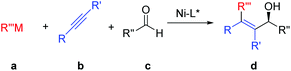Enantioselective nickel-catalyzed alkylative alkyne–aldehyde cross-couplings†
Ming
Nie
,
Wenzhen
Fu
,
Ziping
Cao
and
Wenjun
Tang
*
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, 354 Ling Ling Rd, Shanghai 200032, P. R. China. E-mail: tangwenjun@sioc.ac.cn
First published on 23rd July 2015
Abstract
An efficient asymmetric nickel-catalyzed alkylative alkyne–aldehyde cross-coupling is developed by employing a P-chiral monophosphorus ligand BI-DIME, allowing rapid access to a series of chiral tetra-substituted olefinic allylic alcohols in high yields and good to excellent ees. The three-component reactions enjoy excellent regio- and enantioselectivities, and a broad substrate scope from readily available starting materials.
The development of efficient multicomponent reactions1 for the rapid construction of molecules with complexity from simple feedstock in high yields and excellent selectivities has become a field of significant interest to synthetic organic chemists. Such reactions are often more environmentally benign, energy-saving, and with high atom and step economy. Chiral tetra-substituted olefinic allylic alcohols2 have increasingly become a class of important intermediates in organic synthesis. Their syntheses are often tedious, with the requirement of multiple synthetic steps. The single-step, three-component, nickel-catalyzed alkylative alkyne–aldehyde cross-coupling3,4 from readily available starting materials under mild conditions has provided an appealing method for constructing such structures (Fig. 1). Since Montgomery first reported5 this transformation, enantioselective nickel-catalyzed alkylative alkyne–aldehyde coupling has attracted considerable attention. Due to the lack of efficient chiral nickel catalysts,6,7 only a few highly enantioselective examples were reported with limited substrate scope. Excellent enantioselectivities were achieved by Zhou and coworkers3f by employing a chiral 6,6′-disubstituted spiro phosphoramidite ligand on a series of aryl aldehydes as substrates. In contrast, only one example with alkyl aldehyde was reported at 10 mol% nickel catalyst loading. We report herein an efficient asymmetric nickel-catalyzed alkylative alkyne–aldehyde coupling that has provided high yields and good to excellent enantioselectivities for a series of chiral tetrasubstituted olefinic allylic alcohols with the employment of a P-chiral monophosphorus ligand BI-DIME.
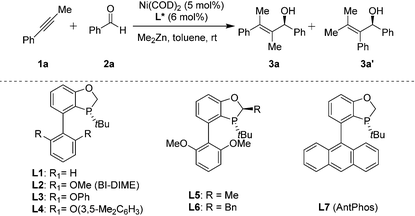
We recently reported8 an asymmetric nickel-catalyzed reductive cyclization of alkynones that has led to a series of tertiary allylic alcohols bearing furan/pyran rings in excellent yields and enantioselectivities with AntPhos/BI-DIME9 as the chiral ligand. Its broad substrate scope for both alkyl and aryl substrates prompted us to investigate the intermolecular alkylative alkyne–aldehyde couplings with the P-chiral phosphorus ligands developed in our laboratory.10 Thus, the ternary reactions between 1-phenyl-1-propyne (1a), benzaldehyde (2a), and dimethylzinc were carried out at room temperature in toluene for 16 h under nitrogen in the presence of Ni(COD)2 (5 mol%) and a P-chiral biaryl monophosphorus ligand (6 mol%). As shown in Table 1, the ligand structure significantly impacts its reactivity and enantioselectivity. When ligand L1 with no substituents on the low aryl ring was employed, the desired coupling product 3a was isolated in 67% yield and 50% ee (entry 1). Nearly no formation of its regio-isomer 3a′ was observed according to 1H NMR. When BI-DIME with two methoxy substituents on the low aryl ring was used as the ligand, a high yield (95%) and excellent enantioselectivity (90%) were observed (entry 2). However, ligands with aryloxy substituents on the low aryl ring provided little or no formation of 3a (entries 3 and 4). Ligands with alkyl groups at the 2 position of the oxophosphole ring also provided diminished reactivities, as both L5 and L6 were less effective (entries 5 and 6). AntPhos, which was highly effective in reductive cyclization of alkynones,8 also proved to be less efficient (entry 7). The solvent also played an important role in the reactivity and enantioselectivity, and toluene was found to be the best among the five solvents studied. We believed that the strong solvation of polar solvents with the nickel catalyst inhibited the cycloaddition step of the catalytic cycle.
| Entriesa | Ligand | Solvent | Yield of 3ab (%) | eec (%) |
|---|---|---|---|---|
| a The reactions were carried out in a solvent (0.1 M) at rt under nitrogen for 16 h with 1a (0.20 mmol), 2a (0.40 mmol), Ni(COD)2 (5 mol%), ligand (6 mol%), and Me2Zn (0.60 mmol). The absolute configuration of 2a was determined by comparison of its optical rotation with reported data.3f b Isolated yields, ratio of 3a/3a′ > 20/1 in all cases. c Determined by chiral HPLC on a Chiralcel OD-H column. | ||||
| 1 | L1 | Toluene | 67 | 50 |
| 2 | L2 | Toluene | 95 | 90 |
| 3 | L3 | Toluene | 12 | 74 |
| 4 | L4 | Toluene | <5 | nd |
| 5 | L5 | Toluene | 36 | 76 |
| 6 | L6 | Toluene | <5 | nd |
| 7 | L7 | Toluene | 46 | 42 |
| 8 | L2 | Dioxane | 78 | 79 |
| 9 | L2 | THF | 25 | 70 |
| 10 | L2 | DCM | 19 | 82 |
| 11 | L2 | DME | 0 | — |
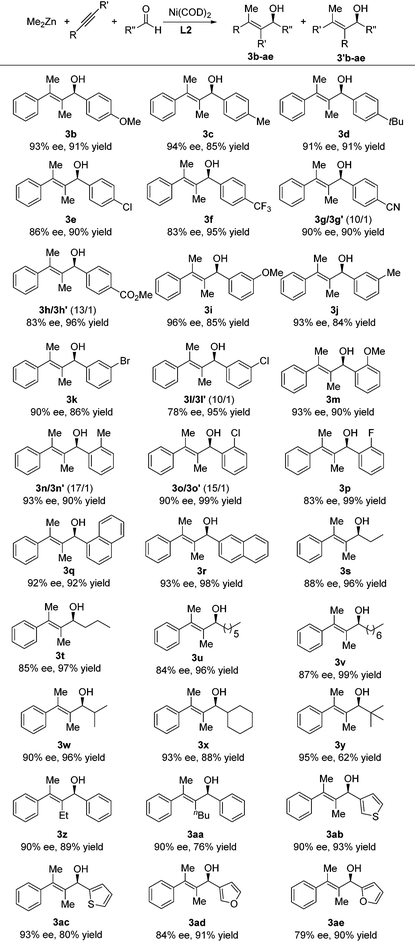
We then looked into the substrate scope of the reductive alkyne–aldehyde cross-coupling. As can be seen in Table 2, a series of chiral allylic alcohols with tetrasubstituted olefin functionality were prepared in high yields (62–99%), high regioselectivities (3b–ae/3′b–ae > 10/1), and good to excellent enantioselectivities (78–96% ee). A range of aryl aldehydes was successfully employed regardless of their electronic properties and substitution patterns. No significant electronic effect of the aryl aldehyde was observed on the enantioselectivity of the coupling (3bvs.3e–h). Besides para- or meta-substituted aryl aldehydes, ortho-substituted aryl aldehydes were also successfully employed for the first time in reductive alkyne–aldehyde cross-couplings to provide excellent ees (3m–p). Both 1- and 2-naphthaldehydes (3q–r) were applicable for this transformation. An array of aliphatic aldehydes (3s–y) was also employed to provide corresponding chiral allylic alcohols in good yields and excellent ees. The enantioselectivities increased with the bulkiness of the aldehyde, as the reaction with pivalaldehyde yielded the alcohol 3y in 95% ee albeit with a moderate yield (62%). 1-Phenyl-1-butyne and 1-phenyl-1-hexyne were also successfully employed to provide the corresponding products in excellent regioselectivities. This was in contrast to Zhou's system3f where a significant amount of regio-isomers was formed. Finally, heteroaryls such as thiophene and furan were well tolerated. Both compounds 3ab and 3ac with thiophene moieties were successfully prepared in excellent ees. A moderate ee (79% or 84%) was achieved when 2- or 3-furanaldehyde was employed as the substrate.
a Reaction conditions: alkynes (0.20 mmol), aldehydes (0.40 mmol), Ni(COD)2 (5 mol%), L2 (6 mol%), and Me2Zn (0.60 mmol), toluene (0.10 M), rt under N2, 16 h, isolated yield. 3b–ae/3′b–ae > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 unless otherwise specified. The absolute configurations were determined by comparison of their optical rotations with reported data or by analogy. 1 unless otherwise specified. The absolute configurations were determined by comparison of their optical rotations with reported data or by analogy.
|
|---|
|
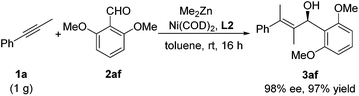
To demonstrate the practicality of this transformation, the reaction of 1-phenyl-1-propyne (1a), 2,6-dimethoxybenzaldehyde (2af), and dimethylzinc with the Ni-(R)-BI-DIME catalyst was carried out in toluene on a 1 gram scale. The desired product 3af was obtained in 97% yield and 98% ee, demonstrating a highly efficient and practical example of enantioselective alkylative alkyne–aldehyde cross-coupling with a di-ortho-substituted aldehyde as the substrate (Scheme 1).
Previous mechanistic studies by Montgomery,3b,11,13a Jamison,7a,12,13b,13c and Houk3b,13 as well as by us8 on nickel-catalyzed reductive/alkylative alkyne–carbonyl cross-coupling has suggested that the stereoselectivity of this three-component transformation is likely to be determined at the cycloaddition step of alkyne–aldehyde with a Ni(0)-(R)-BI-DIME species. To shed light on the stereochemical translation of this reaction, the two conformers I and II of the cycloaddition intermediates derived from 1a, 2a and the Ni-(R)-BI-DIME complex were subjected to DFT calculations (Fig. 2).14 The calculated energy of conformer I was ∼0.5 kcal mol−1 higher than that of conformer II, which was very likely due to a greater steric influence of the tert-butyl group of (R)-BI-DIME over the phenyl group in 1a. The more favorable conformer II led to the chiral alcohol product with the observed stereochemistry. A more sterically crowded Ni(II) structure would provide an even greater energy difference between two conformers. Higher enantioselectivities were therefore achieved when more hindered alkyl aldehydes or di-ortho-substituted aryl aldehydes were employed as the reagents.
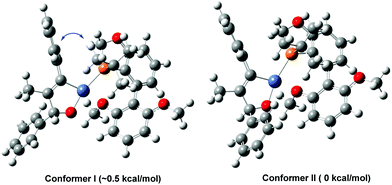 | ||
| Fig. 2 The DFT calculated conformers I and II of the cycloaddition intermediates derived from 1a, 2a, and the Ni-(R)-BI-DIME complex. | ||
In summary, we have developed an efficient asymmetric nickel-catalyzed alkylative alkyne–aldehyde cross-coupling with the use of a P-chiral phosphorus ligand BI-DIME. The three-component reaction enjoys good to excellent regio- and enantioselectivities, and provides a broad substrate scope with good functional group compatibility. A series of chiral allylic alcohols with tetra-substituted olefin moieties were thus formed in high yields and ees. Our further study focuses on expanding its substrate scope and applications to medicinal chemistry and natural product synthesis.
Acknowledgements
We are grateful to the NSFC (21432007, 21272254), STCSM (13J1410900), and the “Thousand Plan” Youth Program.Notes and references
- Selected reviews: (a) D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed; (b) B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef CAS PubMed; (c) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed; (d) A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef CAS PubMed; (e) G. Guillena, D. J. Ramon and M. Yus, Tetrahedron: Asymmetry, 2007, 18, 693 CrossRef CAS PubMed; (f) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Res., 2012, 41, 3969 RSC; (g) J. E. Biggs-Houck, A. Younai and J. T. Shaw, Curr. Opin. Chem. Biol., 2010, 14, 371 CrossRef CAS PubMed; (h) N. R. Candeias, F. Montalbano, P. Cal and P. Gois, Chem. Rev., 2010, 110, 6169 CrossRef CAS PubMed.
- (a) Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon Press, Oxford, 1991 Search PubMed; (b) A. H. Hoveyda, D. A. Evans and G. C. Fu, Chem. Rev., 1993, 93, 1307 CrossRef CAS; (c) D. Banerjee, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2014, 53, 13049 CrossRef CAS PubMed; (d) W. Zi, Y.-M. Wang and D. F. Toste, J. Am. Chem. Soc., 2014, 136, 12864 CrossRef CAS PubMed; (e) E. Vedajs and J. A. Mackay, Org. Lett., 2001, 3, 535 CrossRef PubMed; (f) M. I. Calaza, E. Hupe and P. Knochel, Org. Lett., 2003, 5, 1059 CrossRef CAS PubMed.
- For recent papers on nickel-catalyzed alkylative/reductive alkyne–aldehyde cross-coupling: (a) E. P. Jackson and J. Montgomery, J. Am. Chem. Soc., 2015, 137, 958 CrossRef CAS PubMed; (b) M. T. Haynes, P. Liu, R. D. Baxter, A. J. Nett, K. N. Houk and J. Montgomery, J. Am. Chem. Soc., 2014, 136, 17495 CrossRef CAS PubMed; (c) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed; (d) K. Tanaka and Y. Taiima, Eur. J. Org. Chem., 2012, 3715 CrossRef CAS PubMed; (e) J. Montgomery, Acc. Chem. Res., 2000, 33, 467 CrossRef CAS PubMed; (f) Y. Yang, S.-F. Zhu, C.-Y. Zhou and Q.-L. Zhou, J. Am. Chem. Soc., 2008, 130, 14052 CrossRef CAS PubMed.
- For recent papers on catalytic reductive coupling with other metals: (a) H. A. Reichard, M. Mclaughlin, M. Z. Chen and G. C. Micalizio, Eur. J. Org. Chem., 2010, 391 CrossRef CAS PubMed; (b) J. F. Bower, I. S. Kim, R. L. Patman and M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 34 CrossRef CAS PubMed; (c) E. Skucas, M.-Y. Ngai, V. Komanduri and M. J. Krische, Acc. Chem. Res., 2007, 40, 1394 CrossRef CAS PubMed; (d) A. B. Bahadoor, A. Flyer and G. C. Micalizio, J. Am. Chem. Soc., 2005, 127, 3694 CrossRef CAS PubMed; (e) I. Ojima, M. Tzamarioudaki and C. Y. Tsai, J. Am. Chem. Soc., 1994, 116, 3643 CrossRef CAS.
- (a) E. Oblinger and J. Montgomery, J. Am. Chem. Soc., 1997, 119, 9065 CrossRef CAS; (b) X. Qi and J. Montgomery, J. Org. Chem., 1999, 64, 9310 CrossRef CAS; (c) M. Lozanov and J. Montgomery, J. Am. Chem. Soc., 2002, 124, 2106 CrossRef CAS PubMed; (d) Y. Ni, K. K. Amarasinghe and J. Montgomery, Org. Lett., 2002, 4, 1743 CrossRef CAS PubMed.
- For recent papers on non-enantioselective nickel-catalyzed reductive/alkylative coupling: (a) X.-Q. Tang and J. Montgomery, J. Am. Chem. Soc., 1999, 121, 6098 CrossRef CAS; (b) W.-S. Huang, J. Chan and T. F. Jamison, Org. Lett., 2000, 2, 4221 CrossRef CAS PubMed; (c) K. Takai, S. Sakamoto and T. Isshiki, Org. Lett., 2003, 5, 653 CrossRef CAS PubMed; (d) K. M. Miller, T. Luanphaisarnnont, C. Molinaro and T. F. Jamison, J. Am. Chem. Soc., 2004, 126, 4130 CrossRef CAS PubMed; (e) K. M. Miller and T. F. Jamison, J. Am. Chem. Soc., 2004, 126, 15342 CrossRef CAS PubMed.
- For recent papers on asymmetric nickel-catalyzed reductive/alkylative coupling: (a) K. M. Miller, W.-S. Huang and T. F. Jamison, J. Am. Chem. Soc., 2003, 125, 3442 CrossRef CAS PubMed; (b) K. M. Miller and T. F. Jamison, Org. Lett., 2005, 7, 3077 CrossRef CAS PubMed; (c) Y. Sato, N. Saito and M. Mori, J. Am. Chem. Soc., 2000, 122, 2371 CrossRef CAS; (d) Y. Sato, N. Saito and M. Mori, J. Org. Chem., 2002, 67, 9310 CrossRef CAS PubMed; (e) Y. Yang, S.-F. Zhu, H.-F. Duan, C.-Y. Zhou, L.-X. Wang and Q.-L. Zhou, J. Am. Chem. Soc., 2007, 129, 2248 CrossRef CAS PubMed; (f) M. R. Chaulagain, G. J. Sormunen and J. Montgomery, J. Am. Chem. Soc., 2007, 129, 9568 CrossRef CAS PubMed; (g) C. T. Check, K. P. Jang, C. B. Schwamb, A. S. Wong, M. H. Wang and K. A. Scheidt, Angew. Chem., Int. Ed., 2015, 54, 4264 CrossRef CAS PubMed.
- W. Fu, M. Nie, A. Wang and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 2520 CrossRef CAS PubMed.
- (a) W. Tang, A. G. Capacci, X. Wei, W. Li, A. White, N. D. Patel, J. Savoie, J. J. Gao, S. Rodriguez, B. Qu, N. Haddad, B. Z. Lu, D. Krishnamurthy, N. K. Yee and C. H. Senanayake, Angew. Chem., Int. Ed., 2010, 49, 5879 CrossRef CAS PubMed; (b) W. Tang, S. Keshipeddy, Y. Zhang, X. Wei, J. Savoie, N. D. Patel, N. K. Yee and C. H. Senanayake, Org. Lett., 2011, 13, 1366 CrossRef CAS PubMed; (c) S. Rodriguez, B. Qu, N. Haddad, D. C. Reeves, W. Tang, H. Lee, D. Krishnamurthy and C. H. Senanayake, Adv. Synth. Catal., 2011, 353, 533 CrossRef CAS PubMed; (d) Q. Zhao, C. Li, C. H. Senanayake and W. Tang, Chem. – Eur. J., 2013, 19, 2261 CrossRef CAS PubMed; (e) C. Li, G. Xiao, Q. Zhao, H. Liu, T. Wang and W. Tang, Org. Chem. Front., 2014, 1, 225 RSC.
- (a) W. Tang, N. D. Patel, G. Xu, X. Xu, J. Savoie, S. Ma, M.-H. Hao, S. Keshipeddy, A. G. Capacci, X. Wei, Y. Zhang, J. J. Gao, W. Li, S. Rodriguez, B. Z. Lu, N. K. Yee and C. H. Senanayake, Org. Lett., 2012, 14, 2258 CrossRef CAS PubMed; (b) K. Li, N. Hu, R. Luo, W. Yuan and W. Tang, J. Org. Chem., 2013, 78, 6350 CrossRef CAS PubMed; (c) G. Xu, W. Fu, G. Liu, C. H. Senanayake and W. Tang, J. Am. Chem. Soc., 2014, 136, 570 CrossRef CAS PubMed; (d) G. Xu, Q. Zhao and W. Tang, Chin. J. Org. Chem., 2014, 34, 1919 CrossRef CAS; (e) C. Li, T. Chen, B. Li, G. Xiao and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 3792 CrossRef CAS PubMed; (f) K. Du, P. Guo, Y. Chen, Z. Cao, Z. Wang and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 3033 CrossRef CAS PubMed.
- (a) G. M. Mahandru, G. Liu and J. Montgomery, J. Am. Chem. Soc., 2004, 126, 3698 CrossRef CAS PubMed; (b) G. M. Mahandru, G. Liu and J. Montgomery, J. Am. Chem. Soc., 2004, 126, 15316 CrossRef CAS; (c) H. A. Malik, G. J. Sormunen and J. Montgomery, J. Am. Chem. Soc., 2010, 132, 6304 CrossRef CAS PubMed.
- S. J. Patel and T. F. Jamison, Angew. Chem., Int. Ed., 2003, 42, 1364 CrossRef CAS PubMed.
- (a) P. Liu, J. Montgomery and K. N. Houk, J. Am. Chem. Soc., 2011, 133, 6956 CrossRef CAS PubMed; (b) P. Liu, P. McCarren, P. H.-Y. Cheong, T. F. Jamison and K. N. Houk, J. Am. Chem. Soc., 2010, 132, 2050 CrossRef CAS PubMed; (c) P. McCarren, P. Liu, P. H.-Y. Cheong, T. F. Jamison and K. N. Houk, J. Am. Chem. Soc., 2009, 131, 6654 CrossRef CAS PubMed.
- DFT calculations were performed with the Gaussian 03 package and the geometries were optimized with B3LYP and a mixed basis set of 6-31G(d) for all atoms.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures of cross-coupling reactions, characterization data, and NMR spectra. See DOI: 10.1039/c5qo00148j |
| This journal is © the Partner Organisations 2015 |