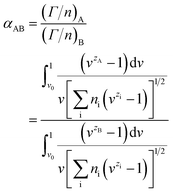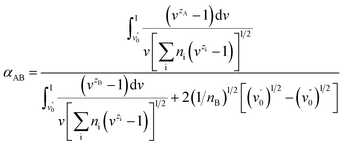Open Access Article
Open Access ArticleA review of the applications of ion floatation: wastewater treatment, mineral beneficiation and hydrometallurgy
Luping Chang
 a,
Yijun Cao
*ab,
Guixia Fan
a,
Chao Li
b and
Weijun Peng
a,
Yijun Cao
*ab,
Guixia Fan
a,
Chao Li
b and
Weijun Peng
 *a
*a
aSchool of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou, Henan 450001, PR China. E-mail: yijuncao@126.com; pwj@zzu.edu.cn
bHenan Province Industrial Technology Research Institute of Resources and Materials, Zhengzhou University, Zhengzhou, Henan 450001, PR China
First published on 28th June 2019
Abstract
Ion flotation was originally used for pre-concentrating precious metals from dilute solutions. To date, it has attracted widespread attention in many fields due to its low energy requirements, simplicity, rapid operation, small space requirements, suitability for a variety of target ions at various levels, small volume of sludge, low residual concentration, and low operating cost. This review focuses on the applications of ion flotation in wastewater treatment, mineral beneficiation, such as rare precious metal recovery, and hydrometallurgy, such as pre-concentrating of rare earth elements and selective separation of multicomponent ions. The outlook of ion flotation is also discussed.
1 Introduction
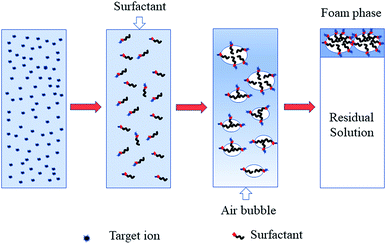
As early as 1937, Langmuir and Schaefer1 observed an interesting and profound phenomenon where insoluble stearic acid on the surface of a solution can adsorb metal ions dissolved in the solution. It was not until 1959 that Sebba2 suggested using this phenomenon as a means of concentrating ions from solution, even very dilute solution, by a flotation method. This process is called ion flotation.3Ion flotation is a specific separation method that involves adding surfactants or collectors with opposite charges to those of the target ions to form a surfactant complex and then collecting the ions by passing gas bubbles through the solution. A small volume of a hydrophobic product that contains concentrated target ions is formed at the top of a flotation machine and recycled, as clearly depicted in Fig. 1.4,5 As a promising separation process, much attention has been paid to ion flotation due to its low energy requirements, simplicity, rapid operation, small space requirements, suitability for a variety of target ions at various levels, small volume of sludge, low residual concentration, relatively low cost, etc.6–9
In the original studies, ion flotation was mostly used for pre-concentrating precious metals from dilute solutions. To date, the ion flotation method has also been applied for wastewater and water treatment,10–12 recovery of precious metals,13,14 pre-concentrating of rare earth elements,15,16 and selective separation of multicomponent ions.17,18
The current state of the application of ion flotation is therefore reviewed in this paper. Basically, this review focuses on the application of ion flotation in wastewater treatment, mineral beneficiation, such as rare precious metal recovery, and hydrometallurgy, such as pre-concentrating of rare earth elements and selective separation of multicomponent ions; the intent of this review is to provide ideas and inspiration to spark rapid development of the application of ion flotation.
2 Wastewater treatment
The rapid development of industry and the increasing productivity of many industrial branches have resulted in the pollution of ground water sources; thus, people worldwide face the problems of lack of fresh water together with the spread of various diseases due to organic and inorganic contaminants.19 Sustainable development of wastewater is especially stressed because wastewater is a renewable resource from which water can be recovered for reuse.20–22 Numerous methods have been developed to remove contaminants from aqueous solutions, such as chemical precipitation, oxidation or reduction, adsorption, coagulation/flocculation, filtration, ion-exchange, electrochemical treatment, reverse osmosis, membrane technology, evaporation and electroflotation.5,23–25 This may be due to the fact that metal ions can enter water bodies and soil, accumulate in animals and plants through the food chain, and eventually accumulate in the human body, seriously endangering human health and life due to their non-degradability, mobility and persistence.26 However, these removal methods have many disadvantages, such as high cost, generation of large amounts of sludge, high reagent or energy requirements, time consumption, incomplete removal of target ions, production of secondary wastes and difficulty of treatment of large volumes of wastewater.27–29 Accordingly, ion flotation has been evaluated as a good alternative treatment process for wastewater treatment due to its low energy requirements, simplicity, rapid operation, small space requirements, etc.Lead is a persistent and toxic contaminant that emanates from acid batteries, painting, printing, ceramic and glass manufacturing, and production of lead additives for gasoline. Long-term exposure can lead to anaemia, cancer, kidney disease, metal retardation, etc.30–32 Craioveanu et al. conducted much research on Pb(II) removal; they noted that high removal efficiency (R% = 99.93) could be obtained under optimum conditions using a naturally occurring compound (caffeic acid) as a collector. The removal mechanism is based on the fact that caffeic acid contains numerous complexing polar groups that can actively react with metallic ions.33 Similar studies were carried out by Peng et al.; they found that the removal of Pb(II) could be greater than 99% and the turbidity of the residual solution could decrease to 1.4 NTU by ion flotation under the optimum operation parameters when using graphene oxide (GO) as the collector (as shown in Fig. 2a). In addition, the GO could be reused after desorption, and the Pb(II) removal remained as high as 84.9% in the sixth regeneration (as shown in Fig. 2b).34 Additionally, they noted that GO was much more efficient than other collectors when used in Pb(II) removal experiments due to the fact that nanoscale GO possesses great numbers of hydroxyl and carboxyl groups that mainly participate in bonding with Pb(II) (as shown in Fig. 2c).23,35
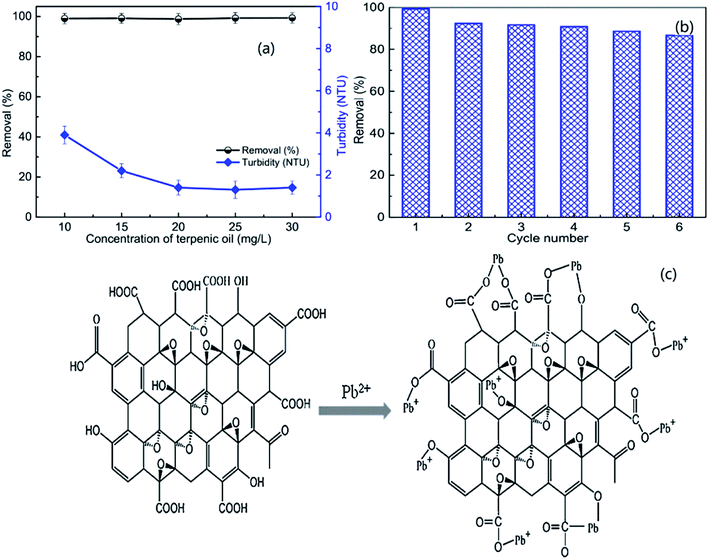 | ||
| Fig. 2 Effects of the dosage of frother (terpenic oil) on the removal of Pb(II) and the turbidity of the residual solution (a); removal of Pb(II) as a function of cycle number (b). Schematic of the mechanism of the adsorption of Pb(II) on the surface of GO (c). Adapted from ref. 34 and 35 with permission from Elsevier. Copyright 2018 and 2016. Adapted from W. Peng, G. Han, Y. Cao, K. Sun and S. Song, Efficiently removing Pb(II) from wastewater by graphene oxide using foam flotation, Colloids Surf., A, 556, 266–272, Copyright (2018), with permission from Elsevier. Reprinted from W. Peng, H. Li, Y. Liu and S. Song, Comparison of Pb(II) adsorption onto graphene oxide prepared from natural graphites: diagramming the Pb(II) adsorption sites, Appl. Surf. Sci., 364, 620–627, Copyright (2016), with permission from Elsevier. | ||
In addition, cadmium may cause destructive trauma to human organs, such as the kidneys, liver, and lungs, and to cardiovascular, immune and reproductive systems. Gratifyingly, in a study by Salmani et al., more than 92.1% of Cd(II) could be efficiently removed from simulated water via ion flotation under the optimum conditions.36
Subsequently, more research on metal ion removal via ion flotation was undertaken by many researchers. They found that ion flotation could not only work on single metal ions but also on multicomponent metal ions. Mahmoud et al. carried out an investigation of the simultaneous removal of cationic ion nickel(II) and anion ion chromium(vi) from simulated wastewaters and aqueous solutions. The results indicated that removals of more than 99.5% were obtained for both nickel(II) and chromium(vi) in a single step via flotation even when the target ions were at high concentrations and the residual concentrations were all below their permissible limits in potable water.37 Ni(II) and Zn(II) ions could be removed simultaneously by ion flotation according to the research of Hoseinian et al. The removal rates of Ni(II) and Zn(II) ions were 88% and 92%, respectively, when SDS was used as the collector and Dowfroth 250 as the frother under the effective parameters investigated by experimental design performed by DX7 software and evaluated in a mechanical flotation cell (as shown in Fig. 3).38 Almost 100% removal of Cd(II) and Zn(II) at pH 5 and 60% to 70% Sr(II) removal at pH 7 to 9 were achieved by Eivazihollagh et al. via ion flotation when 2-dodecyldiethylenetriamine pentaacetic acid (C12-DTPA) served as the collector in combination with two foaming agents: dodecyl trimethyl ammonium chloride (DoTAC) and dimethyl dodecyl amine-N-oxide (DDAO).39 Moreover, Yenidünya studied the removal of Zn(II), Mn(II) and Cu(II) from aqueous solution by ion flotation with sodium dodecyl sulphate in combination with some auxiliary ligands (malic acid, maleic acid and EDTA). He found that the maximum removal rates for Zn(II), Mn(II) and Cu(II) were 90.5%, 99.8% and 73.4%, respectively, within 60 min when the molar ratio between the metal and sodium dodecyl sulphate was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Moreover, by adding auxiliary ligands (malic acid and maleic acid), the recovery efficiencies for all metal ions increased and the flotation times decreased to 40, 20 and 40 min for Zn(II), Mn(II) and Cu(II), respectively, when the molar ratio of metal
5. Moreover, by adding auxiliary ligands (malic acid and maleic acid), the recovery efficiencies for all metal ions increased and the flotation times decreased to 40, 20 and 40 min for Zn(II), Mn(II) and Cu(II), respectively, when the molar ratio of metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sodium dodecyl sulphate
sodium dodecyl sulphate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) auxiliary ligand was 1
auxiliary ligand was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.40 It is evident that ion flotation has a high removal capacity for various metal ions and that the type and dosage of surfactant, solution pH, flotation time, etc. all play important roles in the process. The mechanisms for the removal of metal ions are mainly attributed to electrostatic attraction, ion exchange and surface complexation between the surfactant and target ions; these ions are then separated from the solution by attachment to gas bubbles passing through the solutions.
5.40 It is evident that ion flotation has a high removal capacity for various metal ions and that the type and dosage of surfactant, solution pH, flotation time, etc. all play important roles in the process. The mechanisms for the removal of metal ions are mainly attributed to electrostatic attraction, ion exchange and surface complexation between the surfactant and target ions; these ions are then separated from the solution by attachment to gas bubbles passing through the solutions.
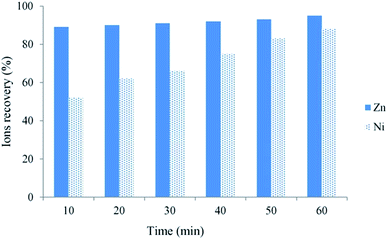 | ||
| Fig. 3 Ni(II) and Zn(II) ion recoveries as a function of flotation time. (SDS = 300 ppm, Dowfroth 250 = 90 ppm, Zn(II) = Ni(II) = 10 ppm, pH 3 and agitating speed = 1000 rpm). Reproduced from ref. 38 with permission from Elsevier, Copyright 2015. Reprinted from F. S. Hoseinian, M. Irannajad and A J. Nooshabadi, Ion flotation for removal of Ni(II) and Zn(II) ions from wastewaters, Int. J. Miner. Process., 143, 131–137, Copyright (2015), with permission from Elsevier. | ||
In addition to heavy metal ions, ion flotation is also commonly used for removing organic and biological pollutants such as oil, triazine herbicides, perfluorooctane sulfonate (PFOS) and perfluorooctanoate, Rhodamine 6G and sulfonylurea herbicides from wastewater or aqueous solution.41–47
Lignin removal research was conducted by Wang et al. through ion flotation using cetyl dimethyl benzyl ammonium as the surfactant. They found that a fraction of more than 0.95 could be removed by continuous ion flotation under the optimum operational conditions.48 The residual concentration of Direct Red could be lowered to below 0.5 ppm after 3 minutes of treatment by ion flotation when Choi et al. used sodium lauryl sulfate as both collector and frother.49 Rhodamine B (RB) and thoron (TH) are widely used for analytical and biological staining purposes and may deleteriously affect water. Removals exceeding 99.5% and 99.9% could be achieved for RB and TH, respectively, when Shakir et al. adopted the anionic surfactant sodium lauryl sulfate (NaLS) and the cationic surfactant cetyltrimethylammonium bromide (CTAB) as collectors.50 More than 96% of cationic dyes could be removed and the enrichment factors in the foam were about 6 under the optimum conditions when Groß et al. used two commercially available biopolymers as alternatives to classical surfactants for dye removal via ion flotation (as shown in Fig. 4). However, for anionic dyes, a further cationic surfactant, DTAB, should be added; the removal can thus be increased from 5% to 70%.51 Hu et al. also used ion flotation technology to remove methylene blue using commercial hydrophobic silica nanoparticles (SNP) (200.0 ± 10.0 nm average particle size) as a collector without using any surfactants. Removal efficiencies of methylene blue and SNPs and volume ratios of 91.1 ± 4.6%, 93.9 ± 4.7%, and 10.5 ± 0.5% could be respectively obtained at pH 9.0, a SNP concentration of 600 mg L−1, an anhydrous ethanol dosage of 8 ml and a flotation column height of 600 mm. In addition, methylene could be effectively separated from methylene blue-adsorbed SNPs with ethanol at pH 2.0 for reuse at least five times (as shown in Fig. 5 and Table 1).52 Tetracyclines are broad-spectrum antibiotics that are most commonly prescribed for human therapy; meanwhile, they are also commonly used in livestock farming. Therefore, they are one of the most commonly detected antibiotics in surface water resources discharged from agriculture wastewater and medical wastewater, and they should be removed as much as possible before discharge.53,54 Saitoh et al. carried out a large number of experiments, and they noted that almost complete removal (>99%) of tetracycline antibiotics could be obtained with the combined use of 20 mg L−1 of an anionic surfactant, sodium dodecyl sulfate (SDS), 6.5 mg L−1 of a cationic polyelectrolyte, poly (allylamine hydrochloride) [PAH], and 1 mg L−1 Al(III). The remaining concentration of SDS was lower than that permitted by Japanese water regulations. Additionally, this process was used to remove other tetracycline and fluoroquinolone antibiotics as well as different acidic and basic pharmaceuticals, even in five minutes.55 Boron and its compounds are important materials in the fields of medicine, material science, the chemical industry, the nuclear industry and agriculture; additionally, boron is an essential micronutrient for humans, animals and some plants. However, it may generate deposits during the recycling of high quality magnesia products from Saline Lake resources because it can be readily absorbed by magnesium hydroxide; this decreases the efficiency of recycling of high quality magnesia products from brine and boron in waste water, which is also poisonous to the environment. 88.69% boron removal efficiency from Da Qaidam brine was obtained when Bai et al. adopted sodium dodecyl benzene sulfonate (SDBS) and D-mannitol as collectors while applying optimized flotation conditions. The removal mechanism is clearly depicted in Fig. 6. Firstly, borate can be formed in alkaline solution by interacting with hydroxide ion:
| B(OH)3 + OH− ⇌ B(OH)4− |
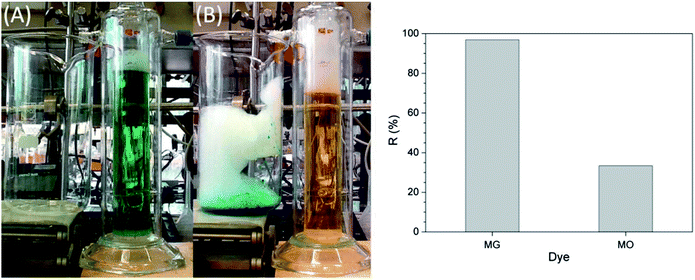 | ||
| Fig. 4 Separation of malachite green (MG) and methyl orange (MO) by flotation with HeSat as the collector. Conditions: cHeSat = 0.5 g L−1, cMG = cMO = 10 mg L−1, 13.4 ml min−1 N2, and t = 4 h. Left: colours of the dye solution before (A) and after (B) the flotation experiment, right: dye removal efficiencies. Reproduced from ref. 51 with permission from Elsevier, Copyright 2017. | ||
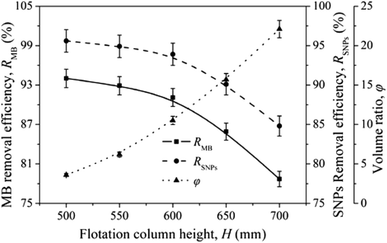 | ||
| Fig. 5 Effects of flotation column height on RMB, RSNPs and φ. Reproduced from ref. 52 with permission from Springer, Copyright 2017. Reprinted by permission from Springer, N. Hu, W. Liu, L. Ding, Z. Wu, H. Yin, D. Huang, H. Li, L. Jin and H. Zheng, Removal of methylene blue from its aqueous solution by froth flotation: hydrophobic silica nanoparticle as a collector, J. Nanopart. Res., Copyright (2017). | ||
| 1 cycle | 2 cycles | 3 cycles | 4 cycles | 5 cycles | |
|---|---|---|---|---|---|
| RMB (%) | 91.1 ± 4.6 | 89.4 ± 4.5 | 88.3 ± 4.4 | 87.5 ± 4.4 | 85.9 ± 4.3 |
| RSNPs (%) | 93.9 ± 4.7 | 93.6 ± 4.7 | 93.3 ± 4.7 | 93.4 ± 4.7 | 93.1 ± 4.7 |
| D (%) | 94.3 ± 4.7 | 94.0 ± 4.7 | 93.5 ± 4.7 | 93.1 ± 4.7 | 92.5 ± 4.6 |
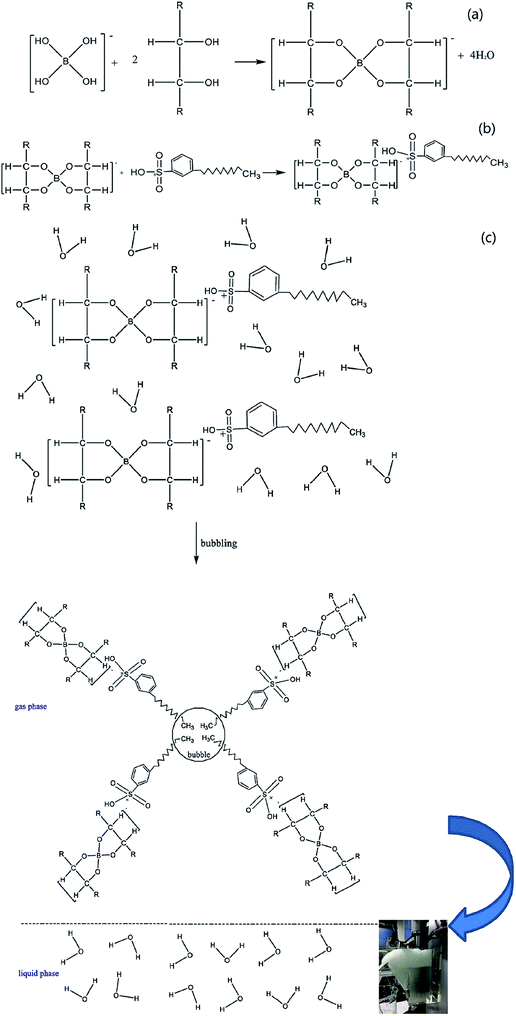 | ||
| Fig. 6 The mechanism of boron removal. Adapted from ref. 56 with permission from Elsevier, Copyright 2018. Reprinted from C. Bai, M. Guo, Z. Liu, Z. Wu and Q. Li, A novel method for removal of boron from aqueous solution using sodium dodecyl benzene sulfonate and D-mannitol as the collector, Desalination, 2018, 431, 47–55, Copyright (2018), with permission from Elsevier. Reprinted with permission by Elsevier.56 | ||
Afterwards, due to its oxophilic character, B(OH)4− can form stable complexes with D-mannitol (as shown in Fig. 6a); then, the negatively charged complexes can be readily absorbed by SDBS due to its electron-acceptor sulfo groups (as shown in Fig. 6b). Finally, the hydrophobic boric complexes can attach to the rising bubbles and reach the top of the flotation cell (as shown in Fig. 6c).56 Briefly speaking, ion flotation can efficiently remove organic and biological pollutants using various types of surfactants as collectors, and the mechanisms of interaction between the target ions and used surfactants vary based on the surfactant and target ions.
Reprinted from M. Groß, M. Tupinamba Lima, M. Uhlig, A. Ebraheme, O. Roeber, B. Olschewski, R. von Klitzing, R. Schomäcker and M. Schwarze, Biopolymers for dye removal via foam separation, Sep. Purif. Technol., 2017, 188, 451–457, Copyright (2017), with permission from Elsevier.
In addition to the above studies, ion flotation has been applied to other target contaminants. However, considering the length of the present article, additional studies on wastewater treatment via ion flotation are summarized in Table 2.
| Target contaminant | Collector | Experimental conditions | Removal (%) | Ref. |
|---|---|---|---|---|
| Cu(II) | EHDABr | pH 7, flow rate 60 ml min−1, time 60 min, Cu(II) 0.066 mM, EHDABr 0.4 mM | 99 | 57 |
| Cu(II) | Xanthates | pH 2.5 to 5.5, 10% excess of xanthate, airflow rate 100 cm3 min−1 | 100% | 58 |
| Cu(II) | Anti and syn 2-hydroxy-3,5-di-tert-butyl-benzaldoxime | pH 8.5 to 9.5, Cu(II) 200 mg L−1, molar ratio of surfactant/metal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 59 |
| Cu(II) | Dry baker's yeast and cetylpyridinium bromide (CPB) | pH 4.5, biosorbent 0.5% w/v, 10 min, CPB 0.01 M, molar ratio CPB/Cu(II) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
97.09 | 60 |
| Cu(II) | Sodium diethyldithiocarbamate (DEDTK) | pH 3, Cu(II) 50 mg L−1, airflow rate 1.8 L min−1, foaming agent 39.6 g m−3 | 96.4 | 61 |
| Cu(II) | Silica nanoparticles (SNP) | pH 6.0, Cu(II) 15 mg L−1, SNP 90 mg L−1, CTAB 35 mg L−1, flotation column height 750 mm | 94.5 ± 4.7 | 62 |
| Pb(II) | SDS and barley husk | pH 8, Pb(II) 50 mg L−1, barley husk 20 mg L−1, SDS 25 mg L−1, airflow rate 1 L min−1 | 95 | 9 |
| Pb(II) | SDS | pH 8, Pb(II) 50 mg L−1, SDS 25 mg L−1, airflow rate 1 L min−1 | 85 | 9 |
| Pb(II) | Sodium lauryl sulfate | pH 8.2, molar ratio of surfactant/metal 2 | 97 | 63 |
| Cd(II) | Sodium trideceth-4 carboxylate (AEC) | pH 7.5, AEC 7.5 mM, molar ratio of surfactant/metal 10 | 99.8 | 64 |
| Cd(II) | Potassium ethyl xanthate (KEtX) | pH 6.2, Cd(II) 0.5 mM, KEtX/Cd(II) molar ratio 3, 30 min | 64 | 65 |
| Cd(II) | KEtX and HDTMA | pH 6.2, Cd(II) 0.5 mM, KEtX/Cd(II) molar ratio 3, collector 0.25 mM, 30 min | 99 | 65 |
| KEtX and SDS | 93 | |||
| Zn(II) | EHDABr | Zn(II) 5 ppm, EHDABr 2.5 mM, flow rate 40 ml min−1, 150 min | 95.98 | 66 |
| Co(II) | EDTA and cetylpyridinium chloride (CPyCl) | Co(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDTA EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CPyCl 1 CPyCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
99 | 28 |
| U(VI) | Cetyl trimethylammonium bromide | U(VI) 0.1 mM, carbonate 0.1 M, collector U 5, gas flow rate 52 ml min−1 | 100 | 67 |
| Ge(IV) | Pyrogallol and DA | pH 4, metal ions![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrogallol pyrogallol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DA 1 DA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
100 | 14 |
| Cr2O72− | EHDABr | pH 5.2, air rate 1600 ml min−1, retention time 150 min | 90 | 68 |
| Cr2O72− | Rhamnolipid (RL) | Cr(VI) 40 ppm, pH 8, airflow rate 50 ml min−1, RL/Cr molar ratio 0.01, Fe/Cr molar ratio 3 | 95 | 69 |
| Ni(II) | R. opacus | pH 5, Ni(II) 5 mg L−1, Al 50 mg L−1, R. opacus 2 g L−1, 15 min | 90 | 70 |
| Al(III) | 93 | |||
| Pb(II) | Sodium alginate and SDBS | pH 5.35, Pb(II) 0.4 mM, Cu(II) 1.5 mM, calcium chloride 4 wt%, flow rate 120 cm3 min−1 | 99 | 71 |
| Cu(II) | 92 | |||
| Zn(II) | SDBS | Cd(II)/Zn(II) 0.01 mM, collector 0.1 mM, surfactant 0.2 mM, 60 min | 90 | 72 |
| Cd(II) | 95.2 | |||
| As(V) | SDS | pH 4.0, SDS 54.13 mg L−1, Fe(III) 134.89 mg L−1, Mo(VI) 48 mg L−1, As(V) 60 mg L−1 | 99.4 | 73 |
| Mo(VI) | 99.9 | |||
| Ca(II) | Calcium aluminate compound | pH above 11.5, CaO 0.75 g L−1, monocalcium aluminate (C70) 2 g L−1, reaction time 6 h | 80 | 74 |
| SO42− | 90 | |||
| Cu(II) | Xanthate and dialkyldithiocarbamate | pH 5, 10% excess of the stoichiometric amount of xanthate, stoichiometric amount of diethyl-dithiocarbamate | >95 | 75 |
| Zn(II) | ||||
| As(V) | ||||
| Pb(II) | Tea saponin | pH 6, tea saponin to metal ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
89.95 | 76 |
| Cu(II) | 81.13 | |||
| Cd(II) | 71.17 | |||
| Hg(II) | S-Octanoyl-cysteine | pH 8, metal ions 5 mg L−1, S-octanoyl-cysteine 0.01 M | 99.9 | 77 |
| As(III) | 99.6 | |||
| Pb(II) | 99.4 | |||
| Cd(II) | 99.2 | |||
| Cr(V) | 99.7 |
As a promising technology for wastewater treatment, ion floatation has attracted increasing attention due to its low energy requirements, rapid operation, small space requirements, low residual concentration, relatively low cost, etc. However, it also has many disadvantages, such as secondary pollution caused by chemical synthetic surfactants, large consumption of biosurfactants, high cost of nanoparticle surfactants, etc. However, to further promote the practical application of ion flotation in wastewater treatment, more work should be undertaken to develop eco-friendly and high-efficiency surfactants. Moreover, recycled surfactants will be very popular due to their low cost and environmental benignity.
3 Recovery of precious metals
Precious metals mainly refer to 8 metal elements, such as gold, silver and platinum, which have beautiful colors and are not susceptible to chemical reactions under normal conditions. Precious metal resources are scarce and non-renewable; thus, arbitrary disposal not only causes waste of resources but also environmental pollution. Therefore, the recovery, purification and reuse of precious metals is conducive to the construction of ecological civilizations and sustainable development.A pilot scale field trial of recovering gold cyanide anions from heap leaching liquor via ion flotation was undertaken with the aid of cetyltrimethyl ammonium bromide (CTAB) as the collector. Almost 100% of the gold could be recycled, and the recyclability of the collector was about 80% after 13 reagent recycle stages.78 Reyes et al. investigated the recovery of silver in spent diluted fixer through ion flotation using a column. A recovery of 97% could be obtained using 0.06 g L−1 of sodium isopropyl xanthate (SIX) and 0.04 g L−1 of frother. Additionally, controlling the pH at 6 helped improve the silver recovery.79 Meanwhile, silver recovery from dilute aqueous solutions containing thiosulphates by ion flotation was also proved to be feasible. A high recovery of silver (almost 100%) could be obtained using dodecylamine as the collector together with ethanol (0.5%) as the frother (as shown in Fig. 7).80
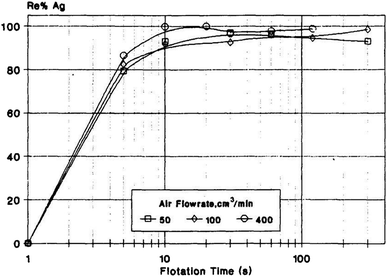 | ||
| Fig. 7 Effects of flotation time and air flowrate on RAg. Reproduced from ref. 80 with permission from Elsevier, Copyright 1995. Reprinted from A. I. Zouboulis, Silver recovery from aqueous streams using ion flotation, Miner. Eng., 8, 1477–1488, Copyright (1995), with permission from Elsevier. | ||
As mentioned above, ion flotation can be used to recover multiple precious metal ions or their cyanide complexes. An experiment of ion flotation on an aqueous solution of gold and silver cyanide anions was conducted with cetyltrimethylammonium bromide as the collector. Almost 100% gold recovery could be obtained, and cetyltrimethylammonium bromide exhibited significant selectivity for gold over silver.81 The recovery of cationic complexes of rhodium and palladium via ion flotation was investigated using sodium dodecyl benzenesulfonate (SDBS) as the surfactant; it was noted that the cationic complexes of rhodium(III) and palladium(II) can be floated with SDBS. Otherwise, two ion flotation procedures were proposed which were rapid, simple and did not require expensive reagents or apparatus to obtain satisfactory separation of binary mixtures of Rh(III), Pd(II) and Pt(iv) (as shown in Fig. 8).13 Au(III), Ir(III), Pd(II) and Pt(iv) could also be recovered by ion flotation when cationic collectors such as pentadecyl trimethyl ammonium bromide (PTMAB) and hexadecyl tripropyl ammonium bromide (HTPAB) were used. The recoveries of each metal were 99.9%, 99.8%, 99.45 and 99.7%, respectively, when floated under optimum conditions. The condensed foam volume was found to be less than 0.5 ml; thus, an enrichment of 200-fold could be obtained, which shows that ion flotation can be an effective method for precious metals recovery.82
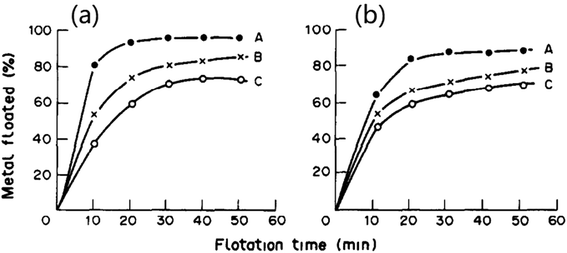 | ||
| Fig. 8 Effects of flotation time and surfactants on RRh (a), RPd (b) recovery: A SDBS, B SDS, C sodium lauryl sulfate (SLS). Reproduced from ref. 13 with permission from Elsevier, Copyright 1991. Reprinted from X. He, Ion flotation of rhodium(III) and palladium(II) with anionic surfactants, Talanta, 38, 319–323, Copyright (1991), with permission from Elsevier. | ||
In summary, ion flotation exhibits great advantages over other technologies in recovering precious metals due to its simplicity, ease of operation, low cost, etc. However, most surfactants used for recovering precious metals have low separation indices. Meanwhile, the surfactants used also bring great environmental hazards, which restricts their industrial application. Therefore, environmentally friendly surfactants with high selectivity are urgently required to expand the practical application of ion flotation.
4 Pre-concentration of rare-earth elements
Rare-earth elements are widely used in semiconductors, electronic components, alloys, metallurgy, glass and ceramic manufacturing, special materials, and other industries; they have long been considered to be a strategic mineral resource.83 However, rare earth industries face the difficulty of separating certain elements from their sum. Ion flotation is a promising technology for ensuring production of concentrates containing 60% to 70% rare earth elements.Rose et al. investigated conditions for concentrating yttrium from solution using α-sulphonated fatty acids via ion flotation, and a maximum recovery of 99.5% was obtained under optimum conditions within 30 minutes; furthermore, they found that by increasing the pH to 8, the extraction rate was 3 times faster with a smaller loss in extraction compared with flotation at pH 2.75.84 Chirkst et al. found that as the pH increased, the distribution coefficients of yttrium(III), cerium(III) and europium(III) sharply increased to almost 100%. The pH values of initial extraction of 4.5 for yttrium(III), 5.5 for cerium(III) and 6.2 for europium(III) indicate that these ions can be recovered and separated from each other by adjusting the pH value via ion flotation with sodium dodecyl sulfate serving as the collector (as shown in Fig. 9). The experimental results fitted well with the calculation of the instability constants of hydroxo complexes, solubility products of hydroxides, and Gibbs energies of formation of the specified compounds.85
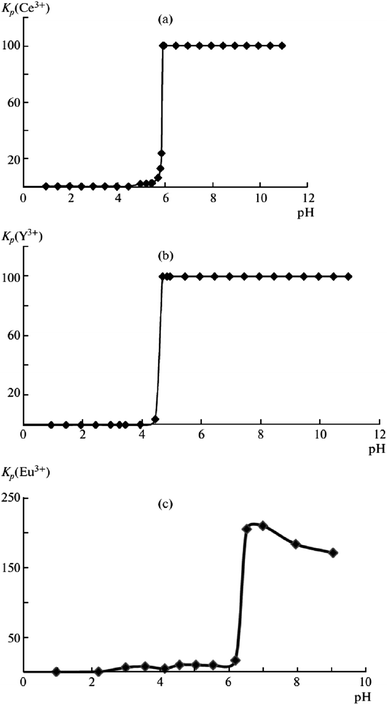 | ||
| Fig. 9 Dependences of the distribution coefficients Kdistr of (a) Ce3+, (b) Y3+, and (c) Eu3+ ions on solution pH. Reproduced from ref. 85 with permission from Springer, Copyright 2009. Reprinted by permission from Springer, D. E. Chirkst, O. L. Lobacheva, I. V. Berlinskii and M. I. Sulimova, Russ. J. Phys. Chem. A, The Thermodynamic Properties of Hydroxo Compounds and the Mechanism of Ion Flotation for Cerium, Europium, and Yttrium, Copyright (2009). | ||
Later, they found that cerium(III) and yttrium(III) could be concentrated individually from solutions of their salts at pH 4.5 to 6 when sodium dodecyl sulfate was used as the surfactant; the experimental results were consistent with the calculated dissociation constant of dodecyl sulfuric acid obtained from experimental potentiometric titration curves of the solutions.15 Additionally, they used sodium dodecyl sulfate as a collector to concentrate lanthanides; upon addition of chloride ions in concentrations of 0.01 to 0.1 M, a decreasing tendency of the distribution coefficients and shifts of the maximum recovery to the region of higher pH values were noted. A maximum distribution coefficient could be obtained at a chloride concentration of 0.01 M, which helped to improve the separation efficiency (as shown in Table 3).16
| Separation coefficient | cNaCl = 0 | cNaCl = 0.01 M | cNaCl = 0.05 M | |||
|---|---|---|---|---|---|---|
| Kmax | pH | Kmax | pH | Kmax | pH | |
| KSm/Ce | 5.53 | 6.70 | 7.54 | 7.00 | 1.51 | 7.60 |
| KEu/Ce | 13.78 | 6.70 | 41.52 | 7.00 | 1.39 | 7.65 |
| KEr/Ce | 40.92 | 6.70 | 6.77 | 6.50 | 2.32 | 6.00 |
| KCe/Yb | 0.74 | 6.00 | 6.73 | 6.30 | 60.15 | 8.56 |
| KCe/Y | 0.54 | 4.50 | 9.80 | 6.50 | 32.41 | 8.56 |
| KEu/Sm | 1.10 | 6.00 | 14.60 | 6.30 | 0.92 | 7.40 |
| KSm/Er | 3.89 | 4.00 | 54.13 | 7.52 | 17.08 | 8.70 |
| KSm/Yb | 1.32 | 5.40 | 7.54 | 7.80 | 25.45 | 8.40 |
| KSm/Y | 1.60 | 3.50 | 25.73 | 6.60 | 13.72 | 8.40 |
| KEu/Er | 1.48 | 5.55 | 25.16 | 7.30 | 6.96 | 8.75 |
| KEu/Yb | 1.21 | 6.10 | 20.69 | 6.35 | 7.57 | 8.90 |
| KEu/Y | 0.92 | 4.60 | 99.45 | 6.70 | 3.49 | 8.40 |
| KEr/Yb | 5.94 | 6.40 | 27.07 | 6.30 | 4.09 | 7.80 |
| KY/Er | 2.55 | 6.20 | 110.03 | 7.80 | 4.28 | 7.40 |
| KY/Yb | 3.04 | 7.00 | 49.73 | 7.80 | 8.62 | 7.80 |
The same phenomenon was also found when floating and concentrating La(III) from nitrate and nitrate-chloride solutions with dodecyl sulfate as the collector. However, the addition of chloride ions did not significantly improve the ion flotation of holmium(III); also, the distribution coefficient increased with the addition of chlorides and reached the maximum at a sodium chloride concentrate of 0.01 M83.
Lobacheva et al. used sodium dodecyl sulfate as a collector to concentrate yttrium(III) and ytterbium(III) cations from diluted aqueous solutions in the presence of chloride ions via ion flotation. A decreasing tendency of the distribution coefficient (Kdistr.) and a shift of the maximum recovery to the range of lower pH values was found for ytterbium ion flotation with the addition of chloride. The Kdistr. of yttrium also decreased, while the pH of maximum recovery shifted to a higher value region. The maximum yttrium recovery (in the form of Y(OH)3) and separation coefficient could be obtained at a chloride concentration of 0.01 M and pH 7.8.86
Maximum recoveries of yttrium and cerium ions could be obtained at pH 5.5 and 7.0, respectively, by ion flotation according to their other research. This allows recovery and separation of cerium and yttrium cations from their salts in the course of processing of lean technogenic raw materials at properly chosen pH values.87
It can be concluded that ion flotation plays a critical role in concentrating and separating rare-earth elements due to its characteristics of low cost, small space requirement, energy saving, etc. Despite its numerous advantages, ion flotation also involves numerous difficulties; for example, when concentrating and separating multicomponent rare-earth elements, it is necessary to adjust the pH value more than once, which is labor-intensive and time-consuming. In addition, the commonly used surfactants are harmful to the environment. Therefore, surfactants with biodegradability and selectivity should be considered as potential alternatives to the surfactants used at present.
5 Selective separation of multicomponent ions
As mentioned above, ion flotation is a promising separation technology for recovering or removing target ions. However, the solution to be treated usually contains more than one ion that needs to be recovered. Separation of one colligend of interest from other ions that may also be collected is quite necessary, either for economic reasons or for compliance with environmental restrictions on waste composition. Therefore, information on the selectivity between different ions is important for designing new ion flotation processes to recover valuable components from solutions and waste treatment. Moreover, it is advantageous to acquire a mechanistic understanding of the selective separation of one ion over others to assist in selecting appropriate collectors and conditions for a specific application.88A great deal of research has been conducted on the selective separation of multicomponent ions by ion flotation, and satisfactory results have been acquired.
At the very beginning, the Gouy–Chapman diffuse layer theory was adopted to define the selective coefficient between two ions. According to this theory, a general discipline can be concluded that the selectivity of a colligend with a higher valence is usually greater than that of other ions with lower valence, which was verified by many studies.89,90
The selective adsorption coefficient between two ions in the Gouy–Chapman model is defined as
There are two assumptions in the above theory; the first is that the dielectric constant is constant over the diffuse double layer and there is no polarization of ions, and the second is that the ions are points of charge with no radius so that there is no selectivity among ions with the same charge. Obviously, selectivity among ions of the same valency does in fact exist. Soon afterwards, Jorne and Rubin made some modifications on the basis of the Gouy–Chapman diffuse layer theory by introducing the difference in the distance of closest approach of ions with different sizes. The selective adsorption coefficient between two ions in the Jorne and Rubin model is defined as
Soon afterwards, they noted that experimental data for the separation of Sr2+ and UO2+ ions in the presence of monobutyl biphenyl sodium sulfonate as a collector fitted well with the above theory, which indicates that the selectivity of ion flotation relies not only on the charge but also on the size of the hydrated ion.18
Huang et al. experimentally studied the ion flotation of copper, cadmium and lead using sodium dodecylbenzene sulfonate on the basis of Jorne and Rubin's model. They found that the order of ion removal was Cu2+ < Cd2+ < Pb2+, which supports the fact that the selectivity depends on the charge and effective radii of the hydrated ion.91
An investigation of the ion flotation of the transition metal cations In(III), Cr(III), Fe(III), Cd(II), Mn(II), Co(II), Zn(II) and Ag(I) with sodium dodecylbenzene sulfonate and sodium dodecyl sulfonate as the collectors was conducted by Walkowiak; the selectivity sequence of Ag(I) < Mn(II) < Zn(II) < Co(II) < Fe(III) < Cr(III) < In(III) was established, which verified that the preferential removal of certain metal ions is closely related to the ratio of ionic charge to ionic radius and the solubility products of the metal-collector compounds. Additionally, the result confirmed the selective foam fractional model proposed by Jorne and Robin, which was based on the Gouy–Chapman diffused double layer theory with the restriction that the closest approach to the surface is determined by the size of the hydrated ions.92
Liu et al. developed two thermodynamically based theoretical models to predict the selectivity coefficients for Ca(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) and Pb(II)
Cu(II) and Pb(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) in ion flotation with sodium dodecylsulfate (SDS) as the collector. Although the surface model predicted the right selectivity order, the selectivity coefficients did not match the experimentally measured selectivity. The selectivity coefficients estimated by the dehydration model were 1.55 and 2.07 for Ca(II)
Cu(II) in ion flotation with sodium dodecylsulfate (SDS) as the collector. Although the surface model predicted the right selectivity order, the selectivity coefficients did not match the experimentally measured selectivity. The selectivity coefficients estimated by the dehydration model were 1.55 and 2.07 for Ca(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) and Pb(II)
Cu(II) and Pb(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II), respectively, which agreed well with the experimentally measured values.88
Cu(II), respectively, which agreed well with the experimentally measured values.88
Liu et al. removed Cd(II), Zn(II) and Cu(II) from solution via ion flotation with rhamnolipid as a surfactant; they found that ions with larger crystalline radii preferentially reacted with the surfactant.93–95 The ratios of the ionic radii for the competitive systems were 1.03, 1.28 and 1.32 for Zn/Cu, Cd/Zn and Cd/Cu, respectively. As shown in Fig. 10, the selectivity coefficient of Cd over Zn, SCd Zn, was determined to be 1.61, with R2 = 0.9854 (as shown in Fig. 10a); the selectivity coefficient of Cd over Cu, SCd Cu, was determined to be 3.05, with a coefficient of determination, R2, of 0.9593 (as shown in Fig. 10b); and the selectivity coefficient of Zn over Cu, SZn Cu, was determined to be 1.677, with R2 = 0.9672 (as shown in Fig. 10c). The obtained selectivity coefficient values were in accordance with the ratios of the ionic radii, which suggests that the selectivity sequence of these metals in ion flotation with rhamnolipid as the collector is Cd(II) > Zn(II) > Cu(II).96
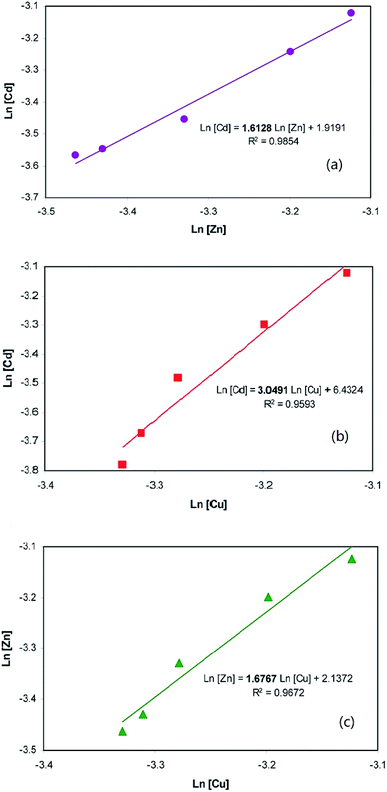 | ||
| Fig. 10 Ion flotation selectivity coefficient determination: Cd and Zn (a), Cd and Cu (b), Zn and Cu (c). Adapted from ref. 96 with permission. Copyright 2013 American Chemical Society. | ||
Furthermore, Chirkst et al. discovered that the distribution coefficients of yttrium(III) and cerium(III) sharply increased to almost 100% at pH 4.5 and 6, respectively; therefore, recovery of yttrium(III) began at pH 4.5 and recovery of cerium(III) began at pH 6. Thus, a general conclusion can be drawn that selective recovery of REMs can be obtained by varying the pH value. Additionally, they noted that the maximum yttrium recovery (in the hydrolysate form of Y(OH)3) and separation coefficient from ytterbium could be obtained at a chloride concentration of 0.01 M and pH 7.8. It can be concluded that pH and hydrolysis also contribute to the selectivity of multicomponent ions.
The above results indicate that the selectivity of multicomponent ions is closely related to the valence, crystalline radii, and hydrated radii of the metal ions, the ratio of ionic charge to ionic radius, pH, and hydrolysis together with the solubility products of the metal-collector compounds. Therefore, the selective separation of multicomponent ions can be easily accomplished with proper collectors, and accurate models should also be developed and established to predict the selectivity of different ions in future work.
Adapted with permission from A. Bodagh, H. Khoshdast, H. Sharafi, H. S. Zahiri and K. A. Noghabi, Removal of Cadmium(II) from Aqueous Solution by Ion Flotation Using Rhamnolipid Biosurfactant as an Ion Collector, Ind. Eng. Chem. Res., 52, 3910–3917. Copyright (2013) American Chemical Society.
6 Summary and outlook
Currently, ion flotation, a promising separation technology, is widely used in the fields of wastewater treatment, mineral beneficiation, such as rare precious metal recovery, and hydrometallurgy, such as pre-concentrating of rare earth elements and selective separation of multicomponent ions. This technology has been widely recognized by the public due to its simplicity, rapidity, economy, good separation yields, and suitability for a variety of target ions at various levels.Much progress has been achieved in the application of ion flotation. Biosurfactants were developed to overcome secondary pollution due to chemical surfactants. Recently, in order to conquer the problem of large consumption of surfactants, nanoparticle collectors were introduced into ion flotation. Moreover, many novel flotation machines have been developed simultaneously, such as a cyclonic state micro-bubble flotation column47 and a modified Jameson cell.97 However, numerous challenges remain to be overcome. Among these, large consumption or high cost of collectors, secondary pollution from chemicals used in ion flotation and low selectivity among ions are the main factors that limit its practical application. In addition, more research should be pursued to develop new, efficient collectors which have the advantages of high efficiency, lower dosage, better selectivity, low cost and environmental friendliness.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (project no. U1704252 and 51804275). Moreover, we also thank the China Postdoctoral Science Foundation (No. 2018M632811), Postdoctoral Fund of Henan Province (No. 174793), Scientific Research Start-up Project of Zhengzhou University (No. 32210793), Key scientific research project plan of Henan Colleges and Universities (No. 19A45001), and Modern Analysis and Computing Center in Zhengzhou University. The literature studies cited in this article are also acknowledged gratefully by the authors.References
- I. Langmuir and V. J. Schaefer, J. Am. Chem. Soc., 2002, 59, 2400–2414 CrossRef.
- F. Sebba, Nature, 1959, 184, 1062–1063 CrossRef CAS.
- S. K. Nicol, K. P. Galvin and M. D. Engel, Miner. Eng., 1992, 5, 1259–1275 CrossRef CAS.
- E. A. Deliyanni, G. Z. Kyzas and K. A. Matis, J. Mol. Liq., 2017, 225, 260–264 CrossRef CAS.
- Ü. Yenial and G. Bulut, J. Mol. Liq., 2017, 241, 130–135 CrossRef.
- J. J. da Rosa and J. Rubio, Miner. Eng., 2005, 18, 701–707 CrossRef CAS.
- E. Carissimi and J. Rubio, Int. J. Miner. Process., 2005, 75, 237–247 CrossRef CAS.
- J. Rubio, M. L. Souza and R. W. Smith, Miner. Eng., 2002, 15, 139–155 CrossRef CAS.
- A. A. Mohammed, S. E. Ebrahim and A. I. Alwared, Journal of Chemistry, 2013, 2013, 1–6 CrossRef.
- S. E. Ghazy, S. M. Elmorsy and A. H. Ragab, J. Appl. Sci. Environ. Manage., 2010, 12, 75–82 Search PubMed.
- R. B. Grieves and G. A. Ettelt, AIChE J., 1967, 13, 1167–1171 CrossRef CAS.
- R. B. Grieves, T. E. Wilson and K. Y. Shih, AIChE J., 1965, 11, 820–824 CrossRef CAS.
- X. C. He, Talanta, 1991, 38, 319–323 CrossRef CAS PubMed.
- A. Hernández-Expósito, J. M. Chimenos, A. I. Fernández, O. Font, X. Querol, P. Coca and F. García Peña, Chem. Eng. J., 2006, 118, 69–75 CrossRef.
- D. E. Chirkst, O. L. Lobacheva, I. V. Berlinskii and M. A. Sulimova, Russ. J. Appl. Chem., 2009, 82, 1370–1374 CrossRef CAS.
- D. E. Chirkst, O. L. Lobacheva and N. V. Dzhevaga, Russ. J. Appl. Chem., 2011, 84, 1476–1482 CrossRef CAS.
- Z. Bahri, B. Rezai and E. Kowsari, Miner. Eng., 2016, 86, 104–113 CrossRef CAS.
- J. Jorné and E. Rubin, Sep. Sci., 1969, 4, 313–324 Search PubMed.
- B. Kawalec-Pietrenko and P. Rybarczyk, Chem. Pap., 2014, 68, 890–898 CAS.
- J. S. Guest, S. J. Skerlos, J. L. Barnard, B. M Bruce, G. T. Daigger, H. Helene, S. J. Jackson, K. Karen, K. Linda and M. Linda, Environ. Sci. Technol., 2009, 43, 6126–6130 CrossRef CAS PubMed.
- T. Oki, S. Kanae and K. Musiake, Membrane, 2003, 28, 206–214 CrossRef.
- D. Zamboulis, E. N. Peleka, N. K. Lazaridis and K. A. Matis, J. Chem. Technol. Biotechnol., 2011, 86, 335–344 CrossRef CAS.
- W. Peng, H. Li, Y. Liu and S. Song, J. Mol. Liq., 2017, 230, 496–504 CrossRef CAS.
- D. S. Patil, S. M. Chavan and J. U. K. Oubagaranadin, J. Environ. Chem. Eng., 2016, 4, 468–487 CrossRef CAS.
- V. Coman, B. Robotin and P. Ilea, Resour. Conserv. Recycl., 2013, 73, 229–238 CrossRef.
- S. S. Ahluwalia and D. Goyal, Bioresour. Technol., 2007, 98, 2243–2257 CrossRef CAS PubMed.
- H. Polat and D. Erdogan, J. Hazard. Mater., 2007, 148, 267–273 CrossRef CAS PubMed.
- M. A. Soliman, G. M. Rashad and M. R. Mahmoud, Radiochim. Acta, 2015, 103 DOI:10.1515/ract-2015-2390.
- A. Paraneeiswaran, S. K. Shukla, T. Subba Rao and K. Prashanth, Chemosphere, 2014, 95, 503–510 CrossRef CAS PubMed.
- V. K. Gupta, A. Shilpi and T. A. Saleh, J. Hazard. Mater., 2010, 185, 17–23 CrossRef PubMed.
- B. A. Fowler, Toxicol. Appl. Pharmacol., 2009, 238, 294–300 CrossRef CAS PubMed.
- Z. Guixia, R. Xuemei, G. Xing, T. Xiaoli, L. Jiaxing, C. Changlun, H. Yuying and W. Xiangke, Dalton Trans., 2011, 40, 10945–10952 RSC.
- G. Craioveanu, L. Stoica and C. Constantin, Sep. Sci. Technol., 2015, 50, 802–812 CrossRef CAS.
- W. Peng, G. Han, Y. Cao, K. Sun and S. Song, Colloids Surf., A, 2018, 556, 266–272 CrossRef CAS.
- W. Peng, H. Li, Y. Liu and S. Song, Appl. Surf. Sci., 2016, 364, 620–627 CrossRef CAS.
- M. H. Salmani, M. Davoodi, M. H. Ehrampoush, M. T. Ghaneian and M. H. Fallahzadah, Iran. J. Environ. Health Sci. Eng., 2013, 10, 16 CrossRef PubMed.
- M. R. Mahmoud and N. K. Lazaridis, Sep. Sci. Technol., 2015, 50, 1421–1432 CrossRef CAS.
- F. S. Hoseinian, M. Irannajad and A. J. Nooshabadi, Int. J. Miner. Process., 2015, 143, 131–137 CrossRef CAS.
- A. Eivazihollagh, J. Tejera, I. Svanedal, H. Edlund, A. Blanco and M. Norgren, Ind. Eng. Chem. Res., 2017, 56, 10605–10614 CrossRef CAS.
- M. Doğutan Yenidünya, Sep. Sci. Technol., 2006, 41, 1741–1756 CrossRef.
- Y.-C. Lee, P.-Y. Wang, S.-L. Lo and C. P. Huang, Sep. Purif. Technol., 2017, 173, 280–285 CrossRef CAS.
- A. Pruss, Water Sci. Technol., 2015, 71, 645–652 CrossRef CAS PubMed.
- J. Yan, Z. Wu, Y. Zhao and C. Jiang, Sep. Purif. Technol., 2011, 80, 300–305 CrossRef CAS.
- L. Zaleschi, M. S. Secula, C. Teodosiu, C. S. Stan and I. Cretescu, Water, Air, Soil Pollut., 2014, 225 DOI:10.1007/s11270-014-2101-z.
- L. Zhang, B. Cao, D. Yao, R. Yu, C. Yu, H. Zhang and A. Yu, J. Sep. Sci., 2015, 38, 1733–1740 CrossRef CAS PubMed.
- L. Zhang, D. Yao, R. Yu, N. Li, H. Zhang and A. Yu, Anal. Methods, 2015, 7, 1977–1983 RSC.
- X. Li, H. Xu, J. Liu, J. Zhang, J. Li and Z. Gui, Sep. Purif. Technol., 2016, 165, 101–106 CrossRef CAS.
- H. W. Mu, M. L. Granstrom, T. E. Wilson and L. K. Wang, Jawra Journal of the American Water Resources Association, 2010, 10, 283–294 Search PubMed.
- S. J. Choi and Y. H. Choi, Sep. Sci. Technol., 2006, 31, 2105–2116 CrossRef.
- K. Shakir, A. F. Elkafrawy, H. F. Ghoneimy, S. G. Elrab Beheir and M. Refaat, Water Res., 2010, 44, 1449–1461 CrossRef CAS PubMed.
- M. Groß, M. Tupinamba Lima, M. Uhlig, A. Ebraheme, O. Roeber, B. Olschewski, R. von Klitzing, R. Schomäcker and M. Schwarze, Sep. Purif. Technol., 2017, 188, 451–457 CrossRef.
- N. Hu, W. Liu, L. Ding, Z. Wu, H. Yin, D. Huang, H. Li, L. Jin and H. Zheng, J. Nanopart. Res., 2017, 19 DOI:10.1007/s11051-017-3762-5.
- K. Sungpyo, E. Peter, J. N. Jensen, W. A Scott and D. S. Aga, Environ. Sci. Technol., 2005, 39, 5816–5823 CrossRef.
- D. Li, M. Yang, J. Hu, L. Ren, Y. Zhang and K. Li, Environ. Toxicol. Chem., 2010, 27, 80–86 CrossRef PubMed.
- T. Saitoh, K. Shibata, K. Fujimori and Y. Ohtani, Sep. Purif. Technol., 2017, 187, 76–83 CrossRef CAS.
- C. Bai, M. Guo, Z. Liu, Z. Wu and Q. Li, Desalination, 2018, 431, 47–55 CrossRef CAS.
- C. McDonald and A. Suleiman, Sep. Sci. Technol., 1979, 14, 219–225 CrossRef CAS.
- N. K. Lazaridis, E. N. Peleka, T. D. Karapantsios and K. A. Matis, Hydrometallurgy, 2004, 74, 149–156 CrossRef CAS.
- L. Stoica and I. Lacatusu, Int. J. Environ. Waste Manage., 2012, 9, 293 CrossRef CAS.
- L. Stoica, A.-M. Stanescu, C. Constantin, O. Oprea and G. Bacioiu, Water, Air, Soil Pollut., 2015, 226 DOI:10.1007/s11270-015-2533-0.
- K. A. Strel'tsov and D. V. Abryutin, Russian Journal of Non-Ferrous Metals, 2010, 51, 85–88 CrossRef.
- N. Hu, W. Liu, L. Jin, Y. Li, Z. Li, G. Liu, D. Huang, Z. Wu and H. Yin, Sep. Purif. Technol., 2017, 184, 257–263 CrossRef CAS.
- A. J. Rubin and W. L. Lapp, Anal. Chem., 1969, 41, 1133–1135 CrossRef CAS.
- J. Lu, Y. Li, S. Zhang and Y. Sun, J. Hazard. Mater., 2015, 286, 466–473 CrossRef CAS PubMed.
- M. R. Mahmoud, N. K. Lazaridis and K. A. Matis, Process Saf. Environ., 2015, 94, 203–211 CrossRef CAS.
- C. W. McDonald and O. A. Ogunkeye, Microchem. J., 1981, 26, 80–85 CrossRef CAS.
- K. Shakir, J. Appl. Chem. Biotechnol., 1973, 23, 339–347 CrossRef CAS.
- R. B. Grieves and S. M. Schwartz, J. Chem. Technol. Biotechnol., Biotechnol., 2010, 16, 14–17 Search PubMed.
- A. Salmani Abyaneh and M. H. Fazaelipoor, J. Environ. Manage., 2016, 165, 184–187 CrossRef CAS PubMed.
- J. E. B. Cayllahua and M. L. Torem, Desalination, 2011, 279, 195–200 CrossRef CAS.
- A. G. Corpuz, P. Pal, F. Banat and M. A. Haija, Sep. Purif. Technol., 2018, 202, 103–110 CrossRef CAS.
- U. Malgorzata, W. Wladyslaw, J. Youngchan, K. Jong Seung and R. A. Bartsch, Anal. Chem., 2003, 75, 2276–2279 CrossRef.
- Y. Zhao, A. I. Zouboulis and K. A. Matis, Sep. Sci. Technol., 1996, 31, 769–785 CrossRef CAS.
- A. D. Guerrero-Flores, A. Uribe-Salas, G. I. Dávila-Pulido and J. M. Flores-Álvarez, Miner. Eng., 2018, 123, 28–34 CrossRef CAS.
- G. A. Stalidis, K. A. Matis and N. K. Lazaridis, Sep. Sci. Technol., 1989, 24, 97–109 CrossRef CAS.
- X. Z. Yuan, Y. T. Meng, G. M. Zeng, Y. Y. Fang and J. G. Shi, Colloids Surf., A, 2008, 317, 256–261 CrossRef CAS.
- M. Taseidifar, F. Makavipour, R. M. Pashley and A. F. M. M. Rahman, Environmental Technology & Innovation, 2017, 8, 182–190 Search PubMed.
- K. P. Galvin, M. D. Engel and S. K. Nicol, Int. J. Miner. Process., 1994, 42, 75–98 CrossRef CAS.
- M. Reyes, F. Patiño, R. Escudero, M. Pérez, M. U. Flores and I. Reyes, J. Mex. Chem. Soc., 2012, 56, 408–416 Search PubMed.
- A. I. Zouboulis, Miner. Eng., 1995, 8, 1477–1488 CrossRef CAS.
- P. K. Galvin, K. S. Nicol and G. A. Waters, Colloids Surf., 1992, 64, 21–33 CrossRef.
- E. W. Berg and D. M. Downey, Anal. Chim. Acta, 1980, 120, 237–248 CrossRef CAS.
- D. E. Chirkst, O. L. Lobacheva and N. V. Dzhevaga, Russ. J. Appl. Chem., 2012, 85, 25–28 CrossRef CAS.
- M. W. Rose and F. Sebba, J. Chem. Technol. Biotechnol., Biotechnol., 2010, 19, 185–187 Search PubMed.
- D. E. Chirkst, O. L. Lobacheva, I. V. Berlinskii and M. I. Sulimova, Russ. J. Phys. Chem. A, 2009, 83, 2022–2027 CrossRef CAS.
- O. Lobacheva, N. Dzhevaga and A. Danilov, Journal of Ecological Engineering, 2016, 17, 38–42 CrossRef.
- O. L. Lobacheva, I. V. Berlinskii and O. V. Cheremisina, Russ. J. Appl. Chem., 2015, 87, 1863–1867 CrossRef.
- Z. Liu and F. M. Doyle, Colloids Surf., A, 2001, 178, 93–103 CrossRef CAS.
- K. Kubota and S. Hayashi, Can. J. Chem. Eng., 1977, 55, 286–292 CrossRef CAS.
- C. Walling, E. E. Ruff and J. L. Thornton, J. Phys. Chem., 1957, 61, 486–490 CrossRef CAS.
- R. C. H. Huang and F. D. Talbot, Can. J. Chem. Eng., 1973, 51, 709–713 CrossRef CAS.
- W. Walkowiak, Sep. Sci. Technol., 1991, 26, 559–568 CrossRef CAS.
- Z. Liu and F. M. Doyle, Miner. Metall. Process., 2001, 18, 167–171 CAS.
- M. Ulewicz, W. Walkowiak and C. Kozłowski, Fizykochem. Probl. Mineralurgii, 2001, 35, 21–29 CAS.
- Z. X. Yuan, T. Y. Meng, M. G. Zeng, Y. Y. Fang and G. J. Shi, Colloids Surf., A, 2008, 317, 256–261 CrossRef.
- A. Bodagh, H. Khoshdast, H. Sharafi, H. Shahbani Zahiri and K. Akbari Noghabi, Ind. Eng. Chem. Res., 2013, 52, 3910–3917 CrossRef CAS.
- M. Santander, L. Valderrama, M. Guevara and J. Rubio, Miner. Eng., 2011, 24, 1010–1015 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |