High-performance Ruddlesden–Popper two-dimensional perovskite solar cells via solution processed inorganic charge transport layers†
Zhihai
Liu
 a,
Lei
Wang
b,
Xiaoyin
Xie
c,
Chongyang
Xu
d,
Jianfeng
Tang
*e and
Wei
Li
a,
Lei
Wang
b,
Xiaoyin
Xie
c,
Chongyang
Xu
d,
Jianfeng
Tang
*e and
Wei
Li
 *e
*e
aSchool of Physics and Electronic Information, Yantai University, Yantai 264005, China
bSchool of Artificial Intelligence, Beijing Technology and Business University, Beijing, 100048, China
cSchool of Chemistry and Chemical Technology, Hubei Polytechnic University, Huangshi 435003, China
dYantai Research Institute and Graduate School of HEU, Harbin Engineering University, Harbin 150001, China
eSchool of Chemistry and Materials Science, Hunan Agricultural University, Changsha 410128, China. E-mail: weili@hunau.edu.cn; jftang@hunau.edu.cn
First published on 7th June 2022
Abstract
Two-dimensional (2D) layered halide perovskites have been shown to enable improved long-term stability in comparison to the well-known three-dimensional hybrid organic–inorganic halide perovskites. The optoelectronic properties of the 2D perovskites are strongly influenced by the chemical nature of the charge transport layer. In this work, we fabricated Ruddlesden–Popper 2D perovskite solar cells (PSCs) using solution processed inorganic NiOx and a C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 (1
C70 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as the hole and electron transport layers, which significantly improved the performance of the 2D PSCs. Time resolved photoluminescence measurements indicate the shortened lifetime of excitons, which demonstrates the excellent charge extraction properties. The PSCs based on these inorganic charge transport materials (CTMs) exhibit an average power conversion efficiency (PCE) of 14.1%, which is higher than that (12.3%) of PSCs using organic CTMs of poly(3,4-ethylenedioxythiophene)
1) mixture as the hole and electron transport layers, which significantly improved the performance of the 2D PSCs. Time resolved photoluminescence measurements indicate the shortened lifetime of excitons, which demonstrates the excellent charge extraction properties. The PSCs based on these inorganic charge transport materials (CTMs) exhibit an average power conversion efficiency (PCE) of 14.1%, which is higher than that (12.3%) of PSCs using organic CTMs of poly(3,4-ethylenedioxythiophene)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(styrenesulfonate) (PEDOT:PSS) and phenyl-C61-butyric acid methyl ester (PCBM). Compared with PEDOT:PSS and PCBM based cases, the PSCs using inorganic CTMs also show improved long-term stability, with the PCE degradation significantly suppressed from 20% to 12% after a measurement of 15 days. The best PSCs using NiOx and C60
poly(styrenesulfonate) (PEDOT:PSS) and phenyl-C61-butyric acid methyl ester (PCBM). Compared with PEDOT:PSS and PCBM based cases, the PSCs using inorganic CTMs also show improved long-term stability, with the PCE degradation significantly suppressed from 20% to 12% after a measurement of 15 days. The best PSCs using NiOx and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 show a high PCE of 14.4%, with a stable power output and negligible hysteresis.
C70 show a high PCE of 14.4%, with a stable power output and negligible hysteresis.
1. Introduction
Recently, organometallic halide perovskite materials have been intensively investigated owing to their unique advantages for optoelectronic applications, such as sufficient light absorption, high charge carrier mobilities, direct band-gaps and solution processibility.1–4 As a result, the power conversion efficiency (PCE) of perovskite solar cells (PSCs) has been significantly improved to 25.5%, which is comparable to that of silicon solar cells.5 In the structure of PSCs, the perovskite absorbers are usually sandwiched between the hole transport layer (HTL) and the electron transport layer (ETL), which further connect with the anode and the cathode.3 For regular n–i–p PSCs, 2,2′,7,7′-tetrakis-(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene (spiro-OMeTAD) and TiO2 are widely used as the HTL and ETL, respectively,6–8 while for inverted p–i–n PSCs, usually organic poly(3,4-ethylenedioxythiophene)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(styrenesulfonate) (PEDOT:PSS) and phenyl-C61-butyric acid methyl ester (PCBM) are used as the hole and electron transport materials, which can be simply deposited through a spin coating operation.6,9,10 However, conventional PSCs using MAPbX3 (MA and X strand for CH3NH3 and halogen) always suffer from a serious problem of poor stability, which is detrimental for commercial applications of PSCs.11,12
poly(styrenesulfonate) (PEDOT:PSS) and phenyl-C61-butyric acid methyl ester (PCBM) are used as the hole and electron transport materials, which can be simply deposited through a spin coating operation.6,9,10 However, conventional PSCs using MAPbX3 (MA and X strand for CH3NH3 and halogen) always suffer from a serious problem of poor stability, which is detrimental for commercial applications of PSCs.11,12
To overcome this problem, developing new perovskite absorbers and charge transport materials (CTMs) with higher intrinsic stability becomes an important aspect. In case of absorbers, recently Ruddlesden–Popper 2-dimensional (2D) perovskites have been widely used for fabricating PSCs because of their tunable band-gaps and better stability.13–16 For example, the (BA)2(MA)n−1PbnI3n+1 (BA = CH3(CH2)3NH3) perovskite series showed direct band-gaps between 1.5 and 2.2 eV,17 which is applicable for fabricating PSCs with high PCE and superior stability. For CTMs, conventional spiro-OMeTAD, PEDOT:PSS and PCBM are organic materials, which show a relatively low stability in air.18–20 Although TiO2 is an inorganic electron transport material with excellent stability, the photocatalytic effect of TiO2 could decompose perovskite into PbI2 under the irradiation of ultraviolet light.21 As a result, some inorganic CTMs have been investigated for improving the stability of PSCs. For example, solution processed NiOx has been widely used as the HTL in PSCs, which is a typical p-type semiconductor with suitable energy levels.22,23 Compared with PEDOT:PSS, PSCs with NiOx showed a superior performance with comparable PCEs and enhanced stability.24–26 Moreover, n-type ZnO and SnO2 are good electron transport materials for replacing TiO2 in PSCs, which could simultaneously improve PCE and stability of PSCs.27,28 Liu and co-workers used inorganic HTL (NiOx) and ETL (ZnO/C60) for fabricating CsPbI2Br-based PSCs, which showed an excellent PCE of 13.3% and enhanced stability.29 Pristine fullerenes (C60 or C70) show higher electron mobilities than fullerene derivatives (such as PCBM), which are good candidates for ETLs in PSCs.30 Although each individual fullerene shows a poor solubility, the mixture of several fullerenes can be well dissolved in organic solvents at high concentrations due to the increased configurational entropy.30,31 As a result, the film of fullerene mixture can be deposited through a solution process (such as spin-coating or spray coating) with a uniform morphology. For example, Lin et al. and Xu et al. reported the use of a fullerene mixture (containing C60 or C70) as ETLs in regular and inverted MAPbX3-based PSCs, achieving high PCEs of 16.7–16.9% with improved stability.31,32 Considering the stability problem, it is very important to investigate the use of an inorganic HTL and ETL in 2D PSCs and their effect on the performance.
In this study, we introduce the use of inorganic solution processable HTL (NiOx) and ETL (fullerene mixture, C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the fabrication of Ruddlesden–Popper type 2D PSCs. Compared with the use of conventional PEDOT:PSS and PCBM, using NiOx and fullerene mixture could improve the charge transport property, which is beneficial for improving the performance of PSCs. As a result, the PSCs based on NiOx and the fullerene mixture exhibited an average PCE of 14.1%, which is much higher than that (12.3%) of PEDOT:PSS and PCBM based PSCs. Based on this technique, stability of the PSCs was also improved with a significantly suppressed PCE degradation from 20% to 12% after a measurement of 15 days. This was mainly induced from the intrinsic high stability of well-crystallized perovskite and inorganic CTMs (NiOx and pristine fullerenes). The highest PCE of 14.4% was achieved for PSCs using NiOx and the fullerene mixture with a stable power output and negligible hysteresis. Our results demonstrate that using inorganic NiOx and the fullerene mixture as the CTMs is an effective approach for boosting the performance of Ruddlesden–Popper 2D PSCs.
1) in the fabrication of Ruddlesden–Popper type 2D PSCs. Compared with the use of conventional PEDOT:PSS and PCBM, using NiOx and fullerene mixture could improve the charge transport property, which is beneficial for improving the performance of PSCs. As a result, the PSCs based on NiOx and the fullerene mixture exhibited an average PCE of 14.1%, which is much higher than that (12.3%) of PEDOT:PSS and PCBM based PSCs. Based on this technique, stability of the PSCs was also improved with a significantly suppressed PCE degradation from 20% to 12% after a measurement of 15 days. This was mainly induced from the intrinsic high stability of well-crystallized perovskite and inorganic CTMs (NiOx and pristine fullerenes). The highest PCE of 14.4% was achieved for PSCs using NiOx and the fullerene mixture with a stable power output and negligible hysteresis. Our results demonstrate that using inorganic NiOx and the fullerene mixture as the CTMs is an effective approach for boosting the performance of Ruddlesden–Popper 2D PSCs.
2. Experimental section
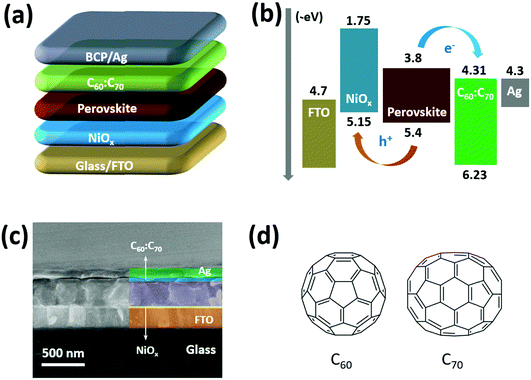
2.1 Device fabrication
Fullerenes (C60 and C70) and PEDOT:PSS (P VP AI4083) were purchased from Nano-C Inc. (USA) and Heraeus Clevios (Germany), respectively. Methylammonium iodide (MAI) and fluorine-doped tin oxide (FTO) glasses were purchased from Advanced Election Technology Co., Ltd (China). Butylammonium iodide (BAI), Ni(Ac)2·4H2O, N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and o-dichlorobenzene (o-DCB) were purchased from Sigma-Aldrich (USA). PbI2 and bathocuproine (BCP) were purchased from Xi’an Polymer Light Technology Corp. (China). As shown in Fig. 1(a), the PSCs were fabricated following a structure of glass/FTO/NiOx/(BA)2(MA)3Pb4I13/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70/BCP/Ag. First, 0.1 mmol Ni(Ac)2·4H2O and 6 μL ethanolamine were dissolved in 1 mL 2-methoxyethanol, which was further stirred at 70 °C overnight. The NiOx precursor was spin coated onto the FTO glasses at 3500 rpm, followed by a thermal annealing at 400 °C for 1 h in air. Then the perovskite precursor (containing BAI
C70/BCP/Ag. First, 0.1 mmol Ni(Ac)2·4H2O and 6 μL ethanolamine were dissolved in 1 mL 2-methoxyethanol, which was further stirred at 70 °C overnight. The NiOx precursor was spin coated onto the FTO glasses at 3500 rpm, followed by a thermal annealing at 400 °C for 1 h in air. Then the perovskite precursor (containing BAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAI
MAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbI2 = 2
PbI2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in a mixed-solvent of DMF and DMSO) was spin coated onto the NiOx layers at 4000 rpm in a N2 filled glovebox. The (BA)2(MA)3Pb4I13 perovskite films were formed after being heated at 100 °C for 10 min. Next, a fullerene mixture solution (C60
4 in a mixed-solvent of DMF and DMSO) was spin coated onto the NiOx layers at 4000 rpm in a N2 filled glovebox. The (BA)2(MA)3Pb4I13 perovskite films were formed after being heated at 100 °C for 10 min. Next, a fullerene mixture solution (C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 = 1
C70 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in o-DCB at 30 mg mL−1) was spin coated onto the perovskite films at 1000, 2000 or 3000 rpm for 30 s. After that, the BCP solution (in propanol at 0.5 mg mL−1) was spin coated on the samples at 4500 rpm. For control PSCs, PEDOT:PSS was spin coated onto the FTO substrates at 4500 rpm and annealed at 150 °C for 10 min. PCBM solution (30 mg mL−1 in o-DCB) was spin coated at 2000 rpm onto the perovskite films. Finally, the Ag electrodes of about 100 nm were thermally evaporated onto the samples under a high vacuum of 10−4 Pa to form an effective working area of 0.1 cm2 for the PSCs, which was defined by a shadow mask.
1 in o-DCB at 30 mg mL−1) was spin coated onto the perovskite films at 1000, 2000 or 3000 rpm for 30 s. After that, the BCP solution (in propanol at 0.5 mg mL−1) was spin coated on the samples at 4500 rpm. For control PSCs, PEDOT:PSS was spin coated onto the FTO substrates at 4500 rpm and annealed at 150 °C for 10 min. PCBM solution (30 mg mL−1 in o-DCB) was spin coated at 2000 rpm onto the perovskite films. Finally, the Ag electrodes of about 100 nm were thermally evaporated onto the samples under a high vacuum of 10−4 Pa to form an effective working area of 0.1 cm2 for the PSCs, which was defined by a shadow mask.
2.2 Characterization
The X-ray diffraction (XRD) spectra were measured using an X-ray diffractometer (Panalytical, the Netherlands). The cross-sectional image of the PSCs and top-view images of the surfaces were acquired using an SU8020 scanning electron microscope (SEM, Hitachi, Japan), which was operated at an acceleration voltage of 8 kV. The photoluminescence (PL) spectra of the films were measured using a spectrometer (FLS920, Edinburgh Instruments, UK), with the excitation wavelength of 372 nm and power density of 9 mW cm−2. The energy levels of NiOx and fullerene mixture were measured using an ultraviolet photoelectron spectroscope (UPS) with the incident light energy of 21.22 eV. The electrochemical impedance spectroscopy (EIS) spectra of the PSCs were performed using an electrochemical work station (Bio-Logic, France). The current density–voltage (J–V) characteristics of the PSCs were measured under an irradiation intensity of 100 mW cm−2 (AM1.5). The incident photon-to-current efficiency (IPCE) spectra of the PSCs were measured using a solar cell IPCE measurement system (Solar Cell Scan 100, Zolix, China).3. Results and discussion
Fig. 1(b) shows the schematic energy alignment of all the functional layers involved in the PSCs, which illustrates the charge transport process under the irradiation of sunlight. As shown in Fig. S1, (ESI†) the valence band of NiOx is about −5.15 eV, which is slightly higher than highest occupied molecular orbital (HOMO, −5.4 eV) level of the (BA)2(MA)3Pb4I13 perovskite and lower than the work function (−4.7 eV) of FTO. The conduction band of fullerene mixture (C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70) is about −4.31 eV (calculated from Fig. S2, ESI†), which is lower than the lowest unoccupied molecular orbital (LUMO, −3.8 eV) level of perovskite and similar to the work function (−4.3 eV) of the Ag electrode. As a result, the energy levels of NiOx and fullerene mixture are suitable for charge extraction and transportation from the perovskite absorber to electrodes. Fig. 1(c) presents the cross-sectional SEM image of the PSCs using NiOx and fullerene mixture, which clearly shows a dense layer-by-layer structure. From the SEM image, the thicknesses of NiOx, perovskite and C60
C70) is about −4.31 eV (calculated from Fig. S2, ESI†), which is lower than the lowest unoccupied molecular orbital (LUMO, −3.8 eV) level of perovskite and similar to the work function (−4.3 eV) of the Ag electrode. As a result, the energy levels of NiOx and fullerene mixture are suitable for charge extraction and transportation from the perovskite absorber to electrodes. Fig. 1(c) presents the cross-sectional SEM image of the PSCs using NiOx and fullerene mixture, which clearly shows a dense layer-by-layer structure. From the SEM image, the thicknesses of NiOx, perovskite and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 layers are about 15, 420 and 40 nm, respectively, which are typical values for PSCs with high performance.13–16,31 The molecular configurations of C60 and C70 are shown in Fig. 1(d), which only contains C–C bonding without other chemical groups.
C70 layers are about 15, 420 and 40 nm, respectively, which are typical values for PSCs with high performance.13–16,31 The molecular configurations of C60 and C70 are shown in Fig. 1(d), which only contains C–C bonding without other chemical groups.
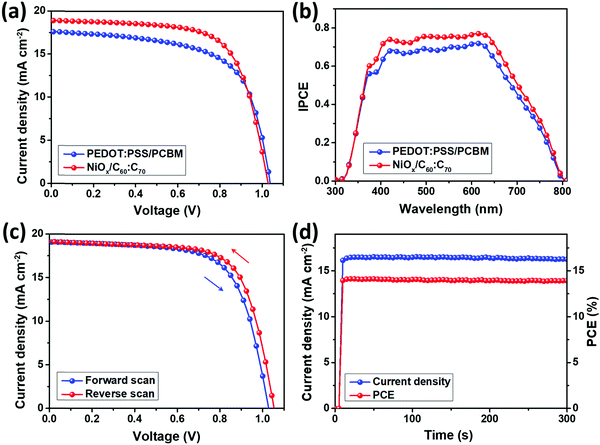
Fig. 2(a) presents the J–V characteristics the PSCs using PEDOT:PSS/PCBM and NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 as the charge transport layers, with the device parameters summarized in Table 1. The control PSCs using PEDOT:PSS and PCBM show an average PCE of 12.3%, with an open-circuit voltage (Voc) of 1.02 V, a short-circuit current density (Jsc) of 17.6 mA cm−2 and a fill factor (FF) of 68.6%. The performance of the control devices is similar with that in previous studies based on (BA)2(MA)3Pb4I13 perovskite,13–17 indicating the good optimization of our experiment. Upon using inorganic NiOx and fullerene mixture, the average PCE of the PSCs was significantly improved to 14.1%, with Jsc and FF simultaneously improved to 18.9 mA cm−2 and 73.8%. The Voc was slightly decreased to 1.01 V, which might be induced by the energy levels of NiOx and pristine fullerenes.31 As shown in Fig. S1 and Table S3, (ESI†) 2000 rpm is an optimal speed for the spin coating of fullerene mixture, which results in the best performance of the PSCs. The PCE variations are shown in Fig. S4, (ESI†) which indicate a stable J–V performance for each batch of the PSCs using different charge transport layers. Fig. 2(b) shows the IPCE spectra of the two kinds of PSCs, from which integrated Jsc can be obtained. The calculated Jsc values from the IPCE spectra are 17.2 and 18.5 mA cm−2 for PSCs using PEDOT:PSS/PCBM and NiOx/C60
C70 as the charge transport layers, with the device parameters summarized in Table 1. The control PSCs using PEDOT:PSS and PCBM show an average PCE of 12.3%, with an open-circuit voltage (Voc) of 1.02 V, a short-circuit current density (Jsc) of 17.6 mA cm−2 and a fill factor (FF) of 68.6%. The performance of the control devices is similar with that in previous studies based on (BA)2(MA)3Pb4I13 perovskite,13–17 indicating the good optimization of our experiment. Upon using inorganic NiOx and fullerene mixture, the average PCE of the PSCs was significantly improved to 14.1%, with Jsc and FF simultaneously improved to 18.9 mA cm−2 and 73.8%. The Voc was slightly decreased to 1.01 V, which might be induced by the energy levels of NiOx and pristine fullerenes.31 As shown in Fig. S1 and Table S3, (ESI†) 2000 rpm is an optimal speed for the spin coating of fullerene mixture, which results in the best performance of the PSCs. The PCE variations are shown in Fig. S4, (ESI†) which indicate a stable J–V performance for each batch of the PSCs using different charge transport layers. Fig. 2(b) shows the IPCE spectra of the two kinds of PSCs, from which integrated Jsc can be obtained. The calculated Jsc values from the IPCE spectra are 17.2 and 18.5 mA cm−2 for PSCs using PEDOT:PSS/PCBM and NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70, which are within 2.5% different from those obtained in the J–V performance. From Fig. 3(c), the best performing PSC using NiOx/C60
C70, which are within 2.5% different from those obtained in the J–V performance. From Fig. 3(c), the best performing PSC using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 shows a high PCE of 14.4%, which is an excellent value for (BA)2(MA)3Pb4I13 based 2D PSCs.13–17,33–35 By reverse scanning, the PCE becomes slightly larger to 14.9%, indicating a small hysteresis of the PSCs. The difference from the J–V performance is mainly induced by the slightly increased FF (75.2%) and Voc (1.05 V) whereas for PEDOT:PSS/PCBM based PSCs shown in Fig. S5, (ESI†) the reverse scanning PCE is 13.5%, which indicates a larger hysteresis. The suppressed hysteresis degree may be induced by the fewer defects in the PSCs upon using NiOx and C60
C70 shows a high PCE of 14.4%, which is an excellent value for (BA)2(MA)3Pb4I13 based 2D PSCs.13–17,33–35 By reverse scanning, the PCE becomes slightly larger to 14.9%, indicating a small hysteresis of the PSCs. The difference from the J–V performance is mainly induced by the slightly increased FF (75.2%) and Voc (1.05 V) whereas for PEDOT:PSS/PCBM based PSCs shown in Fig. S5, (ESI†) the reverse scanning PCE is 13.5%, which indicates a larger hysteresis. The suppressed hysteresis degree may be induced by the fewer defects in the PSCs upon using NiOx and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70, which will be discussed later. Fig. 2(d) shows the steady state power output for the best performing PSCs, which displays a stable Jsc and PCE for 300 s. The Jsc stabilizes between a range of 16.2 to 16.4 mA cm−2, resulting in a PCE output of 14.0–14.1%.
C70, which will be discussed later. Fig. 2(d) shows the steady state power output for the best performing PSCs, which displays a stable Jsc and PCE for 300 s. The Jsc stabilizes between a range of 16.2 to 16.4 mA cm−2, resulting in a PCE output of 14.0–14.1%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 as the charge transport layers
C70 as the charge transport layers
| HTL/ETL | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) | Best PCE (%) |
|---|---|---|---|---|---|
| PEDOT:PSS/PCBM | 1.02 ± 0.01 | 17.6 ± 0.3 | 68.6 ± 1.5 | 12.3 ± 0.3 | 12.6 |
NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 C70 |
1.01 ± 0.01 | 18.9 ± 0.3 | 73.8 ± 1.3 | 14.1 ± 0.3 | 14.4 |
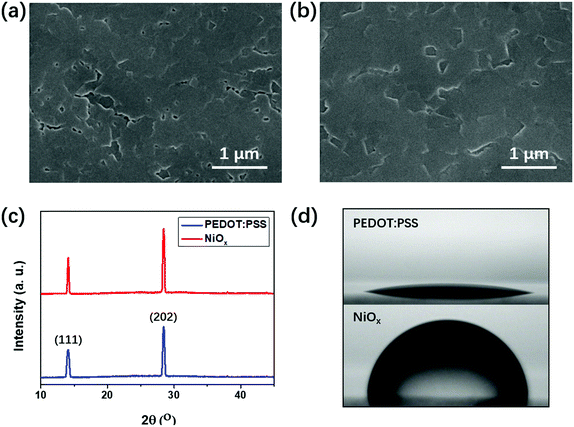
To identify the reason for the performance improvement upon using NiOx and the fullerene mixture, we measured the morphology of the perovskite (BA)2(MA)3Pb4I13 perovskite films formed on different underlying HTLs. From Fig. 3(a and b), both the perovskite films formed on PEDOT:PSS and NiOx HTLs show a uniform morphology with a full surface coverage, which is consistent with those existing in previous studies.14–16 However, the perovskite film formed on NiOx showed enlarged perovskite planes and reduced quantity of pinholes, indicating an improved quality of the (BA)2(MA)3Pb4I13 film. Fig. 3(c) shows the XRD patterns of the two perovskite films on PEDOT:PSS and NiOx, which clearly reveals the crystallinity information for the films. There are two dominant peaks at 14.2° and 28.5°, which represent the crystallographic (111) and (202) planes for the (BA)2(MA)3Pb4I13 film.15 For the NiOx based perovskite film, these characteristic peaks become higher and sharper than those of the perovskite on PEDOT:PSS. The full width at half maximum (FWHM) of the (202) peak for the NiOx based perovskite film is 0.208°, which is narrower than FWHM (0.232°) of the perovskite on PEDOT:PSS. This indicates the improved crystallinity of the perovskite film formed on NiOx. Fig. S6 (ESI†) shows the absorption spectra of the perovskite films formed on NiOx and PEDOT:PSS. The slightly higher absorption of using NiOx is consistent with the improved quality of the perovskite film. The SEM images and XRD spectra demonstrate the improved quality of the perovskite film upon using NiOx. This can be explained from the hydrophilic and hydrophobic nature of the underlying PEDOT:PSS and NiOx, which could significantly influence the crystallization of the upper perovskite films. As shown in Fig. 3(d), the contact angle of NiOx is 80°, much higher than that (12°) of PEDOT:PSS film. This indicates a more hydrophobic property of the NiOx surface, which could induce a larger Gibbs free-energy barrier for suppressing the nucleation of the perovskite at the initial stage.36,37 Moreover, the hydrophobic surface of NiOx would decrease the anchoring effect of perovskite precursor, which further accelerates the movement of the perovskite boundaries at the later stage of crystal growth.36 Consequently, crystallization of the perovskite film was improved upon using hydrophobic underlying NiOx, which further resulted in larger perovskite planes with fewer pinholes. A well crystallized perovskite film with uniform morphology usually contains less charge trapping defects,31 which is highly related to the improved PCE and suppressed hysteresis. From Fig. S7, (ESI†) the SEM images of NiOx and fullerene mixture both show a smooth morphology without significant aggregations, which is beneficial for forming ohmic contact with other functional layers.
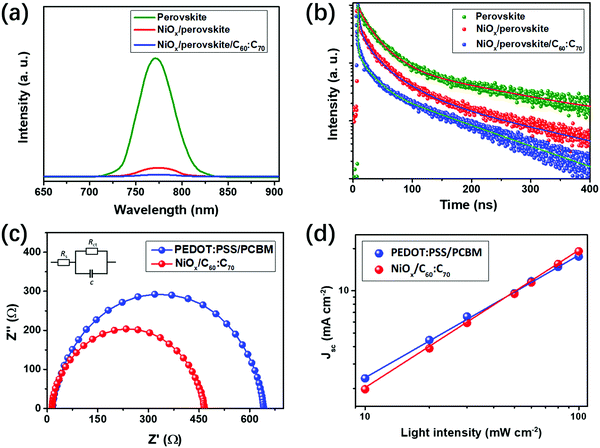
The charge transport property of the PSCs can be revealed from the PL spectra and EIS measurements. As shown in Fig. 4(a), the bare (BA)2(MA)3Pb4I13 perovskite (on glass) exhibits a strong PL signal at 776 nm, which is similar with that in previous studies.15,38 The PL signal of the perovskite on NiOx was dramatically quenched by about 90%, indicating the excellent hole extraction properties of using NiOx. When adding the fullerene mixture as the ETL, the PL signal almost disappeared, indicating the full quenching of excitons in the perovskite absorber. Fig. 4(b) shows the time resolved PL (TR-PL) spectra of the bare perovskite, NiOx/perovskite and NiOx/perovskite/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 films, which reveal the lifetime of the excitons in the devices. With bi-exponential fitting to these curves, parameters of fast (τ1) and slow (τ1) decay times can be obtained, which represent photoluminescence quenching of free carriers and radiative recombination of trapped carriers.34 As shown in Table S2, (ESI†) the τ1 was significantly decreased from 32.3 to 6.8 ns and τ2 was reduced from 304.8 to 38.2 ns upon using NiOx and C60
C70 films, which reveal the lifetime of the excitons in the devices. With bi-exponential fitting to these curves, parameters of fast (τ1) and slow (τ1) decay times can be obtained, which represent photoluminescence quenching of free carriers and radiative recombination of trapped carriers.34 As shown in Table S2, (ESI†) the τ1 was significantly decreased from 32.3 to 6.8 ns and τ2 was reduced from 304.8 to 38.2 ns upon using NiOx and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70, indicating the excellent quenching effect of excitons. The PL and TR-PL spectra for the devices of NiOx/perovskite/C60
C70, indicating the excellent quenching effect of excitons. The PL and TR-PL spectra for the devices of NiOx/perovskite/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 and PEDOT:PSS/perovskite/PCBM are shown in Fig. S8 (ESI†). Compared with the use of PEDOT:PSS and PCBM, using NiOx and C60
C70 and PEDOT:PSS/perovskite/PCBM are shown in Fig. S8 (ESI†). Compared with the use of PEDOT:PSS and PCBM, using NiOx and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 exhibited a lower PL intensity and faster decay time, which indicates the improved charge extracting properties. From the PL and TR-PL measurements, using NiOx and the fullerene mixture could effectively extract charge carriers from the perovskite absorber. The charge transfer property in the PSCs can be conducted from the EIS measurements. As shown in Fig. 4(c), the EIS spectra can be divided into the high (on the left) and low (on the right) frequency ranges, which reveal the series resistance (Rs) and charge transfer resistance (Rct) of the PSCs.38,39 The Rs and Rct values can be obtained by fitting the EIS spectra using the equivalent circuit shown in the inset, which contains these parameters. The Rs and Rct are highly related to the contact resistance of different layers and charge transport property in the PSCs, respectively.38 As indicated from the fitting results, the Rct of PSCs using NiOx/C60
C70 exhibited a lower PL intensity and faster decay time, which indicates the improved charge extracting properties. From the PL and TR-PL measurements, using NiOx and the fullerene mixture could effectively extract charge carriers from the perovskite absorber. The charge transfer property in the PSCs can be conducted from the EIS measurements. As shown in Fig. 4(c), the EIS spectra can be divided into the high (on the left) and low (on the right) frequency ranges, which reveal the series resistance (Rs) and charge transfer resistance (Rct) of the PSCs.38,39 The Rs and Rct values can be obtained by fitting the EIS spectra using the equivalent circuit shown in the inset, which contains these parameters. The Rs and Rct are highly related to the contact resistance of different layers and charge transport property in the PSCs, respectively.38 As indicated from the fitting results, the Rct of PSCs using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 is 425 Ω, which is lower than that (508 Ω) of PEDOT:PSS/PCBM based PSCs. This demonstrates the improved charge transport property of the PSCs upon using NiOx/C60
C70 is 425 Ω, which is lower than that (508 Ω) of PEDOT:PSS/PCBM based PSCs. This demonstrates the improved charge transport property of the PSCs upon using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70, which is in consistent with the PL analysis. Fig. 4(d) shows the dependence of Jsc on the light intensity from 0.1 to 1 sun for the two kinds of PSCs. The calculated slope of NiOx/C60
C70, which is in consistent with the PL analysis. Fig. 4(d) shows the dependence of Jsc on the light intensity from 0.1 to 1 sun for the two kinds of PSCs. The calculated slope of NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 based PSCs is 0.98, which is higher than that (0.93) of PEDOT:PSS/PCBM based ones. This indicates the suppressed bimolecular charge recombination in the PSCs upon using NiOx/C60
C70 based PSCs is 0.98, which is higher than that (0.93) of PEDOT:PSS/PCBM based ones. This indicates the suppressed bimolecular charge recombination in the PSCs upon using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70.40 This phenomenon can also be explained by the improved charge transport property upon using NiOx/C60
C70.40 This phenomenon can also be explained by the improved charge transport property upon using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70, which weakens the charge recombination at the interfaces of NiOx/perovskite/C60
C70, which weakens the charge recombination at the interfaces of NiOx/perovskite/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70. As a result, the improved performance of PSCs using NiOx/C60
C70. As a result, the improved performance of PSCs using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 can be attributed to the improved quality of perovskite absorber and enhanced charge transportation at the interfaces.
C70 can be attributed to the improved quality of perovskite absorber and enhanced charge transportation at the interfaces.
In addition, we compared the stability performance of the PSCs using PEDOT:PSS/PCBM and NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 by storing and testing the samples for 15 days under ambient conditions (25 °C, 30–40% humidity). As shown in Fig. 5(a), the PSCs using PEDOT:PSS and PCBM maintained a PCE of 9.8% after a measurement of 15 days, which corresponded to a PCE degradation of about 20% (initial PCE = 12.3%) whereas the NiOx/C60
C70 by storing and testing the samples for 15 days under ambient conditions (25 °C, 30–40% humidity). As shown in Fig. 5(a), the PSCs using PEDOT:PSS and PCBM maintained a PCE of 9.8% after a measurement of 15 days, which corresponded to a PCE degradation of about 20% (initial PCE = 12.3%) whereas the NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 based PSCs showed a higher PCE of 12.4% after 15 days, leading to a lower PCE degradation of 12%. The PSCs using NiOx/C60
C70 based PSCs showed a higher PCE of 12.4% after 15 days, leading to a lower PCE degradation of 12%. The PSCs using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 showed an excellent long-term stability, which is better than those of using organic charge transport layers14,15,38 in a similar condition. From Fig. 5(b–d), for both cases, the PCE degradation was mainly induced by the degraded Jsc and FF, which are highly related to the series and shunt resistances in the PSCs.41 The degradation of perovskite films could induce a larger contact resistance at the interfaces and more leakage current, which would increase the series resistance and decreases the shunt resistance in PSCs. As discussed above, the perovskite film on NiOx showed a better quality than that on PEDOT:PSS, which could induce a higher intrinsic stability of the PSCs.31,42 On the other hand, the inorganic NiOx and C60
C70 showed an excellent long-term stability, which is better than those of using organic charge transport layers14,15,38 in a similar condition. From Fig. 5(b–d), for both cases, the PCE degradation was mainly induced by the degraded Jsc and FF, which are highly related to the series and shunt resistances in the PSCs.41 The degradation of perovskite films could induce a larger contact resistance at the interfaces and more leakage current, which would increase the series resistance and decreases the shunt resistance in PSCs. As discussed above, the perovskite film on NiOx showed a better quality than that on PEDOT:PSS, which could induce a higher intrinsic stability of the PSCs.31,42 On the other hand, the inorganic NiOx and C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 own better intrinsic stability than organic PEDOT:PSS and PCBM, which could more effectively protect the inner perovskite absorber.29 Moreover, PEDOT:PSS is a hydrophilic material, which can absorb moisture from air and further decompose the perovskite.43 As a result, using hydrophobic NiOx as the HTL can prevent this moisture induced degradation of the PSCs, which is another reason for the improved long-term stability.
C70 own better intrinsic stability than organic PEDOT:PSS and PCBM, which could more effectively protect the inner perovskite absorber.29 Moreover, PEDOT:PSS is a hydrophilic material, which can absorb moisture from air and further decompose the perovskite.43 As a result, using hydrophobic NiOx as the HTL can prevent this moisture induced degradation of the PSCs, which is another reason for the improved long-term stability.
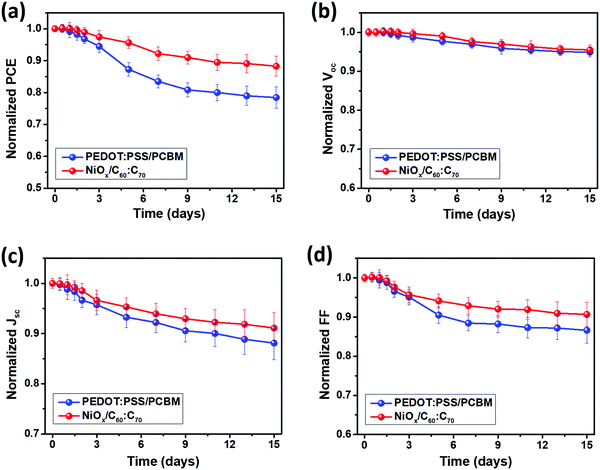 | ||
Fig. 5 Long-term stability of the PSCs using PEDOT:PSS/PCBM and NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 as the charge transport layers, in terms of their normalized PCE (a), Voc (b), Jsc (c) and FF (d) as a function of time. C70 as the charge transport layers, in terms of their normalized PCE (a), Voc (b), Jsc (c) and FF (d) as a function of time. | ||
4. Conclusions
Ruddlesden–Popper 2D PSCs have been intensively studied due to the advantages of high stability and simple processability. In this work, we developed solution-processed inorganic NiOx and fullerene mixture (C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 =1
C70 =1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the HTL and ETL for improving the performance of Ruddlesden–Popper 2D PSCs. Compared with PSCs using conventional PEDOT:PSS and PCBM, the NiOx/C60
1) as the HTL and ETL for improving the performance of Ruddlesden–Popper 2D PSCs. Compared with PSCs using conventional PEDOT:PSS and PCBM, the NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70-based PSCs showed a significantly improved PCE of 14.1% (on average), which was induced by the improved Jsc and FF. Upon using NiOx and fullerene mixture, long-term stability of the PSCs was also improved with the PCE degradation dramatically suppressed from 20% to 12% after being tested for 15 days. The SEM and XRD results demonstrate the improved film quality and crystallinity for perovskite films formed on NiOx. The PL, EIS and light-dependent measurements indicate the excellent charge transport property of using NiOx and fullerene mixture in the PSCs. The best performing PSC using NiOx/C60
C70-based PSCs showed a significantly improved PCE of 14.1% (on average), which was induced by the improved Jsc and FF. Upon using NiOx and fullerene mixture, long-term stability of the PSCs was also improved with the PCE degradation dramatically suppressed from 20% to 12% after being tested for 15 days. The SEM and XRD results demonstrate the improved film quality and crystallinity for perovskite films formed on NiOx. The PL, EIS and light-dependent measurements indicate the excellent charge transport property of using NiOx and fullerene mixture in the PSCs. The best performing PSC using NiOx/C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 exhibits a high PCE of 14.4%, with a stable power output and negligible hysteresis. Our result demonstrates that using solution processed inorganic NiOx and pristine fullerenes as the CTMs is an effective way of fabricating Ruddlesden–Popper 2D PSCs with high efficiency and stability.
C70 exhibits a high PCE of 14.4%, with a stable power output and negligible hysteresis. Our result demonstrates that using solution processed inorganic NiOx and pristine fullerenes as the CTMs is an effective way of fabricating Ruddlesden–Popper 2D PSCs with high efficiency and stability.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Hubei Polytechnic University Talent Introduction Fund (No. KJC2021HXHG01) and the National Natural Foundation of China (No. 51871067). J. F. T acknowledges funding from the Natural Science Foundation of Hunan Province (No. 2020JJ4375) and the Double First-Class Construction Project of Hunan Agricultural University (No. SYL2019063). W. L. acknowledges the Chinese Postdoctoral Science Foundation (No. 2021M701168) and the Science and Technology Innovation Program of Hunan Province (No. 2021RC3089).References
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS.
- W. S. Yang, B.-W. Park, E. H. Jung, N. J. Jeon, Y. C. Kim, D. U. Lee, S. S. Shin, J. Seo, E. K. Kim, J. H. Noh and S. I. Seok, Science, 2017, 356, 1376–1379 CrossRef CAS PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- NREL Best Research-Cell Efficiency Chart (Photovoltaic Research). Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 13 January 2021).
- H. Kim, K.-G. Lim and T.-W. Lee, Energy Environ. Sci., 2016, 9, 12–30 RSC.
- L. Chen, X. Xie, Z. Liu and E.-C. Lee, J. Mater. Chem. A, 2017, 5, 6974–6980 RSC.
- D. Li, D. Zhang, K.-S. Lim, Y. Hu, Y. Rong, A. Mei, N.-G. Park and H. Han, Adv. Funct. Mater., 2021, 31, 2008621 CrossRef CAS.
- J. H. Heo, H. J. Han, D. Kim, T. K. Ahn and S. H. Im, Energy Environ. Sci., 2015, 8, 1602–1608 RSC.
- K. Jiang, F. Wu, G. Zhang, P. C. Y. Chow, C. Ma, S. Li, K. S. Wong, L. Zhu and H. Yan, J. Mater. Chem. A, 2019, 7, 21662–21667 RSC.
- G. Liu, X. Xie, Z. Liu, G. Cheng and E.-C. Lee, Nanoscale, 2018, 10, 11043–11051 RSC.
- J. A. Christians, P. A. Miranda Herrera and P. V. Kamat, J. Am. Chem. Soc., 2015, 137, 1530–1538 CrossRef CAS PubMed.
- H. Tsai, W. Nie, J.-C. Blancon, C. C. Stoumpos, R. Asadpour, B. Harutyunyan, A. J. Neukirch, R. Verduzco, J. J. Crochet, S. Tretiak, L. Pedesseau, J. Even, M. A. Alam, G. Gupta, J. Lou, P. M. Ajayan, M. J. Bedzyk, M. G. Kanatzidis and A. D. Mohite, Nature, 2016, 536, 312–316 CrossRef CAS PubMed.
- Y. Xie, H. Yu, J. Duan, L. Xu and B. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 11190–11196 CrossRef CAS PubMed.
- Z. Liu, L. Wang, C. Xu and X. Xie, Sustainable Energy Fuels, 2021, 5, 2595–2601 RSC.
- X. Zhang, X. Ren, B. Liu, R. Munir, X. Zhu, D. Yang, J. Li, Y. Liu, D. Smilgies, R. Li, Z. Yang, T. Niu, X. Wang, A. Amassian, K. Zhao and S. Liu, Energy Environ. Sci., 2017, 10, 2095–2102 RSC.
- D. H. Cao, C. C. Stoumpos, O. K. Farha, J. T. Hupp and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 7843–7850 CrossRef CAS PubMed.
- K. Lee, J. Ryu, H. Yu, J. Yun, J. Lee and J. Jang, Nanoscale, 2017, 9, 16249–16255 RSC.
- C.-T. Lin, S. Pont, J. Kim, T. Du, S. Xu, X. Li, D. Bryant, M. A. Mclachlan and J. R. Durrant, Sustainable Energy Fuels, 2018, 2, 1686–1692 RSC.
- A. K. Jena, Y. Numata, M. Ikegami and T. Miyasaka, J. Mater. Chem. A, 2018, 6, 2219–2230 RSC.
- T. Leijtens, E. Eperon, S. Pathak, A. Abate, M. M. Lee and H. J. Snaith, Nat. Commun., 2013, 4, 2885 CrossRef PubMed.
- D. D. Girolamo, F. D. Giacomo, F. Matteocci, A. G. Marrani, D. Dini and A. Abate, Chem. Sci., 2020, 11, 7746–7759 RSC.
- X. Yin, Y. Guo, H. Xie, W. Que and L. B. Kong, Sol. RRL, 2019, 3, 1900001 CrossRef.
- C. Liu, W. Li, J. Chen, J. Fan, Y. Mai and R. E. I. Schropp, Nano Energy, 2017, 41, 75–83 CrossRef CAS.
- J. You, L. Meng, T.-B. Song, T.-F. Guo, Y. Yang, W.-H. Chang, Z. Hong, H. Chen, H. Zhou, Q. Chen, Y. Liu, N. D. Marco and Y. Yang, Nat. Nanotechnol., 2016, 11, 75–81 CrossRef CAS PubMed.
- Z. Liu, J. Chang, Z. Lin, L. Zhou, Z. Yang, D. Chen, C. Zhang, S. Liu and Y. Hao, Adv. Energy Mater., 2018, 8, 1703432 CrossRef.
- P. Zhang, J. Wu, T. Zhang, Y. Wang, D. Liu, H. Chen, L. Ji, C. Liu, W. Ahmad, Z. D. Chen and S. Li, Adv. Mater., 2018, 30, 1703737 CrossRef PubMed.
- Q. Jiang, L. Zhang, H. Wang, X. Yang, J. Meng, H. Liu, Z. Yin, J. Wu, X. Zhang and J. You, Nat. Energy, 2017, 2, 16177 CrossRef CAS.
- C. Liu, W. Li, C. Zhang, Y. Ma, J. Fan and Y. Mai, J. Am. Chem. Soc., 2018, 140, 3825–3828 CrossRef CAS PubMed.
- A. D. Z. Mendaza, A. Melianas, S. Rossbauer, O. Backe, L. Nordstierna, P. Erhart, E. Olsson, T. D. Anthopoulos, O. Inganas and C. Muller, Adv. Mater., 2015, 27, 7325–7331 CrossRef CAS PubMed.
- C. Xu, Z. Liu and E.-C. Lee, J. Mater. Chem. C, 2019, 7, 6956–6963 RSC.
- H.-S. Lin, I. Jeon, R. Xiang, S. Seo, J.-W. Lee, C. Li, A. Pal, S. Manzhos, M. S. Goorsky, Y. Yang, S. Maruyama and Y. Matsuo, ACS Appl. Mater. Interfaces, 2018, 10, 39590–39598 CrossRef CAS PubMed.
- P. Liu, N. Han, W. Wang, R. Ran, W. Zhou and Z. Shao, Adv. Mater., 2021, 33, 2002582 CrossRef CAS PubMed.
- R. Liu, Y. Yu, T. Hu, F. Zhang, C. Liu, H. Hou, M. Zhang, X. Chen and H. Yu, J. Power Sources, 2021, 512, 230452 CrossRef CAS.
- A. Caiazzo, K. Datta, J. Jiang, M. C. Gélvez-Rueda, J. Li, R. Ollearo, J. M. Vicent-Luna, S. Tao, F. C. Grozema, M. M. Wienk and R. A. J. Janssen, Adv. Energy Mater., 2021, 11, 2102144 CrossRef CAS.
- C. Bi, Q. Wang, Y. Shao, Y. Yuan, Z. Xiao and J. Huang, Nat. Commnun., 2015, 6, 7747 CrossRef CAS PubMed.
- F. Wang, T. Zhang, Y. Wang, D. Liu, P. Zhang, H. Chen, L. Ji, L. Chen, Z. D. Chen, J. Wu, X. Liu, Y. Li, Y. Wang and S. Li, J. Mater. Chem. A, 2019, 7, 12166–12175 RSC.
- Z. Liu and E.-C. Lee, Sustainable Energy Fuels, 2021, 5, 2354–2361 RSC.
- H. Wei, J. Xiao, Y. Yang, S. Lv, J. Shi, X. Xu, J. Dong, Y. Luo, D. Li and Q. Meng, Carbon, 2015, 93, 861–868 CrossRef CAS.
- T. T. Tong, X. H. Li, S. H. Guo, J. Han and B. Q. Wei, Nano Energy, 2017, 41, 591–599 CrossRef CAS.
- E. Widianto, Shobih, E. S. Rosa, K. Triyana, N. M. Nursam and I. Santoso, Opt. Mater., 2021, 121, 111584 CrossRef CAS.
- Y. Liu, Z. Liu and E.-C. Lee, ACS Appl. Energy Mater., 2019, 2, 1932–1942 CrossRef CAS.
- Z. Liu, P. You, C. Xie, G. Tang and F. Yan, Nano Energy, 2016, 28, 151–157 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and table. See DOI: https://doi.org/10.1039/d2cp02033e |
| This journal is © the Owner Societies 2022 |