Biofouling control in a membrane filtration system by a newly isolated novel quorum quenching bacterium, Bacillus methylotrophicus sp. WY
R. Khanab,
F. Shen*a,
K. Khanab,
L. X. Liuab,
H. H. Wuab,
J. Q. Luoa and
Y. H. Wan*a
aState Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: fshen@ipe.ac.cn; yhwan@ipe.ac.cn; Tel: +86-10-82544991
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 11th March 2016
Abstract
During membrane processes of wastewater treatment, biofouling is a critical issue affecting membrane performance. Interspecies quorum quenching (QQ) by bacterial cells has been reported as a novel approach for mitigating the biofouling via restraining quorum sensing (QS), where secreted signaling molecules, e.g., acyl homoserine lactones (AHLs) being essential for biofilm formation are degraded by QQ bacteria. Herein, we isolated a novel indigenous QQ bacterium, Bacillus methylotrophicus strain WY from real wastewater sludge for controlling membrane biofouling. Strain WY was successfully characterized for QS and QQ activity and possessed a wide-ranging activity for degrading AHLs. More than 90% degradation for C8-HSL, C10-HSL, C12-HSL, C14-HSL, 3-oxo-C6HSL, 3-oxo-C8HSL and 3-oxo-C12HSL were achieved. Further, sodium alginate beads immobilized strain WY cells were applied to the microfiltration process. Compared to the vacant beads, using the beads with stain WY increased membrane flux by 3 to 4 times, demonstrating excellent biofouling control ability of strain WY.
1 Introduction
Membrane processes nowadays are extensively used in the treatment of various kinds of wastewaters. The membrane processes advantages include smaller footprint, simple modular design and ease of operation.1 Despite such advantages, membrane fouling, particularly biofouling has been regarded as one of the main impediments to the efficient application of membrane processes in various types of water treatment system.2,3 Biofouling is generally defined as the deleterious attachment of microbial cells and extracellular polymeric substances (EPS) to a solid surface. Previous works have clearly revealed that the formation of biofilms on the membrane surfaces could trigger irreversible membrane fouling, which decreases membrane performance and ultimately increases operation and maintenance costs.4 Biofouling is regarded as being more problematic than organic fouling as bacterial attachment to the surfaces tends to be irreversibly and form mature biofilms through the production of EPS.5 Many researchers in the past have attempted to control biofouling through various approaches, which are mostly focused on the physical cleaning of membrane surfaces, modification of membranes and incorporation of antibiotics or antimicrobial compounds to membrane surfaces.6–10 Conventional physicochemical approaches to reduce membrane biofouling may be time-consuming and expensive. It seems that biological control of membrane biofouling, which has the advantages of high efficiency, low toxicity and environment friendly, has attracted great consideration.11Recently, a novel biological phenomenon has been investigated for biofouling control based on quorum sensing (QS). QS is bacterial density-dependent cell-to-cell communication, in which some particular signaling molecules (also called autoinducers) are produced and recognized by bacteria.12 QS has been believed to regulate the bacterial gene expression, facilitating some microbial behaviors such as the production of soluble microbial products (SMP), EPS and biofilm formation.13,14 In other words, if the production of signaling molecules is inhibited or regulated, QS or biofouling is expected to be controlled. For instance, acyl homoserine lactone (AHL) is a kind of signaling molecule necessary for biofilm development.15 Yeon et al. used acylase enzyme attached to a magnetic carrier to degrade AHL to inhibit QS in membrane bioreactors for water treatment and found that this approach, called enzymatic quorum quenching (QQ) reduced the membrane biofouling effectively and enhanced the membrane permeability.16 Further, for the degradation of AHL, QQ enzymes were classified into three major types according to their enzymatic mechanisms: AHL-lactonase (lactonlysis), AHL-acylase (amidohydrolysis) and AHL oxidase and reductase (oxidoreduction).17–19 These findings opened a new era of research to control the growth behaviors of microbes and the biofouling in membrane filtration system. However the enzymatic QQ approach has the concern of the enzymatic cost and stability. Bacterial QQ approach was then introduced to overcome the problems mentioned above, because QQ enzymes were discovered in a range of bacteria. For example, Rhodococcus sp. BH4 and recombinant E. coli QQ bacteria, which can produce AHL-lactonase were encapsulated into a hollow fiber membrane module, respectively and the membrane biofouling was successfully controlled.20,21 Moreover, one of the major advantages of QQ approach is that it only influences the biofouling and sludge characteristics, while not impacting membrane performance.22,23 It is worth noting that, in the nature there might be lots of QQ bacteria present.24 Therefore, it is still a matter of research that which QQ strain is more appropriate to inhibit QS and then realize biofouling control in membrane wastewater treatment.25
The main purpose of this work is to isolate novel indigenous QQ bacterial strains for the biofouling control in membrane water treatment system. More than 90 bacterial strains were isolated from real wastewater sludge. All of them were characterized simultaneously for QS and QQ activity: whether they produce their own QS signaling molecules. If not, they were further investigated for the QQ enzymatic activity, which is responsible for the degradation of AHLs. As a result, we isolated a novel QQ strain Bacillus methylotrophicus (B. methylotrophicus). To the best of our knowledge, there is no report about the QQ activity of strain WY and their use in membrane biofouling control. 10 kinds of AHLs with different carbon chain length (with or without oxo group) were used to determine the degradation ability of the strain WY and the degradation mechanism was also investigated through GC-MS. Further, sodium alginate beads immobilized strain WY cells were applied to the microfiltration process and their effect on the membrane biofouling control was researched.
2 Experimental
2.1 Chemicals and bacterial strains
N-(Octanoyl)-DL-homoserine lactone (C8-HSL), N-(3-oxooctanoyl)-L-homoserine lactone (3-oxo-C8-HSL), N-(3-oxodecanoyl)-L-homoserine lactone (3-oxo-C10-HSL), and 5-bromo-4-chloro-3-indolyl-beta-D-galactopy-ranoside (X-gal) were purchased from Sigma-Aldrich Co., USA. The X-gal was dissolved in DMSO (20 mg mL−1). N-(Hexanoyl)-DL-homoserine lactone (C6-HSL), N-(beta-ketocaproyl)-L-homoserine lactone (3-oxo-C6-HSL), N-(decanoyl)-DL-homoserine lactone (C10-HSL), N-(dodecanoyl)-DL-homoserine lactone (C12-HSL), N-(3-oxo-dodecanoyl)-L-homoserine lactone (3-oxo-C12-HSL), N-(tetradecanoyl)-DL-homoserine lactone (C14-HSL) and N-(3-oxo-tetradecanoyl)-L-homoserine lactone (3-oxo-C14-HSL) were purchased from Cayman Chemicals. All the AHLs were dissolved in analytical grade methanol at the concentration of 20 mM as stock solution. LIVE/DEAD BacLight™. Bacterial viability kit was purchased from Thermo Fisher Scientific, U.S. While, Beta Glo Assay System was purchased from Promega, U.S. AHLs were detected by Agrobacterium tumefaciens A136 (A. tumefaciens A136) reporter strain.13 All the other chemicals if used were of the highest grade available unless otherwise stated.2.2 Novel indigenous QQ bacteria
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min), and the whole cell suspension concentration was adjusted to OD600 1.0 using a spectrophotometer (UV757CRT, Lengguang Technology Co., Ltd., Shanghai, China) with PBS buffer (50 mM, pH 6.8). The bacterial isolates (OD600 1.0) were provided with C8-HSL at a final concentration of 2 μM and the degradation was measured after 1 h incubation (30 °C, 200 rpm).
000 rpm, 10 min), and the whole cell suspension concentration was adjusted to OD600 1.0 using a spectrophotometer (UV757CRT, Lengguang Technology Co., Ltd., Shanghai, China) with PBS buffer (50 mM, pH 6.8). The bacterial isolates (OD600 1.0) were provided with C8-HSL at a final concentration of 2 μM and the degradation was measured after 1 h incubation (30 °C, 200 rpm).2.3 Nucleotide sequence accession numbers
The 16S rRNA genes of the QQ positive isolates were amplified by PCR using two universal primers 1492F (5′-GGCTACCTTGTTACGACTT-3′) and 27F (5′-AGAGTTTGATCCTGGCTCAG-3′). The internal transcribed spacer (ITS) sequencing was done by Sunbiotech Co., Ltd. (Beijing, China), and the resulted sequences were searched for the similarity in Genbank via BLAST algorithm. The ITS sequences were automatically aligned using ClustalX 2.0.28 The 16S rRNA sequence of strains Bacillus methylotrophicus, Achromobacter xylosoxidans, Alcaligenaceae bacterium zx5 and Achromobacter xylosoxidans were deposited to Genbank library under the accession number of KF831370, JQ724537, FJ463167 and AB547225 respectively.2.4 GC-MS analysis
To further confirm the inactivation mechanism and inactivation ability of the isolated strain WY, GC-MS analysis was employed. N-(Octanoyl)-DL-homoserine (C8-HSL) was provided to an over-night grown whole cell suspension (OD600 3.0) using PBS buffer (50 mM, pH 6.8) as reaction medium at a final concentration of 20 μM. The reaction mixture was incubated at 30 °C for 12 h with 200 rpm shaking speed. Then, the reaction mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and filtered through 0.22 μm filter, to get cell-free supernatant. The cell-free supernatant was further extracted using the procedure described by Cataldi et al.29 In brief, equal volume of the cell-free supernatant solution was extracted three times with an equal volume of chloroform. The combined organic phases were washed with 4 mL of water and taken to dryness in the oven kept at 25 °C. The residue was re-dissolved in methanol. Non-inoculated LB mixed with sterile MilliQ water and exogenous AHLs were served as negative controls and positive controls, respectively. Analysis was performed using a model TRACE 1300 Gas Chromatograph (Thermo Scientific) with ISQ single quadrupole mass spectrometer. The capillary column used for the analysis was TG-5MS (30 m × 0.25 mm ID × 0.25 μm) supplied by Thermo Scientific. Chromatographic data were collected and recorded by GC-MS Real Time Analysis software. Sample injection was done in split mode (split ratio 5
000 rpm for 5 min and filtered through 0.22 μm filter, to get cell-free supernatant. The cell-free supernatant was further extracted using the procedure described by Cataldi et al.29 In brief, equal volume of the cell-free supernatant solution was extracted three times with an equal volume of chloroform. The combined organic phases were washed with 4 mL of water and taken to dryness in the oven kept at 25 °C. The residue was re-dissolved in methanol. Non-inoculated LB mixed with sterile MilliQ water and exogenous AHLs were served as negative controls and positive controls, respectively. Analysis was performed using a model TRACE 1300 Gas Chromatograph (Thermo Scientific) with ISQ single quadrupole mass spectrometer. The capillary column used for the analysis was TG-5MS (30 m × 0.25 mm ID × 0.25 μm) supplied by Thermo Scientific. Chromatographic data were collected and recorded by GC-MS Real Time Analysis software. Sample injection was done in split mode (split ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The GC injector temperature was set at 270 °C. Mass spectrometry conditions were set as: electron ionization source adjust at 70 eV, MS source temperature 200 °C. The mass spectrometer was run in full scan mode (m/z: 20–400) and in SIM mode at 143 and 114 m/z.
1). The GC injector temperature was set at 270 °C. Mass spectrometry conditions were set as: electron ionization source adjust at 70 eV, MS source temperature 200 °C. The mass spectrometer was run in full scan mode (m/z: 20–400) and in SIM mode at 143 and 114 m/z.
2.5 Bioassay for AHLs molecules quantification
In order to determine the QQ activity of B. methylotrophicus WY, the degradation rates of ten AHL molecules with different carbon chain lengths as mentioned above were measured, respectively. Each of the AHL molecules was provided to the B. methylotrophicus WY (OD600 1.0) whole cell suspension at a final concentration of 1 μM and incubated for 30 min (30 °C, 200 rpm), respectively. Then, degraded AHLs molecules levels were quantitatively determined as per the method reported by Kawaguchi et al. with some slight modifications.30 Briefly, 50 μL of strain WY inactivated products sample mixed with 75 μL of cell-free lysate of A. tumefaciens A136 was loaded in 96 well-plate. After incubation at 30 °C for 1.5 h, oxyluciferin luminescence was generated by adding Beta Glo (Promega, USA). The intensity of luminescence is proportional to the amount of beta galactosidase released from the A. tumefaciens A136 biosensor. Bioluminescence was measured by luminometer (Varioskan Flash Multimode Reader, Thermo Scientific) after 40 min incubation. By using the relationship equations for all of the AHLs, the amounts of AHLs were calculated based on the calibration curve derived from known standard samples of AHLs.2.6 Degradation evaluation of B. methylotrophicus WY according to growth rate in synthetic wastewater medium and LB broth medium
In order to find the QQ activity of strain WY according to its growth phase in LB broth medium and synthetic wastewater (SWW) medium, strain WY was inoculated in fresh LB broth medium and SWW medium, respectively. The composition of SWW was (milligrams per liter): glucose, 400; bacto peptone, 115; yeast extract, 14; (NH4)2SO4, 104.8; KH2PO4, 21.75; MgSO4, 15.63; CaCl2, 2.45; MnSO4, 1.8; FeCl3, 0.075 and NaHCO3, 255.5.31 Culture medium samples were taken at various intervals (10, 12, 16, 20, 23, 27, and 33 h) and the concentrations were adjusted constantly to OD600 1.0 whole cell suspension. All of the samples (adjusted to OD600 1.0, whole cell suspension) taken at various intervals were provided with C12-HSL at a final concentration of 1 μM and incubated for 30 min (200 rpm, 30 °C). Using the procedure described in Section 2.5, the degradation levels were quantified.2.7 Immobilization of strain WY
Immobilization of strain WY was carried out in sodium alginate (Sigma-Aldrich) beads. Sodium alginate is a natural polymer non-toxic to bacteria. An over-night strain WY grown culture in LB broth medium was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g, 10 min), washed with distilled water and was adjusted to volume of 5 mL (OD600 3.0). The entrapment procedure was carried out according to the method described by Konsoula et al. with some slight modifications.32 Briefly, 5 mL of WY suspension having OD600 3.0 was mixed with 95 mL of 4% (w/v) sodium alginate suspension. The mixed suspension was dropped in 3% w/v CaCl2 solution. After making the beads it was kept for 12 h in 3% w/v CaCl2 solution for cross linking, washed with distilled water and dried at room temperature. The average size of beads was approximately 3 to 4 mm.
000 g, 10 min), washed with distilled water and was adjusted to volume of 5 mL (OD600 3.0). The entrapment procedure was carried out according to the method described by Konsoula et al. with some slight modifications.32 Briefly, 5 mL of WY suspension having OD600 3.0 was mixed with 95 mL of 4% (w/v) sodium alginate suspension. The mixed suspension was dropped in 3% w/v CaCl2 solution. After making the beads it was kept for 12 h in 3% w/v CaCl2 solution for cross linking, washed with distilled water and dried at room temperature. The average size of beads was approximately 3 to 4 mm.
2.8 Biofilm growth assay and dead-end microfiltration system
Mixed-species microorganisms cultured from wastewater sludge taken from a local wastewater treatment plant (Gaobeidian, Beijing) were acclimated with the SWW described in Section 2.6. The method used for biofilm growth assay was similar to that proposed by Lee et al.33 Briefly, for biofilm growth on membrane surface, total 45 disc-shaped 47 mm diameter polyvinylidene fluoride (PVDF) membranes (GVWP 04700; Millipore) with a nominal pore size of 0.22 μm were separately attached to the slide glass and placed into a 45 conical flasks each having 50 mL volume. About 49 mL of SWW having 1 mL wastewater sludge (1000 mg L−1 of mixed liquor suspended solid concentration) was added to each conical flask and incubated for 25 days (150 rpm, 25 °C), while SWW was replaced every day, to maintain the biofilm growth intact on each membrane. About 15 beads were added to each membrane: beads having strain WY or vacant beads, in order to evaluate also the physical washing effect and adsorption of AHLs on the alginate vacant beads. Membrane with only SWW without beads was also run. Once after every second day, three membranes were taken out for flux decline test and biofilm observation, respectively.As shown in Fig. 1, a home-made dead-end membrane filtration system was used to test membrane flux, where the PVDF membrane was used and the membrane area was 13.4 cm2. The trans-membrane pressure was kept constant at 50 kPa by applying nitrogen gas at room temperature (25 °C). During the flux test, distilled water was used as feed.
2.9 Membrane surface characterization
Scanning electron microscopy (SEM, JSM-6700LV, JEOL Ltd., Japan) was used to analyze the fouling layer on the membrane surface. For microscopic analysis, samples were prepared according to the method described by Xu et al. with some modifications.34 Briefly, 72 h preformed biofilm membranes were washed with distilled water and kept in 2.5% glutaraldehyde solution at 4 °C for 2 h. The samples were then sequentially dehydrated using ethanol solutions with the concentration of 25%, 50%, 75%, and 95% (v/v) for 15 min and finally 100% ethanol for 20 min. These samples were further dried and observed using the field emission scanning electron microscope operated at 5 kV after sputter coating with a thin layer of gold.The biocake formed on membrane surface was also visually analyzed by confocal laser scanning microscopy (CLSM, SP5; Leica TCS, Germany). SYTO9 (Molecular Probes, USA; ex = 488 nm; em = 515/30 nm) was used to stain the biofilm, which penetrates the bacterial cell membranes and stain the cells green. The stained samples were then kept in dark at room temperature for 20 min. After gently washing three times with distilled water to remove excess dyes, the stained samples were observed under CLSM.
3 Results and discussion
3.1 A novel QQ bacterium, B. methylotrophicus sp. WY
In this study about 90 different pure bacterial cultures were screened from wastewater sludge, taken from local wastewater treatment plants in Gaobeidian (Beijing, China). Screened pure bacterial cultures were respectively investigated for QS activity by bio agar assay at first. We found that only five bacterial isolates (Table 1) were not involved in QS activity (do not produce their own QS signals) as shown in Fig. 2. The five bacterial isolates were further investigated for QQ activity respectively (Fig. 3). Strain 3A2 was found to have no visible QQ activity as shown in Fig. 3e, so we excluded strain 3A2 from the QQ positive isolates.| Strain | 16rDNA with Genbank accession no. | 16rDNA gene sequence homology (%) | QS activity | QQ activity |
|---|---|---|---|---|
| WY | Bacillus methylotrophicus KF831370 | 99.0 | No | Yes |
| S4 | Alcaligenaceae bacterium zx5 FJ463167 | 99.0 | No | Yes |
| 1S3 | Achromobacter xylosoxidans JQ724537 | 99.0 | No | Yes |
| 2A1 | Achromobacter xylosoxidans AB547225 | 99.0 | No | Yes |
| 3A2 | Lysinibacillus sphaericus AB271742 | 99.0 | No | No |
The four QQ positive isolates listed in Table 1 were compared with each other by providing with C8-HSL, and their degradation was inspected. As shown in the Fig. 4, strain WY was found to have the highest QQ activity among the four QQ positive isolates. About 96.3%, 34.3%, 52.7% and 46% degradation were observed at 60 min of incubation for strain WY, S4, 1S3 and 2A1, respectively.
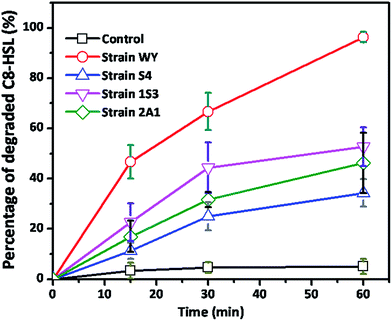 | ||
| Fig. 4 Comparison of the QQ activity among four indigenous QQ bacteria. Non-inoculated LB mixed with sterile MilliQ water served as control. Error bar: standard deviation (n = 3). | ||
In addition, strain WY was Gram-positive, catalase positive while colonies were creamy white convex opaque with regular edges and sticky not to be picked easily. Phylogenetic analysis based on 16S rRNA gene sequences was carried out. The results indicated that the strain WY belongs to the genus B. methylotrophicus (99.0% similarity). The 16S rRNA sequence was deposited in the Genbank under accession number KF831370, and the strain was designated as B. methylotrophicus WY and selected for further detailed study. So far, there is no report about the characteristic QQ activity of this species.
3.2 Degradation mechanism of strain WY
GC-MS analysis was carried out to confirm the degradation mechanism of strain WY. C8-HSL was used as the substrate for strain WY degradation and the degradation products were analyzed. In the MS profile of positive control sample, C8-HSL was identified by a fragmentation abundant (M + H) ion at m/z (mass-to-charge ratio) 143 (Fig. 5b) according to the retention time of 14.26 min (Fig. 5a). When the products of strain WY were analyzed, there were no visible signals at fragmentation pattern of m/z 143 at 14.26 min (Fig. 5a) of retention time. This inferred that all of the C8-HSL has been degraded or inactivated. However, we observed another peak at m/z of 114 (Fig. 5b) according to the retention time of 10.09 min (Fig. 5a). This compound was identified to be N-(octanoyl)-DL-homserine with open lactone ring, which indicated that strain WY most likely produce AHL-lactonase, which breaks the lactone ring of N-(octanoyl)-DL-homserine lactone (Fig. 5c). Though, we do not exclude the possibility that strain WY might produce other AHL degrading enzymes as well.3.3 Quantification of AHL degrading ability of B. methylotrophicus WY
10 kinds of AHLs with different carbon chain length (with or without oxo group) were used as the substrate to further determine the degradation ability of strain WY. As shown in Fig. 6, strain WY is able to degrade all of the AHLs used. C6-HSL, 3-oxo-C14-HSL and 3-oxo-C10-HSL were only 29.1%, 42% and 67% degraded, respectively, while more than 90% degradation was observed for C8-HSL, C10-HSL, C12-HSL, C14-HSL, 3-oxo-C6HSL, 3-oxo-C8-HSL and 3-oxo-C12-HSL. In general trend all the AHLs used with or without oxo group in the acyl chain were readily degraded by strain WY, apart from C6-HSL and 3-oxo-C14-HSL. Zamani et al. reported similar results, where B. cereus U29 belongs to the same group bacillus as strain WY. B. cereus U29 can also produce AHL-lactonase, which degraded C6-HSL at lower amount, while degraded 3-oxo-C8HSL and 3-oxo-C12HSL at highest level.353.4 Evaluation of QQ activity according to growth phase in LB broth medium and SWW medium
The change in the QQ activity was monitored according to the growth phase in LB broth medium and SWW medium, respectively. In the LB medium, highest degradation was detected in the exponential phase up to the early stationary phase, where about 90% to 96% degradation occurred from 16 h to 23 h, as shown in Fig. 7. The reason for this might be the high metabolic activity and rapid growth rate in the exponential phase and early stationary phase. However, little decline in degradation was observed during the late stationary phase degradation, because bacterial cell death start occurring. Furthermore, low metabolic activity and less substrate available for growth of bacteria during the stationary phase might be the reason for the decrease in degradation. Oh et al. also reported similar results for Rhodococcus sp. BH4, which produces AHL-lactonase, degraded AHL molecules at its highest level in the exponential growth phase, while lower degradation was witnessed in the stationary growth phase.36 Further when the growth phase degradation was carried out in SWW, the strain WY readily degraded about 70% to 85% of the provided AHL in the late exponential growth phase and early stationary growth phase. While only 35% degradation was observed during the late stationary growth phase. This less degradation might also be due to the stationary growth and rapid death of bacteria during this phase. As can be seen from the Fig. 7 the degradation efficiency of strain WY in SWW medium is less than that in LB medium, which might be due to the less substrate available in SWW medium.3.5 Biofouling alleviation by immobilized strain WY in membrane water treatment
To investigate the ability of WY for biofouling inhibition, strain WY was immobilized in sodium alginate beads. We first evaluated C8-HSL degradation by strain WY beads in PBS buffer (50 mM, pH 6.8). As shown in the Fig. 8, using the beads with strain WY, about 84% of degradation was observed in the given 60 min. By contrast, about 10% to 12% elimination of C8-HSL were also observed for vacant beads, which might be due to the adsorption of C8-HSL on vacant beads surfaces.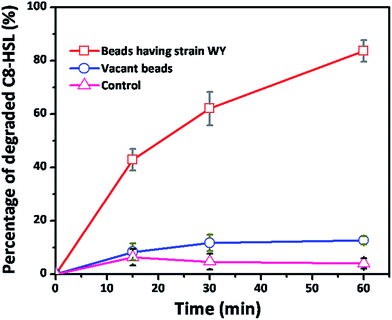 | ||
| Fig. 8 Quantitative QQ activity of beads having strain WY, vacant beads and control. Error bar: standard deviation (n = 4). | ||
Further, we evaluated the ability of entrapped strain WY to mitigate biofouling in simulative membrane water treatment system. Two controls “no addition of beads” and “addition of only vacant beads” showed a rapid flux decline after nine days of incubation period (Fig. 9). A considerably improved flux was observed for the membrane incubated with immobilized WY cells, over period of 25 days, compared to the other two controls. When the incubation time reached 17 days, membranes flux decreased to its respective plateau regardless of the different incubation conditions, which meant the mature biofilm has been formed on membrane surface. Clearly, the plateau flux of the membranes incubated with strain WY was about 3–4 times higher than those without WY. It can also be seen from Fig. 9 that membrane flux decline has been obviously delayed when membranes were incubated with strain WY beads. Furthermore, compared to the control of no beads, slight improved flux was also observed for the control having vacant beads, which was due to physical washing effect and adsorption effect (Fig. 8) by beads.37 The common trend of biofilm cycle consists of initial attachment of single cells, colonization of cells and then formation of a mature complex biofilm on the membrane surfaces.38 More specifically, different bacterial colonies converge into mature biofilm due to QS-dependent mechanism, which ultimately results in a rapid increase in biofilm (biofouling) formation in membrane processes.39 In the present work, for the membrane incubated with WY, the obviously higher membrane flux and the delay in the flux decline was believed to be the role resulted from the inhibition of QS (QS quenching) by QQ mechanism.
 | ||
| Fig. 9 Comparison of the permeate flux of fouled membranes. Experiments were conducted in triplicate. Error bar: standard deviation (n = 3). | ||
3.6 SEM and CLSM analysis of biofouling layer
SEM and CLSM images were captured from the membranes after 72 h incubation under different conditions, as shown in Fig. 10. The pristine PVDF membrane surface was highly porous (Fig. 6a) and no bacterial cells or biomass attached (Fig. 10e). After 72 h incubation with immobilized WY cells, the porous structure was still remained on the membrane surface and only fewer bacterial cells and biomass deposition were observed (Fig. 10b and f). Whereas, high quantity of bacterial cells and bacterial biomass secretion were witnessed clearly on the surfaces of the PVDF membranes incubated with vacant beads and no beads (Fig. 10g and h). As a result, the membrane surface porosity almost disappeared (Fig. 10c and d), proving that the serious biofouling occurred. In short, the QQ effect of immobilized WY cells on disrupting QS system and controlling biofouling formation was further ascertained using SEM and CLSM imaging.3.7 Comparison of various characteristics of B. methylotrophicus sp. WY with previously reported biofouling controlling QQ bacteria
As AHLs molecules are usually used as degradation substrate to determine the QQ activity of bacteria, we compared the degradation rates of various AHLs by the different QQ bacteria (Table 2). It can be seen in the Table 2 that different QQ bacteria have diverse activity to degrade different kind of AHLs and strain WY is comparable to those reported in literatures. As for as biofouling control effect is concerned, since the experimental conditions are different (such as experimental method, membrane material, membrane type, operation mode, immobilization media and strain dosage, etc.), it might be difficult to carry out a direct comparison. Here, we preliminarily compared the biofouling control effect of strain WY with the results in literatures (Table 3). It can be seen from Table 3 that the biofouling control effect of strain WY is also comparable to those reported in literatures.| AHL molecule | Pseudomonas sp. 1A1a | Rhodococcus sp. BH4a | Recombinant E. colic | B. methylotrophicus sp. WYb | |
|---|---|---|---|---|---|
| a Reaction time 10 min, 0.2 μM dosage of each AHLs and reaction medium (TRIS buffer 50 mM, pH 7.0).b Reaction time 30 min, 1 μM dosage of each AHLs and reaction medium (PBS buffer 50 mM, pH 6.8).c Reaction time 30 min, 0.2 μM dosage of C8-HSL only.d Not reported. | |||||
| AHLs degradation rate | C6-HSL | 30–35 | 75–80 | d | 29–30 |
| C8-HSL | 35–40 | 48–53 | 62–67 | 94–95 | |
| C10-HSL | 73–77 | 77–82 | d | 98–99 | |
| C12-HSL | 90–95 | 81–86 | d | 93–94 | |
| C14-HSL | d | d | d | 96–97 | |
| 3-oxo-C6-HSL | 0 | 9–14 | d | 98–99 | |
| 3-oxo-C8-HSL | 0–5 | 37–42 | d | 93–94 | |
| 3-oxo-C10-HSL | 5–10 | 55–60 | d | 99–100 | |
| 3-oxo-C12-HSL | 93–98 | 88–93 | d | 66–67 | |
| 3-oxo-C14-HSL | d | d | d | 41–42 | |
| QQ enzyme | — | AHL-acylase | AHL-lactonase | AHL-lactonase | AHL-lactonase |
| Gram staining | — | Gram-negative | Gram-positive | Gram-negative | Gram-positive |
| Reference | — | 25 | 36 | 21 | This study |
| Strain | Membrane | Experimental method | Operation mode | Biofouling control effect | Reference |
|---|---|---|---|---|---|
| a PE, polyethylene; PVDF, polyvinylidene fluoride; HF, hollow fiber; FS, flat sheet; MBR, membrane bioreactor; MF, microfiltration; TMP, transmembrane pressure.b The effect on the control of the rate of TMP rise-up.c The effect on the control of the rate of flux reduction. | |||||
| Pseudomonas sp. 1A1 | PE/HF | MBR | Constant flux | 2–3 timesb | 25 |
| Rhodococcus sp. BH4 | PVDF/HF | MBR | Constant flux | 2–4 timesb | 21 |
| B. methylotrophicus sp. WY | PVDF/FS | MF | Constant TMP | ∼3 timesc | This study |
4 Conclusions
In this study a novel indigenous QQ bacterium, B. methylotrophicus WY was reported having distinguishing broad QQ activity against AHLs. Strain WY readily degraded more than 90% of all of 10 AHLs molecules provided, except C6-HSL, 3-oxo-C12-HSL and 3-oxo-C14-HSL, which were degraded 29.1%, 67% and 42% respectively. GC-MS analysis revealed that it produces QQ lactonase, which breaks the lactone ring of AHLs. Also strain WY could degrade AHLs in SWW medium readily. Furthermore, immobilized strain WY in sodium alginate beads successfully improved membrane filtration performance substantially over 25 days of period in water treatment system by delaying the biofilm maturation.Acknowledgements
The authors would like to thank the support from the National High-Tech R&D Program of China (2012AA021202, 2014AA021005).References
- F. Meng, S.-R. Chae, A. Drews, M. Kraume, H.-S. Shin and F. Yang, Water Res., 2009, 43, 1489–1512 CrossRef CAS PubMed.
- J. S. Baker and L. Y. Dudley, Desalination, 1998, 118, 81–89 CrossRef CAS.
- H. C. Flemming, G. Schaule, T. Griebe, J. Schmitt and A. Tamachkiarowa, Desalination, 1997, 113, 215–225 CrossRef CAS.
- L.-N. Huang, H. De Wever and L. Diels, Environ. Sci. Technol., 2008, 42, 8360–8366 CrossRef CAS PubMed.
- F. Diagne, R. Malaisamy, V. Boddie, R. D. Holbrook, B. Eribo and K. L. Jones, Environ. Sci. Technol., 2012, 46, 4025–4033 CrossRef CAS PubMed.
- C. X. Liu, D. R. Zhang, Y. He, X. S. Zhao and R. Bai, J. Membr. Sci., 2010, 346, 121–130 CrossRef CAS.
- P. Le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef CAS.
- M. F. Siddiqui, H.-S. Oh, M. Rzechowicz, H. Winters, T. H. Chong and A. G. Fane, J. Ind. Eng. Chem., 2015, 30, 204–211 CrossRef CAS.
- J. W. Lee, W. Jutidamrongphan, K. Y. Park, S. Moon and C. Park, Environ. Eng. Res., 2012, 17, 59–63 CrossRef.
- A. Ramesh, D. J. Lee, M. L. Wang, J. P. Hsu, R. S. Juang, K. J. Hwang, J. C. Liu and S. J. Tseng, Sep. Sci. Technol., 2006, 41, 1345–1370 CrossRef CAS.
- Y. Xiong and Y. Liu, Appl. Microbiol. Biotechnol., 2010, 86, 825–837 CrossRef CAS PubMed.
- M. R. Parsek and E. P. Greenberg, Trends Microbiol., 2005, 13, 27–33 CrossRef CAS PubMed.
- C. Fuqua, S. C. Winans and E. P. Greenberg, Annu. Rev. Microbiol., 1996, 50, 727–751 CrossRef CAS PubMed.
- S. Dobretsov, M. Teplitski and V. Paul, Biofouling, 2009, 25, 413–427 CrossRef CAS PubMed.
- C. M. Waters and B. L. Bassler, Annu. Rev. Cell Dev. Biol., 2005, 21, 319–346 CrossRef CAS PubMed.
- K.-M. Yeon, C.-H. Lee and J. Kim, Environ. Sci. Technol., 2009, 43, 7403–7409 CrossRef CAS PubMed.
- Y.-H. Dong, L.-H. Wang, J.-L. Xu, H.-B. Zhang, X.-F. Zhang and L.-H. Zhang, Nature, 2001, 411, 813–817 CrossRef CAS PubMed.
- Y. H. Lin, J. L. Xu, J. Hu, L. H. Wang, S. L. Ong, J. R. Leadbetter and L. H. Zhang, Mol. Microbiol., 2003, 47, 849–860 CrossRef PubMed.
- S. Uroz, S. R. Chhabra, M. Cámara, P. Williams, P. Oger and Y. Dessaux, Microbiology, 2005, 151, 3313–3322 CrossRef CAS PubMed.
- D. Jahangir, H.-S. Oh, S.-R. Kim, P.-K. Park, C.-H. Lee and J.-K. Lee, J. Membr. Sci., 2012, 411–412, 130–136 CrossRef CAS.
- H.-S. Oh, K.-M. Yeon, C.-S. Yang, S.-R. Kim, C.-H. Lee, S. Y. Park, J. Y. Han and J.-K. Lee, Environ. Sci. Technol., 2012, 46, 4877–4884 CrossRef CAS PubMed.
- T. Maqbool, S. J. Khan, H. Waheed, C.-H. Lee, I. Hashmi and H. Iqbal, J. Membr. Sci., 2015, 483, 75–83 CrossRef CAS.
- W. Jiang, S. Xia, J. Liang, Z. Zhang and S. W. Hermanowicz, Water Res., 2013, 47, 187–196 CrossRef CAS PubMed.
- S. E. Christiaen, G. Brackman, H. J. Nelis and T. Coenye, J. Microbiol. Methods, 2011, 87, 213–219 CrossRef CAS PubMed.
- W.-S. Cheong, C.-H. Lee, Y.-H. Moon, H.-S. Oh, S.-R. Kim, S. H. Lee, C.-H. Lee and J.-K. Lee, Ind. Eng. Chem. Res., 2013, 52, 10554–10560 CrossRef CAS.
- T. M. Chong, C. L. Koh, C. K. Sam, Y. M. Choo, W. F. Yin and K. G. Chan, Sensors, 2012, 12, 4846–4859 CrossRef CAS PubMed.
- R. J. McLean, L. S. Pierson 3rd and C. Fuqua, J. Microbiol. Methods, 2004, 58, 351–360 CrossRef CAS PubMed.
- F. Jeanmougin, J. D. Thompson, M. Gouy, D. G. Higgins and T. J. Gibson, Trends Biochem. Sci., 1998, 23, 403–405 CrossRef CAS PubMed.
- T. R. Cataldi, G. Bianco, M. Frommberger and P. Schmitt-Kopplin, Rapid Commun. Mass Spectrom., 2004, 18, 1341–1344 CrossRef CAS PubMed.
- T. Kawaguchi, Y. P. Chen, R. S. Norman and A. W. Decho, Appl. Environ. Microbiol., 2008, 74, 3667–3671 CrossRef CAS PubMed.
- B. K. Hwang, W. N. Lee, K. M. Yeon, P. K. Park, C. H. Lee, I. S. Chang, A. Drews and M. Kraume, Environ. Sci. Technol., 2008, 42, 3963–3968 CrossRef CAS PubMed.
- Z. Konsoula and M. Liakopoulou-Kyriakides, Enzyme Microb. Technol., 2006, 39, 690–696 CrossRef CAS.
- B. Lee, K.-M. Yeon, J. Shim, S.-R. Kim, C.-H. Lee, J. Lee and J. Kim, Biomacromolecules, 2014, 15, 1153–1159 CrossRef CAS PubMed.
- W. Xu and S. Chellam, Environ. Sci. Technol., 2005, 39, 6470–6476 CrossRef CAS PubMed.
- M. Zamani, K. Behboudi and M. Ahmadzadeh, Biocontrol. Sci. Techn., 2013, 23, 555–573 CrossRef.
- H. S. Oh, S. R. Kim, W. S. Cheong, C. H. Lee and J. K. Lee, Appl. Microbiol. Biotechnol., 2013, 97, 10223–10231 CrossRef CAS PubMed.
- S. Rosenberger, F. P. Helmus, S. Krause, A. Bareth and U. Meyer-Blumenroth, Water Sci. Technol., 2011, 64, 1951–1958 CrossRef CAS PubMed.
- A. Zaky, I. Escobar, A. M. Motlagh and C. Gruden, Desalination, 2012, 286, 296–303 CrossRef CAS.
- M. R. Parsek and E. P. Greenberg, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 8789–8793 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |







