Chemical fixation of CO2/CS2 to access iodoallenyl oxazolidinones and allenyl thiazolidine-thiones†
Xuejian
Li
,
Qinglong
Liu
and
Wangze
Song
 *
*
Cancer Hospital of Dalian University of Technology, School of Chemistry, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024, P. R. China. E-mail: wzsong@dlut.edu.cn
First published on 24th July 2024
Abstract
Constructing heterocyclic compounds by chemical fixation of CO2/CS2 as a C1 building block is a promising approach. An efficient and environmentally friendly synthetic approach has been developed using CO2/CS2 to prepare complicated allenyl heterocycles with high yields and diastereoselectivities in a metal-free manner under mild conditions. NIS promoted CO2 fixation and the cyclization reaction by exclusive 1,4-syn-addition of 1,3-enynes rather than 1,2-addition or 3,4-addition, while CS2 participated in unique 1,4-syn-hydrothiolation of 1,3-enynes to afford allenyl heterocycles with different reaction patterns.
With the increasing of the ever-growing carbon dioxide (CO2) level in the aerosphere, a variety of approaches have been developed for CO2 capture, storage and its utilization in the past few decades.1 With CO2 being an abundant, nontoxic and easily available C1 building block, the fixation and post-transformations into valuable products are very hot topics related to the effective reduction of CO2 emissions nowadays.2 However, chemists have achieved very limited yields for the conversion of CO2 to carboxylic acids, (poly)carbonates, carbamates, ureas and so on.1a,3 Therefore, efficiently recycling CO2 into value-added products remains a formidable challenge especially under atmospheric pressure due to the thermodynamic stability and kinetic inertness of CO2.
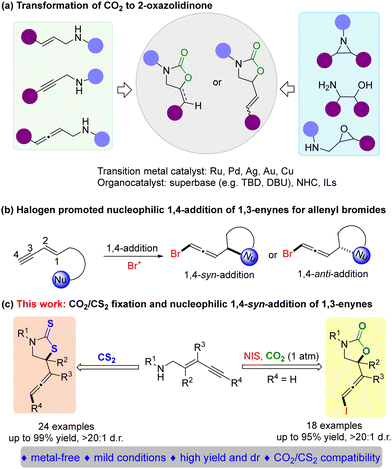
2-Oxazolidinones are some of the important heterocyclic compounds, and have been widely applied as chemical intermediates, chiral auxiliaries, drugs and other bioactive molecules.4 To date, several methods were developed for constructing oxazolidinones using CO2 as a sustainable feedstock such as carbonylations of amino alcohols with phosgene/CO2, carbamations of unsaturated amines/epoxy amines with CO2, coupling reactions between aziridine and CO2, etc.5–7 However, harsh reaction conditions including strong acids/bases, or high pressures, have usually been utilized to activate inert CO2. Recently, several catalytic approaches for the carboxylative cyclization of unsaturated amines with CO2 have been disclosed, including using transition metal catalysts based on Ru, Pd, Ag, Au, and Cu8 and organocatalysts obtained using superbases,9 ionic liquids10 and N-heterocyclic carbene11 (Scheme 1a). Additionally, halogen promoted intramolecular nucleophilic 1,4-syn/anti-addition of 1,3-enynes represents one of the powerful approaches for the stereoselective introduction of allenyl bromides (Scheme 1b).12 Allenes are attractive molecules, and serve as versatile synthons for preparation of a variety of bioactive compounds.13 Therefore, we envisioned that 1,3-enynes could efficiently activate CO2 to undergo nucleophilic 1,4-addition rather than 1,2-addition or 3,4-addition promoted by halogen. Such a designed reaction can not only afford important haloallenyl-containing 2-oxazolidones from simple starting materials, but also expand the diversity of CO2 transformations (Scheme 1c).
 | ||
| Scheme 1 Synthesis of oxazolidinones from CO2. (a) Transformation of CO2 to 2-oxazolidinone. (b) Halogen promoted nucleophilic addition. (c) CO2/CS2 fixation and nucleophilic addition. | ||
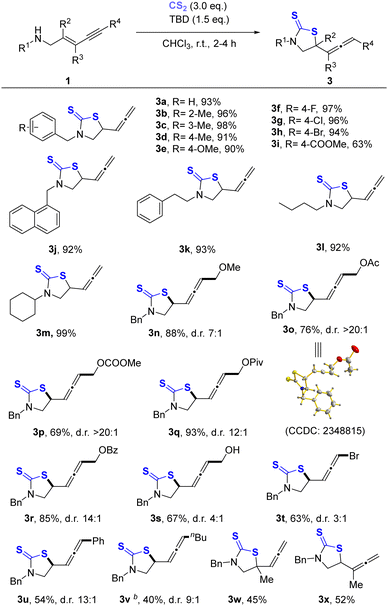
Carbon disulfide (CS2) as an isoelectronic analogue of carbon dioxide is extensively used to prepare versatile organosulfur compounds due to its low cost, stability, and easy availability. A thiazolidine-2-thione skeleton is a useful intermediate in the fields of medicine, agriculture, and fine chemicals.14 Although the synthetic methods for the preparation of thiazolidine-2-thiones using CS2 have been reported,15–17 the reaction between CS2 and 1,3-enynes to afford allenes is still unprecedented. Compared to CO2, CS2 is considered to be more reactive due to the weaker C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond.18 Thus, we rationalized that CS2 could rapidly react with 1,3-enynes even without additional electrophilic reagents. Herein, we proposed a concept for chemical fixation of CO2/CS2 for converting these C1 building blocks to unique iodoallenyl 2-oxazolidones or allenyl thiazolidine-2-thiones by exclusive 1,4-syn-addition of 1,3-enynes.
S double bond.18 Thus, we rationalized that CS2 could rapidly react with 1,3-enynes even without additional electrophilic reagents. Herein, we proposed a concept for chemical fixation of CO2/CS2 for converting these C1 building blocks to unique iodoallenyl 2-oxazolidones or allenyl thiazolidine-2-thiones by exclusive 1,4-syn-addition of 1,3-enynes.
To test the concept, we began our investigation by using 1a as the model substrate, and treated with DBU and NIS under 1 atm CO2 in CHCl3 at room temperature. The iodoallenyl oxazolidinone product 2a was obtained in 28% yield with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table S1, ESI,† entry 1). Then, various bases were screened (Table S1, ESI,† entries 2–6). TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene) could provide better combined yield and diastereoselectivity, generating product 2a in 48% isolated yield and 3
1 dr (Table S1, ESI,† entry 1). Then, various bases were screened (Table S1, ESI,† entries 2–6). TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene) could provide better combined yield and diastereoselectivity, generating product 2a in 48% isolated yield and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Next, different solvents and binary mixed solvents were explored and best results were obtained using a toluene/1,4-dioxane (4
1 dr. Next, different solvents and binary mixed solvents were explored and best results were obtained using a toluene/1,4-dioxane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system (Table S1, entry 25, see the ESI† for optimization details).19
1) system (Table S1, entry 25, see the ESI† for optimization details).19
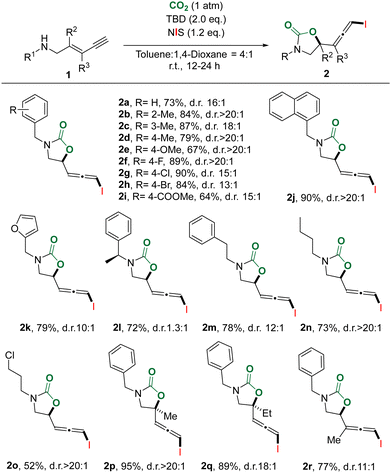
With the optimal reaction conditions in hand, we investigated the substitution effect on the amines and alkenes for the substrate scope (Table 1). Various changes at nitrogen and alkene substituents could be well tolerated. For the diverse substituents of nitrogen, no obvious electronic effects were observed in most cases. The ortho-, meta- and para-methylbenzyl substrates provided desired products (2b–2d) with a high yield (79–87%) and dr (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These results indicate that different substituted patterns may affect the reactivity. The more electron-donating para-methoxy group gave the desired product (2e) in 67% with excellent dr (> 20
1). These results indicate that different substituted patterns may affect the reactivity. The more electron-donating para-methoxy group gave the desired product (2e) in 67% with excellent dr (> 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Therefore, more para-substituted substrates were examined for this CO2 chemical fixation. para-Halo-substrates also showed high yields (84–90%) even though the diastereoselectivities from fluoro- to chloro- and bromo- slightly decreased (2f–2h). The desirable product (2i) was afforded in moderate yield and dr for the more electron-withdrawing ester substrate. To our delight, sterically demanding 1-naphthylmethyl carbamate (2j) was acquired in excellent yield (90%) and diastereoselectivity (dr > 20
1). Therefore, more para-substituted substrates were examined for this CO2 chemical fixation. para-Halo-substrates also showed high yields (84–90%) even though the diastereoselectivities from fluoro- to chloro- and bromo- slightly decreased (2f–2h). The desirable product (2i) was afforded in moderate yield and dr for the more electron-withdrawing ester substrate. To our delight, sterically demanding 1-naphthylmethyl carbamate (2j) was acquired in excellent yield (90%) and diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In contrast, a lower dr was observed for the heterocyclic product (2k) obtained from the furan-2-methyl-substrate. Besides the benzyl group, other alkyl substituted substrates, especially for enantiomerically pure methyl benzyl amine, could also provide corresponding products (2l–2o) in moderate to good yields and dr values. Upon substitution of the alkene near amine by a methyl or an ethyl group, quaternary carbon-containing allenyl oxazolidinones were obtained in very high yields and dr (2p, 2q). In the case of substitution of the olefin away from amine by a methyl group, the product (2r) with a trisubstituted iodoallene was afforded in 77% yield and 11
1). In contrast, a lower dr was observed for the heterocyclic product (2k) obtained from the furan-2-methyl-substrate. Besides the benzyl group, other alkyl substituted substrates, especially for enantiomerically pure methyl benzyl amine, could also provide corresponding products (2l–2o) in moderate to good yields and dr values. Upon substitution of the alkene near amine by a methyl or an ethyl group, quaternary carbon-containing allenyl oxazolidinones were obtained in very high yields and dr (2p, 2q). In the case of substitution of the olefin away from amine by a methyl group, the product (2r) with a trisubstituted iodoallene was afforded in 77% yield and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Unfortunately, neither aryl-substituted substrates nor strongly electron-withdrawing substituents on the amine generated desirable products. These results suggest that the nucleophilicity of nitrogen is very crucial to this transformation. Mixtures of 1,2-addition and 1,4-addition products were obtained using the internal alkynyl substrates (see the ESI† for details).
1 dr. Unfortunately, neither aryl-substituted substrates nor strongly electron-withdrawing substituents on the amine generated desirable products. These results suggest that the nucleophilicity of nitrogen is very crucial to this transformation. Mixtures of 1,2-addition and 1,4-addition products were obtained using the internal alkynyl substrates (see the ESI† for details).
a Standard reaction conditions: 1 (0.1 mmol), TBD (0.2 mmol) and toluene:1,4-dioxane (2 mL, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were stirred for 10 min under a CO2 atmosphere (1 atm) at rt before NIS (0.12 mmol) was added, then the mixture was stirred for 12–24 h with a CO2 balloon (1 atm). 1) were stirred for 10 min under a CO2 atmosphere (1 atm) at rt before NIS (0.12 mmol) was added, then the mixture was stirred for 12–24 h with a CO2 balloon (1 atm).
|
|---|
|
Encouraged by the successful iodoallenylation of (E)-N-benzylpent-2-en-4-yn-1-amine (1a) and CO2, the model reaction of CS2 with 1a was also carefully examined. In contrast, hydrothiolation of 1,3-enyne can proceed smoothly without any activation by electrophilic halogen. It may be due to more suitable nucleophilicity of dithiocarbamate than that of carbamate for this transformation because S was generally considered as a softer nucleophile to preferentially react with a comparable electrophile of 1,3-enyne. Desired allenyl thiazolidine-2-thione 3a was achieved in 96% yield by the combination of 1.5 equiv. TBD and 3.0 equiv. CS2 in CHCl3 at room temperature for 4 h. For more optimal details, please see the ESI.†
The substrate scope for TBD-promoted hydrothiolation of various 1,3-enynes with CS2 is explored in Table 2. It shows that most of the changes for benzyl amine substrates were allowed, and the target products (3a–3h) were obtained in excellent yields (>90%). However, for the strong electron-withdrawing ester group substituted substrate, the product 3i was obtained in 63% yield. Besides the benzyl group, other substituents on the nitrogen including the naphthyl methyl, phenylethyl, n-butyl or cyclohexyl group provided corresponding products (3j–3m) in excellent yields (>90%) as well. In addition, the activated internal alkyne substrates can be well compatible for such hydrothiolation. Disubstituted allenes (3n–3s) containing ether, ester and free hydroxyl groups were obtained in moderate to good yields and dr values. Unfortunately, the desired bromoallene 3t was isolated in 63% yield and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The results (3u, 3v) for aryl and alkyl substituted internal alkyne were unsatisfactory, despite extending the reaction time to 12 h, which may be attributed to the decreased electrophilicity and bulky steric hindrance of 1,3-enynes. With the substitution of alkene by the methyl group, the target products (3w, 3x) were obtained in 45% and 52% yields, respectively. This may also be influenced by steric hindrance and electrophilicity of the substrate. Remarkably, some products undergoing 1,2-addition were also observed (see the ESI† for details).
1 dr. The results (3u, 3v) for aryl and alkyl substituted internal alkyne were unsatisfactory, despite extending the reaction time to 12 h, which may be attributed to the decreased electrophilicity and bulky steric hindrance of 1,3-enynes. With the substitution of alkene by the methyl group, the target products (3w, 3x) were obtained in 45% and 52% yields, respectively. This may also be influenced by steric hindrance and electrophilicity of the substrate. Remarkably, some products undergoing 1,2-addition were also observed (see the ESI† for details).
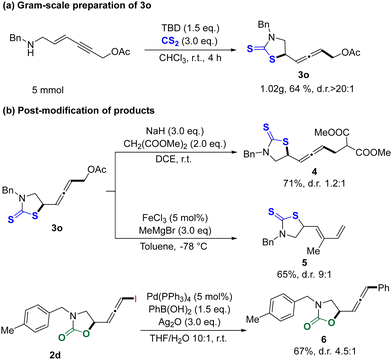
Subsequently, the gram scale synthesis and applicability for such chemical fixation of CO2/CS2 are shown in Scheme 2. As shown in Scheme 2a, allene 3o was smoothly synthesized by gram scale (1.02 g) in 64% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. To highlight the synthetic utilities of present transformation, several post-modifications of the representative products are shown in Scheme 2b. Allene 3o containing –OAc as a leaving group could be applied in the Pd-catalyzed Tsuji–Trost reaction with dimethyl malonate as a nucleophile to obtain a new allene 4 with 71% yield and 1.2
1 dr. To highlight the synthetic utilities of present transformation, several post-modifications of the representative products are shown in Scheme 2b. Allene 3o containing –OAc as a leaving group could be applied in the Pd-catalyzed Tsuji–Trost reaction with dimethyl malonate as a nucleophile to obtain a new allene 4 with 71% yield and 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.20 FeCl3 could promote the nucleophilic addition to allene 3o with MeMgBr as a nucleophile to afford thiazolidine-2-thione 5 containing conjugated diene substitution.21 Moreover, the Suzuki cross-coupling of iodoallene 2d with phenylboronic acid was also performed to give desired product 6 in 67% yield.22
1 dr.20 FeCl3 could promote the nucleophilic addition to allene 3o with MeMgBr as a nucleophile to afford thiazolidine-2-thione 5 containing conjugated diene substitution.21 Moreover, the Suzuki cross-coupling of iodoallene 2d with phenylboronic acid was also performed to give desired product 6 in 67% yield.22
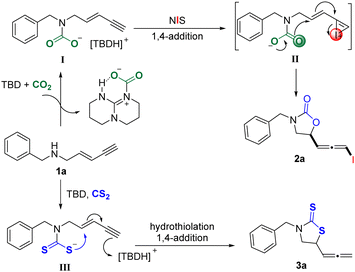
The reaction mechanism is proposed in Scheme 3 on the basis of the current results and literature.9,12 Initially, the nucleophilic addition of 1a to the TBD-CO2 adduct was carried out to obtain the carbamate intermediate I, followed by NIS activation of the alkyne moiety of 1,3-enyne to generate iodonium interm II. Then, the 1,4-syn-addition of 1,3-enyne with carbamate occurred to give the product 2a. Similarly, 1a and CS2 underwent the nucleophilic addition in the presence of TBD to give intermediate III. The hydrothiolation reaction would be immediately performed to form product 3a by 1,4-syn-addition of 1,3-enyne with dithiocarbamate.
In summary, a tandem CO2/CS2 fixation and cyclization reaction was developed to prepare iodoallenyl oxazolidinones and allenyl thiazolidine-thiones and their derivatives with high yields and diastereoselectivities. The remarkable advantages of this strategy include a metal-free manner, mild reaction conditions, high atom-economy and wide substrate scope. Moreover, the two types of allenyl heterocyclic compounds are valuable building blocks for the synthesis of multifunctional molecules or natural products in the future.
This work was supported by grants from the National Natural Science Foundation of China (22375027), the Fundamental Research Funds for the Central Universities (DUT24ZD114, 2023JH2/101700293).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- (a) T. Sakakura, J. Choi and H. Yasuda, Chem. Rev., 2007, 107(6), 2365–2387 CrossRef CAS PubMed; (b) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kuhn, Angew. Chem. Int. Ed., 2011, 50(37), 8510–8537 CrossRef CAS PubMed; (c) E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116(19), 11840–11876 CrossRef PubMed; (d) X. Lu, Springer International Publishing, Switzerland, 2016.
- (a) Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933–5948 CrossRef PubMed; (b) S. Wang and C. Xi, Chem. Soc. Rev., 2019, 48(1), 382–404 RSC; (c) R. Dalpozzo, N. Della Ca, B. Gabriele and R. Mancuso, Catalysts, 2019, 9(6), 511 CrossRef.
- (a) A. Otto, T. Grube, S. Schiebahn and D. Stolten, Energy Environ. Sci., 2015, 8(11), 3283–3297 RSC; (b) M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114(3), 1709–1742 CrossRef CAS PubMed.
- (a) M. Heravi and V. Zadsirjan, Tetrahedron: Asymmetry, 2013, 24(19), 1149–1188 CrossRef CAS; (b) C. Foti, A. Piperno, A. Scala and O. Giuffre, Molecules, 2021, 26(14), 4280–4286 CrossRef CAS PubMed; (c) F. Sun, E. V. Van der Eycken and H. Feng, Adv. Synth. Catal., 2021, 363(23), 5168–5195 CrossRef CAS.
- (a) Z. Zhang, J. Ye, D. Wu, Y. Zhou and D. Yu, Chem. – Asian J., 2018, 13(17), 2292–2306 CrossRef CAS PubMed; (b) C. J. Dinsmore and S. P. Mercer, Org. Lett., 2004, 6(17), 2885–2888 CrossRef CAS PubMed; (c) M. Yoshida, T. Mizuguchi and K. Shishido, Chemistry, 2012, 18(49), 15578–15581 CrossRef CAS PubMed; (d) F. Zhou, S. Xie, X. Gao, R. Zhang, C. Wang, G. Yin and J. Zhou, Green Chem., 2017, 19(16), 908 RSC.
- (a) E. García-Egido, I. Fernández and L. Muñoz, Synth. Commun., 2006, 36(20), 3029–3042 CrossRef; (b) S. Minakata, I. Sasaki and T. Ide, Angew. Chem., Int. Ed., 2010, 49(7), 1309–1311 CrossRef CAS PubMed; (c) J. Ye, L. Song, W. Zhou, T. Ju, Z. Yin, S. S. Yan, Z. Zhang, J. Li and D. Yu, Angew. Chem., Int. Ed., 2016, 55(34), 10022–10026 CrossRef CAS PubMed.
- (a) L. He, X. Dou, Z. Yang and J. Wang, Synlett, 2010,(14), 2159–2163 CrossRef; (b) X. Lin, Z. Yang, L. He and Z. Yuan, Green Chem., 2015, 17(2), 795–798 RSC; (c) X. Tian, X. Jiang, S. Hou, Z. Jiao, J. Han and B. Zhao, Angew. Chem., Int. Ed., 2022, 61(18), e202200123 CrossRef CAS PubMed.
- (a) K. Sekine and T. Yamada, Chem. Soc. Rev., 2016, 45(16), 4524–4532 RSC; (b) T. Zhang, H. Zang, F. Gai, Z. Feng, M. Li and C. Duan, New J. Chem., 2020, 44(35), 15131–15139 RSC; (c) B. Lin, Y. Ruan, Q. Hou, Z. Yuan, Y. Liang and J. Zhang, Org. Biomol. Chem., 2024, 22(27), 5585–5590 RSC.
- (a) S. Zhang and L. He, Aust. J. Chem., 2014, 67(7), 980–988 CrossRef CAS; (b) S. Hajra and A. Biswas, Org. Lett., 2020, 22(13), 4990–4994 CrossRef CAS PubMed; (c) S. Hajra and A. Biswas, Sci. Rep., 2020, 10, 15825 CrossRef CAS PubMed; (d) R. Yousefi, T. J. Struble, J. L. Payne, M. Vishe, N. D. Schley and J. N. Johnston, J. Am. Chem. Soc., 2019, 141(1), 618–625 CrossRef CAS PubMed.
- Z. Yang, L. He, S. Peng and A. Liu, Green Chem., 2010, 12(10), 1850–1854 RSC.
- A. Liu, Y. Dang, H. Zhou, J. Zhang and X. Lu, ChemCatChem, 2018, 10(12), 2686–2692 CrossRef CAS.
- (a) W. Zhang, H. Xu, H. Xu and W. Tang, J. Am. Chem. Soc., 2009, 131(11), 3832–3833 CrossRef CAS PubMed; (b) W. Zhang, N. Liu, C. M. Schienebeck, K. Decloux, S. Zheng, J. B. Werness and W. Tang, Chem. – Eur J., 2012, 18(23), 7296–7305 CrossRef CAS PubMed.
- (a) R. Blieck, M. Taillefer and F. Monnier, Chem. Rev., 2020, 120(24), 13545–13598 CrossRef CAS PubMed; (b) J. Singh, A. Sharma and A. Sharma, Org. Chem. Front., 2021, 8(20), 5651–5667 RSC; (c) J. M. Alonso and P. Almendros, Adv. Synth. Catal., 2023, 365(9), 1332–1384 CrossRef CAS.
- (a) A. W. DeMartino, D. F. Zigler, J. M. Fukuto and P. C. Ford, Chem. Soc. Rev., 2017, 46(1), 21–39 RSC; (b) V. Opletalova, J. Dolezel, K. Kralova, M. Pesko, J. Kunes and J. Jampilek, Molecules, 2011, 16(6), 5207–5227 CrossRef CAS PubMed.
- (a) H. Medini, N. H. Mekni and K. Boujlel, J. Sulfur. Chem., 2015, 36(6), 653–659 CrossRef CAS; (b) A. A. Nechaev, A. A. Peshkov, K. Van Hecke, V. A. Peshkov and E. V. Van der Eycken, Eur. J. Org. Chem., 2017,(6), 1063–1069 CrossRef CAS; (c) A. Biswas and S. Hajra, Adv. Synth. Catal., 2022, 364(17), 3035–3042 CrossRef CAS.
- (a) A. Ziyaei-Halimehjani, K. Marjani and A. Ashouri, Tetrahedron Lett., 2012, 53(27), 3490–3492 CrossRef CAS; (b) K. Safa and M. Alyari, Synthesis, 2015,(02), 256–262 CrossRef CAS.
- (a) M. Anitha and K. C. K. Swamy, Org. Biomol. Chem., 2018, 16(3), 402–413 RSC; (b) Y. Xie, C. Lu, B. Zhao, Q. Wang and Y. Yao, J. Org. Chem., 2019, 84(4), 1951–1958 CrossRef CAS PubMed.
- (a) M. T. C. Ang, L. Phan, A. K. Alshamrani, J. R. Harjani, R. Wang, G. Schatte, N. J. Mosey and P. G. Jessop, Eur. J. Org. Chem., 2015,(33), 7334–7343 CrossRef CAS; (b) K. K. Pandey, Coord. Chem. Rev., 1995, 140, 37–114 CrossRef CAS.
- (a) D. C. Whitehead, R. Yousefi, A. Jaganathan and B. Borhan, J. Am. Chem. Soc., 2010, 132(10), 3298–3300 CrossRef CAS PubMed; (b) L. Zhou, C. K. Tan, X. Jiang, F. Chen and Y. Y. Yeung, J. Am. Chem. Soc., 2010, 132(44), 15474–15476 CrossRef CAS PubMed; (c) H. Wang, H. Qian, J. Zhang and S. Ma, J. Am. Chem. Soc., 2022, 144(28), 12619–12626 CrossRef CAS PubMed.
- Y. Tang, Q. Yu and S. Ma, Org. Chem. Front., 2017, 4(9), 1762–1767 RSC.
- (a) W. J. Kong, S. N. Kessler, H. Wu and J. E. Backvall, Org. Lett., 2023, 25(1), 120–124 CrossRef CAS PubMed; (b) J. Qin, Z. Xiong, C. Cheng and M. Hu Li, J. Org. Lett., 2023, 25(51), 9176–9180 CrossRef CAS PubMed.
- E. M. Woerly, A. H. Cherney, E. K. Davis and M. D. Burke, J. Am. Chem. Soc., 2010, 132(20), 6941–6943 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 2348815. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc02894e |
| This journal is © The Royal Society of Chemistry 2024 |