Enhancement of the O2 gas sensing properties of mesoporous Sr0.9La0.1TiO3 films by increasing the pore connectivity
Chang-Sun Park,
D. B. Mahadik and
Hyung-Ho Park*
Department of Materials Science and Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-749, Republic of Korea. E-mail: hhpark@yonsei.ac.kr; Fax: +82 23125375
First published on 29th July 2015
Abstract
The structural and gas sensing properties of mesoporous Sr0.9La0.1TiO3 films for oxygen sensing applications were investigated as a function of surfactant concentration. Through the synthesis of Sr0.9La0.1TiO3 with a mesoporous structure, the surface area and pore connectivity were increased by increasing the surfactant concentration. Using a thermal treatment, interior pores were exposed to the surface thereby increasing the surface area. This increase in surface area induces an increase in oxygen absorption, which results in an enhanced oxygen sensitivity. The oxygen sensing properties of mesoporous Sr0.9La0.1TiO3 films were analyzed at an oxygen concentration of 30% and an analysis temperature of 550 °C. The results at these conditions showed the maximum oxygen sensitivity (9.43), response time (38 s), and recovery time (181 s). The inclusion of a surfactant during the preparation of mesoporous films was found to be a simple and effective method to enhance the surface area and interior pore connectivity of the films to enhance their gas sensing performance.
Introduction
Gas sensors have attracted enormous attention because of concerns related to environmental pollution and serious health issues.1 The research field of gas sensors has striven to minimize emissions from various industrial sources.2–4 Extensive foundational and technological efforts have been dedicated to fabricate perfect gas sensors. Typically, these sensors are based on various semiconducting metal oxides due to their low cost, high sensitivity, and high compatibility with microelectronic processing. Among the many semiconducting gas sensors, oxygen sensors have played a key role in pollution control from the exhausts of vehicles.5 The exhausts of vehicles are an intensely shaking and high temperature environment. So, oxygen sensors should have high thermal and mechanical stabilities.6,7 Also, oxygen sensors should have high specific surface area to secure high density of surface active sites. A great deal of research have been carried out to improve the properties of oxygen sensors such as nanostructured CeOx and InOx.8,9 However, most of the studies didn't well realize a sufficient measuring stability because a change in sensing properties would be easily induced from the size change in nano-scaled materials during their annealing treatment.Strontium titanate (SrTiO3) is a well-researched, semiconducting oxide sensing material that is often used in oxygen gas sensors due to its low cost and strong stability in a relatively extreme thermal and chemical atmospheres.6,7 The characteristics and applications of SrTiO3 have been comprehensively studied over the past few decades. The electrical conductivities of SrTiO3 can be easily controlled by selecting different doping donors, such as La, Nb, and Fe.10–12 Recently, nanostructured metal oxides have been shown to provide a high specific surface area and high density of surface active sites; therefore, these materials have become promising candidates for gas sensors with high sensitivity, fast response time, and small size. By creating a nanostructured material, the surface area of SrTiO3 can be increased; however, forming SrTiO3 nanostructures via a chemical solution process is difficult due to the different reaction rates of the precursors and the unavoidable high temperature thermal treatment that is required for calcination and phase formation.13 Also, ways of synthesis nanostructures (electrospinning, semipermeable membranes, and so on) are often expensive and difficult to pursue.14,15
In the case of a mesoporous structure, pores between 2 and 50 nm can be achieved with regular distributions.16 The mesoporous structure can be synthesized using an evaporation-induced self-assembly (EISA) process.17 EISA involves the self-assembly process of surfactants, which form micelles upon a gradual increase in the concentration. The EISA process is highly beneficial because it is simple, easy, and cost effective.18,19 However, a mesoporous structure with relatively high mechanical properties is only obtained in the case of an ordered mesoporous structure with closed interior pores.19 In the case of a closed pore structure, the surface area and the surface active sites of the mesoporous structure cannot be enhanced because the effective surface area is limited to only the surface of the mesoporous structure. If a mesoporous structure has an open pore structure, the structure can have a high specific surface area, but the mechanical strength is inevitably weaker. Normally, high surface area and high mechanical strength have been required for gas sensor applications because sensors require high sensitivity and high thermal resistance due to their high working temperatures. Therefore, to be suitable for gas sensor application, an ordered mesoporous structure formed by closed interior pores that also has a high specific surface area is essential. If pore connection among the closed interior pores in an ordered mesoporous structure occurs, the closed interior pores become open pores, thereby increasing the effective specific surface area while maintaining a relatively high mechanical strength.20 Several attempts have been made to increase the effective surface area via interior pore connection. These attempts have including exposing pores by grain growth,21 the generation of cracks by thermal shock,22 and adding large amounts of surfactant.23 In this study, Sr0.9La0.1TiO3 n-type semiconductors were formed in mesoporous films with an interior closed pore structure by a simple EISA process. Additionally, an investigation into the relation between the surfactant amount and the specific surface area through interior closed pore connections were carried out in an attempt to enhance the oxygen gas sensing of Sr0.9La0.1TiO3 mesoporous films. We analyzed how the surface morphology, pore structure, and gas sensing properties can be varied at different surfactant concentrations and annealing temperatures.
Experimental
Commercially available metal acetates (Sr(CH3CO2)2 and La(CH3CO2)3·3H2O, Aldrich) were used to synthesize metal propionate powder, and titaniumtetraisopropoxide (Ti(OPri)4, TTIP, Aldrich, 97%) and a triblock copolymer Brij-S10 (C18H37(OCH2CH2)10OH, MW 711, Aldrich) were used as the Sr0.9La0.1TiO3 precursor and surfactant, respectively. Metal acetates were dissolved in a large excess of propionic acid (C3H6O2, Duksan) and the liberated acetic acid was distilled to prepare the propionates. The excess propionic acid was separated by centrifuge, and then the carboxylates were precipitated with acetone (CH3COCH3, Duksan) and dried in an oven to produce dry metal propionates.24 To prepare Sr and La precursors, we mixed solutions using three steps. First, the weighed propionates of Sr and La were dissolved in propionic acid under stirring for 4 h. Next, Brij-S10 surfactant was dissolved in n-butyl alcohol (CH3(CH2)3OH, Duksan) under stirring for 1 h. Lastly, Sr and La precursors were mixed with a TTIP surfactant solution and stirred for 15 min. The final product has a (Sr0.9La0.1TiO3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brij-S10
Brij-S10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propionic acid
propionic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-butyl alcohol precursor composition that is kept constant at a molar ratio of 1
n-butyl alcohol precursor composition that is kept constant at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Here, x is the Brij-S10 ratio, which was varied as 0, 0.025, 0.05, and 0.075. The as-prepared Sr0.9La0.1TiO3 precursor solutions were spin-coated on clean SiO2 and Si substrates at room temperature. Spin-coating was performed at 2000 rpm for 20 s to spread the solution uniformly over the substrates. The as-prepared films were annealed in a tube furnace at 450 °C, 650 °C, and 850 °C for 4 h to crystallize the films.
20. Here, x is the Brij-S10 ratio, which was varied as 0, 0.025, 0.05, and 0.075. The as-prepared Sr0.9La0.1TiO3 precursor solutions were spin-coated on clean SiO2 and Si substrates at room temperature. Spin-coating was performed at 2000 rpm for 20 s to spread the solution uniformly over the substrates. The as-prepared films were annealed in a tube furnace at 450 °C, 650 °C, and 850 °C for 4 h to crystallize the films.
The microstructures and surface morphology of the prepared thin films were observed by using scanning electron microscopy (SEM: JEOL, JSM 7001F). To investigate the pore ordering and crystal structure of mesoporous Sr0.9La0.1TiO3 films, X-ray diffraction (XRD, Ultima IV, Rigaku) with Cu Kα radiation (λ = 1.5418 Å) was performed. Small-angle and wide-angle patterns were recorded in the 2θ ranges from 0.5° to 5° and from 20° to 60°, respectively. The mesostructure of Sr0.9La0.1TiO3 films was investigated using grazing incidence small-angle X-ray scattering (GISAXS) at the 3C beam line (λ = 1.24 Å and ΔE/E = 2 × 10−4) of the Pohang Light Source (PLS) in the Republic of Korea. The surface area of the Sr0.9La0.1TiO3 films were determined by using N2 adsorption desorption analysis (TriStar 3000 V6.05 A). The refractive indices of the Sr0.9La0.1TiO3 films were obtained using an ellipsometer (Gatan L117 C, 632.8 nm He–Ne laser). Based on these refractive indices, the porosities were calculated using the Lorentz–Lorenz equation.25 The topographies of the surfaces of the mesoporous Sr0.9La0.1TiO3 films were analyzed using atomic force microscopy (AFM, Bruker Nanoscope V).
An oxygen gas sensor was fabricated using a SiO2/Si substrate with Pt interdigitated electrodes (IDE) that were 1 mm × 1 mm size with a gap between electrodes of 5 μm. The thickness of the Pt was 200 nm and the IDE patterns were fabricated using photolithography and dry etching. The response of the fabricated gas sensor to oxygen gas was measured by monitoring the change in sensor resistance while the gas was changed from dry nitrogen to various oxygen concentrations with a constant gas flow rate of 1000 sccm. The film resistance was measured under a dc bias voltage of 1 V using a source measurement unit (Keithley 2635A).
Results and discussion
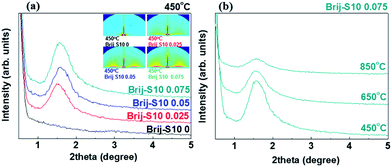
The spin-coated films were annealed at 450 °C for 4 h to remove the surfactant templates and to form a mesoporous structure. The synthesized mesoporous Sr0.9La0.1TiO3 films, made using a simple EISA process, were analyzed using small-angle XRD from 0.5 to 5.0° in order to ascertain the pore structure.Fig. 1(a) shows the small-angle XRD pattern of the mesoporous Sr0.9La0.1TiO3 films that were subjected to different surfactant concentrations after being annealed at 450 °C for 4 h. As observed in the figure, presence of the diffraction peak around 1.6° indicated that the pore structure of the Sr0.9La0.1TiO3 films was ordered. Additionally, the intensity of this diffraction peak increased as the surfactant concentration increased. Using small-angle XRD, the inter-pore distance, which combines the pore size and the wall thickness of the skeleton oxide, was determined to be 5.7 ± 0.1 nm. However, the behaviors of increasing pore size and decreasing wall thickness should be considered with increasing surfactant concentration.26 Using ellipsometry, the porosity of the mesoporous Sr0.9La0.1TiO3 films with surfactant molar ratios of 0, 0.025, 0.05, and 0.075 were calculated to be 5.7%, 10.3%, 17.1%, and 23.7%, respectively. From this result, we confirmed that both the formation of pores and their ordered arrangement were enhanced with increasing surfactant concentration. Increasing the surfactant concentration induces an increased pore size, which is caused by an increased surfactant micelles. The pore arrangements in the mesoporous structure were strongly dependent on the arrangement of surfactant micelles, which mainly depends on micelle size (pore size). This is the case because micelle arrangement in the EISA process is determined by electrostatic interactions between micelles, which are dependent on the micelle size (surfactant concentration). To clarify the surfactant concentration-dependent change in the mesoporous structures, GISAXS analyses were carried out. This information is provided in an inset of Fig. 1(a). The relative GISAXS pattern at 450 °C shows a diffraction pattern, the intensity of which increased with increasing surfactant concentration. This result also agrees well with the small-angle XRD result: increasing surfactant concentration induces increased pore arrangement in the mesoporous structure.
In this work, we deliberately collapsed the pore structure to enhance pore connectivity. In order to track changes in the pore structure of the mesoporous Sr0.9La0.1TiO3 films, small-angle XRD pattern analysis of the Brij-S10 0.075 sample was performed with various annealing temperatures. This information is given in Fig. 1(b). As shown in Fig. 1(b), the diffraction peak intensity decreased with increased annealing temperature. This was caused by the collapse of the ordered mesoporous structure via the grain growth of the Sr0.9La0.1TiO3 wall skeleton oxide.27
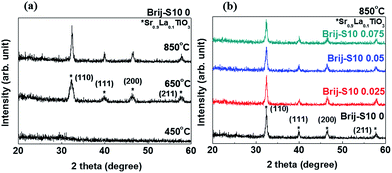
To clarify the annealing temperature-dependent change in the phase and crystal structure, Sr0.9La0.1TiO3 films were analyzed using wide-angle XRD from 20° to 60°. To remove the surfactant templates and form a mesoporous structure, the spin-coated films were annealed at 450 °C for 4 h. At this temperature, the diffraction peaks were not found (Fig. 2(a)), and an amorphous phase for Sr0.9La0.1TiO3 was confirmed. The typical XRD patterns were obtained with the films that were annealed at 650 °C and 850 °C. The sample annealed at 650 °C showed a larger diffraction peak width than that of the sample annealed at 850 °C. This might be due to the increased crystallinity and grain growth (from 8 to 23 nm).28 In order to confirm the effect of the surfactant concentration on the crystal structure of the Sr0.9La0.1TiO3 phase, various amounts of surfactant (Brij-S10) were used when the annealing conditions of the films were selected to be 850 °C for 4 h. Wide-angle XRD analyses for these films were carried, as shown in Fig. 2(b). It is observed that the diffraction peak intensities for the surfactant concentration of 0.05 and 0.075 samples were slightly decreased compared to that of the 0.025 sample. Generally, thermal decomposition of the surfactant induces a broadening in the diffraction peak which was caused by the reduced crystalline state with smaller grains and decreased film density.29
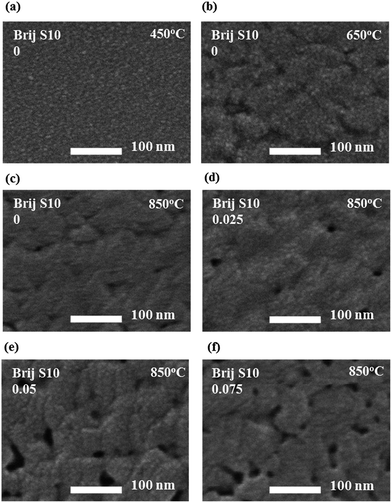
Here, the grain size decreased from 23 nm to 18 nm. The surface morphology, including the surface area, surface pore size and pore arrangement, surface grain size, and surface roughness, were important factors for the semiconductor-based gas sensors because gas sensing is a surface reaction.30 The surface morphologies of mesoporous Sr0.9La0.1TiO3 films, as a function of the increased annealing temperature and surfactant concentration, were analyzed using SEM. These results are presented in Fig. 3. The sample annealed at 450 °C showed a flat and smooth surface morphology. Alternatively, the samples annealed at 650 °C and 850 °C show roughened surfaces and contain pores and flexion in the morphology. This change in surface morphology, induced by a higher annealing temperature, might be due to the crystallization of Sr0.9La0.1TiO3 (shown in the XRD results in Fig. 2(a)). After implementing a mesoporous structure in the Sr0.9La0.1TiO3, surface pores were observed.
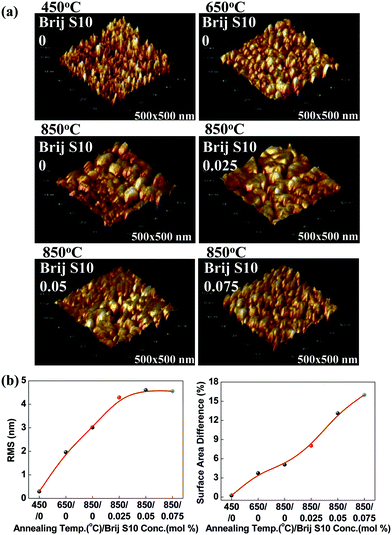
These pores increased in number with increasing surfactant concentration and induced an enhancement in the interpore connection during the grain growth of the wall-structured material and the thermal treatment of the mesoporous structure.21,22 During the growth and agglomeration of surface grains, connected, closed interior pores were exposed to the surface and surface opening occurred. This situation can lead to an increase in the pore connectivity in the mesoporous structure. To clarify the changes in the surface morphology caused by different surfactant concentrations and annealing temperatures, AFM analysis were carried out. These results are given in Fig. 4(a). Also, the plots of root mean square (RMS) surface roughness values and the immediate surface area differences of the films versus annealing temperature and surfactant concentration were shown in Fig. 4(b). AFM analysis was performed on the Sr0.9La0.1TiO3 films, and the surface area differences were obtained from the differences between measurement area and projected area. By comparing the AFM micrographs, the RMS surface roughness and surface area difference were found to be increased with increasing annealing temperature and surfactant concentration. The sudden increase in the RMS surface roughness with annealing temperature might be due to the grain growth of Sr0.9La0.1TiO3 and the reformation of pores, as observed in Fig. 2 and 3. The RMS surface roughness values of Sr0.9La0.1TiO3 films increased until a surfactant concentration of 0.025; above 0.025 the roughness values became saturated. From this result, it can be said that the formation of a porous structure can induce an increase in the surface roughness. However, the RMS value does not strongly depend on changes in the pore size and porosity or collapse of the interior pore structure. Alternatively, the increased difference in the surface area was due to the change of flat surface state into the flexion state by crystallization.31 In the 850 °C annealed films, the surface area difference according to the increased surfactant concentration sharply increased after the collapse of the interior pore structure. N2 adsorption and desorption for Sr0.9La0.1TiO3 films prepared at various annealing temperature and surfactant concentrations were measured to investigate the changes in pore volume and pore diameter. The results were given in Fig. 5 and Table 1.
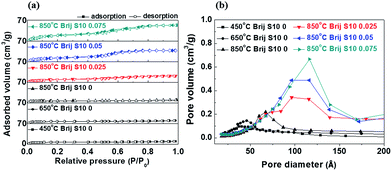 | ||
| Fig. 5 (a) Adsorption and desorption isotherms and (b) pore diameter distribution of Sr0.9La0.1TiO3 films with various annealing temperatures and surfactant concentrations. | ||
| BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | |
|---|---|---|---|
| 450 °C Brij S10 0 | 12.94 | 0.03 | 5.9 |
| 650 °C Brij S10 0 | 16.57 | 0.06 | 7.8 |
| 850 °C Brij S10 0 | 22.3 | 0.1 | 11.2 |
| 850 °C Brij S10 0.025 | 30.57 | 0.17 | 14.1 |
| 850 °C Brij S10 0.05 | 51.25 | 0.25 | 15.5 |
| 850 °C Brij S10 0.075 | 71.4 | 0.34 | 16.5 |
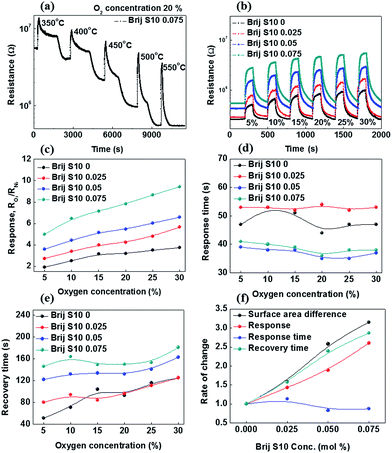
With an annealing temperature increased from 450 to 850 °C, the surface area (12.94 to 22.3 m2 g−1), pore volume (0.03 to 0.1 cm3 g−1) and pore size (5.9 to 11.2 nm) of Sr0.9La0.1TiO3 films formed without surfactant were increased. As mentioned above, the enhancement in textural properties were resulted from the growth and agglomeration of surface grains with temperature. On the other hand, the surfactant concentration increased from 0 to 0.075, the surface area (22.3 to 71.4 m2 g−1) of Sr0.9La0.1TiO3 films was greatly increased because closed interior pores were exposed on the surface and pore connectivity was enhanced during the growth and agglomeration of surface grains. Normally, the gas sensitivity can be increased by increasing the number of absorption sites and reaction area. In this way, the interpore-connected mesoporous structure of Sr0.9La0.1TiO3 films were highly useful for oxygen gas sensing. Therefore, we analyzed the oxygen sensing properties of Sr0.9La0.1TiO3 films in order to confirm the enhancement in oxygen sensing induced by changing the surface area. In order to select the analysis temperature, gas sensing analyses were carried out at an oxygen gas concentration of 20% between 350 °C and 550 °C with an 850 °C annealed Sr0.9La0.1TiO3 film with a surfactant concentration of 0.075, as shown in Fig. 6(a). We were unable to analyze the gas sensing characteristics of the films annealed at 450 °C and 650 °C due to their high resistance. During exposure to oxygen gas, the sensor resistance was increased, because mesoporous Sr0.9La0.1TiO3 films are n-type semiconductors.32 In an n-type semiconductors, when the metal oxide is heated in oxygen deficient state (nitrogen atmosphere), surface oxygen is desorbed on the surface of oxide. And the space charge layer was decreased because desorbed oxygens donate electrons and pass through. As a result, the resistance of mesoporous Sr0.9La0.1TiO3 films decreased at nitrogen atmosphere. This state was initial state for oxygen sensing. After, when oxygen is introduced, oxygen is absorbed on the surface oxide. The absorbed oxygens pull the donor electrons or electrons from the conduction band, O− of O2− remains on the surface, while ions with a (+) charge remain at the space charge layer. Therefore, an electrostatic field that generates a space charge region is formed with blocking charge transfer, and a surface potential is created. This surface potential acts as a potential barrier (depletion area) to prevent the flow of electrons. As a result, when the oxygen is ionized, the surface density and space charge layer decrease through the ionization. Finally, the absorption of oxygen ion occurs, the electrical conductivity decreases by an increase of depletion area. In Fig. 6(a), up to 500 °C, the recovery time was long and the recovery resistance was higher than the initial resistance. However, at an analysis temperature of 550 °C, the recovery time and resistance change were reasonable during oxygen sensing. In order to confirm the oxygen gas sensing properties, gas sensing analyses were carried out and the change of the resistance in the Sr0.9La0.1TiO3 films at various oxygen concentrations were determined, as given in Fig. 6(b). The gas sensing properties are shown in detail in Fig. 6(c–e). By analyzing the difference between the sensor resistance in nitrogen (RN2) and the sensor resistance in oxygen (RO2), we calculated the response of the sensors as RO2/RN2. As shown in Fig. 6(b), the electrical resistance of Sr0.9La0.1TiO3 films increased with an increase in the surfactant concentration due to an increased porosity.33 The change in resistance (response of the sensor) increased with increased oxygen concentration and surfactant concentration.
The oxygen concentration corresponds to the reaction amount and the surfactant concentration corresponds to the specific surface area, i.e., the reaction area. In other words, the response of the sensor strongly depends on the reaction area and the amount of the reaction species. This increased reaction area came from an increase in the interior pore connectivity, which was caused by the collapse and agglomeration of interior pores, as confirmed in Fig. 3 and 4. Also, it is well-known that the response increases with decreasing particle size34 and that the decreased grain size affects the response via the increased Schottky barrier height.35 Of course, the increased oxygen concentration increases the amount of oxygen absorption and sensitivity of Sr0.9La0.1TiO3 films. From the Fig. 6(d), it was found that the response time of Sr0.9La0.1TiO3 films did not change with different oxygen concentrations (i.e., a maximum oxygen sensitivity of 9.43). Alternatively, the response time of Sr0.9La0.1TiO3 films increased until the surfactant concentration reached 0.025 and then decreased at higher surfactant concentrations. This increase in the response time up to a surfactant concentration of 0.025 resulted from the exposure of small pores and grains on the surface, which can be confirmed from the results of Fig. 2–4. Due to these small pores and grains, the absorption rate of oxygen gas and the Schottky barrier height can be increased, which can lead to an increase in the response time.34,35 At surfactant concentrations above 0.025, the response time was reduced, as observed in nanostructured sensors.36 Fig. 6(e) shows that the recovery time of Sr0.9La0.1TiO3 films increased with increased surfactant concentration and oxygen concentration due to an increased amount of an oxygen that is absorbed on the surface and permeated into the connected interior pores. As a result, although the recovery time was increased, the improvement in the oxygen sensitivity of Sr0.9La0.1TiO3 films can be attributed to both the increased surface area and the improved pore connectivity. However, as shown in Fig. 6(f), the changing behavior of the response time was not important compared with the other sensing properties such as the response and recovery times according to the surfactant concentration.
This is caused by the aforementioned fact that the response time is affected by the reduced grain size (toward the Debye length) and the increased Schottky barrier heights.30,35 Alternatively, the well-matched behavior between the recovery time and the difference in surface area was due to the fact that the recovery time is mainly affected by oxygen gas permeation. Finally, in order to confirm the stability of the Sr0.9La0.1TiO3 film sensor (Brij-S10 0.075), gas sensing analysis was carried out over a long period (12 h) at an analysis temperature of 550 °C. The change in the resistance is shown in Fig. 7. It can be seen that the resistance changed little after the 12 h period. Ratios of sensitivity were 5 and 9.5 for oxygen concentrations of 5 and 20%, respectively. These sensitivities were similar to the previous results (Fig. 5(b)). From these results, it can be confirmed that mesoporous Sr0.9La0.1TiO3 with interior pore connectivity demonstrates good stability as an oxygen gas sensor.
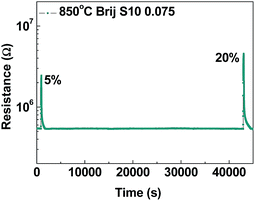 | ||
| Fig. 7 Oxygen gas response (RO2/RN2) of mesoporous Sr0.9La0.1TiO3 films (Brij-S10 0.075) over a long period of time (12 h). | ||
Conclusions
Mesoporous Sr0.9La0.1TiO3 films were synthesized using a metal propionate powder, TTIP as a Sr0.9La0.1TiO3 precursor, and Brij-S10 as a copolymer surfactant. The structural and gas sensing properties of Sr0.9La0.1TiO3 films were investigated as a function of the surfactant concentration. Increased surface area and pore connectivity were induced in mesoporous Sr0.9La0.1TiO3 films by exposing interior pores via controlling the surfactant concentration. When the surfactant concentration was increased, mesoporous Sr0.9La0.1TiO3 films showed an increase in gas sensitivity; however, the recovery time was also increased due to oxygen permeation into the connected-pore structure. Mesoporous Sr0.9La0.1TiO3 was formed using simpler synthesis way as compared to other synthesis ways and showed stable sensing characteristics at high temperature due to the thermal pre-treatment to enhance pore connectivity. Mesoporous Sr0.9La0.1TiO3 films with enhanced pore connectivity were synthesized by a simple and cost effective EISA process; these materials are a promising candidate for oxygen gas sensor of vehicle.Acknowledgements
This work was supported by the Center for Advanced Meta-Materials (CAMM) funded by the Ministry of Science, ICT and Future Planning as Global Frontier Project (CAMM-No. NRF-2014M3A6B3063716). This study was supported by a grant from DAPA and ADD, Republic of Korea. Experiments at PLS were supported in part by MEST and POSTECH.References
- R. Ramamoorthy, P. K. Dutta and S. A. Akbar, J. Mater. Sci., 2003, 38, 4271 CrossRef CAS.
- J. Binder, Sens. Actuators, A, 1992, 31, 60 CrossRef CAS.
- C. B. Alock, Solid State Ionics, 1992, 53–56, 3 CrossRef.
- A. M. Azad, S. A. Akbar, S. G. Mhaisalkar, L. D. Birkefeld and K. S. Goto, J. Electrochem. Soc., 1992, 139, 3690 CrossRef CAS PubMed.
- J. Riegel, H. Neumann and H.-M. Wiedenamann, Solid State Ionics, 2002, 783, 152 Search PubMed.
- J. Gerblinger, K. H. Hardtl, H. Meixner and R. Aigner, High-temperature microsensors, in Sensors, ed. W. Gopel, Comprehensive Survey, Weinheim, 1995 Search PubMed.
- R. Moos, T. Bischoff, W. Menesklou and K. H. Hardtl, J. Mater. Sci., 1997, 32, 4247 CrossRef CAS.
- C.-Y. Chen, K.-H. Chang, H.-Y. Chiang and S.-J. Shih, Sens. Actuators, B, 2014, 204, 31 CrossRef CAS PubMed.
- G. Zhu, L. Guo, X. Shen, Z. Jia, K. Chen and H. Zhou, Sens. Actuators, B, 2015, 220, 977 CrossRef PubMed.
- Z. Yu, C. Ang and L. E. Cross, Appl. Phys. Lett., 1999, 74, 3044 CrossRef CAS PubMed.
- S. X. Zhang, S. B. Ogale, D. C. Kundaliya, L. F. Fu, N. D. Browning, S. Dhar, W. Ramadan, J. S. Higgins, R. L. Greene and T. Venkatesan, Appl. Phys. Lett., 2006, 89, 012501 CrossRef PubMed.
- Ch. Lenser, A. Kuzmin, J. Purans, A. Kalinko, R. Waser and R. Dittmann, J. Appl. Phys., 2012, 111, 076101 CrossRef PubMed.
- D. P. Debecker, W. Hulea and P. H. Mutic, Appl. Catal., A, 2013, 451, 192 CrossRef CAS PubMed.
- H. Bai, Z. Liu and D. D. Sun, J. Am. Ceram. Soc., 2013, 96, 942 CrossRef CAS PubMed.
- G. Xu, S. Deng, Y. Zhang, X. Wei, X. Yang, Y. Liu, G. Shen and G. Han, CrystEngComm, 2014, 16, 2025 RSC.
- J. Rouquerol, D. Avnir, C. W. Fairbridge, D. H. Everett, J. M. Haynes, N. Pernicone, J. D. F. Ramsay, K. S. W. Sing and K. K. Unger, Pure Appl. Chem., 1994, 66, 1739 CrossRef CAS.
- Y. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. Gong, Y. Guo, H. Soyez, B. Dunn, M. H. Huang and J. I. Zink, Nature, 1997, 389, 364 CrossRef CAS.
- E. K. Richman, T. Brezesinski and S. H. Tolbert, Nat. Mater., 2008, 7, 721 CrossRef PubMed.
- D. Grosso, F. Cagnol, G. J. d A. A. Soler-Illia, E. L. Crepaldi, H. Amenitsch, A. Brunet-Bruneau, A. Bourgeois and C. Sanchez, Adv. Funct. Mater., 2004, 14, 309 CrossRef CAS PubMed.
- H. Elbel, J. Nucl. Mater., 1998, 155–157, 480 Search PubMed.
- C.-S. Park, M.-H. Hong and H.-H. Park, J. Ceram. Soc. Jpn., 2014, 122, 608 CrossRef CAS.
- T.-J. Ha, M.-H. Hong, C.-S. Park and H.-H. Park, Sens. Actuators, B, 2013, 181, 874 CrossRef CAS PubMed.
- Z.-Y. Yuan, T.-Z. Ren, A. Vantomme and B.-L. Su, Chem. Mater., 2004, 16, 5096 CrossRef CAS.
- U. Hasenkox, C. Mitze and R. Waser, J. Am. Ceram. Soc., 1997, 80, 2709 CrossRef CAS PubMed.
- M. R. Baklanov and K. P. Mogilnikov, Microelectron. Eng., 2002, 64, 335 CrossRef CAS.
- S.-B. Jung and H.-H. Park, Thin Solid Films, 2006, 494, 320 CrossRef CAS PubMed.
- G. Colon, J. M. Sanchez-Espana, M. C. Hidalgo and J. A. Navio, J. Photochem. Photobiol., A, 2006, 179, 20 CrossRef CAS PubMed.
- J. Yang, D. F. Shao, X. B. Zhu, Z. R. Yang, Y. P. Sun and Y. P. Lee, Appl. Phys. Lett., 2013, 102, 042406 CrossRef PubMed.
- C.-S. Park, U. K. H. Bangi and H.-H. Park, Mater. Lett., 2013, 106, 401 CrossRef CAS PubMed.
- C. Wang, L. Yin, L. Zhang, D. Xiang and R. Gao, Sensors, 2010, 10, 2088 CrossRef CAS PubMed.
- H.-T. Sun, C. Cantalini, L. Lozzi, M. Passacantando, S. Santucci and M. Pelino, Thin Solid Films, 1996, 287, 258 CrossRef CAS.
- Y. Cui, J. R. Salvador, J. Yang, H. Wang, G. Amow and H. Kleinke, J. Electron. Mater., 2009, 38, 1002 CrossRef CAS.
- C.-S. Park, M.-H. Hong, S. Shin, H. H. Cho and H.-H. Park, J. Alloys Compd., 2015, 632, 246 CrossRef CAS PubMed.
- C. Xu, J. Tamaki, N. Miura and N. Yamazoe, Sens. Actuators, B, 1991, 3, 147 CrossRef CAS.
- N. Yamazoe, Sens. Actuators, B, 1991, 5, 7 CrossRef CAS.
- G. Zhang and M. Liu, Sens. Actuators, B, 2000, 69, 144 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |