Pillared clay-supported gold catalysts for CO oxidation
Ling Liu,
Ying Gao,
Pingying Zhao,
Xuewen Wang,
Gang Feng* and
Rongbin Zhang*
Institute of Applied Chemistry, College of Chemistry, Nanchang University, No. 999 Xuefu Road, Nanchang 330031, P. R. China. E-mail: fenggang@ncu.edu.cn; rbzhang@ncu.edu.cn; Tel: +86 136 77080009
First published on 29th January 2016
Abstract
Gold supported on various bentonites was prepared using hydrothermal and co-precipitation methods. The catalysts were characterized by FT-IR, BET, XRD, TEM, SEM, XPS and ICP and tested for CO oxidation reaction. It was found that the Au–Al–Ce pillared bentonite, Au–Al–Ce/Na-LBen (CP) and Au–Al–Ce/Na-LBen (HT), have better catalytic activity and stability than Au/Na-Lben and Au/Al–Ce–Na-LBen. Characterization results show that gold nanoparticles could move into the interlayer spaces of bentonite, which contributes to the high stability of the Au–Al–Ce/Na-LBen (HT) and Au–Al–Ce/Na-LBen (CP) samples. Ce oxide is distributed on the surface of Au–Al–Ce/Na-LBen (HT), while it stays in the interlayers of the bentonite in the Au–Al–Ce/Na-LBen (CP) sample. The pillaring process could increase the d(001) space of the bentonite since the Ce and Au species could move into the interlayer space of the bentonite. The acid-activation process clearly increases the surface area of the bentonite, while the pillaring process increases the surface area of the samples slightly.
1. Introduction
In recent years, gold catalysis has become a hot topic in chemistry.1,2 The catalytic activity of supported gold particles was discovered in the 1980s by Masatake Haruta.3,4 His pioneering work initiated extensive activities in applied and fundamental research in this field.5 Following the pioneering work of Haruta et al. on the application of supported gold nanoparticles as catalysts for low-temperature CO oxidation, gold catalysts have been widely studied.6 The synthesis of gold nanoparticles supported on a variety of metal oxides, such as TiO2, Fe2O3, ZrO2, CeO2, and Al2O3, has been reported.7–9The catalytic performance of Au is defined by three major factors: contact structure, support selection, and particle size, the first of which being the most important because the perimeter interfaces around Au particles act as the site for reaction.10 The use of Au as a catalyst requires careful and unconventional preparation of the Au clusters, focused on achieving very small and stable Au particles (<5 nm).3,11,12 The problem is that Au nanoparticles (NPs) can move and aggregate into larger particles on the support surface, which is one of the key issues impeding the application of Au catalysts.13–15
Clays are layered oxide materials, which widely exits in nature and could be used as catalyst supports.6,16–18 By exchanging the interlayered cations of layered clays with bulky inorganic polyoxocations, followed by calcination, the intercalated polycations increase the basal spacing of the clays and, upon heating, they convert to metal oxide clusters by dehydration and dehydroxylation. In such process the clays became pillared clays. The metal oxide clusters, named pillars, are inserted between the clay layers, yielding temperature stable oxide pillars that permanently keep the layers apart.18 Previous experiments tried to stabilize metal nanoparticles into the interlayer spaces of clay minerals of the smectite group like montmorillonite, hectorite and saponite.1,2,8,15 Several methods were also reported in the literature for preparation of metal nanoparticles in the interlayer space of modified montmorillonite.6,19,20
As a kind of clay, bentonite has been widely used in many industries as filter materials, decolorant, and adsorbents and so on.21,22 The bentonite used as catalyst support remains an important research area, drawing much attention due to its large surface area and moderate reactivity.14,15,23–31
In order to acquire the Au nanoparticles confined in the interlayer space of modified bentonite, the bentonite supported Au–Al–Ce pillaring catalysts were prepared in the present work, and compared with Au nanoparticles catalysts supported on Al–Ce pillared bentonite.19,20,25 The catalyst performance was evaluated for the CO oxidation reaction (2CO + O2 → 2CO2), and the catalysts were characterized by FT-IR, BET, XRD, TEM, SEM, XPS and ICP.
2. Experimental
2.1. Preparation of supports and catalyst
The original clay is a commercial type natural bentonite from Jiangxi province, China. It is a calcium montmorillonite, previously separated through a 80 mesh sieve and dipped in deionized water for 24 h. The bentonite was obtained, centrifuged and dried at 70 °C for 12 h. 10 g bentonite was dissolved in 30% (w/w) H2SO4 solution at 70 °C for 6 h with the ratio of acid/bentonite (w/w) = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The acid-activated bentonite was centrifuged, washed with deionized water until the pH of the solution became 7, and then dried at 80 °C. The dried acid-activated bentonite was grinded into powders and labeled as LBen. Then sodium bentonite was obtained by dispersing LBen to NaCl aqueous solution with constant stirring at 60 °C for 3 h, until a ratio NaCl/clay (mol g−1) = 0.1 was obtained. The clay was then centrifuged and washed with deionized water repeatedly up to the end of chlorides. The bentonite obtained was dried at 80 °C and labeled as Na-Lben.
1. The acid-activated bentonite was centrifuged, washed with deionized water until the pH of the solution became 7, and then dried at 80 °C. The dried acid-activated bentonite was grinded into powders and labeled as LBen. Then sodium bentonite was obtained by dispersing LBen to NaCl aqueous solution with constant stirring at 60 °C for 3 h, until a ratio NaCl/clay (mol g−1) = 0.1 was obtained. The clay was then centrifuged and washed with deionized water repeatedly up to the end of chlorides. The bentonite obtained was dried at 80 °C and labeled as Na-Lben.
Al–Ce pillaring solution had been prepared by AlCl3·6H2O and Ce(NO3)3·6H2O, with the Ce/(Al + Ce) molar ratio of 20% and molar concentration for Al3+ of 0.5 M. Then the dilute NaOH solution (0.05 M) was added into the mixture slowly until the OH−/metal molar ratio reaches 2.6. Hydrothermal treatments were carried out in Teflon coated stainless steel pressure vessels, which were slowly rotated for 24 h at 120 °C. Then, the vessels were cooled to room temperature and opened under atmospheric pressure. The mixtures were added drop-wise to Na-LBen slurry (bentonite/water ration of 2 g/0.1 L), until the reaction mixture/Na-LBen ratio reaches 20 mmol g−1. Then the mixture was stirred continuously for 48 h at 60 °C, after which the mixture was filtered and washed with deionized water until the chlorides were removed. Then, the samples were dried at 80 °C and calcined at 400 °C for 2 h. This sample is labeled as Al–Ce–Na-LBen.
Au/Na-LBen and Au/Al–Ce–Na-LBen catalysts were prepared by deposition–precipitation method with an aqueous of HAuCl4·4H2O as gold precursor. 1 g of Na-LBen and Al–Ce–Na-LBen were mixed with 10 ml HAuCl4 solution separately. The suspension was constantly stirred at 70 °C for 2 h, and then 0.5 M NaOH solution was added to the suspension until the pH of the mixture became 8–9. The suspension was then filtered and washed with large amount of deionized water until the chlorides were removed, dried at 80 °C and calcined at 400 °C for 3 h.
Au–Al–Ce pillaring solutions were prepared by co-precipitation method and hydrothermal treatment, respectively. For the co-precipitation method, AlCl3·6H2O, Ce(NO3)3·6H2O and a certain amount of HAuCl4·4H2O were firstly dissolved into water, with the Al/Ce molar ratios of 20, and the Al3+ molar concentration of 0.5 M. A dilute NaOH solution (0.05 M) was added into the suspensions slowly until the ratio of OH−/metal reaches 2.6. The mixture was stirred at 70 °C for 24 h. Then, the reaction mixture was added drop-wise to Na-LBen slurry (2 g/0.1 L), until the mixture/Na-LBen ratio reaches 20 mmol g−1. Then the mixture was stirred continuously for 48 h at 60 °C, filtered, washed with deionized water until the chlorides were removed, dried at 80 °C and calcined at 400 °C for 3 h. This sample is labeled as Au–Al–Ce/Na-LBen (CP). For the hydrothermal treatment, the AlCl3·6H2O, Ce(NO3)3·6H2O and HAuCl4·4H2O solution and NaOH mixture was kept at 120 °C in Teflon coated stainless steel pressure vessels for 24 h. Then, the mixture was added to the Na-LBen slurry (2 g/0.1 L), until the mixture/Na-LBen ratio reaches 20 mmol g−1 and stirred for 24 h at room temperature. Then, the products were filtered, washed with deionized water until the chlorides were removed, dried at 80 °C and calcined at 400 °C for 3 h. This sample is labeled as Au–Al–Ce/Na-LBen (HT).
2.2. Catalytic activity tests
The catalysts were evaluated for the CO oxidation reaction with a micro-reactor (stainless steel U-shaped tube, 4 mm id) under atmospheric pressure. The sample (100 mg) is placed among quartz sand. A K-type thermocouple was used to control the reaction temperature. The composition of the feed gas contains 3% CO, 3% O2 with N2 as carrier gas and a flow rate of 30 mL min−1, which corresponds to a gas hourly space velocity (GHSV) of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 cat−1 h−1. The catalyst was reduced in situ under hydrogen flow (30 mL min−1) at 150 °C for 2 h before the reaction was performed. The reactants and products were analyzed on-line with a GC7890I gas chromatograph equipped with TDX-01 column and TCD with optimized working conditions of carrier gas flow rate at 35–42 mL min−1, column temperature at 80 °C, injector temperature at 100 °C and detector temperature at 110 °C. Before the products analysis, the reaction over the catalysts at each temperature was stabilized for 30 minutes to get steady data.
000 mL g−1 cat−1 h−1. The catalyst was reduced in situ under hydrogen flow (30 mL min−1) at 150 °C for 2 h before the reaction was performed. The reactants and products were analyzed on-line with a GC7890I gas chromatograph equipped with TDX-01 column and TCD with optimized working conditions of carrier gas flow rate at 35–42 mL min−1, column temperature at 80 °C, injector temperature at 100 °C and detector temperature at 110 °C. Before the products analysis, the reaction over the catalysts at each temperature was stabilized for 30 minutes to get steady data.
2.3. Characterization
The contents of the catalysts were analyzed by ICP-AES (optima 5300DV, PE, USA) quantitative analyses (Au line 267.595 Å, plasma power 1200 W, plasma flow 1.5 L min−1).XPS characterization was carried out on a Perkin-Elmer PHI1600 system using a single Mg–K-X-ray source operating at 300 W and 15 kV of voltage. The spectra were obtained at ambient temperature with an ultrahigh vacuum. The binding energies were calibrated using the C 1s peak of graphite at 284.5 eV as a reference.
XRD analyses were performed on a Bruker AXS D8 Focus diffract meter instrument operated at 40 kV and 40 mA with Cu target Kα-ray irradiation. Scans were collected over a range of 2θ from 2° to 90° with a speed of 2° min−1.
The specific surface area, pore volume, and average pore diameter of the supports and catalysts were measured by BET methods using a Micromeritics ASAP2020C instrument. Before analysis, the samples were degassed for 1 h at 90 °C and 10 h at 200 °C in vacuum.
The morphologies of the samples were investigated by scanning electron microscopy (SEM, FEI Quanta 200F).
Transmission and high resolution transmission electron microscopic images were obtained on a JEOL JSM-2010 electron microscope. The powders were suspended in ethanol for about 10 min under ultrasonic treatment before been deposited on carbon-film-coated copper grids. The average gold particle diameter and its distribution were determined by counting more than 50 particles.
FT-IR spectral data (KBr pellets) were collected in the range of 400–4000 cm−1 on VARIAN 2000 FT-IR.
3. Results and discussion
3.1. Characterization
| Sample | ICP | XPS | ||
|---|---|---|---|---|
| Au (%) | Ce (%) | Au (%) | Ce (%) | |
| Au/Na-LBen | 0.01 | — | 0.04 | — |
| Au/Al–Ce–Na-LBen | 0.32 | 0.018 | 0.11 | 3.23 |
| Au–Al–Ce/Na-LBen (CP) | 0.39 | 0.009 | 0.18 | 0 |
| Au–Al–Ce/Na-LBen (HT) | 0.36 | 0.011 | 0.16 | 6.14 |
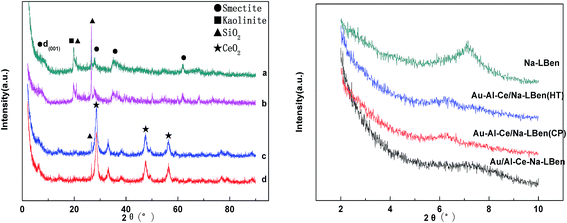 | ||
| Fig. 3 XRD patterns of the catalysts. (a) Au/Al–Ce–Na-LBen, (b) Au/Na-LBen, (c) Au–Al–Ce/Na-LBen (HT), (d) Au–Al–Ce/Na-LBen (CP). | ||
BET surface area, average pore volume, and average pore diameter for all the samples are shown in Table 2. It shows that the original Ben had a BET surface area of 35.5 m2 g−1, after the acid-activation, the LBen became 210.9 m2 g−1, it indicates the acid-activation process could increase the BET surfaces of the bentonite. While, direct ion-exchange process could reduce the BET surface areas of the samples, as indicated by the BET surface area of Ben and LBen (35.5 and 210.9) vs. Na-Ben and Na-LBen (32.8 and 153.8 m2 g−1). In addition, it is found that the BET surface area of Na-LBen increases from 153.8 to 187.3 m2 g−1 for Au–Al–Ce/Na-LBen (CP). It indicates that the pillaring process increases the BET surface area of the samples,33 since the process creates regular porosity. However, the surface area of Al–Ce–Na-Lben decreased from 179.7 to 168.1 m2 g−1 for Au/Al–Ce–Na-LBen. This may because some of the Au nanoparticles go into the interlayers of the bentonite.
| Sample | Average pore diameter (nm) | Pore volume (cm3 g−1) | SBET (m2 g−1) |
|---|---|---|---|
| Ben | 6.68 | 0.059 | 35.5 |
| LBen | 4.23 | 0.223 | 210.9 |
| Na-Ben | 7.07 | 0.058 | 32.8 |
| Na-LBen | 4.61 | 0.177 | 153.8 |
| Al–Ce–Na-LBen | 9.02 | 0.405 | 179.7 |
| Au/Al–Ce–Na-LBen | 9.72 | 0.408 | 168.1 |
| Au–Al–Ce/Na-LBen (HT) | 11.6 | 0.449 | 154.1 |
| Au–Al–Ce/Na-LBen (CP) | 4.46 | 0.209 | 187.3 |
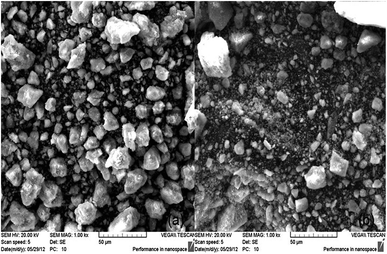
SEM micrographs of Au–Al–Ce/Na-LBen (CP) and Au/Al–Ce–Na-LBen are illustrated in Fig. 5. It is shows that the Au–Al–Ce/Na-LBen (CP) sample has a uniform particles size. While, the particle sizes of Au/Al–Ce–Na-LBen are irregular.
3.2. Catalytic activity and stability tests
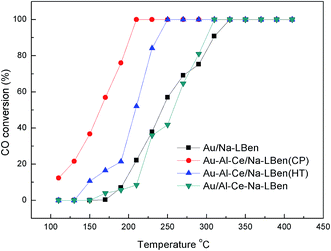
The selectivity of the CO oxidation to CO2 is 100%. The yield of CO2 is equal to the CO conversion. The catalytic performances of the prepared catalysts for CO oxidation are shown in Fig. 1. It should be mentioned that the clay without gold does not show any catalytic activity for the CO oxidation. Our results show that the activity of Au catalysts for CO oxidation highly depends on the type of supports and the preparation methods. In addition, the CO conversion increases as the temperature rises at low temperature. Fig. 1 shows that Au/Na-LBen acquires its best performance until the temperature reaches 330 °C. Au/Al–Ce–Na-LBen acquires its best performance until the temperature reaches 310 °C. Au–Al–Ce/Na-LBen (CP) could reach its best performance at 200 °C (the turnover number of the CO oxidation over Au–Al–Ce/Na-LBen (CP) catalyst is 2.15 × 1016 s−1 m−2 for 100% CO conversion.), vs. 240 °C for Au–Al–Ce/Na-LBen (HT) and 310 °C for Au/Al–Ce–Na-LBen. It indicates that Au–Al–Ce pillaring species and the co-precipitation method contribute to the high CO conversion activity of the catalysts.Some experiments reported that the gold nanoparticles could catalyze the CO oxidation reaction at lower temperature.4,9,34 It should be noted that the catalytic performance of Au is defined by three major factors:10 contact structure, support selection, and particle size, the first of which being the most important because the perimeter interfaces around Au particles act as the site for reaction. The sizes of the gold particles of the Au–Al–Ce/Na-LBen (CP) catalyst are as small as the reported work, while the contact structure and support interaction are different, which may contribute to the activity of the catalyst.
Except for the activity, the stability of the catalyst is another important factor to evaluate the catalytic performance. Fig. 2 shows the durability tests of CO oxidation for the Au–Al–Ce/Na-LBen (CP) and Au/Al–Ce–Na-LBen. The reaction temperature for Au–Al–Ce/Na-LBen (CP) and Au/Al–Ce–Na-LBen are 170 °C and 290 °C, respectively, which obtained ∼80% CO conversions. Fig. 2 shows that no obvious deactivation was observed for the Au–Al–Ce/Na-LBen (CP) catalyst during the 60 h's evaluation. While, the CO conversion over the Au/Al–Ce–Na-LBen decreases from 80% to 60% in 60 h's evaluation. It indicates that the Au–Al–Ce/Na-LBen (CP) shows much better stability than Au/Al–Ce–Na-LBen.
Furthermore, we tried to stop the CO oxidation reaction over the Au–Al–Ce/Na-LBen (CP) catalyst and restart the reaction after the reactor was cooled to room temperature. It shows that Au–Al–Ce/Na-LBen (CP) catalyst could perform as well as the first run.
4. Conclusions
Au/Na-Lben, Au–Al–Ce/Na-LBen (HT), Au–Al–Ce/Na-LBen (CP) and Au/Al–Ce–Na-LBen catalysts are synthesized using hydrothermal and co-precipitation methods for CO oxidation reaction. It is found that Au–Al–Ce/Na-LBen (CP) and Au–Al–Ce/Na-LBen (HT) have better catalytic activity and stability than Au/Na-Lben and Au/Al–Ce–Na-LBen. The samples were characterized using XPS, XRD, BET, TEM, SEM and FT-IR. XPS analysis found that Ce oxide distributes on the surface of Au–Al–Ce/Na-LBen (HT), while stays in the interlayers of the bentonite for the sample Au–Al–Ce/Na-LBen (CP). In addition, the gold nanoparticle could move into the interlayer spaces of bentonite, which contributes to the high stability of the Au–Al–Ce/Na-LBen (HT), Au–Al–Ce/Na-LBen (CP) samples. The XRD characterization indicates that the pillaring process could increase the d(001) space of the bentonite since the Ce and Au species could move into the interlayer space of the bentonite. The BET results show that the acid-activation process obviously increases the surface area of the bentonite, while the pillaring process increases the surface area of the samples slightly. The TEM results indicate that the Au–Al–Ce pillaring species homogeneously dispersed in the interlayer of bentonite for Au–Al–Ce/Na-LBen (CP). SEM micrograph shows that the Au–Al–Ce/Na-LBen (CP) sample has a uniform particles size, while, the particle sizes of Au/Al–Ce–Na-LBen (24 h) are irregular. The FTIR results show that, compared with Na-LBen, some new peaks emerged for the samples with Au, due to the interaction between the supports and nanoparticles.Acknowledgements
We thank the National Natural Science Foundation of China (21166018) and the Education Department of Jiangxi Province of China (GJJ10291, GJJ14121) for their financial support.References
- A. A. Teixeira-Neto, B. Faceto, L. S. Siebra and E. Teixeira-Neto, Effect of the Synthesis Method on the Properties and Catalytic Activities of Au/Pd Nanoparticles Supported on Organophilic Clay, Appl. Clay Sci., 2015, 116, 175–181 CrossRef.
- B. J. Borah, D. Dutta and D. K. Dutta, Controlled Nanopore Formation and Stabilization of Gold Nanocrystals in Acid-Activated Montmorillonite, Appl. Clay Sci., 2010, 49, 317–323 CrossRef CAS.
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, Gold Catalysts Prepared by Coprecipitation for Low-Temperature Oxidation of Hydrogen and of Carbon Monoxide, J. Catal., 1989, 115, 301–309 CrossRef CAS.
- M. Haruta, T. Kobayashi, H. Sano and N. Yamada, Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature Far Below 0 °C, Chem. Lett., 1987, 16, 405–408 CrossRef.
- M. Haruta and M. Date, Advances in the Catalysis of Au Nanoparticles, Appl. Catal., A, 2001, 222, 427–437 CrossRef CAS.
- L. H. Zhu, S. Letaief, Y. Liu, F. Gervais and C. Detellier, Clay Mineral-Supported Gold Nanoparticles, Appl. Clay Sci., 2009, 43, 439–446 CrossRef CAS.
- X. He, L. J. Fu and H. M. Yang, Insight into the Nature of Au-Au2O3 Functionalized Palygorskite, Appl. Clay Sci., 2014, 100, 118–122 CrossRef CAS.
- L. F. Chen, J. C. Hu and R. Richards, Intercalation of Aggregation-Free and Well-Dispersed Gold Nanoparticles into the Walls of Mesoporous Silica as a Robust "Green" Catalyst for N-Alkane Oxidation, J. Am. Chem. Soc., 2009, 131, 914–915 CrossRef CAS PubMed.
- M. Haruta, S. Tsubota, T. Kobayashi, H. Kageyama, M. J. Genet and B. Delmon, Low-Temperature Oxidation of Co over Gold Supported on TiO2, α-Fe2O3, and Co3O4, J. Catal., 1993, 144, 175–192 CrossRef.
- M. Haruta, Catalysis of Gold Nanoparticles Deposited on Metal Oxides, CATTECH, 2002, 6, 102–115 CrossRef CAS.
- Y. T. Yu, Q. H. Zhang and B. Q. Xu, Shape-Controlled Syntheses of Metal Nanoparticles, Prog. Inorg. Chem., 2004, 16, 520–527 CAS.
- M. Haruta, Size- and Support-Dependency in the Catalysis of Gold, Catal. Today, 1997, 36, 153–166 CrossRef CAS.
- Y. Zhang, X. J. Cui, F. Shi and Y. Q. Deng, Nano-Gold Catalysis in Fine Chemical Synthesis, Chem. Rev., 2012, 112, 2467–2505 CrossRef CAS PubMed.
- C. C. Chen and P. L. Kuo, Gold Nanoparticles Prepared Using Polyethylenimine Adsorbed onto Montmorillonite, J. Colloid Interface Sci., 2006, 293, 101–107 CrossRef CAS PubMed.
- T. J. Pinnavaia, S. D. Landau, M. S. Tzou, I. D. Johnson and M. Lipsicas, Layer Cross-Linking in Pillared Clays, J. Am. Chem. Soc., 1985, 107, 7222–7224 CrossRef CAS.
- A. Alvarez, S. Moreno, R. Molina, S. Ivanova, M. A. Centeno and J. A. Odriozola, Gold Supported on Pillared Clays for Co Oxidation Reaction: Effect of the Clay Aggregate Size, Appl. Clay Sci., 2012, 69, 22–29 CrossRef CAS.
- A. Gil, S. A. Korili, R. Trujillano and M. A. Vicente, A Review on Characterization of Pillared Clays by Specific Techniques, Appl. Clay Sci., 2011, 53, 97–105 CrossRef CAS.
- A. Gil, L. M. Gandia and M. A. Vicente, Recent Advances in the Synthesis and Catalytic Applications of Pillared Clays, Catal. Rev.: Sci. Eng., 2000, 42, 145–212 CAS.
- N. R. Sanabria, M. A. Centeno, R. Molina and S. Moreno, Pillared Clays with Al-Fe and Al-Ce-Fe in Concentrated Medium: Synthesis and Catalytic Activity, Appl. Catal., A, 2009, 356, 243–249 CrossRef CAS.
- J. G. Carriazo, R. Molina and S. Moreno, A Study on Al and Al-Ce-Fe Pillaring Species and Their Catalytic Potential as They Are Supported on a Bentonite, Appl. Catal., A, 2008, 334, 168–172 CrossRef CAS.
- M. Nafees and A. Waseem, Organoclays as Sorbent Material for Phenolic Compounds: A Review, Clean: Soil, Air, Water, 2014, 42, 1500–1508 CrossRef CAS.
- A. R. Razakamanantsoa, G. Barast and I. Djeran-maigre, Hydraulic Performance of Activated Calcium Bentonite Treated by Polyionic Charged Polymer, Appl. Clay Sci., 2012, 59–60, 103–114 CrossRef CAS.
- A. Khenifi, B. Zohra, B. Kahina, H. Houari and D. Zoubir, Removal of 2,4-Dcp from Wastewater by Ctab/Bentonite Using One-Step and Two-Step Methods: A Comparative Study, Chem. Eng. J., 2009, 146, 345–354 CrossRef CAS.
- Z. L. Mo, P. Zhang, D. D. Zuo, Y. L. Sun and H. Chen, Synthesis and Characterization of Polyaniline Nanorods/Ce(Oh)3-Pr2O3/Montmorillonite Composites through Reverse Micelle Template, Mater. Res. Bull., 2008, 43, 1664–1669 CrossRef CAS.
- S. F. Zuo and R. X. Zhou, Al-Pillared Clays Supported Rare Earths and Palladium Catalysts for Deep Oxidation of Low Concentration of Benzene, Appl. Surf. Sci., 2006, 253, 2508–2514 CrossRef CAS.
- S. Yao, S. Yuan, J. Xu, Y. Wang, J. Luo and S. Hu, A Hydrogen Peroxide Sensor Based on Colloidal MnO2/Na-Montmorillonite, Appl. Clay Sci., 2006, 33, 35–42 CrossRef CAS.
- J. Feng, R. S. K. Wong, X. Hu and Po L. Yue, Discoloration and Mineralization of Orange Ii by Using Fe3+-Doped TiO2 and Bentonite Clay-Based Fe Nanocatalysts, Catal. Today, 2004, 98, 441–446 CrossRef CAS.
- A. Phukan, J. N. Ganguli and D. K. Dutta, ZnCl2-Zn2+-Montmorillonite Composite: Efficient Solid Acid Catalyst for Benzylation of Benzene, J. Mol. Catal. A: Chem., 2003, 202, 279–287 CrossRef CAS.
- L. M. Gandìa, M. A. Vicente and A. Gil, Preparation and Characterization of Manganese Oxide Catalysts Supported on Alumina and Zirconia-Pillared Clays, Appl. Catal., A, 2000, 196, 281–292 CrossRef.
- K. Bahranowski and E. M. Serwicka, Esr Study of Vanadium-Doped Alumina- and Titania-Pillared Montmorillonites, Colloids Surf., A, 1993, 72, 153–160 CrossRef CAS.
- R. M. Barrer and D. M. MacLeod, Activation of Montmorillonite by Ion Exchange and Sorption Complexes of Tetra-Alkyl Ammonium Montmorillonites, Trans. Faraday Soc., 1955, 51, 1290–1300 RSC.
- S. F. Zuo, Q. Q. Huang and R. X. Zhou, Al/Ce Pillared Clays with High Surface Area and Large Pore: Synthesis, Characterization and Supported Palladium Catalysts for Deep Oxidation of Benzene, Catal. Today, 2008, 139, 88–93 CrossRef CAS.
- E. Booij and J. T. Kloprogge, J. A. R. vanVeen, Large Pore Ree/Al Pillared Bentonites: Preparation, Structural Aspects and Catalytic Properties, Appl. Clay Sci., 1996, 11, 155–162 CrossRef CAS.
- B. L. Moroz, P. A. Pyrjaev, V. I. Zaikovskii and V. I. Bukhtiyarov, Nanodispersed Au/Al2O3 Catalysts for Low-Temperature Co Oxidation: Results of Research Activity at the Boreskov Institute of Catalysis, Catal. Today, 2009, 144, 292–305 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |