown in Fig. 3a, and the optimized performance parameters are summarized in Table 2. The PM6:ABBT-DT device exhibited an open circuit voltage (VOC) of 0.902 V, a short circuit current density (JSC) of 12.29 mA cm−2 and a fill factor (FF) of 56.11%, yielding a poor PCE of 6.24%. However, the PM6:ABBT-BO device achieved a PCE of 10.07% with an VOC of 0.900 V and a significantly higher JSC of 16.41 mA cm−2 and FF of 68.16%. To the best of our knowledge, this is the highest PCE reported to date for small molecule NFAs featuring BN-heteroarenes.The spectra of external quantum efficiency (EQE) are displayed in Fig. 3b. Both devices achieved a broad photo response in the region of 400–800 nm, coinciding well with the combined absorption of PM6 and ABBT-BO/DT. Impressively, a thoroughly stronger photo response was achieved for the PM6:ABBT-BO device, which was in a good agreement with its higher JSC. As a result, JSC values of 15.61 and 12.07 mA cm−2 were deduced for PM6:ABBT-BO and PM6:ABBT-DT, respectively, both of which agree well with the JSC results obtained from the J–V curves (within 5% error), validating the authenticity of device data.
To elucidate the underlying mechanism for the performance difference between ABBT-BO and ABBT-DT based devices, exciton dissociation, charge recombination, carrier lifetime and transport were investigated. Photoluminescence (PL) emission spectra of neat PM6 and PM6:ABBT-BO/DT films are shown in Fig. 3c, and the PL emission of PM6 (excited at 615 nm) was largely quenched in both blend films, indicating efficient exciton dissociation at D/A interfaces. However, it should be noted that the PL quenching efficiency of the PM6:ABBT-BO film (97%) is slightly higher than that of the PM6:ABBT-DT one (95%), showing more sufficient exciton dissociation in the former film. The photocurrent density (Jph) versus effective voltage (Veff) curves were measured to gain further insight into exciton dissociation behaviours in OSCs (Fig. 3d). The Jph/Jsat value is defined as exciton dissociation efficiency, where Jph and Jsat respectively represent the photogenerated and saturation current density. The Jph/Jsat values were calculated to be 97.1% and 90.4% for PM6:ABBT-BO and PM6:ABBT-DT based devices. The higher Jph/Jsat value of the PM6:ABBT-BO based device suggests its more efficient exciton dissociation, which is consistent with the above PL-quenching results.
The curves of JSC or VOCversus the light intensity (Plight) were plotted to study the recombination behaviours in ABBT-BO/DT based devices. As shown in Fig. 3e, the Plight dependence of the VOC was fitted to reveal the trap-assisted recombination. The slope approaching 1KT/q means weaker trap-assisted recombination in the devices (K is the Boltzmann constant, T is the temperature in units of kelvin, and q is the elementary charge). The slopes for PM6:ABBT-BO and PM6:ABBT-DT devices were deduced to be 1.23 and 1.56KT/q, respectively, indicating the severer trap-assisted recombination in PM6:ABBT-DT devices. Additionally, the curves of JSCversus Plight were measured (Fig. 3f) to clarify the bimolecular recombination. The relationship between JSC and Plight can be described as JSC ∝ Plightα, where α is the exponential factor which would be close to 1 when there is negligible bimolecular recombination. The slopes extracted for PM6:ABBT-BO/ABBT-DT devices were 0.974 and 0.959, respectively, indicating the weaker bimolecular recombination in the PM6:ABBT-BO device. The higher exciton dissociation efficiency, and lower trap-assisted and bimolecular recombination together verified the higher FF and JSC for PM6:ABBT-BO devices.
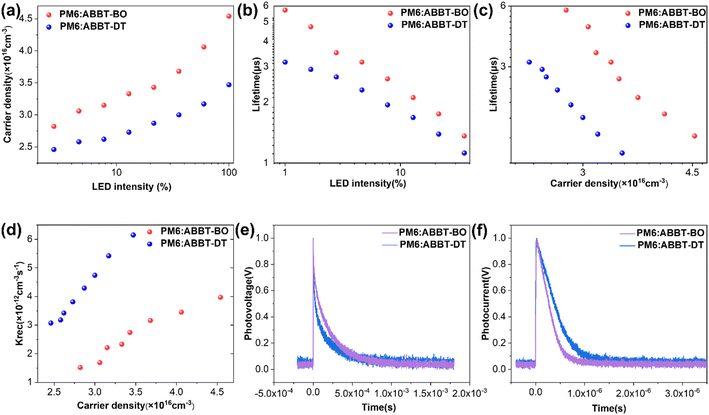
Moreover, the Platform for All-In-One Characterization of Solar Cells and OLED (PAIOS) was used to gain insight into the charge recombination within active layers in operating devices.61–63 The charge density for devices based on ABBT-BO/DT were shown as a function of bias light intensity (Fig. 4a). The charge lifetime (τ) was extracted from the transient photovoltage (TPV) decay dynamics. Obviously, at the same light intensity, the PM6:ABBT-BO device exhibited higher carrier density, suggesting the reduced charge recombination and thus higher photocurrent generation. Additionally, the PM6:ABBT-BO active layer displayed a higher τ value than its ABBT-DT counterpart in a wide range of light intensities (Fig. 4b). Furthermore, charge-extraction (CE) measurements were performed at various light intensities to estimate carrier densities (n) for PM6:ABBT-BO/DT devices(Fig. 4c). An increased n value can be obtained for the ABBT-BO based device, which agrees well with its higher JSC. The bimolecular recombination rate constants krec were then calculated from the τ and n values according to krec = 1/(λ + 1)nτ (λ is the recombination order). As shown in Fig. 4d, krec for the ABBT-DT based device was significantly larger than that for the ABBT-BO based one. All of these results further revealed that the superior performance of the ABBT-BO based device was mainly derived from a higher carrier lifetime and density, and reduced carrier recombination.
The transient photovoltage (TPV) and transient photocurrent (TPC) decay measurements were also carried out to thoroughly understand charge properties including carrier lifetime, recombination and extraction in devices. As shown in Fig. 4d, the transient photovoltage decay lifetime of ABBT-BO and ABBT-DT based devices was 190 and 137 μs, respectively. The longer lifetime indicates the weaker premature recombination of charge carriers in the PM6:ABBT-BO device. The TPC measurement was conducted under short-circuit conditions, which reflects the time from charge generation to charge collection by the electrode.64 A shorter TPC means more efficient carrier transport and extraction progress in OSCs. By fitting the TPC curves as shown in Fig. 4e, the charge extraction time was calculated to be 0.27 and 0.41 μs for ABBT-BO and ABBT-DT based devices, respectively. Overall, the results of TPV and TPC measurements indicated that the improvement of the charge transfer and collection were significantly enhanced in ABBT-BO based devices.
The hole (μh) and electron (μe) mobilities were also measured by the space-charge-limited-current (SCLC) method (Fig. S6 and S7†). The μh/μe of PM6:ABBT-BO and PM6:ABBT-DT devices were 7.20/5.38 × 10−5, and 5.16/3.52 × 10−5 cm2 V−1 s−1, respectively. Accordingly, the μh/μe ratio of 1.34 and 1.47 were calculated for PM6:ABBT-BO and PM6:ABBT-DT devices, respectively. Apparently, PM6:ABBT-BO based devices exhibited higher and more balanced charge transport, contributing to its higher FF and JSC.
Moreover, energy losses (Eloss) in ABBT-BO and ABBT-DT based devices were measured by using the Fourier-transform photocurrent spectrum (FTPS) (Fig. S9†).65 In general, Eloss in solar cells can be divided three parts: (1) ΔE1 is the intrinsic radiative recombination loss in the Shockley–Queisser limit, which is inevitable for all photovoltaic cells. (2) ΔE2 is the additional radiative recombination energy loss, originating from the extra absorption within the bandgap active layer in BHJ OSCs. (3) ΔE3 results from the non-radiative recombination at the D/A interface in OSCs. As shown in Table S1,† the incorporation of different alkyl side chains showed weak impacts on Eloss. Unfortunately, a relatively high value of over 0.70 eV was calculated for both ABBT-BO and ABBT-DT based devices compared to Y6 based devices, which probably resulted in the inferior Jsc and FF of the ABBT-BO/DT based device relative to the state-of-the-art devices.
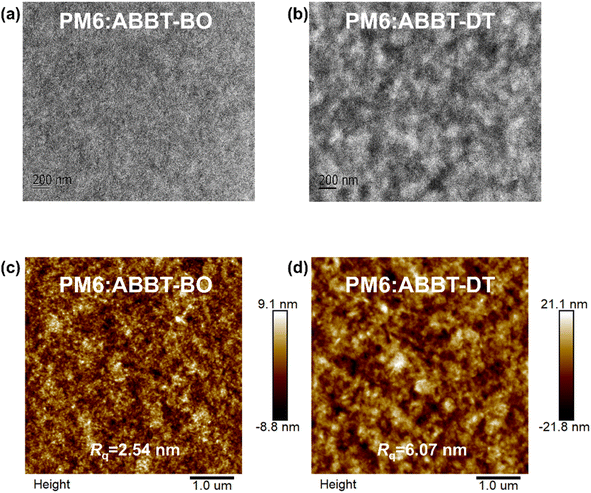
Morphology
It is well recognised that the nanoscale morphology of the active layers is intimately connected with the exciton dissociation, charge transport and recombination in OSCs.66 Transmission electron microscopy (TEM) and atomic force microscopy (AFM) results were obtained as shown in Fig. 5. The homogeneously distributed nanofiber microstructures in the PM6:ABBT-BO film were supposed to be favourable for exciton dissociation and charge transport. In contrast, obvious island-like agglomeration could be observed in the PM6:ABBT-DT film, leading to the oversized phase separation caused by the ordered packing of the longer alkyl chain in ABBT-DT as reported in previous studies.67,68 As shown in the AFM images, the PM6:ABBT-BO film showed a smoother top surface compared to the PM6:ABBT-DT film. A root-mean-square roughness (Rq) of 2.54 nm was observed for the PM6:ABBT-BO film, remarkably larger than 6.07 nm for the PM6:ABBT-DT one. The superior blend morphology of PM6:ABBT-BO was supposed to facilitate exciton dissociation, charge transport and eventually improve the device performance.The Flory–Huggins interaction parameters (χ) were calculated to investigate the miscibility of PM6 with ABBT-BO and ABBT-DT (Fig. S23 and Table S3†). According to the equation: 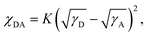 where K is a constant, γ is the surface energy, and D and A refer to donor and acceptor, respectively. The χ parameters were calculated to be 0.10 K and 0.28 K for PM6:ABBT-BO and PM6:ABBT-DT, respectively. The higher χ parameter of PM6 and ABBT-DT prefers to form oversized phase separation, which is consistent with the TEM and AFM images.
where K is a constant, γ is the surface energy, and D and A refer to donor and acceptor, respectively. The χ parameters were calculated to be 0.10 K and 0.28 K for PM6:ABBT-BO and PM6:ABBT-DT, respectively. The higher χ parameter of PM6 and ABBT-DT prefers to form oversized phase separation, which is consistent with the TEM and AFM images.
Grazing-incidence wide angle X-ray scattering (GIWAXS) was performed to reveal the intermolecular packing of ABBT-BO/DT films as well as PM6:ABBT-BO/DT films. Their 2D-GIWAXS patterns and line-cut profiles are presented in Fig. 6 and S9.† For the neat films, PM6 had reflections at approximately 0.99 Å−1 in the out-of-plane (OOP) direction and a weak lamellar stacking peak at approximately 0.30 Å−1 in the in-plane (IP) direction (Table S2†). The π–π stacking peak could be attributed to the reflection at 1.74 Å−1 in the OOP direction, with a d-spacing of 3.22 Å. However, there were weak π–π stacking peaks but strong lamellar stacking peaks at approximately 0.33 Å in the OOP direction for both ABBT-BO and ABBT-DT neat films, indicating the random intermolecular packing mode. For the blend films, the alkyl-to-alkyl lamellar packing in the OOP direction plays a crucial and decisive role in determining the crystalline structure. The π–π stacking peaks of the PM6:ABBT-BO and PM6:ABBT-DT films were located at 1.83 and 1.80 Å−1 in the OOP direction, with a d-spacing of 3.07 and 3.12 Å, respectively. The stronger and intensive π–π stacking peaks indicated that the incorporation of PM6 enhanced the crystallinity of blend films. The crystal coherence length (CCL), which is closely related to the charge transport and device performance, was extracted from the GIWAXS line profiles. The CCL was calculated to be 21.97, 32.50 and 32.84 Å for the π–π stacking peaks located at approximately 1.80 Å−1 for PM6, PM6:ABBT-BO and PM6:ABBT-DT, respectively. The longer CCL of blends films indicates that more ordered domains and tighter π–π stacking were formed, which was beneficial for the charge transport and reduced charge recombination.
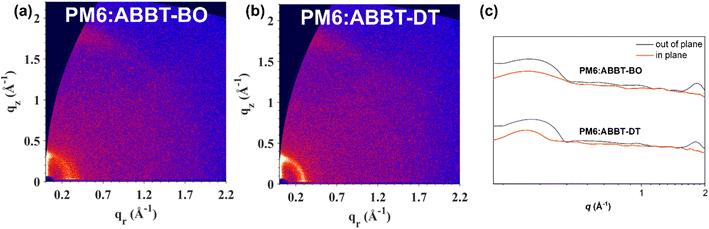 | ||
| Fig. 6 2D GIWAXS patterns of (a) PM6:ABBT-BO, (b) PM6:ABBT-DT and (c) the corresponding out-of-plane (black lines) and in-plane (red lines) line cuts. | ||
Conclusions
In summary, dithieno-fused 1,4-azaborine was innovatively used for constructing two novel A–D–A structural molecules, ABBT-BO and ABBT-DT. These molecules possessed desired solubility, broad absorption, appropriate energy levels, unique ESP distribution, high dielectric coefficient and coplanar conformation, making them promising candidates for NFAs. The OSCs with PM6 as the donor and ABBT-BO/DT as acceptors have been successfully fabricated, and it was found that the length of branched alkyl chains in 1,4-azaborine could make a significant difference to the device performance. Impressively, the ABBT-BO based OSC achieved an optimal PCE of 10.07% with a VOC of 0.900 V, a JSC of 16.41 mA cm−2 and an FF of 68.16%. To the best of our knowledge, it is the highest efficiency reported for OSCs with small molecule NFAs featuring BN-heteroarenes. Deep investigations revealed that the superior performance of the ABBT-BO based device was derived from the improved exciton dissociation, higher and more balanced carrier mobility, and reduced recombination. This study sheds some light on designing novel NFAs featuring 1,4-azaborine for high-performance OSCs.Author contributions
Shihao Chen designed and synthesised the molecules, conducted the experiments and drafted the original manuscript. Minghao Dong fabricated the organic solar cells and contributed to the data analyses. Yuanqing Bai contributed to the theoretical calculations. Yuting Chen and Hongbin Wu carried out the energy loss measurement. Yuang Fu and Xinhui Lu performed the GIWAXS measurement. Lin Shao helped in data curation. Kai Zhang helped in device data analyses. Chunchen Liu and Fei Huang guided and supervised the whole project. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2019YFA0705900) funded by MOST, the Basic and Applied Basic Research Major Program of Guangdong Province (No. 2019B030302007), the National Natural Science Foundation of China (No. U21A6002), and the Guangdong-Hong Kong-Macao Joint Laboratory of Optoelectronic and Magnetic Functional Materials (No. 2019B121205002).References
- G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447 CrossRef CAS PubMed
.
- R. Sun, Q. Wu, J. Guo, T. Wang, Y. Wu, B. Qiu, Z. Luo, W. Yang, Z. Hu, J. Guo, M. Shi, C. Yang, F. Huang, Y. Li and J. Min, Joule, 2020, 4, 407 CrossRef CAS
.
- S. Dong, T. Jia, K. Zhang, J. Jing and F. Huang, Joule, 2020, 4, 2004 CrossRef CAS
.
- Z. Hu, J. Wang, X. Ma, J. Gao, C. Xu, X. Wang, X. Zhang, Z. Wang and F. Zhang, J. Mater. Chem. A, 2021, 9, 6797 RSC
.
- W. Liu, S. Xu, H. Lai, W. Liu, F. He and X. Zhu, CCS Chem., 2022, 1 Search PubMed
.
- X. Meng, Z. Xing, X. Hu and Y. Chen, Chin. J. Polym. Sci., 2022, 40, 1522 CrossRef CAS
.
- C. Yan, S. Barlow, Z. Wang, H. Yan, A. K. Y. Jen, S. R. Marder and X. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef CAS
.
- Q. Wei, W. Liu, M. Leclerc, J. Yuan, H. Chen and Y. Zou, Sci. China: Chem., 2020, 63, 1352 CrossRef CAS
.
- C. Sun, F. Pan, H. Bin, J. Zhang, L. Xue, B. Qiu, Z. Wei, Z.-G. Zhang and Y. Li, Nat. Commun., 2018, 9, 743 CrossRef PubMed
.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 272 CrossRef CAS PubMed
.
- S. Chen, L. Feng, T. Jia, J. Jing, Z. Hu, K. Zhang and F. Huang, Sci. China: Chem., 2021, 64, 1192 CrossRef CAS
.
- P. Han, M. Lin, Q. Jiang, H. Ning, M. Su, L. Dang, F. He and Q. Wu, CCS Chem., 2022, 1 Search PubMed
.
- L. Ye, W. Ye and S. Zhang, J. Semicond., 2021, 42, 101607 CrossRef CAS
.
- P. Bi, S. Zhang, Z. Chen, Y. Xu, Y. Cui, T. Zhang, J. Ren, J. Qin, L. Hong, X. Hao and J. Hou, Joule, 2021, 5, 2408 CrossRef CAS
.
- Y. Cui, Y. Xu, H. Yao, P. Bi, L. Hong, J. Zhang, Y. Zu, T. Zhang, J. Qin, J. Ren, Z. Chen, C. He, X. Hao, Z. Wei and J. Hou, Adv. Mater., 2021, 33, 2102420 CrossRef CAS PubMed
.
- Y. Liu, B. Liu, C.-Q. Ma, F. Huang, G. Feng, H. Chen, J. Hou, L. Yan, Q. Wei, Q. Luo, Q. Bao, W. Ma, W. Liu, W. Li, X. Wan, X. Hu, Y. Han, Y. Li, Y. Zhou, Y. Zou, Y. Chen, Y. Li, Y. Chen, Z. Tang, Z. Hu, Z.-G. Zhang and Z. Bo, Sci. China: Chem., 2022, 65, 224 CrossRef CAS
.
- C. Liu, Y. Bai, Z. Hu and F. Huang, Sci. Sin.: Chim., 2022, 52, 1948 Search PubMed
.
- C. Cui, Acta Polym. Sin., 2021, 52, 663 CAS
.
- F. Huang, Z. Li, G. Song, C. Jiang, Y. Yang, J. Wang, X. Wan, C. Li, Z. Yao and Y. Chen, Adv. Funct. Mater., 2022, 2211140 Search PubMed
.
- S. Li, L. Zhan, F. Liu, J. Ren, M. Shi, C.-Z. Li, T. P. Russell and H. Chen, Adv. Mater., 2018, 30, 1705208 CrossRef PubMed
.
- H. Huang, Q. Guo, S. Feng, C. e. Zhang, Z. Bi, W. Xue, J. Yang, J. Song, C. Li, X. Xu, Z. Tang, W. Ma and Z. Bo, Nat. Commun., 2019, 10, 3038 CrossRef PubMed
.
- X. Liao, W. Xie, Z. Han, Y. Cui, X. Xia, X. Shi, Z. Yao, X. Xu, X. Lu and Y. Chen, Adv. Funct. Mater., 2022, 32, 2204255 CrossRef CAS
.
- Z. X. Giustra and S.-Y. Liu, J. Am. Chem. Soc., 2018, 140, 1184 CrossRef CAS PubMed
.
- J. Miao, Y. Wang, J. Liu and L. Wang, Chem. Soc. Rev., 2022, 51, 153 RSC
.
- C. Dou, J. Liu and L. Wang, Sci. China: Chem., 2017, 60, 450 CrossRef CAS
.
- M. M. Morgan, M. Nazari, T. Pickl, J. M. Rautiainen, H. M. Tuononen, W. E. Piers, G. C. Welch and B. S. Gelfand, Chem. Commun., 2019, 55, 11095 RSC
.
- F. Liu, J. Liu and L. Wang, Org. Chem. Front., 2019, 6, 1996 RSC
.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777 CrossRef CAS PubMed
.
- C. Chen, C.-Z. Du and X.-Y. Wang, Adv. Sci., 2022, 9, 2200707 CrossRef CAS PubMed
.
- S. Pang, Z. Wang, X. Yuan, L. Pan, W. Deng, H. Tang, H. Wu, S. Chen, C. Duan, F. Huang and Y. Cao, Angew. Chem., Int. Ed., 2021, 60, 8813 CrossRef CAS PubMed
.
- X. Liu, S. Pang, L. Zeng, W. Deng, M. Yang, X. Yuan, J. Li, C. Duan, F. Huang and Y. Cao, Chem. Commun., 2022, 58, 8686 RSC
.
- M. Baranac-Stojanović, Chem.–Eur. J., 2014, 20, 16558 CrossRef PubMed
.
- P. M. Maitlis, J. Chem. Soc., 1961, 425 RSC
.
- T. Huang, W. Jiang and L. Duan, J. Mater. Chem. C, 2018, 6, 5577 RSC
.
- P. Jiang, J. Miao, X. Cao, H. Xia, K. Pan, T. Hua, X. Lv, Z. Huang, Y. Zou and C. Yang, Adv. Mater., 2022, 34, 2106954 CrossRef CAS PubMed
.
- T. Wang, Y. Zou, Z. Huang, N. Li, J. Miao and C. Yang, Angew. Chem., Int. Ed., 2022, 61, e202211172 CAS
.
- K. Mitsudo, K. Shigemori, H. Mandai, A. Wakamiya and S. Suga, Org. Lett., 2018, 20, 7336 CrossRef CAS PubMed
.
- Y. Liu, Z. Zhang, S. Feng, M. Li, L. Wu, R. Hou, X. Xu, X. Chen and Z. Bo, J. Am. Chem. Soc., 2017, 139, 3356 CrossRef CAS PubMed
.
- Z. Yao, Y. Li, S. Li, J. Xiang, X. Xia, X. Lu, M. Shi and H. Chen, ACS Appl. Energy Mater., 2021, 4, 819 CrossRef CAS
.
- J. Lee, S.-J. Ko, H. Lee, J. Huang, Z. Zhu, M. Seifrid, J. Vollbrecht, V. V. Brus, A. Karki, H. Wang, K. Cho, T.-Q. Nguyen and G. C. Bazan, ACS Energy Lett., 2019, 4, 1401 CAS
.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140 Search PubMed
.
- S. Feng, M. Li, N. Tang, X. Wang, H. Huang, G. Ran, Y. Liu, Z. Xie, W. Zhang and Z. Bo, ACS Appl. Mater. Interfaces, 2020, 12, 4638 CAS
.
- H. Huang, L. Yang, A. Facchetti and T. J. Marks, Chem. Rev., 2017, 117, 10291 CAS
.
- Z.-P. Yu, Z.-X. Liu, F.-X. Chen, R. Qin, T.-K. Lau, J.-L. Yin, X. Kong, X. Lu, M. Shi, C.-Z. Li and H. Chen, Nat. Commun., 2019, 10, 2152 Search PubMed
.
- H. Huang, Z. Chen, R. P. Ortiz, C. Newman, H. Usta, S. Lou, J. Youn, Y.-Y. Noh, K.-J. Baeg, L. X. Chen, A. Facchetti and T. Marks, J. Am. Chem. Soc., 2012, 134, 10966 CAS
.
- X. Zhang, L. Qin, J. Yu, Y. Li, Y. Wei, X. Liu, X. Lu, F. Gao and H. Huang, Angew. Chem., Int. Ed., 2021, 60, 12475 CAS
.
- Y. Yu, B. Meng, F. Jäkle, J. Liu and L. Wang, Chem.–Eur. J., 2020, 26, 873 CAS
.
- Y. Chen, H. Meng, L. Ding, J. Tang, J. Yi, J. Zhang, Z. Wang, R. Ma, Z. Li, L. Lyu, X. Xu, R. Li, Q. Peng, H. Yan and H. Hu, Chem. Mater., 2022, 34, 10144 CAS
.
- J.-H. Dou, Y.-Q. Zheng, Z.-F. Yao, Z.-A. Yu, T. Lei, X. Shen, X.-Y. Luo, J. Sun, S.-D. Zhang, Y.-F. Ding, G. Han, Y. Yi, J.-Y. Wang and J. Pei, J. Am. Chem. Soc., 2015, 137, 15947 CAS
.
- H. Yao, D. Qian, H. Zhang, Y. Qin, B. Xu, Y. Cui, R. Yu, F. Gao and J. Hou, Chin. J. Chem., 2018, 36, 491 CAS
.
- C. Li, X. Gu, Z. Chen, X. Han, N. Yu, Y. Wei, J. Gao, H. Chen, M. Zhang, A. Wang, J. Zhang, Z. Wei, Q. Peng, Z. Tang, X. Hao, X. Zhang and H. Huang, J. Am. Chem. Soc., 2022, 144, 14731 CAS
.
- X. Song, K. Zhang, R. Guo, K. Sun, Z. Zhou, S. Huang, L. Huber, M. Reus, J. Zhou, M. Schwartzkopf, S. V. Roth, W. Liu, Y. Liu, W. Zhu and P. Müller-Buschbaum, Adv. Mater., 2022, 34, 2200907 CAS
.
- B. Yurash, D. Leifert, G. N. M. Reddy, D. X. Cao, S. Biberger, V. V. Brus, M. Seifrid, P. J. Santiago, A. Köhler, B. F. Chmelka, G. C. Bazan and T.-Q. Nguyen, Chem. Mater., 2019, 31, 6715 CAS
.
- A. J. Stephens, R. Scopelliti, F. F. Tirani, E. Solari and K. Severin, ACS Mater. Lett., 2019, 1, 3 CAS
.
- N. Cho, C. W. Schlenker, K. M. Knesting, P. Koelsch, H.-L. Yip, D. S. Ginger and A. K. Y. Jen, Adv. Energy Mater., 2014, 4, 1301857 Search PubMed
.
- T. Li, K. Wang, G. Cai, Y. Li, H. Liu, Y. Jia, Z. Zhang, X. Lu, Y. Yang and Y. Lin, JACS Au, 2021, 1, 1733 CAS
.
- X. Liu, B. Xie, C. Duan, Z. Wang, B. Fan, K. Zhang, B. Lin, F. J. M. Colberts, W. Ma, R. A. J. Janssen, F. Huang and Y. Cao, J. Mater. Chem. A, 2018, 6, 395 CAS
.
- W. Gao, H. Fu, Y. Li, F. Lin, R. Sun, Z. Wu, X. Wu, C. Zhong, J. Min, J. Luo, H. Y. Woo, Z. Zhu and A. K. Y. Jen, Adv. Energy Mater., 2021, 11, 2003177 CAS
.
- P. K. Tapaswi, M.-C. Choi, Y. S. Jung, H. J. Cho, D. J. Seo and C.-S. Ha, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2316 CAS
.
- Z. Wu, C. Sun, S. Dong, X.-F. Jiang, S. Wu, H. Wu, H.-L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2016, 138, 2004 CAS
.
- Y. Firdaus, L. P. Maffei, F. Cruciani, M. A. Müller, S. Liu, S. Lopatin, N. Wehbe, G. O. N. Ndjawa, A. Amassian, F. Laquai and P. M. Beaujuge, Adv. Energy Mater., 2017, 7, 1700834 Search PubMed
.
- R. Hamilton, C. G. Shuttle, B. O'Regan, T. C. Hammant, J. Nelson and J. R. Durrant, J. Phys. Chem. Lett., 2010, 1, 1432 CAS
.
- J. Yan, Q. Liang, K. Liu, J. Miao, H. Chen, S. Liu, Z. He, H. Wu, J. Wang and Y. Cao, ACS Energy Lett., 2017, 2, 14 CAS
.
- X. Zhou, X. Li, Y. Yan, F. Zhang, J. Zhou, T. Lin, Y. Zhu and D. Xu, Sol. RRL, 2022, 6, 2200424 CAS
.
- S. Liu, J. Yuan, W. Deng, M. Luo, Y. Xie, Q. Liang, Y. Zou, Z. He, H. Wu and Y. Cao, Nat. Photonics, 2020, 14, 300 CAS
.
- S. Chen, L. Hong, M. Dong, W. Deng, L. Shao, Y. Bai, K. Zhang, C. Liu, H. Wu and F. Huang, Angew. Chem., Int. Ed., 2023, 135, e202213869 Search PubMed
.
- Z. Abbas, S. U. Ryu, M. Haris, C. E. Song, H. K. Lee, S. K. Lee, W. S. Shin, T. Park and J.-C. Lee, Nano Energy, 2022, 101, 107574 CAS
.
- Y. Fu, L. Wang, C. Guo, D. Li, J. Cai, B. Zhou, C. Chen, C. Liu, D. Liu, W. Li and T. Wang, ACS Mater. Lett., 2022, 4, 2009 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09188g |
| ‡ S. Chen and M. Dong contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |