Open Access Article
Open Access ArticleBenzothiadiazole-based photosensitizers for efficient and stable dye-sensitized solar cells and 8.7% efficiency semi-transparent mini-modules†
Maxime
Godfroy
 a,
Johan
Liotier
a,
Valid M.
Mwalukuku
a,
Damien
Joly
a,
Quentin
Huaulmé
a,
Lydia
Cabau
a,
Cyril
Aumaitre
a,
Yann
Kervella
a,
Stéphanie
Narbey
b,
Frédéric
Oswald
b,
Emilio
Palomares
a,
Johan
Liotier
a,
Valid M.
Mwalukuku
a,
Damien
Joly
a,
Quentin
Huaulmé
a,
Lydia
Cabau
a,
Cyril
Aumaitre
a,
Yann
Kervella
a,
Stéphanie
Narbey
b,
Frédéric
Oswald
b,
Emilio
Palomares
 cd,
Carlos A.
González Flores
cd,
Carlos A.
González Flores
 e,
Gerko
Oskam
e,
Gerko
Oskam
 ef and
Renaud
Demadrille
ef and
Renaud
Demadrille
 *a
*a
aUniv. Grenoble Alpes, CNRS, CEA, INAC, SyMMES, 17 rue des martyrs, 38000 Grenoble, France. E-mail: renaud.demadrille@cea.fr
bSolaronix SA, Rue de l'Ouriette 129, 1170 Aubonne, Switzerland
cInstitute of Chemical Research of Catalonia (ICIQ), Avenguda Països Catalans, 16, Tarragona 43007, Spain
dICREA, Passeig Lluís Companys, 23, Barcelona E-08010, Spain
eDepartment of Applied Physics, CINVESTAV-IPN, Mérida, Yucatán, 97310, México
fDepartamento de Sistemas Físicos, Químicos y Naturales, Universidad Pablo de Olavide, Carretera de Utrera km 1, Sevilla 41013, Spain
First published on 7th December 2020
Abstract
We report on the synthesis and structure–properties relationships of five benzothiadiazole-based organic dyes designed for use in Dye-Sensitized Solar Cells (DSSCs). These compounds exhibit hues ranging from pink to violet-blue and demonstrate good Power Conversion Efficiencies (PCEs) ranging from 7.0% to 9.8% when employed as photosensitizers with TiO2 mesoporous electrodes. The combination of two of these dyes following a co-sensitization approach led to a PCE of up to 10.9% with an iodine-based liquid electrolyte. We demonstrate, using charge extraction and transient photo-voltage experiments, that the improvement of the performances with the cocktail of dyes is related to better light absorption and passivation of the TiO2 surface. When the volatile electrolyte is swapped for an ionic-liquid, PCEs over 7.5% are reached and the best solar cells retain 80% of their initial performance after 7000 h of light exposure, according to the accelerated aging test ISOS-L2 (65 °C, AM1.5G, under continuous irradiation at 1000 W m−2). Finally, we report excellent performance in five-cell mini-modules with 14 cm2 active area demonstrating a PCE of 8.7%. This corresponds to a power output of circa 123 mW, ranking among the highest performances for such semi-transparent photovoltaic devices.
1. Introduction
During the past two decades, tremendous efforts have been focused on the development of photovoltaic (PV) technologies capable of palliating some of the drawbacks of conventional silicon-based solar panels such as heaviness, opaqueness and large energy consumption during manufacturing.1,2 Technologies integrating organic and hybrid materials such as Dye-Sensitized Solar Cells (DSSCs),3,4 Bulk-Hetero-Junction solar cells (BHJ),5–7 or Perovskite Solar Cells (PSC)8,9 have progressed significantly. Nowadays they appear to be adapted to solve some of these drawbacks.10 In terms of power conversion efficiencies (PCEs), the best DSSCs performances lie in the range of 10 to 14.2%,11–15 which is lower compared to BHJ and PSC, but interestingly they already demonstrated superior long-term stability. Indeed, when non-volatile electrolytes are employed,16–18 their performance can be conserved under harsh testing conditions,16 and a stability corresponding to approximately 10 years of use in real conditions has been demonstrated. Besides, this technology enables the fabrication of semi-transparent solar panels that can be colourful and prepared with non-toxic constituents.19 All these criteria make DSSCs very appealing for Building-Integrated Photovoltaics (BIPV)18,20,21 or Automobile Integrated Photovoltaics (AIPV).22 The performance of a DSSC mainly depends on the molecular structure of the dye used to photosensitize the mesoporous electrode. Historically, Ru(II)–polypyridyl complexes were the first efficient dyes used as photosensitizers in DSSCs (N-719 or N-749)23 but they possess low absorption coefficients in the visible range,24 and contain ruthenium which is a rare and high-cost element. Besides, their plausible toxicity restrains their development at the industrial level. Consequently, metal-free organic dyes based on the donor–(π-spacer)–acceptor structure have been extensively developed, studied and screened as photosensitizers in DSSCs.25,26To improve the photocurrent generation and hence photovoltaic performances of DSSCs, the development of new dyes better matching the solar emission spectrum appeared to be an efficient strategy. A decade of development led to a plethora of new organic dyes, allowing identification of very efficient electron-rich units (such as triarylamine derivatives) and anchoring electron-withdrawing units (such as cyanoacrylic acid or carboxylic acid) for their preparation.22 Many chromophores have been also investigated as π-conjugated spacers (BODIPY,26 isoindigo,27 porphyrins12,28,29) to tune their optoelectronic properties. A few years ago, we reported a benzothiadiazole-containing dye, namely RK1,16 showing a rather simple chemical structure and demonstrating a power conversion efficiency of up to 10.2% with an outstanding stability of more than 9000 hours when subjected to a harsh accelerated ageing test (ISOS-L2). This orange-reddish dye was the first purely organic photosensitizer to be implemented in a large semi-transparent module (active area of 1400 cm2) suitable for BIPV.21 Inspired by the design of this dye, we reported in 2018 another benzothiadiazole-based molecule namely YKP-88, where the TPA unit is bridged to the thiophene ring to form an indeno[1,2-b]thiophene unit. This dye showed an efficiency of over 9% in DSSCs fabricated with classical architectures, and up to 10.3% with inverse opals-based electrodes.30 Consequently, in this work, we investigate further the performance of YKP-88 and report the synthesis of four new analogue compounds whose chemical structures are tuned with the goal to shift its Internal Charge Transfer (ICT) absorption band towards lower energy wavelengths while keeping control of the energy levels of the frontier orbitals. Our objective was to prove efficient and stable dyes following this molecular concept while obtaining various colours. Their optoelectronic properties have been studied by UV-Vis spectroscopy, cyclic voltammetry (CV) and then compared to DFT calculations. We implemented these dyes in DSSCs using opaque TiO2 electrodes and various electrolytes and we report their photovoltaic performances. The combination of YKP-88 with one of the new dyes, following a co-sensitization approach, led to a PCE of up to 10.9% using an iodine-based electrolyte. The reasons behind the improvement of the performances were unravelled thanks to Charge Extraction (CE) and Transient Photo-Voltage (TPV) measurements. We also report, using ionic liquid-based electrolyte, stable solar cells that retain 80% of their initial efficiency for over 7000 hours when subjected to a standard ISOS-L2 accelerating ageing test. Finally, we show that semi-transparent mini-modules with an active surface of 14.08 cm2 (total surface of 23 cm2) can be fabricated with YKP-88. This mini-module reached a PCE of 8.7% with a power output of circa 123 mW.
2. Results and discussion
2.1 Design and synthesis
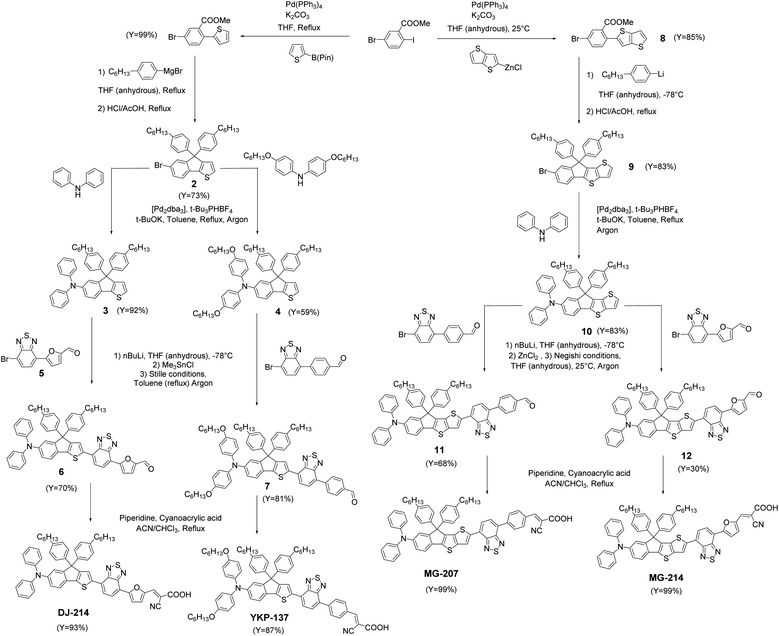
Owing to the good efficiency and stability of DSSCs sensitized with RK1 or YKP-88 and the relatively ease of synthesis of these dyes, we developed in this study new molecules presenting a rather close chemical design. Fig. 1 presents the chemical structures of RK1, YKP-88 and the 4 new dyes synthesized in this work. With the goal to shift the absorption of the new dyes towards longer wavelengths in the visible range, we tuned the push–pull effect within the molecules by increasing the electron-donating strength of the triarylamine (TPA) unit or by improving the planarity of the molecules (see X-ray structures in ESI†) and/or the π-conjugation with the electron-accepting unit. First, the TPA unit was substituted with two alkoxy groups to give YKP-137, second the replacement of the thiophene flanking the indene ring by a thieno[3,2-b]thiophene unit was performed to give MG-207. Then we swapped the benzene spacer between the benzothiadiazole and the anchoring function for a furan unit.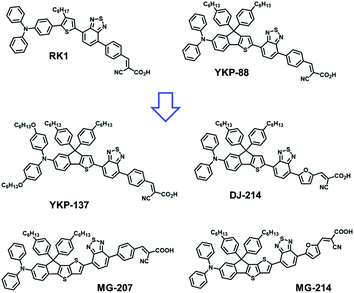 | ||
| Fig. 1 Chemical structure of the previously reported dye RK1, YKP-88 and structures of the four new dyes synthetized in this work. | ||
This replacement led to a better planarization and conjugation of the electron-accepting units.31 Therefore, we obtained DJ-214 and MG-214 that are analogues of YKP-88 and MG-207, respectively. The synthesis routes towards these 4 new dyes are presented in Fig. 2. The synthesis involves, as a precursor, ethyl-5-bromo-2-iodobenzoate that can be coupled with a thiophene unit through a Suzuki coupling or a thieno[3,2-b]thiophene unit through a Negishi coupling to give compounds 1 and 8, respectively. From 1, 6-bromo-4,4-bis(4-hexylphenyl)-4H-indeno[1,2-b]thiophene, 2 can be obtained via Grignard reaction and cyclization under acidic conditions.30 Applying the same approach starting from 8 afforded compound 9. The triarylamine units 3, 4 and 10 were obtained via Buchwald–Hartwig cross-coupling reaction using unsubstituted or alkoxy-substituted diphenylamine. Regioselective lithiation of 3 and 4 followed by a nucleophilic substitution with chloro-trimethylstannane afforded two stannylated intermediates, which were not isolated due to poor chemical stability. The intermediate corresponding to 4 was subsequently used in a Stille cross-coupling reaction with 4-(7-bromobenzo[c][1,2,5]thiadiazol-4-yl)benzaldehyde32 to give compound 7. The stannylated derivative of 3 was reacted with 5-(7-bromobenzo[c][1,2,5]thiadiazol-4-yl)furan-2-carbaldehyde, 5, to afford compound 6. On the other hand, compound 10 was lithiated selectively to allow the formation of an organo-zinc intermediate by nucleophilic substitution with zinc chloride. This organo-zinc intermediate was then involved in a Negishi coupling with 4-(7-bromobenzo[c][1,2,5]thiadiazol-4-yl)benzaldehyde to give compound 11 and with 5-(7-bromobenzo[c][1,2,5]thiadiazol-4-yl)furan-2-carbaldehyde, 5, to give compound 12. The targeted materials YKP-137, DJ-214, MG-207 and MG-214 were obtained via Knoevenagel condensation with cyanoacetic acid in the presence of piperidine (synthetic procedures, 1H-NMR, 13C-NMR, HRMS and elemental analysis are given in ESI†).
2.2 Optoelectronic properties
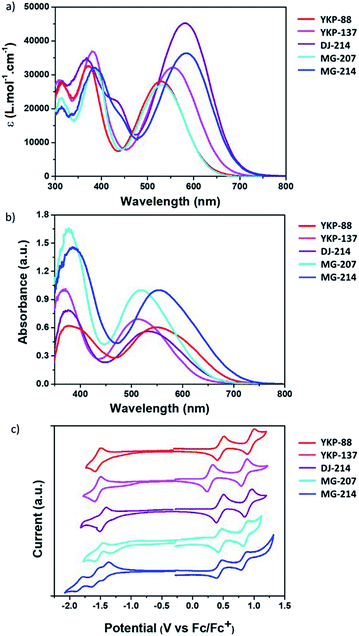
The optical properties of the compounds YKP-137, DJ-214, MG-207 and MG-214 were investigated and compared to those of YKP-88. When the dyes are evaluated in diluted solution (10−5 M in dichloromethane) (see Fig. 3a), they all display intense absorption in the visible range with molar extinction coefficients, at λmax, comprised between 2.7 × 104 and 4.33 × 104 L mol−1 cm−1. The absorption band located at lower energy is attributed to the Internal Charge Transfer (ICT) transition, whereas the other transition (around 400 nm) is attributed to the π–π* transitions of the different aromatic units. As expected, increasing the electronic density difference between the electron donating and the withdrawing part implies a bathochromic shift of the Internal Charge Transfer (ICT) band. Thus, the alkoxy substituents introduced on the TPA unit of YKP-137 induce a red shift of the ICT band by 28 nm compared to YKP-88. On the other hand, the replacement of the thiophene by a thienothiophene unit in MG-207 has a minor effect on the λmax with a small bathochromic shift (+3 nm). However, the shift is more pronounced for the UV absorption band (+12 nm). If the absorption spectra of YKP-88 and MG-207 are compared to the ones of DJ-214 and MG-214 respectively, we can probe the effect of swapping the benzene spacer for a furan. A strong bathochromic shift of λmax is now observed (+52 nm) accompanied by a significant hyperchromic shift. This observation can be explained by the smaller resonance energy of furan compared to benzene (16 kcal mol−1vs. 36 kcal mol−1) leading to more effective conjugation.33 Besides, our results confirm (see Fig. S1 in ESI†) that using a furan spacer, a higher degree of planarity along the molecule is achieved.34 The dihedral angle of 20° between the benzothiadiazole unit and the phenyl in YKP-88 is reduced to 0° between the benzothiadiazole unit and the furan in DJ-214, leading to a perfect co-planarity and a better conjugation with the anchoring function. The introduction of the furan unit also induces the emergence of an absorption shoulder between 400–450 nm.31The new compounds exhibit bright colours ranging from pink to violet-blue in solution. In order to gain more insights into the behaviour of these dyes once adsorbed on mesoporous TiO2, their absorption spectra were measured after grafting on a 2 μm-thick TiO2 mesoporous layer from a 2 mM solution in a 1/1 mixture of ACN and tBuOH (see Table 1). Once the dyes are attached onto TiO2, their absorption spectra are broadened and the ICT band is blue-shifted by 16 nm, 24 nm and 13 nm for YKP-88, YKP-137 and MG-207 respectively, whereas the shift is more important i.e. 32 nm and 30 nm for DJ-214 and MG-214 (see Fig. 3b). A small blue shift is often observed when organic photosensitizers are grafted on the TiO2 surface, which can be ascribed partly to the deprotonation of the carboxylic acid function.35 However, larger shifts and broadening of the spectrum can be associated to the formation of aggregates,36,37 which is consistent with the more planar nature of the furan-containing molecules.
| Dyes | λ maxvisa [nm] | ε [M−1 cm−1] | E opt , [eV] | λ max TiO2c [nm] | HOMOd [eV] | LUMOd [eV] | E elec [eV] |
|---|---|---|---|---|---|---|---|
| a In solution (DCM, 10−5 M) or adsorbed on anatase–TiO2 (2 μm). b Calculated from 1241/λonset. c TiO2 electrode (thickness 2 μm), dyeing solution CHCl3/t-butanol, 0.2 mM dye. d All potentials were obtained by cyclic voltammetry investigations in 0.2 M Bu4NPF6 in CH2Cl2. The platinum electrode diameter was 1 mm and the sweep rate 200 mV s−1. Potentials measured vs. Fc/Fc+ and the values are calculated using half-wave potentials (E1/2) and −4.8 eV as a Fc/Fc+ standard versus vacuum level. e Calculated from Eelec = EHOMO − ELUMO. | |||||||
| YKP-88 | 529 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.99 | 513 | −5.26 | −3.26 | 2.00 |
| DJ-214 | 582 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.79 | 550 | −5.25 | −3.35 | 1.90 |
| YKP-137 | 557 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.87 | 533 | −5.08 | −3.26 | 1.82 |
| MG-207 | 532 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.99 | 519 | −5.25 | −3.30 | 1.95 |
| MG-214 | 584 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.81 | 554 | −5.24 | −3.40 | 1.84 |
To get more insights into the optoelectronic properties of the compounds, cyclic voltammetry experiments were carried out with the objective to determine highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy level positions (see Fig. 3c). We estimated the energy levels from their oxidation and reduction potential after calibration with ferrocene (Fc/Fc+). Similar to YKP-88, the dyes DJ-214, MG-207 and MG-214 exhibit two reversible oxidation waves located at around +0.45 V and +0.95 V. YKP-137 is easier to oxidize because of the presence of alkoxy substituents on the TPA unit and hence its oxidation waves are found around +0.28 V and +0.86 V (see Fig. 3c). It clearly appears that the TPA unit dictates the HOMO energy level position. For all the dyes, the HOMO is around −5.25 eV except for YKP-137 whose HOMO is at −5.08 eV. Regarding the reduction process, it appears that the reduction potentials are similar for YKP-88 and YKP-137 (around −1.55 V), i.e. the dyes showing the same π-conjugated backbone. The reduction waves are shifted towards more positive potentials for the dyes in which the π-conjugated backbone is modified i.e. MG-207, DJ-214 and MG-214 (−1.50 V, −1.45 V and −1.40 V respectively). The LUMO energy levels are lying between −3.26 eV and −3.30 eV for the 3 dyes with a benzene spacer, and as expected, they are shifted towards more negative values for the dyes with a furan spacer.38 This is another manifestation of the higher degree of conjugation between the electron-accepting unit and the benzothiadiazole unit. The HOMO and LUMO energy levels of the dyes are positioned correctly with respect to the conducting band (CB) energy level of the TiO2 and the redox potential of the iodine/iodide electrolyte, to warrant an efficient electron photo-injection from the dye to the TiO2 and a good regeneration of the oxidised dye by the redox mediator. Consequently, we have fabricated DSSCs using this device configuration.
2.3 Dye-Sensitized Solar Cells
Two types of electrolytes containing I−/I3− redox couple were employed, first, a liquid electrolyte (Iodolyte HI-30) to achieve high performances and second, an ionic liquid-based electrolyte (Mosalyte TDE-250) to obtain more robust solar cells. The electrode thicknesses were optimized for each device configuration. Thick electrodes with 10 to 14 μm-thick mesoporous TiO2 films coated with a 3 to 4 μm-thick reflecting layer were prepared and used with the liquid electrolyte. Thinner mesoporous layers (8 μm-thick coated with a 3 μm-thick reflecting layer) were employed to facilitate the complete filling of the pores with Mosalyte TDE-250 because it shows higher viscosity. Some of the devices were fabricated in a double-blind process at Solaronix and at CEA, and the performances are as reported in Table 2 to prove the reliability and reproducibility of the results. The performances were compared to those obtained using RK1 as a reference dye.11,16
| Dyes | Electrolyte | TiO2 electrode (μm) | J sc (mA cm−2) | V oc (mV) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
a Fabricated and tested at CEA.
b Fabricated and tested at Solaronix. First line and second line correspond to best cells; third line corresponds to mean-values and standard deviation calculated from at least 3 devices. Dyeing bath: [dye] = 0.2 mM, [CDCA] = 2 mM in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tBuOH 1 tBuOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (v/v) except for MG-207 and MG-214.
c Dyeing bath: [dye] = 0.2 mM, [CDCA] = 2 mM in CHCl3 1, (v/v) except for MG-207 and MG-214.
c Dyeing bath: [dye] = 0.2 mM, [CDCA] = 2 mM in CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH 1 EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (v/v). 1, (v/v).
|
||||||
| YKP-88 | Iodolyte | 14 + 3a | 17.89 | 735 | 72 | 9.52 |
| YKP-88 | Iodolyte | 14 + 3b | 18.96 | 706 | 71 | 9.54 |
| YKP-88 | Iodolyte | 14 + 3b | 18.66 ± 0.31 | 708 ± 3 | 71 ± 1 | 9.34 ± 0.12 |
| YKP-88 | Mosalyte TDE-250 | 8 + 3b | 17.39 | 642 | 66 | 7.36 |
| YKP-88 | Mosalyte TDE-250 | 8 + 3b | 16.81 ± 0.46 | 645 ± 3 | 67 ± 1 | 7.27 ± 0.04 |
| DJ-214 | Iodolyte | 14 + 3a | 17.03 | 648 | 69 | 7.57 |
| DJ-214 | Iodolyte | 10 + 4b | 17.08 | 670 | 71 | 8.09 |
| DJ-214 | Iodolyte | 10 + 4b | 17.14 ± 0.06 | 666 ± 4 | 71 ± 0 | 8.08 ± 0.02 |
| DJ-214 | Mosalyte TDE-250 | 8 + 3b | 17.21 | 616 | 69 | 7.34 |
| DJ-214 | Mosalyte TDE-250 | 8 + 3b | 16.80 ± 0.41 | 613 ± 4 | 67 ± 3 | 6.87 ± 0.47 |
| YKP-137 | Iodolyte | 14 + 3a | 19.50 | 723 | 68 | 9.55 |
| YKP-137 | Iodolyte | 10 + 4b | 18.56 | 729 | 70 | 9.38 |
| YKP-137 | Iodolyte | 10 + 4b | 18.52 ± 0.05 | 726 ± 4 | 69 ± 1 | 9.21 ± 0.18 |
| YKP-137 | Mosalyte TDE-250 | 8 + 3b | 15.70 | 659 | 63 | 6.59 |
| YKP-137 | Mosalyte TDE-250 | 8 + 3b | 15.61 ± 0.10 | 660 ± 0 | 61 ± 2 | 6.44 ± 0.16 |
| MG-207 | Iodolyte | 14 + 3a | 22.14 | 672 | 66 | 9.78 |
| MG-207 | Iodolyte | 12 + 3b,c | 18.42 | 703 | 73 | 9.41 |
| MG-207 | Iodolyte | 12 + 3b,c | 18.03 ± 0.20 | 704 ± 1 | 72 ± 1 | 9.15 ± 0.14 |
| MG-207 | Mosalyte TDE-250 | 8 + 3b,c | 16.12 | 678 | 69 | 7.52 |
| MG-207 | Mosalyte TDE-250 | 8 + 3b,c | 16.53 ± 0.57 | 674 ± 4 | 67 ± 2 | 7.47 ± 0.04 |
| MG-214 | Iodolyte | 14 + 3a | 16.13 | 623 | 70 | 7.01 |
| MG-214 | Iodolyte | 12 + 3b,c | 14.55 | 660 | 71 | 6.83 |
| MG-214 | Iodolyte | 12 + 3b,c | 14.68 ± 0.19 | 657 ± 2 | 70 ± 1 | 6.82 ± 0.10 |
| MG-214 | Mosalyte TDE-250 | 8 + 3b,c | 15.42 | 609 | 69 | 6.52 |
| MG-214 | Mosalyte TDE-250 | 8 + 3b,c | 15.51 ± 0.49 | 606 ± 1 | 67 ± 1 | 6.32 ± 0.10 |
| RK1 | Iodolyte | 10 + 4a | 18.47 ± 1.39 | 719 ± 11 | 70 ± 1 | 9.28 ± 0.60 |
| RK1 | Mosalyte TDE-250 | 8 + 3b | 15.95 ± 0.18 | 668 ± 1 | 66 ± 1 | 7.21 ± 0.14 |
As can be noticed from this table, when used with a liquid electrolyte based on a low viscosity solvent, the five new dyes exhibit relatively good efficiencies, ranging from 7.01% up to 9.78% for the best cells. Notably, Jsc values over 14.5 mA cm−2 are measured with all the dyes. The performances of the DSSCs sensitized with the compounds embedding a phenyl spacer are higher than the ones arising from the dyes with a furan. One noticeable difference concerns the lower Voc values obtained when a furan spacer is used. Voc mean-values are up to 708 mV, 726 mV and 704 mV for compounds YKP-88, YKP-137 and MG-207 respectively, whereas with DJ-214 and MG-214 they are lowered by circa 40 to 60 mV. The highest Voc is obtained with YKP-137, because of the presence of alkoxy-chains on the TPA that is known to lower the recombination rate.39–41 The loss of Voc when a furan is inserted close to the anchoring group was expected since several teams have reported this phenomenon before. In previous studies, the Voc drop was attributed to an increase of the charge recombination rate42,43 and a conduction band down-shift.31
In our dyes, the substitution with phenyl-hexyl groups of the spiro carbon of the indene unit is expected to reduce the dye aggregation. To verify that the aggregation phenomenon is not critical in our solar cells we have fabricated DSSCs without using CDCA. The use of CDCA as co-adsorbent with organic sensitizers is known to diminish the undesirable formation of dye aggregates on the TiO2 surface.
The results are reported in ESI (Table S5†). We achieved quite comparable performances without CDCA, indicating that aggregation phenomenon is rather limited with these dyes. However, the solar cells show a slightly lower PCE, thus justifying the use of CDCA.
The external quantum efficiency (EQE) was measured for the different solar cells and the spectra are compared in Fig. 4 (J(V) curves are presented in Fig. S19†). The new dyes show a wider photo-response, which is extended towards higher wavelengths compared to YKP-88. The results confirm that the dyes embedding a furan spacer are less efficient to convert photons into electrons. This experiment also confirms the good photovoltaic behaviour of the thieno-thiophene based dyes and that MG-207 is the most efficient dye from this series. IPCE curves and integrated currents of DSSCs are reported in ESI.†
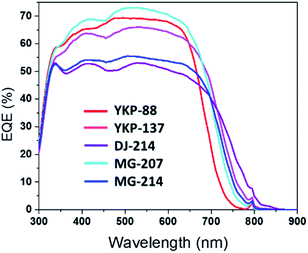 | ||
| Fig. 4 External Quantum Efficiency (EQE) spectra of solar cells fabricated with YKP-88, YKP-137, DJ-214, MG-207 and MG-214. | ||
To summarize, with an iodine-containing liquid electrolyte, the new compounds YKP-137 and MG-207 exhibit efficiencies quite comparable to YKP-88, while the inclusion of a furan spacer in the chemical structure of DJ-214 and MG-214 appears to be clearly detrimental to the performances. When tested with an ionic liquid-based electrolyte, all the dyes yield Jsc values over 15.4 mA cm−2 and two of them i.e.DJ-214 and MG-207 exhibit a PCE over 7.3%. As previously observed, the devices sensitized by the dyes embedding a furan spacer possess lower Voc (decreased by 30–60 mV). It should be noticed that, with PCEs comprised between 6.52% and 7.52%, our best solar cells rank amongst the most efficient using ionic liquid electrolytes.44–46 Besides, these devices are quite robust. For the evaluation of their stability, we subjected them to the standard ISOS-L2 ageing test.47 This test consists of continuous irradiation in a solar simulator with a light intensity of 1000 W m−2 at 65 °C with ambient relative humidity.47 The DSSCs were epoxy-encapsulated and protected with a UV-absorbing polymer (400 nm cut-off). Under these conditions, we found that the solar cells retain more than 85% of their initial performances after 1000 hours. One of the dyes, YKP-88, was then subjected to this test for a longer period. Interestingly, the corresponding device demonstrated a high stability and kept 80% of its initial PCE after 6984 hours (291 days). This T80 corresponds approximately to 7 years in real conditions (see ESI†).
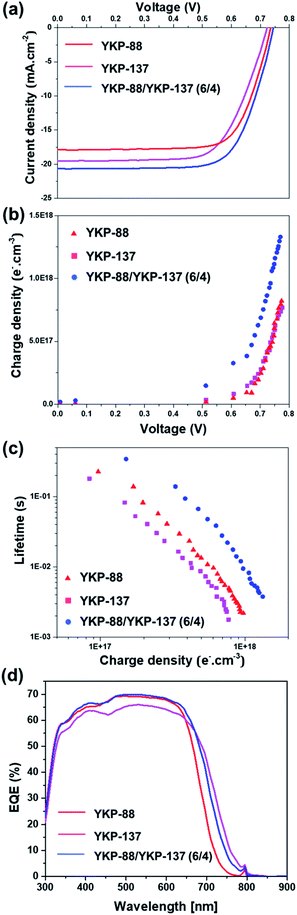
This choice was motivated by their close chemical structure, their good performances and the possibility to benefit from an “umbrella effect” thanks to the alkoxy-groups borne by YKP-137. Indeed, bulky substituents on the TPA unit give rise to a physical protection of the TiO2 surface and prevent the redox electrolyte to recombine with the electrons. The co-sensitized DSSCs were fabricated using dyeing baths containing 0.5 mM of YKP-88/YKP-137 with ratio varying from 8/2 to 2/8 and 5 mM of CDCA. For all compositions, an improvement of the performance was observed with respect to the solar cells sensitized with a single dye (see Table S2 in ESI†). The best performance was achieved for a 6/4 ratio of YKP-88/YKP-137, for which a Jsc of 20.66 mA cm−2, a FF of 71%, a Voc of 745 mV and a PCE of 10.9% was obtained (Fig. 5a).
As expected, the co-sensitization method is an efficient strategy to improve the Jsc because the absorption of the photoelectrode is wider with two dyes, but more interestingly, the Voc is also improved.17 In a DSSC, the Voc is known to correlate with the conduction band edge (Ec) of the semiconductor (TiO2) and the electron density. The latter is in itself dependent on the rate of recombination between TiO2 electrons and oxidized electrolyte species. To shed light on the origin of this improvement, charge extraction (CE) and transient photovoltage (TPV) measurements were conducted for the different cells. CE data (Fig. 5b) shows that the co-sensitized device has a higher charge density with respect to the YKP-88 and the YKP-137-based solar cells. This indicates a TiO2 conduction band shift after co-sensitization compared to other devices. Electron lifetimes measured at identical electron densities are in good agreement with the cell voltages. The electron lifetime (Fig. 5c) for the co-sensitized device is longer than for the solar cells sensitized with a single dye. This suggests a lower propensity for oxide electrons to recombine with redox species in the electrolyte. The benefits of the co-sensitization are also confirmed by the IPCE measurements that clearly show the contribution of the two dyes with an increase in the photon-to-electron conversion efficiency and the extension of the photo-response of the cells towards near-infra red region (Fig. 5d).
2.3 Fabrication and characterization of mini-modules
One goal of this work was to demonstrate the potential of the new dyes for the fabrication of larger area semi-transparent solar cells. Therefore, we fabricated semi-transparent mini-modules with a 23 cm2 overall surface area and an active area of 14.08 cm2 thus representing around 61% of the total area. The mini-modules constitute of five rectangular-shaped cells that are inter-connected in series using a W-type design.49 In order to achieve a good photovoltaic performance while keeping an acceptable semi-transparency in the visible, the thickness of the titania electrode was kept at 7–8 μm without scattering layer.YKP-88 dye was selected as the photosensitizer because of its good performance and stability in laboratory testing devices and the well-known ruthenium dye N-719 was employed as a ref. 50.
The manufacturing was done at Solaronix and is fully described in ESI. The YKP-88 mini-module shows an aesthetic burgundy red tint and possesses an average visible transmittance (AVT) of 26% measured between 380 nm and 740 nm. The first prototype of this 23 cm2 mini-module showed a current, Isc of 58.1 mA, a Voc of 3.63 V, and a FF of 58.26% leading to a PCE of 8.73% and a power output of 122.9 mW when measured under 1 Sun. This corresponds to a surface power density of 53.4 W m−2 (Fig. 6).
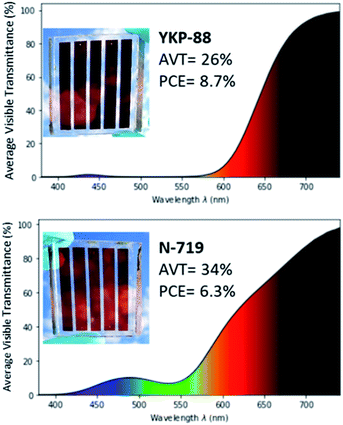 | ||
| Fig. 6 AVT spectrum of an individual cell from the mini-module, picture and performances for the mini-module fabricated with YKP-88 (top) and N-719 (bottom). Figures plotted on python using colour science library.51 | ||
These performances are significantly higher than the ones obtained for the reference N-719 mini-module that exhibited a current, Isc, of 51.8 mA, a Voc of 3.52 V, and a FF of 48.5% leading to a PCE of 6.3% and a power output of 88.5 mW. This device is more transparent in the blue and green region, and shows an AVT of 34%. This may explain the lower Isc and power conversion performance.
Conclusions
We have designed, synthesised, and characterized four new organic dyes for DSSCs application, and compared their performances with our recently reported reference dye YKP-88. The new photosensitizers reveal pink to violet-blue hues and demonstrate PCEs comprised between 7% and 9.8% when used with an iodine-based liquid electrolyte. When an ionic liquid-based electrolyte is employed, the solar cells exhibit PCEs comprised between 6.52% and 7.52%, and retain more than 85% of their initial performance after 1000 hours under ISOS-L2 accelerated ageing conditions. Using one of these molecules i.e.YKP-137 in combination with YKP-88 in a co-sensitization approach, the PCEs of the solar cells are improved up to 10.9% and we demonstrate that better photo-injection of electrons and lower recombination rates are responsible for the simultaneous increase of the Jsc and Voc.Finally, we demonstrate that YKP-88 can be successfully incorporated in solar mini-modules with an active area of 14 cm2 (total area 23 cm2), exhibiting a transparency in the visible of 26% and a power output of circa 123 mW corresponding to a surface power of 53.4 W m−2. This work highlights the potential of this new generation of organic dyes for future applications in semi-transparent solar cells.
Author's contribution
M. G., D. J. and Y. K. performed the synthesis and characterization of the dyes. M. G., S. N., V. M. M. and F. O. fabricated and characterized the solar cells and mini-modules. J. L. performed the DFT calculation and characterized the mini-modules optically. C. A., L. C., and E. P. performed the photo-physical characterization of the solar cells. C. A. G. F. and G. O. carried out a preliminary study on device optimization. Q. H. analysed the data and prepared the first draft of the manuscript. R. D. designed the materials and experiments, supervised the work, analysed the data and wrote the manuscript with contributions of all the co-authors.Conflicts of interest
R. D. and Y. K. are employees of CEA, which holds a patent on this technology (Inventors: R. D., D. J., M. G. and Y. K.; current assignee: Commissariat à l’Energie Atomique et aux Energies Alternatives; number: WO2017194368A1; date of publication: 16/11/2017). S. N is currently an employee of Solaronix that holds a license on this technology and that sells electrodes and chemical components that were used in this study.Acknowledgements
R. D. acknowledges ANR for funding through the ODYCE project (grant agreement number ANR-14-OHRI-0003-01). J. L. acknowledges CEA for funding through a CFR PhD grant. R. D. acknowledges the European Research Council (ERC) for funding. This work was partially funded under the European Union's Horizon 2020 research and innovation programme (grant agreement number 832606; project PISCO). E. P. acknowledges the ICIQ and ICREA for financial support. R. D. acknowledges the LABEX Laboratoire d'Alliances Nanosciences-Energies du Futur (LANEF, ANR-10-LABX-51-44001) for funding and the Hybriden Facility at CEA-Grenoble. C. A. acknowledges the UGA and EDCSV for funding. G. O. acknowledges funding from Conacyt under grant number CB_2017-2018_A1-S-21018. R. D. thanks Jacques Pécaut for solving the X-Ray crystal structures presented in ESI and José Sanchez for his help in measuring the IPCE spectra.Notes and references
- B. Hallam, et al., Eliminating Light-Induced Degradation in Commercial p-Type Czochralski Silicon Solar Cells, Applied Science, 2018, 8, 10 CrossRef.
- S. Pizzini, Towards solar grade silicon: Challenges and benefits for low cost photovoltaics, Sol. Energy Mater. Sol. Cells, 2010, 94, 1528–1533 CrossRef CAS.
- C.-T. Li, Y.-L. Kuo, C. P. Kumar, P.-T. Huang and J. T. Lin, Tetraphenylethylene tethered phenothiazine-based double-anchored sensitizers for high performance dye-sensitized solar cells, J. Mater. Chem. A, 2019, 7, 23225–23233 RSC.
- Y. Ren, et al., A Blue Photosensitizer Realizing Efficient and Stable Green Solar Cells via Color Tuning by the Electrolyte, Adv. Mater., 2020, 32, 2000193 CrossRef CAS.
- P. Cheng, G. Li, X. Zhan and Y. Yang, Next-generation organic photovoltaics based on non-fullerene acceptors, Nat. Photonics, 2018, 12, 131–142 CrossRef CAS.
- L. Meng, et al., Organic and solution-processed tandem solar cells with 17.3% efficiency, Science, 2018, 361, 1094–1098 CrossRef CAS.
- S. A. Hashemi, S. Ramakrishna and A. G. Aberle, Recent progress in flexible–wearable solar cells for self-powered electronic devices, Energy Environ. Sci., 2020, 13, 685–743 RSC.
- A. Cannavale, et al., Perovskite photovoltachromic cells for building integration, Energy Environ. Sci., 2015, 8, 1578–1584 RSC.
- X. Lin, et al., Efficiency progress of inverted perovskite solar cells, Energy Environ. Sci., 2020 10.1039/D0EE02017F.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Solar cell efficiency tables (version 47), Prog. Photovoltaics, 2016, 24, 3–11 Search PubMed.
- K. Kakiage, et al., Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes, Chem. Commun., 2015, 51, 15894–15897 RSC.
- S. Mathew, et al., Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers, Nat. Chem., 2014, 6, 242–247 CrossRef CAS.
- Z. Yao, et al., Dithienopicenocarbazole as the kernel module of low-energy-gap organic dyes for efficient conversion of sunlight to electricity, Energy Environ. Sci., 2015, 8, 3192–3197 RSC.
- Y. Ren, et al., A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency, J. Am. Chem. Soc., 2018, 140, 2405–2408 CrossRef CAS.
- J.-M. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno[3,2-b]indole-Based Organic Dyes with a Promising Porphyrin Sensitizer, Adv. Energy Mater., 2020, 10, 2000124 CrossRef CAS.
- D. Joly, et al., A Robust Organic Dye for Dye Sensitized Solar Cells Based on Iodine/Iodide Electrolytes Combining High Efficiency and Outstanding Stability, Sci. Rep., 2014, 4, 4033 CrossRef CAS.
- P. Wang, et al., Stable and Efficient Organic Dye-Sensitized Solar Cell Based on Ionic Liquid Electrolyte, Joule, 2018, 2, 2145–2153 CrossRef CAS.
- A. Fakharuddin, R. Jose, T. M. Brown, F. Fabregat-Santiago and J. Bisquert, A perspective on the production of dye-sensitized solar modules, Energy Environ. Sci., 2014, 7, 3952–3981 RSC.
- H. Yuan, et al., Outdoor testing and ageing of dye-sensitized solar cells for building integrated photovoltaics, Sol. Energy, 2018, 165, 233–239 CrossRef CAS.
- S. Yoon, S. Tak, J. Kim, Y. Jun, K. Kang and J. Park, Application of transparent dye-sensitized solar cells to building integrated photovoltaic systems, Building and Environment, 2011, 46, 1899–1904 CrossRef.
- D. Joly, et al., Metal-free organic sensitizers with narrow absorption in the visible for solar cells exceeding 10% efficiency, Energy Environ. Sci., 2015, 8, 2010–2018 RSC.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Mueller, P. Liska, N. Vlachopoulos and M. Graetzel, Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline titanium dioxide electrodes, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- S. Aghazada and M. K. Nazeeruddin, Ruthenium Complexes as Sensitizers in Dye-Sensitized Solar Cells, Inorganics, 2018, 6, 52 CrossRef.
- A. Mishra, M. K. R. Fischer and P. Bäuerle, Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS.
- C.-P. Lee, et al., Recent progress in organic sensitizers for dye-sensitized solar cells, RSC Adv., 2015, 5, 23810–23825 RSC.
- S. P. Singh and T. Gayathri, Evolution of BODIPY Dyes as Potential Sensitizers for Dye-Sensitized Solar Cells, Eur. J. Org. Chem., 2014, 4689–4707 CrossRef CAS.
- C. Aumaitre, et al., Visible and near-infrared organic photosensitizers comprising isoindigo derivatives as chromophores: synthesis, optoelectronic properties and factors limiting their efficiency in dye solar cells, J. Mater. Chem. A, 2018, 6, 10074–10084 RSC.
- H.-L. Jia, et al., Efficient cosensitization of new organic dyes containing bipyridine anchors with porphyrins for dye-sensitized solar cells, Sustainable Energy Fuels, 2019, 4, 347–353 RSC.
- J. Yang, et al., Efficient and stable organic DSSC sensitizers bearing quinacridone and furan moieties as a planar π-spacer, J. Mater. Chem., 2012, 22, 24356–24365 RSC.
- L. Xu, et al., Increasing the Efficiency of Organic Dye-Sensitized Solar Cells over 10.3% Using Locally Ordered Inverse Opal Nanostructures in the Photoelectrode, Adv. Funct. Mater., 2018, 28, 1706291 CrossRef.
- S. Qu, et al., New diketo-pyrrolo-pyrrole (DPP) sensitizer containing a furan moiety for efficient and stable dye-sensitized solar cells, Dyes Pigm., 2012, 92, 1384–1393 CrossRef CAS.
- A. Covezzi, et al., 4D–π–1A type β-substituted ZnII-porphyrins: ideal green sensitizers for building-integrated photovoltaics, Chem. Commun., 2016, 52, 12642–12645 RSC.
- R. Venkatraman, S. V. K. Panneer, E. Varathan and V. Subramanian, Aromaticity–Photovoltaic Property Relationship of Triphenylamine-Based D-π-A Dyes: Leads from DFT Calculations, J. Phys. Chem. A, 2020, 124, 3374–3385 CrossRef CAS.
- W. Sharmoukh, J. Cong, B. A. Ali, N. K. Allam and L. Kloo, Comparison between Benzothiadizole–Thiophene- and Benzothiadizole–Furan-Based D–A−π–A Dyes Applied in Dye-Sensitized Solar Cells: Experimental and Theoretical Insights, ACS Omega, 2020, 5, 16856–16864 CrossRef CAS.
- W. Zhang, et al., Rational Molecular Engineering of Indoline-Based D-A-π-A Organic Sensitizers for Long-Wavelength-Responsive Dye-Sensitized Solar Cells, ACS Appl. Mater. Interfaces, 2015, 7, 26802–26810 CrossRef CAS.
- G. A. Sewvandi, et al., Interplay between Dye Coverage and Photovoltaic Performances of Dye-Sensitized Solar Cells Based on Organic Dyes, J. Phys. Chem. C, 2014, 118, 20184–20192 CrossRef CAS.
- T. A. Oudenhoven, Y. Joo, J. E. Laaser, P. Gopalan and M. T. Zanni, Dye aggregation identified by vibrational coupling using 2D IR spectroscopy, J. Chem. Phys., 2015, 142, 212449 CrossRef.
- A. F. Buene, N. Boholm, A. Hagfeldt and B. H. Hoff, Effect of furan π-spacer and triethylene oxide methyl ether substituents on performance of phenothiazine sensitizers in dye-sensitized solar cells, New J. Chem., 2019, 43, 9403–9410 RSC.
- H. Song, X. Li, H. Ågren and Y. Xie, Branched and linear alkoxy chains-wrapped push-pull porphyrins for developing efficient dye-sensitized solar cells, Dyes Pigm., 2017, 137, 421–429 CrossRef CAS.
- F. Lu, et al., Novel D–π–A porphyrin dyes with different alkoxy chains for use in dye-sensitized solar cells, Dyes Pigm., 2016, 125, 116–123 CrossRef CAS.
- A. Yella, et al., Molecular Engineering of a Fluorene Donor for Dye-Sensitized Solar Cells, Chem. Mater., 2013, 25, 2733–2739 CrossRef CAS.
- H. Jia, X. Ju, M. Zhang, Z. Ju and H. Zheng, Effects of heterocycles containing different atoms as π-bridges on the performance of dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2015, 17, 16334–16340 RSC.
- L. Cabau, et al., A single atom change “switches-on” the solar-to-energy conversion efficiency of Zn-porphyrin based dye sensitized solar cells to 10.5%, Energy Environ. Sci., 2015, 8, 1368–1375 RSC.
- M. Gorlov and L. Kloo, Ionic liquid electrolytes for dye-sensitized solar cells, Dalton Trans., 2008, 2655–2666, 10.1039/B716419J.
- Y. Fang, et al., Synthesis of Low-Viscosity Ionic Liquids for Application in Dye-Sensitized Solar Cells, Chem.–Asian J., 2019, 14, 4201–4206 CrossRef CAS.
- W. Zeng, et al., Efficient Dye-Sensitized Solar Cells with an Organic Photosensitizer Featuring Orderly Conjugated Ethylenedioxythiophene and Dithienosilole Blocks, Chem. Mater., 2010, 22, 1915–1925 CrossRef CAS.
- M. V. Khenkin, et al., Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures, Nat. Energy, 2020, 5, 35–49 CrossRef.
- J. M. Cole, G. Pepe, O. K. Al Bahri and C. B. Cooper, Cosensitization in Dye-Sensitized Solar Cells, Chem. Rev., 2019, 119, 7279–7327 CrossRef CAS.
- F. Giordano, E. Petrolati, T. M. Brown, A. Reale and A. Di Carlo, Series-Connection Designs for Dye Solar Cell Modules, IEEE Trans. Electron Devices, 2011, 58, 2759–2764 CAS.
- M. Grätzel, Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells, Inorg. Chem., 2005, 44, 6841–6851 CrossRef.
- T. Mansencal, et al., Colour 0.3.15, 2020, DOI:10.5281/zenodo.3627408.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, spectroscopy, modelling, solar cells. CCDC 2030933 and 2030934. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0se01345e |
| This journal is © The Royal Society of Chemistry 2021 |