Virus templated metallic nanoparticles†
Alaa A. A.
Aljabali
,
J. Elaine
Barclay
,
George P.
Lomonossoff
and
David J.
Evans
*
Department of Biological Chemistry, John Innes Centre, Norwich Research Park, Colney, Norwich, NR4 7UH, UK. E-mail: dave.evans@bbsrc.ac.uk; Fax: +44 (0)1603 450018
First published on 28th September 2010
Abstract
Plant viruses are considered as nanobuilding blocks that can be used as synthons or templates for novel materials. Cowpea mosaic virus (CPMV) particles have been shown to template the fabrication of metallic nanoparticles by an electroless deposition metallization process. Palladium ions were electrostatically bound to the virus capsid and, when reduced, acted as nucleation sites for the subsequent metal deposition from solution. The method, although simple, produced highly monodisperse metallic nanoparticles with a diameter of ca. ≤35 nm. CPMV-templated particles were prepared with cobalt, nickel, iron, platinum, cobalt–platinum and nickel–iron.
Introduction
Metallic nanoparticles have attracted much interest because of their size-dependent magnetic, optical and electrical properties that give them significant potential for applications such as data storage, catalysts and nanomagnets.1 However, the control over the particles’ size and monodispersity remains a major challenge in nanoscience. Further, hollow metallic nanoparticles may offer enhanced properties, which may be useful in catalysis and as drug delivery vehicles.2 Such nanoparticles in their preparation consume less material and may be cheaper to produce than solid nanoparticles. Bionanotechnology focuses, amongst other things, on developing nanoscale materials using biological building blocks. These are highly organized assemblies with a diverse range of shapes and sizes and include viruses, virus-like particles, ferritin and DNA. One of the most promising areas in bionanoscience is the “bottom-up” synthesis of highly monodisperse metallic nanoparticles based on protein templates.3–5 Further, specific peptide sequences, that allow selective deposition, have been identified for the fabrication of nanoparticles for a large number of useful materials from semiconductors to magnetic materials.6Examples of the use of biological entities in the synthesis of nanomaterials by deposition of metals from solution by direct reduction of the metal onto the biological template include: modification of the surface of Tobacco mosaic virus;7 gold templated onto Chilo iridescent virus;8 templated synthesis of metallic coatings on genetically modified bacteriophage M13.9 Recently, we reported the use of Cowpea mosaic virus (CPMV) chimaeras, that incorporated, in their surface-loops, peptides that promoted specific mineralization processes to prepare virus-templated monodisperse nanoparticles of silica10 and iron–platinum.4 Subsequently, we have shown that genetic modification of the capsid is not required but that it is sufficient to chemically couple the appropriate peptide to the virus surface prior to mineralization.5
CPMV is a bipartite, single stranded RNA plant virus with icosahedral symmetry and a diameter of ca. 28 nm. The capsid comprises 60 copies of two proteins, the large (41 kDa) and small subunit (24 kDa). CPMV is stable in the pH range from 3.5–10 and up to a temperature of 60 °C (pH 7.0) for 1 hour and has a very long shelf-life at room temperature.11 These properties make the virus a robust biological scaffold and it has been used as a platform for genetic (epitope presentation)12 and chemical modification with precise positioning and spacing.13 The virus surface has been chemically decorated with a wide range of molecules including fluorescent dyes,14,15 polyethylene glycol chains,16 peptides,4,10 carbohydrates,17 redox-active molecules15,18 and fullerenes.19
In this work, we extend the use of CPMV as a template for fabricating metallic nanoparticles without any prior genetic or chemical modification to the virus capsid and, importantly, without the need of peptides to act as nucleation sites. Electroless deposition (ELD) is an autocatalytic redox process in which metal ions are chemically reduced to metal in the absence of an external current. ELD has become a commonly used process and allows the production of thin layers of metals and alloys with uniform thickness and composition.20 The metallization of CPMV by pre-activation of the capsid surface with palladium(II) followed by ELD of cobalt, nickel, iron, platinum, cobalt–platinum and nickel–iron at room temperature, resulted in monodisperse metallic nanoparticles by a method that is environmentally friendly, simple and quick.
Experimental
General
Sodium tetrachloropalladate, cobalt(II)chloride, nickel(II)chloride hexahydrate, chloroplatinic acid hexahydrate, dimethylaminoborane, sodium hypophosphite, maleic acid and boric acid were purchased from Sigma-Aldrich; ammonium chloride and iron(II) sulfate from BDH; nickel sulfate from Fisher Scientific; and trisodium citrate dihydrate from Riedel-de Häen. All were used as supplied without further purification.UV-visible spectra were recorded on a Perkin Elmer Lambda 25 spectrometer using UVWINLab software. Transmission electron microscopy (TEM) images were obtained on an FEI Tecnai 20 TEM, FEI UK Ltd, Cambridge, using carbon-coated copper EM grids (400 mesh, Agar Scientific). An Oxford Instruments INCA Energy 200Premium was used for energy dispersive X-ray spectroscopy (EDXS) and a NanoSight VM 10 instrument for nanoparticle tracking analysis (NTA). Samples for NTA were prepared with 300 µl (0.2–0.5 mg ml−1) of metallized-CPMV particles suspended in sodium phosphate buffer (10 mM) pH 7.0 and analysed by recording a 30 second video of the nanoparticles' motion when illuminated by laser light mounted under the microscope objective lens. The particle movement was tracked using conventional CCD camera, operating at 30 frames per second. Dynamic light scattering (DLS) was measured on a DynaPro Titan, Wyatt Technology Corporation. Particles (0.1–0.5 mg ml−1, 13 µl) in sodium phosphate buffer (10 mM) pH 7.0, after passing through 0.1 µm filters, were analysed at 21 °C, and data were recorded from 3 datasets. ζ-Potentials were determined on a Malvern Instruments Zetasizer-Nano ZS. For atomic force microscopy (AFM), Ni-CPMV particles were air dried on a clean slide surface; samples were dialysed against Milli-Q water before drying. Images were taken on an Asylum Research MFP 3D mounted on Olympus I × 71 inverted optical microscope.
For immunological detection of CPMV coat proteins, 10 µg each of wild-type CPMV, Co-CPMV, Fe-CPMV, Ni-CPMV, Pt-CPMV, CoPt-CPMV and NiFe-CPMV in 10 mM phosphate buffer were spotted onto a nitrocellulose membrane (Amersham) and air dried for 30 minutes. Nonspecific binding sites were blocked using a solution consisting of 5% skim-milk powder in phosphate buffered saline (PBS) plus 0.025% (v/v) Tween-20. The membrane was then probed with a polyclonal antibody specific to CPMV (G49) followed by a horseradish peroxidise-conjugated anti-rabbit antibody. Both antibodies were diluted in blocking solution. Signals were generated by chemiluminescence using a SuperSignal West Dura substrate kit (Thermo Scientific) and captured on film (Hyperfilm™ ECL, Amersham Biosciences) which was developed using a Curix 60 film processor (Agfa Gevaert). For further general experimental details see the ESI†.
Pre-activation of CPMV with palladium
CPMV particles (4 mg ml−1, 400 µl) suspended in sodium phosphate buffer (10 mM) pH 7.0 were incubated with 400 µl of 3 mM sodium tetrachloropalladate freshly prepared in Milli-Q water. To prevent hydrolysis of palladate, the reaction pH was adjusted to 3.8, by dropwise addition of HCl (1 mM), and 400 µl of aqueous 1 M NaCl were added. The reaction solution was incubated at ambient temperature with gentle stirring for 30 minutes. The Pd2+-CPMV particles produced were purified on PD-10 desalting columns (GE Healthcare) equilibrated with saline solution (500 mM NaCl in Milli-Q water, pH 5.0), the eluted sample was dialysed on 100 kDa molecular weight cut off membranes (Spectrum Labs) against saline solution for 4 hours. The Pd2+-CPMV particle integrity was established by TEM after uranyl acetate staining. Before the electroless deposition of the desired metals, Pd2+-CPMV has first to be reduced with 5 mM of either dimethylaminoborane (DMAB) or sodium hypophosphite (NaH2PO2). The reaction was allowed to proceed at ambient temperature for 30 minutes to generate Pd0 clusters at the surface, which in turn act as nucleation sites for metal deposition. Pd0-CPMV was washed 3–5 times on 100 kDa cut-off columns (Millipore). The Pd0-CPMV particle integrity was ascertained by TEM, EDXS, agarose gel electrophoresis and ζ-potential measurements.ELD of metal coating
Equal volumes of pre-activated Pd0-CPMV (2–5 mg ml−1) in saline solution (500 mM), aqueous sodium chloride solution (1 M) and plating solution (containing metal salt, reducing agent, ammonium chloride and trisodium citrate, Table 1) were stirred for two to three min at ambient temperature. All plating solutions were used within four days of their preparation. Metallized-CPMV particles were purified as described below.| Metal | Pd0-CPMV/mg ml−1 | Metal salt/100 mM | Reductant/500 mM | NH4Cl | Na3 citrate | pH |
|---|---|---|---|---|---|---|
| Co | 3.2 | CoCl2 | DMAB | 900 mM | 170 mM | 8.0 |
| Fe | 4.5 | FeSO4 | NaH2PO2 | 900 mM | 180 mM | 8.1 |
| Ni | 4.0 | NiCl2 | DMAB | 900 mM | 170 mM | 8.0 |
| Pt | 2.3 | H2PtCl4 | NaH2PO2 | 900 mM | 170 mM | 6.0 |
ELD of mixed metal coating
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v/v mixture of pre-activated Pd0-CPMV (3.5 mg ml−1, 1 ml), suspended in saline solution of pH 5.0, aqueous sodium chloride solution (1 M) and a NiFe plating solution, adapted from that published,21 {comprising nickel sulfate (100 mM), ferrous sulfate (100 mM), sodium hypophosphite (100 mM), trisodium citrate (200 mM), maleic acid (50 mM) and boric acid (200 mM)} at pH 6 was gently stirred, at ambient temperature, for two to four min.
1 v/v/v mixture of pre-activated Pd0-CPMV (3.5 mg ml−1, 1 ml), suspended in saline solution of pH 5.0, aqueous sodium chloride solution (1 M) and a NiFe plating solution, adapted from that published,21 {comprising nickel sulfate (100 mM), ferrous sulfate (100 mM), sodium hypophosphite (100 mM), trisodium citrate (200 mM), maleic acid (50 mM) and boric acid (200 mM)} at pH 6 was gently stirred, at ambient temperature, for two to four min.
Purification of metallized particles
After reaction, solutions were spun at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (bench top, Eppendorf) for 20 minutes to remove any large aggregates, the supernatant was collected and purified on PD-10 columns, and eluted samples were concentrated on 100 kDa cut-off columns before being layered onto 5 ml 10–50% sucrose gradients. The gradients were centrifuged at 137
000 rpm (bench top, Eppendorf) for 20 minutes to remove any large aggregates, the supernatant was collected and purified on PD-10 columns, and eluted samples were concentrated on 100 kDa cut-off columns before being layered onto 5 ml 10–50% sucrose gradients. The gradients were centrifuged at 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 1–1.5 hours at 4 °C; 300 µl fractions were collected and dialysed against 10 mM sodium phosphate buffer of pH 7.0 for 15–18 hours. After further concentration on 100 kDa cut-off columns, the concentration of metallized-CPMV was determined photometrically at 260 nm. Recovery of metallized-CPMV was approximately 50–70% based on the initial virus concentration. Particles were characterised by TEM, EDXS, DLS, NTA, AFM, agarose gel electrophoresis, and immunologically.
000g for 1–1.5 hours at 4 °C; 300 µl fractions were collected and dialysed against 10 mM sodium phosphate buffer of pH 7.0 for 15–18 hours. After further concentration on 100 kDa cut-off columns, the concentration of metallized-CPMV was determined photometrically at 260 nm. Recovery of metallized-CPMV was approximately 50–70% based on the initial virus concentration. Particles were characterised by TEM, EDXS, DLS, NTA, AFM, agarose gel electrophoresis, and immunologically.
Results and discussion
It has been previously reported that amine-functionalised surfaces pre-activated with Pd0 clusters promote the ELD of metals.22,23 Incubation of CPMV particles with a sodium tetrachloropalladate solution, followed by chemical reduction with DMAB or NaH2PO2, at pH 3.8, produced islands of palladium clusters on the virus capsid surface, presumably originating at lysine amine side-chains. These then act as nucleation sites for the subsequent ELD of metal.| Pd2+ + 2e− → Pd0 |
| 3M2+ + Me2NHBH3 + 3H2O → 3M + B(OH)3 + Me2NH2+ + 5H+ |
The virus particles were very stable under the reaction conditions employed; neither reducing agents nor ELD solutions disrupted the virus capsid structure. The integrity of the initially isolated Pd2+-CPMV particles was confirmed by uranyl acetate negatively stained TEM (Fig. 1a). DLS shows no significant changes in the particle diameter. The ζ-potential of −10.6 ± 1.5 mV was similar to that of wild-type (∼−12 mV).
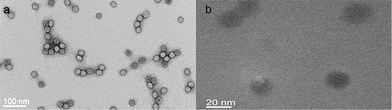 | ||
| Fig. 1 TEM image of (a) Pd2+-CPMV stained with 2% uranyl acetate solution and (b) unstained Pd0-CPMV particles. | ||
On reduction of Pd2+-CPMV, islands of Pd0 clusters distributed over the capsid surface were formed but these did not fully coat the virus. The unstained TEM image for Pd0-CPMV revealed scattered dark clusters on the virion surface (Fig. 1b). Note that only on reduction are the particles visible in the unstained image. On reduction of Pd2+-CPMV to Pd0-CPMV there was no significant change in the particle size (Fig. S1 in the ESI†). EDXS confirmed that palladium is present on the external surfaces of the particles (Fig. S2 in the ESI†). The ζ-potential for Pd0-CPMV particles was considerably more negative (see Table 2 and Fig. S3 in the ESI†) than those for wild-type and Pd2+-CPMV particles; the suspension of Pd0-CPMV particles in buffer is a stable colloid. Further, agarose gel electrophoresis (Fig. S4 in the ESI†) of Pd0-CPMV stained with ethidium bromide showed some fluorescent intensity from a band of different mobility to wild-type, suggesting that the Pd0-CPMV surface has some porosity sufficient to allow intercalation of ethidium bromide with the encapsidated RNA. This is consistent with incomplete coverage of the capsid surface with palladium clusters. When the gel was stained with Coomassie blue, colouration was observed for each of the Pd0-CPMV and the wild-type particles, again consistent with the virus protein surface not being fully coated with palladium.
| CPMVwt diameter/nm | Polydispersity (%) | Metallized particles/nm | Polydispersity (%) | ζ-Potential/mV | |
|---|---|---|---|---|---|
| Ni-CPMV | 27.4 ± 0.4 | 14.1 | 32.4 ± 0.4 | 20.9 | −44.0 ± 2.0 |
| Co-CPMV | 26.8 ± 0.5 | 14.3 | 32.0 ± 0.5 | 13.3 | −41.2 ± 2.5 |
| Pt-CPMV | 27.2 ± 0.5 | 14.0 | 32.6 ± 0.5 | 16.3 | −33.2 ± 2.5 |
| Fe-CPMV | 27.6 ± 0.3 | 9.0 | 31.0 ± 0.3 | 14.0 | −39.7 ± 1.8 |
| NiFe-CPMV | 27.9 ± 0.5 | 10.0 | 31.4 ± 0.5 | 24.6 | −66.3 ± 2.4 |
| CoPt-CPMV | 28.6 ± 0.3 | 12.2 | 34.4 ± 0.3 | 13.6 | −61.7 ± 2.2 |
| Pd0-CPMV | 29.6 ± 0.3 | 13.5 | 30.2 ± 0.1 | 14.7 | −19.0 ± 0.5 |
For the metallization process, the pre-activated Pd0-CPMV particles were dispersed in ELD solutions (Table 1). The reaction time was generally restricted to two minutes and deposition was stopped by washing the particles thoroughly with Milli-Q water. The metallization of CPMV particles was confirmed by TEM (Fig. 2); unstained images reveal dense, monodisperse, metallized nanoparticles of ca. 31–34 nm. Wild-type CPMV cannot be visualised in an unstained TEM image. Control experiments performed under identical conditions, with wild-type CPMV, without CPMV, and without Pd0-CPMV particles, all gave non-specific, visible bulk precipitation of metal with a wide size distribution as observed by TEM. Thus, pre-activation of CPMV is essential for the controlled nanoparticle formation. EDXS (Fig. S5 and S6 in the ESI†) confirmed that cobalt, iron, nickel, and platinum coated the external surfaces of the respective Co-, Fe-, Ni-, Pt-, CoPt- and NiFe-CPMV nanoparticles. The DLS of the particles in buffer (see Fig. S7 and S8 in the ESI†) confirmed the change in the particle size implying that the coating on each particle is approximately 2–3 nm. The DLS polydispersity shows that the particles are monodisperse (Table 2) and that the particles are uniformly metallized on their outer surfaces, in agreement with the observed TEM images.
 | ||
| Fig. 2 Unstained TEM images of (a) Co-CPMV, (b) Fe-CPMV, (c) Ni-CPMV, (d) Pt-CPMV, (e) CoPt-CPMV, and (f) NiFe-CPMV. | ||
The ζ-potential for suspensions of the metallized particles in buffer is considerably more negative than that for wild-type CPMV, and showed that the colloids have good stability (Table 2 and Fig. S9 in the ESI†); the metallized particles are stable in sodium phosphate buffer of pH 7.0 over several months. In addition, the CPMV coat protein of Co-CPMV, Fe-CPMV, Ni-CPMV, Pt-CPMV, CoPt-CPMV and NiFe-CPMV was not detected immunologically (Fig. 3) implying that the coat protein was not accessible to the antibody as it is coated with metal. In addition, when each of the metallized particles were observed by agarose gel electrophoresis with Coomassie blue staining, no protein bands were observed, this is consistent with the virus protein surface not being accessible to the stain as it is coated with metal (Fig. 4). However, when the electrophoretic gel was stained with ethidium bromide, slight fluorescent intensity was observed, in some cases, suggesting that the metal shell may have limited porosity sufficient to allow intercalation of ethidium bromide with the encapsidated RNA.
 | ||
| Fig. 3 Immunological detection of CPMV coat protein of metallized particles spotted on a nitrocellulose membrane probed with polyclonal antibodies raised against CPMV. | ||
 | ||
| Fig. 4 Agarose gel (1.2%) of CPMV particles visualised by ethidium bromide staining (a) and Coomassie blue staining (b). Lane 1, wild-type CPMV; 2, Co-CPMV; 3, Fe-CPMV; 4, Ni-CPMV; 5, Pt-CPMV; 6, CoPt-CPMV; and 7, NiFe-CPMV. | ||
Nanoparticle tracking analysis (NTA, Fig. S10 in the ESI†) was also consistent with metallization of the virus. There was a significant increase in the relative refractive index compared with wild-type particles. The analysis is also consistent with the metallized particles being monodisperse as indicated by the particle size distribution. For Ni-CPMV particles, AFM images confirmed the uniformity of the metallized particles (Fig. S11 in the ESI†).
It has been reported that the use of different reductants, DMAB or hypophosphite, can result in different sized palladium clusters forming on a surface.24 However, we found that there was no significant change in the size of the Pd0-CPMV particles on varying the reductant. However, in the case of metallization with cobalt, for example, the thickness of the metallic coating was dependent on the incubation time with ELD solution, growing from about 2 nm thick after a two minute incubation to 9 nm after 10 minutes (Table 3); hence providing some control over the nanoparticle shell thickness.
| Particle | Diameter/nm | Polydispersity (%) |
|---|---|---|
| CPMVwt | 27.6 ± 0.2 | 9.0 |
| Pd0-CPMV | 30.2 ± 0.2 | 13.9 |
| Co-CPMV (2 minutes) | 32.0 ± 0.5 | 13.3 |
| Co-CPMV (3 minutes) | 37.6 ± 0.2 | 25.1 |
| Co-CPMV (5 minutes) | 39.2 ± 0.3 | 18.5 |
| Co-CPMV (10 minutes) | 46.4 ± 0.5 | 13.3 |
Conclusions
In this work, we have extended the utilisation of the CPMV capsid for templated nanoparticle fabrication. ELD of Co, Ni, Fe, Pt, CoPt and NiFe on Pd0-pre-activated CPMV resulted in the formation of monodisperse, metallic-shells fully coating the virus capsid external surface. The metallization process is simple, quick and efficient and can be performed under conditions that are environmentally friendly.Acknowledgements
The Biotechnology and Biological Sciences Research Council, UK [Core Strategic Grant to the John Innes Centre, D.J.E. and JIC DTG, A.A.A.A.] supported this work. Mike Ridout, Andrew Kirby (Institute of Food Research, Norwich), Richard Evans-Gowing, Bertrand Leze (University of East Anglia), Kim Findlay and Sue Bunnewell (JIC) are thanked for technical assistance.References
- Metal Nanoparticles: Synthesis, Characterization and Applications, ed. D. L. Feldheim and C. A. Foss, Jr, Marcel Dekker, Inc, New York, 2002 Search PubMed; Metallic Nanoparticles (Handbook of Metal Physics, Series ed. P. Misra), ed. J. Blackman, Elsevier, Amsterdam, 2009 Search PubMed.
- S.-W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642–7643 CrossRef CAS; H.-P. Liang, H.-M. Zhang, J.-S. Hu, Y.-G. Guo, L.-J. Wan and C.-L. Bai, Angew. Chem., Int. Ed., 2004, 43, 1540–1543 CrossRef CAS; Y. Vasquez, A. K. Sra and R. E. Schaak, J. Am. Chem. Soc., 2005, 127, 12504–12505 CrossRef CAS; W. Lu, C. Xiong, G. Zhang, Q. Huang, R. Zhang, J. Z. Zhang and C. Li, Clin. Cancer Res., 2009, 15, 876–886 CrossRef CAS.
- M. B. Dickerson, K. H. Sandhage and R. R. Naik, Chem. Rev., 2008, 108, 4935–4978 CrossRef CAS; D. J. Evans, J. Mater. Chem., 2008, 18, 3746–3754 RSC; G. P. Whyburn, Y. Li and Y. Huang, J. Mater. Chem., 2008, 18, 3755–3762 RSC; S. S. Behrens, J. Mater. Chem., 2008, 18, 3788–3798 RSC; I. Yamashita, J. Mater. Chem., 2008, 18, 3813–3820 RSC.
- S. N. Shah, N. F. Steinmetz, A. A. A. Aljabali, G. P. Lomonossoff and D. J. Evans, Dalton Trans., 2009, 8479–8480 RSC.
- A. A. A. Aljabali, S. N. Shah, R. Evans-Gowing, G. P. Lomonossoff and D. J. Evans, Integr. Biol., 2010 Search PubMed , submitted.
- C.-L. Chen and N. L. Rosi, Angew. Chem., Int. Ed., 2010, 49, 1924–1942 CAS.
- M. Knez, A. Kadri, C. Wege, U. Gösele, H. Jeske and K. Nielsch, Nano Lett., 2006, 6, 1172–1177 CrossRef CAS; M. Knez, M. P. Sumser, A. M. Bittner, C. Wege, H. Jeske, D. M. P. Hoffmann, K. Kuhnke and K. Kern, Langmuir, 2003, 20, 441–447; T. L. Schlick, Z. Ding, E. W. Kovacs and M. B. Francis, J. Am. Chem. Soc., 2005, 127, 3718–3723 CrossRef CAS; E. Dujardin, C. Peet, G. Stubbs, J. N. Culver and S. Mann, Nano Lett., 2003, 3, 413–417 CrossRef CAS; R. Tsukamoto, M. Muraoka, M. Seki, H. Tabata and I. Yamashita, Chem. Mater., 2007, 19, 2389–2391 CrossRef CAS; E. Royston, A. Ghosh, P. Kofinas, M. T. Harris and J. N. Culver, Langmuir, 2007, 24, 906–912.
- C. Radloff, R. A. Vaia, J. Brunton, G. T. Bouwer and V. K. Ward, Nano Lett., 2005, 5, 1187–1191 CrossRef CAS.
- C. E. Flynn, S.-W. Lee, B. R. Peelle and A. M. Belcher, Acta Mater., 2003, 51, 5867–5880 CrossRef CAS; C. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, Science, 2004, 303, 213–217 CrossRef CAS; S.-K. Lee, D. S. Yun and A. M. Belcher, Biomacromolecules, 2006, 7, 14–17 CrossRef CAS; Y. Huang, C.-Y. Chiang, S. K. Lee, Y. Gao, E. L. Hu, J. D. Yoreo and A. M. Belcher, Nano Lett., 2005, 5, 1429–1434 CrossRef CAS.
- N. F. Steinmetz, S. N. Shah, J. E. Barclay, J. E. Rallapalli, G. P. Lomonossoff and D. J. Evans, Small, 2009, 5, 813–816 CrossRef CAS.
- G. P. Lomonossoff and J. E. Johnson, Prog. Biophys. Mol. Biol., 1991, 55, 107–137 CrossRef CAS; T. Lin and J. E. Johnson, Adv. Virus Res., 2003, 62, 167–239 CAS; T. Lin, Z. Chen, R. Usha, C. V. Stauffacher, J. B. Dai, T. Schmidt and J. E. Johnson, Virology, 1999, 265, 20–34 CrossRef CAS.
- G. P. Lomonossoff and J. E. Johnson, Curr. Opin. Struct. Biol., 1996, 6, 176–182 CrossRef CAS; G. P. Lomonossoff and W. D. Hamilton, Curr. Top. Microbiol. Immunol., 1999, 240, 177–189 Search PubMed.
- N. F. Steinmetz and D. J. Evans, Org. Biomol. Chem., 2007, 5, 2891–2902 RSC; Viruses and Nanotechnology, in Current Topics in Microbiology and Immunology, ed. M. Manchester and N. F. Steinmetz, Springer-Verlag, Berlin, 2008, vol. 327 Search PubMed; D. J. Evans, Biochem. Soc. Trans., 2009, 37, 665–670 Search PubMed.
- Q. Wang, K. S. Raja, K. D. Janda, T. Lin and M. G. Finn, Bioconjugate Chem., 2003, 14, 38–43 CrossRef CAS; Q. Wang, T. Lin, L. Tang, J. E. Johnson and M. G. Finn, Angew. Chem., Int. Ed., 2002, 41, 459–462 CrossRef CAS; A. Chatterji, W. F. Ochoa, M. Paine, B. R. Ratna, J. E. Johnson and T. Lin, Chem. Biol., 2004, 11, 855–863 CrossRef CAS; N. F. Steinmetz, G. Calder, G. P. Lomonossoff and D. J. Evans, Langmuir, 2006, 22, 10032–10037 CrossRef CAS; F. M. Brunel, J. D. Lewis, G. Destito, N. F. Steinmetz, M. Manchester, H. Stuhlmann and P. E. Dawson, Nano Lett., 2010, 10, 1093–1097 CrossRef CAS.
- N. F. Steinmetz, G. P. Lomonossoff and D. J. Evans, Langmuir, 2006, 22, 3488–3490 CrossRef CAS.
- (a) K. S. Raja, Q. Wang, M. J. Gonzalez, M. Manchester, J. E. Johnson and M. G. Finn, Biomacromolecules, 2003, 4, 472–476 CrossRef CAS; (b) N. F. Steinmetz and M. Manchester, Biomacromolecules, 2009, 10, 784–792 CrossRef CAS.
- K. S. Raja, Q. Wang and M. G. Finn, ChemBioChem, 2003, 4, 1348–1351 CrossRef CAS; E. Kaltgrad, S. S. Gupta, S. Punna, C.-Y. Huang, A. Chang, C.-H. Wong, M. G. Finn and O. Blixt, ChemBioChem, 2007, 8, 1455–1462 CrossRef CAS; A. Miermont, H. Barnhill, E. Strable, X. Lu, K. A. Wall, Q. Wang, M. G. Finn and X. Huang, Chem.–Eur. J., 2008, 14, 4939–4947 CrossRef CAS; R. D. Astronomo, E. Kaltgrad, A. K. Udit, S.-K. Wang, K. J. Doores, C.-Y. Huang, R. Pantophlet, J. C. Paulson, C.-H. Wong, M. G. Finn and D. R. Burton, Chem. Biol., 2010, 17, 357–370 CrossRef CAS.
- N. F. Steinmetz, G. P. Lomonossoff and D. J. Evans, Small, 2006, 2, 530–533 CrossRef CAS.
- N. F. Steinmetz, V. Hong, E. D. Spoerke, P. Lu, K. Breitenkamp, M. G. Finn and M. Manchester, J. Am. Chem. Soc., 2009, 131, 17093–17095 CrossRef CAS.
- Electroless Plating: Fundamentals and Applications, ed. G. Mallory and J. B. Hajdu, American Electroplaters and Surface Finishers Society, Norwich, New York, United States, 1996 Search PubMed; Modern Electroplating, ed. M. Schlesinger and M. Paunowić, John Wiley and Sons, Inc., Canada, 2000 Search PubMed.
- J.-W. Yi, S.-B. Lee, J.-B. Kim and S.-K. Lee, Surf. Coat. Technol., 2010, 204, 1419–1425 CrossRef CAS.
- W. J. Dressick, C. S. Dulcey, J. H. Georger, Jr, G. S. Calabrese and J. M. Calvert, J. Electrochem. Soc., 1994, 141, 210–220 CAS; H. Kind, A. M. Bittner, O. Cavalleri, K. Kern and T. Greber, J. Phys. Chem. B, 1998, 102, 7582–7589 CrossRef CAS.
- J. C. Falkner, M. E. Turner, J. K. Bosworth, T. J. Trentler, J. E. Johnson, T. Lin and V. L. Colvin, J. Am. Chem. Soc., 2005, 127, 5274–5275 CrossRef CAS.
- M. Knez, A. M. Bittner, F. Boes, C. Wege, H. Jeske, E. Maiβ and K. Kern, Nano Lett., 2003, 3, 1079–1082 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental detail, agarose gel electrophoresis results, energy dispersive X-ray spectra, ζ-potential measurements, dynamic light scattering data, nanoparticle tracking analysis and an atomic force microscopy image of Ni-CPMV. See DOI: 10.1039/c0nr00525h |
| This journal is © The Royal Society of Chemistry 2010 |
