Optimization and diagnostic of nonlinear optical features of π-conjugated benzodifuran-based derivatives
B. Kulyk*,
A. P. Kerasidou,
L. Soumahoro,
C. Moussallem,
F. Gohier,
P. Frère and
B. Sahraoui*
Institute of Sciences and Molecular Technologies of Angers (MOLTECH Anjou), UMR CNRS 6200, University of Angers, 2 bd Lavoisier, 49045 Angers, France. E-mail: bohdan_kulyk@yahoo.com; bouchta.sahraoui@univ-angers.fr
First published on 28th January 2016
Abstract
The nonlinear optical parameters of benzodifuran-based derivatives obtained by a green approach are determined under picosecond laser irradiation. The guest–host polymeric films were prepared on the basis of benzodifuran derivatives incorporated into PMMA. The optical absorption spectra of benzodifuran-based compounds in films and solutions were analyzed and their SHG and THG measurements were performed by means of the Maker fringe technique in transmission scheme using the output beam of a mode-locked Nd:YAG/YVO4 laser generating at 1064 nm with 30 ps pulse duration. The compounds with asymmetrical structure and increased π-conjugated chains have shown the highest SHG response of the order of 10−13 m V−1. Z-scan measurements have been carried out at 532 nm and the values of nonlinear optical refractive indexes and absorption coefficients were defined and revealed negative nonlinear refraction and positive nonlinear absorption.
Introduction
Organic materials have drawn remarkable scientific and economic attention in the past decades due to their advantages such as high efficiency, flexibility and low-cost. The promising class of materials is π-conjugated systems with delocalized electrons. The development of π-conjugated materials is motivated by their potential technological applications as organic semiconducting materials for the fabrication of electronic and optoelectronic devices, such as field-effect transistors (OFET), electroluminescent diodes (OLED), photovoltaic cells,1–4 etc. The major goal in the development of organic-based electronic devices resides in the low cost process for their fabrication by using spin coating or inkjet printing.5–8 On the other hand, the flexibility of the organic chemistry allows the access to various π-conjugated structures that can be tailored for the specific electronic or optical properties to optimize the performances of the devices.9–12The development of polymers as materials for nonlinear optics (NLO) has largely extended the range of applications of organic materials in optoelectronics. Composite polymeric materials are indeed inexpensive and can be easily processed into good optical quality thin films, which can be readily integrated into a large variety of devices.13–15 Organic polymer materials are considered as promising materials for applications in photonics.16 Organic nonlinear optical materials offer advantages as compared with NLO inorganic materials, due to their larger electro-optic coefficients, faster response time, larger bandwidth, lower drive voltage, lower dielectric constant and high laser damage threshold.17,18 These advantages are expected to increase the photorefractive figures of merit and hence new interest has started to develop in NLO polymers, in particular with the prospect to be used as a satisfactory alternative to crystals for holographic data storage.19,20
The NLO response of polymer guest–host systems derives from the hyperpolarizability of chromophores (dyes) inside them. NLO chromophores are usually composed of strong electron donor (D) and accepter (A) groups connected by a π-conjugating unit. Chromophores with delocalized π-electron systems capable of exhibiting charge transfer resonances can exhibit quite large molecular hyperpolarizability. When chromophores in a polymer are aligned in a non-centrosymmetric system after poling, it gives rise to NLO properties of polymer system.21–23 On the other hand, in order to obtain a large second-order NLO effect, it is necessary to increase the density of chromophores on one side and to select a chromophore with a large hyperpolarizability value. The NLO effects of some push–pull organic molecules have been extensively investigated in order to incorporate them into materials devoted to technological devices, for example second-order optical nonlinearities are useful in frequency doubling and optical switching with a fast response time.24,25
This research is devoted to the NLO study of π-conjugated molecular systems based on benzodifuran unit connected to furan, thiophene or pentafluorophenyl cycles by azomethine bonds. The azomethine bond (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) is isoelectronic to ethylenic bond (–CH
CH–) is isoelectronic to ethylenic bond (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and represents good alternatives in term of electronic delocalization to their all-carbon counterparts.26 Benzodifuran derivatives have been reported as hole transporting materials in organic electroluminescent cells27 or as electron-donor in organic solar cells.28–31 Several examples of conjugated materials based on azomethine have been described as hole transporting materials32,33 or electrochromic materials34–36 and more recently as electron donor in organic solar cells.37,38 Up to date the nonlinear optical properties of benzodifuran derivatives are not well investigated. Our purpose is to study the NLO response for six types of benzodifuran derivatives by means of second and third harmonic generations (SHG, THG) and Z-scan techniques and extract their NLO parameters. This study is devoted to the understanding of the relationship between chemical structure of benzodifuran-based derivatives and their nonlinear optical properties in order to optimize these molecular materials for application in photonics.
CH–) and represents good alternatives in term of electronic delocalization to their all-carbon counterparts.26 Benzodifuran derivatives have been reported as hole transporting materials in organic electroluminescent cells27 or as electron-donor in organic solar cells.28–31 Several examples of conjugated materials based on azomethine have been described as hole transporting materials32,33 or electrochromic materials34–36 and more recently as electron donor in organic solar cells.37,38 Up to date the nonlinear optical properties of benzodifuran derivatives are not well investigated. Our purpose is to study the NLO response for six types of benzodifuran derivatives by means of second and third harmonic generations (SHG, THG) and Z-scan techniques and extract their NLO parameters. This study is devoted to the understanding of the relationship between chemical structure of benzodifuran-based derivatives and their nonlinear optical properties in order to optimize these molecular materials for application in photonics.
Experimental
The six types of benzodifuran-based derivatives have been synthesized in the framework of planned study. Their chemical structures are shown in Fig. 1. Both the synthesis of benzodifuran and the extension of the conjugated system are performed by condensation reactions in green solvent. The two steps of the synthetic pathway are based on condensation reactions giving only water as by-product. For these two steps, green solvents, such as ethanol or ethyl lactate were used and the purifications were done by precipitation and recrystallization. Detailed synthesis description and electronic properties characterization of various compounds are given elsewhere.39,40 | ||
| Fig. 1 Structure of the conjugated benzodifuran-based derivatives: (a) S1; (b) S2; (c) S3; (d) S4; (e) S5; (f) S6. | ||
The solution of PMMA (Sigma-Aldrich, Mw = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) dissolved in 1,1,2-trichloroethane at concentration of 67 g L−1 was prepared as a host system for our compositions. The glass plates were washed in distilled water using ultrasonic bath, acetone, and ethanol and then dried. The concentration of our composition towards PMMA was 5 wt%. Filtered through a 0.45 μm pore size nylon syringe filter solutions were deposited on glass substrates using the spin-coater at 1000 rpm. Obtained guest–host polymer systems were kept at room temperature during two days in order to eliminate any remaining of solvent. The thickness of deposited films was estimated with the profilometer (Dektak 6M, Veeco) to be about 400 nm.
000 g mol−1) dissolved in 1,1,2-trichloroethane at concentration of 67 g L−1 was prepared as a host system for our compositions. The glass plates were washed in distilled water using ultrasonic bath, acetone, and ethanol and then dried. The concentration of our composition towards PMMA was 5 wt%. Filtered through a 0.45 μm pore size nylon syringe filter solutions were deposited on glass substrates using the spin-coater at 1000 rpm. Obtained guest–host polymer systems were kept at room temperature during two days in order to eliminate any remaining of solvent. The thickness of deposited films was estimated with the profilometer (Dektak 6M, Veeco) to be about 400 nm.
Absorption spectra of the samples were measured by the means of Lambda 950 UV/Vis/NIR spectrophotometer (PerkinElmer) in the range 250–1200 nm. The pure PMMA film on glass and the cuvette filled only with solvent were used on the way of reference beam in spectrometer for the measurements of absorption spectra of our compounds in films and liquids, respectively.
After the deposition by spin-coating, the samples were poled as follows. First, they were heated on a hot plate at selected poling temperature 120 °C, a bit higher than the glass transition temperature Tg. Then an external electric field was provided by applying a voltage of +5 kV to a tungsten needle fixed 1 cm above the polymer surface, while the electrode under the glass substrate was grounded. With the remaining electric field, the heater was switched off, and the sample was cooled down to room temperature. Finally, the corona field was turned off. The whole poling procedure needed about 2.5 hours, including up and down temperature ramping. The scheme of the used corona poling setup is depicted in Fig. 2.
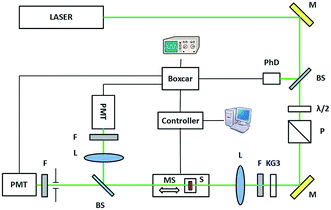
SHG and THG measurements were carried out by means of the rotational Maker fringe technique in the transmission scheme (Fig. 3) for the s- and p-polarized fundamental laser beam. A y-cut crystalline quartz plate has been used as a reference material for SHG measurements and fused silica plate for THG measurements.
As a fundamental beam, we used the output beam of a mode-locked Nd:YAG/YVO4 laser (EKSPLA) generating at λ = 1064 nm with 30 ps pulse duration and 10 Hz repetition rate. The incident polarization was selected with a polarizer and half-wave plate in front of the focusing lens. Before the lens the RG1000 long pass filter was mounted in order to cut the visible region of light. The beam was focused onto the sample with a lens of 250 mm focal length and the beam diameter was 0.4 mm at the sample. A motorized rotation stage with the mounted sample allowed the variation of the incidence angle with a resolution of 0.5–1.0° and was processed by a computer through the controller box. After passing the sample the KG3 filter was used to cut of infrared laser beam. The interference filter at 532 nm (or 355 nm) was used to select the desired wavelength of light. Detector saturation was prevented using linear neutral density filters, whose transmittance value was taken into account during data fitting. The polarization of second and third harmonic was controlled by polarizer placed before the photomultiplier. The second or third harmonic signal was detected by the photomultiplier tube (Hamamatsu), which was connected to a boxcar and processed by a computer. The photodiode was enabled in laser pulse synchronization which was checked by an oscilloscope. The input laser pulses energy was measured by laser power/energymeter (LabMax TOP, COHERENT) to be 116 μJ for SHG and 160 μJ for THG measurements. Finally, we got the angular dependences of SHG/THG or so called Maker-fringes by rotating the sample towards the normal in horizontal plain.
The NLO properties of the compounds were also investigated by means of the Z-scan technique,41,42 using the frequency doubled exit of a 30 ps mode-locked Nd:YVO4 laser with a repetition rate of 10 Hz at 532 nm. The laser beam was focused onto the sample by means of a 20 cm focal length focusing lens, and the spot size of the laser beam at the focus was 17 μm. The Z-scan technique is a simple and effective technique for determining the NLO properties, and it is widely used since it provides simultaneously, by a single measurement, the magnitudes and the signs of the nonlinear absorption and the nonlinear refraction of the investigated sample. During the Z-scan measurements the transmittance of the sample is detected as it moves along the propagation path with the step of 0.25 mm through the focal plane of a focused laser beam. The detection of the transmission has been carried out by appropriate photomultiplier tube, and each experimental point has been the result of a 20 pulse average during the acquisition, performed by boxcar. In particular two different series of measurements are simultaneously carried out giving access to different information: the “open aperture” (OA) Z-scan, where the totality of the transmitted light is collected, and the “closed aperture” (CA) Z-scan where a small part of the transmitted light is collected after passing through a small circular diaphragm. The former allows the determination of the nonlinear absorption, while the latter includes information related to the nonlinear absorption and refraction of the systems. By dividing the CA with the OA the so-called “divided” Z-scan is obtained, which carries information only related with the nonlinear refraction of the system under investigation. The OA Z-scan can exhibit a transmittance “peak” or “valley” around the focal point corresponding to saturable absorption (SA) or reverse saturable absorption (RSA), respectively. The divided Z-scan can exhibit a “peak–valley” or “valley–peak” configuration, which corresponds to negative or positive nonlinear refraction, respectively. The setup of Z-scan technique is shown in Fig. 4.
Results and discussion
The absorption spectra of S1–S6 compounds dissolved in dichloromethane (DCM) at concentration 1 mM and embedded in PMMA films at concentration 5 wt% are given in Fig. 5. Compound S1 with only benzodifuran unit presents a large structured absorption band in UV-region (250–350 nm). For the dissymmetrical compound S2, the extension of the benzodifuran unit with the imino-pentafluorophenyl unit confers to the structure a push–pull character between the donor (amine NH2) and acceptor (imino-pentafluoro –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C6F5) groups. The large and non-structured band at 450 nm is assigned to an internal charge transfer. For the other symmetrical compounds S3–S6, the extension of the conjugated systems lead to the apparition of structured bands in visible region (around 450 nm) with the formation of three maxima corresponding to π–π* absorption bands. The study of the absorption spectra in films at wavelengths below 300 nm is complicated due to the high absorption of glass substrate. At the wavelength more than 550 nm all the samples exhibit high optical transparency.
CH–C6F5) groups. The large and non-structured band at 450 nm is assigned to an internal charge transfer. For the other symmetrical compounds S3–S6, the extension of the conjugated systems lead to the apparition of structured bands in visible region (around 450 nm) with the formation of three maxima corresponding to π–π* absorption bands. The study of the absorption spectra in films at wavelengths below 300 nm is complicated due to the high absorption of glass substrate. At the wavelength more than 550 nm all the samples exhibit high optical transparency.
 | ||
| Fig. 5 Normalized UV-vis absorption spectra of the compounds S1–S6: (a) dissolved in DCM at concentration 1 mM and (b) embedded in PMMA films at concentration 5 wt%. | ||
The determination of the absorption peaks wavelengths in both spectra were done using Lorenzian fitting in the energy scale. The derived values of absorption peaks' positions are given in Tables 1 and 2. As it can be seen from the absorption bands, the vibronic fine structures for compounds S3–S6 are the indication of a high degree of rigidification of the conjugated chain due to the combination of the intrinsically rigid benzodifurane and connected junctions. The lengthening of the conjugated systems leads to a strong batchromic shift corresponding to a decrease of the HOMO–LUMO gap.39
| Sample | λabs, nm | α (532 nm), cm−1 |
|---|---|---|
| S1 | 274, 314 | 0.25 |
| S2 | 263, 297, 448 | 1.85 |
| S3 | 263, 389, 411, 437, 465 | 0.65 |
| S4 | 271, 306, 413, 441, 468, 501 | 4.41 |
| S5 | 274, 418, 448, 475, 508 | 17.97 |
| S6 | 269, 298, 409, 435, 461, 494 | 1.71 |
| Sample | λabs, nm | α, 103 cm−1 | ||
|---|---|---|---|---|
| 1064 nm | 532 nm | 355 nm | ||
| S1 | 320 | ∼0.10 | 0.13 | 3.01 |
| S2 | 306, 457 | 0.36 | 1.08 | |
| S3 | 390, 411, 434, 463 | 0.13 | 0.61 | |
| S4 | 302, 416, 441, 468, 501 | 1.82 | 4.57 | |
| S5 | 422, 448, 476, 510 | 1.35 | 2.45 | |
| S6 | 303, 410, 435, 463, 496 | 0.65 | 1.76 | |
Comparing the absorption in solutions with those obtained for films the negative solvatochromic shift in almost all absorption peaks is observed. It refers to a strong dependence of absorption spectra with the solvent polarity. Since polarities of the ground and excited state of a chromophore are different, a change in the solvent polarity leads to different stabilization of the ground and excited states, and thus, a change in the energy gap between these electronic states.
The calculation of absorption coefficients has been made for S1–S6 compounds (Tables 1 and 2) at the wavelengths of fundamental laser irradiation and SHG and THG generation which are necessary for the calculations of NLO parameters. As it can be seen, the compounds S1–S6 do not have absorption bands neither at the wavelengths of laser irradiation nor at wavelengths of SH and TH generations and the absorption coefficients at these wavelengths are quite low. Therefore, we should not expect any resonant effects in the process of SHG and THG measurements.
The SHG measurements were performed by rotational Maker fringe technique for s- and p-polarized fundamental beam. The S1–S6 compounds embedded in PMMA films at concentration 5 wt% were used in measurements of high harmonic generations. Corona poling was performed before in order to increase a uniaxial orientation of NLO compounds in the polymer films. The Fig. 6 presents the dependences of the second harmonic intensity generated in the S1–S6 guest–host films as the function of incident angle. “M-shape” angular dependence was obtained for all the samples with a maximum signal at about 60° and minimal intensity at normal incidence of the fundamental beam due to the lack of noncentrosymmetry in this direction. The polarization of the second harmonic signal was found to be always p-polarized regardless of the incident polarization.
 | ||
| Fig. 6 SHG intensity as a function of incident angle in S1–S6 films at the s-, and p-polarized ((a) and (b)) fundamental beam. The generated signal is p-polarized. The curves correspond to fitting. | ||
For a thin film sample with a thickness much less than the coherence length, the intensity of SHG in the film was measured and compared to SHG from a quartz plate reference. The quadratic NLO susceptibility χ(2) can be calculated from following equation:43
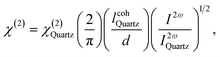 | (1) |
 is coherent length of quartz, d is film thickness, I2ω and I2ωQuartz are the SHG intensities of sample and quartz under the same conditions, respectively.
is coherent length of quartz, d is film thickness, I2ω and I2ωQuartz are the SHG intensities of sample and quartz under the same conditions, respectively.
The obtained quadratic NLO susceptibilities for S1–S6 films are presented in Table 3. For the input–output polarization s–p the values of χ(2) were found to be lower than for polarization p–p, which is caused by symmetry peculiarities of the guest–host polymeric films after poling. However, the tendency in these values is the same in both polarization schemes. The highest SHG parameters are obtained for S2 compound and the lowest for S1. This can be connected with the fact that the molecule of S2 compound has more asymmetrical structure with a push–pull character compared with the others, meanwhile molecule of S1 compound has the smallest structure without extension with azomethine junction. Another important impact on quadratic nonlinearity has the length of π-conjugated chain in molecule as it is responsible for the intramolecular charge transfer. As it can be seen from the molecular structures the S1 and S3 have comparatively short π-conjugated chains and meanwhile they are characterized with the lowest values of χ(2). It is manifested better in p–p polarization scheme, in which laser beam polarization is parallel to the dipole moment of molecules. In addition, these results give some valuable information which of functional groups may enhance NLO response of benzodifuran-based molecule. As it can be noticed, furan or thiophene containing π-conjugated chains connected to the backbone of the molecule by azomethine bonds have higher impact on quadratic nonlinearity of the molecule than butyl ester units or pentafluorophenyl cycles.
| Sample | χ(2), pm V−1 | χ(2), pm V−1 |
|---|---|---|
| s–p | p–p | |
| S1 | 0.09 | 0.12 |
| S2 | 0.13 | 0.37 |
| S3 | 0.09 | 0.14 |
| S4 | 0.12 | 0.31 |
| S5 | 0.09 | 0.19 |
| S6 | 0.09 | 0.19 |
The measurements of THG in S1–S6 films were performed for both s–s and p–p incident and generated polarization. In order not to make confusion we present the Maker fringes only for one sample since these dependences have the similar behaviour for the others. The THG angle dependences collected in Fig. 7 originate from the S4/PMMA film together with glass substrates, since the THG process can occur in any material regardless of its symmetry. However, the effect of glass substrate in THG response was taken into account during data processing and the cubic NLO susceptibility calculation.
 | ||
| Fig. 7 THG intensity from S4 film as a function of incident angle for the polarization of the fundamental beam-generated signal: (a) s–s and (b) p–p. | ||
For the calculation of cubic NLO susceptibilities the model which takes into account optical absorption was used:45
 | (2) |
 is the coherent length of silica, d is the film thickness, lcohSilica and I3ωSilica are the THG intensities of sample and silica at the same conditions, respectively. The calculated values of χ(3) for the S1–S6 films are given in Table 4 and they exceed the latter of pure PMMA film. It is worth to note that cubic NLO susceptibility of these films is several times higher than of silica. As it can be noticed, there is no much difference in values of χ(3) depending on incident polarization due to the fact that maximum value of response was observed at normal incidence as well as THG process is not such dependent on the symmetry of medium as SHG ones. The similar tendency as for SHG can be seen i.e. S1 and S3 compounds are characterized with the lowest χ(3) values, meanwhile S2 and S4–S6 have the higher among which the highest belongs to S4. The molecules with long π-conjugated chains are characterized with comparatively higher values of χ(3) due to delocalized π-electrons, which traveling freely along the conjugated structure of molecules are the key factor to high nonlinearities in organic materials.
is the coherent length of silica, d is the film thickness, lcohSilica and I3ωSilica are the THG intensities of sample and silica at the same conditions, respectively. The calculated values of χ(3) for the S1–S6 films are given in Table 4 and they exceed the latter of pure PMMA film. It is worth to note that cubic NLO susceptibility of these films is several times higher than of silica. As it can be noticed, there is no much difference in values of χ(3) depending on incident polarization due to the fact that maximum value of response was observed at normal incidence as well as THG process is not such dependent on the symmetry of medium as SHG ones. The similar tendency as for SHG can be seen i.e. S1 and S3 compounds are characterized with the lowest χ(3) values, meanwhile S2 and S4–S6 have the higher among which the highest belongs to S4. The molecules with long π-conjugated chains are characterized with comparatively higher values of χ(3) due to delocalized π-electrons, which traveling freely along the conjugated structure of molecules are the key factor to high nonlinearities in organic materials.
| Sample | χ(3), 10−22 m2 V−2 | χ(3), 10−22 m2 V−2 |
|---|---|---|
| s–s | p–p | |
| PMMA | 5.1 | 5.4 |
| S1 | 5.5 | 5.9 |
| S2 | 9.2 | 9.6 |
| S3 | 5.7 | 6.4 |
| S4 | 13.4 | 13.4 |
| S5 | 9.1 | 9.2 |
| S6 | 8.9 | 9.2 |
The absorption and the refraction index of a material under intense laser excitation can be expressed, considering also the nonlinear terms, as:
| α = α0 + βI0 | (3) |
| n = n0 + n2I0, | (4) |
For Z-scan measurements the solutions of S1–S6 compounds in DCM at molar concentration 1 mM were prepared and put in 1 mm quartz cuvettes. In Fig. 8 characteristic divided Z-scans obtained for the compounds S4–S6 are presented. It is obvious that these systems exhibit a “peak–valley” configuration corresponding to negative nonlinear refraction. Simultaneously measured DCM has shown negligible nonlinear refraction in the region of investigated laser energies. On the contrary, compounds S1–S3 showed quite low nonlinear refraction signal at the investigated laser energies and the increase of energy led to impressive influence of DCM in NLO response. Thus, to implement Z-scan measurement for these compounds it is necessary to choose the solvent with lower NLO response or perform the measurement at much higher concentrations.
In Fig. 9 the transmittance difference between the peak and the valley (ΔTp–v) of the divided CA Z-scans is presented as a function of the incident laser energy. A linear behavior can be seen between the ΔTp–v values and the incident laser energy for the samples S4 and S6 and no saturation of the nonlinearity in the utilized energy range is observed. For the sample S5 the linear dependence is observed till 0.40 μJ, at higher energies the saturation of the NLO response is noticeable. Compound S5 among investigated compounds has the highest linear absorption at the laser excitation wavelength (532 nm) (Table 1). This fact has a significant impact on the ΔTp–v values. However, during the analysis of the data and the determination of the NLO parameters the linear absorption has been taken into account through Leff and the calculation has been made for the region of energies where the linearity was observed.
 | ||
| Fig. 9 Dependences of ΔTp–v on the incident laser energy for the compounds S4–S6 at concentration 1 mM in DCM. | ||
The nonlinear absorption of S4–S6 systems has been found to exhibit a significant response. In Fig. 10 the characteristic OA Z-scans obtained for these three molecules can be seen. From these figure it can be found out that these compounds exhibit a RSA attribute corresponding to a decrease of the transmittance around the focal point. Such a behavior is happened in systems in which the excited state absorption is large compared to the ground state absorption.
From the CA, OA curves the nonlinear refraction index (n2) and the nonlinear absorption coefficient (β) can be determined using the following equations:48,49
 | (5) |
 | (6) |
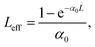 | (7) |
 | (8) |
From the slopes of the linear fits in Fig. 9 the n2 values have been obtained and presented in Table 5. It should be noted that the negative signs of the n2 values obtained for these compounds correspond to self-defocusing behavior. The DCM is characterized with a lower nonlinearity, the value of n2 was found to be about 1.5 × 10−19 m2 W−1 and no noticeable nonlinear absorption was observed at the applied laser energies. Moreover, from the OA curves we have determined the nonlinear absorption parameters β for the compounds S4–S6. Only the compounds with long π-conjugated chains connected to the backbone of the molecule by azomethine bonds have shown considerable third order NLO response. This can be explained by their much higher polarizabilities compared to S1–S3 compounds. As it is known, molecular orientation contribution to the third-order susceptibility quadratically depends on the difference in polarizabilities along the principal dielectric axes of the molecule.50 Among the investigated compounds S5 is characterized with the highest absolute value of NLO refractive index and absorption, about 3 times higher than for S4 and S6. This can be connected with thiophene moieties which are presented only in the structure of S5. The cubic response is higher for the compound containing thiophene groups due to the more effective π-electrons delocalization through this group. Therefore, sulfur ring in a conjugated structure is much more effective than other rings as furan in increasing optical nonlinearity.51 As it is known, the thiophene containing complexes exhibit quite high optical nonlinearities.52,53 Obtained NLO parameters for our compounds are compatible with the latter obtained for other guest–host systems.23,54–57
| Sample | n2, 10−19 m2 W−1 | β, 10−12 m W−1 | Re(χ(3)), 10−21 m2 V−2 | Im(χ(3)), 10−21 m2 V−2 | χ(3), 10−21 m2 V−2 |
|---|---|---|---|---|---|
| S4 | −11 | 6.5 | −7.9 | 2.0 | 8.2 |
| S5 | −33 | 21 | −24 | 6.4 | 25 |
| S6 | −7.8 | 7.8 | −5.6 | 2.4 | 6.1 |
The relations between n2 and Re(χ(3)) as also between β and Im(χ(3)) in the international system (SI) of units are given by the following equations:58,59
 | (9) |
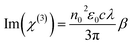 | (10) |
Using these equations the real, imaginary and total values of cubic NLO susceptibilities have been calculated and are presented in Table 5. The values of cubic NLO susceptibility, obtained by Z-scan method, were found to be about one order of magnitude higher than that obtained by THG using Maker fringe method. There are at least three possible reasons which may explain this situation. First is the spectral dependence of NLO susceptibility, e.g. it usually increases with a laser frequency.60–62 Inherently, the χ(3) calculation of our compounds were performed at laser wavelengths 1064 nm and 532 nm for THG and Z-scan, respectively. The second is the amount of mechanisms according to the used technique, which have the impact on NLO response during the measurements. In THG technique the cubic NLO susceptibility has only electronic contribution caused by distortion of electronic clouds in molecules. Meanwhile, in Z-scan, besides electronic contribution in picosecond regime of the laser excitation the χ(3) has also the molecular orientation or/and redistribution contributions. The third reason is connected with different type of investigated samples, e.g. solid polymeric films and liquid solutions, which are, consequently, characterized by their weight and molar concentration of active material. The detailed discussion on the correlation between the chemical structures of benzodifuran-based derivatives and their NLO properties is going to be performed in our further research work after the theoretical quantum chemical calculations on mentioned structures will be done.
Conclusions
In this work the second and third nonlinear optical response of benzodifuran-based derivatives has been determined by means of SHG/THG Maker fringe and Z-scan techniques under picosecond laser pulses. The highest second order NLO susceptibilities after corona poling were obtained for the asymmetric complexes and complexes with a long π-conjugated chains. Calculated cubic NLO susceptibilities were found not to be such dependent on structure of molecules meanwhile few times exceeding the value for silica. According to Z-scan measurements, at least three of benzodifuran-based derivatives exhibit self-defocusing behavior and reverse saturable absorption. The highest NLO refraction and absorption is observed in molecular system which contains thiophene moieties. This material can act as a RSA based optical limiter of picosecond laser pulses at 532 nm. The total third order NLO susceptibilities obtained from Z-scan measurements were found to be about one order of magnitude higher than obtained from THG measurements what is mostly due to the internal mechanisms which have an impact on NLO response in these experimental techniques. Benzodifuran-based derivatives obtained by a green approach seem to be interesting material for photonic applications.Acknowledgements
The authors acknowledge the Pays de la Loire region for its financial support of research work in the framework of the project “Green Chemistry and Organic Semiconductors for Optoelectronics: GREEN SCO”.Notes and references
- A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577 RSC.
- H. Klauk, Chem. Soc. Rev., 2010, 39, 2643 RSC.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- A. Mishra and P. Bauerle, Angew. Chem., Int. Ed., 2012, 51, 2020 CrossRef CAS PubMed.
- A. C. Arias, J. D. MacKenzie, I. McCulloch, J. Rivnay and A. Salleo, Chem. Rev., 2010, 110, 3 CrossRef CAS PubMed.
- M. Mas-Torrent and C. Rovira, Chem. Soc. Rev., 2008, 37, 827 RSC.
- B. Walker, C. Kim and T.-Q. Nguyen, Chem. Mater., 2011, 23, 470 CrossRef CAS.
- W. L. Leong, N. Mathews, B. Tan, S. Vaidyanathan, F. Dotz and S. Mhaisalkar, J. Mater. Chem., 2011, 21, 5203 RSC.
- J. Roncali, Acc. Chem. Res., 2009, 42, 1719 CrossRef CAS PubMed.
- A. Mishra, C.-Q. Ma and P. Bauerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed.
- J. Roncali, Macromol. Rapid Commun., 2007, 28, 1761 CrossRef CAS.
- A. L. Kanibolotsky, I. F. Perepichka and P. J. Skabara, Chem. Soc. Rev., 2010, 39, 2695 RSC.
- W. M. K. P. Wijekoon, K.-S. Lee and P. N. Prasad, Nonlinear Optical Properties of Polymers, in Physical Properties of Polymers Handbook, ed. J. E. Mark, Springer, New York, 2007, p. 795 Search PubMed.
- D. M. Burland, R. D. Miller and C. A. Walsh, Chem. Rev., 1994, 94, 31 CrossRef CAS.
- M. J. Cho, D. H. Choi, P. A. Sullivan, A. J. P. Akelaitis and L. R. Dalton, Prog. Polym. Sci., 2008, 33, 1013 CrossRef CAS.
- I. Papagiannouli, K. Iliopoulos, D. Gindre, B. Sahraoui, O. Krupka, V. Smokal, A. Kolendo and S. Couris, Chem. Phys. Lett., 2012, 554, 107 CrossRef CAS.
- H. Ono, T. Kikuchi and Y. Harato, Appl. Phys. B, 1999, 68, 207 CrossRef CAS.
- Y. Zhang, C. A. Spencer, S. Ghosal, M. K. Casstevens and P. Burzynski, J. Appl. Phys., 1994, 76, 671 CrossRef CAS.
- H. Romer, Theoretical Optics, Wiley-VCH Verlag GmbH, Weinheim, 2005 Search PubMed.
- H. J. Coufal, D. Psaltis and G. T. Sincerbox, Holographic Data Storage, Springer, Berlin, 2000 Search PubMed.
- K. S. Lee, Polymers for Photonics Applications: II, Springer, Berlin, 2003 Search PubMed.
- S. K. Yesodha, C. K. S. Pillai and N. Tsutsumi, Prog. Polym. Sci., 2004, 29, 45 CrossRef CAS.
- H. El Ouazzani, K. Iliopoulos, M. Pranaitis, O. Krupka, V. Smokal, A. Kolendo and B. Sahraoui, J. Phys. Chem. B, 2011, 115, 1944 CrossRef CAS PubMed.
- J. Del Nero, F. M. De Souza and R. B. Capaz, J. Comput. Theor. Nanosci., 2010, 7, 1 CrossRef.
- C. Pakula, C. Hanisch, V. Zaporojtchenko, T. Strunskus, C. Bornholdt, D. Zargarani, R. Herges and F. Faupel, J. Mater. Sci., 2011, 46, 2488 CrossRef CAS.
- A. Bolduc, A. Al Ouahabi, C. Mallet and W. G. Skene, J. Org. Chem., 2013, 78, 9258 CrossRef CAS PubMed.
- H. Tsuji, C. Mitsui, Y. Sato and E. Nakamura, Adv. Mater., 2009, 21, 3776 CrossRef CAS.
- P. Huang, J. Du, M. C. Biewer and M. C. Stephan, J. Mater. Chem. A, 2015, 3, 6244 CAS.
- L. Huo, Y. Huang, B. Fan, X. Guo, Y. Jing, M. Zhang, Y. Li and J. Hou, Chem. Commun., 2012, 48, 3318 RSC.
- C. Moussallem, O. Segut, F. Gohier, M. Allain and P. Frère, ACS Sustainable Chem. Eng., 2014, 2, 1043 CrossRef.
- Z. Du, Y. Chen, W. Chen, S. Qiao, S. Wen, Q. Liu, D. Zhu, M. Sun and R. Yang, Chem.–Asian J., 2014, 9, 2621 CrossRef CAS PubMed.
- D. Sek, A. Iwan, B. Jarzabek, B. Kaczmarczyk, J. Kasperczyk, Z. Mazurak, M. Domanski, K. Karon and M. Lapkowski, Macromolecules, 2008, 41, 6653 CrossRef CAS.
- M. L. Petrus, T. Bein, T. J. Dingemans and P. Docampo, J. Mater. Chem. A, 2015, 3, 12159 CAS.
- T. Tshibaka, S. Bishop, I. U. Roche, S. Dufresne, W. D. Lubell and W. G. Skene, Chem.–Eur. J., 2011, 17, 10879 CrossRef CAS PubMed.
- S. Dufresne, A. Bolduc and W. G. Skene, J. Mater. Chem., 2010, 20, 4861 RSC.
- S. Barik, T. Bletzacker and W. G. Skene, Macromolecules, 2012, 45, 1165 CrossRef CAS.
- M. L. Petrus, R. K. M. Bouwer, U. Lafont, S. Asthanasopoulos, N. C. Greenham and T. J. Dingemans, J. Mater. Chem. A, 2014, 2, 9474 CAS.
- M. L. Petrus, F. S. F. Morgenstern, A. Shadanala, R. H. Friend, N. C. Greenham and T. J. Dingemans, Chem. Mater., 2015, 27, 2990 CrossRef.
- C. Moussallem, F. Gohier, C. Mallet, M. Allain and P. Frère, Tetrahedron, 2012, 68, 8617 CrossRef CAS.
- C. Moussallem, F. Gohier and P. Frère, Tetrahedron Lett., 2015, 56, 5116 CrossRef CAS.
- M. Sheik-Bahae, A. A. Said, T.-H. Wei, D. J. Hagan and E. W. VanStryland, IEEE J. Quantum Electron., 1990, 26, 760 CrossRef CAS.
- P. Aloukos, K. Iliopoulos, S. Couris, D. M. Guldi, C. Sooambar, A. Mateo-Alonso, P. G. Nagaswaran, D. Bonifazi and M. Prato, J. Mater. Chem., 2011, 21, 2524 RSC.
- G. J. Lee, S. W. Cha, S. J. Jeon, J.-I. Jin and J. S. Yoon, J. Korean Phys. Soc., 2001, 39, 912 CAS.
- S. K. Kurtz, J. Jerphagnon and M. M. Choy, Nonlinear dielectric susceptibilities, Landolt-Boernstein New Series, 1979, vol. 11, p. 671 Search PubMed.
- K. Kubodera and H. Kobayashi, Mol. Cryst. Liq. Cryst., 1990, 182, 103 CrossRef.
- F. Kajzar, Y. Okada-Shudo, C. Meritt and Z. Kafafi, Synth. Met., 2001, 117, 189 CrossRef CAS.
- U. Gubler and C. Bosshard, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 10702 CrossRef CAS.
- E. W. Van Stryland and M. Sheik-Bahae, Z-scan Measurements of Optical Nonlinearities in Characterization Techniques and Tabulations for Organic Nonlinear Materials, ed. M. G. Kuzyk and C. W. Dirk, Marcel Dekker, Inc., 1998, p. 655 Search PubMed.
- L. Pálfalvi, B. C. Tóth, G. Almási, J. A. Fülöp and J. Hebling, Appl. Phys. B, 2009, 97, 679 CrossRef.
- R. W. Boyd, Nonlinear Optics, Academic Press, Boston, Amsterdam, 2nd edn, 2003 Search PubMed.
- J. Messier, F. Kajzar and P. Prasad, Organic molecules for nonlinear optics and photonics, Kluwer Academic Publishers, Dordrecht, 1991 Search PubMed.
- Z. Chen, X. Zhoua, Z. Li, L. Niua, J. Yia and F. Zhang, J. Photochem. Photobiol., A, 2011, 218, 64 CrossRef CAS.
- A. Faccinetto, S. Mazzucato, D. Pedron, R. Bozio, S. Destri and W. Porzio, ChemPhysChem, 2008, 9, 2028 CrossRef CAS PubMed.
- K. Iliopoulos, A. El-Ghayoury, H. El Ouazzani, M. Pranaitis, E. Belhadj, E. Ripaud, M. Mazari, M. Sallé, D. Gindre and B. Sahraoui, Opt. Express, 2012, 20, 25311 CrossRef CAS PubMed.
- I. Papagiannouli, K. Iliopoulos, D. Gindre, B. Sahraoui, O. Krupka, V. Smokal, A. Kolendo and S. Couris, Chem. Phys. Lett., 2012, 554, 107 CrossRef CAS.
- B. Sahraoui, X. Nguyen Phu, T. Nozdryn and J. Cousseau, Synth. Met., 2000, 115, 261 CrossRef CAS.
- B. Sahraoui, X. Nguyen Phu, M. Sallé and A. Gorgues, Opt. Lett., 1998, 23, 1811 CrossRef CAS PubMed.
- R. Coso and J. Solis, J. Opt. Soc. Am. B, 2004, 21, 640 CrossRef.
- G. I. Stegeman and R. A. Stegeman, Nonlinear optics: phenomena, materials, and devices, Wiley, Hoboken, 2012 Search PubMed.
- J. Szeremeta, R. Kolkowski, M. Nyk and M. Samoc, J. Phys. Chem. C, 2013, 117, 26197 CAS.
- A. Santhi, V. V. Namboodiri, P. Radhakrishnan and V. P. N. Nampoori, J. Appl. Phys., 2006, 100, 053109 CrossRef.
- S. Couris, E. Koudoumas, A. A. Ruth and S. Leach, J. Phys. B: At., Mol. Opt. Phys., 1995, 28, 4537 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |