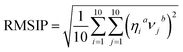The mechanisms of flavonoids inhibiting conformational transition of amyloid-β42 monomer: a comparative molecular dynamics simulation study†
Ling Wang*abd,
Ranran Zenga,
Xiaoqian Panga,
Qiong Gu*c and
Wen Tan*abd
aGuangdong Provincial Key Laboratory of Fermentation and Enzyme Engineering, School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, China. E-mail: went@scut.edu.cn; lingwang@scut.edu.cn
bPre-Incubator for Innovative Drugs & Medicine, School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, China
cResearch Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou 510006, China. E-mail: guqiong@mail.sysu.edu.cn
dKey Laboratory of Industrial Biotechnology of Guangdong Higher Education Institutes, School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, China
First published on 28th July 2015
Abstract
Flavonoids can bind Aβ42 to inhibit the aggregation of Aβ42 monomer. However, the inhibitory mechanism remains unknown. Herein, comparable molecular dynamics simulations for a total of 710 ns were performed to study its mechanism. The in silico experiments revealed that flavonoids halt the conformational transition of Aβ42 monomer by inhibiting β-sheet formation; the flavonoids push the residues D23 and K28 of Aβ42 to be exposed to solvated water, destroy the salt bridge between D23 and K28, induce the conformational distribution of Aβ42 into local minimization energy conformational state, and generate U-shaped Aβ42 configurations, which have more stable helixes and fewer unstable random coils. Moreover, simulation results from the free energy landscape and binding free energy analyses suggest that biflavonoids are superior to monoflavonoids in inhibiting conformational transition of Aβ42 monomer. These findings agree with the experimental data and may help in the design of new agents that will inhibit the conformational transition of Aβ42 so as to treat Alzheimer's disease.
Introduction
Alzheimer's disease (AD) is a progressive, neurodegenerative disorder that is characterized by the progressive, intra-cerebral accumulation of β-amyloid (Aβ) peptides.1 Aβ, which consists of 39–43 residues, is derived from an amyloid precursor protein (APP) through sequential cleavage by β- and γ-secretases.2 Among the variants of Aβ segments, Aβ42 is the predominant component in AD plaques, and displays an enhanced neurotoxicity.3–5Increasing evidence indicates that soluble oligomers are the main toxic agents.6–8 Studies demonstrate that monomeric Aβ42 undergoes spontaneous rearrangement of its initial secondary structure.9,10 Aβ42 is initially in a random coil or helix conformation, and subsequently transforms into β-sheet conformation. This transformation generates oligomeric and polymeric species, which have a higher content of β-sheet structures. Report suggests that the formation of β-sheets drives the amyloid self-assembly process.10 Molecular dynamics (MD) simulations demonstrate that the increased stability of the β-sheet conformation favors Aβ fibrillogenesis.11–14 Therefore, Aβ aggregation inhibitors that prevent β-sheet formation within Aβ are promising agents for treating AD.6,15
Flavonoids have been reported to have anti-fibrillogenic and cytoprotective properties.16–20 Biflavonoids are more potent than monoflavonoids in inhibiting Aβ42 aggregation. Circular dichroism (CD) spectroscopy reveals that biflavonoids have a higher activity than monoflavonoids in inhibiting β-sheet formation within Aβ.21 However, the molecular recognition mechanism remains unknown.
To data, numerous theoretical models, which were generated by molecular docking, MD simulations, and free energy analysis, have been conducted to characterize the inhibition mechanisms of current Aβ inhibitors at atomic level. For example, Liu and coworkers conducted a series of MD simulations and MMPBSA analyses to probe the molecular mechanism of the inhibition effect of (−)-epigallocatechin-3-gallate (EGCG) on the conformational transition of Aβ42 monomer.22 Their computational results suggested that EGCG inhibited conformational transition of Aβ42 monomer by increasing the content of helical structure and reducing the content of β-sheet structure, and the hydrophobic interactions played key role in the binding of EGCG and Aβ42 monomer. Some similar works were conducted to characterize the molecular interaction mechanisms of NQTrp inhibitor and Aβ1–28 monomer,23 FDDNP and ibuprofen and Aβ10–40 monomer,24,25 EGCG and NQTrp and Aβ42 dimer,26,27 and 2002-H20, curcumin, EGCG, NQTrp, and resveratrol and Aβ17–42 trimers.28 In 2013, Zhu and coworkers adopted a strategy for identifying small-molecule binding sites in the Aβ42 monomer in which MD simulations are combined with fragment-based drug design.29 Based on identified binding pockets, the binding models between Aβ42 and curcumin, and Aβ42 and Congo red were characterized using molecular docking and MD simulations. Compared with Zhu's strategy against Aβ42 monomer, Jiang and coworkers discovered fiber-binding compounds using structure-based virtual screening and bioassays.30 Structure–activity relationship studies of the fiber-binding compounds and their derivatives suggested that compound binding increased fiber stability and decreased fiber toxicity. Based on the above studies, it can be concluded that knowledge of inhibition mechanism will allow us to rationally design highly potent and selective Aβ inhibitors for anti-AD drug development.
The goal of this paper is to articulate the mechanism of the molecular recognition between flavonoids and Aβ42 monomer through molecular dynamics (MD) simulations, and to pave a rational way for designing highly effective and selective Aβ42 inhibitors. The study started with investigating the conformational transition of Aβ42 with different binding partners (apigenin, sumaflavone, 2′,8′′-biapigenin, and taiwaniaflavone, ESI Fig. S1†) to probe the driving force and the pathway of Aβ42 folding. Moreover, essential dynamics and free energy landscape analyses were employed to probe the stability of Aβ42 with different binding partners. Finally, the binding free energies for Aβ42 with monoflavonoids, bioflavonoids, and Aβ42 were measured.
Methods
Molecular docking
The structure of Aβ42 used in the docking calculations was retrieved from the Protein Data Bank (PDB ID: 1IYT, model 1).31 Protein atom types and potentials were assigned according to the Amber force field with Kollman-united-atom charges encoded in Sybyl 7.3. The initial structures of apigenin (Ap), sumaflavone (Sum), 2′,8′′-biapigenin (Bia), and taiwaniaflavone (TF) were optimized using the MMFF force field. The Powell method was used for energy minimization via default parameters set in Discovery Studio 2.5.Autodock 4.0 (ref. 32) was employed to identify the potential binding of mono- and biflavonoids to Aβ42 using a Lamarckian genetic algorithm. A grid map with 80 × 80 × 80 equally spaced points (i.e., spaced at 0.375 Å from one another) was generated using the AutoGrid program to evaluate the binding energies between the ligands and receptors. The dimensions were large enough to cover the whole receptor. Docking parameters are set to default values, except for number of GA runs (100) and the energy evaluations (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). All docked poses of each ligand were clustered using a tolerance of 2 Å for the root mean square deviation (RMSD) and ranked on the basis of the binding docking energies. For each compound, the lowest energy conformation in the most populated cluster was chosen for further study.
000). All docked poses of each ligand were clustered using a tolerance of 2 Å for the root mean square deviation (RMSD) and ranked on the basis of the binding docking energies. For each compound, the lowest energy conformation in the most populated cluster was chosen for further study.
Simulation systems
Initial coordinates for the unbound apo form of the Aβ42 in solution and the four Aβ42 complexes with the active inhibitors (i.e., Ap, Sum, Bia, and TF) were taken from the docking results. Each ligand–receptor complex was first put into a suitable box, of which the minimal distance from the peptide to the box wall was 1.2 nm. Then the box was solvated with the TIP3P water model.33 Neutralized counter ions were added to the simulation system. Energy was minimized by steepest descent followed by conjugate gradient algorithms to remove steric clashes within each system. The simulation is equilibrated in two steps lasting 200 ps; the first step involves an NVT ensemble and the second phase involves an NPT ensemble. Each system is simulated for 90 ns and trajectories were saved at 2.0 ps intervals for further analysis. Detailed of all the simulations are summarized in the ESI Table S1.† To ensure the simulations are repeatable and not a stochastic output, two independent simulations with different initial velocity distributions were performed for AP–Aβ42 and TF–Aβ42, and the results are documented in the ESI.†MD simulations
MD simulations were carried out through the GROMACS 4.5.3 package34 with constant number, pressure, and temperature (NPT) and periodic boundary conditions. The AMBER ff03 force field35 was applied for the peptide. Parameters for the four flavonoid ligands were obtained from the ANTECHAMBER module using the Generalized Amber Force Field (GAFF).36 The partial atomic charges for the ligand atoms were assigned using the RESP charge-fitting procedure with input from Hartree–Fock calculations at the 6-31G* level with the Gaussian03 program.37 During the simulations, the pressure and the temperature were coupled to 1 bar with an anisotropic coupling time of 1.0 ps, the temperature was kept at 300 K, and the coupling time was 0.1 ps. Both the pressure and temperature were controlled using Berendsen coupling protocols. The short-range van der Waals interactions were cut off at 1.4 nm. Long-range electrostatic interactions were included on every step using the Particle Mesh Ewald algorithm (PME). All bond lengths were constrained using the LINCS algorithm38 with time steps of 2 fs.MD simulations were run on a 96-CPU D Dawning TC2600 blade server (Dawning, Guangzhou Computer Center, China). Analyses were performed using the tools encoded in the GROMACS package, VMD,39 and in-house handwritten script. The secondary structures were characterized via the DSSP method.40 Structural diagrams were carried out using the Pymol package (http://www.pymol.org/).
Essential dynamics analysis and dynamics similarity metrics
Essential dynamics analysis (also called Principal Component Analysis, PCA)41,42 were employed to address the collective motions of apo–Aβ42 and ligand–Aβ42 complexes. Briefly, PCA is based on the diagonalization of the covariance matrix C, with the elements defined as follows:| Cij = <(ri − 〈ri〉) × (rj − 〈rj〉) (i,j = 1,2,3,…,3N) | (1) |
The similarities and differences among the essential subspaces spanned by the apo–Aβ42 and ligand–Aβ42 (mono- and biflavonoids) complexes were compared via the rooted mean square inner product (RMSIP).43–45 The RMSIP is defined as:
Free energy landscape analysis
The energy landscape for the conformational change of a peptide or ligand–peptide complex can be obtained by an appropriate conformational sampling procedure. Conformations generated by MD simulations were used for energy analysis. To obtain a two-dimensional representation of the energy landscape, the energy landscapes were projected onto the first (PC1) and second (PC2) principal components with the highest eigen values calculated from PCA analysis for apo–peptide and ligand–peptide complexes at 300 K. The free energy landscapes are defined as follows:46–48G(PC1,PC2) = −kBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(PC1,PC2), P(PC1,PC2), |
Binding free energy calculation
Each ligand–receptor complex was simulated for an additional 20 ns to calculate the binding free energies for mono-flavonoid–Aβ42 and biflavonoid–Aβ42 complexes, and to provide insight into the interaction energies and energetic stabilities of the complexes. The simulations were conducted with the AMBER 12 program using the AMBER ff03 force field. The MM/PBSA method in the AMBER 12 suite was used to calculate the binding free energies (see the ESI†).Results and discussion
Initial binding models of flavonoids and Aβ42 from docking
Previously MD simulations indicated that the structural segment of residues 24–40 is crucial for Aβ oligomerization and that four glycine residues (Gly25, Gly29, Gly33 and Gly37) are essential for β-sheet formation.12 Liu and coworkers suggested that the aggregation rate of Aβ correlated positively with the hydrophobic interactions of Aβ monomer and (−)-epigallocatechin-3-gallate (an Aβ42 inhibitor) from MD simulations and free energy analysis.22 A synthesized Aβ40 peptide analog incorporating 2-hydroxy-4-methoxybenzyl (Hmb) at residues 20, 25, 29, 33, or 37 (to protect the backbone amide) did not form fibrils.49 Therefore, small molecules can prevent fibril formation by binding the C-terminus hydrophobic area of Aβ42 monomer.At the beginning, we docked monoflavonoids (AP) and biflavonoids (Sum, Bia, and TF) into Aβ42 (see ESI Fig. S2a and d†). Both mono- and biflavonoids docked at the C-terminus hydrophobic area of Aβ42 (ESI Fig. S2†). Both mono-flavonoid and bi-flavonoids were surrounded by hydrophobic residues, Leu17, Val18, Phe20, Val24, Lys28, Ile31 and Met35, in vacuum. AP can form two hydrogen bonds with Phe20 and Asn27 (ESI Fig. S2a†); Sum forms one hydrogen bond with Phe20 (ESI Fig. S2b†); Bia forms two hydrogen bonds with Val18 and Lys16 (ESI Fig. S2c†); and TF forms two hydrogen bonds with Val18 and Leu34 (ESI Fig. S2d†). These hydrogen bonds may be favorable for the binding of Aβ42 and flavonoids.
Structural and dynamical analyses of apo–Aβ42 and ligand–Aβ42 complexes
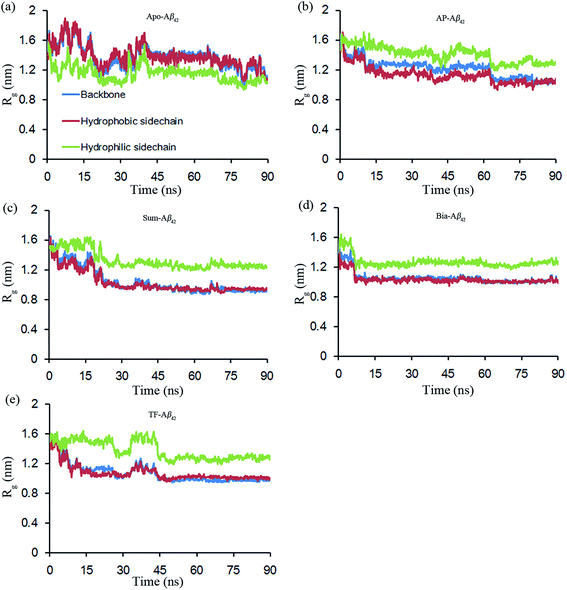
The conformational parameters, including the radius of gyration (Rg) and the end-to-end distance, were monitored as a function of time for all the simulations. The results are depicted in Fig. 1 (parts A and E) and ESI Fig. S3.† All Rg values of apo– and ligand–Aβ42 start with relatively high values and fall to lower values within 45 ns (Bia–Aβ42's Rg value fell within 5 ns, Fig. 1). After, the Rg values are steady; this suggests that the systems have all reached equilibrium status within 45 ns. The end-to-end distances of Asp1-Cα and Ala42-Cα atoms in the equilibrium systems, along with the time, are depicted in the ESI Fig. S3.† The Rg values of the backbone atoms from apo–Aβ42 decrease from ∼1.7 nm (the NMR structure parameter) to ∼1.3 nm within 40 ns. After, the Rg values stay at 1.3 nm (Fig. 1a). The Rg values for monoflavonoid– and biflavonoid–Aβ42 decrease significantly (i.e., from ∼1.7 nm to ∼1.1 nm for monoflavonoid–Aβ42, and from ∼1.7 nm to ∼1.0 nm for biflavonoid–Aβ42). Thus, both monoflavonoid and biflavonoid–Aβ42 have more compact conformations in water. The compact nature of these conformations is particularly salient in biflavonoid–Aβ42 complexes. The average values of the various conformational parameters obtained in the each simulation during the equilibrium period are listed in Table 1. The Rg values of backbone atoms indicate that the conformations of apo–, mono–, and biflavonoid–Aβ42 complexes are changed in water. The monoflavonoid and biflavonoid–Aβ42 conformations are more compact than that of apo–Aβ42. Therefore, we conclude that mono- and biflavonoids can induce Aβ42 to fold into a more compact structure in water; in contrast, the apo–Aβ42 conformation is not as compact. Biflavonoids (Sum, Bia, and TF) are more capable of inducing compact Aβ42 conformation formations than monoflavonoid (AP).| Complex | Rg (backbone) | Rg (hydrophobic side chain) | Rg (hydrophilic side chain) | End-to-end distance |
|---|---|---|---|---|
| a All results were obtained from the equilibrium period (45–90 ns) of the simulations, and are shown in nm. Standard errors of the mean are given in parentheses. | ||||
| Apo–Aβ42 | 1.31(0.11) | 1.31(0.09) | 1.11(0.07) | 2.09(0.39) |
| AP–Aβ42 | 1.14(0.09) | 1.06(0.06) | 1.34(0.09) | 1.53(0.60) |
| Sum–Aβ42 | 0.93(0.03) | 0.94(0.02) | 1.25(0.03) | 1.60(0.15) |
| Bia–Aβ42 | 1.02(0.03) | 1.01(0.02) | 1.24(0.03) | 1.71(0.18) |
| TF–Aβ42 | 0.98(0.01) | 1.01(0.02) | 1.27(0.03) | 1.34(0.21) |
The Rg values of 31 hydrophobic residues side chains atoms in each simulation exhibit similar fluctuations trend with that of all backbone atoms (Fig. 1a and e) and these average values of Rg from equilibrium are also similar (Table 1). These data suggest that the compact conformation folded by Aβ42 is possibly driven by hydrophobic association. It was reported that soluble Aβ is more compact conformation, which can initially interacts with an existing amyloid deposit to form amyloid fibrils, and the driving force for formation of amyloid would be based upon relieving internal stress and increasing bonding energetics via intermolecular interaction (mainly hydrophobic interaction).50–52 Unlike the Rg values of the 31 hydrophobic residues, the Rg values of the 11 hydrophilic residues do not correlate with that of the backbone atoms (Fig. 1a and e). Moreover, the binding of monoflavonoids and biflavonoids result in greater hydrophilic Rg, values (crosscurrent for 31 hydrophobic residues) compared with that of apo–Aβ42. These results suggest that the additional driven force (hydrophobic interactions between monoflavonoids and biflavonoids with Aβ42) induces an extended conformation for hydrophilic residues, and induces a compact conformation for hydrophobic residues.
The end-to-end distance (i.e., the distance between Asp1-Cα and Ala42-Cα) decreases significantly from ∼4.6 nm (see the NMR structure) to ∼2.0 nm within 45 ns, and then remains steady (subject to minor fluctuations). As shown in Fig. 3, the N- and C-termini of Aβ42 are closer to each other after folding. However, the end-to-end distances vary with different ligands (i.e., apo, AP, Sum, Bia, and TF) bind at equilibrium (see ESI Fig. S3† and Table 1). Compared with apo–Aβ42, the Aβ42 termini can be closer when Aβ42 binds with flavonoids (Table 1). Initial docking studies demonstrate that the formed hydrophobic interactions between mono- and biflavonoids with Aβ42 play a key role for the binding affinity. The Rg analysis suggests that the compact conformation of Aβ42 is possibly driven by hydrophobic associate. To sum up, the additional driven force from the hydrophobic interaction between monoflavonoids and biflavonoids with Aβ42 may promote the folding degree of Aβ42 compared with apo–Aβ42 in water.
Effects on Aβ42 conformational transition
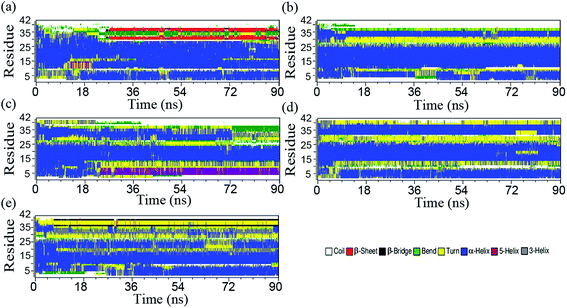
Conformational transition from α-helix to β-sheet is a crucial early step in Aβ amyloidogenesis.12,22,52–57 To characterize the inhibition effect of flavonoids on the conformational transition of Aβ42, the secondary structures of Aβ42 from each simulation were calculated through the DSSP method.40 The profiles of Aβ42 secondary structures with different binding partners along each simulation are shown in Fig. 2a and e. Obviously, the initial long α-helix of Aβ42 disappeared after several nanoseconds for apo–Aβ42 in water (Fig. 2a); in particular, the α-helix of residues 26–40 was converted into several short bends connected with turns or coils during the first 9 ns. Afterward, some α-helixes were occasionally converted into 3-helixes and rapidly returned to an α-helix structure within 9 to 27 ns (Fig. 2a). After 27 ns, residues 30–31 and 37–38 formed β-sheets connected with some bends or turns. However, the central hydrophobic region (residues 10–28) always kept its α-helix throughout the whole 90 ns simulation, with occasional local deviations that converted the α-helix to a 5-helix bend (or turn) structure. The residues 1–10 of Aβ42 mostly adopt a random coil or α-helix, with local deviations involving 5-helix or turns. Statistically, Aβ42 consists of ∼20% coil, ∼7% β-sheet, and 56% helix components in water (Table 2); this agrees with previous theoretical studies,22,53 but is only moderately consistent with NMR observations.3,7,52,58 The helical content, as derived from our apo–Aβ42 simulation, is slightly higher than that of NMR observations. This high helix content may be due to the Amber force filed and the second structure algorithm. The mixed helix/β-sheet conformations existing in aqueous solution indicate the existence of conformational transition, which is consistent with the experimental results.59| Sec-structure | NMR31 | Apo | AP | Sum | Bia | TF |
|---|---|---|---|---|---|---|
| a Statistical standard deviation analysis results of coil, β-sheet, β-bridge, turn, bend, and α-helix components from different simulations systems are 4.33, 3.08, 1.84, 5.50, 2.17, and 3.69, suggesting that the change of coil, β-sheet, turn, and α-helix are significant difference upon different binding partners during MD simulations.b The α-helix is the sum of α-, 5-, and 3-helixes in Fig. 4. | ||||||
| Coil | 9.52 | 19.64 | 18.19 | 16.46 | 9.99 | 10.97 |
| β-Sheet | 0.00 | 6.86 | 0.00 | 0.00 | 0.00 | 0.00 |
| β-Bridge | 0.00 | 0.46 | 0.00 | 0.00 | 0.00 | 4.21 |
| Turn | 9.52 | 9.63 | 17.82 | 18.56 | 21.44 | 24.29 |
| Bend | 9.52 | 6.78 | 5.16 | 7.96 | 3.07 | 3.14 |
| α-Helixb | 71.43 | 56.64 | 58.83 | 57.02 | 65.50 | 57.39 |
The transitional conformations of the monoflavonoid–Aβ42 and biflavonoid–Aβ42 complexes are significantly different from those of apo–Aβ42 (Fig. 2b and e). The residues 10–26 (i.e., the hydrophobic region) of Aβ42 remain in a helical configuration for 90 ns. This is similar to the case of apo–Aβ42. There are three distinct pathways of conformation transition for apo–Aβ42 and flavonoid–Aβ42 complexes. First, β-sheets are not observed during the 90 ns MD simulations for flavonoid–Aβ42 complexes. This suggests that flavonoids inhibit the formation of β-sheets in Aβ42 in water. This result agrees with the CD experimental data.21 Second, residues 30–40 at the C-terminus usually fold into helixes in flavonoid–Aβ42 complexes (Fig. 2). As listed in Table 2, flavonoid–Aβ42 complexes have more helixes than the apo–Aβ42 complex does. Statically more helixes stabilize the Aβ42 conformation, and may decrease the chances of Aβ42 aggregation. Biflavonoid–Aβ42 complexes have fewer random coils than monoflavonoid–Aβ42 and apo–Aβ42 complex have. Experimental data suggest that the high content of random coil structures not only instabilize Aβ conformation, and but also drive the fibrillation of Aβ.52,58 Our simulations indicated that flavonoids can inhibit the β-sheet formation, which may play a key role for inhibiting Aβ42 aggregation. Moreover, biflavonoids induce more helixes, and reduce random coils in Aβ complexes. Biflavonoids are thus better agents for inhibiting Aβ42 aggregation, which is also consistent with experimental results.21
Tarus and coworkers characterized the equilibrium ensemble of the Aβ1–28 monomer with NQTrp inhibitor by means of extensive atomistic MD simulations.23 Their results suggested that the population of α-helix was increased by a factor of 2 and the population of β-sheets was reduced by a factor of 1.5 upon NQTrp binding. Lockhart and coworkers suggested that ibuprofen binding to Aβ10–40 was largely governed by hydrophobic effect, and its binding site in Aβ peptide was entirely composed of hydrophobic amino acids.25 Moreover, conformational ensemble of Aβ monomer in ibuprofen solution revealed two structured regions, 19–25 (R1) and 29–35 (R2), composed of turn/helix and helix structure, respectively. Liu and coworkers simulation results suggested that (−)-epigallocatechin-3-gallate (EGCG) inhibited conformational transition of Aβ42 monomer by increasing the content of helical structure and reducing the content of β-sheet structure.22 Similar to NQTrp, ibuprofen, and EGCG, our simulation results implicates that the helical structure of Aβ42 monomer can be well preserved, and the formation of β-sheet structure is prevented by flavonoids binding.
Flavonoids destroy the D23–K28 salt bridge in Aβ42
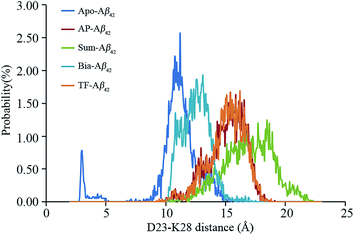
The D23 and K28 salt bridge in the Aβ monomer is important to the formation of Aβ fibrils.7,53,60–62 The salt bridge will be formed when the distance between Cγ at D23 and Nζ at K28 is close to 0.5 nm. The distributions of the distances between D23 and K28 in different ligand–receptor complexes are depicted in Fig. 3. The distance value peak at 0.3 nm for the apo–Aβ42 complex indicates D23–K28 salt bridge formation. Statistically analysis results show that approximately 6.58% structures may form D23–K28 salt bridge. However, monoflavonoid–Aβ42 and biflavonoid–Aβ42 complexes do not have peaks at 0.3 nm (Fig. 3), suggesting that the binding of between monoflavonoid and biflavonoids with Aβ42 may induce the disappearance of D23–K28 salt bridge.Although the D23–K28 salt bridge is unstable, it is crucial for the Aβ aggregation process.60 Our MD simulations demonstrate that approximately 6.58% structures may form D23–K28 salt bridge in apo–Aβ42. Tarus and co-workers60 suggested that the major reason for the D23–K28 salt bridge's instability is that the hydrophilic side chain of D23 and K28 from Aβ10–35 can form many hydrogen bonds with solvate water. These hydrogen bonds disrupt the formation probability of D23–K28 salt bridge. As shown in the ESI Fig. S4,† many hydrogen bonds are formed between the side chain of D23 and K28 from Aβ42 and water molecules. The Rg analyses confirm that hydrophobic interactions induced by monoflavonoids and biflavonoids push the D23 and K28 residues to be exposed to solvated water. This keeps D23 and K28 separate from each other, such that they form more hydrogen bonds with water (Fig. 3 and ESI Fig. S4†).
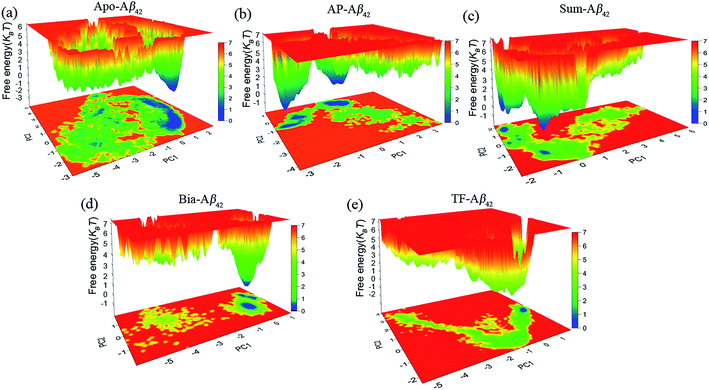
Flavonoids induce conformational distribution of Aβ42 into local minimization energy
We clustered the conformations sampled from MD simulations at 300 K to investigate the differences among apo–Aβ42 and flavonoid–Aβ42 complexes. The relative population distributions of the complex clusters are depicted in Fig. S5.† 2.5 Å is the RMSD threshold for determining whether two conformations belong to the same cluster. Detailed clustering results can be found in the ESI Tables S2 and S3.† The conformations of the flavonoid–Aβ42 complexes are more uniform than that of the apo–Aβ42. For example, the relative populations of the five most popular clusters of the TF–Aβ42 complex are 56.9%, 22.1%, 7.5%, 3.7%, and 2.1%, respectively. In comparison, the relative populations of the five most popular clusters of the apo–Aβ42 are 28.3%, 10.9%, 5.2%, 4.5%, and 2.6%, respectively. The conformations of the apo–Aβ42 are more heterogeneous than that of the monoflavonoid– and biflavonoid–Aβ42 complexes. Therefore, monoflavonoid and biflavonoids can induce Aβ42 to assume more stable conformations.Fig. 4a and e shows the free energy landscapes projected onto the first two principal components of the apo–Aβ42 and flavonoid–Aβ42 complexes. The free energy landscapes vary for different binding complexes. The size and shape of the minimal energy area (in blue) indicate the stability of a complex. Smaller and more centralized blue areas suggest that the corresponding complex is more stable. As indicated in Fig. 4, the stability order of the complexes is: TF–Aβ42 > Bia–Aβ42 > Sum–Aβ42 > AP–Aβ42 > apo–Aβ42. Thus, flavonoids induce Aβ42 to enter the local energy minimal state. Biflavonoids are better than monoflavonoids. This is consistent with experimental observations.21
Binding free energies analysis
To further confirm the interactions between flavonoids and Aβ42, we performed additional 20 ns MD simulations on the central representative conformation of the flavonoid–Aβ42 complexes, which was derived from the clustered conformations generated by previous MD simulations. MMPBSA and MMGBSA were applied for the binding free energy calculation. As listed in Table 3, the binding free energies of biflavonoid–Aβ42 complexes (Sum, Bia, and TF) are superior to that of mono–Aβ42 (AP); this is consistent with bioassay results.21 The three biflavonoids (Sum, Bia, and TF) had similar binding free energy values (−38.81, −36.67, and −37.73 kcal mol−1, respectively). These values are consistent with the bioassay results (which revealed IC50 values of 4.48, 2.90, and 2.41 μM for Sum, Bia, and TF, respectively, IC50 is defined as the concentration of the compound required to reduce the rate of polymerization).21| Energy terms | PB method | GB method | ||||||
|---|---|---|---|---|---|---|---|---|
| AP–Aβ42 | Sum–Aβ42 | Bia–Aβ42 | TF–Aβ42 | AP–Aβ42 | Sum–Aβ42 | Bia–Aβ42 | TF–Aβ42 | |
| a The energy unit is kcal mol−1.b Non-bonded van der Waals.c Non-bonded electrostatics.d Polar component to solvation.e Non-polar component to solvation.f Total gas phase energy.g Sum of nonpolar and polar contributions to solvation.h Final estimated binding free energy calculated from the terms above. Standard errors of the mean are given in parentheses. IC50 values of AP, Sum, Bia, and TF21 are 23.3, 4.48, 2.90, and 2.41 μM, respectively. | ||||||||
| ΔEvdwb | −19.33 (2.78) | −53.99 (3.12) | −50.53 (5.12) | −50.18 (2.52) | −19.33 (2.78) | −53.99 (3.12) | −50.53 (5.12) | −50.18 (2.52) |
| ΔEelec | −2.74 (2.15) | −3.98 (4.84) | −13.31 (5.34) | −8.21 (3.97) | −2.74 (2.15) | −3.98 (4.84) | −13.31 (5.34) | −8.21 (3.97) |
| ΔEele,solvd | 9.94 (6.06) | 24.01 (4.48) | 36.08 (10.47) | 26.26 (3.13) | −10.57 (5.67) | 25.60 (4.31) | 33.77 (9.29) | 26.65 (3.33) |
| ΔEnonpol,solve | −14.76 (1.60) | −32.20 (1.55) | −33.60 (3.56) | −31.36 (1.08) | −2.78 (0.30) | −6.44 (0.23) | −6.59 (0.62) | −5.99 (0.15) |
| ΔGgasf | −22.07 (6.72) | −57.97 (5.56) | −63.84 (12.68) | −58.39 (4.50) | −22.07 (2.28) | −57.97 (5.56) | −63.84 (12.68) | −58.39 (4.51) |
| ΔGsolvg | −4.82 (3.05) | −8.19 (4.49) | 2.48 (1.25) | −5.10 (3.07) | 7.80 (5.61) | 19.16 (4.28) | 27.18 (8.90) | 20.66 (3.31) |
| ΔGbindingh | −26.90 (3.74) | −66.15 (4.32) | −61.36 (6.56) | −63.48 (3.50) | −14.27 (2.35) | −38.81 (2.83) | −36.67 (4.84) | −37.73 (2.45) |
Table 3 indicates that both non-bonded van der Waals (ΔEvdw) and non-bonded electrostatics (ΔEele) are favorable for the formation of the binding complex, but ΔEvdw values are at least 4-fold stronger than ΔEele values (∼13 fold for Sum, ∼4 fold for Bia and ∼6 fold for TF). Non-polar component to solvation (ΔEnonpol,solv) values are also favorable for mono- and biflavonoid bindings. ΔEele values are favorable for the formation of the binding complex (e.g., −8.21 kcal mol−1 for TF), while ΔEele,solv values do not favor TF–Aβ42 complex formation. Similar observations apply to AP–Aβ42, Sum–Aβ42, and Bia–Aβ42 complexes. Therefore, the intermolecular van der Waals and the nonpolar solvation interactions are the dominant forces involved in stabilizing flavonoid–Aβ complexes.
The inhibition activity of a flavonoid against Aβ42 (IC50) is positively correlated with its A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
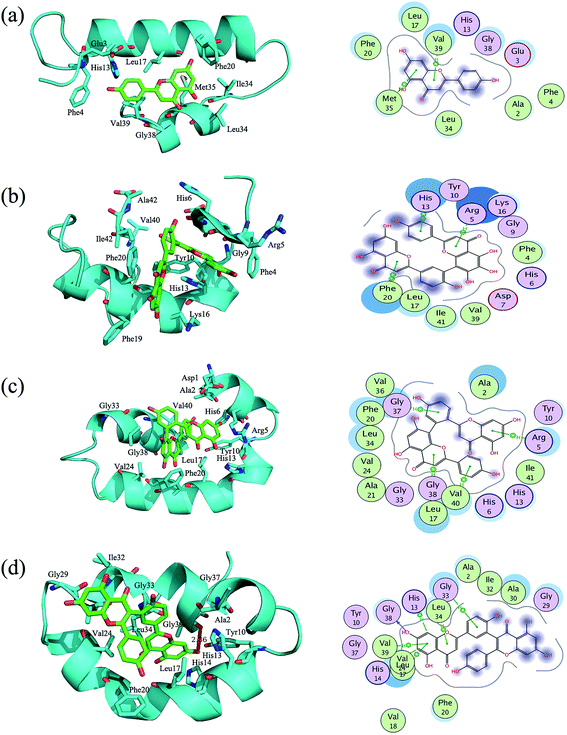
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value (R2 = 0.97, ESI Fig. S6†). This means that flavonoids interact with the hydrophobic residues of Aβ42. This is confirmed by residue-based free energy decomposition analysis (Fig. 5a and d). Generally, if the interaction energy between the residue and the substrate is lower than −1 kcal mol−1, those residues are considered to be important in substrate binding (i.e., they are hot residues).63,64 As shown in Fig. 6, mono- and biflavonoids have different interaction patterns, which indicate that they have different binding modes (Fig. 6a and d). The hot residues are Leu34, Met35, and Val39 for monoflavonoid binding (AP, Fig. 6a). AP binds the similar U-shaped structure of Aβ42 and are surrounded by Val39, Met35, Leu34, Val39, Leu17, and Gly38 (Fig. 6a, left). Met35 and Val39 form an arene–H stack interaction with the aromatic group of the AP molecule (Fig. 6a, right), and Leu34 forms a hydrophobic association with AP. These interactions occur mainly in the side chains (ESI Fig. S7a†). Biflavonoids form more interactions with Aβ42 residues than monoflavonoids do (Fig. 6b–d). Taking TF as an example, the hot residues are His14, Leu17, Val18, Phe20, Val24, Ala30, Gly33, Leu34, Gly38, and Val39 (Fig. 5d). Previously MD simulations and MMPBSA results also demonstrated that interactions between EGCG and Aβ42 monomer were major from the origin of hot residues (Phe19, Phe20, Leu34, Glu22, Lys28, Gly29, Arg5, and Gly38).22 Five arene–H stack interactions are formed, as well as one hydrogen bond between Gly38 and one hydroxyl of TF (Fig. 6d, left). Similar results can be recognized in Sum– and Bia–Aβ42 complexes (Fig. 5b and c, 6b and c). For biflavonoids, the major ligand–protein interactions also involve the Aβ42 side chain residues (see ESI Fig. S7b–d†). The binding free energy calculation and decomposition results (Table 3 and Fig. 5) are consistent with the binding modes analyses, and agree with the bioassay results.
P value (R2 = 0.97, ESI Fig. S6†). This means that flavonoids interact with the hydrophobic residues of Aβ42. This is confirmed by residue-based free energy decomposition analysis (Fig. 5a and d). Generally, if the interaction energy between the residue and the substrate is lower than −1 kcal mol−1, those residues are considered to be important in substrate binding (i.e., they are hot residues).63,64 As shown in Fig. 6, mono- and biflavonoids have different interaction patterns, which indicate that they have different binding modes (Fig. 6a and d). The hot residues are Leu34, Met35, and Val39 for monoflavonoid binding (AP, Fig. 6a). AP binds the similar U-shaped structure of Aβ42 and are surrounded by Val39, Met35, Leu34, Val39, Leu17, and Gly38 (Fig. 6a, left). Met35 and Val39 form an arene–H stack interaction with the aromatic group of the AP molecule (Fig. 6a, right), and Leu34 forms a hydrophobic association with AP. These interactions occur mainly in the side chains (ESI Fig. S7a†). Biflavonoids form more interactions with Aβ42 residues than monoflavonoids do (Fig. 6b–d). Taking TF as an example, the hot residues are His14, Leu17, Val18, Phe20, Val24, Ala30, Gly33, Leu34, Gly38, and Val39 (Fig. 5d). Previously MD simulations and MMPBSA results also demonstrated that interactions between EGCG and Aβ42 monomer were major from the origin of hot residues (Phe19, Phe20, Leu34, Glu22, Lys28, Gly29, Arg5, and Gly38).22 Five arene–H stack interactions are formed, as well as one hydrogen bond between Gly38 and one hydroxyl of TF (Fig. 6d, left). Similar results can be recognized in Sum– and Bia–Aβ42 complexes (Fig. 5b and c, 6b and c). For biflavonoids, the major ligand–protein interactions also involve the Aβ42 side chain residues (see ESI Fig. S7b–d†). The binding free energy calculation and decomposition results (Table 3 and Fig. 5) are consistent with the binding modes analyses, and agree with the bioassay results.
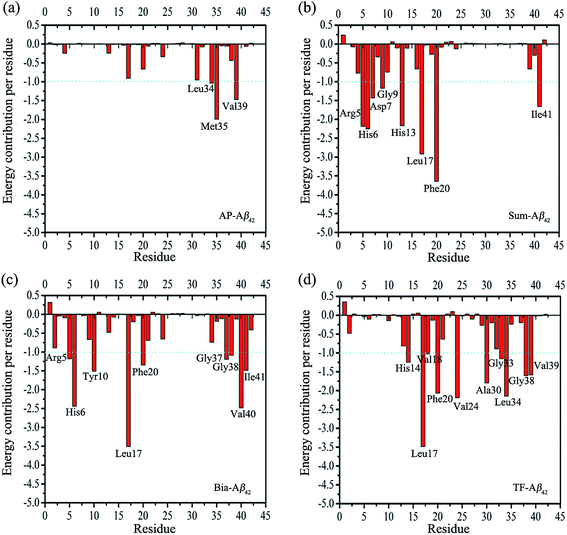 | ||
| Fig. 5 Decomposition of binding free energy on a per-residue basis for each protein–inhibitor complex. The unit for energy contribution per residue is kcal mol−1. | ||
The hydrophobic interactions of flavonoids with Aβ come from the hydrophobic side-chains of Aβ42 (see ESI Fig. S7a and d†). These hydrophobic interactions stabilize the Aβ42 conformation in water, and induce Aβ folding to generate a U-shaped structure, which results in the hydrophilic residues being pushed away to the solvate phase, thus disrupting the D23–K28 salt bridge and preventing β-sheets being formed (Fig. 7a and d).
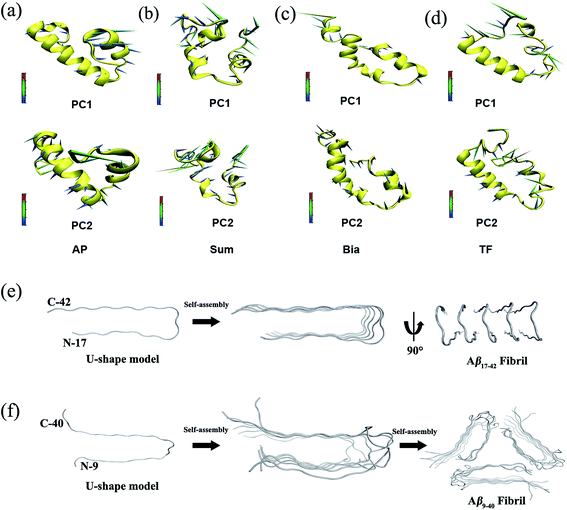 | ||
| Fig. 7 Collective motions obtained by principal component analysis (PCA) on the conformational trajectories of the flavonoid-A complexes derived from the MD simulations. (a) AP–Aβ42, (b) Sum–Aβ42, (c) Bia–Aβ42, and (d) TF–Aβ42. PC1and PC2 accounts for ∼70% of the total movements. The cones and their lengths represent the direction of the protein residue Cα atom and its vibration amplitude, respectively. (e) Quenched hydrogen deuterium-exchange NMR structure of Aβ17–42 fibrils66 (PDB ID: 2BEG). (f) Solid-state NMR structure of Aβ9–40 fibrils67 (PDB ID: 2LMP). A similar U-shaped repeating monomer structure was extracted from the Aβ17–42 fibrils and Aβ9–40 fibrils. | ||
As shown in Fig. 7, U-shaped conformations exist in all flavonoid-A complexes. It seems that flavonoid-induced U-shaped conformation blocks Aβ42 aggregation. It should be noted that the U-shaped conformations are not identical across different complexes. However, the value of the rooted mean square inner product (RMSIP) is greater than 0.5 for all the complexes (see ESI Table S3†). This means that the U-shaped conformation is popular in the flavonoid-induced Aβ42 complexes and show similarity dynamics folding space.
The conformations of the transition state and U-shaped state were representative conformations during the MD simulations (see ESI Fig. S8 and S9†). The transition state conformation is the first snapshot of the β-sheet formed in the apo–Aβ42 complex in water. Liu and coworkers discovered a compound (DC-AB1) inhibiting Aβ40 monomer aggregation based on virtual screening of the Aβ40 transition state.57 The Aβ42 transition state was also used for structure-based virtual screening in our lab.65 Thorsten and coworkers reported a 3D-structural model of the Aβ17–42 fibrils using quenched hydrogen/deuterium-exchange NMR.66 The Aβ17–42 fibrils consist of a similar U-shaped structure derived from the Aβ17–42 monomer (Fig. 7e). Another structural model for Aβ9–40 fibrils was obtained through solid-state NMR and also consists of a similar U-shaped repeating structure derived from the Aβ9–40 monomer (Fig. 7f).67 Therefore, the U-shaped Aβ monomer may be the initial framework for formation of Aβ fibrils (Aβ1–40 or Aβ1–42). These U-shaped Aβ monomers consist of a β-sheet–turn–β-sheet motif that lacks a helical secondary structure (Fig. 7e and f).
Conclusion
The molecular recognitions of flavonoids by Aβ42 monomer have been investigated via MD simulations. The study suggests that the activities of flavonoids halting the conformational transition of Aβ42 are attributed to (i) disrupting the formation of the β-sheet structures in Aβ42, (ii) expanding the more stable secondary structure (i.e., the helix) and reducing random coils, (iii) disrupting the D23–K28 salt bridge in Aβ42, and (iv) inducing Aβ42 conformational change into the local energy minimized state.Binding free energy analyses suggest that hydrophobic interactions are critical for ligand–Aβ42 binding. The hydrophobic interactions lead to (1) the stabilization of an Aβ42 conformation in water, and (2) the inducement of Aβ42 folding, so as to make the D23–K28 salt bridge disappear, and prevent the formation of the β-sheet.
Our study also suggests that compounds with inhibiting activity against Aβ42 monomer also introduce a similar U-shaped Aβ structure with more of helical secondary structures, and which lacks the β-sheet structure. We conclude that if a compound can bind the similar U-shaped structure of the Aβ monomer, it will disrupt the formation of the initial framework (i.e., the β-sheet–turn–β-sheet motif), and inhibit Aβ aggregation. Thus, it would be promising to design Aβ42 inhibitors based on the U-shaped structure of Aβ42.
Acknowledgements
This work was funded in part by China Postdoctoral Science Foundation (No. 2015M572325), the Fundamental Research Funds for the Central Universities (No. 2015ZM049), the Open Project Program of Guangdong Key Laboratory of Fermentation and Enzyme Engineering, SCUT (FJ2015006).Notes and references
- G. G. Glenner and C. W. Wong, Biochem. Biophys. Res. Commun., 2012, 425, 534–539 CrossRef CAS PubMed.
- D. J. Selkoe, Nat. Cell Biol., 2004, 6, 1054–1061 CrossRef CAS PubMed.
- M. Ahmed, J. Davis, D. Aucoin, T. Sato, S. Ahuja, S. Aimoto, J. I. Elliott, W. E. Van Nostrand and S. O. Smith, Nat. Struct. Mol. Biol., 2010, 17, 561–U556 CAS.
- T. E. Golde, C. B. Eckman and S. G. Younkin, Biochim. Biophys. Acta, Mol. Basis Dis., 2000, 1502, 172–187 CrossRef CAS.
- G. W. Small, S. C. Huang, G. Cole, N. Satyamurthy, E. D. Agdeppa, Z. Kiziloglu, V. Kepe, A. Petric, H. V. Vinters, M. E. Phelps and J. R. Barrio, Neurobiol. Aging, 2001, 22, 335 Search PubMed.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- T. Luhrs, C. Ritter, M. Adrian, D. Riek-Loher, B. Bohrmann, H. Doeli, D. Schubert and R. Riek, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17342–17347 CrossRef CAS PubMed.
- R. Kayed, E. Head, J. L. Thompson, T. M. McIntire, S. C. Milton, C. W. Cotman and C. G. Glabe, Science, 2003, 300, 486–489 CrossRef CAS PubMed.
- P. Nguyen and P. Derreumaux, Acc. Chem. Res., 2014, 47, 603–611 CrossRef CAS PubMed.
- W. Zhuang, N. G. Sgourakis, Z. Y. Li, A. E. Garcia and S. Mukamel, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15687–15692 CrossRef CAS PubMed.
- G. H. Wei, A. I. Jewett and J. E. Shea, Phys. Chem. Chem. Phys., 2010, 12, 3622–3629 RSC.
- Y. C. Xu, J. J. Shen, X. M. Luo, W. L. Zhu, K. X. Chen, J. P. Ma and H. L. Jiang, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5403–5407 CrossRef CAS PubMed.
- A. Morriss-Andrews, G. Bellesia and J. E. Shea, J. Chem. Phys., 2012, 137, 145104–145109 CrossRef PubMed.
- B. Urbanc, L. Cruz, S. Yun, S. V. Buldyrev, G. Bitan, D. B. Teplow and H. E. Stanley, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17345–17350 CrossRef CAS PubMed.
- C. L. Moore and M. S. Wolfe, Expert Opin. Ther. Pat., 1999, 9, 135–146 CrossRef CAS.
- F. S. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy and G. M. Cole, J. Biol. Chem., 2005, 280, 5892–5901 CrossRef CAS PubMed.
- K. Ono, H. Naiki and M. Yamada, Curr. Pharm. Des., 2006, 12, 4357–4375 CrossRef CAS.
- K. Ono, Y. Yoshiike, A. Takashima, K. Hasegawa, H. Naiki and M. Yamada, J. Neurochem., 2003, 87, 172–181 CrossRef CAS.
- C. W. Lee, H. J. Choi, H. S. Kim, D. H. Kim, I. S. Chang, H. T. Moon, S. Y. Lee, W. K. Oh and E. R. Woo, Bioorg. Med. Chem. Lett., 2008, 16, 732–738 CrossRef CAS PubMed.
- S. S. Kang, J. Y. Lee, Y. K. Choi, S. S. Song, J. S. Kim, S. J. Jeon, Y. N. Han, K. H. Son and B. H. Han, Bioorg. Med. Chem. Lett., 2005, 15, 3588–3591 CrossRef CAS PubMed.
- A. Thapa, E. R. Woo, E. Y. Chi, M. G. Sharoar, H. G. Jin, S. Y. Shin and I. S. Park, Biochemistry, 2011, 50, 2445–2455 CrossRef CAS PubMed.
- F. F. Liu, X. Y. Dong, L. Z. He, A. P. J. Middelberg and Y. Sun, J. Phys. Chem. B, 2011, 115, 11879–11887 CrossRef CAS PubMed.
- B. Tarus, P. H. Nguyen, O. Berthoumieu, P. Faller, A. J. Doig and P. Derreumaux, Eur. J. Med. Chem., 2014, 16, 43–50 Search PubMed.
- C. Lockhart and D. K. Klimov, Biophys. J., 2012, 103, 2341–2351 CrossRef CAS PubMed.
- C. Lockhart, S. Kim and D. K. Klimov, J. Phys. Chem. B, 2012, 116, 12922–12932 CrossRef CAS PubMed.
- T. Zhang, J. Zhang, P. Derreumaux and Y. Mu, J. Phys. Chem. B, 2013, 117, 3993–4002 CrossRef CAS PubMed.
- T. Zhang, W. X. Xu, Y. G. Mu and P. Derreumaux, ACS Chem. Neurosci., 2014, 5, 148–159 CrossRef CAS PubMed.
- Y. Chebaro, P. Jiang, T. Zang, Y. Mu, P. H. Nguyen, N. Mousseau and P. Derreumaux, J. Phys. Chem. B, 2012, 116, 8412–8422 CrossRef CAS PubMed.
- M. Zhu, A. De Simone, D. Schenk, G. Toth, C. M. Dobson and M. Vendruscolo, J. Chem. Phys., 2013, 139, 035101 CrossRef PubMed.
- L. Jiang, C. Liu, D. Leibly, M. Landau, M. L. Zhao, M. P. Hughes and D. S. Eisenberg, eLife, 2013, 2, e00857 Search PubMed.
- O. Crescenzi, S. Tomaselli, R. Guerrini, S. Salvadori, A. M. D'Ursi, P. A. Temussi and D. Picone, Eur. J. Biochem., 2002, 269, 5642–5648 CrossRef CAS.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, J. Comput. Chem., 2009, 30, 2785–2791 CrossRef CAS PubMed.
- D. J. Price and C. L. Brooks, J. Chem. Phys., 2004, 121, 10096–10103 CrossRef CAS PubMed.
- M. J. Abraham and J. E. Gready, J. Comput. Chem., 2011, 32, 2031–2040 CrossRef CAS PubMed.
- Y. Duan, C. Wu, S. Chowdhury, M. C. Lee, G. Xiong, W. Zhang, R. Yang, P. Cieplak, R. Luo, T. Lee, J. Caldwell, J. Wang and P. Kollman, J. Comput. Chem., 2003, 24, 1999–2012 CrossRef CAS PubMed.
- W. Zhang, T. J. Hou, X. B. Qiao and X. J. Xu, J. Phys. Chem. B, 2003, 107, 9071–9078 CrossRef CAS.
- G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria and M. A. Robb, Gaussian 03, Revision E.01, Gaussian, Inc., Pittsburgh PA, 2004 Search PubMed.
- B. Hess, J. Chem. Theory Comput., 2008, 4, 116–122 CrossRef CAS.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS.
- W. Kabsch and C. Sander, Biopolymers, 1983, 22, 2577–2637 CrossRef CAS PubMed.
- A. Amadei, A. B. Linssen and H. J. Berendsen, Proteins, 1993, 17, 412–425 CrossRef CAS PubMed.
- M. Stepanova, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 76, 051918 CrossRef.
- A. Amadei, M. A. Ceruso and A. Di Nola, Proteins, 1999, 36, 419–424 CrossRef CAS.
- A. Merlino, L. Vitagliano, M. A. Ceruso, A. Di Nola and L. Mazzarella, Biopolymers, 2002, 65, 274–283 CrossRef CAS PubMed.
- A. Merlino, L. Vitagliano, M. A. Ceruso and L. Mazzarella, Biophys. J., 2004, 86, 2383–2391 CrossRef CAS.
- E. Papaleo, P. Mereghetti, P. Fantucci, R. Grandori and L. De Gioia, J. Mol. Graphics Modell., 2009, 27, 889–899 CrossRef CAS PubMed.
- G. Colombo, G. Morra, M. Meli and G. Verkhivker, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7976–7981 CrossRef CAS PubMed.
- W. Li, J. Zhang, Y. Su, J. Wang, M. Qin and W. Wang, J. Phys. Chem. B, 2007, 111, 13814–13821 CrossRef CAS PubMed.
- A. B. Clippingdale, M. Macris, J. D. Wade and C. J. Barrow, J. Pept. Res., 1999, 53, 665–672 CrossRef CAS.
- J. Talafous, K. J. Marcinowski, G. Klopman and M. G. Zagorski, Biochemistr, 1994, 33, 7788–7796 CrossRef CAS.
- S. Zhang, N. Casey and J. P. Lee, Folding Des., 1998, 3, 413–422 CrossRef CAS.
- K. I. S. Zhang, M. J. Lachenmann, J. W. Peng, S. Li, E. R Stimson, Y. Lu, A. M. Felix, J. E. Maggio and J. P. Lee, J. Struct. Biol., 2000, 130, 130–141 CrossRef PubMed.
- C. Yang, X. Zhu, J. Li and R. Shi, J. Mol. Model., 2010, 16, 813–821 CrossRef CAS PubMed.
- L. O. Tjernberg, A. Tjernberg, N. Bark, Y. Shi, B. P. Ruzsicska, Z. Bu, J. Thyberg and D. J. Callaway, Biochem. J., 2002, 366, 343–351 CAS.
- M. Coles, W. Bicknell, A. A. Watson, D. P. Fairlie and D. J. Craik, Biochemistry, 1998, 37, 11064–11077 CrossRef CAS PubMed.
- W. B. Stine Jr, K. N. Dahlgren, G. A. Krafft and M. J. LaDu, J. Biol. Chem., 2003, 278, 11612–11622 CrossRef PubMed.
- D. Liu, Y. Xu, Y. Feng, H. Liu, X. Shen, K. Chen, J. Ma and H. Jiang, Biochemistry, 2006, 45, 10963–10972 CrossRef CAS PubMed.
- G. Bitan, M. D. Kirkitadze, A. Lomakin, S. S. Vollers, G. B. Benedek and D. B. Teplow, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 330–335 CrossRef CAS PubMed.
- M. D. Kirkitadze, M. M. Condron and D. B. Teplow, J. Mol. Biol., 2001, 312, 1103–1119 CrossRef CAS PubMed.
- B. Tarus, J. E. Straub and D. Thirumalai, J. Am. Chem. Soc., 2006, 128, 16159–16168 CrossRef CAS PubMed.
- K. L. Sciarretta, D. J. Gordon, A. T. Petkova, R. Tycko and S. C. Meredith, Biochemistry, 2005, 44, 6003–6014 CrossRef CAS PubMed.
- W. M. Berhanu and U. H. E. Hansmann, PLoS One, 2012, 7, e41479 CAS.
- H. Chen, Y. Zhang, L. Li and J. G. Han, J. Phys. Chem. B, 2012, 116, 10219–10233 CrossRef CAS PubMed.
- W. Li, Y. Tang, H. Liu, J. Cheng, W. Zhu and H. Jiang, Proteins, 2008, 71, 938–949 CrossRef CAS PubMed.
- Y. Y. Cao, L. Wang, H. Ge, X. L. Lu, Z. Pei, Q. Gu and J. Xu, Mol. Diversity, 2013, 17, 515–524 CrossRef CAS PubMed.
- T. Luhrs, C. Ritter, M. Adrian, D. Riek-Loher, B. Bohrmann, H. Dobeli, D. Schubert and R. Riek, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17342–17347 CrossRef CAS PubMed.
- A. K. Paravastu, R. D. Leapman, W. M. Yau and R. Tycko, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18349–18354 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12328c |
| This journal is © The Royal Society of Chemistry 2015 |