Open Access Article
Open Access ArticleLeucomethylene blue probe detects a broad spectrum of reactive oxygen and nitrogen species†
Christopher Cremer‡
 a,
Jean Michél Merkes‡ab,
Christina L. Bub
a,
Jean Michél Merkes‡ab,
Christina L. Bub a,
Dirk Rommelc,
Frederic W. Patureau
a,
Dirk Rommelc,
Frederic W. Patureau a and
Srinivas Banala
a and
Srinivas Banala *abd
*abd
aInstitute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, Aachen, Germany
bInstitute for Experimental Molecular Imaging, University Clinic, RWTH Aachen, Forckenbeckstrasse 55, Germany. E-mail: sbanala@ukaachen.de
cDWI-Leibnitz Institute for Interactive Materials and Institute for Technical and Macromolecular Chemistry, RWTH Aachen University, Forckenbeckstrasse 50, Aachen, Germany
dFraunhofer Institute for Digital Medicine MEVIS, Max-von-Laue-Str. 2, 28359 Bremen, Germany
First published on 29th September 2021
Abstract
Reactive oxygen and nitrogen species (ROS, RNS) are ubiquitous in biology with a variety of physiological and pathological functions. Here we describe a broad spectrum ROS/RNS detecting fluorogenic probe with red fluorescence emission and up to 100-fold gain. Hence these modified probes are useful for in vivo non-invasive quantification of ROS/RNS.
Reactive oxygen species (ROS: O2˙−, H2O2, HO˙, OCl−, and HO2˙) and reactive nitrogen species (RNS: ONOO−, NO˙, NO3−) are classes of short-lived molecules produced in biological environments, e.g. in cellular metabolism, and in neutrophil actions.1,2 They function as messengers, pathogen neutralizers, and play key roles in inducing inflammation,3,4 and cancers.5–7 Therefore, early non-invasive detection of ROS/RNS enable the onset of diseases. Several strategies, methods and probes are employed currently for the detection of ROS and RNS.2,8–10 Electron paramagnetic resonance (EPR)11 and fluorescence-based techniques are prevalent,12 followed by photometric, chemiluminescent and electrochemical methods. In all these techniques, the applied probes change their properties post-reaction (or trapping) with ROS/RNS, and enable the detection.
Typically, many of above probes detect only one of the particular ROS/RNS species, and “overlook” those formed in earlier or subsequent metabolic steps, e.g. in EPR only radical species, in fluorescence and colorimetric techniques one type of ROS/RNS species. However, in biological systems the primarily produced ROS species (e.g. O2˙−) undergoes a variety of dissociation pathways forming secondary ROS species (see Fig. 1) and cause oxidative burden.13 Thus, to estimate an overall oxidative stress, it is necessary to accurately assess the pathogenic levels production of all ROS/RNS species using a single probe.
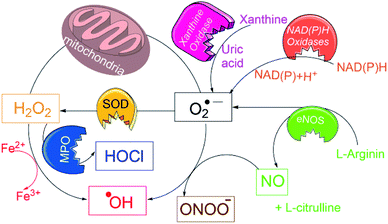 | ||
| Fig. 1 Schematic overview for generation of a variety of ROS/RNS species in biological conditions and their interconversions. | ||
Highly studied ROS detecting fluorogenic probes, 2′-7′-dichlorodihydrofluorescein (DCFH), its diacetate (DCFH-DA),14 and dihydrorhodamine (DHR),15 produce O2˙− from O2 after reaction with one-electron-oxidizing ROS/RNS species, via the radical ion (DCFH˙− or DHR˙−). This catalytic formation of O2˙−, and its further conversion to other reactive species leads to an inaccurate detection of cellular ROS.16 Further, the absorption and emissions wavelengths are incompatible for in vivo use.17 It is optimal to have probes exhibiting optical properties in red-to-near infrared (NIR) range, where tissue auto fluorescence and phototoxicity are minimal. Towards such red-to-NIR emitting probes, previous studies employed reduced hydrocyanines, which undergo HO˙ and O2˙− mediated oxidation yielding red-to-NIR emitting cyanine dyes.18 However the polymethine chain in cyanine itself is sensitive to oxidative cleavage with ROS species,19,20 thus could lead to underestimation of ROS. Thus, new probes are of interest, which show high stability towards ROS, simultaneously exhibit fluorescence in red-to-NIR range.
Methylene blue (MB) dye has attracted our attention, as it exhibits absorption and emission in the biocompatible region, at 665 nm and 686 nm, respectively.21 Further, MB core is chemically modifiable,22,23 and FDA-approved for therapeutic use,24,25 and has been applied in a variety of applications, in disinfecting blood,26 in treatment a variety of cancers,27 as an antidote for methemoglobinemia28 and in the photodynamic inactivation of bacteria,25 funguses29 and viruses.30,31 These biological uses coupled with in vivo suitable optical properties make MB an attractive core the detection of ROS/RNS.
Recently, a reduced leucomethylene blue (LMB) was applied to selectively detect HOCl by formylating 10-N site (N10-CHO) of LMB.32 In another study, the N10-site was modified with an enzyme33 or light cleavable synthon,34 which upon applying the respective trigger, yielded LMB and spontaneously oxidized to MB. We therefore are interested in modifying the LMB for detection of a broad spectrum of ROS/RNS species.
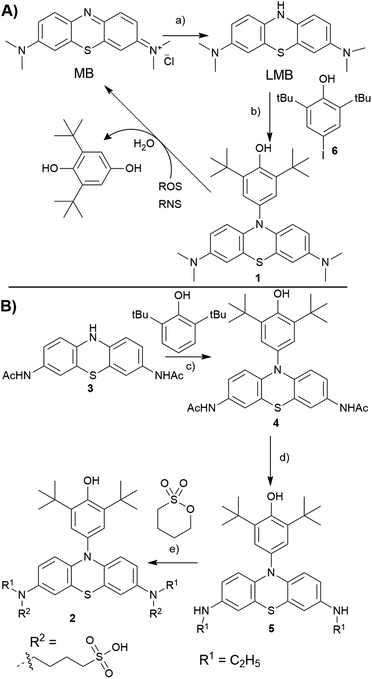
In our previous studies, we have identified that 2,6-di-tert-butyl phenol (BHP) moiety substituted dyes like porphyrins,35 BODIPYs36 reacted with a variety of ROS/RNS species, undergoing changes in their optical properties. The reactivity of BHP towards a variety of ROS/RNS is similar to that of the antioxidant 2,6-di-tert-butyl hydroxy toluene (BHT, a food additive),37 as both share the same reactive site. Extending this strategy to LMB, we conceived to prepare a N10-BHP appended LMB (BHP-LMB; 1, 2) for the ROS detection. As the BHP unit is hydrophobic, we approached a modification of LMB with polar groups like 1-butanesulfonate. Here we report the synthesis, and ROS detection characteristics as well as imaging applications of both constructs.
Towards the synthesis of the target BHP-LMB 1, we employed a Buchwald–Hartwig C–N coupling, using reduced LMB and 4-iodo-2,6-tert-butylphenol (6). This method is appealing for its simplicity, as the C–N coupling chemistry has been extensively studied.38 The C–N coupling reaction was performed in toluene with Pd2(dba)3 catalyst at 120 °C under nitrogen atmosphere (Scheme 1A). The BHP-LMB 1 could be isolated in 10% yield after column chromatography. Low yield was due to solubility of LMB in toluene, further sensitivity towards dissolved oxygen. Thus for purification of the coupling product 1, N2-gas was employed in chromatography, and the dye 1 could be obtained as pale-turquoise coloured solid after removal of the solvent. The HPLC and NMR showed >98% purity. The air-oxidation can be prevented by using reducing agents like citric acid, especially for spectroscopic measurements.
For the hydrophilic 1-butanesulfonylated BHP-LMB 2, we have developed a second generation multi-step synthetic route starting from phenothiazine (PTZ). Towards 2, we have first prepared a 2,8-diaminoacyl phenothiazine derivative (PTZ-NHAc, 3) from 3,7-dinitro-10H-phenothiazine-5-oxide (see ESI†) as reported previously.39 Then, for introducing the BHP unit at 3, a convenient metal-free dehydrogenative amination method was applied based on a recently published route (Scheme 1B).40 An advantage of this route is also the high stability of 3 and 4 towards oxygen. The following reduction of the acetyl groups in 4 was achieved using BH3·SMe2 complex, obtaining mono ethyl BHP-PTZ derivative (5), which sets the stage for the final reaction towards the desired product 2. Treating 5 with 1,4-butanesultone under basic conditions, by nucleophilic ring opening of the sultone, yielded 1-butanesulfonate attached BHP-LMB (2), along with mono sultone addition product. The hydrophilic product 2 was isolated by preparative RP-HPLC (reverse phase, with CH3CN/H2O gradient) as white solid after lyophilisation.
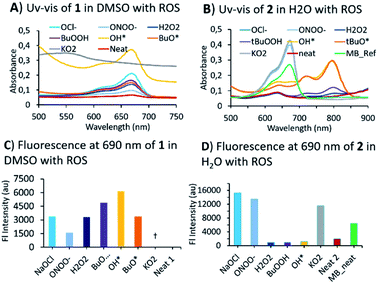
Having obtained two BHP-tethered probes 1 & 2, we performed a screening with a variety of ROS/RNS by optical methods (Fig. 2; Fig. S1 and S2 in ESI† for titrations). The absorption (UV-vis) spectra of compounds were obtained, 1 in DMSO and of 2 in water; both BHP-LMBs showed a similar reactivity towards ROS, yielding MB as the final compound, though some peculiarities were observed. The O2˙− was found to be highly aggressive especially in DMSO with 1, compared to 2 in water. In DMSO, the O2˙− reaction yielded MB instantly, that underwent decomposition in the presence of excess O2˙−. We investigated this reaction by NMR spectroscopy to identify any intermediates towards MB, using 1 titration with O2˙− and MB with O2˙−, but found no stable species (Fig. S5†). However, during the treatment with OH˙ and tBuO˙ species, it was found that 2 in water gave a persistent far-red absorbing species which was not observed in DMSO with 1 (Fig. S3† versus Fig. S1†). Similar spectral features were observed with H2O2 and tBuOOH towards 2 in H2O albeit with a lower intensity, but not with 1 in DMSO. This is presumably, a relatively stable BHP-radical appended LMB formed in water but not in DMSO (Fig. S3†). For anionic ROS species, O2˙−, ONOO− and OCl−, the absorption and fluorescence emission are consistent in both solvents with a small discrepancy in the gain ratio. This can be attributed to a different rate of reaction for formation of MB. In treating 1 with RNS species, NO2− and NO3− in DMSO, resulted MB (Fig. S2†). These optical characteristics confirm that 1 and 2 exhibit a good sensitivity towards most of the ROS/RNS species and are thus suitable for estimating overall oxidative stress.
Mechanistic investigations into the formation of intermediates were conducted by HPLC and NMR (Fig. 3). Here, 1 was treated with ONOO−, OCl−, O2˙− as they were found to be consistent in both solvents. Adding ONOO− and OCl− in excess to 1 gave a clean MB product without any trace of other species (Fig. 3A). Treatment of 1 with a minimal amount of O2˙− showed existence of both 1 and MB traces in HPLC. For NMR titrations, to a DMSO-d6 solution of 1, ROS species in D2O were added. For addition of O2˙−, the chemical shifts corresponding to MB are downfield shifted slightly compared to ONOO−, OCl−. Furthermore, the NMR signals of the cleaved BHP unit were upfield shifted for O2˙− than ONOO−, OCl− addition (Fig. 3B). This could indicate that the formed by-product, tBu-hydroquinol, might have been further oxidized to a tBu-quinone with O2˙−, but with ONOO−, OCl− persistent tBu-hydroquinol. Nevertheless, as no other intermediate species were detected in HPLC, and all ROS produced MB as the final compound, 1 could be suitable for detecting all the ROS species.
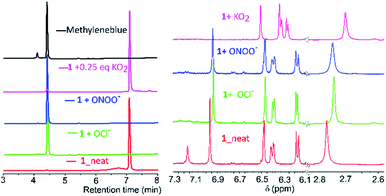 | ||
| Fig. 3 (A) HPLC chromatograms (detection at 254 nm; along with MB and neat 1) and (B) NMR-spectra (in DMSO-d6) for addition of O2˙−, ONOO−, OCl− species to 1 in D2O/H2O to the DMSO-d6. | ||
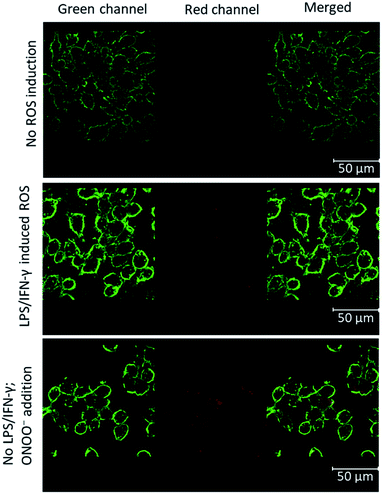
Having characterized the ROS reactions of 1 and 2 in the formation of MB, we focused on imaging the cellular produced ROS by confocal laser scanning microscopy. For this, we chose the established ROS-producing macrophage cell line J774.A1, which express ROS when treated with lipopolysaccharide (LPS) and interferon-γ (IFN-γ).
The cells attached to coverslips were treated with LPS and IFN-γ, incubated for 24 hours to induce ROS. To these activated cells in 1 mL medium, 10 nmol of BHP-LMB 1 or 2 was added as DMSO or phosphate buffered saline (PBS) solution, respectively, and incubated for 6 h. The cells were fixated with 4% formalin, washed PBS and mounted on glass slides with Mowiol for microscopy. The membrane labelling WGA-488 probe was used additionally for reference. For control cells, no ROS induction was carried out with LPS/IFN-γ (Fig. 4, top). In addition, the hydrophobic 1 incorporated controlled cells, after formalin fixation, were treated with ONOO− for 30 min, and washed with PBS (Fig. 4, bottom) to characterize the activation of intracellularly accumulated 1. This confirmed intra-cellularization of 1 and trapping the cellular produced ROS (Fig. 4, middle panel) or the added ROS by BHP-LMB 1 yielding red-emitting MB. A similar procedure was performed with 2, and microscopy imaging confirmed that there was no fluorescence (Fig. S4†) indicating the dye 2 was not intracellularized.
In summary, here we described broad-spectrum ROS/RNS detecting probes based on 2,6-di-tert-butyl phenol (BHP) appended leuco-methylene blue (BHP-LMBs, 1, 2). Hydrophobic 1 and hydrophilic 2 are sensitive towards a variety of ROS and RNS, producing the red fluorescent MB. In vitro titration with a variety of ROS showed formation of MB from 1 in DMSO, and also a persistent far red-absorbing radical species from 2 in H2O. The NMR and HPLC analysis of 1 in DMSO with O2˙−, ONOO− and OCl− showed no stable intermediates en route to MB. Then 1 was employed in detecting cellular produced ROS by fluorescence microscopy, which confirmed its intracellularization and suitability for detecting cellular generated ROS. The hydrophilic 2 found to be not intracellularized, and hence it may be used for extracellularly diffused ROS detection. These results suggest the suitability of 1, 2 in broad spectrum in vivo detection of ROS, which will be explored in future studies.
Author contributions
The manuscript was written through contributions of all authors. C. C., C. L. B. performed synthesis and analysis. J. M. M, S. B. performed cellular experiments, spectroscopic studies, and D. R. the microscopy. S. B. proposed and coordinated the research with F. W. P, analysed the results and wrote the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. B. acknowledges financial support from Excellent Initiative of the German federal and state governments through the I3TM Seed Fund and I3TM Step2Projects, F. W. P. acknowledges the financial support from European Research Council for project 716136: "2O2ACTIVATION".Notes and references
- Reactive Oxygen Species in Biology and Human Health, ed.S. I. Ahmad, CRC Press, New York, 2016 Search PubMed.
- J.-T. Hou, K.-K. Yu, K. Sunwoo, W. Y. Kim, S. Koo, J. Wang, W. X. Ren, S. Wang, X.-Q. Yu and J. S. Kim, Chem, 2020, 6, 832–866 CAS.
- S. Dikalov, K. K. Griendling and D. G. Harrison, Hypertension, 2007, 49, 717–727 CrossRef CAS PubMed.
- S. Di Meo, T. T. Reed, P. Venditti and V. M. Victor, Oxid. Med. Cell. Longevity, 2016, 2016, 1245049 Search PubMed.
- P. T. Schumacker, Cancer Cell, 2006, 10, 175–176 CrossRef CAS PubMed.
- S. Kumari, A. K. Badana, G. Murali Mohan, G. Shailender and R. R. Malla, Biomarker Insights, 2018, 13, 1177271918755391 CrossRef PubMed.
- C. R. Reczek and N. S. Chandel, Annu. Rev. Cancer Biol., 2017, 1, 79–98 CrossRef.
- X. Bai, K. K.-H. Ng, J. J. Hu, S. Ye and D. Yang, Annu. Rev. Biochem., 2019, 88, 605–633 CrossRef CAS PubMed.
- Y. Zhang, M. Dai and Z. Yuan, Anal. Methods, 2018, 10, 4625–4638 RSC.
- E. M. Espinoza, J. J. Røise, I. C. Li, R. Das and N. Murthy, J. Nucl. Med., 2021, 62, 457–461 CrossRef CAS PubMed.
- P. L. Zamora and V. F. A. Røise, in Measuring Oxidants and Oxidative Stress in Biological Systems, ed. L. Berliner and N. L. Parinandi, Springer, Cham, 2020, vol. 34, pp. 13–38 Search PubMed.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X.-P. He and T. D. James, Acc. Chem. Res., 2019, 52, 2582–2597 CrossRef CAS PubMed.
- P. Wardman, Free Radical Biol. Med., 2007, 43, 995–1022 CrossRef CAS PubMed.
- S. L. Hempel, G. R. Buettner, Y. Q. O'Malley, D. A. Wessels and D. M. Flaherty, Free Radical Biol. Med., 1999, 27, 146–159 CrossRef CAS PubMed.
- P. Wardman, in Methods in Enzymology, ed. E. Cadenas and L. Packer, Academic Press, 2008, vol. 441, pp. 261–282 Search PubMed.
- M. G. Bonini, C. Rota, A. Tomasi and R. P. Mason, Free Radical Biol. Med., 2006, 40, 968–975 CrossRef CAS PubMed.
- B. Kalyanaraman, V. Darley-Usmar, K. J. A. Davies, P. A. Dennery, H. J. Forman, M. B. Grisham, G. E. Mann, K. Moore, L. J. Roberts and H. Ischiropoulos, Free Radical Biol. Med., 2012, 52, 1–6 CrossRef CAS PubMed.
- K. Kundu, S. F. Knight, N. Willett, S. Lee, W. R. Taylor and N. Murthy, Angew. Chem., Int. Ed., 2009, 48, 299–303 CrossRef CAS PubMed.
- Q. Zheng, S. Jockusch, Z. Zhou and S. C. Blanchard, Photochem. Photobiol., 2014, 90, 448–454 CrossRef CAS PubMed.
- G. W. Byers, S. Gross and P. M. Henrichs, Photochem. Photobiol., 1976, 23, 37–43 CrossRef CAS PubMed.
- J. P. Tardivo, A. Del Giglio, C. S. de Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, R. de Fátima Turchiello and M. S. Baptista, Photodiagn. Photodyn. Ther., 2005, 2, 175–191 CrossRef CAS PubMed.
- S. P. Massie, Chem. Rev., 1954, 54, 797–833 CrossRef CAS.
- E. P. Guttmann, Berl. Klin. Wochenschr., 1891, 28, 953–956 Search PubMed.
- M. Wainwright and K. B. Crossley, Chemother. J., 2002, 14, 431–443 CrossRef CAS PubMed.
- R. Boltes Cecatto, L. Siqueira de Magalhães, M. Fernanda Setúbal Destro Rodrigues, C. Pavani, A. Lino-dos-Santos-Franco, M. Teixeira Gomes and D. Fátima Teixeira Silva, Photodiagn. Photodyn. Ther., 2020, 31, 101828 CrossRef CAS PubMed.
- H. Mohr, B. Lambrecht and A. Selz, Immunol. Invest., 1995, 24, 73–85 CrossRef CAS PubMed.
- X. Jin, H. Xu, J. Deng, H. Dan, P. Ji, Q. Chen and X. Zeng, Photodiagn. Photodyn. Ther., 2019, 28, 146–152 CrossRef CAS PubMed.
- Y. Yusim, D. Livingstone and A. Sidi, J. Clin. Anesth., 2007, 19, 315–321 CrossRef CAS PubMed.
- J. J. Shen, G. B. E. Jemec, M. C. Arendrup and D. M. L. Saunte, Photodiagn. Photodyn. Ther., 2020, 31, 101774 CrossRef CAS PubMed.
- T.-W. Wong, H.-J. Huang, Y.-F. Wang, Y.-P. Lee, C.-C. Huang and C.-K. Yu, J. Antimicrob. Chemother., 2010, 65, 2176–2182 CrossRef CAS PubMed.
- Q. Huang, W.-L. Fu, B. Chen, J.-F. Huang, X. Zhang and Q. Xue, J. Photochem. Photobiol., B, 2004, 77, 39–43 CrossRef CAS.
- P. Wei, W. Yuan, F. Xue, W. Zhou, R. Li, D. Zhang and T. Yi, Chem. Sci., 2018, 9, 495–501 RSC.
- J. Bae, L. E. McNamara, M. A. Nael, F. Mahdi, R. J. Doerksen, G. L. Bidwell, N. I. Hammer and S. Jo, Chem. Commun., 2015, 51, 12787–12790 RSC.
- H. M. Dao, C.-H. Whang, V. K. Shankar, Y.-H. Wang, I. A. Khan, L. A. Walker, I. Husain, S. I. Khan, S. N. Murthy and S. Jo, Chem. Commun., 2020, 56, 1673–1676 RSC.
- J. M. Merkes, M. Rueping, F. Kiessling and S. Banala, ACS Sens., 2019, 4, 2001–2008 CrossRef CAS PubMed.
- J. M. Merkes, A. Hasenbach, F. Kiessling, S. Hermann and S. Banala, Unpublished work, 2021.
- W. A. Yehye, N. A. Rahman, A. Ariffin, S. B. Abd Hamid, A. A. Alhadi, F. A. Kadir and M. Yaeghoobi, Eur. J. Med. Chem., 2015, 101, 295–312 CrossRef CAS PubMed.
- P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed.
- R. Jin, C. L. Bub and F. W. Patureau, Org. Lett., 2018, 20, 2884–2887 CrossRef CAS PubMed.
- R. Jin and F. W. Patureau, Org. Lett., 2016, 18, 4491–4493 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06498c |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2021 |