Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents†
Rajkumar Bandia,
Bhagavanth Reddy Gangapurama,
Ramakrishna Dadigalaa,
Ravikumar Eslavathb,
Surya S. Singhb and
Veerabhadram Guttena*a
aDepartment of Chemistry, Osmania University, Hyderabad 500007, Telangana State, India. E-mail: gvbhadram@gmail.com
bDepartment of Biochemistry, Osmania University, Hyderabad 500007, Telangana State, India
First published on 14th March 2016
Abstract
Here, we report a novel, facile and green approach for the synthesis of highly fluorescent carbon dots (CDs) with 28% quantum yield by utilizing onion waste as a precursor and by employing a simple autoclave. Optical and physicochemical properties of as synthesized CDs were studied using transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), dynamic light scattering (DLS), X-ray diffraction (XRD), Raman, thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR), UV-visible, fluorescence spectroscopy and elemental analysis. These CDs exhibited high aqueous dispersibility, excitation dependent fluorescence emission and excellent stability to various effects like pH, high ionic strength and continuous irradiation. It was observed that the presence of Fe3+ ions could result in a strong fluorescence quenching, hence these CDs were applied as a fluorescent probe for the selective & sensitive detection of Fe3+ ions and a good linear correlation (R2 = 0.996) in the range of 0–20 μM with a detection limit of 0.31 μM was obtained. Cytotoxicity studies performed on both normal (HEK-293 cells) and cancerous (HeLa) cells revealed their excellent biocompatibility and they were further employed as fluorescent probes for multicoloured (blue, green and red) imaging of HeLa cells. Impressed by their high selectivity, photo stability and excellent biocompatibility, the Fe3+ detection capability of these CDs was further evaluated in HeLa cells and applied for real water samples.
Introduction
Luminescent carbon dots (CDs) have grabbed the attention of the scientific community with their spectacular properties like wavelength-tuneable fluorescence emission, low toxicity, high biocompatibility, aqueous solubility, high stability and functionalizability,1,2 since they were serendipitously discovered during the purification of carbon nanotubes.3 These unique and novel properties made them find wide applicability in the fields of photocatalysis, biosensors, bioimaging, optoelectronics, and drug delivery.4–6 Impressed by their multimodal applications, numerous approaches such as laser ablation, arc discharge, thermal treatment, electrochemical oxidation, combustion, ultra-sonication, hydrothermal and microwave treatment have been developed for the synthesis of CDs by using various precursors.7–9 However these methods suffer from one or the other limitations such as severe synthetic conditions, post treatment steps, low yield, low fluorescence quantum yields and time consuming. Hence developing a simple synthetic approach to produce highly fluorescent CDs is necessary to use them practically with high efficiency.Recently many efforts have been devoted to develop green synthetic strategies for the production of CDs which explored the usage of various natural resources like milk,10 ginger,11 cabbage,12 orange juice,13 banana juice,14 soy milk,15 winter melon,16 vegetables,17 potato,18 coffee grounds,19 soya bean grounds20 and honey21 as precursors. In this context, usage of waste materials as precursors for the synthesis of fluorescent CDs would be more fascinating as it offers waste management besides the production of CDs.
Onions rank sixth among the world's leading vegetable crops with 85 million tonnes of global production (FAO statistics 2013). They are highly versatile and are an indispensable commodity in cuisines all over the world. Recently the demand for processed onions increased rapidly, which lead to the production of huge amounts of onion waste from onion processing industries. Further, the disposal of onion waste is also a major challenge which cannot be effectively carried out using conventional waste disposal methods.22
In an attempt to manage these wastes in a more convenient way and to develop a simple synthetic approach for CDs preparation, we used onion waste as the precursor for CDs synthesis. Furthermore to make this method more economical and scalable, simple autoclave was employed. To the best of our knowledge usage of simple autoclave for the synthesis of CDs is not yet reported. The obtained CDs possessed favourable optical properties and good quantum yield (28%). Besides the detailed analysis of the optical and physicochemical properties of CDs, we also evaluated their ability as a fluorescent probe for the label free detection of Fe3+ in real water samples & biological systems and successfully employed them as multi-coloured cellular imaging agents.
Results and discussion
Synthesis of CDs
The synthetic procedure is illustrated in Scheme 1 and more details are available in ESI.† Onion waste is rich source of dietary fibre, alk(en)yl cysteine sulphoxides and non-structural carbohydrates (NSC) which include glucose, fructose, sucrose and fructooligosaccharides.23 When the aqueous extract of onion waste along with ethylene diamine (EDA) is treated in an autoclave at 120 °C and 15 lbs pressure, the NSC of onion waste undergoes carbonization and passivation to produce fluorescent CDs, which is quite similar to the previous reports on preparation of CDs from carbohydrate sources.24,25 In order to obtain the best CDs from this simple procedure, several experiments were performed and the synthetic conditions were optimized by selecting quantum yield (QY) as a parameter. As shown in Fig. S1† 2 h of reaction time and 200 μL of EDA (for 20 mL of extract) yielded highly fluorescent CDs with a maximum QY of 28%. It is worth mentioning that the yield of CDs produced by this method was found to be 6.06%, which is higher than previous reports20,26,27 and makes this method suitable for large scale application.Physicochemical characterization
The morphology and particle size distribution of onion waste derived CDs was assessed by TEM and DLS analysis shown in Fig. 1. TEM images (Fig. 1a) depicts that the particles were well dispersed and uniform showing spherical morphology with an average diameter of about 15 nm. Corresponding DLS size distribution analysis (Fig. 1b) shows that the CDs have narrow size distribution in the range of 7–25 nm. The high resolution TEM (HRTEM) image (inset of Fig. 1a) indicates the crystallinity of CDs, with a lattice parameter of 0.21 nm, which may be attributed to the sp2 (1120) graphitic crystal phase of graphene.12 Compared to the previous reports,28,29 size of these CDs appears to be a little large which can be attributed to the mild reaction conditions used for the synthesis.30 An intense peak in the XRD pattern (Fig. S2†) at 2θ = 22.79° can be ascribed to the (002) diffraction pattern of graphitic carbon and the interlayer spacing d for corresponding peak (0.39 nm) is larger than that of graphite (0.34 nm). The increase in d value indicates an increase in the amorphous nature, and can be attributed to the introduction of oxygen containing functional groups.31 Raman spectra of CDs (Fig. S3†) exhibited two broad peaks at around 1334 and 1578 cm−1, which can be attributed to the D band (sp3 hybridized) and G band (sp2 hybridized) of graphite, respectively.26,32 The relative intensity of the disordered D band and crystalline G band is found to be 0.68 for these CDs. These results imply their structural similarity with graphite.26 Elemental analysis results showed that the CDs were composed of 55.04% C, 5.92% H, 9.34% N, and 29.65% O (calculated). XPS and FTIR analysis were performed to obtain clear information about the surface functional groups of as synthesized CDs. In the XPS survey spectrum (Fig. 2a) three distinct peaks centred at 285 eV, 400 eV and 530 eV were observed, which can be attributed to C 1s, N 1s and O 1s respectively. Deconvolution of high resolution C 1s peak gave 4 component peaks (Fig. 2b) with binding energies 284.9, 285.8, 286.9 and 288.9 eV showing the presence of C–C, C–N, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O respectively.33 N 1s spectrum can be resolved into 2 peaks (Fig. 2c) which shows the presence of C–N–C (398.5 eV) and N–H (400.2 eV) bonds. In the FTIR spectrum (shown in Fig. 2d) characteristic bands of –O–H and –N–H stretching vibrations were observed at 3200–3400 cm−1 region. The peak at 1656 cm−1 corresponds to carbonyl stretching, while the peak at 1340 cm−1 can be ascribed to C–N stretching vibrations. The peaks at 1618 and 1533 cm−1 corresponds to C
O respectively.33 N 1s spectrum can be resolved into 2 peaks (Fig. 2c) which shows the presence of C–N–C (398.5 eV) and N–H (400.2 eV) bonds. In the FTIR spectrum (shown in Fig. 2d) characteristic bands of –O–H and –N–H stretching vibrations were observed at 3200–3400 cm−1 region. The peak at 1656 cm−1 corresponds to carbonyl stretching, while the peak at 1340 cm−1 can be ascribed to C–N stretching vibrations. The peaks at 1618 and 1533 cm−1 corresponds to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of polycyclic aromatic hydrocarbons indicating the presence of sp2 hybridization.34 Furthermore the peaks at 1124 cm−1 and 1050 cm−1 represents stretching and bending vibrations of C–O bonds in carboxyl groups.35 From these results it is evident that the CDs derived from onion waste are composed of multiple functional groups like –OH, –COOH and –NH, which makes them highly soluble in water and can act as linkers for the attachment of biomolecules, serve as nano carriers. The zeta potential of CDs was measured to be −8.71 mV (Fig. S4†) indicating that the CDs are negatively charged due to the presence of carboxyl and hydroxyl groups. Thermal stability of CDs was verified by TGA analysis and the obtained thermogram showing a two-step degradation pattern is illustrated in Fig. S5.† Initial weight loss of 5–6% up to 140 °C is due to the evaporation of water molecules and other weakly bounded molecules on the surface of CDs.36 This slight weight loss also suggests that the CDs are stable up to this temperature. Significant weight loss in the range of 140–500 °C clearly indicates that the surface functional groups of CDs gradually degraded in this temperature range. In consistence with previous reports32,37 the curve levelled off beyond this temperature.
C stretching of polycyclic aromatic hydrocarbons indicating the presence of sp2 hybridization.34 Furthermore the peaks at 1124 cm−1 and 1050 cm−1 represents stretching and bending vibrations of C–O bonds in carboxyl groups.35 From these results it is evident that the CDs derived from onion waste are composed of multiple functional groups like –OH, –COOH and –NH, which makes them highly soluble in water and can act as linkers for the attachment of biomolecules, serve as nano carriers. The zeta potential of CDs was measured to be −8.71 mV (Fig. S4†) indicating that the CDs are negatively charged due to the presence of carboxyl and hydroxyl groups. Thermal stability of CDs was verified by TGA analysis and the obtained thermogram showing a two-step degradation pattern is illustrated in Fig. S5.† Initial weight loss of 5–6% up to 140 °C is due to the evaporation of water molecules and other weakly bounded molecules on the surface of CDs.36 This slight weight loss also suggests that the CDs are stable up to this temperature. Significant weight loss in the range of 140–500 °C clearly indicates that the surface functional groups of CDs gradually degraded in this temperature range. In consistence with previous reports32,37 the curve levelled off beyond this temperature.
 | ||
| Fig. 2 XPS and FTIR spectra of CDs: (a) XPS wide scan (b) C 1s spectrum (c) N 1s spectrum (d) FTIR spectrum. | ||
Optical properties
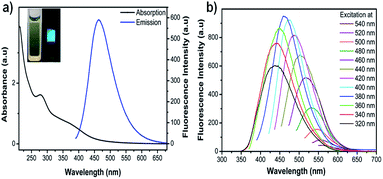
To explore the optical properties of CDs, UV-visible and photoluminescence spectral studies were carried out. CDs aqueous solution is pale yellow coloured and transparent under daylight, while it emits bright blue fluorescence under UV light, which could be easily observed with naked eyes (inset of Fig. 3a). Fig. 3a shows the absorption and emission spectra of as synthesized CDs. In the absorption spectrum, a peak at 280 nm and a hump around 370 nm characteristic for aromatic π–π* and carbonyl n–π* transitions respectively,38 were observed with absorption tail extending to the visible region. The emission spectra of CDs showed a strong emission peak at about 464 nm (λex = 380 nm) with a full width at half maximum (FWHM) of 87 nm which is consistent with previous reports39,40 and further confirms their narrow size distribution.Fluorescence spectra of CDs in water at different excitation wavelengths is illustrated in Fig. 3b, which clearly displays their excitation dependent fluorescence emission. With a gradual increase (20 nm) in the excitation wavelength from 320 to 540 nm, a red shift in the emission peak from 426 nm to 570 nm is observed. The fluorescence intensity progressively increased with increase in λex from 320 to 380 nm while further increase in λex to 540 nm caused a decline. This excitation tuneable emission property is considered to be the versatile characteristic of CDs and makes them as fluorescent probes for multicolour imaging.18,41,42 The fluorescence quantum yield of CDs at λex 380 nm was determined to be 28% using quinine sulphate as reference (Fig. S6†). This value is considered to be high compared with other CDs obtained from natural resources.12,16,27 The reasons for this high QY may be the high nitrogen content and in situ surface passivation by formation of amide bonds, which is evident from XPS, FTIR and elemental analysis. It has been reported that N-passivation of CDs creates a favourable energy trap to enhance their photoluminescence.43 The exact origin of fluorescence in CDs is still uncertain and based on previous research four possible mechanisms were proposed which include the quantum confinement effect or conjugated π-domains, surface/edge state, molecule state and the crosslink enhanced emission.44 And a recent study on time-resolved emission revealed that the commonly observed red-shifting phenomena originates from an individual entity with an ensemble of energy substates but not from a quantum confinement due to mixture of particles or due to the presence of multichromophoric groups.45
Fluorescence emission of CDs was measured at different pH values by adjusting the solution pH with 0.1 N HCl and 0.1 N NaOH solutions. It was observed that with change in the pH, fluorescence intensity changed with a constant emission wavelength and maximum fluorescence emission was observed in the pH range of 5–8 (Fig. S7†). Photo stability studies indicates that there was negligible decrease in the fluorescence intensity even after continuous exposure to Xe lamp for one hour (Fig. S8A†) and studies on the effect of ionic strength revealed that CDs have tolerance to high salt concentrations (2 M) (Fig. S8B†). Good photo stability, higher fluorescence emission in physiological pH and their ability to sustain higher ionic strengths makes this material a potential agent for biosensing and bioimaging applications. Solubility and fluorescent properties of CDs in various organic solvents were also studied. It is worth saying that CDs were found to be soluble in various organic solvents such as methanol, dimethylformamide (DMF), acetonitrile & dimethyl sulfoxide (DMSO) and exhibited prominent fluorescence emission, which further extends their applicability. The fluorescence intensity of CDs using these solvents (Fig. S9†) was in the order of water > methanol > DMF > acetonitrile > DMSO with a slight shift in their emission peak which may be because of the difference in their interaction with solvents. High polarity of water makes it an appropriate solvent for CDs with strong fluorescence emission.37
Metal ion sensing
Fe3+ is an important metal ion in life because of its essential functions in oxygen transport, oxygen metabolism, electronic transfer and many catalytic processes.46,47 Even though, iron has an essential role in living systems, at higher concentrations it shows adverse effects on cells and tissues by readily participating in oxidation–reduction reactions forming reactive oxidative intermediates.48 Hence development of a sensor for sensitive and selective detection of Fe3+ in environmental and biological samples became a matter of considerable interest.To evaluate the ability of CDs synthesized from onion waste as a fluorescent probe for analytical applications, effect of different metal ions on their fluorescence intensity was studied by treating 0.1 mg mL−1 CDs solution with Na+, K+, Mn2+, Ba2+, Fe2+, Cu2+, Sn2+, Cr3+, Al3+, Pb2+, Ni2+, Mg2+, Zn2+, Hg2+, Cd2+, Ca2+, and Fe3+ metal ions each at a concentration of 100 μM. As shown in Fig. 4a only Fe2+, Cu2+, Cr3+, Hg2+, Pb2+, Cd2+, and Ni2+ ions caused a slight decrease in the fluorescence intensity ascribing to the nonspecific interactions of these metal ions with the functional groups of CDs. On the other hand, Fe3+ caused a drastic decrease in the fluorescence intensity of CDs, which makes them highly selective fluorescent probes for Fe3+ ions. This high selectivity can be ascribed to the special coordination between Fe3+ ions and phenolic hydroxyl and/or amine groups of the CDs which has been widely used for the detection of Fe3+ ions or coloured reactions in traditional organic chemistry.49,50 Fluorescence response of CDs towards Fe3+ ions was measured as a function of time and illustrated in Fig. S10A.† After the addition of Fe3+, fluorescence intensity of CDs decreased rapidly and reached a static value within 3 min of interaction and this 3 min time duration is selected as the incubation period for Fe3+ detection and used throughout the assay.
Sensitivity of CDs towards Fe3+ ions was appraised by measuring their fluorescence response to different concentrations (0–80 μM) of Fe3+ ions. Steady decrease in the fluorescence intensity with increase in Fe3+ concentration (Fig. 4b) revealed that the fluorescence intensity of CDs is sensitive to Fe3+ ions. Fig. S10B† represents the relative fluorescence response (F0/F) of CDs as a function of Fe3+ concentration. Stern–Volmer plot was further employed to describe the fluorescence quenching efficiency of Fe3+ ions, which exhibited a good linear correlation (R2 = 0.996) in the concentration range of 0–20 μM (Fig. 4c) and the equation for the same can be depicted as
| F0/F = 0.0952C + 1.036 |
Fluorescence intensity of CDs in the presence and absence of Fe3+ ions at different pH values is shown in Fig. S11.† CDs displayed a very good response to Fe3+ ions (higher quenching efficiency of Fe3+ ions) over a pH range of 4–8, which extends their applicability over this wide pH range. Protonation of CDs and strong interaction of Fe3+ with OH− might be the reasons for the poor response of CDs at low and high pH values respectively.37 Addition of most commonly interfering ions (each at a concentration of 50 μM) along with Fe3+ ions (50 μM) did not cause any significant change in the relative fluorescence of CDs, compared with that of Fe3+ alone (Fig. S12†). These observations indicate that the CDs are highly selective to Fe3+ ions even in the presence of other ions and in the broad pH range, hence can be used for practical applications. As a proof of concept, real water samples (tap and lake water) spiked with 10 μM and 15 μM Fe3+ were also analysed by the same procedure. As shown in Table S1† good recoveries were obtained for both samples.
Cytotoxicity and multicolour imaging
Cytotoxicity studies play a crucial role in determining the aptness of a material for biological applications and low cytotoxicity is the key requirement for an ideal multifunctional biomaterial with the capability to act as bioimaging agent.7,8 Hence, inherent cytotoxicity of CDs was evaluated by measuring the relative viabilities of normal (HEK-293) and cancer (HeLa) cells treated with CDs of different concentrations, using MTT assay. Fig. 5 depicts the viability of HEK-293 and HeLa cells after incubation with CDs at a concentration of 0–1 mg mL−1 for 24 h. As shown in Fig. 5 the viabilities of both normal and cancer cells still remain ≈90% even at a concentration of 1 mg mL−1, which denotes that these CDs are less toxic and highly biocompatible. It is worth mentioning that the CDs did not induce any cytotoxicity (cell viability > 95%) up to a concentration of 0.4 mg mL−1, which is much higher than that required for bioimaging applications. These results indicate that these CDs can serve as potential candidates for both invitro and invivo applications. | ||
| Fig. 5 In vitro cell viability of HEK and HeLa cells treated with various concentrations of CDs as estimated by the MTT assay. | ||
Impressed by their excellent fluorescent properties and high biocompatibility, studies were further extended to demonstrate their bioimaging potential. For this purpose, HeLa cells were first incubated in DME medium containing CDs at a concentration of 200 μg mL−1 for 6 hours at 37 °C, then the cells were washed and imaged under confocal laser scanning microscope. As shown in Fig. 6 the cells were brightly illuminated with multicolours depending on the excitation lasers used, that is blue, green and red coloured emission under the excitation of 405, 488 and 561 nm respectively, while there was no detectable fluorescence emission for control cells (without CDs treatment) under the same exposure conditions. Further observation of these images clearly indicates that the cells retained good morphology and fluorescence emission was mainly confined to the cytoplasmic region leaving the nuclear region feebly stained. This implies that the CDs were highly biocompatible, can be easily internalized and were able to label both cytoplasm and cell membrane. As the size exclusion of nuclear pore limit for the passive diffusion of macromolecules is about 9 nm and only a little fraction of CDs have this size limit, the weak fluorescence emission in the nuclear region could suggest that these CDs entered the nucleus through passive diffusion.54
Taking the advantage of their magnificent imaging potential and high sensitivity to Fe3+ ions, we further employed them for intracellular detection of Fe3+. For this purpose, after 6 h incubation with CDs, HeLa cells were further incubated for 2 h with 0.1 mM Fe3+ ions, washed thrice with PBS and imaged. Similar experiments were performed with 0.1 mM Fe2+ for comparison. As shown in Fig. S13† stronger emission was observed in the presence of Fe2+ ions than that of Fe3+, indicating that Fe3+ could quench the fluorescence of CDs in biological systems also thereby making CDs an efficient probe for intracellular Fe3+ detection. These results confirmed that the CDs synthesized from onion waste can act as potential candidate for cell imaging with multi-coloured emission and can be used for the intracellular detection of Fe3+.
Conclusions
Utilizing onion waste as the precursor and a simple autoclave, we have successfully developed a facile one-step method for the synthesis of fluorescent CDs. As disposal of onion waste is a major environmental problem, our method could offer a solution to this issue besides the production of fluorescent CDs. Utilization of a simple autoclave, waste as the starting material and good production yield (6.06%), makes this method economical and scalable. As synthesized CDs exhibited outstanding properties like high aqueous dispersibility, good chemical and photo stability, strong fluorescence emission with good quantum yield (28%) and excellent biocompatibility. Further they were successfully applied as fluorescent probe for the sensitive and selective detection of Fe3+ ions. Bioimaging studies with HeLa cells revealed their potential as multicolour imaging agents. All these properties make this material a potential candidate for cell imaging, tracking, drug delivery and other biomedical applications and can be used in both invitro and invivo conditions.Acknowledgements
One of the authors (Rajkumar Bandi) gratefully acknowledges CSIR-INDIA for Junior Research Fellowship. The authors would like to thank CDFD Hyderabad for extending their Confocal Laser Scanning Microscope facility. The authors also grateful to SAIF, the DST unit on nanoscience, IIT Madras for providing analytical facilities. Sincere thanks to Department of Chemistry, Osmania University (OU) and CFRD-OU for providing infrastructure and other necessary facilities.Notes and references
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2014, 44, 362 RSC.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921 RSC.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736 CrossRef CAS PubMed.
- K. A. S. Fernando, S. Sahu, Y. Liu, W. K. Lewis, E. A. Guliants, A. Jafariyan, P. Wang, C. E. Bunker and Y.-P. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 8363 CAS.
- X. Li, M. Rui, J. Song, Z. Shen and H. Zeng, Adv. Funct. Mater., 2015, 25, 4929–4947 CrossRef CAS.
- H. Shi, J. Wei, L. Qiang, X. Chen and X. Meng, J. Biomed. Nanotechnol., 2014, 10, 2677 CrossRef CAS PubMed.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- A. L. Himaja, P. S. Karthik and S. P. Singh, Chem. Rec., 2015, 15, 595 CrossRef CAS PubMed.
- D. Wang, X. Wang, Y. Guo, W. Liu and W. Qin, RSC Adv., 2014, 4, 51658 RSC.
- C.-L. Li, C.-M. Ou, C.-C. Huang, W.-C. Wu, Y.-P. Chen, T.-E. Lin, L.-C. Ho, C.-W. Wang, C.-C. Shih, H.-C. Zhou, Y.-C. Lee, W.-F. Tzeng, T.-J. Chiou, S.-T. Chu, J. Cang and H.-T. Chang, J. Mater. Chem. B, 2014, 2, 4564 RSC.
- A.-M. Alam, B.-Y. Park, Z. K. Ghouri, M. Park and H.-Y. Kim, Green Chem., 2015, 17, 3791 RSC.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835 RSC.
- B. De and N. Karak, RSC Adv., 2013, 3, 8286 RSC.
- C. Zhu, J. Zhai and S. Dong, Chem. Commun., 2012, 48, 9367 RSC.
- X. Feng, Y. Jiang, J. Zhao, M. Miao, S. Cao, J. Fang and L. Shi, RSC Adv., 2015, 5, 31250 RSC.
- X. Niu, G. Liu, L. Li, Z. Fu, H. Xu and F. Cui, RSC Adv., 2015, 5, 95223 RSC.
- V. N. Mehta, S. Jha, R. K. Singhal and S. K. Kailasa, New J. Chem., 2014, 38, 6152 RSC.
- P.-C. Hsu, Z.-Y. Shih, C.-H. Lee and H.-T. Chang, Green Chem., 2012, 14, 917 RSC.
- H. Wang, Y. Liu, M. Li, H. Huang, H. M. Xu, R. J. Hong and H. Shen, RSC Adv., 2013, 3, 20662 RSC.
- X. Yang, Y. Zhuo, S. Zhu, Y. Luo, Y. Feng and Y. Dou, Biosens. Bioelectron., 2014, 60, 292 CrossRef CAS PubMed.
- F. Salak, S. Daneshvar, J. Abedi and K. Furukawa, Fuel Process. Technol., 2013, 112, 86 CrossRef CAS.
- V. Benítez, E. Mollá, M. a. Martín-Cabrejas, Y. Aguilera, F. J. López-Andréu, L. a. Terry and R. M. Esteban, Food Bioprocess Technol., 2013, 6, 1979 CrossRef.
- D. Kim, Y. Choi, E. Shin, Y. K. Jung and B.-S. Kim, RSC Adv., 2014, 4, 23210 RSC.
- Y. Liu, Y. Liu, S.-J. Park, Y. Zhang, T. Kim, S. Chae, M. Park and H.-Y. Kim, J. Mater. Chem. A, 2015, 3, 17747–17754 CAS.
- S. Y. Park, H. U. Lee, E. S. Park, S. C. Lee, J. W. Lee, S. W. Jeong, C. H. Kim, Y. C. Lee, Y. S. Huh and J. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 3365 CAS.
- H. U. Lee, S. Y. Park, E. S. Park, B. Son, S. C. Lee, J. W. Lee, Y.-C. Lee, K. S. Kang, M. Il Kim, H. G. Park, S. Choi, Y. S. Huh, S.-Y. Lee, K.-B. Lee, Y.-K. Oh and J. Lee, Sci. Rep., 2014, 4, 4665 Search PubMed.
- M. Xue, M. Zou, J. Zhao, Z. Zhan and S. Zhao, J. Mater. Chem. B, 2015, 3, 6783 RSC.
- Z. Zhang, W. Sun and P. Wu, ACS Sustainable Chem. Eng., 2015, 3, 1412 CrossRef CAS.
- Y. Liang, H. Zhang, Y. Zhang and F. Chen, Anal. Methods, 2015, 7, 7540 RSC.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. a. Marti, T. Hayashi, J. J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844 CrossRef CAS PubMed.
- A. Mewada, S. Pandey, S. Shinde, N. Mishra, G. Oza, M. Thakur, M. Sharon and M. Sharon, Mater. Sci. Eng., C, 2013, 33, 2914 CrossRef CAS PubMed.
- Q.-Q. Shi, Y.-H. Li, Y. Xu, Y. Wang, X.-B. Yin, X.-W. He and Y.-K. Zhang, RSC Adv., 2014, 4, 1563 RSC.
- J. Niu, H. Gao, L. Wang, S. Xin, G. Zhang, Q. Wang, L. Guo, W. Liu, X. Gao and Y. Wang, New J. Chem., 2014, 38, 1522 RSC.
- S.-L. Hu, K.-Y. Niu, J. Sun, J. Yang, N.-Q. Zhao and X.-W. Du, J. Mater. Chem., 2009, 19, 484 RSC.
- A. Mewada, S. Pandey, M. Thakur, D. Jadhav and M. Sharon, J. Mater. Chem. B, 2014, 2, 698 RSC.
- A. Sachdev and P. Gopinath, Analyst, 2015, 140, 4260 RSC.
- A. Sachdev, I. Matai and P. Gopinath, RSC Adv., 2014, 4, 20915 RSC.
- H. Xu, X. Yang, G. Li, C. Zhao and X. Liao, J. Agric. Food Chem., 2015, 63, 6707 CrossRef CAS PubMed.
- Y. Y. Zhang, M. Wu, Y. Q. Wang, X. W. He, W. Y. Li and X. Z. Feng, Talanta, 2013, 117, 196 CrossRef CAS PubMed.
- K. M. Tripathi, A. K. Sonker, S. K. Sonkar and S. Sarkar, RSC Adv., 2014, 4, 30100 RSC.
- B. S. B. Kasibabu, S. L. D'souza, S. Jha, R. K. Singhal, H. Basu and S. K. Kailasa, Anal. Methods, 2015, 7, 2373 RSC.
- J. Feng, W. Wang, X. Hai, Y.-L. Yu and J.-H. Wang, J. Mater. Chem. B, 2015, 4, 387 RSC.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2015, 8, 355 CrossRef CAS.
- S. Khan, A. Gupta, N. C. Verma and C. K. Nandi, Nano Lett., 2015, 15, 8300 CrossRef CAS PubMed.
- T. a. Rouault, Nat. Chem. Biol., 2006, 2, 406 CrossRef CAS PubMed.
- P. Aisen, M. Wessling-Resnick and E. a. Leibold, Curr. Opin. Chem. Biol., 1999, 3, 200 CrossRef CAS PubMed.
- S.-P. Wu, Y.-P. Chen and Y.-M. Sung, Analyst, 2011, 136, 1887 RSC.
- E. F. Wesp and W. R. Brode, J. Am. Chem. Soc., 1934, 56, 1037 CrossRef CAS.
- D. Wang, L. Wang, X. Dong, Z. Shi and J. Jin, Carbon, 2012, 50, 2147 CrossRef CAS.
- Q. Yang, L. Wei, X. Zheng and L. Xiao, Sci. Rep., 2015, 5, 17727 CrossRef CAS PubMed.
- Z. Zhou, M. Zhou, A. Gong, Y. Zhang and Q. Li, Talanta, 2015, 143, 107 CrossRef PubMed.
- Q. Xu, P. Pu, J. Zhao, C. Dong, C. Gao, Y. Chen, J. Chen, Y. Liu and H. Zhou, J. Mater. Chem. A, 2015, 3, 542 CAS.
- N. Zhou, S. Zhu, S. Maharjan, Z. Hao, Y. Song, X. Zhao, Y. Jiang, B. Yang and L. Lu, RSC Adv., 2014, 4, 62086 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/c6ra01669c |
| This journal is © The Royal Society of Chemistry 2016 |