C, N co-doping promoted mesoporous Au/TiO2 catalyst for low temperature CO oxidation
Liang Li*,
Binghan Wu,
Gengnan Li and
Yongsheng Li
Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: liliang@ecust.edu.cn; Fax: +86-021-64250740; Tel: +86-021-64252599
First published on 14th March 2016
Abstract
Mesoporous C, N co-doped TiO2 was fabricated by a one pot pyrolysis method using ammonium titanyl oxalate as the precursor. When deposited with Au, the resulting materials possessed a relatively high surface area and highly dispersed gold nano-particles, exhibiting high catalytic activities for CO oxidation. The doping of C and N into meso-structured TiO2 increases the number of surface defect which could improve the absorption of oxygen, tune the metal-support interaction and promote the catalytic activities for CO oxidation.
1. Introduction
CO is the main component of outdoor air contamination, by which the problem that is caused is becoming more and more serious and detrimental to the environment and humans' health. It generated mainly by industrial processes and the popularity of the automobile. In general conditions, disposal of CO needs a high temperature and energy consumption. Catalytic oxidation is found to be an effective method to eliminate this kind of air pollutant. Among various developed catalysts, supported noble metals have achieved intense attention, such as Pd,1,2 Pt3 and Ag,4–6 which have been shown to be highly active for CO oxidation. Gold has been believed non-effective until Haruta found support gold with particle size less than 5 nm possessed high catalytic activity for CO oxidation.7,8Besides, the synergism between active noble metal component and support also plays an important role in determining the catalytic activities.9–14 In this regard, TiO2 as support has been the focus for its properties of bulk defect and reduced surface, which are significant in catalysis.15 Au/TiO2 catalyst has been extensively studied in the past two decades. Rodolfo et al. prepared Au/TiO2 using urea as precipitator to promote the gold dispensability. The influence of thermal treatment in preparation on the size of gold particles supported on TiO2 had been well studied.16,17 Dekkers et al. found that the pretreatment, oxidation or reduction, impacted the Au/TiO2 catalytic activity for CO oxidation.18 All these literatures suggested that the activity of the TiO2 supported gold catalysts is strongly influenced by the preparation method and the pretreatment conditions. These may be concerned with the surface defects of support, which could promote the absorption of the oxygen and tune metal-support interaction resulting in the outstanding catalyst for CO oxidation. The modification of TiO2 through doping could also add the oxygen vacancies and change the TiO2 structure. For so long, doped TiO2 has been widely applied for photo-catalysis and studied in depth, very few studies has been reported about doped TiO2 as support for CO catalytic oxidation.19–23
Herein, we report the facile synthesis of C, N co-doped mesoporous TiO2 supported Au catalyst for CO oxidation. In such a protocol, ammonium titanyl oxalate was used as precursor. After calcination and deposited with Au, the catalytic activities for CO oxidation was evaluated. The as-prepared materials processed high surface area and exhibited high catalytic activities for CO oxidation. The doping of C and N into meso-structured TiO2 increases the number of surface defect which improve the absorption of the oxygen, tune the metal-support interaction and promote the catalytic activities.
2. Experimental
2.1 Materials preparation
Ammonium titanyl oxalate precursor was prepared as reported before.19 In a typical procedure, 25 ml concentrated hydrochloric acid (d = 1.19 g cm−3), 11 ml TiCl4 and 0.21 mol oxalic acid were mixed with 35 ml water. After dissolving at 80 °C, 55 ml of 25% ammonia solution was added to neutralize the mixture until the white precipitate dissolved. Then, 55 ml ethanol was added. The precipitate was filtered and washed with dilute ethanol. The resulting crystals were purified by recrystallization. C, N co-doped mesoporous TiO2 was obtained through calcination of the precursor at 250 °C, 300 °C and 350 °C respectively for 4 h with the heating rate of 0.5 °C min−1.A series of C, N co-doped mesoporous TiO2 supported Au catalysts were synthesized by deposition–precipitation using urea as precipitant. 0.5 g support was added into 25 ml solution containing calculated content of HAuCl4. Then urea was added into the mixture under stirring at 80 °C for 8 h. The precipitate was obtained by centrifugation, washed with deionized water, dried at 80 °C and calcined at 200 °C for 4 h.
2.2 Characterization
Powder X-ray diffraction (XRD) patterns were performed on a Bruker D8 Focus powder diffract meter with Cu Kα radiation (λ = 0.15405 nm) operated at 40 kV. Transmission Electron Microscopy (TEM) observations were performed on a field emission JEM-2100 (JEOL) electron microscope operated at 300 kV equipped with a Gatan-666 electron energy loss spectrometer and energy dispersive X-ray spectrometer. Nitrogen adsorption and desorption isotherms were measured on a Micromeritics ASAP 2020M analyzer at 77 K. The samples were degased at 373 K in vacuum for 6 h. The specific surface area and pore size distribution were calculated using the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively. H2 temperature-programmed reduction (H2-TPR) analysis was analyzed using a Micromeritics Chemisorb 2750 apparatus. For each analysis, accurate amounts of calcined sample (50 mg) were purged in a flow of pure argon at 473 K for 30 min to remove traces water (heating rate 10 °C min−1). After cooling to room temperature, H2-TPR experiments were performed using a 5 vol% H2/Ar mixture at a flow rate of 25 ml min−1. The sample was heated from ambient temperature to 700 °C at heating rate of 10 °C min−1 and H2 consumption was detected by a thermal conductivity detector (TCD). Laser Raman spectra of the samples were collected under ambient conditions on a LabRAM HR-800 spectrometer. A laser beam (λ = 532 nm) was used for the excitation. The laser beam intensity and spectrum slit width were 2 mW and 3.5 cm−1, respectively. X-ray photoelectron spectroscopy signals were collected on a VG Micro MK II instrument using monochromatic Al Kα X-ray at 1486.6 eV operated at 200 W. All the elemental binding energies were referenced to the C (1s) line situated at 284.6 eV. In situ diffuse reflectance infrared Fourier transform spectroscopy analysis (DRIFTS) was recorded on a Nicolet iS10 instrument equipped with a MCT detector. A chamber fitted with KBr windows was utilized as reactor. The typical gas mixture was 5 vol% CO, 20.0 vol% O2 balanced with N2.2.3 CO oxidation test
The catalytic test for CO oxidation was carried out in fixed-bed quartz tubular reactor (i.d. = 6 mm) containing 200 mg of catalyst samples without any pretreatment. A standard reaction gas containing 1.0 vol% CO, 20.0 vol% O2, and a high-purity N2 (99.99%) gas was used as sources to form desired gas mixture. The feed gas at a flow rate of 50 ml min−1 was introduced into the reactor, corresponding to a space velocity of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml h−1 gcat−1. The activity of catalyst was tested after the steady operation for 10 min. The conversion of CO was measured by online gas chromatograph (GC) equipped with a FID. The CO and CO2 gas were catalytically converted to CH4 by a Ni catalyst prior to FID measurement.
000 ml h−1 gcat−1. The activity of catalyst was tested after the steady operation for 10 min. The conversion of CO was measured by online gas chromatograph (GC) equipped with a FID. The CO and CO2 gas were catalytically converted to CH4 by a Ni catalyst prior to FID measurement.
3. Results and discussion
3.1 Physicochemical properties of Au/TiO2 catalysts
A series of C, N co-doped mesoporous TiO2 were fabricated directly through pyrolysis of ammonium titanyl oxalate, the resulting materials were denoted as CNTi-250, CNTi-300 and CNTi-350 presenting the calcination temperature of 250 °C, 300 °C and 350 °C, respectively, after deposed with Au using deposition–precipitation method, the actual content of Au on TiO2 supports were analyzed by ICP-AES, which are all about 0.98 wt%. It proved that almost all of the Au content has been reduced and deposed on the TiO2 support. The commercial TiO2 (P25) supported Au catalyst with the same Au content (Au/P25) was also fabricated for comparison.The crystalline structure of C, N co-doped mesoporous TiO2 and the resulting Au/TiO2 catalyst were firstly characterized by X-ray diffraction (XRD) analysis. Fig. 1A shows the XRD patterns of the TiO2 supports fabricated at different temperatures. After pyrolysis, all the materials lost their original crystalline structures. The XRD pattern of the sample calcined at 250 °C only shows a broad diffraction halo at about 25° indicating an amorphous structure. While the materials calcined at 300 °C and 350 °C turned into anatase TiO2 (PDF card no. 21-1276) crystallites, and the intensity of the diffraction peak increase with the pyrolysis temperature, suggesting the increased crystallization. The XRD patterns of Au/TiO2 catalysts are described in Fig. 1B. A widen peak could be found emerged at 2 theta angle of 38° for that calcined at 250 °C, which could be assigned as (111) diffraction peak for Au (PDF card no. 4-784), proving the successful deposition of Au. The estimate Au particle size by Scherrer equation is only about 3 nm. However, due to the overlapping between diffraction peaks of Au (111) and anatase TiO2 (004), there only shows a relative increased peak intensity for that calcined at 300 and 350 °C.
The C, N co-doped TiO2 possesses meso-porous structure as detected by adsorption/desorption isotherms of nitrogen at 77 K (Fig. 2). With the increase of the calcination temperature, surface area decreases due to the growth of anatase TiO2 nanocrystals, which is in accordance with the above XRD analysis. When loaded with gold, mesoporous structure was still maintained. The typical Langmuir IV isotherms indicate the meso-porous structure and the specific surface area along with pore size show no obvious change, which demonstrates that gold nanoparticles have been uniformly dispersed on the supports. The morphology and Au distribution of the as-synthesized catalysts were characterized by the transmission electron microscopy (TEM), as shown in Fig. 3. Compared with Au/P25 catalyst (Fig. 3a), porous structure can be observed in all the Au/C, N co-doped TiO2 catalysts, which confirm that the loading process didn't influence their porous structure. Besides, Au NPs are highly dispersed within the mesoporous TiO2 supports. From the High Resolution Transmission Electron Microscopy (HRTEM) investigation of Au/CNTi-350 °C (Fig. 3e), the identification of Au and TiO2 is evidenced. The (111) plane of metallic Au and the (1 0 1) plane of TiO2 can be clearly distinguished in great agreement with XRD results. The average size of Au particles in Au/CNTi-350 catalyst obtained by measuring 10 particles from different regions is about 3.8 nm in diameter. The small size of gold nanoparticles consists well with above XRD results. The good dispersion and small Au NPs imply the enhanced catalytic performance of Au/C, N co-doped TiO2 catalysts. For comparison, the particle size distribution of Au within Au/P25 was also calculated. It is almost the same as that of Au/CNTi-350 due to the same deposition method (Fig. 4).
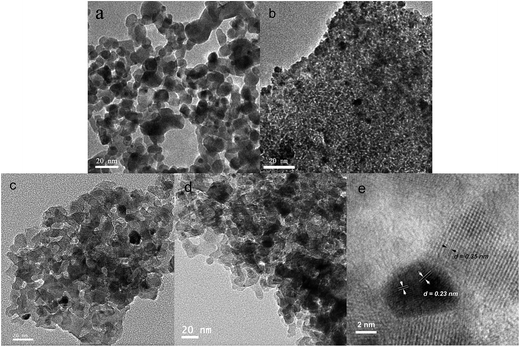 | ||
| Fig. 3 TEM images of Au/P25 (a), Au/CNTi-250 (b), Au/CNTi-300 (c), Au/CNTi-350 (d) and HRTEM image of Au/CNTi-350 (e). | ||
H2 temperature programmed reduction (H2-TPR) was performed to investigate the reducibility of Au/C, N co-doped TiO2 catalysts. For comparison, the profiles of each support and Au/P25 are also present, as shown in Fig. 5. For C, N co-doped TiO2 support, the dominant peak at about 600 °C can be identified as reduction of Ti4+ to Ti3+ according to the literatures.24 When deposed with Au catalyst, the main reduction peaks are all shifted to the relative lower temperatures indicating the activation of the TiO2 supports. Furthermore, the reduction temperature shows some decrease with the increased pyrolysis temperature for the preparation of the CNTi support. The Au/CNTi-350 processes the lowest reduction temperature at about 410 °C. On the other hand, pure TiO2 (commercial TiO2, P25) can hardly react with H2, it only shows a very weak signal and no reduction peak as expected. Although the main reduction single is enhanced and emerged at about 500 °C after deposed with Au, the quantitative measurement of the H2 consumption for Au/P25 is only 1/8 for that Au/CNTi-350. Comparatively, the C, N co-doped TiO2 is easily reduced than pure TiO2 (P25) whatever deposed with Au or not. Thus, Au/CNTi-350 has the highest redox properties and should have much higher catalytic activity during CO oxidation process.
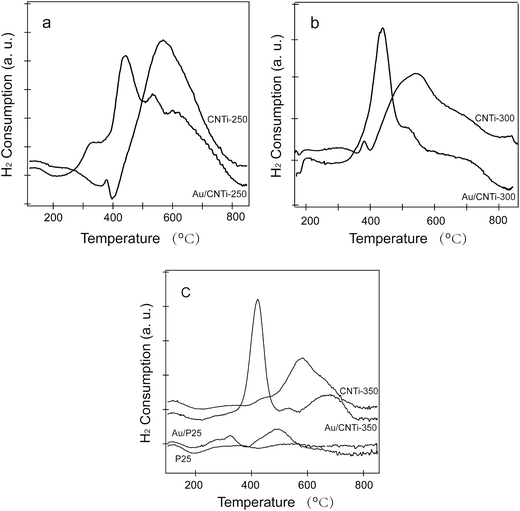 | ||
| Fig. 5 H2-TPR profiles of C, N co-doped TiO2 supports calcined at different temperatures and the corresponding Au/TiO2 catalysts. | ||
3.2 Catalytic properties
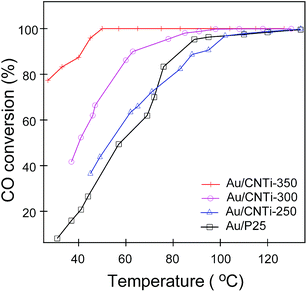
To evaluate the catalytic activities of Au/CNTi catalysts, the CO oxidation reaction was performed and the results are depicted in Fig. 6. It clearly shows the gradually enhanced catalytic activity with the increased calcination temperature. As the supports' calcination temperatures increase form 250 °C to 350 °C, the complete CO conversion temperature drop from 120 °C to 50 °C. Poor crystallinity of Au/CNTi-250 may account for its depressed catalytic activity. Meanwhile, Au/CNTi-350 possesses the excellent catalytic performance and CO conversion could reach to 80% even at room temperature (25 °C). For that Au deposed on commercial TiO2 (Au/P25), 1% CO could be complete oxidized at about 120 °C, much higher than that for Au/CNTi-350. Comparatively, Au/CNTi-350 shows the highest catalytic activities for CO oxidation. It has been proved previously that the TiO2 generated from the pyrolysis of ammonium titanyl oxalate could be doped with C and N heteroatoms during calcination process.19 The homogeneously doped heteroatoms may produce a lot of oxygen vacancies within the TiO2 structure and active the oxygen species in CO oxidation process. When combined with Au nanoparticles, the resulting material should have high catalytic activities for CO oxidation.To prove above speculation, Raman spectra were firstly used to preliminary investigate the oxygen deficiency within TiO2 structure, as shown in Fig. 7. Typically, anatase TiO2 has five typical Raman active modes at around 144 cm−1 (Eg), 197 cm−1 (Eg), 397 cm−1 (B1g), 518 cm−1 (A1g + B1g) and 640 cm−1 (Eg), respectively, while rutile phase locate at 144 cm−1 (B1g), 448 cm−1 (Eg), 613 cm−1 (A1g) and 827 cm−1 (B2g), respectively. The Au/P25 and as prepared Au/CNTi-350 °C all displayed clearly Raman feature at around 144 cm−1, 197 cm−1, 397 cm−1, 518 cm−1 and 640 cm−1, which can be clearly assigned as vibrational mode of anatase phase. Comparing to Au/P25, the Raman peaks feature for Au/CNTi-350 sample is somewhat wider and rougher and a newly emerged peak at about 810 cm−1 could be found. Besides, a slight Raman shift is also observed in Eg mode peaks, which support the assumption that some C or (and) N are inserted within titania framework and the formation of oxygen deficient structure.25 It should be note that the observed shifted and widened peak is also feasible for the decreased crystallite size. However, the rough of the Raman feature and the newly emerged Raman peak all could be assigned as the doping of the heteroatoms (N and C) and the formation of oxygen vacancy.26,27
To get further inside into its structure, especially the heteroatoms doping behavior, XPS analysis (Fig. 8) was used to investigate the surface chemical composition and the states of the elements in the as-prepared materials. Interestingly, N could be in situ doped during pyrolysis process even the calcination temperature lower than 400 °C. Fig. 8a shows the typical XPS spectra for the N 1s region of the sample Au/CNTi-350 and its fitting curves. There are two possibilities for the peak centered at 399.4 eV. One is N–H or adsorbed NH3 (the binding energy 398.7–399.7 eV), the other is Ti–O–N or Ti–N–O oxynitride. To exclude the possibility of NH3, the calcined samples in the present work were also analyzed by FTIR. No bands characteristic of N–H or NH3 were found. Thus, this peak can only be assigned as Ti–O–N or Ti–N–O oxynitride, indicating the successfully incorporated N atoms into the anatase TiO2 structure.28,29
 | ||
| Fig. 8 XPS spectra of TiO2 support calcined at 350 °C and corresponding Au loaded catalyst Au/CNTi-350. The Au XPS spectrum for Au/P25 is also presented for comparison. | ||
The C 1s XPS spectrum of the sample Au/CNTi-350 is shown in Fig. 8b. The spectrum shows a strong peak at 284.6 eV companied with two weak shoulders at around 286.3 eV and 288.4 eV, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O bonds, respectively. Generally, the C–O band at 288.4 eV could be considered as the formation of a Ti–O–C band due to the substitution of carbon for some of the lattice titanium atoms. It should be noted that this peak may also be contributed by the surface carbon black. For C/TiO2 system, carbon doping can lead to the formation of oxygen vacancies and Ti3+ defects and result in a slight shift of the Ti 2p peaks toward the lower binding energy. Hence, the red shift of the Ti 2p peak can be used to highlight the successful carbon doping into the TiO2 lattice. Thus, further analysis of the XPS Ti 2p spectra can help to investigate the carbon doping state. Fig. 8c shows the Ti 2p XPS spectra of the sample CNTi-350 and Au deposed samples. The core level binding energies of Ti 2p are about 458.0 eV for Ti 2p3/2 and 464.2 eV for Ti 2p1/2. The core level of Ti 2p XPS spectra clearly exhibit shifting towards lower binding energies compared with pure TiO2 (458.7 eV for Ti 2p3/2 and 464.5 eV for Ti 2p1/2) clearly revealing the successful carbon-doping behavior. Besides, the XPS spectrum of Au 4f5/2 and 4f7/2 at 86.8 and 83.2 eV with a constant separation of spin orbit coupling of 3.6 eV can be clearly observed for the as-prepared Au/CNTi-350 catalyst. It clearly indicates that the Au species are mainly present in the metallic state. The negative shift of Au0 4f7/2 (0.8 eV) may come from the strong interactions between Au and the TiO2 framework.30,31 To exclude the effect of the chemical state of Au species to the catalytic properties for CO oxidation, the XPS spectrum of Au element within Au/P25 was also present in Fig. 8d for comparison. It clearly shows that the Au species exist in metallic state just the same as that within Au/CNTi-350 structure. The presence of characteristic Au XPS binding energy bands also give a clearly evidence that Au has been successfully incorporated on the surface of TiO2 support.
O, C–O bonds, respectively. Generally, the C–O band at 288.4 eV could be considered as the formation of a Ti–O–C band due to the substitution of carbon for some of the lattice titanium atoms. It should be noted that this peak may also be contributed by the surface carbon black. For C/TiO2 system, carbon doping can lead to the formation of oxygen vacancies and Ti3+ defects and result in a slight shift of the Ti 2p peaks toward the lower binding energy. Hence, the red shift of the Ti 2p peak can be used to highlight the successful carbon doping into the TiO2 lattice. Thus, further analysis of the XPS Ti 2p spectra can help to investigate the carbon doping state. Fig. 8c shows the Ti 2p XPS spectra of the sample CNTi-350 and Au deposed samples. The core level binding energies of Ti 2p are about 458.0 eV for Ti 2p3/2 and 464.2 eV for Ti 2p1/2. The core level of Ti 2p XPS spectra clearly exhibit shifting towards lower binding energies compared with pure TiO2 (458.7 eV for Ti 2p3/2 and 464.5 eV for Ti 2p1/2) clearly revealing the successful carbon-doping behavior. Besides, the XPS spectrum of Au 4f5/2 and 4f7/2 at 86.8 and 83.2 eV with a constant separation of spin orbit coupling of 3.6 eV can be clearly observed for the as-prepared Au/CNTi-350 catalyst. It clearly indicates that the Au species are mainly present in the metallic state. The negative shift of Au0 4f7/2 (0.8 eV) may come from the strong interactions between Au and the TiO2 framework.30,31 To exclude the effect of the chemical state of Au species to the catalytic properties for CO oxidation, the XPS spectrum of Au element within Au/P25 was also present in Fig. 8d for comparison. It clearly shows that the Au species exist in metallic state just the same as that within Au/CNTi-350 structure. The presence of characteristic Au XPS binding energy bands also give a clearly evidence that Au has been successfully incorporated on the surface of TiO2 support.
The result discussed above confirms the successful doping of C and N heteroatoms within TiO2 structure. To shed the light on the effect of doping behavior on the reaction mechanism for CO oxidation, in situ DRIFTS analysis was performed to investigate CO oxidation process over Au/CNTi-350 catalyst (Fig. 9). When exposed under the condition including 5% CO and 95% N2 at room temperature, a series of infrared absorption peaks could be found as shown in Fig. 8a. According to the literatures, the bands at 1340 cm−1 and 1540 cm−1 can be assigned as νas(OCO) and νs(OCO) of [COOH].32–34 Peak at 1630 cm−1 is associated with [OH] groups.35,36 Other three peaks located at 2048 cm−1, 2119 cm−1 and 2200 cm−1, respectively, are concerned with an absorbed CO. The first two are attributed to adsorbed CO on different sites of metallic gold, while the third could be assigned as CO adsorption on titania.37–41 With the time adding from 2 to 60 min, the CO adsorption peaks gradually increased indicating the increased adsorption quantity of CO. Besides, it should be noted that the adsorption peak of OH shows slight decrease companied with the increase of COOH, suggesting one of the CO2 formation mechanism. OH group on the surface combine with absorbed CO to form the intermediate COOH, and then react with surface/lattice O to form CO2. The adsorption peak at 2340 cm−1 proved this mechanism. Upon 20% O2 was introduced into the feed gas (Fig. 8b), the CO adsorption bands, whatever on Au (2048 cm−1 and 2119 cm−1) or TiO2 (2200 cm−1), are all decrease with the increased reaction time. This may be caused not only by the competitive adsorption between oxygen and CO on the catalyst surface, but also concerned with the consumption of the absorbed CO to from CO2. All these clearly indicate that the reaction mechanism has not any change when C and N heteroatoms were doped within TiO2 structure.42 The enhanced CO oxidation properties only concerned with the oxygen deficiency within TiO2 structure originated by doping of the heteroatoms (C and N).
 | ||
| Fig. 9 In situ DRIFTS analysis of Au/CNTi-350 catalyst under different conditions: CO + N2 (A), CO + N2 + O2 (B). | ||
4. Conclusion
The meso-structured Au catalysts loaded on C, N co-doped TiO2 have been successfully synthesized by decomposition of ammonium titanyl oxalate and deposition–precipitation method. The as-prepared materials owned relatively high surface areas and highly dispersed gold particles. Doping of carbon and nitrogen generated more oxygen vacancies, which in favor of enhanced catalytic activity for CO oxidation. The Au catalyst supported on C, N co-doped TiO2 calcined at 350 °C possessed the highest catalytic activity during CO oxidation process. The total conversion temperature could be achieved at as low as 50 °C.Acknowledgements
This study was supported by National Basic Research Program of China 2013CB933201.References
- H. Q. Zhu, Z. F. Qin, W. J. Shan, W. J. Shen and J. G. Wang, J. Catal., 2004, 225, 267–277 CrossRef CAS.
- L. Q. Liu, F. Zhou, L. G. Wang, X. J. Qi, F. Shi and Y. Q. Deng, J. Catal., 2010, 274, 1–10 CrossRef CAS.
- M. D. Graham, I. G. Kevrekidis, K. Asakura, J. Lauterbach, K. Krischer, H.-H. Rotermund and G. Ertl, Science, 1994, 264, 80–82 CAS.
- R. Xu, X. Wang, D. S. Wang, K. B. Zhou and Y. D. Li, J. Catal., 2006, 237, 426–430 CrossRef CAS.
- S. F. Chen, J. P. Li, K. Qian, W. P. Xu, Y. Lu, W. X. Huang and S. H. Yu, Nano Res., 2010, 3, 244–255 CrossRef CAS.
- P. Bera, K. C. Patil and M. S. Hegde, Phys. Chem. Chem. Phys., 2000, 2, 3715–3719 RSC.
- M. Haruta, T. Kobayashi, H. Sano and N. Yamada, Chem. Lett., 1987, 16, 405–408 CrossRef.
- M. Haruta, N. Yamada, T. Kobayashi and S. Lijima, J. Catal., 1989, 115, 301–309 CrossRef CAS.
- M. Meng, Y. Q. Zha, J. Y. Luo, T. D. Hu, Y. N. Xie, T. Liu and J. Zhang, Appl. Catal., A, 2006, 301, 145–151 CrossRef CAS.
- V. Idakiev, T. Tabakova, K. Tenchev, Z. Y. Yuan, T. Z. Ren and B. L. Su, Catal. Today, 2007, 128, 223–229 CrossRef CAS.
- L. S. Zhong, J. S. Hu, Z. M. Cui, L. J. Wan and W. G. Song, Chem. Mater., 2007, 19, 4557–4562 CrossRef CAS.
- B. Zhang, J. Fang, J. G. Li, J. J. Lau, M. Davide, Z. Y. Zhong, J. P. Xie and N. Yan, Chem.–Asian J., 2016, 11, 532–539 CrossRef CAS PubMed.
- J. Fang, J. G. Li, B. Zhang, X. Yuan, A. Hiroyuki, T. Tsunehiro, T. Kentaro, J. P. Xie and N. Yan, Nanoscale, 2015, 7, 6325–6333 RSC.
- J. Y. Park, L. R. Baker and G. A. Somorjai, Chem. Rev., 2015, 115, 2781–2817 CrossRef CAS PubMed.
- M. V. Ganduglia-Pirovano, A. Hofmann and J. Sauer, Surf. Sci. Rep., 2007, 62, 219–270 CrossRef CAS.
- R. Zanella, S. Giorgio, C. H. Shin, C. R. Henry and C. Louis, J. Catal., 2004, 222, 357–367 CrossRef CAS.
- R. Zanella and C. Louis, Catal. Today, 2005, 107, 768–777 CrossRef.
- M. A. P. Dekkers, M. J. Lippits and B. E. Nieuwenhuys, Catal. Lett., 1998, 56, 195–197 CrossRef CAS.
- L. Li, J. J. Shi, G. N. Li, Y. Y. Yuan, Y. S. Li, W. R. Zhao and J. L. Shi, New J. Chem., 2013, 37, 451–457 RSC.
- S. Klosek and D. Raftery, J. Phys. Chem. B, 2001, 105, 2815–2819 CrossRef CAS.
- T. Umebayashi, T. Yamaki, S. Tanaka and K. Asai, Chem. Lett., 2003, 32, 330–331 CrossRef CAS.
- Q. C. Xu, D. V. Wellia, S. Yan, D. W. Liao, T. M. Lim, T. Timothy and T. Yang, J. Hazard. Mater., 2011, 188, 172–180 CrossRef CAS PubMed.
- K. C. Goddeti, S. M. Kim, Y. K. Lee, S. H. Kim and J. Y. Park, Catal. Lett., 2014, 144, 1411–1417 CrossRef CAS.
- J. A. Wang, A. Cuan, J. Salmones, N. Nava, S. Castillo, M. Moran-Pineda and F. Rojas, Appl. Surf. Sci., 2004, 230, 94–105 CrossRef CAS.
- X. D. Jiang, Y. P. Zhang, J. Jiang, Y. S. Rong, Y. C. Wang, Y. C. Wu and C. X. Pan, J. Phys. Chem. C, 2012, 116, 22619–22624 CAS.
- K. M. Parida, N. Sahu, A. K. Tripathi and V. S. Kamble, Environ. Sci. Technol., 2010, 44, 4155–4160 CrossRef CAS PubMed.
- S. Carrettin, Y. Hao, V. A. Guerrero, B. C. Gates, S. Trasobares, J. J. Calvino and A. Corma, Chem.–Eur. J., 2007, 13, 7771–7779 CrossRef CAS PubMed.
- G. B. Soares, B. Bravin, C. M. P. Vaz and C. Ribeiro, Appl. Catal., B, 2011, 103, 287–293 CrossRef.
- Z. Z. Zhang, X. X. Wang, J. L. Long, Q. Gu, Z. X. Ding and X. Z. Fu, J. Catal., 2010, 276, 201–214 CrossRef CAS.
- J. Li and H. C. Zeng, Chem. Mater., 2006, 18, 4270–4277 CrossRef CAS.
- H. X. Li, Z. F. Bian, J. Zhu, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 4538–4539 CrossRef CAS PubMed.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jing, Y. T. Cui, J. Y. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- Y. Denkwitz, A. Karpenko, V. Plzak, R. Leppelt, B. Schumacher and R. J. Behm, J. Catal., 2007, 246, 74–90 CrossRef CAS.
- S. Zhang, X. S. Li, B. B. Chen, X. B. Zhu, C. Shi and A. M. Zhu, ACS Catal., 2014, 4, 3481–3489 CrossRef CAS.
- K. I. Choi and M. A. Vannice, J. Catal., 1991, 127, 465–488 CrossRef CAS.
- K. I. Choi and M. A. Vannice, J. Catal., 1991, 131, 36–50 CrossRef CAS.
- M. Haruta, Catal. Surv. Asia, 1997, 1, 61–73 CrossRef CAS.
- X. S. Huang, H. Sun, L. C. Wang, Y. M. Liu, K. N. Fan and Y. Cao, Appl. Catal., B, 2009, 90, 224–232 CrossRef CAS.
- M. A. Bollinger and M. A. Vannice, Appl. Catal., B, 1996, 8, 417–443 CAS.
- I. Lee, J. B. Joo, Y. D. Yin and F. Zaera, Angew. Chem., 2011, 123, 10390–10393 CrossRef.
- F. Boccuzzi, A. Chiorino, M. Manzoli, P. Lu, T. Akita, S. Ichikawa and M. Haruta, J. Catal., 2001, 202, 256–267 CrossRef CAS.
- M. C. Raphulu, J. Mcpherson, E. van der Lingen, J. A. Anderson and M. S. Scurrell, Gold Bull., 2010, 43, 21–28 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |