Mn-based tunnel-structured Na0.44MnO2 cathode materials for high-performance sodium-ion batteries: electrochemical mechanism, synthesis and modifications
Dong
Wang
,
Liumei
Teng
and
Weizao
Liu
 *
*
College of Materials Science and Engineering, Chongqing University, Chongqing, 400044, China. E-mail: liuwz@cqu.edu.cn
First published on 11th December 2024
Abstract
Sodium-ion batteries (SIBs) have emerged as promising and mature alternatives to lithium-ion batteries (LIBs) in the post-LIB era, necessitating the development of cost-effective and high-performance cathode materials. The unique crystal texture of Mn-based tunnel-structured cathode materials offers outstanding cycling stability, rate capability and air stability, making them a highly attractive option for sodium-ion storage applications. This comprehensive review summarizes recent advancements in the understanding of sodium-ion storage mechanism, synthesis techniques, and modification strategies for Mn-based tunnel-structured cathode materials, thereby significantly contributing to the advancement of high-performance cathodes for SIBs.
1. Introduction
Sodium-ion batteries (SIBs), characterized by their abundant resources, high performance, and low cost, are considered a promising complementary technology to lithium-ion batteries (LIBs). This makes SIBs particularly suitable for grid-scale energy storage, offering significant potential for broad applications.1–6 Over the past decade, significant progress has been made in the development of electrochemical energy storage technologies for SIBs. Both fundamental research and commercialization efforts have gained significant momentum, leading to promising advancements in this rapidly evolving field.7 Continuous innovations in material iteration and device engineering optimization for SIBs further enhance their potential for future growth in the energy storage market. In the SIB system, the choice of cathode material is a critical factor that influences both battery performance and cost.8 One key advantage of SIBs is their ability to utilize a wide range of cathode materials. Currently, a series of cathode materials have been specifically developed for SIBs, encompassing transition metal oxides,9–12 polyanionic compounds,13 Prussian blue analogues (PBAs),14 and organic cathode materials.15 This diverse array enables researchers and engineers to tailor the overall battery performance according to specific requirements, such as high energy density, long cycle life, or fast-charging capabilities.16One of the key challenges in advancing SIB technologies lies in the enhancement and fine-tuning of cathode materials. These materials must be optimized to effectively accommodate sodium ions while facilitating rapid and reversible sodium-ion insertion/extraction process.17–20 Ensuring cycling stability is crucial for the practical implementation of SIBs in large-scale energy storage systems. Due to their advantages of abundant manganese reserves, environment friendliness and low cost, the study of Na–Mn–O cathode materials has become a key focus in the research of SIBs.21 The manganese (Mn)-based layer-structured cathode materials can be further categorized into P2-Na0.67MnO2 and O3-NaMnO2 phase structures based on the stacking sequences of oxygen atoms and the positions occupied by sodium ions within the manganate-based oxide structure.22 Here, P and O denote the coordination environment of Na+ in trigonal prismatic and octahedral configurations, respectively. Moreover, 2 and 3 represent the number of transition metal layers within each cyclic unit of the layered structure.23 Among these manganese oxides, the Mn-based tunnel-structured cathode material Na4Mn9O18 (Na0.44MnO2) has been extensively researched as a promising cathode material for SIBs due to its unique S-type tunnel structure, which facilitates rapid de-embedding of sodium ions, along with outstanding cycling stability and superior rate capability than layer-type cathode.24–26 This type of Mn-based tunnel structure material was first reported by Parant et al. in 1971,27 but it was not until 1994 that Doeff and his colleagues studied this tunnel-type structure as an electrode material for electrochemical sodium-ion de-/intercalation process.28 The Mn-based tunnel crystal structure depicted in Fig. 1(a) predominantly comprises five distinct manganese-ion sites and three different sodium-ion sites. The Na0.44MnO2 exhibits the same crystal structure as Na4Mn4Ti5O18 and adopts an orthorhombic structure arrangement (space group: Pbam). The Mn(1) and Mn(2) sites indicated are occupied by Mn3+, while the remaining Mn(3), Mn(4), and Mn(5) sites are occupied by Mn4+. The entire framework is composed of double and triple linear chain structures with shared edges of MnO6 octahedra, as well as a single chain structure with shared edges of MnO5 tetrahedra.29–31 Through the interconnected angles of these polyhedra, adjacent chain structures combine to form a “pentagonal” one-dimensional tunnel configuration, along with another S-shaped two-dimensional tunnel configuration. The sodium-ion sites distributed within these two types of tunnel structures can be categorized into two groups: Na(1) site located within the pentagonal tunnel, and Na(2) and Na(3) sites belonging to the S-type tunnel.32,33 Sodium ions at the Na(2) and Na(3) positions within the wide S-shaped tunnel structure exhibit rapid diffusion ability along the c-axis direction, while sodium ions within the smaller pentagonal tunnel are generally considered immobile under conventional electrochemical conditions. Consequently, there is limited availability for extracting sodium ions from this crystal structure, which restricts its theoretical electrochemical capacity (121 mA h g−1) as a high-energy-density electrode.34,35 However, its exceptional sodium ion diffusion kinetics and structural stability continue to be extensively investigated in non-aqueous and aqueous SIBs.36–39 The size of the tunnel should be adjusted to accommodate the requirements of Na+ insertion/extraction, while Mn-based materials inevitably encounter Jahn–Teller distortion induced by Mn3+. These factors contribute to structural deterioration and Mn dissolution of the tunnel structure cathode materials, resulting in low-rate performance and limited cycle stability.
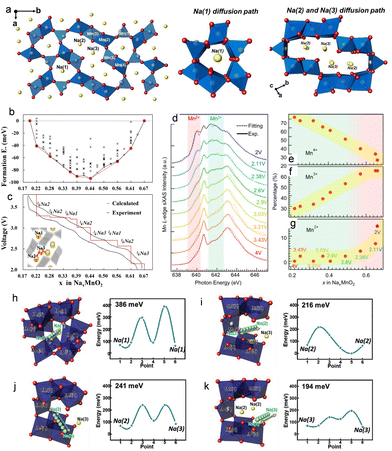 | ||
| Fig. 1 (a) Crystal-structure diagram of Mn-based tunnel-structure Na0.44MnO2. Reproduced with permission from ref. 40, Copyright (2021), Wiley-VCH. (b) Seven stable intermediate phases during the cycles of NaxMnO2 (x = 0.19–0.44) obtained from the calculated formation energies. (c) Contrasting graph of the calculated voltage profile along the minimum energy path on formation energies and the initial charging/discharging curve from experimental data. Reproduced with permission from ref. 41, Copyright (2012), the American Chemical Society. (d) Mn L-edge sXAS data collected on NaxMnO2 cathode at different voltages. (e)–(g) Concentration evolution of Mn4+, Mn3+ and Mn2+ at varying sodium-ion concentrations from NaxMnO2 cathode. Reproduced with permission from ref. 42, Copyright (2015), Elsevier. Sodium-ion diffusion energy barriers for the tunnel-structured Na0.44MnO2 demonstrated for the Na-site pairs (h) Na(1)–Na(1); (i) Na(2)–Na(2); (j) Na(2)–Na(3); and (k) Na(3)–Na(3). Reproduced with permission from ref. 43, Copyright (2020), Wiley-VCH. | ||
Therefore, a comprehensive review is imperative to elucidate and summarize the electrochemical mechanism, synthesis methods, modification strategies, and other pertinent aspects of Mn-based tunnel structure cathode materials for SIBs. This will enable researchers in the field of energy storage materials to gain a lucid understanding of the research progress and future development directions in Mn-based tunnel structure cathode materials, thereby further advancing the fundamental investigation and industrialization of high-performance SIBs.
2. Sodium-ion storage mechanism
The unique atomic arrangement in the tunnel-type structure of Na–Mn–O contributes to its exceptional crystal structure stability, distinguishing it from layer-structure compounds, such as the P2-type layer or O3-type layer, which undergo significant cell volume expansion and eventually structure collapse, leading to inadequate cyclability.7,44 Therefore, it is crucial to investigate the sodium-ion storage mechanism in Mn-based tunnel cathode materials despite their lower reversible capacity. The larger S-shaped tunnel structure has Na(2) and Na(3) sites that significantly contribute to the redox reaction during charge/discharge processes, while the smaller pentagram tunnel structure contains Na(1) sites where minimal sodium-ion insertion and de-intercalation occur electrochemically.40 Mn ions exist in two distinct environments: all Mn4+ ions and half of the Mn3+ ions occupy octahedral sites (MnO6), while the remaining Mn3+ ions are situated within a square-pyramidal environment (MnO5). The crystal particle within this structure comprises both Mn3+ and Mn4+ oxidation states, which are charge-neutral as represented by the chemical composition Na+4Mn3+4Mn4+5O2−18. During the charging and discharging processes, the range of variation for the molar ratio of Na/TM primarily lies from 0.22 to 0.66. The charging process primarily involves the following chemical reactions: Na0.44MnO2 − 0.22 Na+ − 0.22 e− → Na0.22MnO2. Conversely, the corresponding discharge process can be denoted as Na0.44MnO2 + 0.22 Na+ + 0.22 e− → Na0.66MnO2.Baudrin et al.45 used the potentiostatic intermittent titration technique (PITT), along with in situ X-ray diffraction (in situ XRD) measurements, to clearly demonstrate the complexity of the sodium-ion insertion/de-insertion process. They observed at least six distinct biphasic phenomena in 2–3.8 V (vs. Na+/Na) within a composition range of 0.18 < x < 0.64 in the compound NaxMnO2. Kim et al.41 conducted density functional theory (DFT) calculations to investigate the structural and electrochemical properties of Na0.44MnO2 cathode material. They identified seven intermediate phases and two-phase reactions, which were consistent with the experimental data (Fig. 1(b) and (c)). Furthermore, they highlighted that despite the presence of Mn3+ ions throughout all electrochemical cycles, the crystal structure of Na0.44MnO2 does not undergo structural transformations due to its unique arrangement of an oxygen lattice along the a-axis rather than cubic close-packed layers. Additionally, they found that the migration of Mn ions into neighboring vacant sodium-ion sites is unfavorable, and structures with varying sodium-ion content ranging from 0.22 to 0.66 in the crystal structure are more energetically stable compared to nearby Na–Mn–O compounds. These factors contribute significantly to maintaining structural stability and ensuring superior cycling performance during the sodium-ion extraction/insertion processes for tunnel-structure cathode materials. Afterwards, Kim and his colleagues46 further investigated the sodium-ion intercalation/de-intercalation behavior of Na0.44MnO2 tunnel-structure cathode materials by utilizing electrochemical impedance spectroscopy (EIS) measurements. They discovered that the apparent sodium-ion diffusion coefficients in organic electrolyte systems ranged from 5.75 × 10−16 to 2.14 × 10−14 cm2 s−1. Additionally, the CV curve exhibited at least six redox reaction processes that aligned with the corresponding charge/discharge curves. Aurbach et al.43 combined various characterization methods, including X-ray diffraction data, 3D bond valence difference maps and X-ray diffraction data. The barrier-energy calculations of sodium diffusion were used to study the sodium-ion intercalation mechanism. The ion migration during the contributions of Na(2) and Na(3) sites in the S-shaped tunnel structure was further confirmed (Fig. 1(h)–(k)). In addition to investigating the evolution of the crystal structure within the bulk structure during the charging and discharging processes, comprehending the alterations in surface structure also plays a pivotal role in influencing electrochemical performance. Yang et al.42 conducted an extensive analysis utilizing surface-sensitive soft X-ray absorption spectroscopy (sXAS) to quantitatively explore the transformation of Mn on the surface area of tunnel-structure cathode materials Na0.44MnO2 with varying potentials and electrochemical cycle numbers. They discovered that below an electrochemical potential of 2.6 V, the specific formation of Mn2+ occurs solely on the outside layer of crystal particles for 10 nm, which is not detected in the bulk material (Fig. 1(d)–(g)). Moreover, after prolonged cycles, some of these surface compounds, including Mn2+, become electrochemically inactive, contributing to the degradation of discharge capacity.
The electrochemical sodium-ion storage mechanism of Mn-based tunnel structure cathode materials has been extensively investigated by researchers using in/ex situ characterization means and theoretical calculations. These studies have focused on understanding the electrochemistry behavior of Mn-based tunnel structure materials during the charge and discharge processes from the perspectives of crystal structure, local structure and electrochemical property. However, further attention can be given to the crystal structure and surface chemical environment evolution of cathode materials after long cycles and variations in sodium-ion transport channels in the tunnel-type crystal texture.
3. Material synthesis
Researchers have employed various synthesis methods to prepare Mn-based tunnel structure materials to investigate their sodium-ion storage properties and electrochemistry mechanism as a cathode in SIBs, including the high-temperature solid-phase method, sol–gel method, co-precipitation method,47 and hydrothermal method. The following section presents the commonly utilized synthesis methods in Mn-based tunnel structure materials.3.1 Solid-phase method
The high-temperature solid phase method is one of the common synthesis solutions for transition-metal-oxide cathode materials. The synthesis approach possesses the benefits of a straightforward procedure, convenient access to raw materials, manageable regulation of reaction conditions, and economical production expenses. Consequently, it has extensive application in the synthesis of diverse inorganic materials for both fundamental scientific investigations and industrial production. However, there are certain drawbacks associated with this approach, including high energy consumption, lack of uniformity in material composition, tendency for particle agglomeration, wide distribution of particle diameters, and a significant proportion of larger-sized grains. The synthesis of NaxMnO2 by applying the high-temperature solid phase method is mainly based on the uniform mixing of sodium source and manganese source according to the designed stoichiometric ratio, and the precursor preparation is formed through mechanical mixing. The chemical reaction of crystallization progress occurs under high-temperature conditions; finally, the Mn-based tunnel cathode material is obtained. By adjusting the synthesis parameters, Baudrin et al.45 successfully prepared a highly crystalline pure Na0.44MnO2 cathode material using the solid-phase method. The specific procedure involved thoroughly grinding Na2CO3 and MnCO3 in a mortar, followed by heating in air at 300 °C for 8 hours, and then at 800 °C for 9 hours with intermittent grinding. Electrochemical testing and in situ XRD technique characterization revealed significant changes in the polarization effect and specific capacity of the Na0.44MnO2 cathode during sodium ion insertion/removal progress. Avci et al.48 utilized a mixture of Na2O2 and MnO2, which were uniformly blended using an agate mortar in a glove box filled with Ar gas. The resulting mixture was then sintered at 750 °C for 24 hours in air. The growth of tunnel-structured nanorods initiates at 650 °C, with their orientation being perpendicular to the grain boundaries (Fig. 2(a)). Besides, it was observed that the presence of excess Na is crucial for the formation of rod-shaped tunnel structure Na0.44MnO2 (Fig. 2(b)).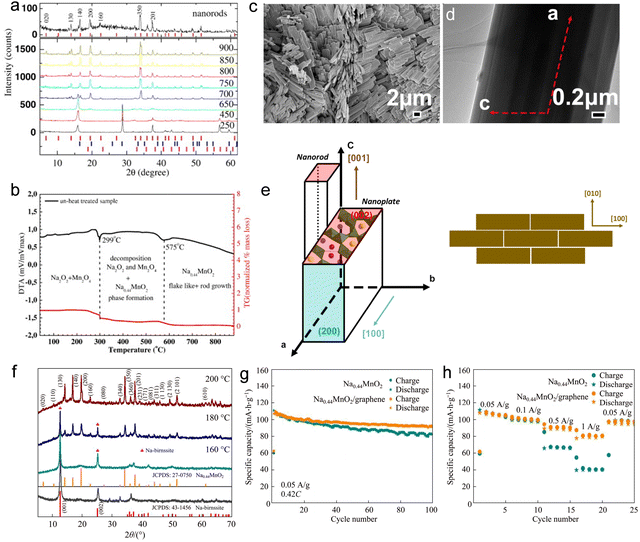 | ||
| Fig. 2 (a) Ex situ XRD patterns of Na0.44MnO2 sample during heat treatment progress. (b) Differential thermal analysis (DTA) and thermogravimetric analysis (TG) data of the heating progress on the composition of Na2O2 and MnO2. Reproduced with permission from ref. 48, Copyright (2015), Elsevier. (c) SEM image; (d) TEM image; and (e) schematic of the crystal particle growth and structure formation of nanorods and nanoplates. Reproduced with permission from ref. 49, Copyright (2016), Elsevier. (f) XRD patterns of tunnel-structured nanorods, Na0.44MnO2, prepared via hydrothermal synthesis at different hydrothermal reaction temperatures. The electrochemical performance of Na0.44MnO2/graphene and pristine Na0.44MnO2 cathode: (g) cycle performance at a current density of 50 mA g−1 and voltage range of 2.0–4.0 V; (h) rate capability. Reproduced with permission from ref. 50, Copyright (2019), Springer Nature. | ||
3.2 Liquid-phase method
The sol–gel method involves the utilization of inorganic salts and other raw materials to dissolve them in a solution, form a sol, and then cure it into a gel. This process is employed to prepare precursors for cathode materials. Subsequently, these precursors are subjected to heat treatment in a furnace, resulting in the desired chemical products. Utilizing the sol–gel method can effectively address the issue of the large grain size commonly encountered during solid-phase synthesis progress. Moreover, this approach enables the production of tunnel-type structure cathode materials with small particle sizes ranging from nanometers to microns. However, it is important to note that there are few drawbacks associated with synthesizing Mn-based tunnel cathode materials using the sol–gel method. These include higher costs due to expensive raw materials and the potential release of gases and organic compounds during both drying and high-temperature calcination processes, which may have adverse effects on environmental conditions in large-scale production. Xu and his colleagues51 synthesized a Na0.44MnO2 cathode material with a unique morphology of elongated submicron slabs. Through precise control of the fabrication process and the use of specific chelating agents, they successfully manipulated the crystal structure and morphology of the resulting product. The resultant cathode material demonstrated exceptional dispersibility, ultra-long length, and significantly reduced thickness in the direction of sodium-ion deintercalation. Furthermore, the cathode material prepared using this method exhibited a high capacity exceeding 120 mA h g−1. Li et al.49 successfully synthesized Na0.44MnO2 nanoplates with a monocrystalline orthorhombic structure using the sol–gel method assisted by templates. These nanoplates exhibited exceptional characteristics, such as high crystallinity, a pure phase, and a uniform size distribution (Fig. 2(c) and (d)). By subjecting polystyrene and citric acid to high-temperature calcination, a reductive carbothermal environment was created that effectively hindered the formation of one-dimensional particles and restricted particle growth along the [001] direction. The lamellar structure of Na0.44MnO2 material provided mechanical stability and facilitated short diffusion paths for efficient insertion/de-insertion of sodium ions due to its suitable channels and controlled morphology with limited crystal growth along the [001] direction (Fig. 2(e)).The hydrothermal method is a widely utilized wet chemical synthesis method in which target products are synthesized under high temperature and high pressure by dissolving chemical raw materials in a solution. The particles of cathode materials produced using this method exhibit uniformity, and the morphology of the resulting products can be controlled by adjusting reaction conditions and reactants. Additionally, the synthesized materials possess high purity. However, it should be noted that the hydrothermal method requires extensive reaction time, yields lower output, and necessitates advanced equipment, technology, and safety. In terms of synthesizing Na0.44MnO2 using a hydrothermal synthesis approach, soluble sodium salt and metallic manganese oxide serve as primary raw materials, while water or other solvents act as mediums for conducting chemical reactions within a high-pressure reactor. Wang et al.50 successfully synthesized Na0.44MnO2 nanorods by optimizing the experimental parameters of the hydrothermal method (Fig. 2(f)). The conductivity of the material was increased by combining graphene with Na0.44MnO2 nanorods. Na0.44MnO2/graphene exhibits even better electrochemical performance, with an electrochemical discharge capacity of 106.9 mA h g−1 and superior rate capability. After 100 cycles, the capacity retention rate of the Na0.44MnO2/graphene cathode material reached 85.9% (Fig. 2(g) and (h)).
3.3 Other synthesis methods
In addition, some other preparation methods have been employed for the synthesis of Mn-based tunnel structure cathode materials, including the electrospinning technique, spray pyrolysis method, combustion method and molten salt method. Electrospinning is a flexible and practical method for producing fibers of varying diameters, ranging from nanometers to micrometers, with lengths that can extend up to kilometers. Wang et al.52 reported the successful creation of two distinct hierarchical structures of Na0.44MnO2 using an optimized electrospinning technique, followed by controlled annealing processes. The structure consists of single-crystalline nanorods (NR) and demonstrates excellent cyclic stability, maintaining a reversible specific capacity of 120 mA h g−1 even after undergoing 140 cycles. However, the structure composed of nanofibers (NF) exhibits remarkable rate performance with a reversible specific capacity of 69.5 mA h g−1 at a high current density of 10C, which can be attributed to its unique one-dimensional ultralong and continuous fibrous network architecture. Axelbaum et al.53 utilized a cost-effective and scalable technique, namely spray pyrolysis, to synthesize Na–Mn–O cathode material possessing tunnel structures. In the initial electrochemistry cycle, a discharge capacity of 115 mA h g−1 was achieved, while the material demonstrated favorable cycling stability and rate capability. Solution combustion synthesis is a highly promising technique for the controlled preparation of various functional oxide materials.54 This method involves an exothermic and self-sustaining redox reaction, which occurs upon heating a mixture of aqueous metal nitrates and organic fuels, such as glycine and urea. Zhu et al.55 successfully synthesized tunnel-structured Na0.44MnO2 using a simple and controllable solution combustion synthesis method, followed by the progress of calcination. The optimized Na0.44MnO2 electrode exhibited excellent electrochemical performance, including a high reversible capacity of 117 mA h g−1 at 0.1C, good rate capability, and outstanding cyclability with a high capacity retention of 100 mA h g−1 after undergoing 300 cycles at 4C rate. In addition, the synthesis of a high-performance Na0.44MnO2 cathode is attempted using molten salt chemistry.56 Na2CO3 and Mn2O3 are employed as reactants, while sodium chloride is utilized as the molten salt. The resulting product demonstrates exceptional long-term cycling stability, with a capacity retention rate of 85.4% after 500 cycles. This outstanding electrochemical performance is further confirmed through continuous cycling tests in full-cell, where the capacity retention rate remains at 80.5% after 300 cycles at a 1C rate. The future direction of development not only involves further investigating the mechanism of crystal structure and morphology transformation in the synthesis processes of reported methods on cathode materials but also entails exploring innovative synthesis strategies suitable for Mn-based tunnel structure cathode material preparation.4. Modification strategy
4.1 Bulk phase structure doping
Although the Mn-based S-shaped tunnel structure cathode has higher crystal structure stability than the layer structure cathode, the long-term cycle process still faces the issue of discharge capacity attenuation.32 The cell volume expansion caused by sodium ions entering the tunnel structure also leads to lattice changes and phase transitions in the Mn-based cathode material, making it challenging to achieve superior electrochemical stability. The continuous embedding/removal of sodium ions in the Mn-based tunnel cathode material, as well as the sodium-ion diffusion ability in the matrix framework of the tunnel-structure cathode material gradually reduces, restricting its specific capacity value and rate performance upon discharge progress. To address these issues, the ion doping strategy has been extensively adapted to reform the intrinsic crystal structure of tunnel-type architecture cathode materials and improve their corresponding electrochemical performance.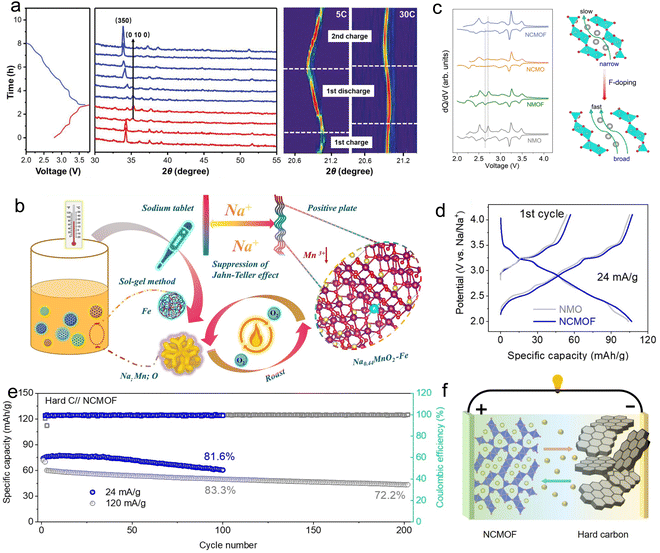 | ||
| Fig. 3 (a) Ex situ XRD data of Mg-doped tunnel cathode during charge/discharge progress at 0.2C, 5C, and 30C. Reproduced with permission from ref. 58, Copyright (2021), Wiley-VCH. (b) Synthesis progress of Fe-doped tunnel cathode materials and systematic elucidation of the Fe doping mechanism. Reproduced with permission from ref. 59, Copyright (2024), Elsevier. (c) Boosting effect on the electrochemical property of synergetic anion–cation co-doping in Na0.44MnO2 tunnel-phase cathode. (d) Charge/discharge curves during the initial cycle in voltage ranging from 2.0 to 4.1 V. (e) Cycle performance of the full cell. (f) Schematic diagram of the assembled full cell by Cu/F co-doping tunnel cathode and hard carbon anode. Reproduced with permission from ref. 60, Copyright (2024), Elsevier. | ||
The cation co-doping methods were also employed to facilitate Na+ insertion/extraction behavior and stabilize the tunnel crystal structure to further enhance its comprehensive electrochemical properties. Through the implementation of metal ion co-doping techniques, a favorable balance and enhancement in overall material properties encompassing structure, morphology, discharge capacity, cycle life, and rate performance are anticipated in the tunnel-type structure cathode. The Mg-doped Mn-based materials with a tunnel structure exhibit a higher discharge capacity than the original tunnel structure cathode, while non-electrochemically active Ti-doped tunnel structure materials sacrifice part of the electrochemically active element in favor of crystal structure stabilization, resulting in a lower initial discharge capacity. Remarkably, the synergistic effect of trace amounts of Mg and Ti leads to a more stable crystal structure and superior electrochemical performance of the co-doped cathode material Na0.44Mn0.895Ti0.1Mg0.005O2. By simultaneously introducing magnesium ions and titanium ions into the crystal structure, it is possible to expand the cell lattice, resulting in enhanced structure stability, enlarged tunnel size, reduced length-to-diameter ratio for facilitating Na+ insertion/extraction, and increased ion diffusion rate. The cathode Na0.44Mn0.895Ti0.1Mg0.005O2 exhibits an impressive specific capacity of 80.0 mA h g−1 at a high rate of 20C, demonstrating a remarkable capacity retention rate of 67% even after the ultra-long cycle of 2000 times.61 Qian's research group62 introduced three kinds of metal elements (Al, Ti, and Co) into the Mn-ion sites simultaneously to form a medium-entropy substituted tunnel-type Na0.44Mn0.97 Al0.01Ti0.01Co0.01O2 material. The medium-entropy substitution increases the spacing of lattice fringes and replaces Mn ions in the Na0.44MnO2 effectively. The optimized cathode material exhibits excellent rate performance, achieving a rated capacity of up to 80 mA h g−1 at 20C and maintaining a high capacity of 78 mA h g−1 after 2000 cycles at 10C.
Although various heterogeneous ions are substituted at different ion sites in Mn-based tunnel-type cathode materials, the modified materials exhibit exceptional electrochemical performance. It is crucial to further comprehend the ion-doping mechanism for enhancing the crystal structure properties of Mn-based cathode materials and guiding the development of novel strategies for ion substitution modification strategy. Furthermore, unexplored elements can be employed for trace ion substitution to facilitate further investigation of modifications in the crystal structure and electrochemical properties.
4.2 Surface modification
Surface modification is a highly practical tailoring method for mitigating side reactions on the outer surface of cathode materials, effectively enhancing chemical stability and electrochemical properties. Park et al.69 successfully designed and fabricated a relatively uniform dispersion of hybrid coating networks consisting of Al2O3 and MWCNTs onto the surface of tunnel-structure crystal particles, which exerted an inhibitory effect on the formation of damaging NaF-based solid–electrolyte interface, thereby facilitating rapid sodium ion transfer across the tunnel structure cathode/electrolyte interface. The rate capability and long-term cyclability of the tunnel-structure cathode were significantly enhanced through the incorporation of Al2O3/MWCNT. The upper limitation of the operating potential set for the tunnel-structure cathode in most previous studies is typically kept at about 4.0 V to prevent rapid discharge capacity degradation during cycle progress. Wang et al.70 conducted a layer of Al2O3 coating on the surface of a tunnel-structure cathode to enhance its electrochemical properties at a high cut-off voltage. The Al2O3-coated tunnel-structure cathode materials were prepared using a simple effective chemical coating method, and the corresponding coating amount was optimized to achieve this goal of enhancing high-voltage tolerance. The Al2O3-coated strategy effectively prevents the tunnel-structure cathode from undergoing direct contact reactions with the electrolyte and mitigating cell volume expansion during electrochemical processes. The resulting submicron rods with a 2 wt% Al2O3 coating exhibited increased electrochemical performance in the voltage range of 2.0–4.5 V, delivering an initial discharge capacity of 109.8 mA h g−1 at 0.4C and maintaining a capacity retention rate of 93.2% even after 200 cycles. Considering the analogous chemical properties between In2O3 and Al2O3, as well as its superior inertness and electrolyte resistance compared to Al2O3. Feng et al.71 employed a precipitation method to coat In2O3 onto Na0.44MnO2 cathode material synthesized using a solid-state reaction method. Their findings demonstrate significant improvement in the electrochemical performance of 1 wt% In2O3-coated Na0.44MnO2, compared to pristine Na0.44MnO2, with an increase in capacity retention from 70.3% to 86.7% after cycling for 400 cycles at 1C under a high cut-off voltage of 4.5 V. These electrochemistry property enhancements can be attributed to the protective effect of In2O3 coating, which effectively mitigates undesired structure changes occurring during cycle processes and suppresses complex side reactions between the tunnel-structure cathode and electrolyte.In addition to the conventional oxide coating tactics, Myung and his colleagues72 propose a melt-impregnation technique at 350 °C to incorporate (NH4)2MoO4 with unfavorable surface sodium residues on Na0.44MnO2 crystal particles, resulting in formatting an electro-conducting Na2MoO4 nanolayer on the tunnel-type Na0.44MnO2 compound's surface (Fig. 4(a)). The 1.5 wt% Na2MoO4-coated tunnel-structure cathode achieved long-term cycle stability at an ultra-high rate of 60C at the current density of 7.2 A g−1 and maintained a capacity of approximately 56 mA h g−1 without experiencing notable capacity decay for 1000 cycles (Fig. 4(b)). This remarkable cycle achievement can be attributed to the multifunctional effects of Na2MoO4 coating on the active compounds, facilitating electron transfer and providing protection coating against HF attack in the organic electrolyte environment during electrochemical reactions (Fig. 4(c)). In contrast, Huang et al.73 adopted a different approach by utilizing a Na-rich layer oxide (Na2TiO3) as a multifunctional coating layer to modify the cathode material composed of tunnel-structure nanorods (Fig. 4(d)–(f)). The optimized 3 wt% Na2TiO3-coated Na0.44MnO2 exhibits an exceptionally high capacity-retention rate of up to 96.7%. Besides, this modified cathode exhibited ultra-stability rate cycle performance, achieving a capacity of 80.2 mA h g−1 at a rate of 20C and retaining 97.7% of its capacity after 900 cycles. Na2TiO3 coating acts as a reservoir for sodium ions and serves as an effective protective layer against electrolyte etching on the outer structure of Na0.44MnO2. Additionally, it should be noted that a 2D-layered Na2TiO3 coating functions to provide extra pathways other than [001] for sodium-ion diffusion along radial directions within nanorods, thereby reducing migration distances to improve the rate performance of tunnel-structure compounds. Although the cathode material of the Mn-based tunnel structure exhibits favorable air stability, it is still susceptible to the side effects of surface residual alkali. In a recent investigation, Xiao et al.74 successfully mitigated this issue by constructing a Ti-containing epitaxial stabilization layer. Meanwhile, they prepared a substantial quantity of industrial-grade Na0.44Mn0.85Ti0.15O2, and the assembled coin full cell and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 battery retained superior electrochemical properties (Fig. 4(g)). Although surface modification has yielded favorable results in enhancing the properties of Mn-based tunnel-structure cathode materials, the current range of reported coating materials and specific coating method remains limited. Therefore, it is imperative to utilize experiments and theoretical calculations to explore some new coating agents to potentially uncover unforeseen enhancements in electrochemical properties.
650 battery retained superior electrochemical properties (Fig. 4(g)). Although surface modification has yielded favorable results in enhancing the properties of Mn-based tunnel-structure cathode materials, the current range of reported coating materials and specific coating method remains limited. Therefore, it is imperative to utilize experiments and theoretical calculations to explore some new coating agents to potentially uncover unforeseen enhancements in electrochemical properties.
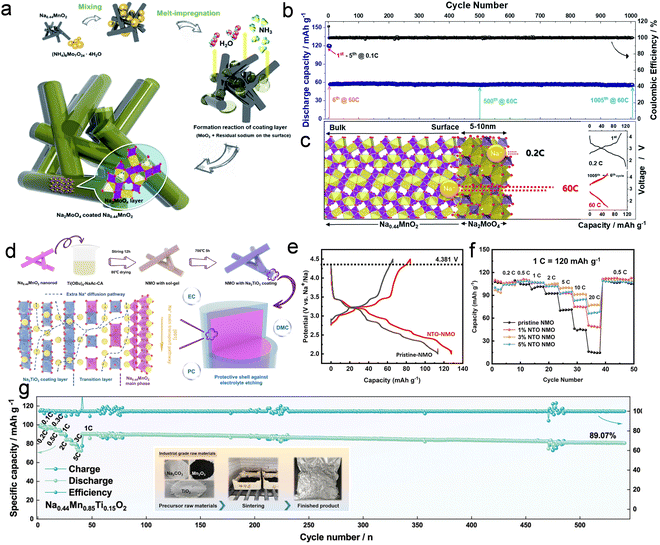 | ||
| Fig. 4 (a) Formation process for the Na2MoO4 coating on the surface of crystal particle in Na0.44MnO2 cathode. (b) Initial 5 cycles at 0.1C and long-term cyclability performance at 60C for the coated tunnel-structure cathode Na0.44MnO2. (c) Schematic of sodium-ion transfer behavior through the Na2MoO4 coating layer during the charge/discharge process. Reproduced with permission from ref. 72, Copyright (2019), the Royal Society of Chemistry. (d) Synthesis process of Na2TiO3-coated tunnel-structure cathode. (e) Charge/discharge curves of pristine tunnel structure and 3 wt% Na2TiO3-coated tunnel structure cathode at 0.1C from 2 to 4.5 V. (f) Rate property of varying amounts of Na2TiO3-coated between 2 and 4.3 V. Reproduced with permission from ref. 73, Copyright (2023), the American Chemical Society. (g) Rate and cycle performance of industrial-grade cathode material Na0.44Mn0.85Ti0.15O2 and the inset graph corresponding to the optical picture of the industrial sample. Reproduced with permission from ref. 74, Copyright (2024), Wiley-VCH. | ||
4.3 Composite structure construction
Although the Mn-based tunnel structure cathode material offers outstanding structural stability and electrochemical cycle stability, its sodium-ion storage capacity is relatively lower than that of the Mn-based layer-type structure cathode. To address this property limitation, a composite phase cathode material with a combined Mn-based layer-structure Na0.7MnO2 with high capacity and tunnel-structure Na0.44MnO2 with high cycle stability has been developed to obtain a synergistic effect.75,76 The reported literature presents two main distinct approaches for obtaining such layer-tunnel composite structure cathode materials: (1) the incorporation of heterogeneous ions into the Mn-based tunnel structure material leads to a partial transformation of tunnel-type structure into a layer-type structure during synthesis progress, resulting in the final formation of a hybrid phase consisting of both tunnel and layer structures. (2) Meticulous design of an appropriate sodium-ion content within the layer-tunnel composite structure and subsequent necessary modifications accordingly.Chen et al.77 synthesized a series of Na0.44Mn1−xCoxO2 cathode materials by doping Co3+, resulting in Co3+ substitution into a tunnel-structure cathode for a lower ratio of Mn3+. It was observed that an increase in Co content with a strong preference for octahedral coordination ions led to a transition from a tunnel structure to a layer structure and the formation of a layer-tunnel composite structure. The part of the tunnel-type crystal structure could transform into the P2-type layer structure when the sodium content is 0.44 in Na0.44Mn0.92Co0.08O2 compound, with further increase in Co content for Na0.44Mn0.89Co0.11O2 compound, in which the P2 layer structure would be more thermodynamically stable in the final chemical product. Subsequently, the same research team introduced nickel ions and magnesium ions into the Mn-ion site of the tunnel structure Na0.44MnO2.78 It was observed that as the introduction of nickel ions or magnesium ions increased in the structure, the Mn-based tunnel structure underwent a crystal structure transformation into a layer structure. Within a certain range of heterogeneous metal ion contents, a stabilized layer-tunnel composite structure can be achieved using a conventional thermopolymerization method. The specific discharge capacity of Na0.44Mn0.89Mg0.11O2 with tunnel-layer hybrid phase composition can achieve 188 mA h g−1 within the voltage range of 2.0–4.2 V at 0.1C, exhibiting a capacity retention of 81% after 70 cycles. Nickel-ion or magnesium-ion substitutions result in smoother charge/discharge curves compared to that of Na0.44MnO2 and can significantly enhance the overall capacity albeit with a slight reduction in specific capacity retention when compared to the pristine tunnel-structure cathode. Avci et al.79 synthesized a composite-phase material by substituting trace amounts of Co into Na0.44MnO2, resulting in a layer-tunnel composite phase cathode (Na0.44MnO2/Na0.7MnO2.05) that effectively prevents phase transformations and structure degradation for superior electrochemical performance. The 1% Co-substituted composite material alleviates the Jahn–Teller effect and significantly improves the stability of its crystal structure. Furthermore, the same research team created another series of tunnel/P2 hybrid structures through a one-step heat treatment with Ni substitution at Mn sites.80 These structures maintained high cycling stability and Coulombic efficiency similar to pristine T-Na0.44MnO2 while suppressing phase transition during charge/discharge cycles, ultimately resulting in superior cycling performance. Electrochemical analysis reveals that Na0.44MnO2 exhibits a capacity retention of 77% after 100 cycles at a rate of 0.3C, while Ni-substituted cathode materials demonstrate capacity retentions of approximately 86.4% and 77.3%.
Cao81 then introduced a small amount of high-valence tungsten (W) ions into Na0.44MnO2, resulting in the efficient conversion of the tunnel structure to the layer structure for the first time. Compared with other reported doped metal ions, W ions can induce the phase transformation of Na0.44MnO2 at a relatively low doping concentration. When the proportion of tungsten (W) in the total amount of transition metal achieves 1%, the tunnel structure Na0.44MnO2 undergoes a phase transformation, which is fully converted to pure P2-layer Na0.44Mn0.99W0.01O2 during synthesis, demonstrating an enhanced reversible capacity of 195.5 mA h g−1, along with exceptional cycling stability for capacity retention of 80% after 200 cycles. This provides a novel approach for engineering transition metal oxides to induce the conversion from tunnel structure to layer structure. In addition to metal–cation substitution, Liu et al.68 also systematically explored and investigated the introduction of fluorine ions into the structural oxygen in Mn-based tunnel structure cathode materials. They found that Na0.44MnO1.93F0.07 cathode exhibited a layer structure and tunnel structure composite phase within the temperature range of 800–1100 °C after optimizing temperature conditions to obtain cathode materials with relatively superior electrochemical performance. The optimized Na0.44MnO1.93F0.07 cathode exhibits higher electrochemical activity than that of the primary tunnel-phase cathode Na0.44MnO2, resulting in an exceptional discharge capacity of 149 mA h g−1 at a rate of 0.5C. Furthermore, the cathode demonstrates remarkable cycling stability with a capacity retention of around 79% over 400 cycles at a rate of 5C.
In addition to the aforementioned crystal structure transformation from an Mn-based tunnel structure to a layer structure, this results in the easy formation of the cathode material in the layer-tunnel composite structure. The Na/TM ratio is commonly 0.44 in the Mn-based tunnel structure, which enhances the thermodynamic stability of the tunnel structure during formation. Meanwhile, the Na/TM ratio in Mn-based layer oxide structures typically ranges around 0.7, making it easier to form a composite structure consisting of both layer and tunnel structures by directly setting the Na/TM ratio between 0.4 and 0.70.16,82 In 2017, Wu et al.83 successfully constructed a layer-tunnel composite cathode material Na0.6MnO2 by carefully designing a Na–Mn–O system cathode material with a Na/Mn ratio of 0.6, which exhibited excellent electrochemical performance. The underlying mechanism of the layer-tunnel composite structure cathode material is also elucidated (Fig. 5(a) and (b)). Subsequent studies regulated the composition ratio of layer-tunnel composite structures through metal Ni-ion substitution.84 Additionally, other transition metal ions, such as Cu,85 Fe,86 Zr87 and Mg,88 have been explored owing to their application as substitution ions for Mn-ion sites or Na-ion sites in layer-tunnel composite structure research. Xiao et al.89 utilized the thermal polymerization technique combined with the solid-state method to fabricate cathode materials featuring a composite structure of layer-tunnel intergrowth. The well-established layer-tunnel intergrowth structure cathode exhibited a satisfying reversible discharge capacity of 198.2 mA h g−1 at a rate of 0.2C, resulting in a remarkable high-energy density of 520.4 W h kg−1. Wang et al.90 investigated the regulatory mechanism of different elements on cathode materials with layer-tunnel composite structures and found that introducing iron ions promoted an increase in the proportion of layer structures, while titanium-ion introduction increased the proportion of tunnel structure within layer-tunnel composites. Introducing equal proportions of iron ions and titanium ions still facilitated transformation from layer to tunnel structure during the high-temperature calcination synthesis stage (Fig. 5(c)). This further indicates that different metal ions exhibit certain variances in regulating the layer-tunnel composite structure cathode materials, which can enable targeted phase regulation by utilizing distinct metal ions into the crystal texture. Since then, some studies on the design and construction of the particle layers of layer-tunnel cathode materials have been reported.91 In a recent study, Xiao et al.92 successfully prepared a range of Ti-substituted Na2/3Mn1−xTixO2 cathode materials by manipulating the dynamic structural evolution, leading to a transition from the P2-Na2/3MnO2 layered structure to tunnel structure with increasing Ti-ion content (Fig. 5(d)). The optimized Ti-substituted cathode material Na2/3Mn8/9Ti1/9O2 demonstrated a discharge capacity of 202.9 mA h g−1 at 0.1C within a voltage range of 1.5–4.3 V, resulting in an energy density of 536.6 W h kg−1 and exhibiting 71.0% capacity retention after undergoing 300 cycles at 1C. Furthermore, advanced characterization techniques, including in situ XRD analysis, confirmed a highly reversible phase transition process between P2/tunnel-OP4/tunnel structures and an interlocking effect between the layer and tunnel structures as well as remarkable moisture stability even after exposure to water treatment.
 | ||
| Fig. 5 (a) Sodium-ion migration barriers and the distance between oxygen and sodium migration in tunnel-type structure and layer-type structure NaxMnO2. (b) Schematic of the synergistic effect of the layer-tunnel composite structure. Reproduced with permission from ref. 83, Copyright (2017), the American Chemical Society. (c) Fe/Ti-ion doping induced a crystal structure formation process to achieve controlled synthesis of the layer-tunnel composite structure cathode. Reproduced with permission from ref. 90, Copyright (2020), Elsevier. (d) Schematic of the dynamic evolution of the atomic arrangement for Na2/3Mn1−xTixO2 cathode materials with different Ti-ion contents. Reproduced with permission from ref. 92, Copyright (2024), Elsevier. | ||
Overall, significant progress has been made in layer-tunnel composite structure cathode materials, especially because breaking through the theoretical capacity of the original tunnel structure cathode materials is not sufficiently high. Some more accurate tuning strategies for tunnel-layer composite structure cathode materials need to be further developed, and in-depth studies in this area have great research potential. The modification strategies extensively utilized for cathode materials featuring an Mn-based tunnel structure Na0.44MnO2 are comprehensively illustrated in Fig. 6.
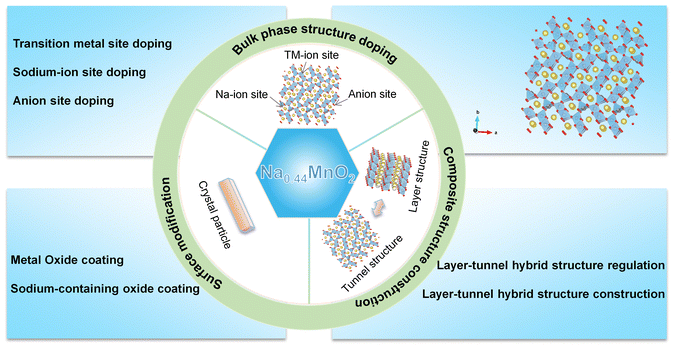 | ||
| Fig. 6 Summary diagram of the modification strategies for tunnel-structured Na0.44MnO2 cathode material in SIBs. | ||
5. Conclusion and perspective
Due to the promising application prospects of SIBs in large-scale energy storage systems and the substantial market demand in the near future, the research and development of high-performance and cost-effective cathode materials will play a pivotal role in optimizing both the overall cost and performance of SIBs. As a potential candidate for cathode material, Mn-based tunnel structure cathode material Na0.44MnO2 possesses remarkable advantages, such as exceptional stability, high-power output, and low production costs. Despite significant progress achieved in developing SIBs utilizing Mn-based tunnel-structure cathode materials, there is still room for further improvement and enhancement of their inherent merits, including a stable crystal structure, outstanding cycle durability, and impressive rate capability. However, challenges such as insufficient specific capacity persist, resulting from limited sodium-ion mobility within the intrinsic structure, instability of the crystal structure over extended cycles, and capacity degradation caused by the Jahn–Teller effect. In this comprehensive review article, we systematically summarize and discuss various aspects related to electrochemical sodium-ion storage mechanisms and controlled synthesis techniques for material fabrication, and modification strategies employed for Mn-based tunnel structures and propose some potential directions for future research trends.Overall, the future research and development of tunnel structure cathode materials can be concentrated on the following aspects: (1) developing more effective modification strategies to mitigate the impact of the Jahn–Teller effect on trivalent manganese ions within the tunnel structure, while optimizing the surface structure of crystal particles to control the formation of inactive substances. Further utilization of versatile characterization strategies and research methodologies is vital for elucidating the underlying electrochemical mechanisms of these modification methods, thereby facilitating the attainment of more stable crystal structures and enhanced electrochemical properties. (2) Designing high-sodium-content Mn-based cathode materials or exploring additional sodium sources for chemical pre-sodiation solutions to compensate for insufficient sodium-ion content in tunnel-structure cathodes can enhance the initial Coulomb efficiency when directly assembling a full cell with a hard carbon anode. Alternatively, conducting a targeted search for chemically presodiated hard carbon anodes that exhibit good compatibility with the cathode material of the Mn-based tunnel structure can further optimize the first Coulomb efficiency in the full cell, thereby maintaining the specific discharge capacity performance of the tunnel-structure cathode materials. (3) Developing scalable synthesis strategies for producing Mn-based tunnel-type cathode materials with a stable crystal structure and uniform morphological characteristics, which contribute to improved electrochemical performance and cost reduction. These efforts will facilitate the development of low-cost Mn-based tunnel-structure cathode materials from fundamental research to practical industry applications.
Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Postdoctoral Fellowship Program of CPSF: (grant no. GZC20233310), China Postdoctoral Science Foundation (grant no. 2024M753844), Natural Science Foundation of Chongqing (grant no. CSTB2024NSCQ-MSX0585), and The Special Grant for Chongqing Postdoctoral Research Project (grant no. 2023CQBSHTB3007).Notes and references
- Z. W. Yang Yang, C. Du, B. Wang, X. Li, S. Wu, X. Li, X. Zhang, X. Wang, Yaoshen Niu, Feixiang Ding, Xiaohui Rong, Yaxiang Lu, Nian Zhang, Jg Xu, Ruijuan Xiao, Qinghua Zhang, Xuefeng Wang, Wen Yin, J. Zhao, Liquan Chen, Jianyu Huang and Yong-Sheng Hu, Science, 2024, 385, 744–752 CrossRef PubMed.
- X. Jin, X. Zhang, P. Zhang, S. Rohani, H. Li, M. He, L. Teng, Q. Liu and W. Liu, Hydrometallurgy, 2024, 224, 106243 CrossRef CAS.
- C. Zhao, Q. Wang, Z. Yao, J. Wang, B. Sánchez-Lengeling, F. Ding, X. Qi, Y. Lu, X. Bai, B. Li, H. Li, A. Aspuru-Guzik, X. Huang, C. Delmas, M. Wagemaker, L. Chen and Y. S. Hu, Science, 2020, 370, 708–711 CrossRef CAS.
- M. He, W. Liu and Z. Yang, Chem. Commun., 2023, 59, 10785–10788 RSC.
- M. He, X. Jin, X. Zhang, X. Duan, P. Zhang, L. Teng, Q. Liu and W. Liu, Green Chem., 2023, 25, 6561–6580 RSC.
- M. He, X. Zhang, H. Li, X. Jin, L. Teng, Q. Liu and W. Liu, J. Environ. Chem. Eng., 2023, 11, 111099 CrossRef CAS.
- Y. Liu, D. Wang, H. Li, P. Li, Y. Sun, Y. Liu, Y. Liu, B. Zhong, Z. Wu and X. Guo, J. Mater. Chem. A, 2022, 10, 3869–3888 RSC.
- S. Jia, S. Kumakura and E. McCalla, Energy Environ. Sci., 2024, 17, 4343–4389 RSC.
- D. Wang, C. Zhu, Y. Liu, C. Hu, H. Yang, Z. Li, T. Chen, B. Zhong, Z. Wu and X. Guo, ACS Appl. Mater. Interfaces, 2024, 16, 24442–24452 CrossRef CAS.
- R. Umezawa, Y. Tsuchiya, T. Ishigaki, H. B. Rajendra and N. Yabuuchi, Chem. Commun., 2021, 57, 2756–2759 RSC.
- Y. Liang, H. Xu, K. Jiang, J. Bian, S. Guo and H. Zhou, Chem. Commun., 2021, 57, 2891–2894 RSC.
- J. Wang, Y. F. Zhu, Y. Su, J. X. Guo, S. Chen, H. K. Liu, S. X. Dou, S. L. Chou and Y. Xiao, Chem. Soc. Rev., 2024, 53, 4230–4301 RSC.
- N. Zhang, X. Dong, Q. Yan, J. Wang, F. Jin, J. Liu, D. Wang, H. Liu, B. Wang and S. Dou, Energy Storage Mater., 2024, 72, 103734 CrossRef.
- X. Zhou, J. Hu, S. Ajmal, D. Xiang, Z. Sun, W. Chen, M. Zhu, P. Chen and P. Li, Chem. Commun., 2023, 59, 12152–12155 RSC.
- B. J. Xin and X. L. Wu, Battery Energy, 2024, 3, 20230074 CrossRef.
- D. Wang, Y. H. Liu, Z. G. Wu, Y. L. Liu, C. Q. Zhu, B. H. Zhong and X. D. Guo, Chem. Eng. J., 2023, 462, 141994 CrossRef CAS.
- T. Zhang, M. Ren, Y. Huang, F. Li, W. Hua, S. Indris and F. Li, Angew. Chem., Int. Ed., 2024, 63, e202316949 CrossRef CAS.
- D. Wang, Y. Liu, Z. Wu, X. Liu, J. Qu, H. Liu, Y. Ming, Y. Zhong, B. Zhong and X. Guo, Chem. Commun., 2020, 56, 2921–2924 RSC.
- J. Liu, W. Huang, R. Liu, J. Lang, Y. Li, T. Liu, K. Amine and H. Li, Adv. Funct. Mater., 2024, 34, 2315437 CrossRef CAS.
- K. H. Wong, M. Zhang, T. Yang, Q. Ma, S. Dai, J. Wei, G. K. Veerasubramani, A. A. AlHammadi, G. Karanikolos, E. Bekyarova, A. Elkamel and A. Yu, Energy Storage Mater., 2024, 71, 103549 CrossRef.
- H. Hirsh, M. Olguin, H. Chung, Y. Li, S. Bai, D. Feng, D. Wang, M. Zhang and Y. S. Meng, J. Electrochem. Soc., 2019, 166, A2528–A2535 CrossRef CAS.
- Y. Wang, T. Yang, J. Liu, T. Zhang, Y. Zhang, Y. Gao, Z. Sun, S. Jia, L. Yang and Z. Chen, Nano Energy, 2024, 131, 110260 CrossRef CAS.
- Y. Cao, M. Xiao, X. Sun, W. Dong and F. Huang, Chemistry, 2023, 29, e202202997 CrossRef CAS PubMed.
- J. Feng, S. Luo, K. Cai, S. Yan, Q. Wang, Y. Zhang and X. Liu, Chin. Chem. Lett., 2022, 33, 2316–2326 CrossRef CAS.
- Y. Wang, L. Mu, J. Liu, Z. Yang, X. Yu, L. Gu, Y. S. Hu, H. Li, X. Q. Yang, L. Chen and X. Huang, Adv. Energy Mater., 2015, 5, 1501005 CrossRef.
- C. Liu, L. Jia, J. Li, Y. Wu and B. Zhang, Mater. Lett., 2022, 308, 131102 CrossRef CAS.
- R. O. Jean-Paul Parant, M. Devalette, C. Fouassier and E. Paul Hagenmuller, J. Solid State Chem., 1971, 3, 1–11 CrossRef.
- M. Y. P. Marca, M. Doeff, Y. Ma and L. C. De Jonghe, J. Electrochem. Soc., 1994, 141, 145–147 CrossRef.
- S. Xu, Y. Wang, L. Ben, Y. Lyu, N. Song, Z. Yang, Y. Li, L. Mu, H. T. Yang, L. Gu, Y. S. Hu, H. Li, Z. H. Cheng, L. Chen and X. Huang, Adv. Energy Mater., 2015, 5, 1501156 CrossRef.
- X. Zhang, J. Chen, J. Ye, T. Zhang and Z. Hou, Adv. Energy Mater., 2023, 13, 2204413 CrossRef CAS.
- S. Guo, H. Yu, D. Liu, W. Tian, X. Liu, N. Hanada, M. Ishida and H. Zhou, Chem. Commun., 2014, 50, 7998–8001 RSC.
- W.-J. Shi, H.-X. Li, D. Zhang, F.-H. Du, Y.-H. Zhang, Z.-Y. Wang, X.-H. Zhang and P.-F. Zhang, Chem. Eng. J., 2023, 477, 146976 CrossRef CAS.
- C. H. Rim, C. H. Jang, K. H. Kim, C. Ryu and C. J. Yu, Phys. Chem. Chem. Phys., 2022, 24, 22736–22745 RSC.
- S. Chakrabarty, J. A. Dar, A. Joshi, A. Paperni, S. Taragin, A. Maddegalla, G. Sai Gautam, A. Mukherjee and M. Noked, J. Mater. Chem. A, 2024, 12, 25109 RSC.
- B. Mandal, S. Chakrabarti and A. K. Thakur, Comput. Mater. Sci., 2024, 238, 112934 CrossRef CAS.
- S. Maddukuri, A. Nimkar, M. S. Chae, T. R. Penki, S. Luski and D. Aurbach, Front. Energy Res., 2021, 8, 615677 CrossRef.
- Z. Guo, Y. Zhao, Y. Ding, X. Dong, L. Chen, J. Cao, C. Wang, Y. Xia, H. Peng and Y. Wang, Chem, 2017, 3, 348–362 CAS.
- K. Wang, G. Guo, X. Tan, L. Zheng and H. Zhang, Chem. Eng. J., 2023, 451, 139059 CrossRef CAS.
- M. Soleimanzade, M. Radaelli, J. Manidi, M. Bahdanchyk and A. Vicenzo, Batteries, 2023, 9, 428 CrossRef CAS.
- M. S. Chae, Y. Elias and D. Aurbach, ChemElectroChem, 2021, 8, 798–811 CrossRef CAS.
- H. Kim, D. J. Kim, D.-H. Seo, M. S. Yeom, K. Kang, D. K. Kim and Y. Jung, Chem. Mater., 2012, 24, 1205–1211 CrossRef CAS.
- R. Qiao, K. Dai, J. Mao, T.-C. Weng, D. Sokaras, D. Nordlund, X. Song, V. S. Battaglia, Z. Hussain, G. Liu and W. Yang, Nano Energy, 2015, 16, 186–195 CrossRef CAS.
- M. S. Chae, H. J. Kim, H. Bu, J. Lyoo, R. Attias, B. Dlugatch, M. Oliel, Y. Gofer, S. T. Hong and D. Aurbach, Adv. Energy Mater., 2020, 10, 2000564 CrossRef CAS.
- Q. Liu, Z. Hu, M. Chen, Q. Gu, Y. Dou, Z. Sun, S. Chou and S. X. Dou, ACS Appl. Mater. Interfaces, 2017, 9, 3644–3652 CrossRef CAS PubMed.
- L. L. F. Sauvage, J.-M. Tarascon and E. Baudrin, Inorg. Chem., 2007, 46, 3289–3294 CrossRef.
- D. J. Kim, R. Ponraj, A. G. Kannan, H.-W. Lee, R. Fathi, R. Ruffo, C. M. Mari and D. K. Kim, J. Power Sources, 2013, 244, 758–763 CrossRef CAS.
- W. J. Shi, D. Zhang, X. M. Meng, C. X. Bao, S. D. Xu, L. Chen, X. M. Wang, S. B. Liu and Y. C. Wu, ACS Appl. Mater. Interfaces, 2020, 12, 14174–14184 CrossRef CAS.
- S. Demirel, E. Oz, E. Altin, S. Altin, A. Bayri, P. Kaya, S. Turan and S. Avci, Mater. Charact., 2015, 105, 104–112 CrossRef CAS.
- X. He, J. Wang, B. Qiu, E. Paillard, C. Ma, X. Cao, H. Liu, M. C. Stan, H. Liu, T. Gallash, Y. S. Meng and J. Li, Nano Energy, 2016, 27, 602–610 CrossRef CAS.
- Y. Zhang, Y. Ouyang, L. Liu, J. Xia, S. Nie, W. Liu and X.-Y. Wang, J. Cent. South Univ., 2019, 26, 1510–1520 CrossRef CAS.
- M. Xu, Y. Niu, C. Chen, J. Song, S. Bao and C. M. Li, RSC Adv., 2014, 4, 38140–38143 RSC.
- B. Fu, X. Zhou and Y. Wang, J. Power Sources, 2016, 310, 102–108 CrossRef CAS.
- K.-Y. Shen, M. Lengyel, L. Wang and R. L. Axelbaum, MRS Commun., 2017, 7, 74–77 CrossRef CAS.
- C. Ferrara, C. Tealdi, V. Dall’Asta, D. Buchholz, L. Chagas, E. Quartarone, V. Berbenni and S. Passerini, Batteries, 2018, 4, 8 CrossRef.
- N. Sheng, C.-g Han, Y. Lei and C. Zhu, Electrochim. Acta, 2018, 283, 1560–1567 CrossRef CAS.
- H. Zhao, L. Wu, J. Tian, D. Zhang, X. Li, S. Xu, L. Chen, Q. Yi, K. Dai and H. Guo, New J. Chem., 2023, 47, 3892–3902 RSC.
- W.-J. Shi, Y.-M. Zheng, X.-M. Meng, S.-B. Liu, S.-D. Xu, L. Chen, X.-M. Wang and D. Zhang, ChemElectroChem, 2021, 8, 798–811 CrossRef.
- X. L. Li, J. Bao, Y. F. Li, D. Chen, C. Ma, Q. Q. Qiu, X. Y. Yue, Q. C. Wang and Y. N. Zhou, Adv. Sci., 2021, 8, 2004448 CrossRef CAS.
- H. Zhang, Y. Xiang, B. Liu, G. Li, C. Dun, H. Huang, Q. Zou, L. Xiong and X. Wu, J. Colloid Interface Sci., 2024, 661, 389–400 CrossRef CAS PubMed.
- T. Cui, X. Li, Y. Si and Y. Fu, Energy Storage Mater., 2024, 65, 103161 CrossRef.
- H. Zhonge, J. Yuxuan, J. Wang, L. Yuhua, Z. Wenqing, J. Hongyuqi, S. Yongqiang, W. Xianwen and X. Yanhong, J. Solid State Chem., 2024, 329, 124415 CrossRef.
- J. Chen, Z. Hou, L. Zhang, W. Mao, T. Zhang, X. Zhang and Y. Qian, Inorg. Chem. Front., 2023, 10, 841–849 RSC.
- N. Floros, C. Michel, M. Hervieu and B. Raveau, J. Solid State Chem., 2001, 162, 34–41 CrossRef CAS.
- J. Zhang, H. Yuan, Y. Huang, S. Kan, Y. Wu, M. Bu, Y. Liu, P. He and H. Liu, Chem. Eng. J., 2021, 417, 128097 CrossRef CAS.
- Z.-G. Wu, Y.-J. Zhong, J.-T. Li, K. Wang, X.-D. Guo, L. Huang, B.-H. Zhong and S.-G. Sun, RSC Adv., 2016, 6, 54404–54409 RSC.
- R. Ruiz, C. Perez-Vicente and R. Alcantara, Dalton Trans., 2024, 53, 4814–4822 RSC.
- Q.-C. Wang, Q.-Q. Qiu, N. Xiao, Z.-W. Fu, X.-J. Wu, X.-Q. Yang and Y.-N. Zhou, Energy Storage Mater., 2018, 15, 1–7 CrossRef.
- W.-J. Shi, Y.-W. Yan, C. Chi, X.-T. Ma, D. Zhang, S.-D. Xu, L. Chen, X.-M. Wang and S.-B. Liu, J. Power Sources, 2019, 427, 129–137 CrossRef CAS.
- T.-E. Fan, S.-M. Liu, X. Tang, Z.-H. Zou, J.-Q. Xie and M.-Y. Wang, Ionics, 2021, 27, 1137–1142 CrossRef CAS.
- Y. Zhang, L. Liu, S. Jamil, J. Xie, W. Liu, J. Xia, S. Nie and X. Wang, Appl. Surf. Sci., 2019, 494, 1156–1165 CrossRef CAS.
- W. Liu, Q. Ren, M. Yang, L. Liu, Y. Zhang, D. Su, J. Wen, Q. Wang, X. Wang and Y. Feng, J. Alloys Compd., 2022, 896, 163087 CrossRef CAS.
- J. U. Choi, J. H. Jo, C.-H. Jo, M. K. Cho, Y. J. Park, Y. Jin, H. Yashiro and S.-T. Myung, J. Mater. Chem. A, 2019, 7, 13522–13530 RSC.
- Y. Cao, M. Xiao, W. Dong, T. Cai, Y. Gao, H. Bi and F. Huang, ACS Appl. Mater. Interfaces, 2023, 15, 40469–40477 CrossRef CAS.
- H. Liu, L. Kong, H. Wang, J. Li, J. Wang, Y. Zhu, H. Li, Z. Jian, X. Jia, Y. Su, S. Zhang, J. Mao, S. Chen, Y. Liu, S. Chou and Y. Xiao, Adv. Mater., 2024, e2407994, DOI:10.1002/adma.202407994.
- Y. Su, N. N. Zhang, J. Y. Li, Y. Liu, H. Y. Hu, J. Wang, H. Li, L. Y. Kong, X. B. Jia, Y. F. Zhu, S. Chen, J. Z. Wang, S. X. Dou, S. Chou and Y. Xiao, ACS Appl. Mater. Interfaces, 2023, 15, 44839–44847 CrossRef CAS.
- J. Wang, Q.-Q. Sun, J. Yu, J.-X. Guo, N.-K. Mo, H.-W. Li, Y. Su, S. Zhao, Y.-F. Zhu, H. Chu, S. Dou and Y. Xiao, Composites, Part B, 2024, 284, 111664 CrossRef CAS.
- Y.-T. Zhou, X. Sun, B.-K. Zou, J.-Y. Liao, Z.-Y. Wen and C.-H. Chen, Electrochim. Acta, 2016, 213, 496–503 CrossRef CAS.
- Y. Shao, Y.-T. Zhou, M.-M. Deng, Z.-F. Tang, J.-Y. Liao, H. J. M. Bouwmeester and C.-H. Chen, J. Solid State Electrochem., 2019, 23, 2979–2988 CrossRef CAS.
- E. Oz, S. Altin and S. Avci, ACS Omega, 2023, 8, 27170–27178 CrossRef CAS PubMed.
- E. Oz, S. Altin and S. Avci, J. Solid State Chem., 2023, 318, 123741 CrossRef CAS.
- Q. Ding, W. Zheng, A. Zhao, Y. Zhao, K. Chen, X. Zhou, H. Zhang, Q. Li, X. Ai, H. Yang, Y. Fang and Y. Cao, Adv. Energy Mater., 2023, 13, 2203802 CrossRef CAS.
- T.-R. Chen, Z.-G. Wu, W. Xiang, E.-H. Wang, C.-J. Wu, M.-Z. Chen, X.-D. Guo and B.-H. Zhong, Ceram. Int., 2017, 43, 6303–6311 CrossRef CAS.
- Z. G. Wu, J. T. Li, Y. J. Zhong, X. D. Guo, L. Huang, B. H. Zhong, D. A. Agyeman, J. M. Lim, D. H. Kim, M. H. Cho and Y. M. Kang, ACS Appl. Mater. Interfaces, 2017, 9, 21267–21275 CrossRef CAS.
- H. Chen, Z. Wu, Z. Zheng, T. Chen, X. Guo, J. Li and B. Zhong, Electrochim. Acta, 2018, 273, 63–70 CrossRef CAS.
- T. R. Chen, T. Sheng, Z. G. Wu, J. T. Li, E. H. Wang, C. J. Wu, H. T. Li, X. D. Guo, B. H. Zhong, L. Huang and S. G. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 10147–10156 CrossRef CAS.
- Z. Sun, B. Peng, L. Zhao, J. Li, L. Shi and G. Zhang, Energy Storage Mater., 2021, 40, 320–328 CrossRef.
- J. Qu, T. Sheng, Z.-G. Wu, T.-R. Chen, H. Chen, Z.-G. Yang, X.-D. Guo, J.-T. Li, B.-H. Zhong and X.-S. Dou, J. Mater. Chem. A, 2018, 6, 13934–13942 RSC.
- J. Qu, D. Wang, Z. G. Yang, Z. G. Wu, L. Qiu, X. D. Guo, J. T. Li, B. H. Zhong, X. C. Chen and S. X. Dou, ACS Appl. Mater. Interfaces, 2019, 11, 26938–26945 CrossRef CAS.
- Y. Xiao, P. F. Wang, Y. X. Yin, Y. F. Zhu, X. Yang, X. D. Zhang, Y. Wang, X. D. Guo, B. H. Zhong and Y. G. Guo, Adv. Energy Mater., 2018, 8, 1800492 CrossRef.
- D. Wang, C. Shi, Y.-P. Deng, Z. Wu, Z. Yang, Y. Zhong, Y. Jiang, B. Zhong, L. Huang, X. Guo and Z. Chen, Nano Energy, 2020, 70, 104539 CrossRef CAS.
- D. Wang, Y.-P. Deng, Y. Liu, Y. Jiang, B. Zhong, Z. Wu, X. Guo and Z. Chen, Nano Energy, 2023, 110, 108340 CrossRef CAS.
- Z.-C. Jian, Y.-F. Liu, Y.-F. Zhu, J.-Y. Li, H.-Y. Hu, J. Wang, L.-Y. Kong, X.-B. Jia, H.-X. Liu, J.-X. Guo, M.-Y. Li, Y.-S. Xu, J.-F. Mao, S.-L. Zhang, Y. Su, S.-X. Dou, S.-L. Chou and Y. Xiao, Nano Energy, 2024, 125, 109528 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |
