The electrochemical catalytic behavior of pyrogallol at an 8-hydroxyquinoline-aluminum complex modified carbon paste electrode and its detection in tomato†
Amin Baoa,
Nan Xiaoa,
Yongchun Zhu*a,
Shigang Xinb and
Hongbo Zhangb
aChemistry Department, College of Chemistry and Life Science, Shenyang Normal University, Shenyang 110034, China. E-mail: yongchunzhu@126.com; Tel: +86 024 86593377
bLaboratory Centre, Shenyang Normal University, Shenyang 110034, China
First published on 6th January 2015
Abstract
Tomato (Solanum lycopersicum) fruit is an excellent food for promoting human health and wellbeing due to its containing polyphenols as antioxidants. Pyrogallol among the polyphenols is a very important chemical reagent to be used in detection of peroxidase activity with auto-oxidation behavior. A simple cyclic voltammetric method for determination of pyrogallol in tomato has been developed based on the 8-hydroxyquinoline-aluminum complex modified carbon paste electrode with microextraction and electrochemical catalytic behavior. The amount of pyrogallol in tomato is about 2.8 mg/100 g and recovery is about 84.4%. The standard addition recovery is not stable, with the decrease with time due to the existence of peroxidases in the tomato suspension solution and oxygen from air, which follows a first order reaction with an apparent first order reaction constant of 0.0852 min−1.
Introduction
Pyrogallol is an important kind of polyphenol with a strong reduction property, and has been widely used as a useful antioxidant and scavenger of reactive oxygen species (ROS)1–5 and free radicals.6,7 Free radicals are chemical species possessing an unpaired electron that can be considered as fragments of molecules which are generally very reactive. Oxidative stress is a general term used to describe the steady state level of oxidative damage in a cell, tissue or organ, caused by ROS. Oxidative stress is the cause of many human diseases like diabetes, thyroid disorders, hypertension, arthritis etc.8–10 Antioxidants are compounds which act as inhibitors of the oxidative damage.11,12 Therefore the determination of pyrogallol is very important in chemistry, the environment, the clinic, and biological systems. There are several methods which have been developed for the determination of pyrogallol, such as UV spectrophotometry,13 chromatography,14 chemical luminescence,15,16 electrochemistry.17,18 In all of these methods, the autoxidation property of pyrogallol in aqueous solution must be considered before detection, because it not only changes the molecular form, but also decreases its autoxidation activity. There are several methods had been used to improve the stability and prevent the autoxidation before the measurements, such as extraction19 and adsorption.20 Carbon paste electrode (CPE) is commonly considered as a three-phase system, including of a solid phase of carbon material powder, a liquid phase of organic binder and a liquid phase or aqueous solution as working solution.21 The micro organic binder serves as micro-extraction phase to extract and stabilize pyrogallol from aqueous solution before the electrochemical measurements. The graphite powder serves as the micro solid phase, which can be chemically modified by some catalyst to accelerate the electrochemical reaction. The flexible modifications of CPE further take the advantage of electro-catalytic property in electroanalytical and electrochemical studies.22–26 8-Hydroxyquinoline aluminum complexes are semiconducting materials with unique spherical structure, high stability, film-forming property and good electronic conductivity,27 and have been widely used in the developments of organic electroluminescent devices as electron transfer medium.28–31 Modification of a CPE with 8-hyroxyquinoline-aluminum complex may offer us more chance to study the electrochemical behavior of pyrogallol due to the semiconducting properties, electron transfer and strong interaction of 8-hyroxyquinoline-aluminum.Tomato is a common vegetable of abundance of antioxidants such as vitamin C and other polyphenol moieties,32–34 which serves as reductive species to justify the oxidation stress in tomato.35 Pyrogallol may induce oxidative stress in biological systems.36,37 What will be happened if the pyrogallol is added into tomato system? Whether or not a tomato meat suspension solution can prevent or accelerate pyrogallol autoxidation reaction? In the present paper, graphite powder surface was modified with 8-hydroxyquinoline-aluminum complex, and used to build up a carbon paste electrode together with methyl silicone oil. The modified carbon paste electrode shows an electrochemical catalytic oxidation for pyrogallol. The 8-hydroxyquinoline-aluminum modifier and methyl silicone show a solid phase micro extraction and a liquid phase micro extraction for pyrogallol from aqueous solution, and stabilize the pyrogallol molecule for the electrochemical catalytic oxidation with three oxidation peaks. The extraction dynamics and thermodynamics are also investigated. The amount of pyrogallol in tomato was determined and the dynamics of pyrogallol autoxidation in tomato suspension solution was also monitored. Some interesting results were described here in this paper.
Experimental
Instruments and reagents
All electrochemical experiments were carried out on a CHI620b electrochemical instrument (Chen Hua Instruments Inc., Shanghai, China) with a three-electrode system, including a home-made platinum electrode as the counter electrode, a 8-hydroxyquinoline aluminum complex modified carbon paste electrode as the working electrode and a KCl saturated calomel electrode (Model 232, Thunder Magnetic Equipment Factory, Shanghai, China) as the reference electrode, and all potential reported here was respect to this reference electrode.Polyamide resin (Yan Hai Chemical Co., Tianjin, China), epoxy resin (Star Chemical Resin Factory, Wuxi, China) methyl silicone oil (Dong Ling Fine Chemicals, Shenyang, China) and graphite powder (spectrographic grade pure) all were used for the preparation of basic carbon electrode. 8-Hydroxyquinoline (analytical grade pure) and aluminum chloride (analytical grade pure) were used for the modification of carbon electrode. Potassium chloride (analytical grade pure) was used for the preparation of supporting electrolyte solution. Pyrogallol and concentrated hydrochloric acid were all analytical pure and purchased from Sinopharm Chemical Reagent Co. Ltd., Shenyang. All solutions were prepared with ultrapure water (18.2 M Ω, Billerica Co., USA) for the experiment, and all experiments were carried out at room temperature.
The preparation of basic carbon electrode
The basic carbon electrode was prepared as described in a previous work.22 Graphite powder, epoxy resin and polyamide resin was mixed into paste, filled into a clean glass tube about 6–7 cm in length and 5.0 mm in diameter. A piece of copper wire was inserted into the paste from other end of the tube as electronic lead. The electrode was solidified in air for about 72 hours. After the solidification, the top of the electrode was polished on sand paper, and tipped out some paste from the top of the electrode, and left a cavity about 1 mm depth to hold carbon paste for the working electrode. The prepared carbon electrode with resistance of 10–100 Ω was used as the basic carbon paste electrode in all the experiments.Preparation of 8-hydroxyquinoline-aluminum modified CPE
The 8-hydroxyquinoline-aluminum (Alq) complexes modified carbon paste electrodes were prepared with different molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The molar ratios of the complexes were simply controlled in addition of the initial reactants of aluminum chloride and 8-hydroxyquinoline in the synthesis of the three complexes. For example, the Alq complex with 1
3. The molar ratios of the complexes were simply controlled in addition of the initial reactants of aluminum chloride and 8-hydroxyquinoline in the synthesis of the three complexes. For example, the Alq complex with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was prepared by mixing 1.0 g of graphite powder with 0.50 mL of 3.0 mM of stock solution of 8-hydroxyquinoline with 95% ethanol, and 1.50 mL of 1.0 mM AlCl3 solution with one drop of concentrated HCl stock solution. The mixture solution was heated at about 70–100 °C and stirred to evaporate solvents rapid. After all solvent was evaporated out, the resulted dry powder was cooled naturally at room temperature as the Alq complex modified graphite powder. The Alq modified graphite powder was mixed with methyl silicone oil at mass ratio of 2
1 molar ratio was prepared by mixing 1.0 g of graphite powder with 0.50 mL of 3.0 mM of stock solution of 8-hydroxyquinoline with 95% ethanol, and 1.50 mL of 1.0 mM AlCl3 solution with one drop of concentrated HCl stock solution. The mixture solution was heated at about 70–100 °C and stirred to evaporate solvents rapid. After all solvent was evaporated out, the resulted dry powder was cooled naturally at room temperature as the Alq complex modified graphite powder. The Alq modified graphite powder was mixed with methyl silicone oil at mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on a clean glass plate into a paste with glass bar. The carbon paste was filled into the cavity of the basic carbon electrode, polished on glassy paper into mirror-like surface, and served as the modified carbon paste electrode (modified CPE) in all of this work. After an electrochemical experiment, the paste was clean out from the cavity, and new paste was filled in for the next experiment. In this way, the CPEs can keep in renewable.
1 on a clean glass plate into a paste with glass bar. The carbon paste was filled into the cavity of the basic carbon electrode, polished on glassy paper into mirror-like surface, and served as the modified carbon paste electrode (modified CPE) in all of this work. After an electrochemical experiment, the paste was clean out from the cavity, and new paste was filled in for the next experiment. In this way, the CPEs can keep in renewable.
Tomato sample and preparation of its suspension solution
Tomato meat suspension solution was prepared prior to measurement from a fresh ripened red tomato (Lycopersicon esculentum) bought from a market. The ripened tomato was washed clearly with drinking water. A small piece of tomato meat about 3.0 g was cut off the inside of a tomato with a knife, put into a 50 mL clean beaker, add 10 mL 0.15 M KCl solution, mashed and pulped into a suspension solution with glassy bar.Measurement procedure
The measurements were carried out on a CHI electrochemical instrument with three electrodes (working electrode, counter electrode and reference electrode) in an electrolyte solution including 5.00 mL 0.5 M of potassium chloride solution, 5.00 mL different concentration of pyrogallol solution or tomato suspension solution and some concentrated hydrogen chloride solution to control the solution acidity. Cyclic voltammetric experiments were performed under suitable experimental conditions of initial potential of −0.20 V, high potential of 1.0 V, scan rate in the range of 0.003–0.1 V s−1, sample interval of 0.001 V, quiet time of 2 s and sensitivity of 1.0 μA V−1. After each experiment, the used carbon paste was renewed before the next experiment.Results and discussion
Electrocatalytic oxidation of pyrogallol at modified CPE
Fig. 1A shows a typical cyclic voltammogram of pyrogallol at Alq (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) complex modified carbon paste electrode with three oxidation peaks located at 0.448 V (47.72 μA), 0.520 V (no obvious peak current), and 0.883 V (10.57 μA), and one reduction peak at 0.146 V (4.727 μA, curve 1).
1) complex modified carbon paste electrode with three oxidation peaks located at 0.448 V (47.72 μA), 0.520 V (no obvious peak current), and 0.883 V (10.57 μA), and one reduction peak at 0.146 V (4.727 μA, curve 1).
But at carbon paste electrode without modification, it shows two oxidation peaks located at 0.507 V (31.20 μA), 0.886 V (11.25 μA), and one reduction peak at 0.162 V (2.779 μA, curve 2), while the carbon fiber electrode has no obvious redox peak in the background electrolyte solution as shown in Fig. 1A curve 3. Comparison of the cyclic voltammetric curve 1 and 2, it can be seen that the first oxidation process of pyrogallol at the modified CPE is an obvious electrochemical catalytic oxidation with a great increase in oxidation peak current about 53% and a small negative shift of oxidation potential about 59 mV.
Cyclic voltammetric experiments with different scan rates were performed on the modified and unmodified CPEs. The first oxidation peak currents were plotted against square root of scan rates, the curves were obtained as shown in Fig. 1B. The first oxidation peak currents at modified CPE increase with square root of scan rates with the regression equation of ipa = 10.51 + 127.3v1/2 − 169.9v, R2 = 0.992, SD = 0.48.
While the oxidation peak current at unmodified CPE shows a similar tendency as at the modified CPE with ipa = 8.294 + 108.3v1/2 − 139.6v, R2 = 0.997, SD = 0.23. These relationships between oxidation peak currents and scan rates at CPE and modified CPE indicate that the oxidation process is partly controlled by diffusion and partly controlled by surface. The coefficients of v1/2 and v at the modified CPE are 17.6% and 21.7% larger than those at unmodified CPE, respectively, which means then interaction of pyrogallol molecule with modified CPE is stronger than that with the unmodified CPE, and organic phase micro extraction of the methyl silicone oil greatly prevents the autoxidation of pyrogallol in aqueous solution, and the solid phase micro extraction of the modified graphite powder catalyses the first oxidation step and stabilizes the product of this step. Both of the modified CPE and unmodified CPE show the similar liquid phase micro extraction behavior, but the modified CPE gives out one more oxidation peak current with more negative shift of the first oxidation potential.
The influence of complex ratios
The coordination ratio of Alq complex is an important factor influencing the molecular structure, properties of the modified film, and activity of its modified electrode. The modified CPEs with different molar ratio of Alq complexes were set into the electrolyte solution for the cyclic voltammetric experiments. The obtained cyclic voltammograms were shown in Fig. 2. A typical CV curve of pyrogallol at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio complex modified CPE was the same as described in Fig. 1A. The CV curve of pyrogallol at the 1
1 molar ratio complex modified CPE was the same as described in Fig. 1A. The CV curve of pyrogallol at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio complex modified CPE shows two oxidation peaks at 0.556 V (28.14 μA) and 0.887 V (6.12 μA) and one reduction peak at 0.164 V (2.32 μA) (curve 2 in Fig. 2). The CV curve of pyrogallol at the 1
2 molar ratio complex modified CPE shows two oxidation peaks at 0.556 V (28.14 μA) and 0.887 V (6.12 μA) and one reduction peak at 0.164 V (2.32 μA) (curve 2 in Fig. 2). The CV curve of pyrogallol at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio complex modified CPE shows two oxidation peaks at 0.518 V (20.97 μA) and 0.881 V (5.91 μA) and one reduction peak at 0.165 V (2.42 μA) (curve 3 in Fig. 2). These results indicate that the electrocatalytic activity of pyrogallol at modified CPE with Alq complex in 1
3 molar ratio complex modified CPE shows two oxidation peaks at 0.518 V (20.97 μA) and 0.881 V (5.91 μA) and one reduction peak at 0.165 V (2.42 μA) (curve 3 in Fig. 2). These results indicate that the electrocatalytic activity of pyrogallol at modified CPE with Alq complex in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio is better than other complexes, and gives out highest oxidation peak current and negative oxidation potential shift. As we known, the graphite surface is mainly planar. The planar structural molecules and electron transfer mediator of 1
1 molar ratio is better than other complexes, and gives out highest oxidation peak current and negative oxidation potential shift. As we known, the graphite surface is mainly planar. The planar structural molecules and electron transfer mediator of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Alq complex is more suitable to contact with planar surface, and for the electron transfer from molecule to electrode, so it shows better catalytic activity than others.
1 Alq complex is more suitable to contact with planar surface, and for the electron transfer from molecule to electrode, so it shows better catalytic activity than others.
The influence of the acidity in solution
The acidity of solution greatly influences the electrochemical activity of pyrogallol as well as the catalytic activity of modified electrode. Pyrogallol is likely to take part in auto-oxidation reaction in basic solution,38 but in acidic solution (in pH range of 2.0–8.0), it shows very good electrochemical activity at pH 3.0.38 Peroxidases in tomato have the optimal activity at pH about 8.0.39 So the decrease of solution pH will decrease peroxidase activity and increase the electrochemical activity of pyrogallol. In order to control the solution pH in the range of pH < 3.0, a concentrated hydrogen chloride solution (12 M) was added into the electrolyte solution. Cyclic voltammetric experiments were carried out at different volume of 12 M HCl added into the solution. The acidity in the solution was calculated as pH. The original CV curves for different pH as shown in Fig. S1. (ESI†). The obtained oxidation peak current linear decreases with pH as shown in Fig. 3A with regression equation of ipa = 57.51 − 9.14pH, R2 = 0.982, SD = 0.394 in the range of pH 1.25–0.53, but further decrease of pH the oxidation peak current decreases, so in practice, we used pH = 0.53 as the suitable pH in the following experiments.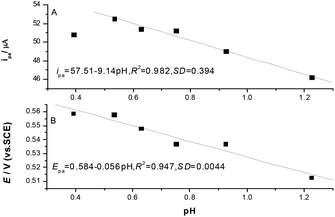 | ||
| Fig. 3 The plots of anodic peak currents (A) and anodic peak potentials (B) against solution pH including 0.5 M KCl and 1.00 mM pyrogallol. | ||
The obtained oxidation peak potential decreases also with solution pH (in Fig.3B) with a regression equation of Epa = 0.584 − 0.056pH, R2 = 0.947, SD = 0.0044.
From the slope of the equation we can see that the oxidation process is companied by proton transfer process, which is similar to the ref. 38.
The influence of initial potential
Initial potential is the potential added to electrode before cyclic voltammetric experiment is performed, which commonly used to test the influence of potential on the electrochemical system. The cyclic voltammetric experiments with deferent initial potentials were carried out as described about. The oxidation peak current obtained in the cyclic voltammograms was plotted against initial potential as shown in Fig. 4. The curve in the initial potential range of −0.40–0.50 V can be regressed into a Bolzman equation as the following.
 | (1) |
 | ||
Fig. 4 The anodic peak currents at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex ratio of Alq modified carbon paste electrode to the added initial potential in the acidity solution containing 0.5 M KCl and 1.00 mM pyrogallol. 1 complex ratio of Alq modified carbon paste electrode to the added initial potential in the acidity solution containing 0.5 M KCl and 1.00 mM pyrogallol. | ||
This regression equation gives some information about the oxidation process. The equilibrium potential of the oxidation reaction is located at about 0.539 V, which is far away from the first oxidation peak potential (0.448 V). The minimum current at equilibrium potential is about −16.57 μA, and the half peak potential about the equilibrium potential is 0.064 V. Negative potential added to the electrode can be prevent the pyrogallol near the electrode oxidized or auto-oxidized before the experiment, but positive potential will induced the auto-oxidation of pyrogallol. In practice, initial potential of −0.20 V was used in the following experiments.
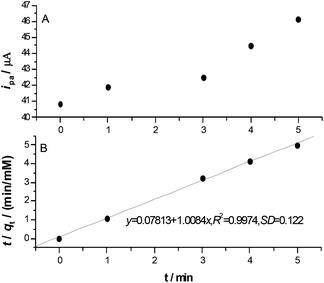
The influence of preconcentration time
The preconcentration time is the time interval from setting electrodes in an electrolyte solution to starting potential scanning, which is also the time interval for reactant diffusion and adsorption at the electrode surface. The cyclic voltammetric experiments were performed at different preconcentration times. The original CV curves for different preconcentration times as shown in Fig. S2. (ESI†). The obtained oxidation peak current was plotted against preconcentration time, and a linear relationship was obtained approximately in the time range of 0–5 min as shown in Fig. 5A. With the increase of preconcentration time longer than 5 min, the system becomes not stable.According to the pseudo-second order adsorption model,39–41 one pyrogallol molecule may occupy two active adsorption sites and transfer electrons with the sites. The dynamic equation41 can be expressed as,
 | (2) |
 | (3) |
The eqn (3) can be integrated from qt = 0 to qe, and time from t = 0 to t, and rewritten as,
 | (4) |
In our case, qt = ipa,t/ipa,f, and ipa,f = ipa,5, so qe = 1.0. The plotting of t/qt against t is a straight line as shown in Fig. 5B. The linear regression equation was obtained as,
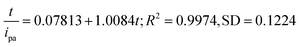 | (5) |
From the slope and intersect of eqn (5), the pseudo-second order rate constant was calculated as k = 12.8 mM−1 min−1. This rate constant is the micro extraction dynamic rate constant, and indicates one pyrogallol molecule adsorbed at two adsorption sites on the modified electrode surface.
The influence of pyrogallol concentration
The cyclic voltammetric experiments were carried out in the electrolyte solution including different concentration of pyrogallol at the modified CPE. The original CV curves for pyrogallol concentration in the range of 0.5–500 μM as shown in Fig. S3. (ESI†). The logarithm of obtained oxidation peak current were linear proportional to logarithm of pyrogallol concentration as shown in Fig. 6 (at the modified and unmodified CPEs).The regression equations is log(ipa, μA) = −0.941 + 1.024![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(c, μM), R2 = 0.996, SD = 0.104 for modified CPE, and log(ipa, μA) = −0.594 + 0.816
log(c, μM), R2 = 0.996, SD = 0.104 for modified CPE, and log(ipa, μA) = −0.594 + 0.816![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(c, μM), R2 = 0.997, SD = 0.051 for unmodified CPE, respectively. This behavior belongs to the Freundlich adsorption kinetic model.42
log(c, μM), R2 = 0.997, SD = 0.051 for unmodified CPE, respectively. This behavior belongs to the Freundlich adsorption kinetic model.42
According to the Freundlich adsorption theory, the amount of adsorption can be expressed as,
 | (6) |
The logarithm form of the eqn (6) becomes,
 | (7) |
The Freundlich constants of Kf and 1/n can be obtained as 0.115 and 1.02 for the modified CPE and 0.255 and 0.816 for the unmodified CPE. Higher the 1/n value, more favorable is the adsorption. These results indicate the pyrogallol molecule has the slightly larger adsorption at modified CPE than at the unmodified CPE. These results further clear show that methyl silicone oil in CPE serves as a liquid–liquid micro extractant and stabilizes the pyrogallol molecule in aqueous solution. The Alq modifier is responsible for the solid phase micro extraction, which occurs before the electrochemical catalytic oxidation of pyrogallol molecule. The Alq modifier stabilizes not only the pyrogallol but also the intermediate of the oxidation process.
The solid phase micro extraction process of pyrogallol on the 8-hydroxyquinoline-aluminum modified carbon electrode may be described as the Scheme 1.
The two Alq complexes on the graphite powder surface interactions and form a two-centre complex, which can weakly interact with two nearby hydroxyl groups of pyrogallol, and leave one hydroxyl to be oxidized to quinone bond firstly. If only one hydroxyl group in pyrogallol molecule interact with modifier, the rest two may be oxidized quickly, and the two oxidation peak mixed together into one, and only shows two oxidation peaks in the cases of modifiers with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molecular ratios.
3 molecular ratios.
The determination of pyrogallol in tomato
The fresh prepared tomato suspension solution was diluted 10 times. 5.0 mL of the diluted suspension solution and then add 5.0 mL 0.5 M KCl and 0.25 mL concentrated HCl were mixed as the measurement solution. The electrode was set up in the solution and performed the cyclic voltammetric experiments under the suitable experimental conditions. Three samples were measured, and the average oxidation peak current was obtained as 0.442 μA (n = 3), the concentration of pyrogallol in the tomato solution was calculated as 3.74 μM. It calculated that the tomatoes contained the content of pyrogallol is 2.8 mg/100 g. The result is much lower than literature result of 16.0 mg/100 g (ref. 33) of total phenolic compounds in tomato.Tomato is abundance of polyphenols,34 but also includes lot of peroxidises.43 Addition of reductant will induce peroxidase and result in auto-oxidation of pyrogallol.35,36 If pyrogallol solution was added into the tomato suspension solution, it will induce peroxidase from tomato meat and induce the auto-oxidation of pyrogallol. In this case, the addition recovery cannot be tested in this suspension tomato solution sample, but kinetics of the auto-oxidation process can be easily to be monitored as described followings.
Recovery of the method was tested with a mixture of 5.00 mL tomato suspension solution, 5.00 mL 0.05 mM pyrogallol solution and 40.00 mL 0.20 M KCl solution. The average current was obtained as 0.693 μA (0.7105, 0.6928, 0.6800, 0.688 μA), and the recovery was calculated as 84.4%. During the experiments we found that the concentration in the mixture solution changes with time, and not got a stable result, that means there are some reactions occur in the solution during the time. In order to insight into the reaction process, the first oxidation peak current of pyrogallol was measured following the method described about with a 5.00 mL mixture solution taken from the mixture solution every 5–10 min. The oxidation peak current measured above is plotted against time, and an exponential decay curve was obtained as shown in Fig. 7. The exponential decay curve was regressed as,
 | (8) |
 | ||
Fig. 7 The oxidation current of pyrogallol at modified CPE with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Alq complex in dilute tomato solution after addition of 1.00 μM pyrogallol with time. 1 Alq complex in dilute tomato solution after addition of 1.00 μM pyrogallol with time. | ||
This is a typical first order reaction dynamic equation, from which the apparent first order rate constant was obtained as 0.0852 min−1. This is the auto-oxidation reaction rate induced by peroxidase in tomato suspension solution and oxygen from air.
Conclusions
A simple electrochemical method for determination of pyrogallol has been developed based on the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Alq complex modified carbon paste electrode with microextraction and electrochemical catalytic behavior, and used for the detection of pyrogallol in tomato. The amount of pyrogallol in tomato is about 2.8 mg/100 g and recovery is about 84.4%. The standard addition recovery is not stable with the decrease with time due to the exist of peroxidases in the tomato suspension solution and oxygen from air, which follows a first order reaction behavior with the apparent first order reaction constant of 0.0852 min−1.
1 Alq complex modified carbon paste electrode with microextraction and electrochemical catalytic behavior, and used for the detection of pyrogallol in tomato. The amount of pyrogallol in tomato is about 2.8 mg/100 g and recovery is about 84.4%. The standard addition recovery is not stable with the decrease with time due to the exist of peroxidases in the tomato suspension solution and oxygen from air, which follows a first order reaction behavior with the apparent first order reaction constant of 0.0852 min−1.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 20875063), the project Foundation of Education Department of Liaoning Province (2004-c022) and the project Foundation of Technology Bureau of Shenyang (2007GX-32).Notes and references
- J. Alanko, A. Riutta, P. Holm, I. Mucha, H. Vapaatalo and T. Metsa-Ketela, Free Radic Biol. Med., 1999, 26, 193–201 CrossRef CAS.
- J. D. Lambert, S. Sang and C. S. Yang, Chem. Res. Toxicol., 2007, 20, 583–585 CrossRef CAS PubMed.
- K. Kondo, M. Kurihara, N. Miyata, T. Suzuki and M. Toyoda, Arch. Biochem. Biophys., 1999, 362, 79–86 CrossRef CAS PubMed.
- L. M. Nutter, Y. Y. Wu, E. O. Ngo, E. E. Sierra, P. L. Gutierrez and Y. J. Abul-Hajj, Chem. Res. Toxicol., 1994, 7, 23–28 CrossRef CAS.
- A. W. Boots, H. Li, R. P. F. Schins, R. Duffin, J. W. M. Heemskerk, A. Bast and G. Haenen, Toxicol. Appl. Pharmacol., 2007, 222, 89–96 CrossRef CAS PubMed.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radical Biol. Med., 1996, 20, 933–956 CrossRef CAS.
- T. Yokozawa, C. P. Chen, E. Dong, T. Tanaka, G. I. Nonaka and I. Nishioka, Biochem. Pharmacol., 1998, 56, 213–222 CrossRef CAS.
- M. Ajitha and K. Rajnarayana, Indian drugs, 2001, 38, 545–552 CAS.
- G. Paolisso and D. Giugliano, International Diabetes Monitor., 1998, 10, 1–5 Search PubMed.
- N. F. Wiernssperger, Diabetes Metab., 2003, 29, 579–585 Search PubMed.
- S. R. Naik, Indian Drugs, 2003, 40, 501–508 Search PubMed.
- J. M. Mates, C. P. Gomez and I. N. Castro, Clin. Biochem., 1999, 32, 595–603 CrossRef CAS.
- M. Mudasir, M. Mugiyanti and N. Hadipranoto, Indos. J. Chem., 2002, 2, 161–166 Search PubMed.
- K. Agrawal, J. G. Ebel, C. Altier and K. Bischoff, J. Vet. Diagn. Invest., 2003, 25, 112–119 CrossRef PubMed.
- K. V. Dyke, M. Sacks and N. Qazi, J. Biolumin Chemilumin., 1998, 13, 339–348 CrossRef.
- N. P. Evmiridis, A. G. Vlessidis and N. C. Thanasoulias, Bioinorg. Chem. Appl., 2007, 2007, 1–10 CrossRef PubMed.
- G. Ziyatdinova, A. Gainetdinova, M. Morozov, H. Budnikova, S. Grazhulene and A. Red'kind, J. Solid State Electrochem., 2012, 16, 127–134 CrossRef CAS PubMed.
- D. Moscone and M. Mascini, Anal. Chim. Acta, 1988, 211, 195–204 CrossRef CAS.
- J. Sangeetha and K. Vijayalakshmi, Int. J. Pharm. Sci. Drug Res., 2011, 3, 116–122 CAS.
- S. Zhong, J. Su, L. Chen, J. Tong, W. Jia, X. Li and H. Zou, Int. J. Electrochem., 2013, 2013, 1–7 CrossRef PubMed.
- J. D. Wadhawan, R. G. Evans and R. G. Compton, J. Electroanal. Chem., 2002, 533, 71–84 CrossRef CAS.
- J. Hao, Y. Zhu and G. Li, Microchim. Acta, 2009, 167, 267–272 CrossRef CAS.
- K. Vytras, I. Švancara and R. Metelka, J. Serb. Chem. Soc., 2009, 74, 1021–1033 CrossRef CAS.
- M. Siswana, K. I. Ozoemena and T. Nyokong, Talanta, 2006, 69, 1136–1142 CrossRef CAS PubMed.
- Z. Zhai, J. Wu, W. Sun and K. Jiao, J. Chin. Chem. Soc, 2009, 56, 561–567 CAS.
- H. Karimi-Maleh, M. Keyvanfard, K. Alizad, M. Fouladgar, H. Beitollahi, A. Mokhtari and F. Gholami-Orimi, Int. J. Electrochem. Sci., 2011, 6, 6141–6150 CAS.
- A. Meyers and M. Weck, Macromolecules, 2003, 36, 1766–1768 CrossRef CAS.
- J. E. Knox, M. D. Halls, H. P. Hratchianz and H. B. Schlegel, Phys. Chem. Chem. Phys., 2006, 8, 1371–1377 RSC.
- P. Desai, P. Shakya, T. Kreouzis and W. P. Gillin, J. Appl. Phys., 2007, 102, 073710 CrossRef PubMed.
- A. Curioni, M. Boero and W. Andreoni, Chem. Phys. Lett., 1998, 294, 263–271 CrossRef CAS.
- J. D. Anderson, E. M. McDonald and P. A. Lee, et al., J. Am. Chem. Soc., 1998, 120, 9646–9655 CrossRef CAS.
- E. Cieślik, A. Gręda and W. Adamus, Food Chem., 2006, 94, 135–142 CrossRef PubMed.
- S. Georgé, F. Tourniaire, H. Gautier, P. Goupy, E. Rock and C. Caris-Veyrat, Food Chem., 2011, 124, 1603–1611 CrossRef PubMed.
- J. J. Evans, Plant Physiol., 1968, 43, 1037–1041 CrossRef CAS PubMed.
- P. Thipyapong and J. C. Steffens, Plant Physiol., 1997, 115, 409–418 CAS.
- Y. K. Gupta, M. Sharma and G. Chaudhary, Methods Find. Exp. Clin. Pharmacol., 2002, 24, 497–500 CrossRef CAS.
- S. Matić, S. Stanić, D. Bogojević, M. Vidaković, N. Grdović, J. Arambašić, S. Dinić, A. Uskoković, G. Poznanović, S. Solujić, M. Mladenović, J. Marković and M. Mihailović, Can. J. Physiol. Pharmacol, 2011, 89, 401–411 CrossRef PubMed.
- P. Feng, S. Wang, W. Su and S. Cheng, J. Chin. Chem. Soc., 2011, 59, 1–8 Search PubMed.
- S. Azizian, J. Colloid Interface Sci., 2004, 276, 47–52 CrossRef CAS PubMed.
- Y. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- Y. Ho, J. Hazard. Mater., 2006, 136, 681–689 CrossRef CAS PubMed.
- A. H. Sulaymon, T. J. Mohammed and J. Al-Najar, Can. J. Chem. Eng. Tech., 2012, 3, 86–92 Search PubMed.
- J. J. Evans, Plant Physiol., 1970, 45, 66–69 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c4ra14842h |
| This journal is © The Royal Society of Chemistry 2015 |