Open Access Article
Open Access ArticleTransient release of radioactive iodine from the fission of UF4 in 2LiF–BeF2 salt
Junxia Geng abc,
Zhongqi Zhao
abc,
Zhongqi Zhao abc,
Zhiqiang Chengabc,
Wenxin Liab,
Qiang Douabc,
Haiying Fuabc,
Jifeng Huab,
Xiangzhou Caiabc,
Jingen Chen*abc and
Qingnuan Li*abc
abc,
Zhiqiang Chengabc,
Wenxin Liab,
Qiang Douabc,
Haiying Fuabc,
Jifeng Huab,
Xiangzhou Caiabc,
Jingen Chen*abc and
Qingnuan Li*abc
aShanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China. E-mail: liqingnuan@sinap.ac.cn; chenjingen@sinap.ac.cn
bCAS Innovative Academies in TMSR Energy System, Chinese Academy of Sciences, Shanghai 201800, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 28th June 2021
Abstract
In this study, the behavior of fission product iodine released from the melting process of a mixture consisting of UF4 irradiated with neutrons and 2LiF–BeF2 (FLiBe) salt was studied. The experiment showed that a large amount of iodine was released immediately during melting and captured by Ni metal foils. The transient release of iodine observed in this experiment is attributed to the redox reaction between the hot atoms of the fission product iodine that cumulated due to long-time irradiation. The effect of the redox status of the molten salt on the transient release of iodine was also investigated. Based on this investigation, it was proposed that the activity ratios of 131I to salt-seeking fission products in the fuel salt, as an effective diagnostic criterion, may be used for the surveillance of the redox potential of fuel salts in a molten salt reactor.
Introduction
Nuclear fission produces a variety of fission products. In a solid fuel reactor, these fission products are restricted to fuel elements together with fissile and fertile materials. Differently from the solid fuel reactor, the fission products and fission materials circulate in the primary loop of the reactor, together with FLiBe molten salt, used as coolants in the molten salt reactor (MSR).1 Thus, the effect of the fission products on MSR such as neutron economy, delayed neutron fraction, control of the reactor and corrosion of structure materials has received considerable attention.2,3 The study of the behavior and distribution of the fission products in MSR becomes a significant research project in the development of MSR.4,5Up to now, the chemistry of fission products in aqueous solutions has been investigated quite well.6 These research studies have provided strong scientific and technical supports for the development and optimization of spent fuel reprocessing. Similarly, the study of the chemistry of fission products in molten salts should be important not only for the processing of irradiated fuels but also for the design, operation, and safety control of the MSR. Unfortunately, reports on the chemistry of fission products in molten salts rarely exist. In the 1960s, Oak Ridge National Laboratory (ORNL) started the research and development of the molten salt reactor. The 8 MWt Molten Salt Reactor Experiment (MSRE) facility was built in 1966 and successfully operated for nearly 4 years.7,8 During the operation of the MSRE, the chemistry of fission products in molten salts was one of the important research subjects conducted by the chemistry division of ORNL. Based on their behavior and distribution, in the research at ORNL, the fission products were divided into three categories: (1) noble gas fission products (Kr and Xe), which are slightly soluble in salts and easily escapable from the fuel salt at the MSRE operating temperature, (2) salt-seeking fission products (Rb, Cs, Sr, Ba, Y, Zr and the lanthanides), which could exist stably in the fuel salt in the form of fluorides and (3) noble metal fission products (Nb, Mo, Tc, Ru, Rh, Pd, Ag, Sb, and Te), which are unstable in salt and whose behavior was very complicated. In most cases, they tend to be reduced into metals and deposited on various surfaces of the MSRE materials such as graphite as the neutron moderator and Hastelloy-N as the fuel container. The research at ORNL found that their behavior and distribution are often affected by the redox potential of the fuel salt. Thus, their behavior and dependence on the redox potential, especially for 95Nb, has become one of the key research objects.9,10
Iodine isotopes are significant fission products because of their high yield and wide mass numbers involved in mass distribution. Moreover, they are also the precursor nuclei both for delayed neutron emitters and neutron poison 135Xe. Their behavior was correlated closely with the operation and control of the MSR. Compared with the typical salt-seeking and noble metal fission products, however, less iodine-related investigations were reported by ORNL. Using γ-ray spectrometry, ORNL obtained some information about the behavior and distribution of the fission product iodine via detection of the fuel salt, gas vapor and the alloy/graphite surveillance specimens inserted into the pump bowl.7,11 According to the reports from ORNL, the fission product iodine was categorized sometime in the halogen group, like bromine, but in most cases, categorized as a subgroup together with Sb and Te in the noble metal fission product group. Regardless of how the fission product iodine was grouped, the results from the analysis for γ-spectra appeared very often to be contradictory. ORNL pointed that fission product iodine seemed to exist steadily, in the form of I−, in fuel salts at the redox potential of the MSRE.11 However, the radioactive measurements of the fuel salt samples indicated that the 131I radioactivity relative to the inventory of irradiated UF4 was between 8% and 113%, with most values ranging from 30% to 60%.7 The phenomenon of the iodine loss from the fuel salt was very similar to that of the noble metal fission product. However, the analysis of the γ-spectra for the surfaces of alloy and graphite specimens indicated no noticeable deposition of fission product iodine to be found except for the great deposition of typical noble metal fission products such as Mo, Te, Nb and Ru. Considering the high volatility of the iodine element, the gas samples from the pump bowl and the off-gas system were carefully measured many times with γ-ray detectors; however, the existence of fission product iodine was not found except for occasionally detectable in the pump bowl gas samples.11,12 It can be seen that, iodine is indeed an elusive fission product in MSR, and it is interesting and necessary to understand the reason for iodine loss up to about one-fourth to one-third.
In this work, the UF4 powder was irradiated by a photo neutron source driven by a 15 MeV electron linear accelerator. The behaviors of iodine and some fission products in the molten salt compound consisting of irradiated UF4 and FLiBe were investigated under experimental conditions. To understand the reason for the loss of fission product iodine, as observed at MSRE operation, the emphasis of this work was placed on the unexpected release of iodine from the molten salts at the beginning of melting. Moreover, a new way to examine the redox potential of the molten salt was proposed on the basis of the activity measurement of 131I.
Experimental
Reagents and materials
UF4 was purchased from China National Nuclear Corporation. Li metal (99.9% purity), LiI (99.99% purity) and CeF4 (99.99% purity) were purchased from Alfa Aesar Co., Ltd. All the reagents were used without further purification. Ar gas with 99.999% purity was purchased from Shanghai Louyang Gas Co. Ltd. The 2LiF–BeF2 (FLiBe) eutectic salt of 99.9% purity was prepared according to the procedures described in our previous work, and its oxygen content was determined to be less than 400 ppm. Graphite and Hastelloy C276 specimens were used as the surveillance specimens, and the size was 20 × 5 × 2 mm for both of them. The reason for choosing the Hastelloy-C276 alloy as the surveillance specimen in this work was its similarity in components (Ni-58.10%, Mo-15.82%, Cr-15.98%, Fe-5.95%, and W-3.41%) to the Hastelloy-N alloy used in the MSRE. A 60Co standard source and a 152Eu standard source used to calibrate the gamma-ray spectrometer were purchased from Eckert & Ziegler BEBIG GmbH, Germany.Equipment
A self-designed reaction apparatus placed in a glove box in Ar atmosphere was used to investigate the behavior of iodine, and the detailed description has been reported in our previous works.10,13,14 A graphite crucible with an inner diameter of 25 mm and a height of 60 mm was used as the reaction crucible.A photo neutron source driven by a 15 MeV electron linear accelerator was self-designed and constructed by Shanghai Institute of Applied Physics, CAS (SINAP);15 its maximum neutron output of the photo neutron source was about 1.2 × 1011 n s−1, and the average energy of the neutrons was about 1 MeV.16 The detector used for γ-spectrum measurement (count the nuclides) was an n-type ORTEC GEM304P Hyper Pure Germanium (HPGe) detector, and the energy resolution was 1.85 keV at 1.33 MeV γ-ray. Before measurement of the γ-spectra, the energy calibration and the efficiency calibration of the detectors were obtained using the 60Co and 152Eu standard sources.
Experimental method
First, 20 g UF4 powder was loaded into a customized PMMA box and then installed on the photo neutron source. The neutron flux was estimated to be about 1 × 108 n s−1 cm−2 on the target, with total fluence being about 3 × 1013 n. After four-day irradiation and 24 hour cooling, irradiated UF4 was well mixed, and about 0.5 g powder was sampled accurately and measured using a calibrated γ-ray spectrometer to obtain the original activities (denoted as inventory) of 237U and fission products.About 4 g of irradiated UF4 was mixed with 26 g FLiBe eutectic salt in a graphite crucible placed into the reaction apparatus. Two pieces of surveillance specimens (one for graphite and one for Hastelloy C276 alloy) were inserted into the molten salt to examine the possible deposition of fission products on the surface of the Hastelloy C276 alloy and crucible. Before heating, the reaction apparatus was covered with a Ni foil to collect the fission products released from the molten salt. The temperature of the apparatus was then raised up to 650 °C in 2 hours and maintained at 650 °C throughout the experiment. In the process of heating and holding, the Ni foil on the top of the apparatus was removed at intervals as required. At the same time, a small portion of salt was taken out from the crucible using a quartz tube with an inner diameter of 5 mm and weighted accurately. After sampling, a new Ni foil was covered again on the reaction apparatus instead. The Ni foils caught fission products and salt samples were subjected to measure the γ-ray spectra. In order to investigate the effect of the redox potential on the release of iodine, Li metal and CeF4 were used to change the redox condition of the FLiBe molten salt. Sampling inspection of salt and Ni surveillance specimens was carried out after 24 hours of addition of the Li metal and CeF4.
Measurement and data analysis
All the samples of Ni foils and salt samples were counted using a calibrated γ-ray spectrometer. The counting times varied from 1 hour to 24 hours, depending on the activity of interesting nuclides. The γ-ray spectra were analyzed using the Gamma Vision Program provided by ORTEC Co. Assignments of radioactive nuclides were made mainly on the basis of their characteristic energies. If necessary, half-life and concordance with other γ-rays emitted by the presumed nuclide were used to help the assignments of nuclides. The basic nuclear data used for nuclide assignment and activity calculation were provided by ENDF/B-VII.1 (USA, 2011), and they are listed in Table 1. The uncertainties in activities experimentally determined in this work were standard deviations, including mainly the statistic errors in the γ-ray counts, a 5–6% error in detector efficiency and a 5% error in sample geometry. The typical uncertainties for 131I were about 10–15%.| Nuclides | T1/2 (day) | Energy (keV) | Emission intensity | Activity (Bq g−1 UF4) |
|---|---|---|---|---|
| 237U | 6.75 | 208.0 | 0.212 | 5.50 × 104 |
| 143Ce | 1.377 | 293.3 | 0.428 | 2.26 × 103 |
| 140Ba | 12.75 | 537.3 | 0.2439 | 8.72 × 102 |
| 95Zr | 64.02 | 756.7 | 0.5446 | 2.43 × 102 |
| 131I | 8.021 | 364.5 | 0.817 | 9.00 × 102 |
| 132I | 0.096 | 667.7 | 0.987 | 2.88 × 103 |
| 133I | 0.867 | 529.9 | 0.87 | 4.21 × 103 |
| 99Mo | 2.748 | 181.1 | 0.0599 | 2.51 × 103 |
| 103Ru | 39.26 | 497.1 | 0.91 | 3.11 × 102 |
| 132Te | 3.204 | 228.2 | 0.88 | 1.46 × 103 |
| 135Xe | 0.381 | 249.8 | 0.9 | 3.12 × 103 |
| 147Nd | 10.98 | 531.02 | 0.1337 | 3.33 × 102 |
Results and discussions
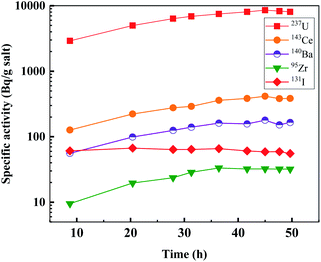
After complete dissolution of irradiated UF4 in the FLiBe molten salt, a certain amount of molten salt was sampled using a quartz tube, weighed, and then counted using a γ-ray spectrometer. The initial specific activities at 4 day irradiation and 1 day cooling for over all 12 nuclides were obtained from the measurement and calculation of γ-ray spectra, and the results are also listed in Table 1. In addition, the specific activities of 95Zr, 131I, 143Ce, 140Ba and 237U, after decay correction, are shown in Fig. 1, as a function of the melting time.As shown in Fig. 1, the specific activities of the nuclides 95Zr, 140Ba, 143Ce and 237U in molten salt increased gradually and reached a steady state after about 40 hours. The result was consistent with ORNL's study that 95Zr, 140Ba and 143Ce fell into the group of salt-seeking fission products and could stably exist in the fuel salt.7 131I appeared to reach dissolution equilibrium more quickly in the molten salt, which may be attributed to its lower melting point. According to the report of ORNL, iodine was grouped as a noble metal fission product. However, iodine could form iodide and exist in a steady form in the molten salt of the MSRE.
After the dissolution equilibrium, total activities of each radioactive nuclide in the FLiBe molten salt could be calculated on the basis of their specific activities measured in the salt sample. When comparing the total activities with the data of inventories in irradiated UF4, some interesting information could be obtained. Fig. 2 shows the total activities of 95Zr, 143Ce, 140Ba and 237U in the molten salt and the relative data of inventories in irradiated UF4. 95Zr, 143Ce and 140Ba, belonging to the salt-seeking fission products, should completely dissolve and stably exist in the fuel salt. Consequently, the total activity of each nuclide in the FLiBe molten salt, like that of 237U, was in agreement with those of the inventories in irradiated UF4, as expected.
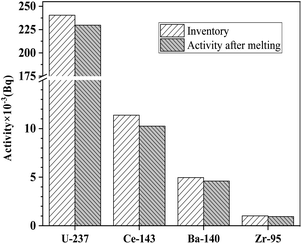 | ||
| Fig. 2 Comparison of total activities in the salt with the inventories for 237U, 143Ce, 140Ba and 95Zr. | ||
However, the noble metal fission products (99Mo, 103Ru and 132Te), iodine and noble gas fission products (131I, 132I, 133I and 135Xe) exhibited different behaviors, and their total activities in the FLiBe molten salt were much lower than the inventories. Due to little solubility in the fluoride salt and high volatility, noble gas 135Xe was easily released from the molten salt. Hence, no 135Xe fission product existed in the molten salt any longer, as shown in Fig. 3. According to the report of ORNL,9 the noble metal fission products tended to deposit, in a form of metal, on the surface of structural materials at the redox potential of the MSRE. Thus, the deposition of 99Mo, 103Ru and 132Te on the wall of graphite crucible would be responsible for the decrease in total activities observed in this work. It is interesting to note that the total activities of 131I, 132I and 133I in salt, similarly to these of 99Mo, 103Ru and 132Te, also became low when compared to the inventories. Although iodine was grouped under noble metal fission products, the decrease in the total activities of the fission product iodine in the salt could not be explained by their deposition on the surface of solid materials.
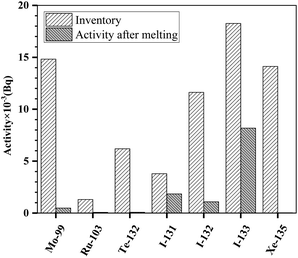 | ||
| Fig. 3 Activities after melting and the inventories of 99Mo, 103Ru, 132Te, 131I, 132I, 133I and 135Xe. | ||
To investigate the underlying cause for the decrease in iodine activity in the molten salt, two pieces of graphite and Hastelloy C276, used as the surveillance specimens, were inserted into the salt for several hours. After taken out, these specimens were measured using a γ-ray spectrometer. After correction for the additional activity induced by the nuclides in the salt adsorbed on specimens, the real activity of the nuclides deposited on the surface of the specimen was examined. The result indicated that all the activities were due to noble metal fission products 99Mo, 103Ru, 132Te and its daughter 132I, with no activities of 131I and 133I being observed. Moreover, three samples of salt, after cooling, were taken out from the upper, middle and lower layers of the molten salt in the crucible. The measurements for γ-activity indicated that there was no obvious difference in the specific activity of 131I in the samples from three different regions, implying homogeneous distribution of 131I in the molten salt. Since no deposition on the surface of the specimens and no precipitation at the bottom of the molten salt for fission product 131I were observed, unique possible explanation for iodine loss observed by ORNL should be their release, in the form of volatile gas I2 from the molten salt.
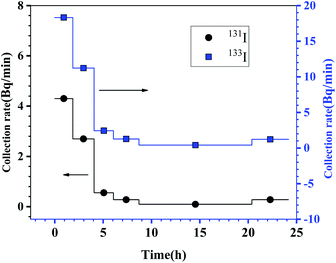
To verify the explanation for the iodine loss, the irradiated UF4 and FLiBe salt were mixed in the crucible. Prior to heating, a piece of Ni foil was placed on the top of the reaction apparatus. In the experimental process, the Ni foil covered on the apparatus was replaced in turn at certain time intervals. Within the 25 hour experiment, six Ni foils were used and activities on these Ni foils were measured. The measurements showed that a great amount of 131I and 133I activities existed on the Ni foils. According to their activities on the Ni foil and collection time, the collection rates of 131I and 133I, expressed as Bq min−1, are given in Fig. 4. Surprisingly, more or less, a large amount of iodine was released within the initial two hours and captured by the first piece of the Ni foil, with the collection rates of 131I and 133I being up to 4.4 Bq min−1 and 18.3 Bq min−1, respectively. With the continuing experimental process, the collection rates of 131I and 133I decreased rapidly. After 8 hours, the collection rates decreased to 0.2 Bq min−1 and 0.8 Bq min−1, about 4% of the peak values. Then, the collection rates of 131I and 133I were maintained at a stable level close to the background for the rest time of the experiment. Exemplified by 131I, the activity of 131I on the first two Ni foils accounted for more than 80% of the total collection activity on six Ni foils, and about 43% of 131I activity lost in the molten salt, indicating that the release of iodine from the molten salt occurred mainly in the first 4 hours from the beginning of the melting process. It could be seen clearly that the release of fission product iodine exhibited typically transient characteristics. In other words, the release of a part of iodine occurred intensively within a short time at the beginning of melting. After the transient release, the remaining iodine was able to exist stably in the molten salt in the form of iodide, with no distinct decrease in its activity being observed during the continuous heating of the molten salt. The latter has been confirmed for times by ORNL.17
The transient release of a large amount of fission product iodine could be explained by hot atom chemistry.18,19 It is well known that, after capturing a neutron, the fissioning nucleus will split into two high-speed fission fragments. During the interaction of the primary fission fragments with the surrounding medium, various number of extranuclear electrons of these fission fragments would be striped sequentially, and form the highly exciting ions with various charges. When the fission fragments were retarded by the medium, the highly exciting iodine ions would, via relaxing, form the relatively stable iodine ions with a variety of chemical valence ranging from −1 to + 7. The coexistence of fission product iodine with different chemical valence has been confirmed in aqueous solution systems.20 Therefore, the chemical valence for the iodine carrier added in the aqueous solution must be adjusted to be identical to that of fission product iodine in the classical chemistry procedure of separation and yield determination. It was reasonable to believe that the fission product iodine generated in this work also had different chemical valences in the molten salt. When the mixture of irradiated UF4 and FLiBe eutectic salt was heated, a chemical interaction between the iodine isotopes with different valences would occur to produce high-volatile elemental iodine as follows:
| IF5 + 5I− = 3I2 + 5F− | (1) |
Then the released iodine was captured by the Ni foils and easily detected using a γ-ray spectrometer.
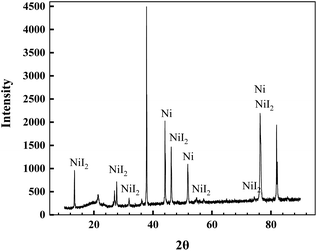
To demonstrate capture of the elemental iodine by a Ni foil, an additional experiment using natural iodine was carried out. CeF4, as an oxidation reagent, was added into the FLiBe molten salt containing 10 wt% of LiI, and then the mixture was heated at 650 °C for 24 hours. After cooling, the collections on the Ni foil were characterized by XRD, and the XRD pattern is shown in Fig. 5. As can be seen, the existence of NiI2 could be certainly verified, although the peaks located at 38 and 82 have not yet been matched. The demonstrating experiment indicated the ability of the Ni foil to capture the released I2, which was formed by the reaction as follows:
| Ni + I2 = NiI2 | (2) |
Consequently, the additional experiment provided further support for the transient release of fission product iodine, as a form of I2.
This work clearly revealed the release of a large amount of iodine that resulted from the effect of the hot atom chemistry. The UF4 used in this work has been irradiated as long as about 70 hours, and an abundant amount of fission product iodine with different chemical valences was generated. During the melting process, iodine accumulated in the long time was released in a short time from the salt, making it easy to be detected. A similar process should also occur in the MSRE. Differently from the observations of this study, however, both the generation of fission product iodine and the redox reactions between iodine species with different chemical valences would continuously occur to follow the nuclear fission. Because of no opportunity for the transient release of fission product iodine, the slow release certainly made its detection very difficult. This should be one of the main reasons responsible for the unknown fate of about one-fourth to one-third of fission product iodine observed in the MSRE operation by ORNL.7
The significant release of iodine from the fuel salt of the MSR might affect the neutron economics and the fraction of delayed-neutron precursor. Therefore, the behavior of iodine and its surveillance should be taken into account for the design and operation of MSRs. Furthermore, information about the release of fission product iodine from the reactor might have potential applications in the operation and control of the MSR. In this work, a Li metal reductant and a CeF4 powder oxidant were added into the FLiBe molten salt containing irradiated UF4 respectively. The effect of the variation in the redox potential of the molten salt on the iodine release from the salt was examined (see Table 2). Compared with 54.7% of release rate of iodine for the salt without addition of any reductant or oxidant, the transient release of iodine decreased to 23.1% when the Li metal was added into the salt. Conversely, the addition of the CeF4 oxidant made the transient release increase to 87.2%. The reason was easily intelligible. The addition of the Li metal reductant consumed directly a part of fission product iodine with high valence, resulting in more iodine, in the form of I−, remain in the salt. When the CeF4 oxidant was added into the system, the I− ions would be oxidized into elemental iodine I2 followed by release from the salt. It could be inferred that the amount of iodine that remained in the salt, or that released from the salt as well, was correlated closely with the variation in the redox potential that the molten salt underwent.
| Molten salt system | Additives (mg) | Release rate of 131I | Ratio of activities | ||
|---|---|---|---|---|---|
| 131I/140Ba | 131I/143Ce | 131I/95Zr | |||
| a No corresponding experimental data were obtained due to the short half-life of 143Ce. | |||||
| UF4 | — | — | 0.83 | 0.41 | 3.56 |
| UF4 + FLiBe | — | 54.7% | 0.40 | 0.17 | 1.82 |
| UF4 + FLiBe | Li (20 mg) | 23.1% | 0.61 | —a | 2.43 |
| UF4 + FLiBe | CeF4 (20 mg) | 87.2% | 0.106 | 0.035 | 0.444 |
According to the operation experience of the MSRE in ORNL, the fission process would gradually increase the redox potential of the fuel salt,7,21–23 leading to the corrosion of the structural material of the MSR. In order to mitigate the material corrosion, the beryllium metal was required to be added into the fuel salt periodically to control the redox potential. Recently, the study of the redox potential and its control has received increasing attention.10,13 Zhang et al. pointed out the major phenomena that affect the redox potential and material corrosion, and main technologies for the redox potential measurement, including electro-chemical sensors and optical spectroscopy.22
ORNL pointed out that the fission product 95Nb in the fuel salt could be used as an in-line indicator of the redox condition of the fuel salt, because the transition in the chemical species of 95Nb between soluble fluoride and the insoluble metal is dependent on the redox potential of the fuel salt.9 Our previous investigation indicated that, however, it was difficult to correctly distinguish 95Nb existing as soluble fluoride from those in the form of insoluble metal particles,10 because the fuel salt was circulated at high velocity in the primary loop of MSR. Consequently, the experimental result given by ORNL was fragmentary, scattered, and even anomalous sometimes.9 Since the amount of iodine that remained in the salt was correlated closely with the variation in the redox potential, the activity of fission product iodine that remained in the fuel salt might be used as another indicator, to monitor the redox potential of the fuel salt in the MSR.
For a given reactor, the yields of fission products are known and fixed, and the ratio of activities for various fission products can be estimated correctly. Although ORNL classified the fission product iodine as a noble metal, in fact, after partly released in the form of elemental iodine from the molten salt, iodine that remained in the salt exhibited a similar behavior to the salt-seeking fission products and existed stably in the form of I− in the salt. As a result, the redox condition of the molten salt in the MSR could be characterized by the activity ratios of 131I to the salt-seeking fission products in the salt. As shown in Table 2, all of the activity ratios for 131I to 140Ba, 143Ce and 95Zr in salt increased with the addition of Li metal reductants when compared with the reference sample. On the contrary, these ratios decreased with the addition of the CeF4 oxidant. The highly positive correlation between the activity ratios of 131I to salt-seeking fission products in the salt illustrated the possible application of 131I as an indicator for monitoring the redox potential of the molten salt.
Compared with the 95Nb redox indicator proposed by ORNL, the application of 131I as a redox indicator has several advantages. First, the fission product 131I in the salt exists in the unique form of I−, unlike the case of 95Nb, where careful difference of soluble 95Nb from insoluble 95Nb is necessary but very difficult. Second, the measurement of the activity ratios of 131I to the salt-seeking fission products relies only on the relative measurement, which is more reliable and feasible than the absolute measurement involved in the determination of 95Nb activity in the salt. Finally, the half-life of 131mTe, the precursor of 131I, is about 25 min, much shorter than that of 95Zr (65 days), the precursor of 95Nb, thus the evaluations of the redox potential for the salt based on the measurement of activity ratios of 131I to the salt-seeking fission products have less effect resulting from the radioactive growth-decay. In order to validate the application of 131I in the surveillance of the redox potential of the salt in the MSR, it is certainly necessary to carry out further investigation.
Conclusions
The experiments indicated that a large amount of fission product iodine was released in a short time after melting the mixture of irradiated UF4 and FLiBe eutectic salt. The behavior of transient release of iodine was attributed to the redox reaction between fission product iodine cumulated due to long-time irradiation. A similar process would occur continuously in the MSR, but with no condition of transient release. As a result, the detection of iodine released from the salt was not easy more or less, and this should be one of the main reasons responsible for unknown loss up to about one-fourth to one-third of fission product iodine observed by ORNL in the operation of MSRE.Because of the close correlation between the release of iodine from the salt and the redox potential of the molten salt, it was pointed that the amount of 131I remaining in the fuel salt may be used as another indicator for the redox potential of the MSR. The activity ratios of 131I to the salt-seeding fission products such as 95Zr, 140Ba and 143Ce would be preferable for the characterization of the redox potential of the MSR.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their sincere thanks to Free Electron Group, and appreciate their encouragement. This work was supported by the “Strategic Priority Research Program”, “Frontier Science Key Program” and the “National Natural Science Foundation of China” of the Chinese Academy of Sciences (grant nos. XDA02030000, QYZDYSSW-JSC016 and 21771188).References
- J. Serp, M. Alibert, O. Beneš, S. Delpech, O. Feynberg, V. Ghetta, D. Heuer, D. Holcomb, V. Ignatiev, J. L. Kloosterman, L. Luzzi, E. Merle-Lucotte, J. Uhlíř, R. Yoshioka and Z. Dai, Nucl. Energy, 2014, 77, 308–319 CrossRef CAS.
- H. E. McCoy, Oak Ridge National Lab, ORNL-TM-1997, 1967.
- A. M. Wheeler, V. Singh, L. F. Miller and O. Chvála, Prog. Nucl. Energy, 2021, 132, 103616 CrossRef CAS.
- S. A. Walker and W. Li, Ann. Nucl. Energy, 2021, 158, 108250 CrossRef CAS.
- J. Kalilainen, S. Nichenko and J. Krepel, J. Nucl. Mater., 2020, 533, 152134 CrossRef CAS.
- C. H. Castaño, Nuclear Energy Encyclopedia: Science, Technology, and Applications, 2011, pp. 121–126 Search PubMed.
- E. L. Compere, S. S. Kirslis, E. G. Bohlmann, F. F. Blankenship and W. R. Grimes, Oak Ridge National Lab, ORNL-4865, 1975.
- P. N. Haubenreich and J. R. Engel, Nucl. Appl. Technol., 1970, 8, 118–136 CrossRef CAS.
- E. Thoma, Oak Ridge National Lab, ORNL-4658, 1971.
- Z. Cheng, X. Wang, Z. Zhao, J. Geng, J. Hu, J. Chen, X. Cai, W. Li, Q. Dou and Q. Li, Radiochim. Acta, 2021, 109, 311–317 CrossRef.
- W. R. Grimes, Nucl. Technol., 1970, 8, 137–155 CAS.
- A. Houtzeel and F. F. Dyer, Oak Ridge National Lab, ORNL-TM-3151, 1972.
- Z. Zhao, J. Hu, Z. Cheng, J. Geng, W. Li, Q. Dou, J. Chen, Q. Li and X. Cai, RSC Adv., 2021, 11, 7436–7441 RSC.
- Z. Cheng, Z. Zhao, J. Geng, X. Wang, J. Hu, J. Chen, X. Cai, W. Li, Q. Dou and Q. Li, Radiochim. Acta, 2021, 109, 357–365 CrossRef.
- Z. Lin, G. Sun, J. Chen, G. Liu and Z. Dai, Nucl. Sci. Tech., 2012, 23, 272–276 CAS.
- H. Wang, J. Chen, X. Zhou, Z. Lin, Y. Ma, G. Zhang, C. Li, D. Fang, S. Zhang, G. Zhang, X. Cao, C. Zhong, F. Lu, Y. Cao, R. Hu, J. Jin, J. Hu, W. Chen, J. Huang, N. Wang, J. Han, G. Kang, L. Du, Y. Wang, L. Zhu, L. Chang and C. Zhou, Nucl. Tech., 2014, 37, 100522 Search PubMed.
- M. Taira, Y. Arita and M. Yamawaki, Open Access J. Sci. Technol., 2017, 5, 101315 Search PubMed.
- G. N. Walton, Nuclear fission, Q. Rev. Chem. Soc., 1961, 15, 71–98 RSC.
- D. Hall and G. N. Walton, J. Inorg. Nucl. Chem., 1961, 19, 16–26 CrossRef CAS.
- G. W. Keiholtz and C. J. Barton, Oak Ridge National Lab, ORNL-NSIC-4, 1965.
- D. A. Petti, G. R. Smolik, M. F. Simpson, J. P. Sharpe, R. A. Anderl, S. Fukada, Y. Hatano, M. Hara, Y. Oya, T. Terai, D.-K. Sze and S. Tanaka, Fusion Eng. Des., 2006, 81, 1439–1449 CrossRef CAS.
- J. S. Zhang, C. W. Forsberg, M. F. Simpson, S. Q. Guo, S. T. Lam, R. O. Scarlat, F. Carotti, K. J. Chan, P. M. Singh, W. Doniger, K. Sridharan and J. R. Keiser, Corros. Sci., 2018, 14, 44–53 CrossRef.
- C. F. Baes, J. Nucl. Mater., 1974, 51, 149–162 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |