Vinyl esters as effective acetaldehyde surrogates in [4 + 1] cycloaddition based multicomponent cascade†
Manoj Kumar,
Sourav Bagchi and
Anuj Sharma*
Department of Chemistry, Indian Institute of Technology Roorkee, Roorkee-247667, India. E-mail: anujsharma.mcl@gmail.com; anujsfcy@iitr.ac.in; Fax: +91 13 3227 3560; Tel: +91 13 3228 4751
First published on 11th June 2015
Abstract
A new multicomponent cascade has been designed by utilizing vinyl esters as effective acetaldehyde surrogate. While most of the cyclic α-ketoenols underwent a facile three component conversion to annulated furans, phenols such as naphthol and sesamol got simply acylated under the same reaction conditions. This microwave assisted method provides clean access to a wide range of functionalised furans in a short span of time.
Introduction
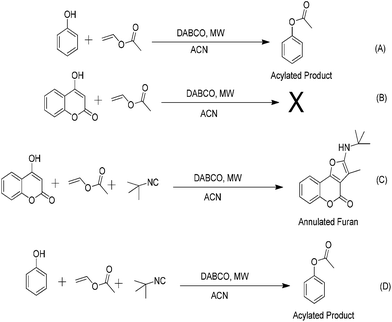
Having both “ester” and “olefinic” functionalities, the chemistry of vinyl esters looks quite impressive.1,2 As an ester, it has been utilized as an innocuous and mild acylating agent for several important transformations and this indeed is the most explored area of its known chemistry (Fig. 1).1 By contrast, there are only few reports concerning its use as an activated olefinic input which have remained limited to Povarov and Markovnikov type additions (Fig. 1).2Interestingly, another very important yet scarcely explored use of vinyl acetate is through its cleavage into an enolate and its subsequent role in synthesis. In fact, nearly all it's acylation reactions are accompanied by simultaneous generation of this “enolate half”.1 Except a couple of reports, the chemistry of this enolate functionality and ensuing aldehyde has remained largely unexplored (Fig. 1).3
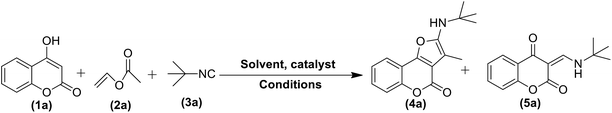
We recently devised a general method for vinyl ester mediated transesterification of phenols under organocatalytic conditions (Fig. 2, Scheme A).4 During this investigation, we found that this otherwise very clean and successful reaction didn't work well with cyclic α-ketoenols and an unidentifiable mixture was obtained in all the cases (Fig. 2, Scheme B). We speculated that unlike phenols, acylation was not privileged with cyclic enols and latent “enolate” rather than “acyl component” might prefer to react with α-acidic ketoenols. Hence, in this case, there were chances to use vinyl ester as an acetaldehyde surrogate rather than an acylating agent. To test this possibility and as a continuation to our previous work, we herein present the first report of organocatalysed 4 + 1 cycloaddition involving α-ketoenols, isocyanides and vinyl acetate as acetaldehyde equivalent under microwave irradiation (Fig. 2, Scheme C).5a–5e
Same conditions on phenols provided transesterification product without any intervention from the isocyanide component (Fig. 2, Scheme D).
Moreover, this work can be posed as an example of “Single Reactant Replacement (SRR)” as suggested by Ganem.6
Results and discussion
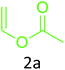
To confirm the feasibility of the above three component condensation, 4-hydroxycoumarin (1a), vinyl acetate (2a) and t-butylisonitrile (3a) were selected as model substrates and reacted together. In preliminary investigation, all the three reactants were resorted to either simple grinding without a solvent, stirring in acetonitrile or heating at varied temperatures, unfortunately, all these methods failed to provide the desired product 4a and mostly the starting material was recovered (Table 1, entries 1–3) in addition to formation of the respective enaminone 5a7 (formed via direct addition of 1a and 3a) upon heating (Table 1, entry 3).| Entry | Catalyst (mol%) | Solvent | Conditions | Yieldb (%) | |
|---|---|---|---|---|---|
| 4a | 5a | ||||
| a General condition: 4-hydroxycoumarine 1a (1 mmol), vinyl acetate 2a (1 mmol), tert-butyl isocyanide 3a (1 mmol). Anton Paar Monowave 300 Microwave reactor, irradiation power: 850 W, ramp time: 1 min. 60 °C.b Isolated yield.c 1.5 mmol of vinyl acetate was used.d 2.5 mmol of vinyl acetate was used. h = hours, min = minutes, MW = microwave conditions. DIPEA = N,N-diisopropylethylamine, DMAP = 4-dimethylaminopyridine, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DABCO = 1,4-diazabicyclo[2.2.2]octane, THF = tetrahydrofuran, IPA = 2-propanol. | |||||
| 1 | Grinding (rt, 1 h) | 0 | Trace | ||
| 2 | CH3CN | Stirring (rt, 5 h) | 0 | Trace | |
| 3 | CH3CN | Heating (60 °C, 10 h) | 0 | 25 | |
| 4 | DABCO (10) | CH3CN | Heating (60 °C, 10 h) | 38 | 16 |
| 5 | DABCO (10) | CH3CN | MW (120 °C, 10 min) | 58 | 22 |
| 6 | Morpholine | CH3CN | MW (120 °C, 10 min) | 42 | 28 |
| 7 | Piperidine | CH3CN | MW (120 °C, 10 min) | 52 | 25 |
| 8 | DBU | CH3CN | MW (120 °C, 10 min) | 38 | 22 |
| 9 | DMAP | CH3CN | MW (120 °C, 10 min) | 42 | 25 |
| 10 | DIPEA | CH3CN | MW (120 °C, 10 min) | 36 | 28 |
| 11 | DABCO (20) | CH3CN | MW (120 °C, 10 min) | 62 | 18 |
| 12 | DABCO (30) | CH3CN | MW (120 °C, 10 min) | 65 | 14 |
| 13 | DABCO (40) | CH3CN | MW (120 °C, 10 min) | 66 | 10 |
| 14 | DABCO (30) | CH3CN | MW (160 °C, 10 min) | 69 | 22 |
| 15 | DABCO (30) | CH3CN | MW (120 °C, 5 min) | 61 | 21 |
| 16 | DABCO (30) | CH3CN | MW (120 °C, 20 min) | 69 | 26 |
| 17 | DABCO (30) | 1,4 Dioxane | MW (120 °C, 10 min) | 61 | 12 |
| 18 | DABCO (30) | Toluene | MW (120 °C, 10 min) | 51 | 28 |
| 19 | DABCO (30) | THF | MW (120 °C, 10 min) | 57 | 21 |
| 20 | DABCO (30) | IPA | MW (120 °C, 10 min) | 69 | 10 |
| 21c | DABCO (30) | IPA | MW (120 °C, 10 min) | 77 | Trace |
| 22d | DABCO (30) | IPA | MW (120 °C, 10 min) | 79 | Trace |
From these initial failures, it was obvious that a catalyst was necessary to assist cleavage of the enol ester and subsequent generation of the enolate. DABCO was picked up as a catalyst of choice based on our recent experience of it being effective for the cleavage of vinyl acetates.
Introduction of DABCO in catalytic amount (10 mol%) in acetonitrile created a marked change in the reaction profile with the formation of a fluorescent compound at a higher Rf alongside enaminone 5a and some un-reacted starting material (Table 1, entry 4).7 To our delight, this fluorescent compound turned out to be the desired furan 4a by spectroscopic analysis. The same reaction worked even better at a higher temperature under microwave conditions (Table 1, entry 5). Not only did the yield improve under microwave irradiation, the compound got crystallized in the reaction vial after cooling, which did not happen under conventional heating. Replacing DABCO with other catalysts like morpholine, piperidine, DBU, DMAP and DIPEA resulted in lower yields of the product 4a (Table 1, entries 6–10). Some further sets of experiments helped realize the fact that sub-stoichiometric loading of DABCO upto 30% and a temperature of 120 °C under microwave irradiation was better than other conditions (Table 1, entries 11–16). While most of the other solvents were found comparable or inferior to acetonitrile, use of isopropanol as solvent further improved the yield of 4a (Table 1, entries 17–20). Finally, an additional amount of vinyl acetate seemed to not only improve the yield, but also suppress the competitive formation of enaminone 5a (Table 1, entries 13 and 21, 22). Hence the optimized condition for the above reaction involved microwave heating of 4-hydroxycoumarin (1a, 1 mmol), vinyl acetate (2a, 1.5 mmol) and t-butylisonitrile (3a, 1 mmol) at 120 °C for 10 minutes in isopropanol using DABCO (30 mol%) as a catalyst.
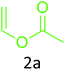
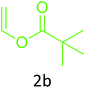
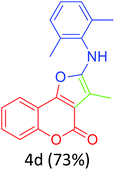
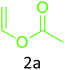
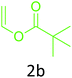
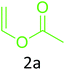
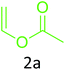
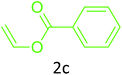
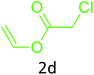
Having achieved optimized conditions, we next decided to investigate the scope and limitations of the reaction (Table 2). A number of C–H acids such as 4-hydroxycoumarins (1a), 1-hydroxy-3H-benzo[f]chromen-3-ones (1b), 2-hydroxy-1,4-naphthoquinone (Lawsone, 1c), 4-hydroxy-6-methylpyrone (1d), 2,5-dihydroxycyclohexa-2,5-diene-1,4-dione (1e), 4-hydroxyquinolin-2-(1H)-one (1f), 5,5-dimethylcyclohexane-1,3-dione (dimedone, 1g), cyclohexane-1,3-dione (1h) and vinyl esters (2a to 2d) and were taken which resulted in a diverse range of products (4a to 4s) as shown in Table 2.
| Serial no. | C–H acids 1(a-h) | Vinyl esters 2(a-d) | Isocyanides 3(a-f) | Products 4(a-s)b |
|---|---|---|---|---|
| a General condition: C–H acid (1 mmol), vinyl ester (1.5 mmol), isocyanide (1 mmol), DABCO (30 mol%), IPA (2 ml) as a solvent. Anton PaarMonowave 300 Microwave reactor, irradiation power: 850 W, ramp time: 1 min. 60 °C, holding time: 10 min 120 °C.b Isolated yield. nd = not determined. | ||||
| 1 |  |
 |
 |
 |
| 2 |  |
 |
 |
 |
| 3 |  |
 |
 |
 |
| 4 |  |
 |
 |
 |
| 5 |  |
 |
 |
 |
| 6 |  |
 |
 |
 |
| 7 |  |
 |
 |
 |
| 8 |  |
 |
 |
 |
| 9 |  |
 |
 |
 |
| 10 | 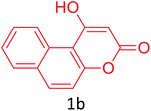 |
 |
 |
 |
| 11 | 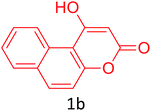 |
 |
 |
 |
| 12 |  |
 |
 |
 |
| 13 |  |
 |
 |
 |
| 14 |  |
 |
 |
 |
| 15 |  |
 |
 |
 |
| 16 |  |
 |
 |
 |
| 17 | 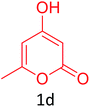 |
 |
 |
 |
| 18 | 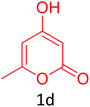 |
 |
 |
 |
| 19 | 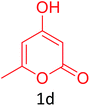 |
 |
 |
 |
| 20 |  |
 |
 |
 |
| 21 |  |
 |
 |
 |
| 22 | 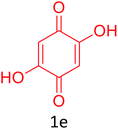 |
 |
 |
 |
| 23 |  |
 |
 |
 |
| 24 | 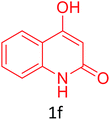 |
 |
 |
 |
| 25 | 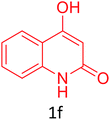 |
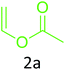 |
 |
 |
| 26 |  |
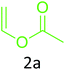 |
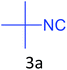 |
 |
| 27 |  |
 |
 |
 |
| 28 | 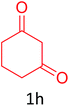 |
 |
 |
 |
The method seemed to be well tolerant to all the reactants. While vinyl pivalate (2b), Vinyl benzoate (2c) were found comparable to vinyl acetate (2a), vinyl chloroacetate (2d) provided higher yield probably because of higher electrophilicity of ester carbonyl.
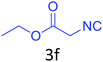
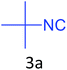
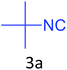
Both cyclic and branched isonitriles such as tert-butylisonitrile (3a), 1,1,3,3-tetramethylbutylisonitrile (3b) and cyclohexylisonitrile (3c) afforded the desired products in moderate to good yield. Aromatic isonitrile such as 2,6-dimethylphenylisocyanide (3d), aralkyl isonitrile such as benzylisonitrile (3e) and α-isocyano ester such as ethyl isocyanoacetate (3f) were also successfully tested.
Unfortunately, in the case of 5,5-dimethylcyclohexane-1,3-dione (dimedone, 1g) and cyclohexane-1,3-dione (1h), the reaction resulted in failures and each time a prospective product spot degraded into an unidentifiable mixture before it was purified (4q to 4s). There are reports of some electron-rich furans to be sensitive towards atmospheric oxidation and it may have happened in our case.8
In order to examine the effectiveness of vinyl acetates as aldehyde component, a comparative experiment was carried out using 4-hydroxycoumarins (1a), acetaldehyde (2a′) and tert-butylisonitrile (3a) under similar set of conditions. The respective product (4a) got formed in relatively smaller amount (55%) after column purification. This result is in contrast to vinyl acetate, where direct deposition of solid product was observed in reaction vial after microwave heating (Fig. 3). The result indicates the advantage of vinyl ester over acetaldehyde for this particular transformation.
A plausible mechanism that is also consistent with the previous literature3b is depicted in Fig. 4. Initial nucleophilic attack from DABCO leads to the cleavage of vinyl acetate to intermediate A and enolate B. Hydrolysis of intermediate results in conversion of B into enol and subsequently to corresponding aldehyde regenerating DABCO in the process. Aldehyde, thus generated undergoes a subsequent three component condensation to an iminolactone D, by a well known [4 + 1] cycloaddition route. This N-substituted iminolactone, by [1,3] proton shift yield the desired product.
We finally explored some other useful transformation to broaden the utility and scope of this interesting chemistry (Fig. 5). After modulating several conditions, in two examples [multicomponent synthesis of 1,8-dioxodecahydroacridines (6a and 6b) and bis(3-hydroxycyclohex-2-enone) 7a], vinyl esters were found equally effective. These results points to the fact that the usefulness of vinyl esters as acetaldehyde surrogate is quite general in nature.
Conclusions
In summary, a new multicomponent cascade has been devised, which depends on enolate functionality arising from vinyl esters. The utility of this reaction was demonstrated by synthesizing a diverse array of functionalized furans. Additional benefits of this microwave assisted method include, direct accumulation of pure product, no need of laborious and time consuming columns, short reaction time and preparative simplicity. The underlying chemistry looks impressive and may have potential applicability in number of other useful transformations.Experimental section
General experimental detail
NMR spectra were recorded on a Jeol Resonance ECX-400II. Chemical shifts are reported in parts per million and are referenced to TMS. Spectra were processed using MestReNova6 software. Mass spectrometry (HRMS) was performed using a Bruker daltronics microTOF-QII® spectrometer using ESI ionization, with less than 5 ppm error for all HRMS analyses. Analytical thin layer chromatography (TLC) was performed on a silica gel plate (Merck® 60F254). IR spectra were done on PerkinElmer FT-IR spectrometer (Spectrum Two). Melting points were performed with Ambassador® and Digital Melting point apparatus (Nutronics), Popular India. All chemicals were purchased from Sigma-Aldrich® and used without further purification.Microwave irradiation experiment
All microwave experiments were carried out in a dedicated Anton Paar Monowave 300 reactor®, operating at a frequency of 2.455 GHz with continuous irradiation power of 0 to 300 W. The reactions were performed in a G10 borosilicate glass vial sealed with Teflon septum and placed in a microwave cavity. Initially, microwave of required power was used and temperature was being ramped from room temperature to a desired temperature. Once this temperature was attained, the process vial was held at this temperature for required time. The reactions were continuously stirred. Temperature was measured by an IR sensor. After the experiments a cooling jet cooled the reaction vessel to ambient temperature.General procedure for the microwave-assisted three component reaction
4-Hydroxycoumarins 1a (1.0 mmol), vinyl acetate 2a (1.5 mmol), isonitrile 3a (1.0 mmol), DABCO (30 mol%) in isopropanol was taken in G10 process vial capped with Teflon septum. After a pre-stirring of 1 or 2 minutes, the vial was subjected to microwave irradiation with the initial ramp time of 1 minute at 70 °C. The temperature was then raised to 120 °C with the holding time of 10 minutes. The reaction mixture was cooled down to 0–5 °C by a cooling air jet. The product got crystallized in the reaction vial, which was then filtered off, in most of the cases, was pure enough for spectral elucidation by 1H NMR, 13C NMR and HRMS.Acknowledgements
This work was financially supported by Department of Science and Technology (DST), New Delhi, Govt. of India (Grant no. SR/FT/CS-55/2011). M. K. and S. B. would like to thank CSIR and MHRD for the award of research fellowship.Notes and references
- (a) J. Otera, Chem. Rev., 1993, 93, 1449 CrossRef CAS; (b) Y. Ishii, M. Takeno, Y. Kawasaki, A. Muromachi, Y. Nishiyama and S. Sakaguchi, J. Org. Chem., 1996, 61, 3088 CrossRef CAS PubMed; (c) A. Orita, A. Mitsutome and J. Otera, J. Org. Chem., 1998, 63, 2420 CrossRef CAS; (d) Y. Shirae, T. Mino, T. Hasegawa, M. Sakamotoa and T. Fujitab, Tetrahedron Lett., 2005, 46, 5877 CrossRef CAS PubMed; (e) M. Trost and T. Mino, J. Am. Chem. Soc., 2003, 125, 2410 CrossRef PubMed; (f) P. Ilankumaran and J. G. Verkade, J. Org. Chem., 1999, 64, 9063 CrossRef CAS; (g) J. W. J. Bosco and A. K. Saikia, Chem. Commun., 2004, 1116 RSC; (h) A. Kamal, M. Naseer, A. Khan, K. Srinivasa Reddy, Y. V. V. Srikanth and T. Krishnaji, Tetrahedron Lett., 2007, 48, 3813 CrossRef CAS PubMed; (i) M.-H. Lin and T. V. R. Babu, Org. Lett., 2000, 2, 997 CrossRef CAS; (j) Y. Kita, H. Maeda, K. Omori, T. Okuno and Y. Tamura, Synlett, 1993, 273 CrossRef CAS; (k) T. Itoh, S. Han, Y. Matsushita and S. Hayase, Green Chem., 2004, 6, 437 RSC; (l) V. Framis, F. Camps and P. Clapés, Tetrahedron Lett., 2004, 45, 5031 CrossRef CAS PubMed; (m) P. M Dinh, J. A Howarth, A. R Hudnott, J. M. J. Williams and W. Harris, Tetrahedron Lett., 1996, 37, 7623 CrossRef.
- (a) N. Isambert, M. Cruz, M. J. Arévalo, E. Gómez and R. Lavilla, Org. Lett., 2007, 21, 4200 Search PubMed; (b) M. Kidwai, N. K. Mishra and A. Jahan, Chin. Chem. Lett., 2011, 22, 417 CrossRef CAS PubMed.
- (a) P. K. Mahata, O. Barun, H. Ila and H. Junjappa, Synlett, 2000, 1345 CAS; (b) V. Nadaraj, S. Kalaivani and S. T. Selvi, Indian J. Chem., 2007, 46B, 1703 CAS.
- M. Kumar, S. Bagchi and A. Sharma, New J. Chem. Search PubMed submitted.
- (a) M. Kumar, T. Kaur, V. K. Gupta and A. Sharma, RSC Adv., 2015, 5, 17087 RSC; (b) V. Nair, R. S. Menon, A. U. Vinod and S. Vijji, Tetrahedron Lett., 2002, 43, 2293 CrossRef CAS; (c) A. Shaabani, M. B. Teimouri, S. Samadi and K. Soleimani, Synth. Commun., 2005, 35, 535 CrossRef CAS; (d) J. Wu, Chem. Lett., 2006, 118 CrossRef; (e) M. Adib, M. Mahdavi, S. Bagherzadeh and H. R. Bijanzadeh, Synlett, 2009, 2542 CrossRef CAS PubMed.
- B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed.
- M. T. Maghsoodlou, N. Hazeri, H.-K. M. Sayyed, V. Solimani, G. Marandi and Z. Razmjoo, J. Chem. Res., 2008, 4, 198 CrossRef.
- M. N. Pennell, R. W. Foster, P. G. Turner, H. C. Hailes, C. J. Tameband and T. D. Sheppard, Chem. Commun., 2014, 50, 1302 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental detail, microwave irradiation experiment, general procedure, 1H, 13C NMR and HRMS spectral data and recorded spectra. See DOI: 10.1039/c5ra10073a |
| This journal is © The Royal Society of Chemistry 2015 |