Open Access Article
Open Access ArticleEnantioselective aerobic oxidative cross-dehydrogenative coupling of glycine derivatives with ketones and aldehydes via cooperative photoredox catalysis and organocatalysis†
Xiaorong
Yang
,
Zhixiang
Xie
 ,
Ying
Li
,
Ying
Li
 and
Yuan
Zhang
and
Yuan
Zhang
 *
*
State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, 222 Tianshui South Road, Lanzhou 730000, P. R. China. E-mail: zhangyuan@lzu.edu.cn
First published on 18th April 2020
Abstract
The combination of photoredox catalysis and enamine catalysis has enabled the development of an enantioselective aerobic oxidative cross-dehydrogenative coupling between glycine derivatives and simple ketones or aldehydes, which provides an efficient approach for the rapid synthesis of enantiopure unnatural α-alkyl α-amino acid derivatives in good yield with excellent diastereo- (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (up to 97% ee). This process includes the direct photoinduced oxidation of glycine derivatives to an imine intermediate, followed by the asymmetric Mannich-type reaction with an enamine intermediate generated in situ from a ketone or aldehyde and a chiral secondary amine organocatalyst. This mild method allows the direct formation of a C–C bond with simultaneous installation of two new stereocenters without wasteful removal of functional groups.
1) and enantioselectivities (up to 97% ee). This process includes the direct photoinduced oxidation of glycine derivatives to an imine intermediate, followed by the asymmetric Mannich-type reaction with an enamine intermediate generated in situ from a ketone or aldehyde and a chiral secondary amine organocatalyst. This mild method allows the direct formation of a C–C bond with simultaneous installation of two new stereocenters without wasteful removal of functional groups.
Introduction
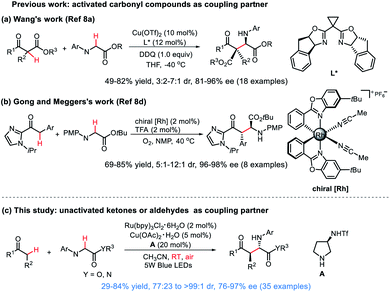
The oxidative cross-dehydrogenative coupling (CDC) reaction has emerged rapidly as one of the most straightforward and atom-economical strategies for forming C–C bonds through the direct coupling of two C(sp3)–H bonds, as it does not require additional prefunctionalization of substrates, and thus largely reduces the number of reaction steps.1–3 Among these reactions, pioneered by Li and co-workers,4 the direct α-C(sp3)–H functionalization of readily available glycine derivatives has proved to be a powerful tool for the rapid synthesis of unnatural α-substitued α-amino acids which have significant applications in the synthesis of biologically active natural products and peptides,5 and therefore received intense attention in recent years.6,7 Despite the advances, the stereocontrol of such transformations is highly challenging,8 mainly because the C(sp3)–H oxidation process commonly requires harsh reaction conditions (e.g. stoichiometric amounts of strong oxidants, high temperature), which are often incompatible with the chiral catalyst system. The carbonyl moiety remains among the most utilized functional groups in organic chemistry. The direct enantioselective coupling of carbonyl compounds9 with glycine derivatives has long been considered an elusive goal for practitioners of asymmetric catalysis. In 2011, Wang et al. reported a pioneering work on asymmetric CDC reaction of glycine esters with β-ketoesters in the presence of a chiral bisoxazoline/Cu(II) catalyst, producing various α-alkyl α-amino acids with good enantioselectivities (Scheme 1a).8a However, poor diastereoselectivities were obtained; moreover, stoichiometric amounts of strong oxidant (DDQ) was needed to complete the transformation. Recently, the Gong and Meggers research group reported an elegant oxidative asymmetric catalytic CDC reaction of 2-acyl imidazoles with glycine ester by using molecular oxygen as the terminal oxidant (Scheme 1b).8d However, the report was limited to only a single glycine ester.Moreover, both of these two reports are restricted to the use of highly activated carbonyl compounds as nucleophilic partners. Huang and Xie had explored the development of an asymmetric CDC reaction between glycine ester with acetone mediated by TBHP under cooperative transition metal/chiral amine catalysis, however, either the enantioselectivity (15% ee) or yield (47%) was poor.10 So far, to our knowledge, a catalytic enantioselective CDC reaction of glycine derivatives with simple ketones or aldehydes which proceeds with both high diastereo- and enantioselectivity is unprecedented. In this respect, we wish to develop the first direct catalytic enantioselective CDC reaction of glycine derivatives with simple ketones or aldehydes (Scheme 1c). This atom economic transformation provides an efficient enantio- and diastereoselective access to chiral α-alkyl α-amino acids under mild conditions.
In the past few years, visible-light photoredox catalysis has attracted increasing interest in organic synthesis community, owing to its mild, clean, easy to handle, and environmentally benign characteristics.11 Recently visible-light photoredox catalysis has emerged as a promising tool to trigger the CDC reaction through single-electron transfer (SET) pathways.12–14 Despite a few recent successes have verified the feasibility of visible-light induced enantioselective CDC reaction,15 however, such photocatalytic CDC reactions are mostly focused on the asymmetric oxidative coupling of α-C(sp3)–H bonds in cyclic amines (such as tetrahydroisoquinolines, tetrahydro-β-carbolines and 3,4-dihydroquinoxalin-2-ones). To date, to the best of our knowledge, there have been no reports on an asymmetric photoredox catalytic α-C(sp3)–H functionalization of glycine derivatives, probably due to the poor reactivity and difficulty of controlling the stereoselectivity. With our continuous interests in the visible light-driven α-C(sp3)–H functionalization of glycine derivatives,16 herein, we describe the first visible-light-induced highly enantioselective oxidative cross coupling of glycine derivatives with simple ketones and aldehydes.
Results and discussion
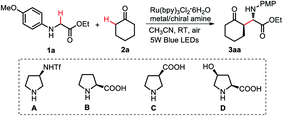
This new visible light-induced enantioselective CDC protocol was first examined using glycine ester (1a) and cyclohexanone (2a) as the model substrates, along with photocatalyst Ru(bpy)3Cl2·6H2O, chiral amine A and Cu(OAc)2·H2O, under the irradiation of a 5 W blue LED bulb (Table 1, for details see ESI†). After preliminary manipulation of the reaction conditions, we were pleased to find that the desired asymmetric CDC reaction could be realized in the presence of 10 mol% Cu(OAc)2·H2O and 20 mol% chiral amine A, to provide the coupling product 3aa with 76% yield and excellent enantioselectivity (Table 1, entry 1, 96% ee). Encouraged by this result, we next screened a range of chiral amine catalysts (Table 1, entries 1–4) and Lewis acids (Table 1, entries 5–8). The results indicated that chiral amine A and Cu(OAc)2·H2O are the optimal catalysts for this transformation (Table 1, entry 1). Different photocatalysts and solvents were also screened (for details, see ESI†). The results indicated that the reactions with Ru(bpy)3Cl2·6H2O as the photocatalyst in CH3CN performed the best in terms of yield and ee value. Moreover, decreasing the Ru(bpy)3Cl2·6H2O loading to 2 mol% (Table 1, entry 9) or decreasing the Cu(OAc)2·H2O loading to 5 mol% (Table 1, entry 10) resulted in a slightly increase in both efficiency and enantioselectivity. In addition, a similar outcome was obtained when the reaction was conducted under O2 (Table 1, entry 12). Finally, control experiments revealed that both the photocatalyst and Cu(OAc)2·H2O were essential to this reaction (Table 1, entries 13 and 14), and no reaction occurred in dark or under argon conditions (Table 1, entries 15 and 16).| Entry | Amine/metal | Yieldb (%) | d.r.c (anti/syn) | eed (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), Ru(bpy)3Cl2·6H2O (5 mol%), Lewis acid (10 mol%), CH3CN (2.0 mL), 5 W blue LEDs light irradiation under air at room temperature for 2 h, then 2a (0.5 mmol) and chiral amine catalyst (20 mol%) were added. b Combined yield of isolated 3aa (syn and anti). c Determined by HPLC on a chiral stationary phase. d Determined by HPLC on a chiral stationary phase. e 2 mol% of Ru(bpy)3Cl2·6H2O was used. f 5 mol% of Cu(OAc)2·H2O was used. g 1.5 mL CH3CN was used. h 10 mol% of A was used. i Reaction was carried out under O2 atmosphere. j Reaction was performed without Ru(bpy)3Cl2·6H2O. k Reaction was carried out in the dark. l Reaction was carried out under argon. n.d. = not determined. n.r. = no reaction. | ||||
| 1 | A/Cu(OAc)2·H2O | 76 | 97/3 | 96 |
| 2 | B/Cu(OAc)2·H2O | n.d. | — | — |
| 3 | C/Cu(OAc)2·H2O | 57 | 98/2 | 88 |
| 4 | D/Cu(OAc)2·H2O | n.d. | — | — |
| 5 | A/Zn(OAc)2 | n.d. | — | — |
| 6 | A/Cu(OTf)2 | 63 | 98/2 | 86 |
| 7 | A/CuSO4 | 68 | 97/3 | 92 |
| 8 | A/CuI | 63 | 97/3 | 92 |
| 9e | A/Cu(OAc)2·H2O | 80 | 97/3 | 96 |
| 10e,f,g | A/Cu(OAc)2·H2O | 81 | 98/2 | 97 |
| 11e,f,h | A/Cu(OAc)2·H2O | 47 | 95/5 | 94 |
| 12e,f,g,i | A/Cu(OAc)2·H2O | 82 | 98/2 | 97 |
| 13j | A/Cu(OAc)2·H2O | n.r. | — | — |
| 14 | A/- | n.d. | — | — |
| 15e,f,k | A/Cu(OAc)2·H2O | n.r. | — | — |
| 16e,f,l | A/Cu(OAc)2·H2O | n.r. | — | — |
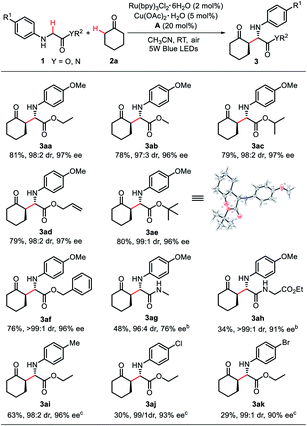
Having identified the optimal reaction conditions, we next investigated the scope of glycine derivative component in this new visible-light induced enantioselective CDC protocol. As revealed in Scheme 2, various glycine esters, such as methyl ester, isopropyl ester, allyl ester, t-butyl ester and benzyl ester, reacted well with cyclohexanone (2a) under the optimized conditions, and the corresponding coupling products 3aa–3af were provided in good yields (76–81%) with excellent diastereo- and enantioselectivities (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to >99
3 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 96–97% ee). In addition to glycine esters, glycine amide and glycine derived dipeptide can also be utilized in this transformation, providing the corresponding products 3ag–3ah in moderate yields (34–48%) with good diastereo- and enantioselectivities (96
1 dr, 96–97% ee). In addition to glycine esters, glycine amide and glycine derived dipeptide can also be utilized in this transformation, providing the corresponding products 3ag–3ah in moderate yields (34–48%) with good diastereo- and enantioselectivities (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to >99
4 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 76–91% ee). Glycine esters with variations on the phenyl moiety were also suitable for this transformation and gave the products 3ai–3ak in moderate yield (29–63%) and excellent diastereo- and enantioselectivity (98
1 dr, 76–91% ee). Glycine esters with variations on the phenyl moiety were also suitable for this transformation and gave the products 3ai–3ak in moderate yield (29–63%) and excellent diastereo- and enantioselectivity (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 99
2 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 90–96% ee). The absolute configuration was determined by single-crystal X-ray diffraction of 3ae.17
1 dr, 90–96% ee). The absolute configuration was determined by single-crystal X-ray diffraction of 3ae.17
Next, the substrate scope of the ketone component was explored (Scheme 3). We first explored the desymmetrization process of 4-substituted cyclohexanones. It is difficult to simultaneously control the diastereo- and enantioselectivities of C2 and C4 position. Gratifyingly, all of the substrates gave the desired coupling products in good yields with excellent dr and ee values (3ba–3bc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 97
5 to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr, 97% ee). Meanwhile, 4-ketalized cyclohexa-1,4-diones, 3,3-difluorocyclohexanone and 3,3-dimethylcyclohexanone were all excellent substrates for this transformation with good to excellent dr and ee values (3bd–3bg, 89
3 dr, 97% ee). Meanwhile, 4-ketalized cyclohexa-1,4-diones, 3,3-difluorocyclohexanone and 3,3-dimethylcyclohexanone were all excellent substrates for this transformation with good to excellent dr and ee values (3bd–3bg, 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 to 97
11 to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr, 77–96% ee). Furthermore, in addition to cyclohexanones, other cyclic ketones, such as tetrahydrothiapyrone, tetrahydropyranone and cycloheptanone can also be utilized in this transformation, providing the corresponding products 3bh–3bj in good yields with high dr and ee values (88
3 dr, 77–96% ee). Furthermore, in addition to cyclohexanones, other cyclic ketones, such as tetrahydrothiapyrone, tetrahydropyranone and cycloheptanone can also be utilized in this transformation, providing the corresponding products 3bh–3bj in good yields with high dr and ee values (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to 98
12 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr, 96–97% ee). Acyclic ketones had also been examined to give the desired adducts (3bk–3bl) with excellent enantioselectivities but lower yields. In particular, 2-butanone was found to undergo oxidative cross coupling regioselectively at the ethyl moiety, and no other isomer was observed. The poor yield was due to the unknown byproducts.
2 dr, 96–97% ee). Acyclic ketones had also been examined to give the desired adducts (3bk–3bl) with excellent enantioselectivities but lower yields. In particular, 2-butanone was found to undergo oxidative cross coupling regioselectively at the ethyl moiety, and no other isomer was observed. The poor yield was due to the unknown byproducts.
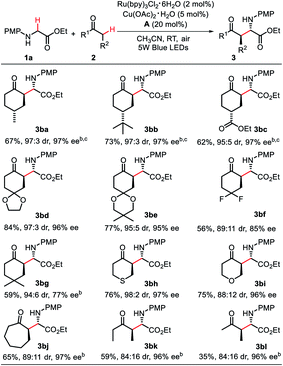 | ||
| Scheme 3 Scope of the ketone component. a Reaction conditions: 1a (0.2 mmol), Ru(bpy)3Cl2·6H2O (2 mol%), Cu(OAc)2·H2O (5 mol%), CH3CN (3 mL), 5 W blue LEDs light irradiation under air at room temperature for 2 h, then, A (20 mol%) and 2 (1 mmol) were added. Yield is that of the product isolated by flash column chromatography on silica gel. The ee and dr were determined by HPLC. b 30 mol% A was used. c dr: ratio of the shown stereoisomer with all the other isomers, for details, see ESI.† | ||
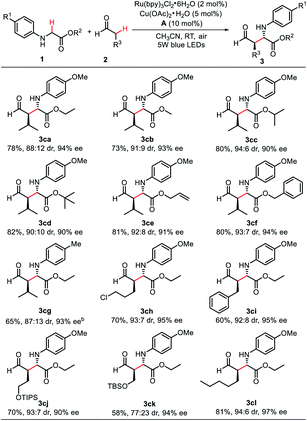
The scope of the aldehyde component in this protocol has also been investigated. As indicated in Scheme 4, an array of glycine esters reacted well with isovaleraldehyde under the optimized conditions (10 mol% A was used), leading to the desired products (3ca–3cg) in 65–82% yields with good to excellent dr and ee values (87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 to 94
13 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr, 90–94% ee). A broad array of aliphatic aldehydes smoothly underwent this transformation, generating the desired coupling products 3ch–3cl in moderate to good yield (58–81%) with excellent ee values (90–97% ee). Additional chemical functionalities groups (e.g., chlorine, protected alcohols) were found to be inert to these mild conditions without a deleterious effect on either the efficiency or enantioselectivity, which give the potential synthetic utility for further modification.
6 dr, 90–94% ee). A broad array of aliphatic aldehydes smoothly underwent this transformation, generating the desired coupling products 3ch–3cl in moderate to good yield (58–81%) with excellent ee values (90–97% ee). Additional chemical functionalities groups (e.g., chlorine, protected alcohols) were found to be inert to these mild conditions without a deleterious effect on either the efficiency or enantioselectivity, which give the potential synthetic utility for further modification.
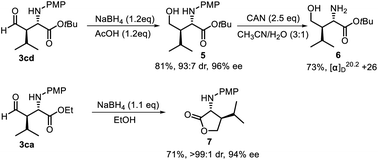
To further demonstrate the synthetic value of this visible light-induced asymmetric CDC process, transformations of the products were conducted (Scheme 5). In the presence of NaBH4 and AcOH, 3cd was transformed to alcohol 5 in 81% yield and 96% ee. Subsequently, the PMP group of 5 was removed by the treatment with ammonium cerium(IV) nitrate (CAN), leading to chiral amino alcohol 6 in 73% yield. In addition, 3ca could also be selectively transformed into chiral γ-butyrolactone derivative 7 in 71% yield and 94% ee (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) with NaBH4 as the reducing reagent.
1 dr) with NaBH4 as the reducing reagent.
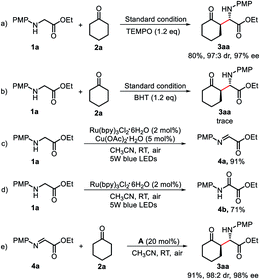
A series of control experiments were conducted to gain some insight into the mechanism of this reaction (Scheme 6). First, when the radical scavenger TEMPO (1.2 equiv.) was added to the standard reaction system, the reaction proceeded without interruption, both the yield and the stereoselectivity of the desired product 3aa was almost the same as those in standard conditions, and the radical captured by TEMPO was not detected either, indicating that this transformation was not a radical process. However, only trace product 3aa was observed when 2,6-di-tert-butyl-4-methylphenol (BHT) was added to the reaction. The observed BHT inhibition may be probably due to the preferential oxidization of the electron-rich phenol, hence suppressing the desired pathway. Next, upon irradiation of 1a with visible-light in the presence of Ru(bpy)3Cl2·6H2O and Cu(OAc)2·H2O, the imine 4a could be isolated in 91% yield. While in the absence of Cu(OAc)2·H2O, the oxidative product 4b was obtained in 71% yield, and only trace 4a could be observed. Subsequently, the obtained imine 4a could readily react with cyclohexanone under the catalysis of chiral amine A, affording 3aa in 91% yield (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr, 98% ee). These results revealed that 4a is a significant intermediate of this reaction. Moreover, Wu et al. recently reported that Cu(OTf)2–glycine complex could be acted as the photoredox species.18 We therefore carried out additional studies to explore the role of Cu(II) in this transformation. As shown in Fig. S1,† both substrate 1a and Cu(II) salt showed no absorption in the range of visible light, and no bathochromic shift was observed in the UV-vis spectrum of the mixture of 1a and Cu(II) salt (The Cu(II) salt used here refers to Cu(OAc)2·H2O, for details, see ESI†). In addition, no reaction was observed in the absence of Ru(bpy)3Cl2·6H2O (Table 1, entry 13). These results indicated that Cu(II)–glycine complex was not the photoredox species of this reaction. The role of Cu(II) in this transformation should be to suppress the formation of oxidative product 4b and stabilize the imine intermediate 4a as well.14c,19
2 dr, 98% ee). These results revealed that 4a is a significant intermediate of this reaction. Moreover, Wu et al. recently reported that Cu(OTf)2–glycine complex could be acted as the photoredox species.18 We therefore carried out additional studies to explore the role of Cu(II) in this transformation. As shown in Fig. S1,† both substrate 1a and Cu(II) salt showed no absorption in the range of visible light, and no bathochromic shift was observed in the UV-vis spectrum of the mixture of 1a and Cu(II) salt (The Cu(II) salt used here refers to Cu(OAc)2·H2O, for details, see ESI†). In addition, no reaction was observed in the absence of Ru(bpy)3Cl2·6H2O (Table 1, entry 13). These results indicated that Cu(II)–glycine complex was not the photoredox species of this reaction. The role of Cu(II) in this transformation should be to suppress the formation of oxidative product 4b and stabilize the imine intermediate 4a as well.14c,19
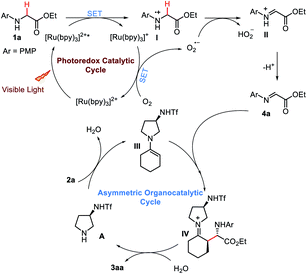
On the basis of these observations and the precedent literatures, a possible mechanism for this enantioselective photocatalytic CDC reaction was proposed (Scheme 7). Initially, the irradiation of [Ru(bpy)3]2+ with visible-light would render the *[Ru(bpy)3]2+ excited state, which should readily accept a single electron from 1a to produce [Ru(bpy)3]+ and the radical cation I. Rapid oxidation of [Ru(bpy)3]+ by O2 would then close the photoredox catalytic cycle while regenerating [Ru(bpy)3]2+. Meanwhile, the active species O2−˙ formed during this process may abstract a hydrogen atom from radical cation I to produce the iminium ion II, which then eliminates a proton and forms imine 4a. Within the same time frame, condensation of 2a with chiral amine A should form a nucleophilic enamine intermediate III, which would then intercept the electrophile 4a to enantioselectively forge the new C–C bond.20 Finally, subsequent hydrolysis of iminium intermediate IV would regenerate the organocatalyst A while providing the enantioenriched coupling product 3aa.
Conclusions
In conclusion, we have developed the first visible-light-induced asymmetric cross-dehydrogenative coupling between glycine derivatives and simple ketones or aldehydes through a novel cooperative photoredox catalysis and asymmetric organocatalysis. This operationally simple and mild protocol provides a powerful means for the synthesis of diverse chiral unnatural α-alkyl α-amino acid derivatives with good yield and excellent diastereo- (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (up to 97% ee). Significantly, the strategy was also successfully applied in the enantioselective alkylated modification of dipeptide. The process is noteworthy in that it represents an unprecedented report on visible-light photoredox catalytic C(sp3)–H asymmetric functionalization of glycine derivatives. Moreover, this protocol employs molecular oxygen as the sustainable oxidant, thereby avoids the undesired byproducts associated with typical oxidation processes. The further extension of substrate scope and application of this novel CDC process to the synthesis of complex molecules are in progress in our laboratory.
1) and enantioselectivities (up to 97% ee). Significantly, the strategy was also successfully applied in the enantioselective alkylated modification of dipeptide. The process is noteworthy in that it represents an unprecedented report on visible-light photoredox catalytic C(sp3)–H asymmetric functionalization of glycine derivatives. Moreover, this protocol employs molecular oxygen as the sustainable oxidant, thereby avoids the undesired byproducts associated with typical oxidation processes. The further extension of substrate scope and application of this novel CDC process to the synthesis of complex molecules are in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (No. 21572092) and Natural Science Foundation of Gansu Province (18JR4RA003) for financial support.Notes and references
- For reviews on CDC reactions, see: (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335–344 CrossRef PubMed; (b) C. J. Scheuermann, Chem. - Asian J., 2010, 5, 436–451 CrossRef PubMed; (c) M. Klussmann and D. Sureshkumar, Synthesis, 2011, 3, 353–369 CrossRef; (d) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed; (e) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780–1824 CrossRef CAS PubMed; (f) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464–3484 RSC; (g) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74–100 CrossRef CAS PubMed; (h) Y. Qin, L. Zhu and S. Luo, Chem. Rev., 2017, 117, 9433–9520 CrossRef CAS PubMed.
- For reviews on asymmetric CDC reactions, see: (a) C. Zheng and S.-L. You, RSC Adv., 2014, 4, 6173–6214 RSC; (b) Y. Qin, J. Lv and S. Luo, Tetrahedron Lett., 2014, 55, 551–558 CrossRef CAS; (c) Y.-L. Zhao, Y. Wang, Y.-C. Luo, X.-Z. Fu and P.-F. Xu, Tetrahedron Lett., 2015, 56, 3703–3714 CrossRef CAS.
- For selected examples on asymmetric CDC reactions, see: (a) Z. P. Li and C. J. Li, Org. Lett., 2004, 6, 4997–4999 CrossRef CAS PubMed; (b) C. Guo, J. Song, S.-W. Luo and L.-Z. Gong, Angew. Chem., Int. Ed., 2010, 49, 5558–5562 CrossRef CAS PubMed; (c) G. Zhang, Y. Ma, S. Wang, Y. Zhang and R. Wang, J. Am. Chem. Soc., 2012, 134, 12334–12337 CrossRef CAS PubMed; (d) A. J. Neel, J. P. Hehn, P. F. Tripet and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 14044–14047 CrossRef CAS PubMed; (e) X. Liu, Z. Meng, C. Li, H. Lou and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 6012–6015 CrossRef CAS PubMed; (f) S. Sun, C. Li, P. E. Floreancig, H. Lou and L. Liu, Org. Lett., 2015, 17, 1684–1687 CrossRef CAS PubMed; (g) T. Huang, X. Liu, J. Lang, J. Xu, L. Lin and X. Feng, ACS Catal., 2017, 7, 5654–5660 CrossRef CAS; (h) S. Biswas, K. Kubota, M. Orlandi, M. Turberg, D. H. Miles, M. S. Sigman and F. D. Toste, Angew. Chem., Int. Ed., 2018, 57, 589–593 CrossRef CAS PubMed; (i) A. Lee, R. C. Betori, E. A. Crane and K. A. Scheidt, J. Am. Chem. Soc., 2018, 140, 6212–6216 CrossRef CAS PubMed; (j) K. Mori, R. Isogai, Y. Kamei, M. Yamanaka and T. Akiyama, J. Am. Chem. Soc., 2018, 140, 6203–6207 CrossRef CAS PubMed.
- L. Zhao and C.-J. Li, Angew. Chem., Int. Ed., 2008, 47, 7075–7078 CrossRef CAS PubMed.
- J. X. Ji, J. Wu and A. S. C. Chan, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11196–11200 CrossRef CAS PubMed.
- For selected examples on the synthesis of α-amino acids via α-C(sp3)–H functionalization of glycine derivatives, see: (a) K. Li, G. Tan, J. Huang, F. Song and J. You, Angew. Chem., Int. Ed., 2013, 52, 12942–12945 CrossRef CAS PubMed; (b) C. Huo, Y. Yuan, M. Wu, X. Jia, X. Wang, F. Chen and J. Tang, Angew. Chem., Int. Ed., 2014, 53, 13544–13547 CrossRef CAS; (c) Z.-Q. Zhu, P. Bai and Z.-Z. Huang, Org. Lett., 2014, 16, 4881–4883 CrossRef CAS PubMed; (d) M. Salman, Z.-Q. Zhu and Z.-Z. Huang, Org. Lett., 2016, 18, 1526–1529 CrossRef CAS PubMed; (e) X. Jia, X. Liu, Y. Shao, Y. Yuan, Y. Zhu, W. Hou and X. Zhang, Adv. Synth. Catal., 2017, 359, 4399–4404 CrossRef CAS.
- For selected examples on the synthesis of azo-heterocyclic compounds via α-C(sp3)–H functionalization of glycine derivatives, see: (a) H. Richter and O. G. Mancheño, Org. Lett., 2011, 13, 6066–6069 CrossRef CAS PubMed; (b) X. Jia, F. Peng, C. Qing, C. Huo and X. Wang, Org. Lett., 2012, 14, 4030–4033 CrossRef CAS PubMed; (c) Y.-J. Li, X. Li, S.-X. Zhang, Y.-L. Zhao and Q. Liu, Chem. Commun., 2015, 51, 11564–11567 RSC; (d) X. Jia, Y. Zhu, Y. Yuan, X. Zhang, S. Lu, L. Zhang and L. Luo, ACS Catal., 2016, 6, 6033–6036 CrossRef CAS; (e) H. Li, S. Huang, Y. Wang and C. Huo, Org. Lett., 2018, 20, 92–95 CrossRef CAS PubMed; (f) J. Xie, Y. Huang, H. Song, Y. Liu and Q. Wang, Org. Lett., 2017, 19, 6056–6059 CrossRef CAS PubMed.
- (a) G. Zhang, Y. Zhang and R. Wang, Angew. Chem., Int. Ed., 2011, 50, 10429–10432 CrossRef CAS PubMed; (b) X.-H. Wei, G.-W. Wang and S.-D. Yang, Chem. Commun., 2015, 51, 832–835 RSC; (c) Z. Xie, X. Liu and L. Liu, Org. Lett., 2016, 18, 2982–2985 CrossRef CAS PubMed; (d) Y. Tan, W. Yuan, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2015, 54, 13045–13048 CrossRef CAS PubMed.
- For selected examples on asymmetric α-coupling of carbonyls, see: (a) F. Benfatti, M. G. Capdevila, L. Zoli, E. Benedetto and P. G. Cozzi, Chem. Commun., 2009, 39, 5919–5921 RSC; (b) B. Zhang, S.-K. Xiang, L.-H. Zhang, Y. Cui and N. Jiao, Org. Lett., 2011, 13, 5212–5215 CrossRef CAS PubMed; (c) J. Zhang, B. Tiwari, C. Xing, X. Chen and Y. R. Chi, Angew. Chem., Int. Ed., 2012, 51, 3649–3652 CrossRef CAS; (d) W. Cao, X. Liu, R. Peng, P. He, L. Lin and X. Feng, Chem. Commun., 2013, 49, 3470–3472 RSC; (e) G. Zhang, Y. Ma, S. Wang, W. Kong and R. Wang, Chem. Sci., 2013, 4, 2645–2651 RSC; (f) Z. Meng, S. Sun, H. Yuan, H. Lou and L. Liu, Angew. Chem., Int. Ed., 2014, 53, 543–547 CrossRef CAS PubMed; (g) Z. Xie, X. Zan, S. Sun, X. Pan and L. Liu, Org. Lett., 2016, 18, 3944–3947 CrossRef CAS PubMed.
- J. Xie and Z.-Z. Huang, Angew. Chem., Int. Ed., 2010, 49, 10181–10185 CrossRef CAS PubMed.
- (a) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (b) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828–6838 CrossRef CAS PubMed; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (d) N. Corrigan, S. Shanmugam, J. Xu and C. Boyer, Chem. Soc. Rev., 2016, 45, 6165–6212 RSC; (e) M. D. Kärkäs, J. A. Porco Jr and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef PubMed; (f) D. Ravelli, S. Protti and M. Fagnoni, Chem. Rev., 2016, 116, 9850–9913 CrossRef CAS PubMed.
- For an elegant review, see: L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687–7697 RSC.
- For selected examples on the photocatalytic α-C(sp3)–H functionalization of tertiary amines, see: (a) A. G. Condie, J. C. González-Gómez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464–1465 CrossRef CAS PubMed; (b) M. Rueping, C. Vila, R. M. Koenigs, K. Poscharny and D. C. Fabry, Chem. Commun., 2011, 47, 2360–2362 RSC; (c) Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052–19055 CrossRef CAS PubMed; (d) Z.-J. Feng, J. Xuan, X.-D. Xia, W. Ding, W. Guo, J.-R. Chen, Y.-Q. Zou, L.-Q. Lu and W.-J. Xiao, Org. Biomol. Chem., 2014, 12, 2037–2040 RSC; (e) X. Liu, X. Ye, F. Bures, H. Liu and Z. Jiang, Angew. Chem., Int. Ed., 2015, 54, 11443–11447 CrossRef CAS PubMed; (f) L. Niu, S. Wang, J. Liu, H. Yi, X.-A. Liang, T. Liu and A. Lei, Chem. Commun., 2018, 54, 1659–1662 RSC.
- For examples on the photocatalytic α-C(sp3)–H functionalization of secondary amines, see: (a) Z.-Q. Wang, M. Hu, X.-C. Huang, L.-B. Gong, Y.-X. Xie and J.-H. Li, J. Org. Chem., 2012, 77, 8705–8711 CrossRef CAS PubMed; (b) S. Zhu and M. Rueping, Chem. Commun., 2012, 48, 11960–11962 RSC; (c) X.-W. Gao, Q.-Y. Meng, M. Xiang, B. Chen, K. Feng, C.-H. Tung and L.-Z. Wu, Adv. Synth. Catal., 2013, 355, 2158–2164 CrossRef CAS; (d) X.-W. Gao, Q.-Y. Meng, J.-X. Li, J.-J. Zhong, T. Lei, X.-B. Li, C.-H. Tung and L.-Z. Wu, ACS Catal., 2015, 5, 2391–2396 CrossRef; (e) W. Dong, B. Hu, X. Gao, Y. Li, X. Xie and Z. Zhang, J. Org. Chem., 2016, 81, 8770–8776 CrossRef PubMed; (f) Y.-H. He, Y. Xiang, D.-C. Yang and Z. Guan, Green Chem., 2016, 18, 5325–5330 RSC; (g) C. Wang, M. Guo, R. Qi, Q. Shang, Q. Liu, S. Wang, L. Zhao, R. Wang and Z. Xu, Angew. Chem., Int. Ed., 2018, 57, 15841–15846 CrossRef PubMed.
- (a) D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2012, 134, 8094–8097 CrossRef PubMed; (b) G. Bergonzini, C. S. Schindler, C.-J. Wallentin, E. N. Jacobsen and C. R. J. Stephenson, Chem. Sci., 2014, 5, 112–116 RSC; (c) I. Perepichka, S. Kundu, Z. Hearne and C.-J. Li, Org. Biomol. Chem., 2015, 13, 447–451 RSC; (d) D. Uraguchi, N. Kinoshita, T. Kizu and T. Ooi, J. Am. Chem. Soc., 2015, 137, 13768–13771 CrossRef CAS PubMed; (e) G. Wei, C. Zhang, F. Bures, X. Ye, C.-H. Tan and Z. Jiang, ACS Catal., 2016, 6, 3708–3712 CrossRef CAS; (f) J. J. Murphy, D. Bastida, S. Paria, M. Fagnoni and P. Melchiorre, Nature, 2016, 532, 218–222 CrossRef CAS PubMed; (g) C. Wang, J. Qin, X. Shen, R. Riedel, K. Harms and E. Meggers, Angew. Chem., Int. Ed., 2016, 55, 685–688 CrossRef CAS PubMed; (h) E. Larionov, M. M. Mastandrea and M. A. Pericàs, ACS Catal., 2017, 7, 7008–7013 CrossRef CAS; (i) Q. Yang, L. Zhang, C. Ye, S. Luo, L.-Z. Wu and C.-H. Tung, Angew. Chem., Int. Ed., 2017, 56, 3694–3698 CrossRef CAS PubMed; (j) H. Hou, S. Zhu, I. Atodiresei and M. Rueping, Eur. J. Org. Chem., 2018, 2018, 1277–1280 CrossRef CAS; (k) J. Rostoll-Berenguer, G. Blay, M. Carmen Muñoz, J. R. Pedro and C. Vila, Org. Lett., 2019, 21, 6011–6015 CrossRef CAS PubMed.
- (a) X. Yang, L. Li, Y. Li and Y. Zhang, J. Org. Chem., 2016, 81, 12433–12442 CrossRef CAS PubMed; (b) S. Li, X. Yang, Y. Wang, H. Zhou, B. Zhang, G. Huang, Y. Zhang and Y. Li, Adv. Synth. Catal., 2018, 360, 4452–4456 CrossRef CAS; (c) Y. Zhang, X. Yang, H. Zhou, S. Li, Y. Zhu and Y. Li, Org. Chem. Front., 2018, 5, 2120–2125 RSC; (d) Y. He, B. Yan, H. Tao, Y. Zhang and Y. Li, Org. Biomol. Chem., 2018, 16, 3816–3823 RSC; (e) H. Zhou, X. Yang, S. Li, Y. Zhu, Y. Li and Y. Zhang, Org. Biomol. Chem., 2018, 16, 6728–6734 RSC.
- H. Zhang, M. Mifsud, F. Tanaka and C. F. Barbas III, J. Am. Chem. Soc., 2006, 128, 9630–9631 CrossRef CAS PubMed.
- Q.-Y. Meng, X.-W. Gao, T. Lei, Z. Liu, F. Zhan, Z.-J. Li, J.-J. Zhong, H. Xiao, K. Feng, B. Chen, Y. Tao, C.-H. Tung and L.-Z. Wu, Sci. Adv., 2017, 3, e1700666 CrossRef PubMed.
- Z. Li and C.-J. Li, J. Am. Chem. Soc., 2004, 126, 11810–11811 CrossRef CAS PubMed.
- For a recent elegant review on the asymmetric oxidative enamine transformations, see: L. Zhu, D. Wang, Z. Jia, Q. Lin, M. Huang and S. Luo, ACS Catal., 2018, 8, 5466–5484 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, optimization of reaction conditions, X-ray crystal structure for compound 3ae, and spectral data for all products. See DOI: 10.1039/d0sc00683a |
| This journal is © The Royal Society of Chemistry 2020 |